Oral Semaglutide, the First Ingestible Glucagon-Like Peptide-1 Receptor Agonist: Could It Be a Magic Bullet for Type 2 Diabetes?
Abstract
:1. Introduction
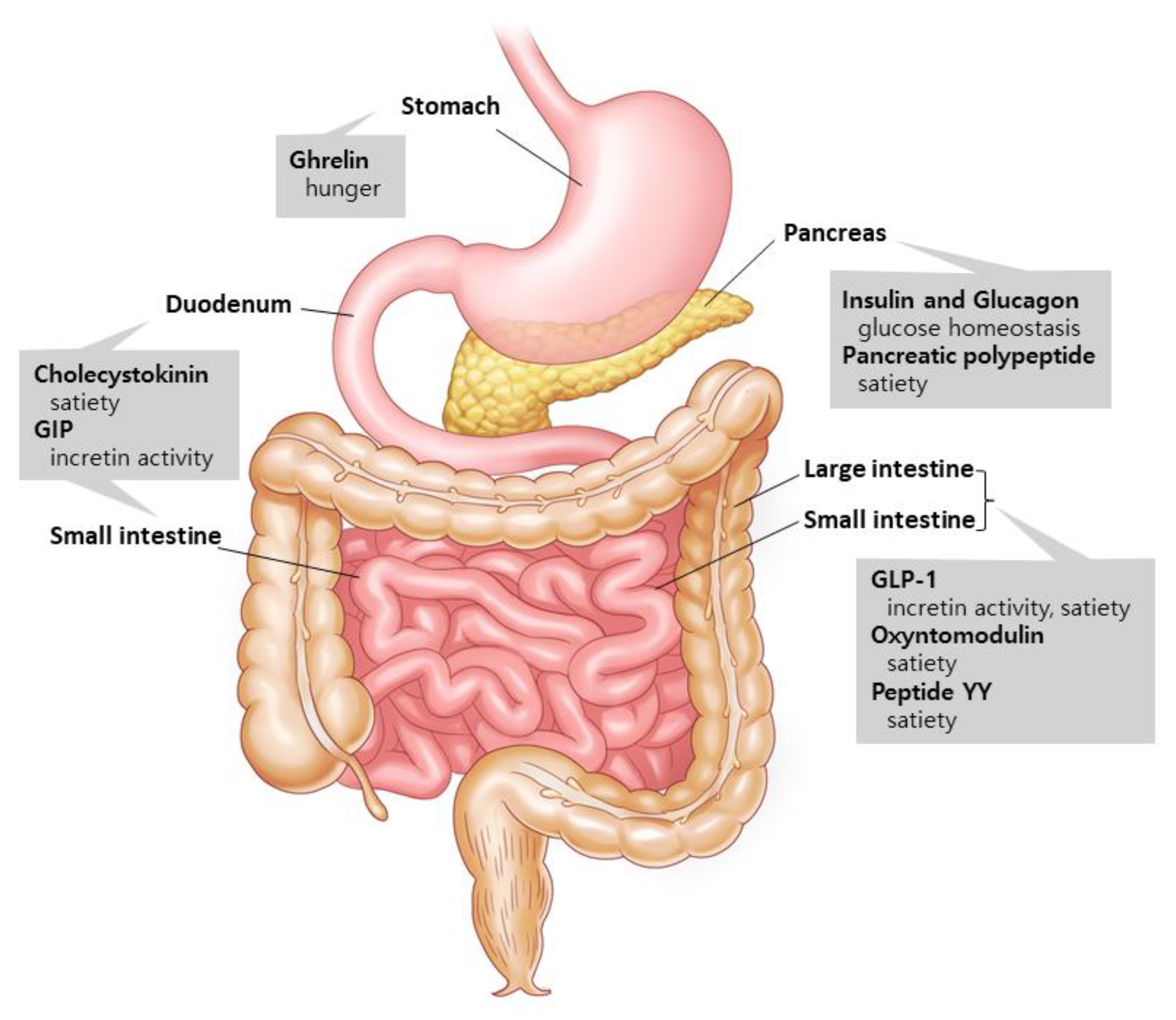
1.1. Gut Hormones: The Metabolism Regulators
1.2. Glucagon-like Peptide-1 (GLP-1): An Innovator for Gut Hormone Therapies
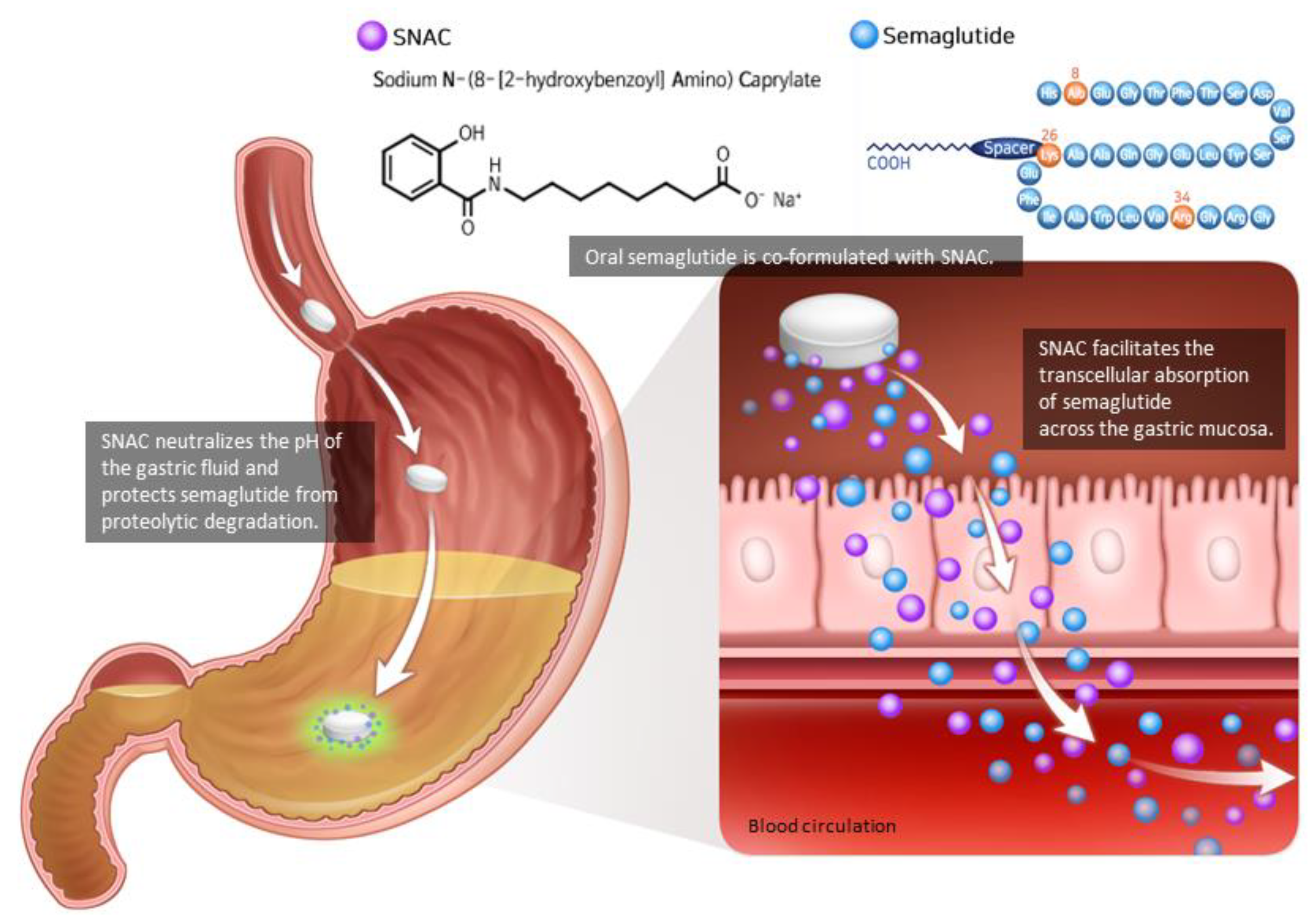
2. Pharmacodynamics and Pharmacokinetics of Oral Semaglutide
3. Clinical Efficacy and Safety: Summary of Peptide Innovation for Early Diabetes Treatment (PIONEER) Trials
3.1. Placebo-Controlled Trials
3.1.1. Monotherapy
3.1.2. Combination Therapy
3.2. Active-Comparator Trials
3.2.1. Sodium Glucose Co-Transporter 2 (SGLT-2) Inhibitors
3.2.2. DPP-4 Inhibitors
3.2.3. Injectable GLP-1RAs
4. Special Populations
5. Cardiovascular Outcomes with Oral Semaglutide
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Marx, N.; Davies, M.J.; Grant, P.J.; Mathieu, C.; Petrie, J.R.; Cosentino, F.; Buse, J.B. Guideline recommendations and the positioning of newer drugs in type 2 diabetes care. Lancet Diabetes Endocrinol. 2021, 9, 46–52. [Google Scholar] [CrossRef]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 487–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hur, K.Y.; Moon, M.K.; Park, J.S.; Kim, S.K.; Lee, S.H.; Yun, J.S.; Baek, J.H.; Noh, J.; Lee, B.W.; Oh, T.J.; et al. 2021 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association. Diabetes Metab. J. 2021, 45, 461–481. [Google Scholar] [CrossRef]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.G.; Bloom, S.R. Gut hormones and the regulation of energy homeostasis. Nature 2006, 444, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Lee, H.W.; Choi, H.J. GLP-1 Based Combination Therapy for Obesity and Diabetes. J. Obes. Metab. Syndr. 2017, 26, 155–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavrieli, A.; Mantzoros, C.S. Novel Molecules Regulating Energy Homeostasis: Physiology and Regulation by Macronutrient Intake and Weight Loss. Endocrinol. Metab. 2016, 31, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Brennan, I.M.; Luscombe-Marsh, N.D.; Seimon, R.V.; Otto, B.; Horowitz, M.; Wishart, J.M.; Feinle-Bisset, C. Effects of fat, protein, and carbohydrate and protein load on appetite, plasma cholecystokinin, peptide YY, and ghrelin, and energy intake in lean and obese men. Am. J. Physiol. Gastrointest Liver Physiol. 2012, 303, G129–G140. [Google Scholar] [CrossRef]
- Yu, J.H.; Kim, M.S. Molecular mechanisms of appetite regulation. Diabetes Metab. J. 2012, 36, 391–398. [Google Scholar] [CrossRef]
- Day, J.W.; Ottaway, N.; Patterson, J.T.; Gelfanov, V.; Smiley, D.; Gidda, J.; Findeisen, H.; Bruemmer, D.; Drucker, D.J.; Chaudhary, N.; et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol. 2009, 5, 749–757. [Google Scholar] [CrossRef]
- Wynne, K.; Park, A.; Small, C.; Meeran, K.; Ghatei, M.; Frost, G.; Bloom, S. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: A randomised controlled trial. Int. J. Obes. 2006, 30, 1729–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankar, S.S.; Shankar, R.R.; Mixson, L.A.; Miller, D.L.; Pramanik, B.; O’Dowd, A.K.; Williams, D.M.; Frederick, C.B.; Beals, C.R.; Stoch, S.A.; et al. Native Oxyntomodulin Has Significant Glucoregulatory Effects Independent of Weight Loss in Obese Humans with and without Type 2 Diabetes. Diabetes 2018, 67, 1105–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gribble, F.M.; Reimann, F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat. Rev. Endocrinol. 2019, 15, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Tasyurek, H.M.; Altunbas, H.A.; Balci, M.K.; Sanlioglu, S. Incretins: Their physiology and application in the treatment of diabetes mellitus. Diabetes Metab. Res. Rev. 2014, 30, 354–371. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 5–21. [Google Scholar] [CrossRef]
- Kieffer, T.J.; Habener, J.F. The glucagon-like peptides. Endocr. Rev. 1999, 20, 876–913. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Heimesaat, M.M.; Orskov, C.; Holst, J.J.; Ebert, R.; Creutzfeldt, W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Investig. 1993, 91, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Christensen, M.; Vedtofte, L.; Holst, J.J.; Vilsbøll, T.; Knop, F.K. Glucose-dependent insulinotropic polypeptide: A bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes 2011, 60, 3103–3109. [Google Scholar] [CrossRef] [Green Version]
- Nauck, M.; Kemmeries, G.; Holst, J.; Meier, J. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 2011, 60, 1561–1565. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef] [Green Version]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Spain, C.V.; Wright, J.J.; Hahn, R.M.; Wivel, A.; Martin, A.A. Self-reported Barriers to Adherence and Persistence to Treatment with Injectable Medications for Type 2 Diabetes. Clin. Ther. 2016, 38, 1653–1664.e1. [Google Scholar] [CrossRef] [Green Version]
- Guerci, B.; Chanan, N.; Kaur, S.; Jasso-Mosqueda, J.G.; Lew, E. Lack of Treatment Persistence and Treatment Nonadherence as Barriers to Glycaemic Control in Patients with Type 2 Diabetes. Diabetes Ther. 2019, 10, 437–449. [Google Scholar] [CrossRef] [Green Version]
- Kapitza, C.; Nosek, L.; Jensen, L.; Hartvig, H.; Jensen, C.B.; Flint, A. Semaglutide, a once-weekly human GLP-1 analog, does not reduce the bioavailability of the combined oral contraceptive, ethinylestradiol/levonorgestrel. J. Clin. Pharmacol. 2015, 55, 497–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorli, C.; Harashima, S.I.; Tsoukas, G.M.; Unger, J.; Karsbøl, J.D.; Hansen, T.; Bain, S.C. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): A double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017, 5, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Ahrén, B.; Masmiquel, L.; Kumar, H.; Sargin, M.; Karsbøl, J.D.; Jacobsen, S.H.; Chow, F. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): A 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017, 5, 341–354. [Google Scholar] [CrossRef]
- Ahmann, A.J.; Capehorn, M.; Charpentier, G.; Dotta, F.; Henkel, E.; Lingvay, I.; Holst, A.G.; Annett, M.P.; Aroda, V.R. Efficacy and Safety of Once-Weekly Semaglutide Versus Exenatide ER in Subjects with Type 2 Diabetes (SUSTAIN 3): A 56-Week, Open-Label, Randomized Clinical Trial. Diabetes Care 2018, 41, 258–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aroda, V.R.; Bain, S.C.; Cariou, B.; Piletič, M.; Rose, L.; Axelsen, M.; Rowe, E.; DeVries, J.H. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): A randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017, 5, 355–366. [Google Scholar]
- Pratley, R.E.; Aroda, V.R.; Lingvay, I.; Lüdemann, J.; Andreassen, C.; Navarria, A.; Viljoen, A. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): A randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018, 6, 275–286. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [Green Version]
- Beglinger, C.; Poller, B.; Arbit, E.; Ganzoni, C.; Gass, S.; Gomez-Orellana, I.; Drewe, J. Pharmacokinetics and pharmacodynamic effects of oral GLP-1 and PYY3-36: A proof-of-concept study in healthy subjects. Clin. Pharmacol. Ther. 2008, 84, 468–474. [Google Scholar] [CrossRef]
- Andersen, A.; Knop, F.K.; Vilsbøll, T. A Pharmacological and Clinical Overview of Oral Semaglutide for the Treatment of Type 2 Diabetes. Drugs 2021, 81, 1003–1030. [Google Scholar] [CrossRef] [PubMed]
- Buckley, S.T.; Bækdal, T.A.; Vegge, A.; Maarbjerg, S.J.; Pyke, C.; Ahnfelt-Rønne, J.; Madsen, K.G.; Schéele, S.G.; Alanentalo, T.; Kirk, R.K.; et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci. Transl. Med. 2018, 10, eaar7047. [Google Scholar] [CrossRef] [PubMed]
- Bækdal, T.A.; Breitschaft, A.; Donsmark, M.; Maarbjerg, S.J.; Søndergaard, F.L.; Borregaard, J. Effect of Various Dosing Conditions on the Pharmacokinetics of Oral Semaglutide, a Human Glucagon-Like Peptide-1 Analogue in a Tablet Formulation. Diabetes Ther. 2021, 12, 1915–1927. [Google Scholar] [CrossRef]
- Baekdal, T.A.; Thomsen, M.; Kupčová, V.; Hansen, C.W.; Anderson, T.W. Pharmacokinetics, Safety, and Tolerability of Oral Semaglutide in Subjects with Hepatic Impairment. J. Clin. Pharmacol. 2018, 58, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Granhall, C.; Søndergaard, F.L.; Thomsen, M.; Anderson, T.W. Pharmacokinetics, Safety and Tolerability of Oral Semaglutide in Subjects with Renal Impairment. Clin. Pharm. 2018, 57, 1571–1580. [Google Scholar] [CrossRef] [Green Version]
- Bækdal, T.A.; Breitschaft, A.; Navarria, A.; Hansen, C.W. A randomized study investigating the effect of omeprazole on the pharmacokinetics of oral semaglutide. Expert Opin. Drug Metab. Toxicol. 2018, 14, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Bækdal, T.A.; Borregaard, J.; Hansen, C.W.; Thomsen, M.; Anderson, T.W. Effect of Oral Semaglutide on the Pharmacokinetics of Lisinopril, Warfarin, Digoxin, and Metformin in Healthy Subjects. Clin. Pharm. 2019, 58, 1193–1203. [Google Scholar] [CrossRef] [Green Version]
- Jordy, A.B.; Albayaty, M.; Breitschaft, A.; Anderson, T.W.; Christiansen, E.; Houshmand-Øregaard, A.; Manigandan, E.; Bækdal, T.A. Effect of Oral Semaglutide on the Pharmacokinetics of Levonorgestrel and Ethinylestradiol in Healthy Postmenopausal Women and Furosemide and Rosuvastatin in Healthy Subjects. Clin Pharm. 2021, 60, 1171–1185. [Google Scholar] [CrossRef]
- Hauge, C.; Breitschaft, A.; Hartoft-Nielsen, M.L.; Jensen, S.; Bækdal, T.A. Effect of oral semaglutide on the pharmacokinetics of thyroxine after dosing of levothyroxine and the influence of co-administered tablets on the pharmacokinetics of oral semaglutide in healthy subjects: An open-label, one-sequence crossover, single-center, multiple-dose, two-part trial. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1139–1148. [Google Scholar]
- Aroda, V.R.; Saugstrup, T.; Buse, J.B.; Donsmark, M.; Zacho, J.; Davies, M.J. Incorporating and interpreting regulatory guidance on estimands in diabetes clinical trials: The PIONEER 1 randomized clinical trial as an example. Diabetes Obes. Metab. 2019, 21, 2203–2210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aroda, V.R.; Rosenstock, J.; Terauchi, Y.; Altuntas, Y.; Lalic, N.M.; Morales Villegas, E.C.; Jeppesen, O.K.; Christiansen, E.; Hertz, C.L.; Haluzík, M. PIONEER 1: Randomized Clinical Trial of the Efficacy and Safety of Oral Semaglutide Monotherapy in Comparison with Placebo in Patients with Type 2 Diabetes. Diabetes Care 2019, 42, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Aroda, V.R.; Buse, J.B.; Cariou, B.; Harris, S.B.; Hoff, S.T.; Pedersen, K.B.; Tarp-Johansen, M.J.; Araki, E. Efficacy, Safety, and Tolerability of Oral Semaglutide Versus Placebo Added to Insulin with or without Metformin in Patients with Type 2 Diabetes: The PIONEER 8 Trial. Diabetes Care 2019, 42, 2262–2271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodbard, H.W.; Rosenstock, J.; Canani, L.H.; Deerochanawong, C.; Gumprecht, J.; Lindberg, S.; Lingvay, I.; Søndergaard, A.L.; Treppendahl, M.B.; Montanya, E. Oral Semaglutide Versus Empagliflozin in Patients with Type 2 Diabetes Uncontrolled on Metformin: The PIONEER 2 Trial. Diabetes Care 2019, 42, 2272–2281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenstock, J.; Allison, D.; Birkenfeld, A.L.; Blicher, T.M.; Deenadayalan, S.; Jacobsen, J.B.; Serusclat, P.; Violante, R.; Watada, H.; Davies, M. Effect of Additional Oral Semaglutide vs Sitagliptin on Glycated Hemoglobin in Adults with Type 2 Diabetes Uncontrolled with Metformin Alone or with Sulfonylurea: The PIONEER 3 Randomized Clinical Trial. Jama 2019, 321, 1466–1480. [Google Scholar] [CrossRef] [Green Version]
- Pieber, T.R.; Bode, B.; Mertens, A.; Cho, Y.M.; Christiansen, E.; Hertz, C.L.; Wallenstein, S.O.R.; Buse, J.B. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): A multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019, 7, 528–539. [Google Scholar] [CrossRef]
- Pratley, R.; Amod, A.; Hoff, S.T.; Kadowaki, T.; Lingvay, I.; Nauck, M.; Pedersen, K.B.; Saugstrup, T.; Meier, J.J. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): A randomised, double-blind, phase 3a trial. Lancet 2019, 394, 39–50. [Google Scholar] [CrossRef]
- Yamada, Y.; Katagiri, H.; Hamamoto, Y.; Deenadayalan, S.; Navarria, A.; Nishijima, K.; Seino, Y. Dose-response, efficacy, and safety of oral semaglutide monotherapy in Japanese patients with type 2 diabetes (PIONEER 9): A 52-week, phase 2/3a, randomised, controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 377–391. [Google Scholar] [CrossRef]
- Yabe, D.; Nakamura, J.; Kaneto, H.; Deenadayalan, S.; Navarria, A.; Gislum, M.; Inagaki, N. Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10): An open-label, randomised, active-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2020, 8, 392–406. [Google Scholar] [CrossRef]
- Mosenzon, O.; Blicher, T.M.; Rosenlund, S.; Eriksson, J.W.; Heller, S.; Hels, O.H.; Pratley, R.; Sathyapalan, T.; Desouza, C. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): A placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019, 7, 515–527. [Google Scholar] [CrossRef] [Green Version]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Research Study Comparing a New Medicine Oral Semaglutide to Placebo in People with Type 2 Diabetes (PIONEER 11). Available online: https://clinicaltrials.gov/ct2/show/NCT04109547 (accessed on 11 August 2021).
- A Research Study Comparing a New Medicine Oral Semaglutide to Sitagliptin in People with Type 2 Diabetes (PIONEER 12). Available online: https://clinicaltrials.gov/ct2/show/NCT04017832 (accessed on 11 August 2021).
- A Heart Disease Study of Semaglutide in Patients with Type 2 Diabetes (SOUL). Available online: https://clinicaltrials.gov/ct2/show/NCT03914326 (accessed on 11 August 2021).
- Rybelsus (Semaglutide) [US Prescribing Information]. Available online: http://www.novo-pi.com/rybelsus.pdf (accessed on 6 August 2021).
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef] [PubMed]
| Time (wk) | No. of Patients (Japanese) | Comparator | Baseline HbA1c (%) | Mean Reduction in HbA1c (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Semaglutide | Comparator | |||||||
| 3 mg | 7 mg | 14 mg | ||||||
| PIONEER 1 | 26 | 703 (116) | Placebo | 8.0 | −0.9 * | −1.2 * | −1.4 * | −0.3 |
| PIONEER 2 | 52 | 822 (0) | Empagliflozin 25 mg | 8.1 | −1.3 * | −0.9 | ||
| PIONEER 3 | 78 | 1864 (207) | Sitagliptin 100 mg | 8.3 | −0.6 | −1.0 * | −1.3 * | −0.8 |
| PIONEER 4 | 52 | 711 (75) | Liraglutide 1.8 mg or placebo | 8.0 | −1.2 | −1.1 (liraglutide) −0.2 (placebo) | ||
| PIONEER 5 | 26 | 324 (0) | Placebo (renal) | 8.0 | −1.0 * | −0.2 | ||
| PIONEER 7 | 52 | 504 (0) | Sitagliptin 100 mg | 8.3 | −1.3 * (flexible dosing) | −0.8 | ||
| PIONEER 8 | 52 | 731 (194) | Placebo (add-on to insulin) | 8.2 | −0.6 * | −0.9 * | −1.3 * | −0.1 |
| PIONEER 9 | 52 | 243 (243) | Liraglutide 0.9 mg or placebo | 8.2 | −0.9 ¶ | −1.4 ¶ | −1.5 ¶ | −1.2 (liraglutide) −0.1 (placebo) |
| PIONEER 10 | 52 | 458 (458) | Dulaglutide 0.75 mg | 8.3 | −0.9 * | −1.4 | −1.7 * | −1.4 |
| Time (wk) | No. of Patients (Japanese) | Comparator | Baseline Weight (kg) | Mean Reduction in Weight (kg) | ||||
|---|---|---|---|---|---|---|---|---|
| Semaglutide | Comparator | |||||||
| 3 mg | 7 mg | 14 mg | ||||||
| PIONEER 1 | 26 | 703 (116) | Placebo | 88.1 | −1.5 | −2.3 | −3.7 * | −1.4 |
| PIONEER 2 | 52 | 822 (0) | Empagliflozin 25 mg | 91.6 | −3.8 | −3.7 | ||
| PIONEER 3 | 78 | 1864 (207) | Sitagliptin 100 mg | 91.2 | −1.2 * | −2.2 * | −3.1 * | −0.6 |
| PIONEER 4 | 52 | 711 (75) | Liraglutide 1.8 mg or placebo | 94.0 | −4.4 * | −3.1 (liraglutide) −0.2 (placebo) | ||
| PIONEER 5 | 26 | 324 (0) | Placebo (renal) | 90.8 | −3.4 * | −0.9 | ||
| PIONEER 7 | 52 | 504 (0) | Sitagliptin 100 mg | 88.6 | −2.6 * (flexible dosing) | −0.7 | ||
| PIONEER 8 | 52 | 731 (194) | Placebo (add-on to insulin) | 85.9 | −1.4 * | −2.4 * | −3.7 * | −0.4 |
| PIONEER 9 | 52 | 243 (243) | Liraglutide 0.9 mg or placebo | 71.1 | −0.3 | −0.8 | −2.6 †,¶ | −0.6 (placebo) 0.0 (liraglutide) |
| PIONEER 10 | 52 | 458 (458) | Dulaglutide 0.75 mg | 72.1 | −0.0 * | −0.9 * | −1.6 * | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.S.; Jung, C.H. Oral Semaglutide, the First Ingestible Glucagon-Like Peptide-1 Receptor Agonist: Could It Be a Magic Bullet for Type 2 Diabetes? Int. J. Mol. Sci. 2021, 22, 9936. https://doi.org/10.3390/ijms22189936
Kim HS, Jung CH. Oral Semaglutide, the First Ingestible Glucagon-Like Peptide-1 Receptor Agonist: Could It Be a Magic Bullet for Type 2 Diabetes? International Journal of Molecular Sciences. 2021; 22(18):9936. https://doi.org/10.3390/ijms22189936
Chicago/Turabian StyleKim, Hwi Seung, and Chang Hee Jung. 2021. "Oral Semaglutide, the First Ingestible Glucagon-Like Peptide-1 Receptor Agonist: Could It Be a Magic Bullet for Type 2 Diabetes?" International Journal of Molecular Sciences 22, no. 18: 9936. https://doi.org/10.3390/ijms22189936
APA StyleKim, H. S., & Jung, C. H. (2021). Oral Semaglutide, the First Ingestible Glucagon-Like Peptide-1 Receptor Agonist: Could It Be a Magic Bullet for Type 2 Diabetes? International Journal of Molecular Sciences, 22(18), 9936. https://doi.org/10.3390/ijms22189936






