Luteinizing Hormone Regulation of Inter-Organelle Communication and Fate of the Corpus Luteum
Abstract
1. Introduction
2. Molecular Changes Occurring during Corpus Luteum Formation
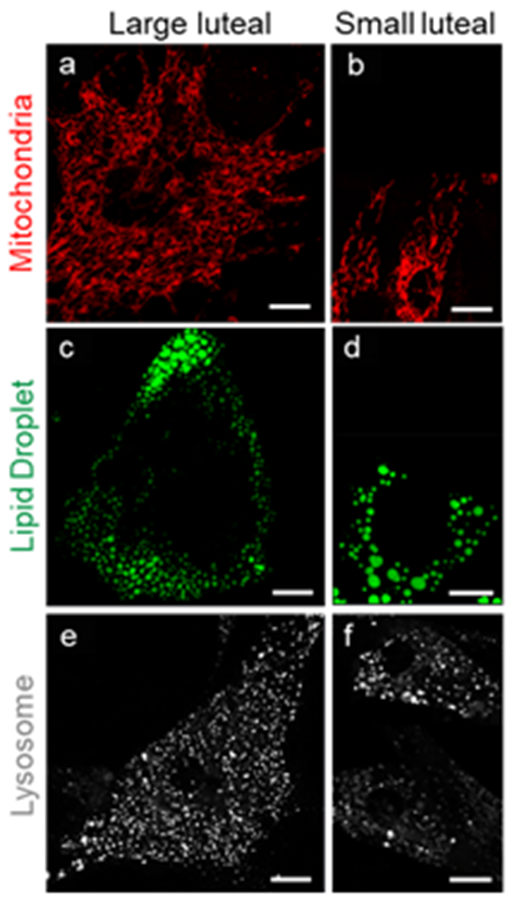
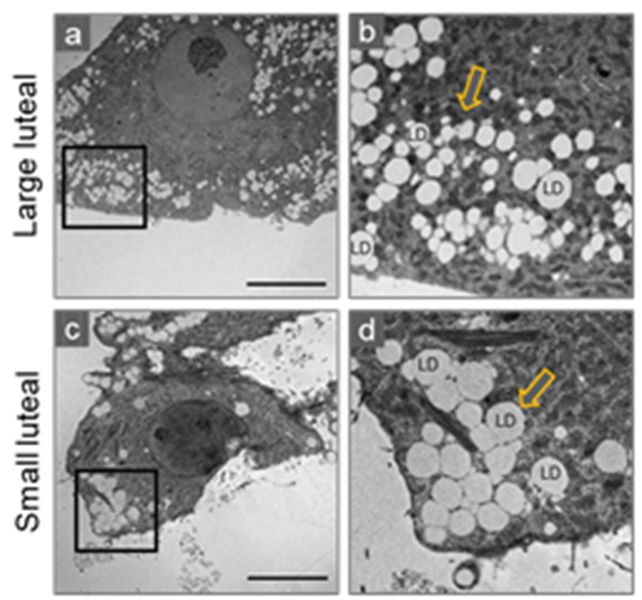
3. Morphological Characteristic of Small and Large Luteal Cells
4. Organelle Communication: Perspectives on Mitochondria, Endoplasmic Reticulum, Lipid Droplets, and Lysosomes in the Maintenance of Luteal Function
4.1. Mitochondria
4.2. Mitochondrial Associated Membranes
4.3. Mitochondria-Actin
4.4. Endoplasmic Reticulum (ER)
Lipid Droplets
4.5. Lipid Droplets and Mitochondria: Peri-Droplet Mitochondria
4.6. Lysosomes
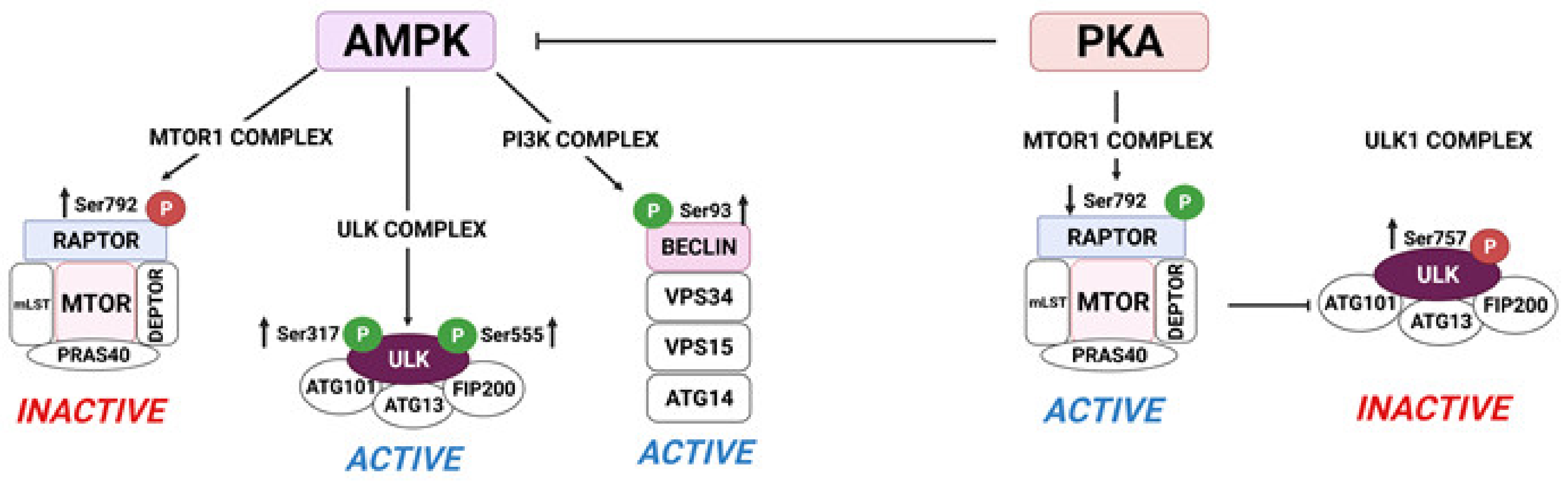
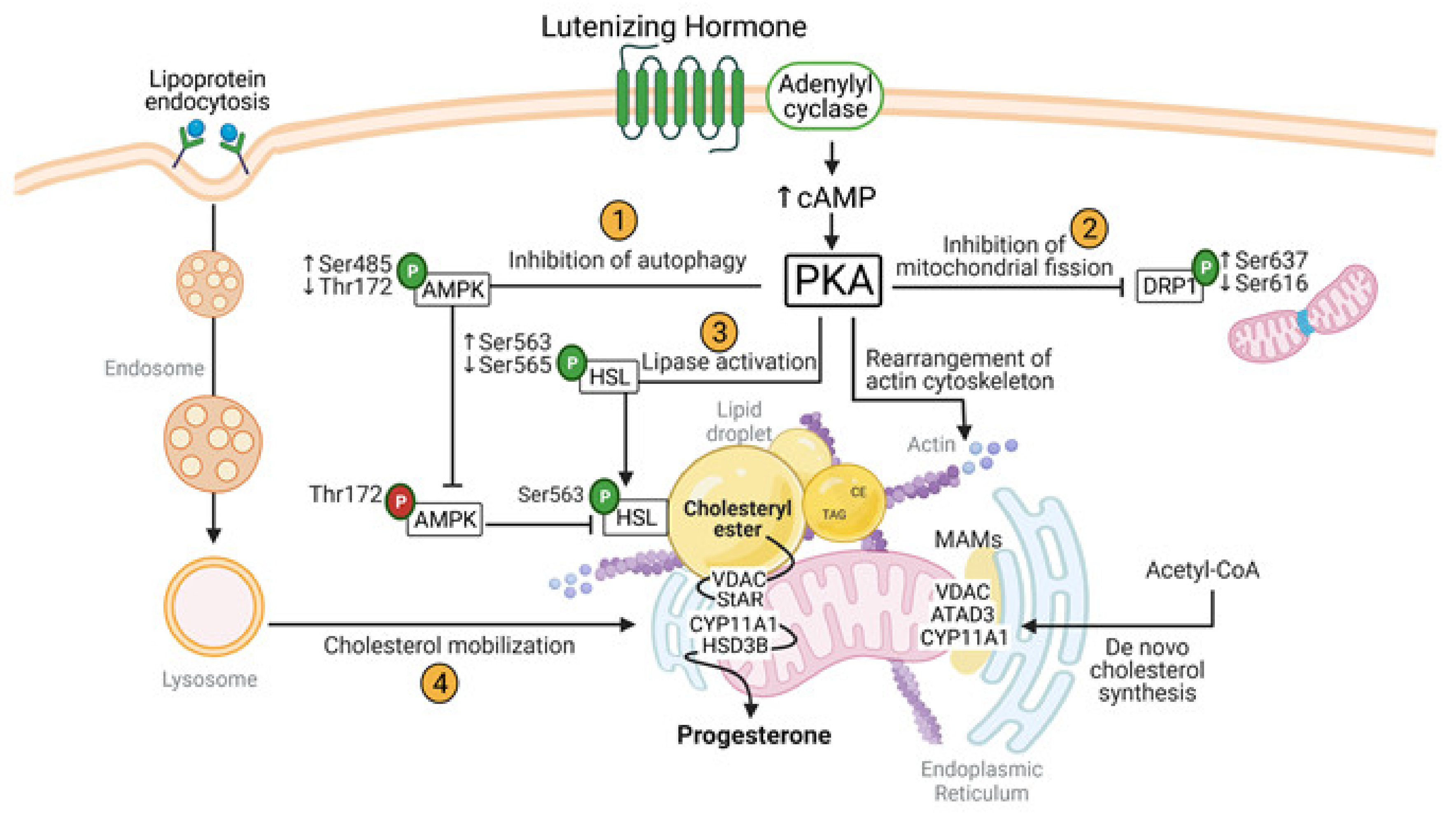
5. Metabolic Events Induced by LH in the Corpus Luteum: The Crosstalk of PKA/MTOR and AMPK Signaling in the Regulation of Metabolic Events in Luteal Cells
5.1. AMPK Attenuates Progesterone Production via Post-Translational Modifications of Enzymes Involved in Lipolysis in the Luteal Cells
5.2. MTOR Regulates Translation and Autophagy in the Luteal Cells
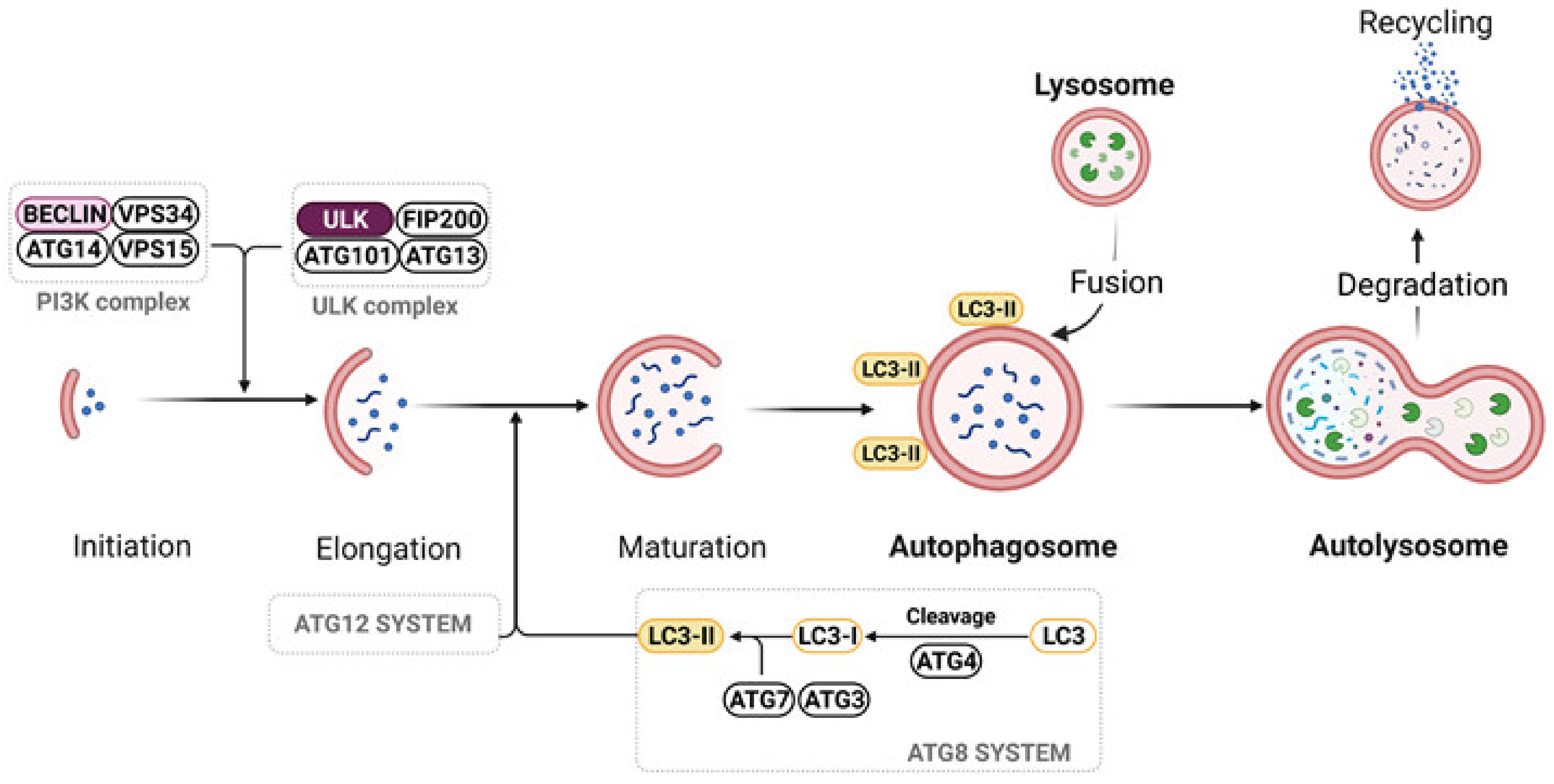
5.3. Autophagy
6. Summary
- What is the repertoire of intracellular signaling events triggered by luteotropic and luteolytic agents? Where are these signals located in the cell and how do they affect cellular function?
- Can advanced cellular and organelle imaging techniques be used to improve our understanding of inter-organelle communication? Can this information be used to better understand cellular metabolism and control of steroidogenesis?
- Will analysis of the follicular and luteal proteome reveal actionable targets for improvement or control of fertility? Can we identify specific hormone-responsive protein kinases and their substrates in follicular and luteal cell types?
- How do hormones control post-translational changes other than phosphorylation (methylation, acetylation, ubiquitylation, etc.) and what is their contribution to ovarian function?
- What important cellular metabolic changes occur during the follicular to luteal transition? Can these be targeted to rescue or terminate luteal function?
- What are the intracellular metabolic changes induced by luteotropic and luteolytic agents? Can metabolites be identified that can regulate steroidogenesis and cellular fate?
Author Contributions
Funding
Conflicts of Interest
References
- Casarini, L.; Crépieux, P. Molecular Mechanisms of Action of FSH. Front. Endocrinol. 2019, 10, 305. [Google Scholar] [CrossRef]
- Gutiérrez, C.G.; Campbell, B.K.; Webb, R. Development of a long-term bovine granulosa cell culture system: Induction and maintenance of estradiol production, response to follicle-stimulating hormone, and morphological characteristics. Biol. Reprod. 1997, 56, 608–616. [Google Scholar] [CrossRef]
- Riccetti, L.; Sperduti, S.; Lazzaretti, C.; Casarini, L.; Simoni, M. The cAMP/PKA pathway: Steroidogenesis of the antral follicular stage. Minerva Ginecol. 2018, 70, 516–524. [Google Scholar] [CrossRef]
- Abedel-Majed, M.A.; Romereim, S.M.; Davis, J.S.; Cupp, A.S. Perturbations in Lineage Specification of Granulosa and Theca Cells May Alter Corpus Luteum Formation and Function. Front. Endocrinol. 2019, 10, 832. [Google Scholar] [CrossRef] [PubMed]
- Meidan, R.; Girsh, E.; Blum, O.; Aberdam, E. In vitro differentiation of bovine theca and granulosa cells into small and large luteal-like cells: Morphological and functional characteristics. Biol. Reprod. 1990, 43, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Sanders, S.L.; Stouffer, R.L. Localization of steroidogenic enzymes in macaque luteal tissue during the menstrual cycle and simulated early pregnancy: Immunohistochemical evidence supporting the two-cell model for estrogen production in the primate corpus luteum. Biol. Reprod. 1997, 56, 1077–1087. [Google Scholar] [CrossRef][Green Version]
- Shrestha, K.; Rodler, D.; Sinowatz, F.; Meidan, R. Chapter 16–Corpus Luteum Formation. In The Ovary, 3rd ed.; Leung, P.C.K., Adashi, E.Y., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 255–267. [Google Scholar]
- Diskin, M.G.; Morris, D.G. Embryonic and early foetal losses in cattle and other ruminants. Reprod. Domest. Anim. 2008, 43, 260–267. [Google Scholar] [CrossRef]
- Lonergan, P. Influence of progesterone on oocyte quality and embryo development in cows. Theriogenology 2011, 76, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.M.; Mainigi, M.; Strauss, J.F. Secretory products of the corpus luteum and preeclampsia. Hum. Reprod. Update 2021, 27, 651–672. [Google Scholar] [CrossRef]
- Davis, J.S.; Rueda, B.R. The corpus luteum: An ovarian structure with maternal instincts and suicidal tendencies. Front. Biosci. 2002, 7, d1949–d1978. [Google Scholar] [CrossRef] [PubMed]
- Daya, S. Luteal support: Progestogens for pregnancy protection. Maturitas 2009, 65 (Suppl. 1), S29–S34. [Google Scholar] [CrossRef] [PubMed]
- Nardo, L.G.; Sallam, H.N. Progesterone supplementation to prevent recurrent miscarriage and to reduce implantation failure in assisted reproduction cycles. Reprod. Biomed. Online 2006, 13, 47–57. [Google Scholar] [CrossRef]
- Park, C.J.; Lin, P.C.; Zhou, S.; Barakat, R.; Bashir, S.T.; Choi, J.M.; Cacioppo, J.A.; Oakley, O.R.; Duffy, D.M.; Lydon, J.P.; et al. Progesterone Receptor Serves the Ovary as a Trigger of Ovulation and a Terminator of Inflammation. Cell Rep. 2020, 31, 107496. [Google Scholar] [CrossRef] [PubMed]
- Csapo, A.I.; Pulkkinen, M.O.; Ruttner, B.; Sauvage, J.P.; Wiest, W.G. The significance of the human corpus luteum in pregnancy maintenance. I. Preliminary studies. Am. J. Obstet. Gynecol. 1972, 112, 1061–1067. [Google Scholar] [CrossRef]
- Niswender, G.D.; Juengel, J.L.; Silva, P.J.; Rollyson, M.K.; McIntush, E.W. Mechanisms controlling the function and life span of the corpus luteum. Physiol. Rev. 2000, 80, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Papa, P.C.; Kowalewski, M.P. Factors affecting the fate of the canine corpus luteum: Potential contributors to pregnancy and non-pregnancy. Theriogenology 2020, 150, 339–346. [Google Scholar] [CrossRef]
- Stouffer, R.L.; Hennebold, J.D. Chapter 23–Structure, Function, and Regulation of the Corpus Luteum. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T.M., Zeleznik, A.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 1023–1076. [Google Scholar]
- Davis, J.S.; Weakland, L.L.; Farese, R.V.; West, L.A. Luteinizing hormone increases inositol trisphosphate and cytosolic free Ca2+ in isolated bovine luteal cells. J. Biol. Chem. 1987, 262, 8515–8521. [Google Scholar] [CrossRef]
- Davis, J.S.; Weakland, L.L.; West, L.A.; Farese, R.V. Luteinizing hormone stimulates the formation of inositol trisphosphate and cyclic AMP in rat granulosa cells. Evidence for phospholipase C generated second messengers in the action of luteinizing hormone. Biochem. J. 1986, 238, 597–604. [Google Scholar] [CrossRef]
- Janowski, T.; Fingerhut, J.; Kowalewski, M.P.; Zduńczyk, S.; Domosławska, A.; Jurczak, A.; Boos, A.; Schuler, G.; Hoffmann, B. In vivo investigations on luteotropic activity of prostaglandins during early diestrus in nonpregnant bitches. Theriogenology 2014, 82, 915–920. [Google Scholar] [CrossRef]
- Gregoraszczuk, E.L.; Michas, N. Progesterone and estradiol secretion by porcine luteal cells is influenced by individual and combined treatment with prostaglandins E2 and F2alpha throughout the estrus cycle. Prostaglandins Other Lipid Mediat. 1999, 57, 231–241. [Google Scholar] [CrossRef]
- Przygrodzka, E.; Kaczmarek, M.M.; Kaczynski, P.; Ziecik, A.J. Steroid hormones, prostanoids, and angiogenic systems during rescue of the corpus luteum in pigs. Reproduction 2016, 151, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Arosh, J.A.; Banu, S.K.; McCracken, J.A. Novel concepts on the role of prostaglandins on luteal maintenance and maternal recognition and establishment of pregnancy in ruminants. J. Dairy Sci. 2016, 99, 5926–5940. [Google Scholar] [CrossRef]
- Skarzynski, D.J.; Okuda, K. Inter- and intra-cellular mechanisms of prostaglandin F2alpha action during corpus luteum regression in cattle. Soc. Reprod. Fertil. Suppl. 2010, 67, 305–324. [Google Scholar] [CrossRef] [PubMed]
- Wiltbank, M.C.; Salih, S.M.; Atli, M.O.; Luo, W.; Bormann, C.L.; Ottobre, J.S.; Vezina, C.M.; Mehta, V.; Diaz, F.J.; Tsai, S.J.; et al. Comparison of endocrine and cellular mechanisms regulating the corpus luteum of primates and ruminants. Anim. Reprod. 2012, 9, 242–259. [Google Scholar] [PubMed]
- Boiti, C.; Canali, C.; Zerani, M.; Gobbetti, A. Changes in refractoriness of rabbit corpora lutea to a prostaglandin F2 alpha analogue, alfaprostol, during pseudopregnancy. Prostaglandins Other Lipid Mediat. 1998, 56, 255–264. [Google Scholar] [CrossRef]
- Diaz, F.J.; Crenshaw, T.D.; Wiltbank, M.C. Prostaglandin f(2alpha) induces distinct physiological responses in porcine corpora lutea after acquisition of luteolytic capacity. Biol. Reprod. 2000, 63, 1504–1512. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Shiao, T.F.; Bergfelt, D.R.; Ginther, O.J. Prostaglandin F2 alpha receptors in the early bovine corpus luteum. Biol. Reprod. 1995, 52, 74–78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pate, J.L. Roadmap to pregnancy during the period of maternal recognition in the cow: Changes within the corpus luteum associated with luteal rescue. Theriogenology 2020, 150, 294–301. [Google Scholar] [CrossRef]
- Stocco, C.; Telleria, C.; Gibori, G. The molecular control of corpus luteum formation, function, and regression. Endocr. Rev. 2007, 28, 117–149. [Google Scholar] [CrossRef] [PubMed]
- Zerani, M.; Polisca, A.; Boiti, C.; Maranesi, M. Current Knowledge on the Multifactorial Regulation of Corpora Lutea Lifespan: The Rabbit Model. Animals 2021, 11, 296. [Google Scholar] [CrossRef]
- Galvão, A.M.; Skarzynski, D.; Ferreira-Dias, G. Chapter Eleven–Luteolysis and the Auto-, Paracrine Role of Cytokines From Tumor Necrosis Factor α and Transforming Growth Factor β Superfamilies. In Vitamins and Hormones; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 107, pp. 287–315. [Google Scholar]
- Miller, W.L. Steroidogenesis: Unanswered Questions. Trends Endocrinol. Metab. 2017, 28, 771–793. [Google Scholar] [CrossRef] [PubMed]
- Conley, A.J.; Bird, I.M. The role of cytochrome P450 17 alpha-hydroxylase and 3 beta-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the delta 5 and delta 4 pathways of steroidogenesis in mammals. Biol. Reprod. 1997, 56, 789–799. [Google Scholar] [CrossRef]
- Gwynne, J.T.; Strauss, J.F., 3rd. The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr. Rev. 1982, 3, 299–329. [Google Scholar] [CrossRef] [PubMed]
- Christenson, L.K.; Devoto, L. Cholesterol transport and steroidogenesis by the corpus luteum. Reprod. Biol. Endocrinol. 2003, 1, 90. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. Disorders in the initial steps of steroid hormone synthesis. J. Steroid Biochem. Mol. Biol. 2017, 165, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.C.; Midgley, A.R., Jr.; Richards, J.S. Hormonal regulation of ovarian cellular proliferation. Cell 1978, 14, 71–78. [Google Scholar] [CrossRef]
- Christenson, L.K.; Stouffer, R.L. Proliferation of microvascular endothelial cells in the primate corpus luteum during the menstrual cycle and simulated early pregnancy. Endocrinology 1996, 137, 367–374. [Google Scholar] [CrossRef][Green Version]
- Murphy, B.D.; Gévry, N.; Ruiz-Cortés, T.; Coté, F.; Downey, B.R.; Sirois, J. Formation and early development of the corpus luteum in pigs. Reprod. Suppl. 2001, 58, 47–63. [Google Scholar] [CrossRef]
- Farin, C.E.; Moeller, C.L.; Sawyer, H.R.; Gamboni, F.; Niswender, G.D. Morphometric analysis of cell types in the ovine corpus luteum throughout the estrous cycle. Biol. Reprod. 1986, 35, 1299–1308. [Google Scholar] [CrossRef]
- Zheng, J.; Fricke, P.M.; Reynolds, L.P.; Redmer, D.A. Evaluation of growth, cell proliferation, and cell death in bovine corpora lutea throughout the estrous cycle. Biol. Reprod. 1994, 51, 623–632. [Google Scholar] [CrossRef][Green Version]
- Espey, L.L.; Ujioka, T.; Russell, D.L.; Skelsey, M.; Vladu, B.; Robker, R.L.; Okamura, H.; Richards, J.S. Induction of early growth response protein-1 gene expression in the rat ovary in response to an ovulatory dose of human chorionic gonadotropin. Endocrinology 2000, 141, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- MacLean, J.A., 2nd; Rao, M.K.; Doyle, K.M.; Richards, J.S.; Wilkinson, M.F. Regulation of the Rhox5 homeobox gene in primary granulosa cells: Preovulatory expression and dependence on SP1/SP3 and GABP. Biol. Reprod. 2005, 73, 1126–1134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Richards, J.S.; Russell, D.L.; Robker, R.L.; Dajee, M.; Alliston, T.N. Molecular mechanisms of ovulation and luteinization. Mol. Cell Endocrinol. 1998, 145, 47–54. [Google Scholar] [CrossRef]
- Wissing, M.L.; Kristensen, S.G.; Andersen, C.Y.; Mikkelsen, A.L.; Høst, T.; Borup, R.; Grøndahl, M.L. Identification of new ovulation-related genes in humans by comparing the transcriptome of granulosa cells before and after ovulation triggering in the same controlled ovarian stimulation cycle. Hum. Reprod. 2014, 29, 997–1010. [Google Scholar] [CrossRef]
- Xu, J.; Stouffer, R.L.; Searles, R.P.; Hennebold, J.D. Discovery of LH-regulated genes in the primate corpus luteum. Mol. Hum. Reprod. 2005, 11, 151–159. [Google Scholar] [CrossRef]
- Espey, L.L.; Richards, J.S. Temporal and spatial patterns of ovarian gene transcription following an ovulatory dose of gonadotropin in the rat. Biol. Reprod. 2002, 67, 1662–1670. [Google Scholar] [CrossRef]
- Kfir, S.; Basavaraja, R.; Wigoda, N.; Ben-Dor, S.; Orr, I.; Meidan, R. Genomic profiling of bovine corpus luteum maturation. PLoS ONE 2018, 13, e0194456. [Google Scholar] [CrossRef]
- Agca, C.; Ries, J.E.; Kolath, S.J.; Kim, J.H.; Forrester, L.J.; Antoniou, E.; Whitworth, K.M.; Mathialagan, N.; Springer, G.K.; Prather, R.S.; et al. Luteinization of porcine preovulatory follicles leads to systematic changes in follicular gene expression. Reproduction 2006, 132, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Christenson, L.K.; Gunewardena, S.; Hong, X.; Spitschak, M.; Baufeld, A.; Vanselow, J. Research resource: Preovulatory LH surge effects on follicular theca and granulosa transcriptomes. Mol. Endocrinol. 2013, 27, 1153–1171. [Google Scholar] [CrossRef]
- Likszo, P.; Skarzynski, D.J.; Moza Jalali, B. Proteomic Analysis of Porcine Pre-ovulatory Follicle Differentiation Into Corpus Luteum. Front. Endocrinol. 2019, 10, 774. [Google Scholar] [CrossRef]
- Romereim, S.M.; Summers, A.F.; Pohlmeier, W.E.; Zhang, P.; Hou, X.; Talbott, H.A.; Cushman, R.A.; Wood, J.R.; Davis, J.S.; Cupp, A.S. Transcriptomes of bovine ovarian follicular and luteal cells. Data Brief 2017, 10, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Fitz, T.A.; Mayan, M.H.; Sawyer, H.R.; Niswender, G.D. Characterization of two steroidogenic cell types in the ovine corpus luteum. Biol. Reprod. 1982, 27, 703–711. [Google Scholar] [CrossRef]
- Chegini, N.; Ramani, N.; Rao, C.V. Morphological and biochemical characterization of small and large bovine luteal cells during pregnancy. Mol. Cell Endocrinol. 1984, 37, 89–102. [Google Scholar] [CrossRef]
- Fields, M.J.; Dubois, W.; Fields, P.A. Dynamic features of luteal secretory granules: Ultrastructural changes during the course of pregnancy in the cow. Endocrinology 1985, 117, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Plewes, M.R.; Krause, C.; Talbott, H.A.; Przygrodzka, E.; Wood, J.R.; Cupp, A.S.; Davis, J.S. Trafficking of cholesterol from lipid droplets to mitochondria in bovine luteal cells: Acute control of progesterone synthesis. FASEB J. 2020, 34, 10731–10750. [Google Scholar] [CrossRef]
- Talbott, H.A.; Plewes, M.R.; Krause, C.; Hou, X.; Zhang, P.; Rizzo, W.B.; Wood, J.R.; Cupp, A.S.; Davis, J.S. Formation and characterization of lipid droplets of the bovine corpus luteum. Sci. Rep. 2020, 10, 11287. [Google Scholar] [CrossRef] [PubMed]
- Alila, H.W.; Dowd, J.P.; Corradino, R.A.; Harris, W.V.; Hansel, W. Control of progesterone production in small and large bovine luteal cells separated by flow cytometry. J. Reprod. Fertil. 1988, 82, 645–655. [Google Scholar] [CrossRef]
- Mamluk, R.; Chen, D.; Greber, Y.; Davis, J.S.; Meidan, R. Characterization of messenger ribonucleic acid expression for prostaglandin F2 alpha and luteinizing hormone receptors in various bovine luteal cell types. Biol. Reprod. 1998, 58, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Vallese, F.; Barazzuol, L.; Maso, L.; Brini, M.; Calì, T. ER-Mitochondria Calcium Transfer, Organelle Contacts and Neurodegenerative Diseases. Adv. Exp. Med. Biol. 2020, 1131, 719–746. [Google Scholar] [CrossRef] [PubMed]
- Rimessi, A.; Pedriali, G.; Vezzani, B.; Tarocco, A.; Marchi, S.; Wieckowski, M.R.; Giorgi, C.; Pinton, P. Interorganellar calcium signaling in the regulation of cell metabolism: A cancer perspective. Semin. Cell Dev. Biol. 2020, 98, 167–180. [Google Scholar] [CrossRef]
- Ikonen, E.; Zhou, X. Cholesterol transport between cellular membranes: A balancing act between interconnected lipid fluxes. Dev. Cell 2021, 56, 1430–1436. [Google Scholar] [CrossRef]
- Hariri, H.; Ugrankar, R.; Liu, Y.; Henne, W.M. Inter-organelle ER-endolysosomal contact sites in metabolism and disease across evolution. Commun. Integr. Biol. 2016, 9, e1156278. [Google Scholar] [CrossRef] [PubMed]
- Grimm, S. The ER-mitochondria interface: The social network of cell death. Biochim. Biophys. Acta 2012, 1823, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Pesaresi, P.; Schneider, A.; Kleine, T.; Leister, D. Interorganellar communication. Curr. Opin. Plant Biol. 2007, 10, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Kirshenbaum, L.A. Regulation of mitochondrial dynamics and cell fate. Circ. J. 2014, 78, 803–810. [Google Scholar] [CrossRef]
- Yu, R.; Lendahl, U.; Nistér, M.; Zhao, J. Regulation of Mammalian Mitochondrial Dynamics: Opportunities and Challenges. Front. Endocrinol. 2020, 11, 374. [Google Scholar] [CrossRef]
- Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Reddy, P.H.; Iijima, M.; Sesaki, H. Mitochondrial division and fusion in metabolism. Curr. Opin. Cell Biol. 2015, 33, 111–118. [Google Scholar] [CrossRef]
- Ramachandran, R. Mitochondrial dynamics: The dynamin superfamily and execution by collusion. Semin. Cell Dev. Biol. 2018, 76, 201–212. [Google Scholar] [CrossRef]
- Schrepfer, E.; Scorrano, L. Mitofusins, from Mitochondria to Metabolism. Mol. Cell 2016, 61, 683–694. [Google Scholar] [CrossRef]
- Chen, H.; Detmer, S.A.; Ewald, A.J.; Griffin, E.E.; Fraser, S.E.; Chan, D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003, 160, 189–200. [Google Scholar] [CrossRef]
- Chen, L.; Liu, T.; Tran, A.; Lu, X.; Tomilov, A.A.; Davies, V.; Cortopassi, G.; Chiamvimonvat, N.; Bers, D.M.; Votruba, M.; et al. OPA1 mutation and late-onset cardiomyopathy: Mitochondrial dysfunction and mtDNA instability. J. Am. Heart Assoc. 2012, 1, e003012. [Google Scholar] [CrossRef]
- Ferguson, S.; Raimondi, A.; Paradise, S.; Shen, H.; Mesaki, K.; Ferguson, A.; Destaing, O.; Ko, G.; Takasaki, J.; Cremona, O.; et al. Coordinated Actions of Actin and BAR Proteins Upstream of Dynamin at Endocytic Clathrin-Coated Pits. Dev. Cell 2009, 17, 811–822. [Google Scholar] [CrossRef]
- Wakabayashi, J.; Zhang, Z.; Wakabayashi, N.; Tamura, Y.; Fukaya, M.; Kensler, T.W.; Iijima, M.; Sesaki, H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J. Cell Biol. 2009, 186, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.M.; Brasnjo, G.; Hayashi, M.; Wolfel, M.; Collesi, C.; Giovedi, S.; Raimondi, A.; Gong, L.W.; Ariel, P.; Paradise, S.; et al. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science 2007, 316, 570–574. [Google Scholar] [CrossRef]
- Smirnova, E.; Shurland, D.L.; Ryazantsev, S.N.; van der Bliek, A.M. A human dynamin-related protein controls the distribution of mitochondria. J. Cell Biol. 1998, 143, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Santel, A.; Frank, S. Shaping mitochondria: The complex posttranslational regulation of the mitochondrial fission protein DRP1. IUBMB Life 2008, 60, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Zaja, I.; Bai, X.; Liu, Y.; Kikuchi, C.; Dosenovic, S.; Yan, Y.; Canfield, S.G.; Bosnjak, Z.J. Cdk1, PKCδ and calcineurin-mediated Drp1 pathway contributes to mitochondrial fission-induced cardiomyocyte death. Biochem. Biophys. Res. Commun. 2014, 453, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, N.; Ishihara, N.; Jofuku, A.; Oka, T.; Mihara, K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J. Biol. Chem. 2007, 282, 11521–11529. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Luo, C.L.; Tao, L.Y. Dynamin-related protein 1 (Drp1) mediating mitophagy contributes to the pathophysiology of nervous system diseases and brain injury. Histol. Histopathol. 2017, 32, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Cieplak, P.; Cho, D.-H.; Godzik, A.; Lipton, S.A. S-nitrosylation of Drp1 links excessive mitochondrial fission to neuronal injury in neurodegeneration. Mitochondrion 2010, 10, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-R.; Blackstone, C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J. Biol. Chem. 2007, 282, 21583–21587. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.R.; Blackstone, C. Drp1 phosphorylation and mitochondrial regulation. EMBO Rep. 2007, 8, 1088–1089. [Google Scholar] [CrossRef] [PubMed]
- Plewes, M.R.; Hou, X.; Talbott, H.A.; Zhang, P.; Wood, J.R.; Cupp, A.S.; Davis, J.S. Luteinizing hormone regulates the phosphorylation and localization of the mitochondrial effector dynamin-related protein-1 (DRP1) and steroidogenesis in the bovine corpus luteum. FASEB J. 2020, 34, 5299–5316. [Google Scholar] [CrossRef] [PubMed]
- Otera, H.; Wang, C.; Cleland, M.M.; Setoguchi, K.; Yokota, S.; Youle, R.J.; Mihara, K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J. Cell Biol. 2010, 191, 1141–1158. [Google Scholar] [CrossRef]
- Ong, S.B.; Kalkhoran, S.B.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Mitochondrial fusion and fission proteins as novel therapeutic targets for treating cardiovascular disease. Eur. J. Pharmacol. 2015, 763, 104–114. [Google Scholar] [CrossRef]
- Hall, A.R.; Burke, N.; Dongworth, R.K.; Hausenloy, D.J. Mitochondrial fusion and fission proteins: Novel therapeutic targets for combating cardiovascular disease. Br. J. Pharmacol. 2014, 171, 1890–1906. [Google Scholar] [CrossRef]
- Marsboom, G.; Toth, P.T.; Ryan, J.J.; Hong, Z.; Wu, X.; Fang, Y.H.; Thenappan, T.; Piao, L.; Zhang, H.J.; Pogoriler, J.; et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ. Res. 2012, 110, 1484–1497. [Google Scholar] [CrossRef]
- Sharp, W.W.; Fang, Y.H.; Han, M.; Zhang, H.J.; Hong, Z.; Banathy, A.; Morrow, E.; Ryan, J.J.; Archer, S.L. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: Therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J. 2014, 28, 316–326. [Google Scholar] [CrossRef]
- Nakamura, T.; Lipton, S.A. Protein S-Nitrosylation as a Therapeutic Target for Neurodegenerative Diseases. Trends Pharmacol. Sci. 2016, 37, 73–84. [Google Scholar] [CrossRef]
- Zhou, J.; Li, G.; Zheng, Y.; Shen, H.-M.; Hu, X.; Ming, Q.-L.; Huang, C.; Li, P.; Gao, N. A novel autophagy/mitophagy inhibitor liensinine sensitizes breast cancer cells to chemotherapy through DNM1L-mediated mitochondrial fission. Autophagy 2015, 11, 1259–1279. [Google Scholar] [CrossRef]
- Olichon, A.; Emorine, L.J.; Descoins, E.; Pelloquin, L.; Brichese, L.; Gas, N.; Guillou, E.; Delettre, C.; Valette, A.; Hamel, C.P.; et al. The human dynamin-related protein OPA1 is anchored to the mitochondrial inner membrane facing the inter-membrane space. FEBS Lett. 2002, 523, 171–176. [Google Scholar] [CrossRef]
- Frezza, C.; Cipolat, S.; Martins de Brito, O.; Micaroni, M.; Beznoussenko, G.V.; Rudka, T.; Bartoli, D.; Polishuck, R.S.; Danial, N.N.; De Strooper, B.; et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 2006, 126, 177–189. [Google Scholar] [CrossRef] [PubMed]
- McBride, H.; Soubannier, V. Mitochondrial function: OMA1 and OPA1, the grandmasters of mitochondrial health. Curr. Biol. 2010, 20, R274–R276. [Google Scholar] [CrossRef] [PubMed]
- MacVicar, T.; Langer, T. OPA1 processing in cell death and disease–the long and short of it. J. Cell Sci. 2016, 129, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.; Scott, J.D. AKAP signalling complexes: Focal points in space and time. Nat. Rev. Mol. Cell Biol. 2004, 5, 959–970. [Google Scholar] [CrossRef]
- Dodge-Kafka, K.L.; Langeberg, L.; Scott, J.D. Compartmentation of cyclic nucleotide signaling in the heart: The role of A-kinase anchoring proteins. Circ. Res. 2006, 98, 993–1001. [Google Scholar] [CrossRef]
- Pidoux, G.; Witczak, O.; Jarnaess, E.; Myrvold, L.; Urlaub, H.; Stokka, A.J.; Kuntziger, T.; Tasken, K. Optic atrophy 1 is an A-kinase anchoring protein on lipid droplets that mediates adrenergic control of lipolysis. EMBO J. 2011, 30, 4371–4386. [Google Scholar] [CrossRef]
- Rogne, M.; Chu, D.T.; Küntziger, T.M.; Mylonakou, M.N.; Collas, P.; Tasken, K. OPA1-anchored PKA phosphorylates perilipin 1 on S522 and S497 in adipocytes differentiated from human adipose stem cells. Mol. Biol. Cell 2018, 29, 1487–1501. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, L.; Rajki, A.; Katona, D.; Szanda, G.; Spät, A. Extramitochondrial OPA1 and adrenocortical function. Mol. Cell Endocrinol. 2013, 381, 70–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Plewes, M.R.; Davis, J.S. Unpublished work. University of Nebraska Medical Center: Omaha, NE, USA, 2021; to be submitted. [Google Scholar]
- Plewes, M.R.; Zhang, P.; Hou, X.; Davis, J.S. Effect of Mitochondrial Dynamin Like GTPase on Steroidogenesis in the Corpus Luteum. In Proceedings of the 52nd Annual Meeting of the Society for the Study of Reproduction, San Jose, CA, USA, 18–21 July 2019. [Google Scholar]
- Skulachev, V.P. Functions of mitochondria: From intracellular power stations to mediators of a senescence program. Cell Mol. Life Sci. 2009, 66, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Kleizen, B.; Braakman, I. Protein folding and quality control in the endoplasmic reticulum. Curr. Opin. Cell Biol. 2004, 16, 343–349. [Google Scholar] [CrossRef]
- Fagone, P.; Jackowski, S. Membrane phospholipid synthesis and endoplasmic reticulum function. J. Lipid Res. 2009, 50, S311–S316. [Google Scholar] [CrossRef]
- Di Mattia, T.; Tomasetto, C.; Alpy, F. Faraway, so close! Functions of Endoplasmic reticulum-Endosome contacts. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158490. [Google Scholar] [CrossRef]
- Hayashi, T.; Rizzuto, R.; Hajnoczky, G.; Su, T.P. MAM: More than just a housekeeper. Trends Cell Biol. 2009, 19, 81–88. [Google Scholar] [CrossRef]
- Van Vliet, A.R.; Verfaillie, T.; Agostinis, P. New functions of mitochondria associated membranes in cellular signaling. Biochim. Biophys. Acta 2014, 1843, 2253–2262. [Google Scholar] [CrossRef]
- Annunziata, I.; d’Azzo, A. Interorganellar membrane microdomains: Dynamic platforms in the control of calcium signaling and apoptosis. Cells 2013, 2, 574–590. [Google Scholar] [CrossRef] [PubMed]
- Sassano, M.L.; van Vliet, A.R.; Agostinis, P. Mitochondria-Associated Membranes As Networking Platforms and Regulators of Cancer Cell Fate. Front. Oncol. 2017, 7, 174. [Google Scholar] [CrossRef]
- Hugenroth, M.; Bohnert, M. Come a little bit closer! Lipid droplet-ER contact sites are getting crowded. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118603. [Google Scholar] [CrossRef]
- Issop, L.; Rone, M.B.; Papadopoulos, V. Organelle plasticity and interactions in cholesterol transport and steroid biosynthesis. Mol. Cell Endocrinol. 2013, 371, 34–46. [Google Scholar] [CrossRef]
- Rone, M.B.; Midzak, A.S.; Issop, L.; Rammouz, G.; Jagannathan, S.; Fan, J.; Ye, X.; Blonder, J.; Veenstra, T.; Papadopoulos, V. Identification of a dynamic mitochondrial protein complex driving cholesterol import, trafficking, and metabolism to steroid hormones. Mol. Endocrinol. 2012, 26, 1868–1882. [Google Scholar] [CrossRef]
- Issop, L.; Fan, J.; Lee, S.; Rone, M.B.; Basu, K.; Mui, J.; Papadopoulos, V. Mitochondria-associated membrane formation in hormone-stimulated Leydig cell steroidogenesis: Role of ATAD3. Endocrinology 2015, 156, 334–345. [Google Scholar] [CrossRef]
- Strauss, J.F. Chapter 5–Organization of Ovarian Steroidogenic Cells and Cholesterol Metabolism. In The Ovary, 3rd ed.; Leung, P.C.K., Adashi, E.Y., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 83–94. [Google Scholar]
- Prasad, M.; Kaur, J.; Pawlak, K.J.; Bose, M.; Whittal, R.M.; Bose, H.S. Mitochondria-associated endoplasmic reticulum membrane (MAM) regulates steroidogenic activity via steroidogenic acute regulatory protein (StAR)-voltage-dependent anion channel 2 (VDAC2) interaction. J. Biol. Chem. 2015, 290, 2604–2616. [Google Scholar] [CrossRef]
- Moore, A.S.; Holzbaur, E.L.F. Mitochondrial-cytoskeletal interactions: Dynamic associations that facilitate network function and remodeling. Curr. Opin. Physiol. 2018, 3, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Nobes, C.D.; Hall, A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 1999, 144, 1235–1244. [Google Scholar] [CrossRef]
- Bamburg, J.R. Proteins of the ADF/cofilin family: Essential regulators of actin dynamics. Annu. Rev. Cell Dev. Biol. 1999, 15, 185–230. [Google Scholar] [CrossRef]
- Niwa, R.; Nagata-Ohashi, K.; Takeichi, M.; Mizuno, K.; Uemura, T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell 2002, 108, 233–246. [Google Scholar] [CrossRef]
- Ohta, Y.; Kousaka, K.; Nagata-Ohashi, K.; Ohashi, K.; Muramoto, A.; Shima, Y.; Niwa, R.; Uemura, T.; Mizuno, K. Differential activities, subcellular distribution and tissue expression patterns of three members of Slingshot family phosphatases that dephosphorylate cofilin. Genes Cells 2003, 8, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.B.; Maizels, E.T.; Flynn, M.P.; Jones, J.C.; Shelden, E.A.; Bamburg, J.R.; Hunzicker-Dunn, M. Luteinizing hormone receptor-stimulated progesterone production by preovulatory granulosa cells requires protein kinase A-dependent activation/dephosphorylation of the actin dynamizing protein cofilin. Mol. Endocrinol. 2010, 24, 1765–1781. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.; Freeman, D.A. Effect of cholesterol transport inhibitors on steroidogenesis and plasma membrane cholesterol transport in cultured MA-10 Leydig tumor cells. Endocrinology 1990, 126, 2267–2276. [Google Scholar] [CrossRef]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Guzel, E.; Arlier, S.; Guzeloglu-Kayisli, O.; Tabak, M.S.; Ekiz, T.; Semerci, N.; Larsen, K.; Schatz, F.; Lockwood, C.J.; Kayisli, U.A. Endoplasmic Reticulum Stress and Homeostasis in Reproductive Physiology and Pathology. Int. J. Mol. Sci. 2017, 18, 792. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Nose, E.; Takahashi, N.; Hirota, Y.; Hirata, T.; Yoshino, O.; Koga, K.; Fujii, T.; Osuga, Y. Evidence of the activation of unfolded protein response in granulosa and cumulus cells during follicular growth and maturation. Gynecol. Endocrinol. 2015, 31, 783–787. [Google Scholar] [CrossRef]
- Lin, P.; Yang, Y.; Li, X.; Chen, F.; Cui, C.; Hu, L.; Li, Q.; Liu, W.; Jin, Y. Endoplasmic reticulum stress is involved in granulosa cell apoptosis during follicular atresia in goat ovaries. Mol. Reprod. Dev. 2012, 79, 423–432. [Google Scholar] [CrossRef]
- Park, H.J.; Park, S.J.; Koo, D.B.; Kong, I.K.; Kim, M.K.; Kim, J.M.; Choi, M.S.; Park, Y.H.; Kim, S.U.; Chang, K.T.; et al. Unfolding protein response signaling is involved in development, maintenance, and regression of the corpus luteum during the bovine estrous cycle. Biochem. Biophys. Res. Commun. 2013, 441, 344–350. [Google Scholar] [CrossRef]
- Braakman, I.; Bulleid, N.J. Protein folding and modification in the mammalian endoplasmic reticulum. Annu. Rev. Biochem. 2011, 80, 71–99. [Google Scholar] [CrossRef]
- Chambers, J.E.; Marciniak, S.J. Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 2. Protein misfolding and ER stress 2. Protein misfolding and ER stress. Am. J. Physiol. Cell Physiol. 2014, 307, C657–C670. [Google Scholar] [CrossRef]
- Takahashi, N.; Harada, M.; Hirota, Y.; Zhao, L.; Azhary, J.M.; Yoshino, O.; Izumi, G.; Hirata, T.; Koga, K.; Wada-Hiraike, O.; et al. A Potential Role for Endoplasmic Reticulum Stress in Progesterone Deficiency in Obese Women. Endocrinology 2017, 158, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, T.S.; Park, C.K.; Lee, S.H.; Kim, J.M.; Lee, K.S.; Lee, I.K.; Park, J.W.; Lawson, M.A.; Lee, D.S. hCG-induced endoplasmic reticulum stress triggers apoptosis and reduces steroidogenic enzyme expression through activating transcription factor 6 in Leydig cells of the testis. J. Mol. Endocrinol. 2013, 50, 151–166. [Google Scholar] [CrossRef]
- Ullah, S.; Zhang, M.; Yu, H.; Mustafa, S.; Shafiq, M.; Wei, Q.; Wang, W.; Jan, M.; Mao, D. Heat exposure affected the reproductive performance of pregnant mice: Enhancement of autophagy and alteration of subcellular structure in the corpus luteum. Reprod. Biol. 2019, 19, 261–269. [Google Scholar] [CrossRef]
- Azhary, J.M.K.; Harada, M.; Kunitomi, C.; Kusamoto, A.; Takahashi, N.; Nose, E.; Oi, N.; Wada-Hiraike, O.; Urata, Y.; Hirata, T.; et al. Androgens Increase Accumulation of Advanced Glycation End Products in Granulosa Cells by Activating ER Stress in PCOS. Endocrinology 2020, 161, bqaa015. [Google Scholar] [CrossRef]
- Takahashi, N.; Harada, M.; Hirota, Y.; Nose, E.; Azhary, J.M.; Koike, H.; Kunitomi, C.; Yoshino, O.; Izumi, G.; Hirata, T.; et al. Activation of Endoplasmic Reticulum Stress in Granulosa Cells from Patients with Polycystic Ovary Syndrome Contributes to Ovarian Fibrosis. Sci. Rep. 2017, 7, 10824. [Google Scholar] [CrossRef] [PubMed]
- Dimova, R.; Tankova, T. The role of vaspin in the development of metabolic and glucose tolerance disorders and atherosclerosis. Biomed. Res. Int. 2015, 2015, 823481. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, P.; Mlyczyńska, E.; Dupont, J.; Rak, A. Novel Insights on the Corpus Luteum Function: Role of Vaspin on Porcine Luteal Cell Angiogenesis, Proliferation and Apoptosis by Activation of GRP78 Receptor and MAP3/1 Kinase Pathways. Int. J. Mol. Sci. 2020, 21, 6823. [Google Scholar] [CrossRef]
- Barbosa, A.D.; Siniossoglou, S. Function of lipid droplet-organelle interactions in lipid homeostasis. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1459–1468. [Google Scholar] [CrossRef]
- Sztalryd, C.; Brasaemle, D.L. The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1221–1232. [Google Scholar] [CrossRef]
- Welte, M.A.; Gould, A.P. Lipid droplet functions beyond energy storage. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1260–1272. [Google Scholar] [CrossRef]
- Fujimoto, T.; Parton, R.G. Not just fat: The structure and function of the lipid droplet. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef]
- Bloor, W.R.; Okey, R.; Corner, G. The relation of the lipids to physiological activity i. The changes in the lipid content of the corpus luteum of the sow. J. Biol. Chem. 1930, 86, 291–306. [Google Scholar] [CrossRef]
- Claesson, L. Quantitative relationship between gonadotrophic stimulation and lipid changes in the interstitial gland of the rabbit ovary. Acta Physiol. Scand. Suppl. 1954, 31, 23–51. [Google Scholar] [PubMed]
- Deane, H.W.; Andrews, J.S. A comparison of the staining of lipid droplets in the mouse ovary by the Schiff reaction and by the Ashbel-Seligman carbonyl reaction. J. Histochem. Cytochem. 1953, 1, 283–295. [Google Scholar] [CrossRef]
- Dorlikar, A.; Dhamani, A.; Charde, P.; Mohite, A. Ultrastructure of ovarian preantral follicles and corpus luteum in Indian flying fox Pteropus giganteus (Brunnich). Edorium J. Cell Biol. 2014, 1, 1–11. [Google Scholar] [CrossRef]
- Green, J.A.; Maqueo, M. Ultrastructure of the human ovary. I. The luteal cell during the menstrual cycle. Am. J. Obstet. Gynecol. 1965, 92, 946–957. [Google Scholar] [CrossRef]
- Guraya, S.S. Histochemical study of granulosa and theca interna during follicular development, ovulation, and corpus luteum formation and regression in the human ovary. Am. J. Obset. Gynecol. 1968, 101, 448–457. [Google Scholar] [CrossRef]
- Kapoor, K. Lipid Distribution Variations in Different Stages of Cyclic Corpus Luteum of Indian Buffalo. J. Anim. Res. 2018, 8, 379–385. [Google Scholar] [CrossRef]
- Parry, D.M.; Willcox, D.L.; Thorburn, G.D. Ultrastructural and cytochemical study of the bovine corpus luteum. J. Reprod. Fertil. 1980, 60, 349–357. [Google Scholar] [CrossRef]
- Seachord, C.L.; VandeVoort, C.A.; Duffy, D.M. Adipose differentiation-related protein: A gonadotropin- and prostaglandin-regulated protein in primate periovulatory follicles. Biol. Reprod. 2005, 72, 1305–1314. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strauss, J.F., 3rd; Seifter, E.; Lien, E.L.; Goodman, D.B.; Stambaugh, R.L. Lipid metabolism in regressing rat corpora lutea of pregnancy. J. Lipid Res. 1977, 18, 246–258. [Google Scholar] [CrossRef]
- Waterman, R.A. Changes in lipid contents and fatty acid compositions in ovine corpora lutea during the estrous cycle and early pregnancy. Biol. Reprod. 1988, 38, 605–615. [Google Scholar] [CrossRef]
- Khanthusaeng, V.; Thammasiri, J.; Bass, C.S.; Navanukraw, C.; Borowicz, P.; Redmer, D.A.; Grazul-Bilska, A.T. Lipid droplets in cultured luteal cells in non-pregnant sheep fed different planes of nutrition. Acta Histochem. 2016, 118, 553–559. [Google Scholar] [CrossRef]
- Armstrong, D.T.; Flint, A.P. Isolation and properties of cholesterol esterstorage granules from ovarian tissues. Biochem. J. 1973, 134, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Heath, E.; Weinstein, P.; Merritt, B.; Shanks, R.; Hixon, J. Effects of prostaglandins on the bovine corpus luteum: Granules, lipid inclusions and progesterone secretion. Biol. Reprod. 1983, 29, 977–985. [Google Scholar] [CrossRef]
- Kraemer, F.B.; Shen, W.J. Hormone-sensitive lipase: Control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J. Lipid Res. 2002, 43, 1585–1594. [Google Scholar] [CrossRef]
- Zechner, R.; Zimmermann, R.; Eichmann, T.O.; Kohlwein, S.D.; Haemmerle, G.; Lass, A.; Madeo, F. FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012, 15, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.J.; Patel, S.; Natu, V.; Hong, R.; Wang, J.; Azhar, S.; Kraemer, F.B. Interaction of hormone-sensitive lipase with steroidogenic acute regulatory protein: Facilitation of cholesterol transfer in adrenal. J. Biol. Chem. 2003, 278, 43870–43876. [Google Scholar] [CrossRef] [PubMed]
- Talbott, H.; Davis, J.S. Lipid Droplets and Metabolic Pathways Regulate Steroidogenesis in the Corpus Luteum. In The Life Cycle of the Corpus Luteum; Meidan, R., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 57–78. [Google Scholar]
- Manna, P.R.; Cohen-Tannoudji, J.; Counis, R.; Garner, C.W.; Huhtaniemi, I.; Kraemer, F.B.; Stocco, D.M. Mechanisms of action of hormone-sensitive lipase in mouse Leydig cells: Its role in the regulation of the steroidogenic acute regulatory protein. J. Biol. Chem. 2013, 288, 8505–8518. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, R.E.; Vandenboom, R.; Roy, B.D.; Peters, S.J. Skeletal muscle PLIN3 and PLIN5 are serine phosphorylated at rest and following lipolysis during adrenergic or contractile stimulation. Physiol. Rep. 2013, 1, e00084. [Google Scholar] [CrossRef]
- Raclot, T.; Holm, C.; Langin, D. Fatty acid specificity of hormone-sensitive lipase. Implication in the selective hydrolysis of triacylglycerols. J. Lipid Res. 2001, 42, 2049–2057. [Google Scholar] [CrossRef]
- Raclot, T.; Holm, C.; Langin, D. A role for hormone-sensitive lipase in the selective mobilization of adipose tissue fatty acids. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2001, 1532, 88–96. [Google Scholar] [CrossRef]
- Benador, I.Y.; Veliova, M.; Mahdaviani, K.; Petcherski, A.; Wikstrom, J.D.; Assali, E.A.; Acín-Pérez, R.; Shum, M.; Oliveira, M.F.; Cinti, S.; et al. Mitochondria Bound to Lipid Droplets Have Unique Bioenergetics, Composition, and Dynamics that Support Lipid Droplet Expansion. Cell Metab. 2018, 27, 869–885.e866. [Google Scholar] [CrossRef] [PubMed]
- Inpanathan, S.; Botelho, R.J. The Lysosome Signaling Platform: Adapting With the Times. Front. Cell Dev. Biol. 2019, 7, 113. [Google Scholar] [CrossRef]
- Settembre, C.; Ballabio, A. Lysosome: Regulator of lipid degradation pathways. Trends Cell Biol. 2014, 24, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Thelen, A.M.; Zoncu, R. Emerging Roles for the Lysosome in Lipid Metabolism. Trends Cell Biol. 2017, 27, 833–850. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, H.; Yang, F.; Zhang, H.; Zeng, S. FSH Promotes Progesterone Synthesis by Enhancing Autophagy to Accelerate Lipid Droplet Degradation in Porcine Granulosa Cells. Front. Cell Dev. Biol. 2021, 9, 626927. [Google Scholar] [CrossRef]
- Alonso-Pozos, I.; Rosales-Torres, A.M.; Avalos-Rodríguez, A.; Vergara-Onofre, M.; Rosado-García, A. Mechanism of granulosa cell death during follicular atresia depends on follicular size. Theriogenology 2003, 60, 1071–1081. [Google Scholar] [CrossRef]
- Escobar, M.L.; Echeverría, O.M.; Ortíz, R.; Vázquez-Nin, G.H. Combined apoptosis and autophagy, the process that eliminates the oocytes of atretic follicles in immature rats. Apoptosis 2008, 13, 1253–1266. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Wu, Y.; Zhao, S.; Liu, Z.X.; Zeng, S.M.; Zhang, G.X. Lysosomes are involved in induction of steroidogenic acute regulatory protein (StAR) gene expression and progesterone synthesis through low-density lipoprotein in cultured bovine granulosa cells. Theriogenology 2015, 84, 811–817. [Google Scholar] [CrossRef]
- Aboelenain, M.; Kawahara, M.; Balboula, A.Z.; Montasser Ael, M.; Zaabel, S.M.; Okuda, K.; Takahashi, M. Status of autophagy, lysosome activity and apoptosis during corpus luteum regression in cattle. J. Reprod. Dev. 2015, 61, 229–236. [Google Scholar] [CrossRef]
- Gregoraszczuk, E.L.; Sadowska, J. Lysosomal acid phosphatase activity and progesterone secretion by porcine corpora lutea at various periods of the luteal phase. Folia. Histochem. Cytobiol. 1997, 35, 35–39. [Google Scholar] [PubMed]
- Wang, Z.; El Zowalaty, A.E.; Li, Y.; Andersen, C.L.; Ye, X. Association of luteal cell degeneration and progesterone deficiency with lysosomal storage disorder mucolipidosis type IV in Mcoln1-/- mouse model. Biol. Reprod. 2019, 101, 782–790. [Google Scholar] [CrossRef]
- Bowdridge, E.C.; Goravanahally, M.P.; Inskeep, E.K.; Flores, J.A. Activation of Adenosine Monophosphate-Activated Protein Kinase Is an Additional Mechanism That Participates in Mediating Inhibitory Actions of Prostaglandin F2Alpha in Mature, but Not Developing, Bovine Corpora Lutea. Biol. Reprod. 2015, 93, 7. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Arvisais, E.W.; Davis, J.S. Luteinizing hormone stimulates mammalian target of rapamycin signaling in bovine luteal cells via pathways independent of AKT and mitogen-activated protein kinase: Modulation of glycogen synthase kinase 3 and AMP-activated protein kinase. Endocrinology 2010, 151, 2846–2857. [Google Scholar] [CrossRef] [PubMed]
- Przygrodzka, E.; Hou, X.; Zhang, P.; Plewes, M.R.; Franco, R.; Davis, J.S. PKA and AMPK Signaling Pathways Differentially Regulate Luteal Steroidogenesis. Endocrinology 2021, 162, bqab015. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.E.; Armstrong, D.T.; Gilchrist, R.B. Bovine cumulus cell-oocyte gap junctional communication during in vitro maturation in response to manipulation of cell-specific cyclic adenosine 3′,5′-monophosophate levels. Biol. Reprod. 2004, 70, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Tosca, L.; Chabrolle, C.; Uzbekova, S.; Dupont, J. Effects of metformin on bovine granulosa cells steroidogenesis: Possible involvement of adenosine 5′ monophosphate-activated protein kinase (AMPK). Biol. Reprod. 2007, 76, 368–378. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Willows, R.; Sanders, M.J.; Xiao, B.; Patel, B.R.; Martin, S.R.; Read, J.; Wilson, J.R.; Hubbard, J.; Gamblin, S.J.; Carling, D. Phosphorylation of AMPK by upstream kinases is required for activity in mammalian cells. Biochem. J. 2017, 474, 3059–3073. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.J.; Azhar, S.; Kraemer, F.B. Lipid droplets and steroidogenic cells. Exp. Cell Res. 2016, 340, 209–214. [Google Scholar] [CrossRef]
- Kim, S.J.; Tang, T.; Abbott, M.; Viscarra, J.A.; Wang, Y.; Sul, H.S. AMPK Phosphorylates Desnutrin/ATGL and Hormone-Sensitive Lipase To Regulate Lipolysis and Fatty Acid Oxidation within Adipose Tissue. Mol. Cell Biol. 2016, 36, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Pernicova, I.; Korbonits, M. Metformin—mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef]
- Tosca, L.; Crochet, S.; Ferré, P.; Foufelle, F.; Tesseraud, S.; Dupont, J. AMP-activated protein kinase activation modulates progesterone secretion in granulosa cells from hen preovulatory follicles. J. Endocrinol. 2006, 190, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Tosca, L.; Solnais, P.; Ferré, P.; Foufelle, F.; Dupont, J. Metformin-induced stimulation of adenosine 5′ monophosphate-activated protein kinase (PRKA) impairs progesterone secretion in rat granulosa cells. Biol. Reprod. 2006, 75, 342–351. [Google Scholar] [CrossRef]
- Martin, D.E.; Hall, M.N. The expanding TOR signaling network. Curr. Opin. Cell Biol. 2005, 17, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR signaling in growth and metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef]
- Guertin, D.A.; Sabatini, D.M. Defining the role of mTOR in cancer. Cancer Cell 2007, 12, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Guan, K.L. Expanding mTOR signaling. Cell Res. 2007, 17, 666–681. [Google Scholar] [CrossRef]
- Jacinto, E.; Loewith, R.; Schmidt, A.; Lin, S.; Rüegg, M.A.; Hall, A.; Hall, M.N. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004, 6, 1122–1128. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Ali, S.M.; Kim, D.H.; Guertin, D.A.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004, 14, 1296–1302. [Google Scholar] [CrossRef]
- González, A.; Hall, M.N. Nutrient sensing and TOR signaling in yeast and mammals. Embo. J. 2017, 36, 397–408. [Google Scholar] [CrossRef]
- Grolleau, A.; Bowman, J.; Pradet-Balade, B.; Puravs, E.; Hanash, S.; Garcia-Sanz, J.A.; Beretta, L. Global and specific translational control by rapamycin in T cells uncovered by microarrays and proteomics. J. Biol. Chem. 2002, 277, 22175–22184. [Google Scholar] [CrossRef]
- Alessi, D.R.; James, S.R.; Downes, C.P.; Holmes, A.B.; Gaffney, P.R.; Reese, C.B.; Cohen, P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 1997, 7, 261–269. [Google Scholar] [CrossRef]
- Li, Y.; Corradetti, M.N.; Inoki, K.; Guan, K.L. TSC2: Filling the GAP in the mTOR signaling pathway. Trends Biochem. Sci. 2004, 29, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Zhu, T.; Guan, K.L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
- Inoki, K.; Ouyang, H.; Zhu, T.; Lindvall, C.; Wang, Y.; Zhang, X.; Yang, Q.; Bennett, C.; Harada, Y.; Stankunas, K.; et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 2006, 126, 955–968. [Google Scholar] [CrossRef]
- Roy, L.; McDonald, C.A.; Jiang, C.; Maroni, D.; Zeleznik, A.J.; Wyatt, T.A.; Hou, X.; Davis, J.S. Convergence of 3′,5′-cyclic adenosine 5′-monophosphate/protein kinase A and glycogen synthase kinase-3beta/beta-catenin signaling in corpus luteum progesterone synthesis. Endocrinology 2009, 150, 5036–5045. [Google Scholar] [CrossRef]
- Kayampilly, P.P.; Menon, K.M. Follicle-stimulating hormone increases tuberin phosphorylation and mammalian target of rapamycin signaling through an extracellular signal-regulated kinase-dependent pathway in rat granulosa cells. Endocrinology 2007, 148, 3950–3957. [Google Scholar] [CrossRef] [PubMed]
- Moravek, M.B.; Shang, M.; Menon, B.; Menon, K. HCG-mediated activation of mTORC1 signaling plays a crucial role in steroidogenesis in human granulosa lutein cells. Endocrine 2016, 54, 217–224. [Google Scholar] [CrossRef]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Yang, Z.; Klionsky, D.J. An overview of the molecular mechanism of autophagy. Curr. Top. Microbiol. Immunol. 2009, 335, 1–32. [Google Scholar] [CrossRef] [PubMed]
- How Do I Cite BioRender? Available online: https://help.biorender.com/en/articles/3619405-how-do-i-cite-biorender (accessed on 1 September 2021).
- Wang, Y.; Zhang, H. Regulation of Autophagy by mTOR Signaling Pathway. In Autophagy: Biology and Diseases: Basic Science; Qin, Z.-H., Ed.; Springer Singapore: Singapore, 2019; pp. 67–83. [Google Scholar]
- Li, Y.; Chen, Y. AMPK and Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Egan, D.; Kim, J.; Shaw, R.J.; Guan, K.L. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 2011, 7, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Choi, J.; Jo, M.; Lee, E.; Choi, D. The role of autophagy in corpus luteum regression in the rat. Biol. Reprod. 2011, 85, 465–472. [Google Scholar] [CrossRef]
- Grzesiak, M.; Michalik, A.; Rak, A.; Knapczyk-Stwora, K.; Pieczonka, A. The expression of autophagy-related proteins within the corpus luteum lifespan in pigs. Domest. Anim. Endocrinol. 2018, 64, 9–16. [Google Scholar] [CrossRef]
- Meng, L.; Jan, S.Z.; Hamer, G.; van Pelt, A.M.; van der Stelt, I.; Keijer, J.; Teerds, K.J. Preantral follicular atresia occurs mainly through autophagy, while antral follicles degenerate mostly through apoptosis. Biol. Reprod. 2018, 99, 853–863. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, Z.; Lin, Q.; Xu, R.; Chen, J.; Wang, Y.; Zhang, Y.; Tang, Y.; Shi, C.; Liu, Y.; et al. HIF-1α/BNIP3-Mediated Autophagy Contributes to the Luteinization of Granulosa Cells During the Formation of Corpus Luteum. Front. Cell. Dev. Biol. 2020, 8, 619924. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, Z.; Zhang, H.; Wang, Y.; Zhang, Y.; Zhao, J.; Yang, H.; Wang, Z. Autophagy Attenuation Hampers Progesterone Synthesis during the Development of Pregnant Corpus Luteum. Cells 2019, 9, 71. [Google Scholar] [CrossRef]
- Wen, X.; Liu, L.; Li, S.; Lin, P.; Chen, H.; Zhou, D.; Tang, K.; Wang, A.; Jin, Y. Prostaglandin F2α Induces Goat Corpus Luteum Regression via Endoplasmic Reticulum Stress and Autophagy. Front. Physiol. 2020, 11, 868. [Google Scholar] [CrossRef] [PubMed]
- Baddela, V.S.; Sharma, A.; Michaelis, M.; Vanselow, J. HIF1 driven transcriptional activity regulates steroidogenesis and proliferation of bovine granulosa cells. Sci. Rep. 2020, 10, 3906. [Google Scholar] [CrossRef]
- Gawriluk, T.R.; Rucker, E.B. BECN1, corpus luteum function, and preterm labor. Autophagy 2015, 11, 183–184. [Google Scholar] [CrossRef]
- Przygrodzka, E.; Monaco, C.; Plewes, M.R.; Li, G.; Wood, J.R.; Cupp, A.S.; Davis, J.S. Protein Kinase A and 5′ AMP-ActivatedProtein Kinase Signaling Pathways Exert Opposite Effects on Induction ofAutophagy in Luteal Cells. 2021; to be submitted. [Google Scholar]
- Doherty, J.; Baehrecke, E.H. Life, death and autophagy. Nat. Cell Biol. 2018, 20, 1110–1117. [Google Scholar] [CrossRef]
- Djavaheri-Mergny, M.; Maiuri, M.C.; Kroemer, G. Cross talk between apoptosis and autophagy by caspase-mediated cleavage of Beclin 1. Oncogene 2010, 29, 1717–1719. [Google Scholar] [CrossRef]
- Shi, C.S.; Kehrl, J.H. TRAF6 and A20 regulate lysine 63-linked ubiquitination of Beclin-1 to control TLR4-induced autophagy. Sci. Signal. 2010, 3, ra42. [Google Scholar] [CrossRef]
- Zalckvar, E.; Berissi, H.; Mizrachy, L.; Idelchuk, Y.; Koren, I.; Eisenstein, M.; Sabanay, H.; Pinkas-Kramarski, R.; Kimchi, A. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep. 2009, 10, 285–292. [Google Scholar] [CrossRef]
- Wei, Y.; Pattingre, S.; Sinha, S.; Bassik, M.; Levine, B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell 2008, 30, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhu, S.; Chen, P.; Hou, W.; Wen, Q.; Liu, J.; Xie, Y.; Liu, J.; Klionsky, D.J.; Kroemer, G.; et al. AMPK-Mediated BECN1 Phosphorylation Promotes Ferroptosis by Directly Blocking System Xc- Activity. Curr. Biol. 2018, 28, 2388–2399.e5. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przygrodzka, E.; Plewes, M.R.; Davis, J.S. Luteinizing Hormone Regulation of Inter-Organelle Communication and Fate of the Corpus Luteum. Int. J. Mol. Sci. 2021, 22, 9972. https://doi.org/10.3390/ijms22189972
Przygrodzka E, Plewes MR, Davis JS. Luteinizing Hormone Regulation of Inter-Organelle Communication and Fate of the Corpus Luteum. International Journal of Molecular Sciences. 2021; 22(18):9972. https://doi.org/10.3390/ijms22189972
Chicago/Turabian StylePrzygrodzka, Emilia, Michele R. Plewes, and John S. Davis. 2021. "Luteinizing Hormone Regulation of Inter-Organelle Communication and Fate of the Corpus Luteum" International Journal of Molecular Sciences 22, no. 18: 9972. https://doi.org/10.3390/ijms22189972
APA StylePrzygrodzka, E., Plewes, M. R., & Davis, J. S. (2021). Luteinizing Hormone Regulation of Inter-Organelle Communication and Fate of the Corpus Luteum. International Journal of Molecular Sciences, 22(18), 9972. https://doi.org/10.3390/ijms22189972




