Disease-Dependent Antiapoptotic Effects of Cannabidiol for Keratinocytes Observed upon UV Irradiation
Abstract
1. Introduction
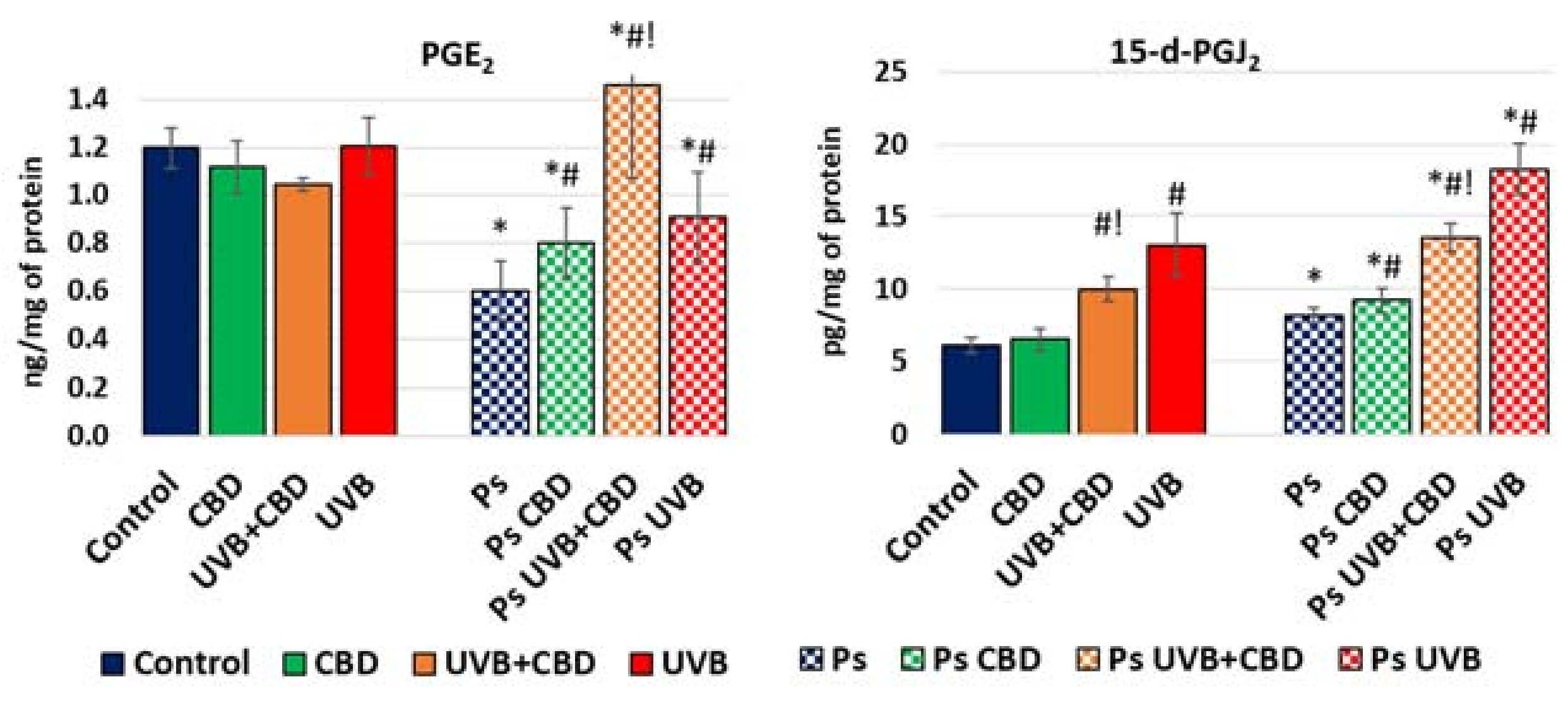
2. Results
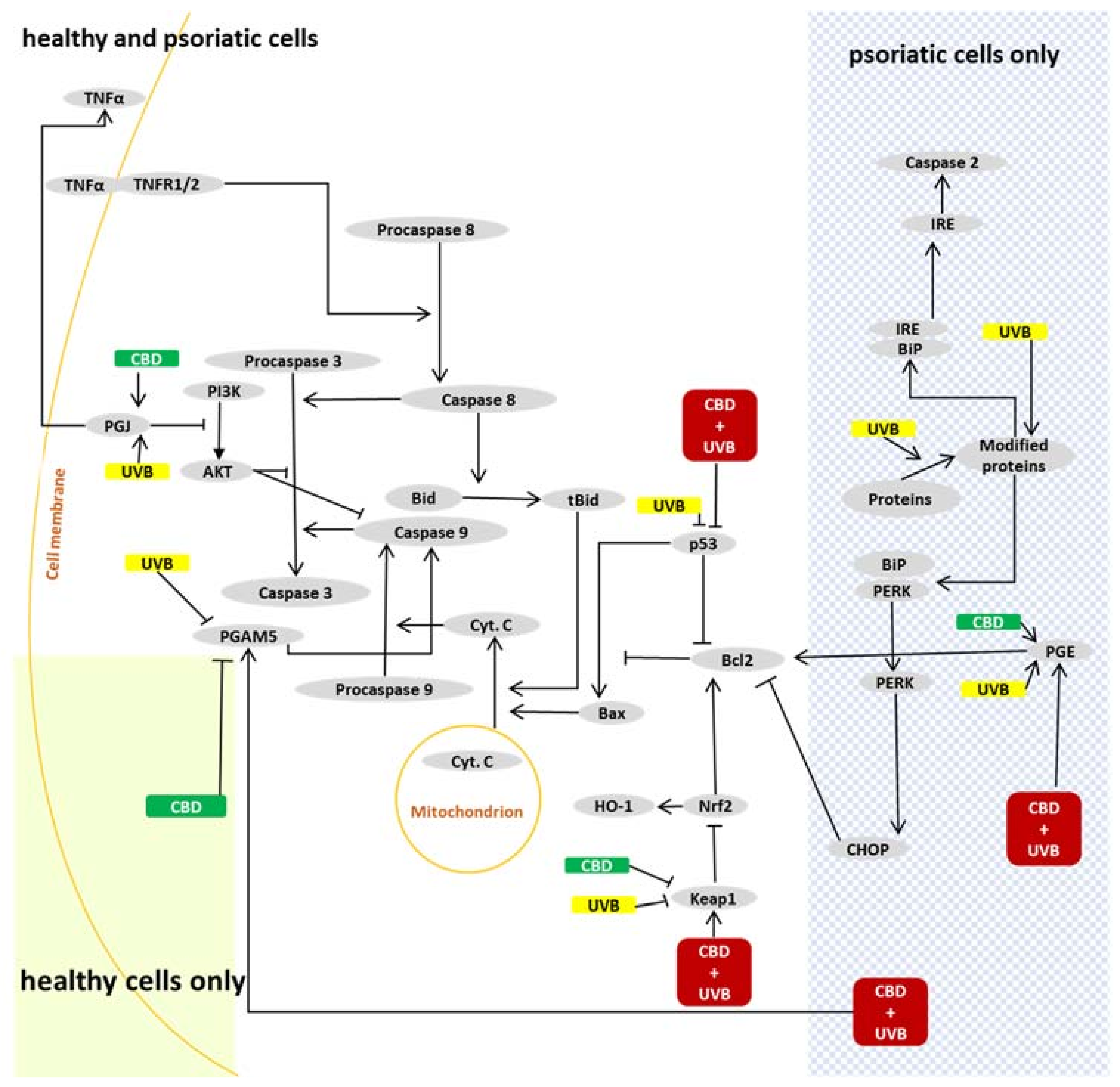
3. Discussion
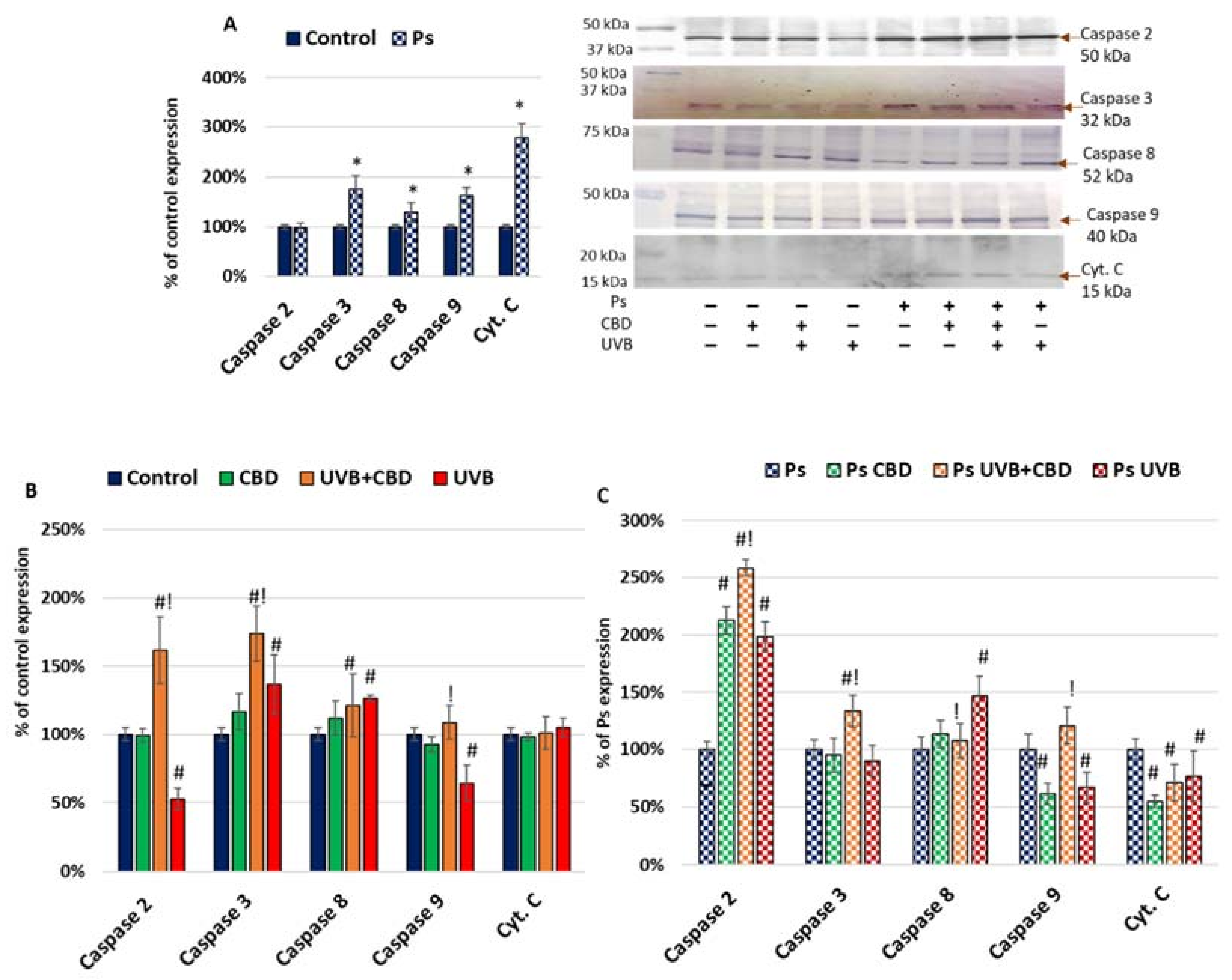
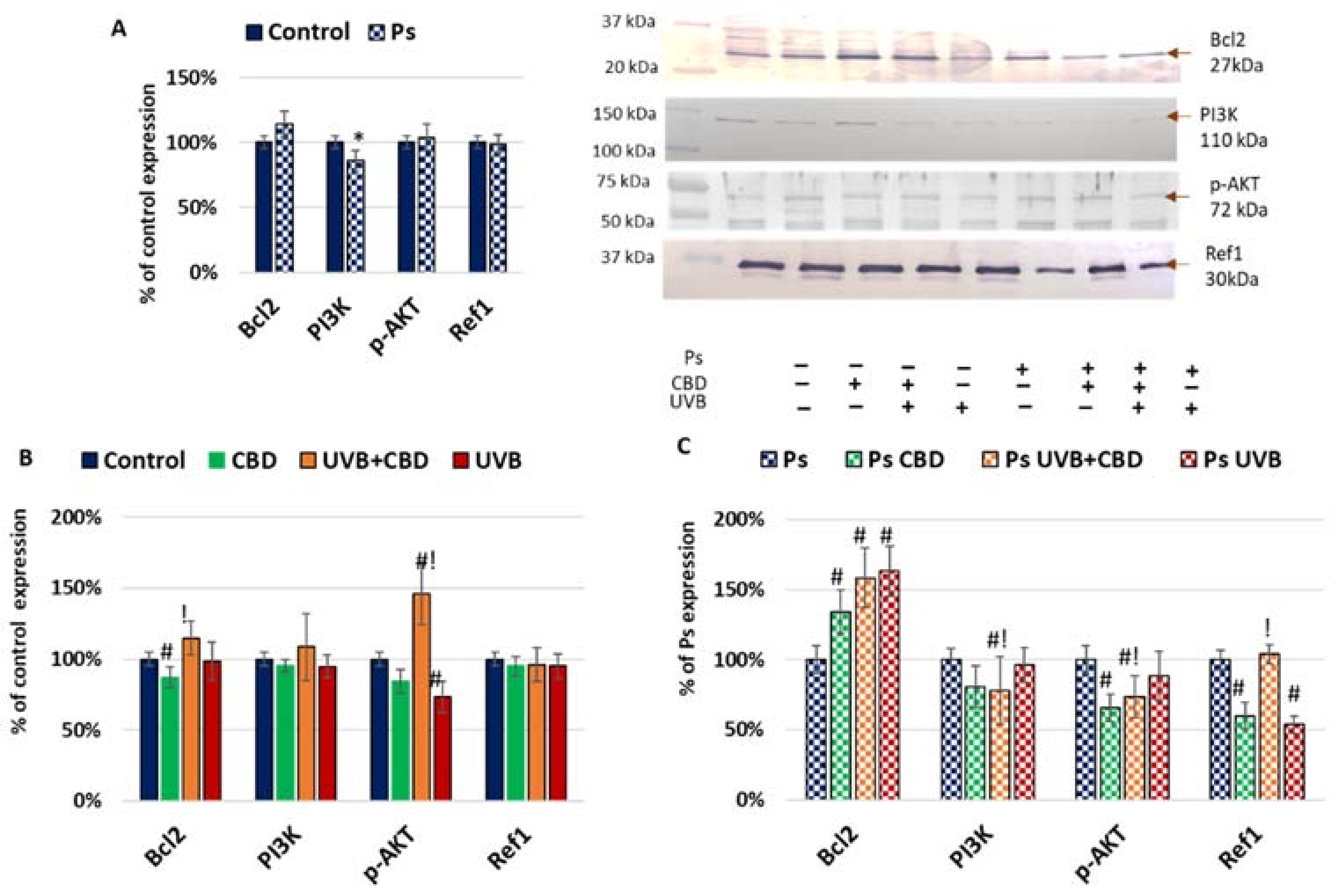
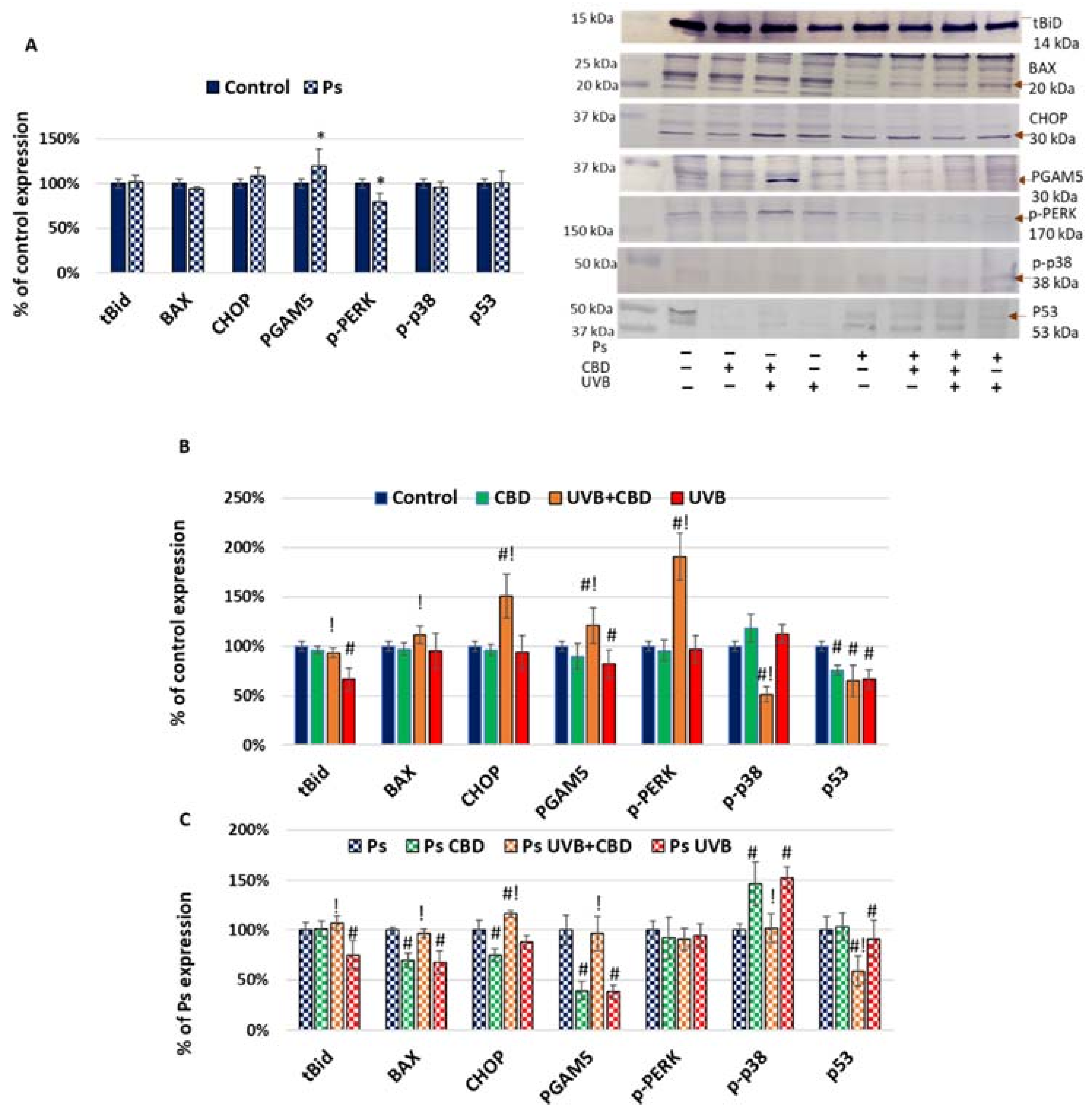
3.1. Apoptosis in Psoriatic Keratinocytes
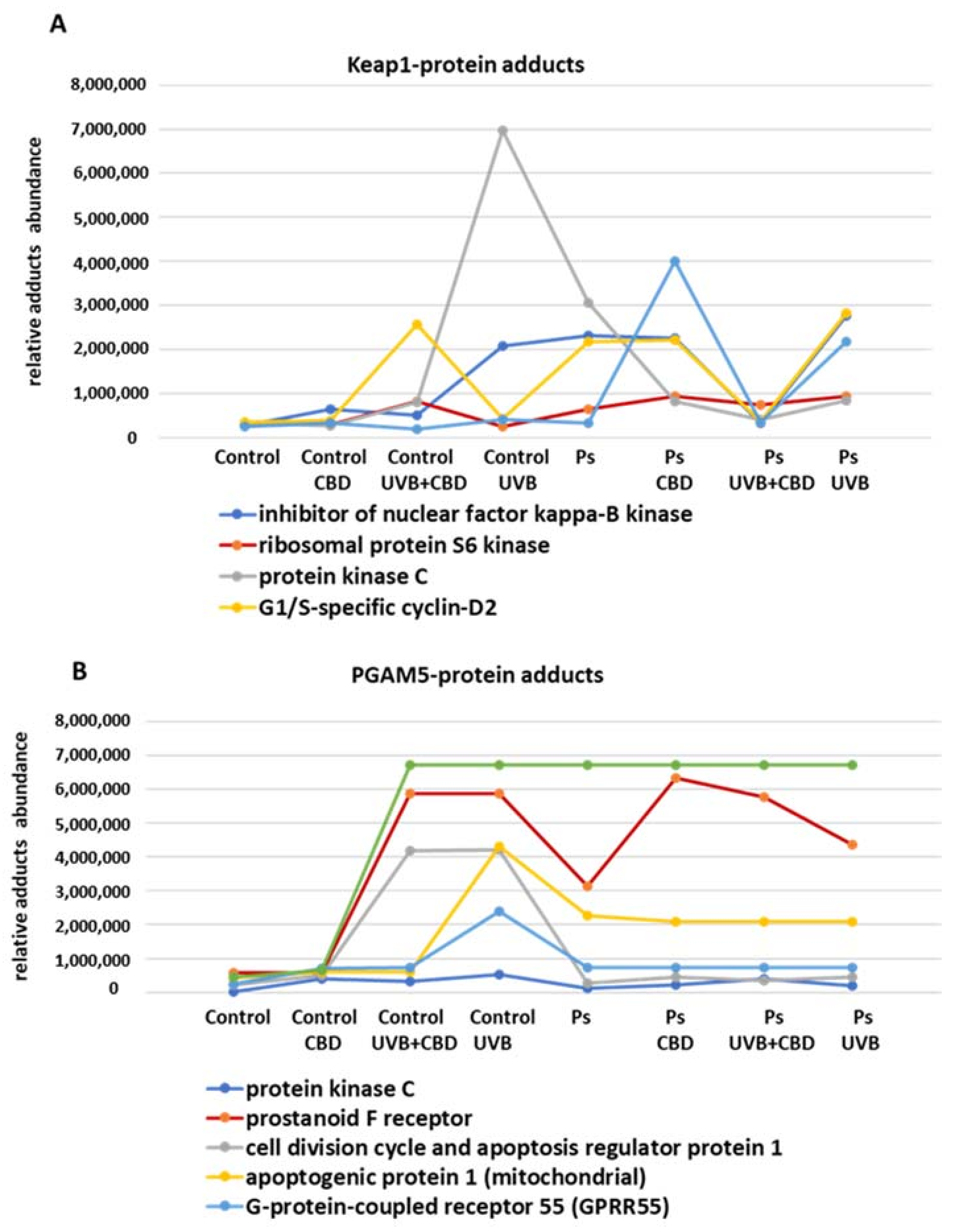
3.2. Effects of CBD or UVB Radiation on Apoptosis of Keratinocytes
3.3. Effects of CBD on Apoptosis in UVB-Irradiated Keratinocytes
4. Materials and Methods
4.1. Material
4.1.1. Patient/Donor Selection and Cell Line Acquisition
4.1.2. Cell Culture and Treatment
- Control keratinocytes (Control)—cultured in the medium described above;
- Control keratinocytes treated with CBD (CBD)—cells cultured for 24 h in the medium described above supplemented with 4 µM CBD;
- Control keratinocytes irradiated with UVB (UVB)—cells treated with UVB (60 mJ/cm2) and next cultured for 24 h in the medium described above;
- Control keratinocytes irradiated with UVB and treated with CBD (UVB+CBD)—cells treated with UVB and next cultured for 24 h in the medium described above supplemented with 4 µM CBD as in CBD group.
- ■
- Psoriatic keratinocytes (Ps)—cultured in the medium described above;
- ■
- Psoriatic keratinocytes treated with CBD (Ps CBD)—cells cultured for 24 h in the medium described above supplemented with 4 µM CBD;
- ■
- Psoriatic keratinocytes irradiated with UVB (Ps UVB)—cells treated with UVB and next cultured for 24 h in the medium described above;
- ■
- Psoriatic keratinocytes irradiated with UVB and treated with CBD (Ps UVB+CBD)—cells treated with UVB and next cultured for 24 h in the medium described above supplemented with 4 µM CBD as in Ps CBD group.
4.2. Methods
4.2.1. Determination of Protein Expression
4.2.2. Isolation of Keratinocyte Protein Adducts
4.2.3. Protein Adduct Identification by MS/MS
4.2.4. Determination of Prostaglandin Levels
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furue, K.; Ito, T.; Tsuji, G.; Nakahara, T.; Furue, M. The CCL20 and CCR6 Axis in Psoriasis. Scand. J. Immunol. 2020, 91, e12846. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell Death: A Review of the Major Forms of Apoptosis, Necrosis and Autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as Anticancer Mechanism: Function and Dysfunction of Its Modulators and Targeted Therapeutic Strategies. Aging (Albany NY) 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Sano, R.; Reed, J.C. ER Stress-Induced Cell Death Mechanisms. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2013, 1833, 3460–3470. [Google Scholar] [CrossRef]
- Wójcik, P.; Žarković, N.; Gęgotek, A.; Skrzydlewska, E. Involvement of Metabolic Lipid Mediators in the Regulation of Apoptosis. Biomolecules 2020, 10, 402. [Google Scholar] [CrossRef]
- Wu, C.-C.; Bratton, S.B. Regulation of the Intrinsic Apoptosis Pathway by Reactive Oxygen Species. Antioxid. Redox Signal. 2013, 19, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Viant, C.; Guia, S.; Hennessy, R.J.; Rautela, J.; Pham, K.; Bernat, C.; Goh, W.; Jiao, Y.; Delconte, R.; Roger, M.; et al. Cell Cycle Progression Dictates the Requirement for BCL2 in Natural Killer Cell Survival. J. Exp. Med. 2017, 214, 491–510. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, K.R.; McMillan, J.D.; Kumar, A. Emerging Roles of ER Stress and Unfolded Protein Response Pathways in Skeletal Muscle Health and Disease. J. Cell Physiol. 2018, 233, 67–78. [Google Scholar] [CrossRef]
- Bendavit, G.; Aboulkassim, T.; Hilmi, K.; Shah, S.; Batist, G. Nrf2 Transcription Factor Can Directly Regulate MTOR. J. Biol. Chem. 2016, 291, 25476–25488. [Google Scholar] [CrossRef]
- Luo, J.-L.; Kamata, H.; Karin, M. IKK/NF-ΚB Signaling: Balancing Life and Death—A New Approach to Cancer Therapy. J. Clin. Investig. 2005, 115, 2625–2632. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-F.; Kuo, H.-P.; Liu, M.; Chou, C.-K.; Xia, W.; Du, Y.; Shen, J.; Chen, C.-T.; Huo, L.; Hsu, M.-C.; et al. KEAP1 E3 Ligase-Mediated Down-Regulation of NF-ΚB Signaling by Targeting IKKβ. Mol. Cell 2009, 36, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.; You, D.-J.; Lee, C.; Ahn, C.; Seong, J.Y.; Hwang, J.-I. Suppression of NF-KappaB Signaling by KEAP1 Regulation of IKKbeta Activity through Autophagic Degradation and Inhibition of Phosphorylation. Cell Signal. 2010, 22, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Tami Wong, B.S.; Leon Hsu, B.A.; Wilson Liao, M.D. Phototherapy in Psoriasis: A Review of Mechanisms of Action. J. Cutan. Med. Surg. 2013, 17, 6–12. [Google Scholar] [CrossRef]
- Lee, C.-H.; Wu, S.-B.; Hong, C.-H.; Yu, H.-S.; Wei, Y.-H. Molecular Mechanisms of UV-Induced Apoptosis and Its Effects on Skin Residential Cells: The Implication in UV-Based Phototherapy. Int. J. Mol. Sci. 2013, 14, 6414–6435. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Cencioni, M.T.; Bisicchia, E.; Bardi, M.D.; Gasperini, C.; Borsellino, G.; Centonze, D.; Battistini, L.; Maccarrone, M. Distinct Modulation of Human Myeloid and Plasmacytoid Dendritic Cells by Anandamide in Multiple Sclerosis. Ann. Neurol. 2013, 73, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.C.; Rosas, E.C. Cannabinoid Receptors as Regulators of Neutrophil Activity in Inflammatory Diseases. Neutrophils 2018. [Google Scholar] [CrossRef]
- Martinelli, G.; Magnavacca, A.; Fumagalli, M.; Dell’Agli, M.; Piazza, S.; Sangiovanni, E. Cannabis Sativa and Skin Health: Dissecting the Role of Phytocannabinoids. Planta Med. 2021. [Google Scholar] [CrossRef]
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic Potential of Cannabidiol (CBD) for Skin Health and Disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927–942. [Google Scholar] [CrossRef]
- Jarocka-Karpowicz, I.; Biernacki, M.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Effects on Phospholipid Metabolism in Keratinocytes from Patients with Psoriasis Vulgaris. Biomolecules 2020, 10, 367. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Dondelinger, Y.; Aguileta, M.A.; Goossens, V.; Dubuisson, C.; Grootjans, S.; Dejardin, E.; Vandenabeele, P.; Bertrand, M.J.M. RIPK3 Contributes to TNFR1-Mediated RIPK1 Kinase-Dependent Apoptosis in Conditions of CIAP1/2 Depletion or TAK1 Kinase Inhibition. Cell Death Differ. 2013, 20, 1381–1392. [Google Scholar] [CrossRef]
- Atalay, S.; Gęgotek, A.; Wroński, A.; Domigues, P.; Skrzydlewska, E. Therapeutic Application of Cannabidiol on UVA and UVB Irradiated Rat Skin. A Proteomic Study. J. Pharm. Biomed. Anal. 2021, 192, 113656. [Google Scholar] [CrossRef]
- Atalay, S.; Dobrzyńska, I.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Protects Keratinocyte Cell Membranes Following Exposure to UVB and Hydrogen Peroxide. Redox Biol. 2020, 36, 101613. [Google Scholar] [CrossRef]
- Siddiqui, W.A.; Ahad, A.; Ahsan, H. The Mystery of BCL2 Family: Bcl-2 Proteins and Apoptosis: An Update. Arch. Toxicol. 2015, 89, 289–317. [Google Scholar] [CrossRef]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-Talk between Apoptosis and Autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Knatko, E.V.; Ibbotson, S.H.; Zhang, Y.; Higgins, M.; Fahey, J.W.; Talalay, P.; Dawe, R.S.; Ferguson, J.; Huang, J.T.-J.; Clarke, R.; et al. Nrf2 Activation Protects against Solar-Simulated Ultraviolet Radiation in Mice and Humans. Cancer Prev. Res. (Phila) 2015, 8, 475–486. [Google Scholar] [CrossRef]
- Hirota, A.; Kawachi, Y.; Itoh, K.; Nakamura, Y.; Xu, X.; Banno, T.; Takahashi, T.; Yamamoto, M.; Otsuka, F. Ultraviolet A Irradiation Induces NF-E2-Related Factor 2 Activation in Dermal Fibroblasts: Protective Role in UVA-Induced Apoptosis. J. Investig. Dermatol. 2005, 124, 825–832. [Google Scholar] [CrossRef]
- Jia, H.-Y.; Zhang, K.; Lu, W.-J.; Xu, G.-W.; Zhang, J.-F.; Tang, Z.-L. LncRNA MEG3 Influences the Proliferation and Apoptosis of Psoriasis Epidermal Cells by Targeting MiR-21/Caspase-8. BMC Mol. Cell Biol. 2019, 20, 46. [Google Scholar] [CrossRef]
- Huang, T.-H.; Lin, C.-F.; Alalaiwe, A.; Yang, S.-C.; Fang, J.-Y. Apoptotic or Antiproliferative Activity of Natural Products against Keratinocytes for the Treatment of Psoriasis. Int. J. Mol. Sci. 2019, 20, 2558. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nadella, P.; Kim, D.J.; Brodmerkel, C.; Correa da Rosa, J.; Krueger, J.G.; Suárez-Fariñas, M. Histological Stratification of Thick and Thin Plaque Psoriasis Explores Molecular Phenotypes with Clinical Implications. PLoS ONE 2015, 10, e0132454. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, M.X. A Clinical Review of Phototherapy for Psoriasis. Lasers Med. Sci. 2018, 33, 173–180. [Google Scholar] [CrossRef]
- Rane, M.J.; Song, Y.; Jin, S.; Barati, M.T.; Wu, R.; Kausar, H.; Tan, Y.; Wang, Y.; Zhou, G.; Klein, J.B.; et al. Interplay between Akt and P38 MAPK Pathways in the Regulation of Renal Tubular Cell Apoptosis Associated with Diabetic Nephropathy. Am. J. Physiol. Ren. Physiol. 2010, 298, F49–F61. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J. Caspases in Apoptosis and Beyond. Oncogene 2008, 27, 6194–6206. [Google Scholar] [CrossRef] [PubMed]
- Rupniewska, Z.; Bojarska-Junak, A. Apoptosis: Mitochondrial membrane permeabilization and the role played by Bcl-2 family proteins. Postepy Hig. Med. Dosw. (Online) 2004, 58, 538–547. [Google Scholar] [PubMed]
- Li, X.; Miao, X.; Wang, H.; Xu, Z.; Li, B. The Tissue Dependent Interactions between P53 and Bcl-2 in Vivo. Oncotarget 2015, 6, 35699–35709. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Kanneganti, T.-D. Caspase-7: A Protease Involved in Apoptosis and Inflammation. Int. J. Biochem. Cell Biol. 2010, 42, 21–24. [Google Scholar] [CrossRef]
- Mérino, D.; Lalaoui, N.; Morizot, A.; Schneider, P.; Solary, E.; Micheau, O. Differential Inhibition of TRAIL-Mediated DR5-DISC Formation by Decoy Receptors 1 and 2. Mol. Cell. Biol. 2006, 26, 7046–7055. [Google Scholar] [CrossRef] [PubMed]
- Chima, M.; Lebwohl, M. TNF Inhibitors for Psoriasis. Semin. Cutan. Med. Surg. 2018, 37, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Koyani, C.N.; Windischhofer, W.; Rossmann, C.; Jin, G.; Kickmaier, S.; Heinzel, F.R.; Groschner, K.; Alavian-Ghavanini, A.; Sattler, W.; Malle, E. 15-Deoxy-Δ12,14-PGJ2 Promotes Inflammation and Apoptosis in Cardiomyocytes via the DP2/MAPK/TNFα Axis. Int. J. Cardiol. 2014, 173, 472–480. [Google Scholar] [CrossRef][Green Version]
- Joo, H.W.; Kang, Y.R.; Kwack, M.H.; Sung, Y.K. 15-Deoxy Prostaglandin J2, the Nonenzymatic Metabolite of Prostaglandin D2, Induces Apoptosis in Keratinocytes of Human Hair Follicles: A Possible Explanation for Prostaglandin D2-Mediated Inhibition of Hair Growth. Naunyn-Schmiedebergs Arch. Pharmacol. 2016, 389, 809–813. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, M.S.; Park, J.Y.; Woo, J.S.; Kim, Y.K. 15-Deoxy-Δ12,14-Prostaglandin J2 Induces Apoptosis via JNK-Mediated Mitochondrial Pathway in Osteoblastic Cells. Toxicology 2008, 248, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, P.; Biernacki, M.; Wroński, A.; Łuczaj, W.; Waeg, G.; Žarković, N.; Skrzydlewska, E. Altered Lipid Metabolism in Blood Mononuclear Cells of Psoriatic Patients Indicates Differential Changes in Psoriasis Vulgaris and Psoriatic Arthritis. Int. J. Mol. Sci. 2019, 20, 4249. [Google Scholar] [CrossRef]
- Ambrożewicz, E.; Wójcik, P.; Wroński, A.; Łuczaj, W.; Jastrząb, A.; Žarković, N.; Skrzydlewska, E. Pathophysiological Alterations of Redox Signaling and Endocannabinoid System in Granulocytes and Plasma of Psoriatic Patients. Cells 2018, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Lenhausen, A.M.; Wilkinson, A.S.; Lewis, E.M.; Dailey, K.M.; Scott, A.J.; Khan, S.; Wilkinson, J.C. Apoptosis Inducing Factor Binding Protein PGAM5 Triggers Mitophagic Cell Death That Is Inhibited by the Ubiquitin Ligase Activity of X-Linked Inhibitor of Apoptosis. Biochemistry 2016, 55, 3285–3302. [Google Scholar] [CrossRef]
- Guo, X.; Sesaki, H.; Qi, X. Drp1 Stabilizes P53 on the Mitochondria to Trigger Necrosis under Oxidative Stress Conditions in Vitro and in Vivo. Biochem. J. 2014, 461, 137–146. [Google Scholar] [CrossRef]
- Liu, A.; Zhao, W.; Zhang, B.; Tu, Y.; Wang, Q.; Li, J. Cimifugin Ameliorates Imiquimod-Induced Psoriasis by Inhibiting Oxidative Stress and Inflammation via NF-ΚB/MAPK Pathway. Biosci. Rep. 2020, 40, BSR20200471. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kang, D.H.; Lee, D.H.; Bae, S.H. PF-4708671, a Specific Inhibitor of P70 Ribosomal S6 Kinase 1, Activates Nrf2 by Promoting P62-Dependent Autophagic Degradation of Keap1. Biochem. Biophys. Res. Commun. 2015, 466, 499–504. [Google Scholar] [CrossRef]
- Dobrzyńska, I.; Szachowicz-Petelska, B.; Skrzydlewska, E.; Figaszewski, Z.A. Effects of UVB Radiation on the Physicochemical Properties of Fibroblasts and Keratinocytes. J. Membr. Biol. 2016, 249, 319–325. [Google Scholar] [CrossRef]
- Kim, K.M.; Im, A.-R.; Park, S.K.; Shin, H.S.; Chae, S.-W. Protective Effects of Timosaponin AIII against UVB-Radiation Induced Inflammation and DNA Injury in Human Epidermal Keratinocytes. Biol. Pharm. Bull. 2019, 42, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Soonthornchai, W.; Tangtanatakul, P.; Meephansan, J.; Ruchusatsawat, K.; Reantragoon, R.; Hirankarn, N.; Wongpiyabovorn, J. Down-Regulation of MiR-155 after Treatment with Narrow-Band UVB and Methotrexate Associates with Apoptosis of Keratinocytes in Psoriasis. Asian Pac. J. Allergy Immunol. 2019. [Google Scholar] [CrossRef]
- Morita, A. Current Developments in Phototherapy for Psoriasis. J. Dermatol. 2018, 45, 287–292. [Google Scholar] [CrossRef]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol Enhances Anandamide Signaling and Alleviates Psychotic Symptoms of Schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef]
- Chan, J.Z.; Duncan, R.E. Regulatory Effects of Cannabidiol on Mitochondrial Functions: A Review. Cells 2021, 10, 1251. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, D.; Zhuang, J.; Zhang, F.; Xu, C. Caspase-8 and Caspase-9 Functioned Differently at Different Stages of the Cyclic Stretch-Induced Apoptosis in Human Periodontal Ligament Cells. PLoS ONE 2016, 11, e0168268. [Google Scholar] [CrossRef]
- Deshmukh, J.; Pofahl, R.; Haase, I. Epidermal Rac1 Regulates the DNA Damage Response and Protects from UV-Light-Induced Keratinocyte Apoptosis and Skin Carcinogenesis. Cell Death Dis. 2017, 8, e2664. [Google Scholar] [CrossRef] [PubMed]
- Noh, D.; Choi, J.G.; Huh, E.; Oh, M.S. Tectorigenin, a Flavonoid-Based Compound of Leopard Lily Rhizome, Attenuates UV-B-Induced Apoptosis and Collagen Degradation by Inhibiting Oxidative Stress in Human Keratinocytes. Nutrients 2018, 10, 1998. [Google Scholar] [CrossRef] [PubMed]
- Rasheva, V.I.; Domingos, P.M. Cellular Responses to Endoplasmic Reticulum Stress and Apoptosis. Apoptosis 2009, 14, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Collier, A.E.; Wek, R.C.; Spandau, D.F. Translational Repression Protects Human Keratinocytes from UVB-Induced Apoptosis through a Discordant EIF2 Kinase Stress Response. J. Investig. Dermatol. 2015, 135, 2502–2511. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Ahmed, H.; Yang, P.; Czinn, S.J.; Blanchard, T.G. Endoplasmic Reticulum Stress and IRE-1 Signaling Cause Apoptosis in Colon Cancer Cells in Response to Andrographolide Treatment. Oncotarget 2016, 7, 41432–41444. [Google Scholar] [CrossRef]
- Petrovici, A.R.; Simionescu, N.; Sandu, A.I.; Paraschiv, V.; Silion, M.; Pinteala, M. New Insights on Hemp Oil Enriched in Cannabidiol: Decarboxylation, Antioxidant Properties and In Vitro Anticancer Effect. Antioxidants 2021, 10, 738. [Google Scholar] [CrossRef]
- Niture, S.K.; Jaiswal, A.K. Nrf2-Induced Antiapoptotic Bcl-XL Protein Enhances Cell Survival and Drug Resistance. Free Radic. Biol. Med. 2013, 57, 119–131. [Google Scholar] [CrossRef]
- Murata, K.; Oyama, M.; Ogata, M.; Fujita, N.; Takahashi, R. Oral Administration of Jumihaidokuto Inhibits UVB-Induced Skin Damage and Prostaglandin E2 Production in HR-1 Hairless Mice. J. Nat. Med. 2021, 75, 142–155. [Google Scholar] [CrossRef]
- Coras, R.; Kavanaugh, A.; Boyd, T.; Huynh, Q.; Pedersen, B.; Armando, A.M.; Dahlberg-Wright, S.; Marsal, S.; Jain, M.; Paravar, T.; et al. Pro- and Anti-Inflammatory Eicosanoids in Psoriatic Arthritis. Metabolomics 2019, 15, 65. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Bode, A.M.; Zhao, Q.; Cho, Y.-Y.; Zhu, F.; Ma, W.-Y.; Dong, Z. The Cannabinoid Receptors Are Required for UV-Induced Inflammation and Skin Cancer Development. Cancer Res. 2008, 68, 3992–3998. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, E.; Martín-Ruiz, A.; Martín, P.; Calvo, V.; Provencio, M.; García, J.M. CB2 Cannabinoid Receptor Activation Promotes Colon Cancer Progression via AKT/GSK3β Signaling Pathway. Oncotarget 2016, 7, 68781–68791. [Google Scholar] [CrossRef]
- Derkinderen, P.; Ledent, C.; Parmentier, M.; Girault, J.A. Cannabinoids Activate P38 Mitogen-Activated Protein Kinases through CB1 Receptors in Hippocampus. J. Neurochem. 2001, 77, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Y.; Zhao, H.; Zheng, Q.; Xiao, L.; Zhao, M. CB2 Receptor Activation Ameliorates the Proinflammatory Activity in Acute Lung Injury Induced by Paraquat. BioMed Res. Int. 2014, 2014, e971750. [Google Scholar] [CrossRef] [PubMed]
- Ruhaak, L.R.; Felth, J.; Karlsson, P.C.; Rafter, J.J.; Verpoorte, R.; Bohlin, L. Evaluation of the Cyclooxygenase Inhibiting Effects of Six Major Cannabinoids Isolated from Cannabis Sativa. Biol. Pharm. Bull. 2011, 34, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Black, A.T.; Gray, J.P.; Shakarjian, M.P.; Mishin, V.; Laskin, D.L.; Heck, D.E.; Laskin, J.D. UVB Light Upregulates Prostaglandin Synthases and Prostaglandin Receptors in Mouse Keratinocytes. Toxicol. Appl. Pharmacol. 2008, 232, 14–24. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as Novel Anti-Inflammatory Drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Fumagalli, M.; Pacchetti, B.; Piazza, S.; Magnavacca, A.; Khalilpour, S.; Melzi, G.; Martinelli, G.; Dell’Agli, M. Cannabis Sativa L. Extract and Cannabidiol Inhibit in Vitro Mediators of Skin Inflammation and Wound Injury. Phytother. Res. 2019, 33, 2083–2093. [Google Scholar] [CrossRef]
- Sun, Y.; He, L.; Wang, T.; Hua, W.; Qin, H.; Wang, J.; Wang, L.; Gu, W.; Li, T.; Li, N.; et al. Activation of P62-Keap1-Nrf2 Pathway Protects 6-Hydroxydopamine-Induced Ferroptosis in Dopaminergic Cells. Mol. Neurobiol. 2020, 57, 4628–4641. [Google Scholar] [CrossRef]
- Casares, L.; García, V.; Garrido-Rodríguez, M.; Millán, E.; Collado, J.A.; García-Martín, A.; Peñarando, J.; Calzado, M.A.; de la Vega, L.; Muñoz, E. Cannabidiol Induces Antioxidant Pathways in Keratinocytes by Targeting BACH1. Redox Biol. 2020, 28, 101321. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Heinemann, K.; Merkord, J.; Rohde, H.; Salamon, A.; Linnebacher, M.; Hinz, B. COX-2 and PPAR-γ Confer Cannabidiol-Induced Apoptosis of Human Lung Cancer Cells. Mol. Cancer Ther. 2013, 12, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Pucci, M.; Rapino, C.; Di Francesco, A.; Dainese, E.; D’Addario, C.; Maccarrone, M. Epigenetic Control of Skin Differentiation Genes by Phytocannabinoids. Br. J. Pharmacol. 2013, 170, 581–591. [Google Scholar] [CrossRef]
- Rieder, S.A.; Chauhan, A.; Singh, U.; Nagarkatti, M.; Nagarkatti, P. Cannabinoid-Induced Apoptosis in Immune Cells as a Pathway to Immunosuppression. Immunobiology 2010, 215, 598–605. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Chu, R.-M.; Wang, C.-C.; Lee, C.-Y.; Lin, S.-H.; Jan, T.-R. Cannabidiol-Induced Apoptosis in Primary Lymphocytes Is Associated with Oxidative Stress-Dependent Activation of Caspase-8. Toxicol. Appl. Pharmacol. 2008, 226, 260–270. [Google Scholar] [CrossRef]
- Gęgotek, A.; Domingues, P.; Wroński, A.; Ambrożewicz, E.; Skrzydlewska, E. The Proteomic Profile of Keratinocytes and Lymphocytes in Psoriatic Patients. Proteom. Clin. Appl. 2019, 13, 1800119. [Google Scholar] [CrossRef] [PubMed]
- Behrens, S.; Grundmann-Kollmann, M.; Schiener, R.; Peter, R.U.; Kerscher, M. Combination Phototherapy of Psoriasis with Narrow-Band UVB Irradiation and Topical Tazarotene Gel. J. Am. Acad. Dermatol. 2000, 42, 493–495. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In Vitro Cytotoxicity Assays: Comparison of LDH, Neutral Red, MTT and Protein Assay in Hepatoma Cell Lines following Exposure to Cadmium Chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Regulates the Expression of Keratinocyte Proteins Involved in the Inflammation Process through Transcriptional Regulation. Cells 2019, 8, 827. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Seada, L.S. Quantitation of Bcl-2 Protein in Bladder Cancer Tissue by Enzyme Immunoassay: Comparison with Western Blot and Immunohistochemistry. Clin. Chem. 1998, 44, 1423–1429. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gęgotek, A.; Domingues, P.; Wroński, A.; Wójcik, P.; Skrzydlewska, E. Proteomic Plasma Profile of Psoriatic Patients. J. Pharm. Biomed. Anal. 2018, 155, 185–193. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik, P.; Gęgotek, A.; Žarković, N.; Skrzydlewska, E. Disease-Dependent Antiapoptotic Effects of Cannabidiol for Keratinocytes Observed upon UV Irradiation. Int. J. Mol. Sci. 2021, 22, 9956. https://doi.org/10.3390/ijms22189956
Wójcik P, Gęgotek A, Žarković N, Skrzydlewska E. Disease-Dependent Antiapoptotic Effects of Cannabidiol for Keratinocytes Observed upon UV Irradiation. International Journal of Molecular Sciences. 2021; 22(18):9956. https://doi.org/10.3390/ijms22189956
Chicago/Turabian StyleWójcik, Piotr, Agnieszka Gęgotek, Neven Žarković, and Elżbieta Skrzydlewska. 2021. "Disease-Dependent Antiapoptotic Effects of Cannabidiol for Keratinocytes Observed upon UV Irradiation" International Journal of Molecular Sciences 22, no. 18: 9956. https://doi.org/10.3390/ijms22189956
APA StyleWójcik, P., Gęgotek, A., Žarković, N., & Skrzydlewska, E. (2021). Disease-Dependent Antiapoptotic Effects of Cannabidiol for Keratinocytes Observed upon UV Irradiation. International Journal of Molecular Sciences, 22(18), 9956. https://doi.org/10.3390/ijms22189956







