Nitrogen-Doped Carbon Aerogels Derived from Starch Biomass with Improved Electrochemical Properties for Li-Ion Batteries
Abstract
1. Introduction
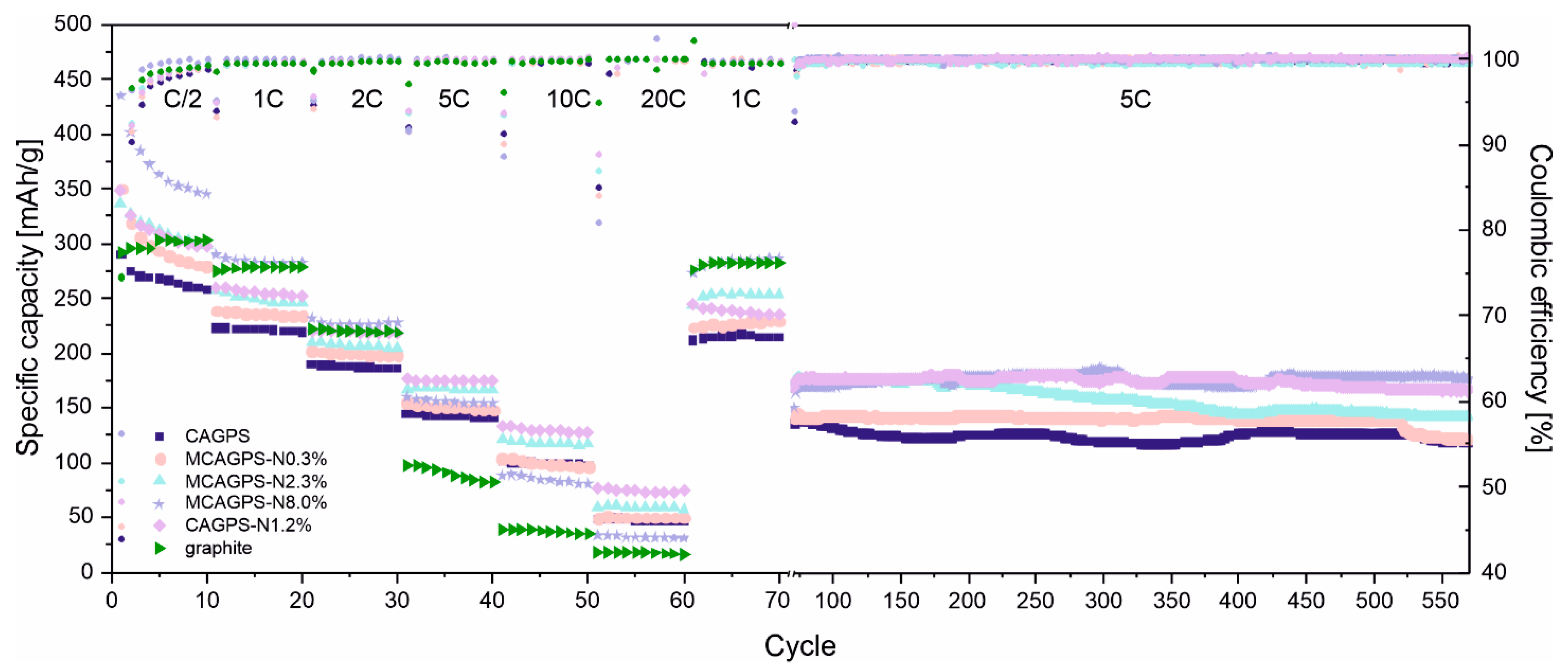
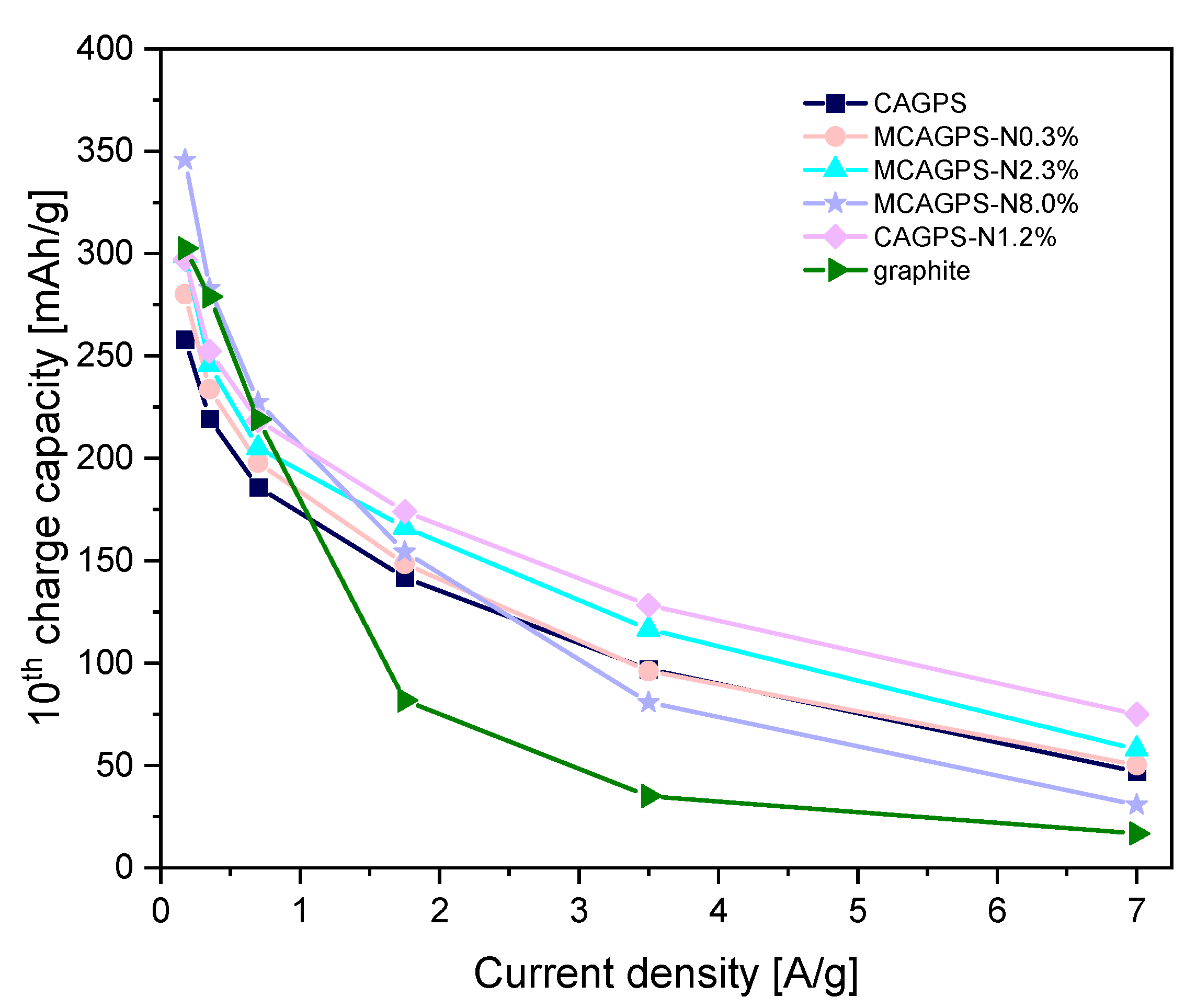
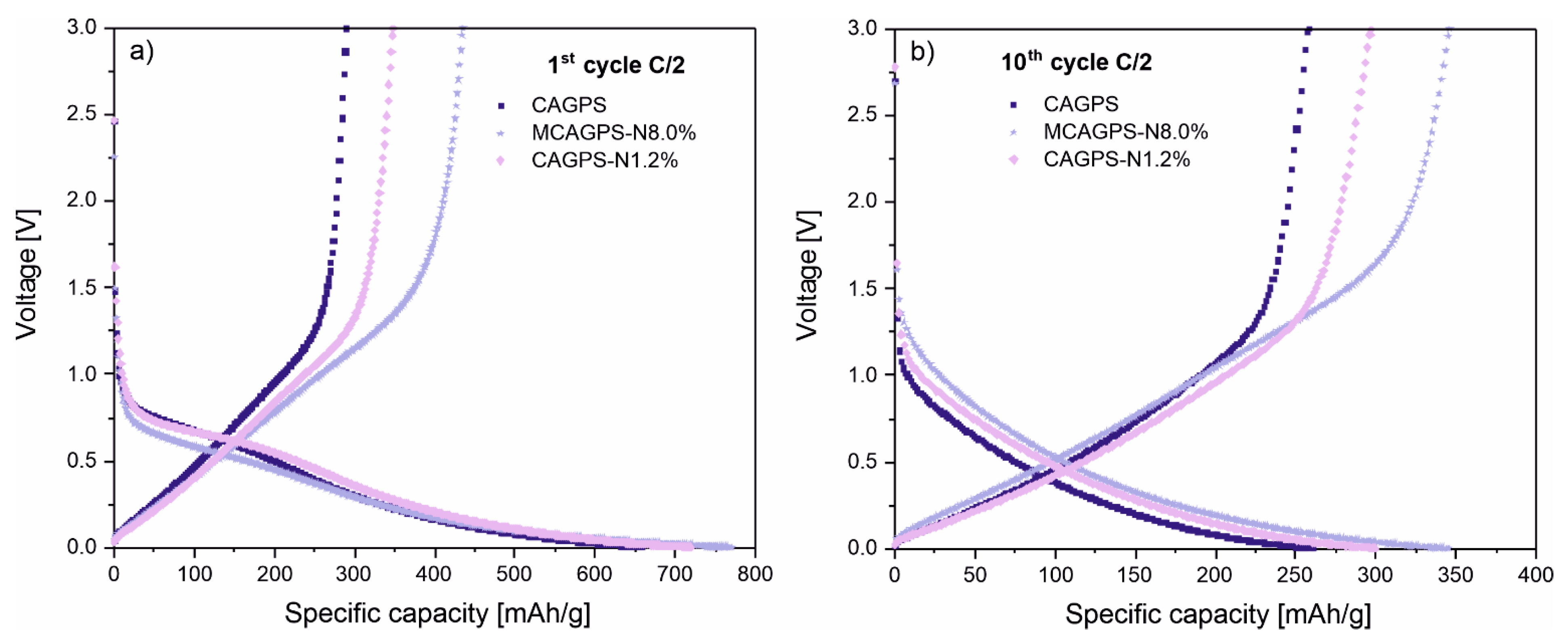
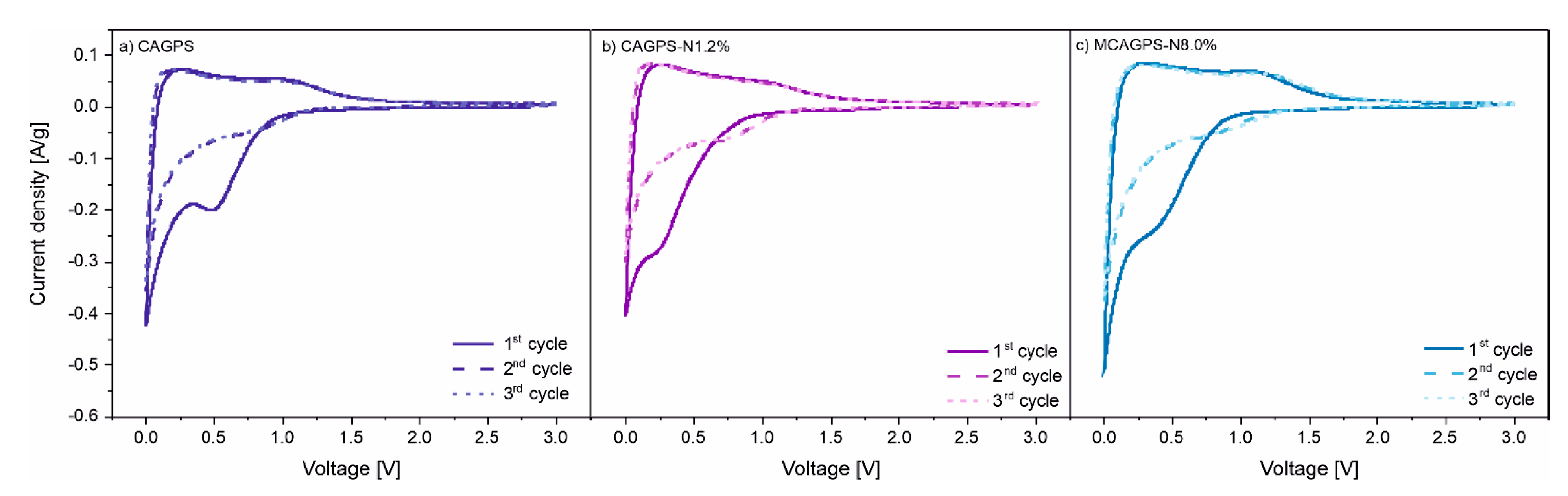
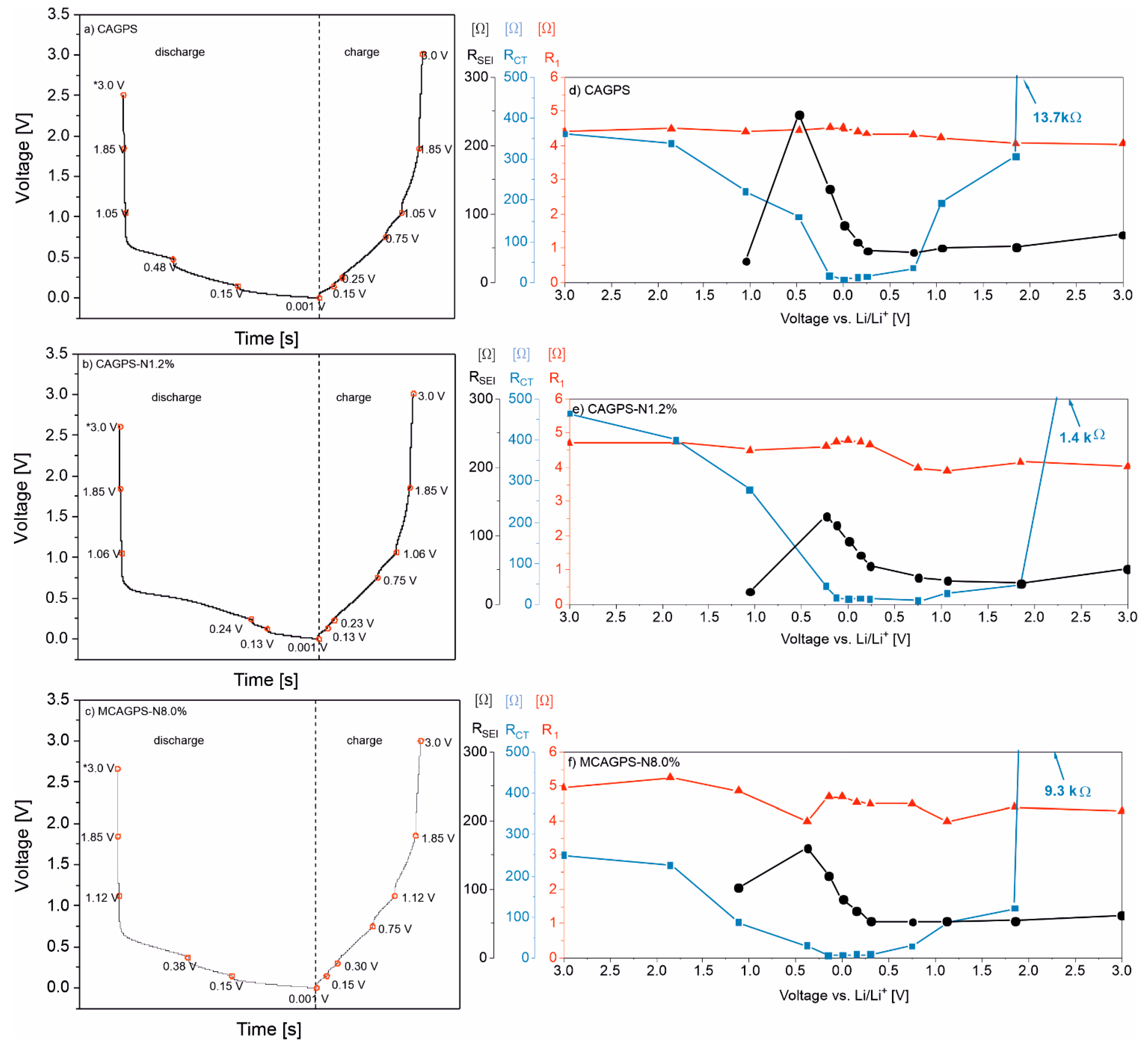
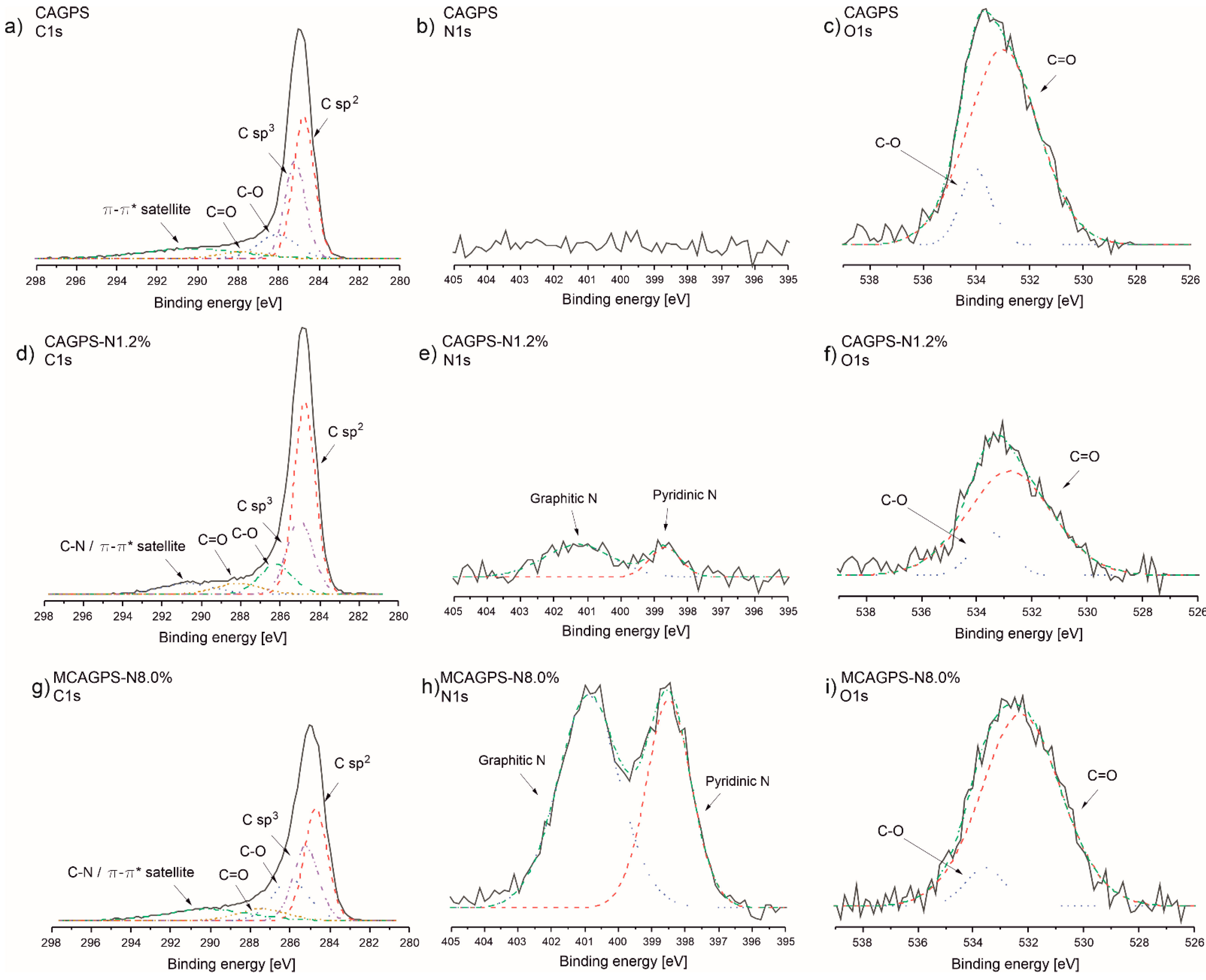
2. Results
3. Discussion
4. Materials and Methods
4.1. Synthesis of Carbon Aerogel Derived from Potato Starch (CAGPS) and N-Doped Carbon Aerogel Materials (MCAGPS-N and CAGPS-N)
4.2. Materials Characterization
4.3. Electrochemical Characterization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lebedeva, N.; Di Persio, F.; Boon-Brett, L. Lithium ion Battery Value Chain and Related Opportunities for Europe; EUR 28534 EN; Publications Office of the European Union: Luxembourg, 2017; ISBN 978-92-79-66948-4. [Google Scholar]
- Graphite Market—Growth, Trends, and Forecast (2020–2025). Available online: https://www.reportlinker.com/p05826223/Graphite-Electrode-Market-Growth-Trends-and-Forecast.html?utm_source=GNW (accessed on 20 April 2020).
- Bhardwaj, S.; Sharon, M.; Ishihara, T.; Jayabhaye, S.; Afre, R.; Soga, T.; Sharon, M. Carbon Material from Natural Sources as an Anode in Lithium Secondary Battery. Carbon Lett. 2007, 8, 285–291. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, Y.; Song, N.; Li, X. Biomass-derived renewable carbon materials for electrochemical energy storage. Mater. Res. Lett. 2017, 5, 69–88. [Google Scholar] [CrossRef]
- Liu, L.; Yang, L.; Wang, P.; Wang, C.Y.; Cheng, J.; Zhang, G.; Gu, J.J.; Cao, F.F. Porous nitrogen-doped carbon derived from peanut shell as anode material for lithium ion battery. Int. J. Electrochem. Sci. 2017, 12, 9844–9854. [Google Scholar] [CrossRef]
- Li, W.; Chen, M.; Wang, C. Spherical hard carbon prepared from potato starch using as anode material for Li-ion batteries. Mater. Lett. 2011, 65, 3368–3370. [Google Scholar] [CrossRef]
- Chen, M.; Yu, C.; Liu, S.; Fan, X.; Zhao, C.; Zhang, X.; Qiu, J. Micro-sized porous carbon spheres with ultra-high rate capability for lithium storage. Nanoscale 2015, 7, 1791–1795. [Google Scholar] [CrossRef]
- Wei, R.; Xu, R.; Zhang, K.; Liang, F.; Yao, Y. Biological enzyme treatment of starch-based lithium-ion battery silicon-carbon composite. Nanotechnology 2021, 32, 045605. [Google Scholar] [CrossRef]
- Jabbour, L.; Bongiovanni, R.; Chaussy, D.; Gerbaldi, C.; Beneventi, D. Cellulose-based Li-ion batteries: A review. Cellulose 2013, 20, 1523–1545. [Google Scholar] [CrossRef]
- Wang, S.X.; Yang, L.; Stubbs, L.P.; Li, X.; He, C. Lignin-derived fused electrospun carbon fibrous mats as high performance anode materials for lithium ion batteries. ACS Appl. Mater. 2013, 5, 12275–12282. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xiao, C.; Xing, Y.; Xu, H.; Zhang, S. Carbon nanofibers/nanosheets hybrid derived from cornstalks as a sustainable anode for Li-ion batteries. J. Mater. Chem. A 2015, 33, 6742–6746. [Google Scholar] [CrossRef]
- Jin, C.; Hu, J.; Wu, J.; Liang, H.; Li, J. Innovative and Economically Beneficial Use of Corn and Corn Products in Electrochemical Energy Storage Applications. ACS Sustain. Chem. Eng. 2021, 9, 10678–10703. [Google Scholar] [CrossRef]
- Kesavan, T.; Sasidharan, M. Palm spathe derived N-doped carbon nanosheets as a high performance electrode for Li-ion batteries and supercapacitors. ACS Sustain. 2019, 7, 12160–12169. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, J.; Ai, W.; Fan, Z.; Shen, X.; Zou, C.; Liu, J.; Zhang, H.; Yu, T. Evolution of disposable bamboo chopsticks into uniform carbon fibers: A smart strategy to fabricate sustainable anodes for Li-ion batteries. Energy Environ. Sci. 2014, 7, 2670–2679. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Muruganantham, R.; Tai, S.H.; Chang, B.K.; Wu, S.C.; Chueh, Y.L.; Liu, W.R. Coffee grounds-derived carbon as high performance anode materials for energy storage applications. J. Taiwan Inst. Chem. Eng. 2019, 97, 178–188. [Google Scholar] [CrossRef]
- Campbell, B.; Ionescu, R.; Favors, Z.; Ozkan, C.S.; Ozkan, M. Bio-derived, binderless, hierarchically porous carbon anodes for Li-ion batteries. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bakierska, M.; Molenda, M.; Majda, D.; Dziembaj, R. Functional starch based carbon aerogels for energy applications. Procedia Eng. 2014, 98, 14–19. [Google Scholar] [CrossRef]
- Bakierska, M.; Chojnacka, A.; Świętosławski, M.; Natkański, P.; Gajewska, M.; Rutkowska, M.; Molenda, M. Multifunctional carbon aerogels derived by sol–gel process of natural polysaccharides of different botanical origin. Materials 2017, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Bakierska, M.; Lis, M.; Pacek, J.; Świętosławski, M.; Gajewska, M.; Tąta, A.; Proniewicz, E.; Molenda, M. Bio-derived carbon nanostructures for high-performance lithium-ion batteries. Carbon 2019, 145, 426–432. [Google Scholar] [CrossRef]
- Kubicka, M.; Bakierska, M.; Chudzik, K.; Rutkowska, M.; Pacek, J.; Molenda, M. Electrochemical properties and structure evolution of starch-based carbon nanomaterials as Li-ion anodes with regard to thermal treatment. Polymers 2019, 11, 1527. [Google Scholar] [CrossRef]
- Kubicka, M.; Bakierska, M.; Chudzik, K.; Molenda, M. A Strategy to Optimize the Performance of Bio-Derived Carbon Aerogels by a Structuring Additive. Nanomaterials 2020, 10, 1811. [Google Scholar] [CrossRef]
- Ventosa, E.; Xia, W.; Klink, S.; La Mantia, F.; Muhler, M.; Schuhmann, W. Influence of surface functional groups on lithium ion intercalation of carbon cloth. Electrochim. Acta 2012, 65, 22–29. [Google Scholar] [CrossRef]
- Yang, J.; Wang, S.C.; Zhou, X.Y.; Xie, J. Electrochemical behaviors of functionalized carbon nanotubes in LiPF6/EC+DMC electrolyte. Int. J. Electrochem. Sci. 2012, 7, 6118–6126. [Google Scholar]
- Hui, X.; Geng, L.; Cheng-Bing, Y.; Chong, L.; Chao, L. Effects of the Functional Group on the Lithium Irons Across the Port of Carbon Nanotube. Comput. Theor. Nanosci. 2016, 13, 4687–4693. [Google Scholar] [CrossRef][Green Version]
- Ding, Z.; Trouillet, V.; Dsoke, S. Are functional groups beneficial or harmful on the electrochemical performance of activated carbon electrodes? J. Electrochem. Soc. 2019, 166, A1004–A1014. [Google Scholar] [CrossRef]
- Piedboeuf, M.L.C.; Job, N.; Aqil, A.; Busby, Y.; Fierro, V.; Celzard, A.; Detrembleur, C.; Léonard, A.F. Understanding the influence of surface oxygen groups on the electrochemical behavior of porous carbons as anodes for lithium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 36054–36065. [Google Scholar] [CrossRef]
- Frackowiak, E.; Lota, G.; Machnikowski, J.; Vix-Guterl, C.; Béguin, F. Optimisation of supercapacitors using carbons with controlled nanotexture and nitrogen content. Electrochim. Acta 2006, 51, 2209–2214. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Z.; Zhang, Y.; Wang, B.; Peng, H. The recent progress of nitrogen-doped carbon nanomaterials for electrochemical batteries. J. Mater. Chem. A 2018, 6, 12932–12944. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, H.; Xu, X.; Lv, Y.; Shi, J.; Liu, W.; Chen, S.; Wang, X. Nitrogen-doped porous carbons derived from a natural polysaccharide for multiple energy storage devices. Sustain. Energy Fuels 2018, 2, 381–391. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Q.; Sun, B.; Gu, J.; Yu, B.; Zhang, W.; Zhang, D. N-doped catalytic graphitized hard carbon for high-performance lithium/sodium-ion batteries. Sci. Rep. 2018, 8, 9934. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wei, W.; Wang, Y.; Ren, J. Novel sponge-like N-doped graphene film as high-efficiency electrode for Li-ion battery. Appl. Surf. Sci. 2019, 485, 529–535. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Yang, R.X.; Dutta, S.; Ok, Y.S.; Wu, K.C.W. Recent progress in the development of biomass-derived nitrogen-doped porous carbon. J. Mater. Chem. A 2021, 9, 3703–3728. [Google Scholar] [CrossRef]
- Kaźmierczak-Raźna, J.; Pietrzak, R.; Nowicki, P. Synthesis of new carbon-nitrogen composites based on waste sweet drinks. Physicochem. Probl. Miner. Process. 2019, 55, 1366–1374. [Google Scholar]
- Kaźmierczak-Raźna, J.; Półrolniczak, P.; Wasiński, K.; Pietrzak, R.; Nowicki, P. Comparison of physicochemical, sorption and electrochemical properties of nitrogen-doped activated carbons obtained with the use of microwave and conventional heating. Adsorption 2019, 25, 405–417. [Google Scholar] [CrossRef]
- Wan, H.; Hu, X. Nitrogen doped biomass-derived porous carbon as anode materials of lithium ion batteries. Solid State Ion. 2019, 341, 115030. [Google Scholar] [CrossRef]
- Huang, J.; Lin, Y.; Ji, M.; Cong, G.; Liu, H.; Yu, J.; Yang, B.; Li, C.; Zhu, C.; Xu, J. Nitrogen-doped porous carbon derived from foam polystyrene as an anode material for lithium-ion batteries. Appl. Surf. Sci. 2020, 504, 144398. [Google Scholar] [CrossRef]
- Majeed, S.; Zhao, J.; Zhang, L.; Anjum, S.; Liu, Z.; Xu, G. Synthesis and electrochemical applications of nitrogen-doped carbon nanomaterials. Nanotech. Rev. 2013, 2, 615–635. [Google Scholar] [CrossRef]
- Boehm, H.P. Catalytic Properties of Nitrogen-Containing Carbons. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 219–265. [Google Scholar]
- Lahaye, J.; Nanse, G.; Bagreev, A.; Strelko, V. Porous structure and surface chemistry of nitrogen containing carbons from polymers. Carbon 1999, 37, 585–590. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M.; Hromádková, J.; Kovářová, J.; Kalendová, A. The carbonization of colloidal polyaniline nanoparticles to nitrogen-containing carbon analogues. Polym. Int. 2010, 59, 875–878. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, M.S.; Xu, K.; Allen, J.; Jow, T.R. Understanding solid electrolyte interface film formation on graphite electrodes. Electrochem. Solid-State Lett. 2001, 4, 206–208. [Google Scholar] [CrossRef]
- Qin, D.; Liu, Z.; Zhao, Y.; Xu, G.; Zhang, F.; Zhang, X. A sustainable route from corn stalks to N, P-dual doping carbon sheets toward high performance sodium-ion batteries anode. Carbon 2018, 130, 664–671. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Srivastava, A.; Gowda, S.R.; Gullapalli, H.; Dubey, M.; Ajayan, P.M. Synthesis of nitrogen-doped graphene films for lithium battery application. ACS Nano 2010, 4, 6337–6342. [Google Scholar] [CrossRef]
- Wang, H.G.; Wang, Y.; Li, Y.; Wan, Y.; Duan, Q. Exceptional electrochemical performance of nitrogen-doped porous carbon for lithium storage. Carbon 2015, 82, 116–123. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, X.; Qie, L.; Huang, Y. High-performance lithium storage in nitrogen-enriched carbon nanofiber webs derived from polypyrrole. Electrochim. Acta 2013, 106, 320–326. [Google Scholar] [CrossRef]
- Matter, P.H.; Zhang, L.; Ozkan, U.S. The role of nanostructure in nitrogen-containing carbon catalysts for the oxygen reduction reaction. J. Catal. 2006, 239, 83–96. [Google Scholar] [CrossRef]
- Kiuchi, H.; Kondo, T.; Sakurai, M.; Guo, D.; Nakamura, J.; Niwa, H.; Miyawaki, J.; Kawai, M.; Oshimad, M.; Harada, Y. Characterization of nitrogen species incorporated into graphite using low energy nitrogen ion sputtering. Phys. Chem. Chem. Phys. 2016, 18, 458–465. [Google Scholar] [CrossRef]
- Guo, W.; Li, X.; Xu, J.; Liu, H.K.; Ma, J.; Dou, S.X. Growth of highly nitrogen-doped amorphous carbon for lithium-ion battery anode. Electrochim. Acta 2016, 188, 414–420. [Google Scholar] [CrossRef]
- Wu, Z.S.; Ren, W.; Xu, L.; Li, F.; Cheng, H.M. Doped graphene sheets as anode materials with superhigh rate and large capacity for lithium ion batteries. ACS Nano 2011, 5, 5463–5471. [Google Scholar] [CrossRef]
- Bhattacharjya, D.; Park, H.Y.; Kim, M.S.; Choi, H.S.; Inamdar, S.N.; Yu, J.S. Nitrogen-doped carbon nanoparticles by flame synthesis as anode material for rechargeable lithium-ion batteries. Langmuir 2014, 30, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Shao, X.; Cao, D. Nitrogen-doped graphene nanosheets as anode materials for lithium ion batteries: A first-principles study. J. Mater. Chem. 2012, 22, 8911–8915. [Google Scholar] [CrossRef]
- Lin, Z.; Song, M.K.; Ding, Y.; Liu, Y.; Liu, M.; Wong, C. Facile preparation of nitrogen doped graphene as a metal-free catalyst for oxygen reduction reaction. Phys. Chem. Chem. Phys. 2012, 14, 3381–3387. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Shao, L.; Chen, J.J.; Bao, W.J.; Wang, F.B.; Xia, X.H. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphene oxide with melamine and its excellent electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef]
- Cai, D.; Wang, S.; Lian, P.; Zhu, X.; Li, D.; Yang, W.; Wang, H. Superhigh capacity and rate capability of high-level nitrogen-doped graphene sheets as anode materials for lithium-ion batteries. Electrochim. Acta 2013, 90, 492–497. [Google Scholar] [CrossRef]

| Sample | Description of the Sample | Chemical Composition | |||
|---|---|---|---|---|---|
| C (wt. %) | H (wt. %) | N (wt. %) | Others (wt. %) | ||
| MCAGPS-N8.0% * | MOA_PS ** + 20 wt. % of melamine pyrolysed in Ar | 79.02 | 1.775 | 7.96 | 11.245 |
| MCAGPS-N2.3% | MOA_PS + 5 wt. % of melamine pyrolysed in Ar | 87.66 | 1.563 | 2.28 | 8.497 |
| MCAGPS-N0.3% | MOA_PS + 1 wt. % of melamine pyrolysed in Ar | 89.11 | 1.869 | 0.30 | 8.721 |
| CAGPS-N1.2% *** | OA_PS **** pyrolysed in N2 | 92.55 | 1.405 | 1.18 | 4.865 |
| CAGPS ***** | OA_PS pyrolysed in Ar | 93.96 | 1.201 | 0.04 | 4.799 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubicka, M.; Bakierska, M.; Chudzik, K.; Świętosławski, M.; Molenda, M. Nitrogen-Doped Carbon Aerogels Derived from Starch Biomass with Improved Electrochemical Properties for Li-Ion Batteries. Int. J. Mol. Sci. 2021, 22, 9918. https://doi.org/10.3390/ijms22189918
Kubicka M, Bakierska M, Chudzik K, Świętosławski M, Molenda M. Nitrogen-Doped Carbon Aerogels Derived from Starch Biomass with Improved Electrochemical Properties for Li-Ion Batteries. International Journal of Molecular Sciences. 2021; 22(18):9918. https://doi.org/10.3390/ijms22189918
Chicago/Turabian StyleKubicka, Marcelina, Monika Bakierska, Krystian Chudzik, Michał Świętosławski, and Marcin Molenda. 2021. "Nitrogen-Doped Carbon Aerogels Derived from Starch Biomass with Improved Electrochemical Properties for Li-Ion Batteries" International Journal of Molecular Sciences 22, no. 18: 9918. https://doi.org/10.3390/ijms22189918
APA StyleKubicka, M., Bakierska, M., Chudzik, K., Świętosławski, M., & Molenda, M. (2021). Nitrogen-Doped Carbon Aerogels Derived from Starch Biomass with Improved Electrochemical Properties for Li-Ion Batteries. International Journal of Molecular Sciences, 22(18), 9918. https://doi.org/10.3390/ijms22189918







