Abstract
Recent studies have demonstrated the feasibility of islet implantation into the alveoli. However, until today, there are no data on islet behavior and morphology at their transplant site. This study is the first to investigate islet distribution as well insulin production at the implant site. Using an ex vivo postmortem swine model, porcine pancreatic islets were isolated and aerosolized into the lung using an endoscopic spray-catheter. Lung tissue was explanted and bronchial airways were surgically isolated and connected to a perfusor. Correct implantation was confirmed via histology. The purpose of using this new lung perfusion model was to measure static as well as dynamic insulin excretions following glucose stimulation. Alveolar islet implantation was confirmed after aerosolization. Over 82% of islets were correctly implanted into the intra-alveolar space. The medium contact area to the alveolar surface was estimated at 60 +/− 3% of the total islet surface. The new constructed lung perfusion model was technically feasible. Following static glucose stimulation, insulin secretion was detected, and dynamic glucose stimulation revealed a biphasic insulin secretion capacity during perfusion. Our data indicate that islets secrete insulin following implantation into the alveoli and display an adapted response to dynamic changes in glucose. These preliminary results are encouraging and mark a first step toward endoscopically assisted islet implantation in the lung.
1. Introduction
Type I diabetes mellitus (T1DM) is an autoimmune disorder characterized by the destruction of β-cells of pancreatic Langerhans islets. A variety of secondary diseases can emerge as a result of disrupted insulin secretions and imbalanced glycemic control. For affected patients, human pancreatic islet allografts are a potential curative treatment [1,2]. The limited availability of quality human islets has encouraged attempts to perform islet xenotransplantation [3,4], which requires the use of non-human xenogeneic pancreatic islets donors. With respect to continuous experimental and clinical research on this topic for the past three decades, islet xenotransplantations have delivered promising results in both animal studies and clinical trials [5,6,7,8]. The scientific community has focused on tackling a wide range of challenges when attempting xenograft transplantations in a new host. Some of these challenges include possible interactions with the host’s immune system, endurance and longevity of the graft at its implantation site, vascular supply, and complications during and following transplantations. Additionally, the risk of disease transfer from the graft must be considered, including porcine endogenous retrovirus in the case of swine grafts [9]. This becomes an even greater issue with higher levels of immune suppression.
Moreover, with a wide range of potential implantation sites, it is quite a challenge to choose an optimal location with respect to the aforementioned challenges. As demonstrated in a recent study, islets can potentially be implanted within the alveolar space by means of aerosolization [10]. In recent years, the scientific community has expressed increased interest in aerosol-based and catheter-guided medical and pharmacological applications [11,12,13,14]. Recent studies have demonstrated the capability of aerosol-based applications to carry and deliver complex pharmaceutical particles [15,16,17]. The implantation of islets into the alveolar space can potentially solve some of the challenges clinicians and researchers currently face. One of the most important challenges is hypoxia at the transplant site. In fact, hypoxia has been identified as the most critical factor for survival and function of transplanted islets [18,19,20]. Thus, achieving optimal oxygenation of the transplanted islets is a key factor when developing new transplantation devices harvesting islets or when directly implanting islets at a chosen location [21,22]. Alveolar islet transplantation (AIT) is a new promising concept with the potential to overcome the problem of oxygen deprivation and distance to the vascular network while providing islets with nutritional supply. Until today, specific technical and applicational aspects for the transplantation have barely been studied [10]. By means of a swine lung model, this study aims to analyze islet cell distribution and as well as the extent of insulin production at the transplantation site following AIT.
2. Results
The application of the islets into the lung by means of an endoscopically guided catheter was possible. Both during preparation as well as while connecting the syringe containing the islet cell suspension to the MC, a tendency for islet sedimentation within the syringe was observed. Hence, the injection of the suspension had to be swift to prevent increased cell sedimentation within the syringe. No congestion of the MC nozzle was observed during the injection phase. After aerosolization was completed, thoracotomy was performed and ICG-marked islet-graft bearing lung tissue was explanted for further analyzes.
2.1. Islet Size Distribution and Morphology Following Aerosolization
The size of islets used was grouped into five different cohorts. The percent distribution ranged from 50–100 μm, 100–150 μm, 150–200 μm, 200–250 μm, and 300–350 μm. The total islets within these cohorts were initially (control) 14 ± 3%, 41 ± 7%, 32 ± 11%, 4 ± 0.1%, and 0.7 ± 0.7%. After aerosolization, islet size distribution changed as follows: 27 ± 8%, 45 ± 9%, 18 ± 4%, 7 ± 2%, and 0.00 ± 0.00%, respectively (Figure 1). The control group (8.33 ± 0.12%) had a significantly higher amount of large islets (250–300 μm) compared to aerosolized islets (p < 0.01) and a considerably lower amount of small islets (50–100 μm) (p < 0.05). The total diameter of islets changed in the described manner, and minimal changes in their round shape were observed. Larger aerosolized islets tended to have a more oval shape after aerosolization than islets in the control group. Islet cell equivalence was significantly lower after aerosolization while the total islet cell count was only slightly reduced. This further indicates that some of the islets were fragmented into smaller particles.
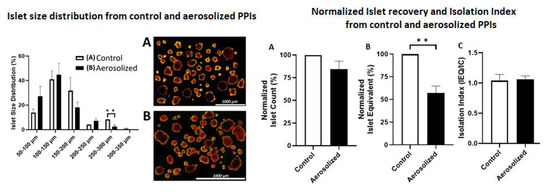
Figure 1.
Size distribution and islet cell morphology before and after aerosolization. Left: Percentual size distribution (diameter in micrometer) of islets before (white column) and after aerosolization (black column). Right: light microscopy of islets before (A) and after (B) aerosolization. ** = p < 0.01.
2.2. Placement of Aerosolized Islets in the Lungs and Structural Alteration following Transplantation
After aerosolization, several areas were collected for further histological analyzes by using the ICG signal as an index. In the histologic analysis, islets were clearly identified within the lung tissue (Figure 2B). Islets were detected at two major sites within the lung tissue (Figure 3A and Figure 4A,B). In the analyzed sections, a total of 84 islets were detected. Most islets (n = 69, 82.2%) were in the intra-alveolar space (Figure 3A). The intra-alveolar site includes both the alveolus cavity as well as the alveolar sac (sacculus). A smaller number of islets (n = 15, 17.8%) was detected in the small bronchiole. No conglomeration of islets was observed at any location. At the bronchial site, we observed a partial structural collapse of six islets. This amount corresponds to 40% of all islets at this location. This finding contrasted with the alveolar site, where only two islets (3%) showed disintegration and collapse. In some cases, implantation at the small bronchiole site resulted in either partial or total obstruction of that bronchiole. This partial or total obstruction was observed in eight islets (53.3%). Most islets (82.2%) were detected in the alveolar cavity as well as the alveolar sacculus. In most of these implantations, islets were at the alveolar site (69.1%). Islets at that location were in close contact with the alveolar wall. The total contact surface with the alveolar wall at the alveolar site was estimated at 60 ± 3% of the total islet diameter. A minority of islets implemented in the alveolar sacs (13.1%) resulted in loose contact with the surrounding tissue wall.
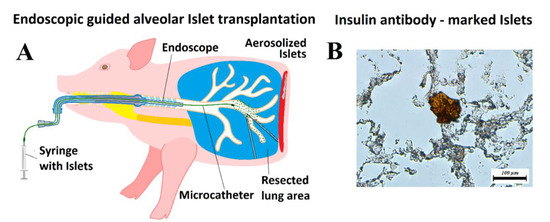
Figure 2.
Islet transplantation. (A) Model of endoscopic guided alveolar islet transplantation (AIT) in a swine model. (B) Histology of lung tissue after AIT with verification of islet cell implantation using anti-insulin antibody-staining (C27C9, Rabbit mAB).
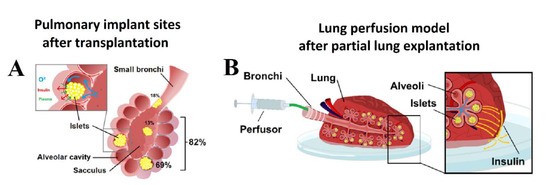
Figure 3.
(A) Pulmonary transplant sites after AIT. Spatial distribution of islets at different locations in percent of total implanted Islets. (B) Pulmonary perfusion model for the detection of insulin production after transplantation. Continuous or pulsatile glucose stimulation can be achieved via alveolar exposure to glucose. The carrier volume (saline) of the glucose transports insulin through the lung tissue passing the visceral pleura where its concentration can be detected.
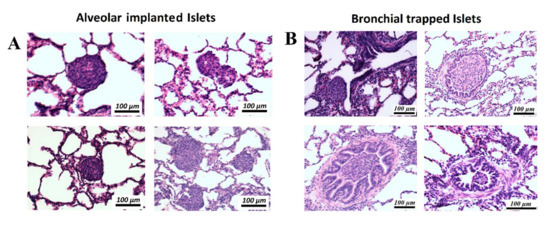
Figure 4.
Histology (hematoxylin and eosin, H&E-staining) of implanted islets in different regions within the lung cavity. (A) Islets are either located in the alveolar cavity or at the center of a sacculus where multiple alveolar structures merge. (B) Islets trapped in different locations within the small bronchial system with partial or complete obstruction of the bronchial airway.
2.3. Static and Dynamic Glucose Stimulation after Transplantation into the Lungs
On average, transplanted islets secreted 1.42 ± 0.36 mU insulin/L in L1 glucose media in the static glucose stimulation test. The amount of insulin secreted increased to 2.12 ± 0.28 mU insulin/L in H glucose media and decreased to 1.48 ± 0.27 mU insulin/L in L2 glucose media. However, this increase in the amount of insulin secreted in H glucose media was not significantly different when compared to either L1 or L2 glucose media (p = NS, Figure 3A). The stimulation index of transplanted islets was 1.53 ± 0.16 (Figure 5A). In the dynamic glucose stimulation test, the transplanted islets in the lungs showed a biphasic glucose stimulated insulin secretion capacity during perfusion (Figure 4A). The total insulin secreted by islets transplanted into the lungs expressed as AUC was significantly higher in H, with 28 mM glucose solution (141.20 ± 18.84 (mU insulin/L) × minutes), compared to the first L1 with 2.8 mM (49.58 ± 6.74 (mU insulin/L) × minutes) and second L2 with 2.8 mM glucose solution (82.86 ± 5.29 (mU insulin/L) × minutes) (p < 0.01, p < 0.05, respectively, Figure 5B).
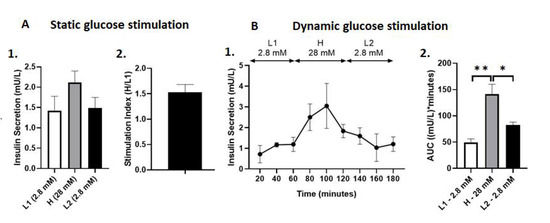
Figure 5.
(A) Static glucose stimulated insulin secretion of PPIs after aerosolization into the lungs. (1) Insulin secretion (mU/L) in glucose media at varying concentrations. (2) Stimulation Index, calculated as the insulin secretion in H media over L1 media. Data are expressed as mean +/− SEM. (B) Dynamic glucose stimulated insulin secretion of PPIs after aerosolization into the lungs. (1) Dynamic insulin secretion (mU/L) throughout 180 min of perfusion assay. (2) Total insulin secretion expressed as AUC. * p < 0.05, ** p < 0.01. Data are expressed as mean +/− SEM.
3. Discussion
3.1. The Delivery of Pharmacological Substances into the Lung Using Aerosolization
The delivery of pharmacological substances into the lung by means of aerosolization is an established concept in pulmonary medicine. Besides topical applications and oral intake, both aerosolization and inhalation represent two of the least invasive options of drug delivery. Therefore, multiple trials have been conducted with the aim to establish the concept of insulin uptake using lung inhalation and absorption as a viable alternative to current methods of insulin delivery [23,24]. However, significant challenges, including fluctuation in insulin uptake, have been observed following each inhalation. The extent of drug absorption depends on many factors, including composition of mucosal tissue and individual patients’ characteristics. The complexity of insulin glucose pathophysiology, including approximating the inhaled dose, mechanisms of absorption, and insulin metabolism within the lung, has impaired efforts to establish inhaled insulin as a real alternative for patients [25,26]. Following a series of studies, the FDA approved Exubera, a product designed for intrapulmonary insulin delivery by Pfizer. However, Pfizer was forced to withdraw the drug from the market due to a variety of associated problems, including its high cost, associated side effects, as well as errors in dosage [27].
3.2. Islet Cell Transplantation into the Lung Using Aerosolization
To the authors’ knowledge, no attempts have been made to investigate actual islet transplantation in the lung by means of aerosolization except for one study which discussed the potential of such a concept [10]. Endoscopically assisted AIT could reduce complication rates, dosing errors, as well as the need for repetitive inhalations as compared to inhaled insulin. At the same time, this concept offers minimal risk for potential complications when compared to conventional surgical transplantations. While bronchoscopy is not completely risk-free, the occurrence of severe complications is still considered rare [28,29]. Aerosolization of islets while preserving their mechanical stability has been previously demonstrated as technically feasible [10]. However, there are no data on the behavior of islets following AIT from an anatomical and physiological perspective. Our preliminary data are encouraging and not only indicate that AIT is feasible, but also confirm the possibility of insulin production, secretion, and diffusion in the alveoli. The next challenge is to find the optimal dosage of islets, which can now be estimated thanks to our data. However, further studies must address remaining questions on the endurance and longevity of transplanted islets. Furthermore, the interaction between lung tissue and islets must be further analyzed. The volume of islets required for transplantation seems rather insignificant compared to the total lung volume. However, some important questions remain unanswered at this point and must be addressed in future studies. These include estimations of the area of the lung occupied by the transplanted cells, as well as the extent of occupied lung tissue required for proper functioning of this concept, insight on how transplanted islets may affect respiratory function, and the amount of islets transplanted to ensure sufficient insulin production. While this concept may cause some unwanted interactions, many benefits could also be expected.
We know that the alveoli contain the highest oxygen levels in the body and could support long-term endurance of the graft. Simultaneously, surface contact with adjacent alveoli is limited as we demonstrated that approximately 60% of the islet surface is in direct contact with the alveoli. Thus, the presented surface antigen is minimally exposed to passing cells from the immune system. Besides, this in-lumen location serves as a partial barrier to the immune system itself since it would require passing cells to enter the alveolar cavity to exert their effects.
4. Material and Methods
4.1. Porcine Pancreatic Islet Isolation and Culture
All animal procedures were performed in accordance with the University of California, Irvine Institutional Animal Care, and followed committee guidelines. On postnatal days 8–15, pre-weaned Yorkshire pigs were used as pancreatic islet donors as previously described [30]. In short, porcine pancreata were procured, isolated for 10 min, and stored in Hank’s balanced Salt solution (HBSS) on ice. The pancreata were subjected to less than one hour of cold ischemia and then chopped into 1 mm3 pieces. Using a Sigma Type V Collagenase (2.5 mg/mL, dissolved in HBBS, Sigma-Aldrich, St. Louis, MO, USA), chopped tissues were digested at 37 °C in a 100 rpm shaking water bath. To quench the digestion, HBSS supplemented with 1% porcine serum (Gibco-Thermo Fisher Scientific, Waltham, MA, USA) was used. Digested tissues were then filtered through a 500 μm metal mesh. After filtration, an islet maturation media composed of 50% Ham’s F-12 medium (Corning Inc., Midland, NC, USA), 50% medium 199 (Corning Inc., Midland, NC, USA), 10 mM HEPES (Sigma-Aldrich, St. Louis, MO, USA), 5 mM L-glutathione (Sigma-Aldrich, St. Louis, MO, USA), 0.6 mL/L ITS+3 (Sigma-Aldrich, St. Louis, MO, USA), 10 mM nicotinamide (Sigma-Aldrich, St. Louis, MO, USA), 100 μg/mL gentamicin sulfate (Corning Inc., Midland, NC, USA), 10 μM Trolox (Sigma-Aldrich, St. Louis, MO, USA), 200 U/L heparin (Sagent Pharmaceuticals, Schaumburg, IL, USA), 0.1 mM pefabloc (Santa Cruz Biotechnology, Santa Cruz, CA, USA), 2 mM L-glutamine (Alfa Aesar, Carlsbad, CA USA), 2.5 mM calcium chloride dihydrate (Fisher Scientific, Waltham, MA, USA), 1000 U/L DNase (Sigma-Aldrich, St. Louis, MO, USA), antibiotic/antimycotic solution (Corning Inc., Midland, NC, USA), and 10% porcine serum (Gibco-Thermo Fisher Scientific, Waltham, MA, USA) were used to culture islets for 7 days in T-150 untreated suspension flasks (Corning Inc., Midland, NC, USA) inside a 37 °C, 5% CO2 humidified incubator (Thermo Forma Series II 3120, Water Jacketed CO2 Incubators, Waltham, MA, USA). One day after culture, a complete media change was performed. On days 3 and 5, a half media change was performed. On day 7, porcine pancreatic islets (PPIs) were collected for further assessment. Due to limitations in organizing and scheduling, islet implantation could not be performed on the same day as islet cell explantation. Therefore, islets were temporarily placed in a maturation medium.
4.2. Microcatheter (MC)
A suspension of islets, dissolved in 10 mL 0.9% saline solution with 0.5 mL indocyanine green dye (ICG, IC-Green-™, Akorn Inc., IL, Buffalo Green, USA), was filled into a sterile syringe. Then, 10 mL of each islet sample was delivered into the small opening of a microcatheter (MC, PW-205V Olympus Surgical Technologies Europe, Hamburg, Germany) with constant flow. The MC has a connecting device that consists of a pressure line that connects the shaft to a nozzle, which has a small opening. Using a 10 mL syringe and high manual pressure (1 mL/s), these pneumatic nebulizers generated a polydisperse aerosol stream out of the nozzle [31,32]. The MC device itself was introduced to an endoscope. It was possible to conduct the aerosolization procedure under visual control. The islet suspension was then aerosolized into the bronchial system using the MC with the described endoscopic support (Figure 2A).
4.3. Islet Recovery and Isolation Index
To determine islet cell count (IC) and equivalence (IEQ), an aliquot of 100 μL IEQ was collected for staining with 1 mL dithizone (DTZ, MP Biomedicals, Solon, OH, USA) for 5 min [30]. A standard stereo microscope (Max Erb, Santa Ynez, USA) attached to a 10× eyepiece granule was used to count the stained islets. Islet recovery was determined based on the percentage of IC and IEQ normalized to control islets without aerosolization [33] (Figure 1). Islet fragmentation was estimated based on the isolation index, which was determined as the ratio of IEQ divided by IC, as previously mentioned [34].
4.4. Islet Transplantation into the Lungs
Three Yorkshire pigs at 30 min postmortem were used for these experiments. They were first placed in a supine position, and an endoscope was inserted into the trachea. The endoscope with the MC was slowly advanced into the right bronchus and subsequently into the bronchial system (Figure 2A). A suspension of islets, which was dissolved into 10 mL 0.9% saline solution with 0.5 mL ICG dye, was aerosolized into the bronchial system. After aerosolization was completed, thoracotomy was performed and ICG-marked islet-graft bearing lung tissue was explanted. Part of the explanted lung was used to verify implantation using an insulin antibody (Cell Signaling Technology, Danvers, MA, USA) (Figure 2B). After confirming the presence of islets within the deep lung tissue, further histologic analyses were conducted using hematoxylin and eosin (H&E) staining to analyze the distribution of implanted islets within the tissue.
4.5. Histological Analysis
To further investigate the implantation sites following islet cell transplantation, a part of ICG-marked lung tissue was subjected to histological analysis. The tissue samples with transplanted islets were fixed for 48 h in 10% neutral buffered formalin. Formalin-fixed samples were prepared for paraffin processing by serial dehydration in increasing concentrations of ethanol solution using a tissue processor (Leica TP1020, Leica Microsystems, Buffalo Grove, IL, USA). After preparation, tissues were embedded in paraffin wax using a tissue embedder (Leica EG 1150C, Leica Microsystems, Buffalo Grove, IL, USA). Paraffin-embedded tissue blocks were cut into 5 μm thin sections using a microtome (Leica RM 2255, Leica Microsystems, Buffalo Grove, IL, USA). The 5 μm sections were stained with hematoxylin and eosin (H&E) according to standard protocol. Slides were imaged using an inverted microscope (Nikon Ti-E Widefield microscope, Nikon Instruments Inc., Melville, NY, USA).
4.6. The Lung Perfusion Model
A resected lung area with islets marked with ICG was placed in a Petri dish with a thin layer of physiological saline solution. The bronchial branch of the resected area was tightly connected to a small tube attached to a perfusor. Lungs were placed in a perfusion chamber at 37 °C and 5% CO2. An initial volume as physiological saline solution was applied to ensure there was no leakage from any damaged bronchial branch distal to the main branch. After leaving the implanted islets to rest in the lung tissue for 60 min, a glucose solution was filled into the perfusor. Both static and dynamic insulin secretion tests were conducted.
4.7. Static Glucose Stimulated Islet Insulin Secretion
A glucose-stimulated insulin release (GSIR) assay was used to determine the insulin secretion of transplanted islets [30] in the following order of glucose media: low glucose (2.8 mM; L1), high glucose (28 mM; H), second low glucose (2.8 mM; L2), and high glucose plus 3-isobutyl-1-methylxanthine (28 mM + 0.1 mM IBMS; H+). The fluid medium in the Petri dish was continuously monitored to detect insulin concentrations at any given time. A standard porcine insulin enzyme-linked immunosorbent assay (Porcine Insulin ELISA, Mercodia, Winston Salem NC, USA) was used to quantify the insulin concentration from the supernatant of the medium and then analyzed on a microplate reader (Tecan Infinite F200, Tecan, Morrisville, NC, USA). As such, dividing the amount of insulin in the high glucose media over the first low glucose media was used to calculate the stimulation index (SI).
4.8. Dynamic Glucose Stimulated Islet Insulin Secretion
Dynamic perfusion assay experiments were performed based on previous studies [35,36]. A syringe pump was used to circulate glucose media at a rate of 0.2 mL/min. Islet-graft bearing lungs were stabilized with 2.8 mM glucose media for 45 min and then exposed to the following sequence of glucose media: 2.8 mM glucose media (L1—2.8 mM) for 60 min, 28 mM glucose media (H—2.8 mM) for 60 min, and 2.8 mM glucose media again (L2—2.8 mM) for 60 min. Serial perifusate samples were collected every 20 min and analyzed for insulin concentration. The area under the curve (AUC) was used to calculate the total insulin secretion from each glucose media concentration [37].
4.9. Statistical Analysis
All data are presented as mean ± standard error of mean (SEM). Statistical significance was evaluated using an unpaired two-sample Student’s t-test. p-values < 0.05 were considered statistically significant. GraphPad Prism (GraphPad Software 8.0.1, GraphPad Inc., La Jolla, CA, USA) was used to analyze all data.
5. Conclusions
This study is the first to provide significant data on AIT procedures. However, many questions on the behavior and efficacy of islets at the transplantation site remain unanswered, impacting the planning of any clinical trials. Further studies, as well as in vivo experiments, are required to assess the biological efficacy of this method with regards not only to endurance and longevity, but also associated complications of this novel concept.
Author Contributions
H.L.: Study design, laboratory analysis, and data acquisition. T.K.: Study design, data analysis, manuscript drafting, and submission. S.L.: Study design, data acquisition, and manuscript drafting. M.A.: Study design, laboratory analysis, data acquisition, and manuscript drafting. P.F.: Data interpretation and critical revision for important intellectual content of the manuscript. S.S.: Data interpretation and critical revision for important intellectual content of the manuscript. M.K.L.: Study design, data interpretation, graphic design, and manuscript drafting. J.R.T.L.: Supervision of the study, drafting, and critical revision for important intellectual content of the manuscript. W.K.: Supervision of the study, drafting, and critical revision for important intellectual content of the manuscript. V.K.: Supervision of the study, drafting and critical revision for important intellectual content of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research, authorship, or publication of this article.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of California, Irvine (Approval Case number AUP-17-241).
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant and supporting data is presented within the manuscript.
Acknowledgments
We thank the Department of Surgery at UC Irvine for their support.
Conflicts of Interest
The authors declare no potential conflict of interest with respect to research, authorship, or publication of this article.
Ethics Approval
This study was approved by our institutional review board (IACUC-Approval case number AUP-17-241).
Statement of Human and Animal Rights
This article does not contain any studies with human subjects.
Abbreviations
| AIT | Alveolar Islet Transplantation |
| HBBS | Hank’s balanced Salt solution |
| H&E | Hematoxylin and Eosin |
| IC | Islet count |
| ICG | Indocyanine green |
| IEQ | Islet equivalence |
| MC | Microcatheter, PW-205V Olympus |
| PPI | Porcine pancreatic islets |
References
- Shapiro, A.M.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.; Pokrywczynska, M.; Ricordi, C. Clinical pancreatic islet transplantation. Nat. Rev. Endocrinol. 2017, 13, 268–277. [Google Scholar] [PubMed]
- Dufrane, D.; Gianello, P. Pig islet xenotransplantation into non-human primate model. Transplantation 2008, 86, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Soto-Gutierrez, A.; Wertheim, J.A.; Ott, H.C.; Gilbert, T.W. Perspectives on whole-organ assembly: Moving toward transplantation on demand. J. Clin. Investig. 2012, 122, 3817–3823. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, P.O.; Espes, D.; Sedigh, A.; Rotem, A.; Zimerman, B.; Grinberg, H.; Goldman, T.; Barkai, U.; Avni, Y.; Westermark, G.T.; et al. Transplantation of macroencapsulated human islets within the bioartificial pancreas betaAir to patients with type 1 diabetes mellitus. Am. J. Transplant. 2018, 18, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Rickels, M.R.; Robertson, R.P. Pancreatic Islet Transplantation in Humans: Recent Progress and Future Directions. Endocr. Rev. 2019, 40, 631–668. [Google Scholar] [CrossRef]
- O’Connell, P.J.; Cowan, P.J.; Hawthorne, W.J.; Yi, S.; Lew, A.M. Transplantation of xenogeneic islets: Are we there yet? Curr. Diab. Rep. 2013, 13, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.T.; Wang, W.L.; Yu, L.; Wang, B. Pig-islet xenotransplantation: Recent progress and current perspectives. Front. Surg. 2014, 1, 7. [Google Scholar] [CrossRef]
- Fishman, J.A. Infection in xenotransplantation. BMJ 2000, 321, 717–718. [Google Scholar]
- Lau, H.; Khosrawipour, T.; Alexander, M.; Li, S.; Mikolajczyk, A.; Nicpon, J.; Schubert, J.; Bania, J.; Lakey, J.R.T.; Khosrawipour, V. Islet Transplantation in the Lung via Endoscopic Aerosolization: Investigation of Feasibility, Islet Cluster Cell Vitality, and Structural Integrity. Cell Transplant. 2020, 29, 963689720949244. [Google Scholar] [CrossRef]
- Schubert, J.; Khosrawipour, V.; Chaudhry, H.; Arafkas, M.; Knoefel, W.T.; Pigazzi, A.; Khosrawipour, T. Comparing the cytotoxicity of taurolidine, mitomycin C, and oxaliplatin on the proliferation of in vitro colon carcinoma cells following pressurized intra-peritoneal aerosol chemotherapy (PIPAC). World J. Surg. Oncol. 2019, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Khosrawipour, T.; Schubert, J.; Khosrawipour, V.; Chaudhry, H.; Grzesiak, J.; Arafkas, M.; Mikolajczyk, A. Particle stability and structure on the peritoneal surface in pressurized intra-peritoneal aerosol chemotherapy (PIPAC) analyzed by electron microscopy: First evidence of a new physical concept for PIPAC. Oncol. Lett. 2019, 17, 4921–4927. [Google Scholar]
- Mikolajczyk, A.; Khosrawipour, V.; Schubert, J.; Grzesiak, J.; Chaudhry, H.; Pigazzi, A.; Khosrawipour, T. Effect of Liposomal Doxorubicin in Pressurized Intra-Peritoneal Aerosol Chemotherapy (PIPAC). J. Cancer 2018, 9, 4301–4305. [Google Scholar] [CrossRef] [PubMed]
- Khosrawipour, T.; Schubert, J.; Kulas, J.; Migdal, P.; Arafkas, M.; Bania, J.; Khosrawipour, V. Creating nanocrystallized chemotherapy: The differences in pressurized aerosol chemotherapy (PAC) via intracavitary (IAG) and extracavitary aerosol generation (EAG) regarding particle generation, morphology and structure. J. Cancer 2020, 11, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk, A.; Khosrawipour, V.; Schubert, J.; Chaudhry, H.; Pigazzi, A.; Khosrawipour, T. Particle Stability During Pressurized Intra-peritoneal Aerosol Chemotherapy (PIPAC). Anticancer Res. 2018, 38, 4645–4649. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk, A.; Khosrawipour, V.; Schubert, J.; Plociennik, M.; Nowak, K.; Fahr, C.; Chaudhry, H.; Khosrawipour, T. Feasibility and Characteristics of Pressurized Aerosol Chemotherapy (PAC) in the Bladder as a Therapeutical Option in Early-stage Urinary Bladder Cancer. In Vivo 2018, 32, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Khosrawipour, V.; Mikolajczyk, A.; Paslawski, R.; Plociennik, M.; Nowak, K.; Kulas, J.; Arafkas, M.; Khosrawipour, T. Intrathoracic aerosol chemotherapy via spray-catheter. Mol. Clin. Oncol. 2020, 12, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Henriksnäs, J.; Svensson, J.; Carlsson, P.O. Oxygenation of islets and its role in transplantation. Curr. Opin. Organ. Transplant. 2009, 14, 688–693. [Google Scholar] [CrossRef]
- Komatsu, H.; Kandeel, F.; Mullen, Y. Impact of Oxygen on Pancreatic Islet Survival. Pancreas 2018, 47, 533–543. [Google Scholar] [CrossRef]
- Komatsu, H.; Cook, C.; Wang, C.H.; Medrano, L.; Lin, H.; Kandeel, F.; Tai, Y.C.; Mullen, Y. Oxygen environment and islet size are the primary limiting factors of isolated pancreatic islet survival. PLoS ONE 2017, 12, e0183780. [Google Scholar]
- Juang, J.H.; Hsu, B.R.; Kuo, C.H. Islet transplantation at subcutaneous and intramuscular sites. Transplant. Proc. 2005, 37, 3479–3481. [Google Scholar] [CrossRef] [PubMed]
- Delaune, V.; Berney, T.; Lacotte, S.; Toso, C. Intraportal islet transplantation: The impact of the liver microenvironment. Transpl. Int. 2017, 30, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Fuso, L.; Pitocco, D.; Incalzi, R.A. Inhaled insulin and the lung. Curr. Med. Chem. 2007, 14, 1335–1347. [Google Scholar] [CrossRef]
- Setji, T.L.; Hong, B.D.; Feinglos, M.N. Technosphere insulin: Inhaled prandial insulin. Expert Opin. Biol. Ther. 2016, 16, 111–117. [Google Scholar] [CrossRef]
- Yeung, S.; Traini, D.; Lewis, D.; Young, P.M. Dosing challenges in respiratory therapies. Int. J. Pharm. 2018, 548, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, M. Insulin disposition in the lung following oral inhalation in humans: A meta-analysis of its pharmacokinetics. Clin. Pharmacokinet. 2004, 43, 539–552. [Google Scholar] [CrossRef]
- Henkin, R.I. Inhaled insulin-intrapulmonary, intranasal, and other routes of administration: Mechanisms of action. Nutrition 2010, 26, 33–39. [Google Scholar] [CrossRef]
- Costa, A.S.; Scordamaglio, P.R.; Suzuki, I.; Palomino, A.L.M.; Jacomelli, M. Indications, clinical outcomes and complications of 1,949 flexible bronchoscopies. Einstein 2018, 16, eAO4380. [Google Scholar] [CrossRef]
- Ergan, B.; Nava, S. The use of bronchoscopy in critically ill patients: Considerations and complications. Expert Rev. Respir. Med. 2018, 12, 651–663. [Google Scholar] [CrossRef]
- Lamb, M.; Laugenour, K.; Liang, O.; Alexander, M.; Foster, C.E.; Lakey, J.R. In vitro maturation of viable islets from partially digested young pig pancreas. Cell Transplant. 2014, 23, 263–272. [Google Scholar] [CrossRef]
- Khosrawipour, V.; Mikolajczyk, A.; Schubert, J.; Khosrawipour, T. Pressurized Intra-peritoneal Aerosol Chemotherapy (PIPAC) via Endoscopical Microcatheter System. Anticancer Res. 2018, 38, 3447–3452. [Google Scholar] [CrossRef] [PubMed]
- Göhler, D.; Khosrawipour, V.; Khosrawipour, T.; Diaz-Carballo, D.; Falkenstein, T.A.; Zieren, J.; Stintz, M.; Giger-Pabst, U. Technical description of the microinjection pump (MIP®) and granulometric characterization of the aerosol applied for pressurized intraperitoneal aerosol chemotherapy (PIPAC). Surg. Endosc. 2017, 31, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.; Corrales, N.; Alexander, M.; Mohammadi, M.R.; Li, S.; Smink, A.M.; de Vos, P.; Lakey, J.R.T. Necrostatin-1 supplementation enhances young porcine islet maturation and in vitro function. Xenotransplantation 2020, 27, e12555. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, D.; Iken, M.; Brendel, M.D.; Bretzel, R.G.; Brandhorst, H. Successful pancreas preservation by a perfluorocarbon-based one-layer method for subsequent pig islet isolation. Transplantation 2005, 79, 433–437. [Google Scholar] [CrossRef]
- Buchwald, P.; Tamayo-Garcia, A.; Manzoli, V.; Tomei, A.A.; Stabler, C.L. Glucose-stimulated insulin release: Parallel perifusion studies of free and hydrogel encapsulated human pancreatic islets. Biotechnol. Bioeng. 2018, 115, 232–245. [Google Scholar] [CrossRef]
- Henquin, J.C.; Nenquin, M. Dynamics and Regulation of Insulin Secretion in Pancreatic Islets from Normal Young Children. PLoS ONE 2016, 11, e0165961. [Google Scholar]
- Hui, H.; Khoury, N.; Zhao, X.; Balkir, L.; D’Amico, E.; Bullotta, A.; Nguyen, E.D.; Gambottl, A.; Perfetti, R. Adenovirus-Mediated XIAP Gene Transfer Reverses the Negative Effects of Immunosuppressive Drugs on Insulin Secretion and Cell Viability of Isolated Human Islets. Diabetes 2005, 54, 424. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).