In Vitro Heme Coordination of a Dye-Decolorizing Peroxidase—The Interplay of Key Amino Acids, pH, Buffer and Glycerol
Abstract
1. Introduction
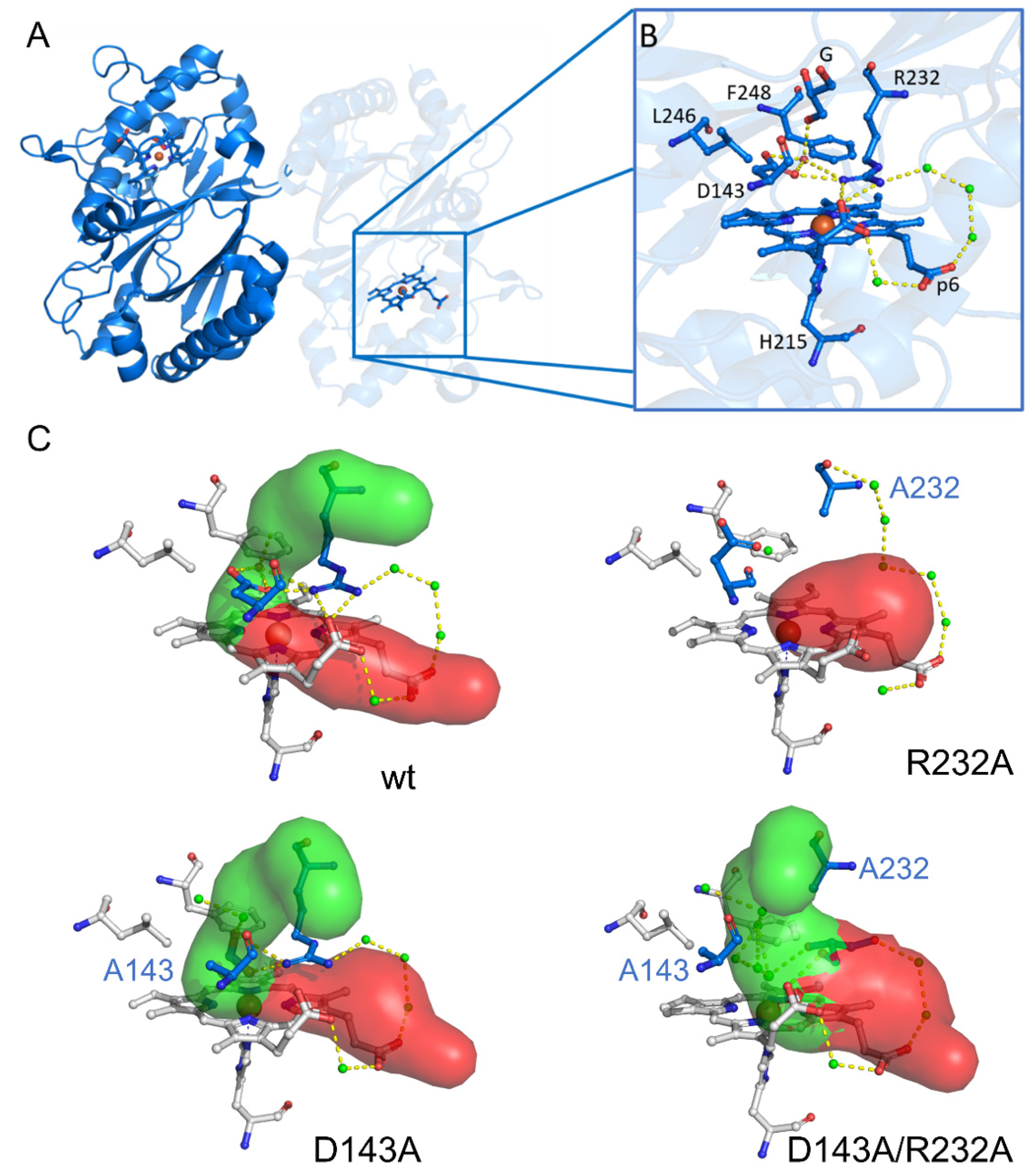
2. Results
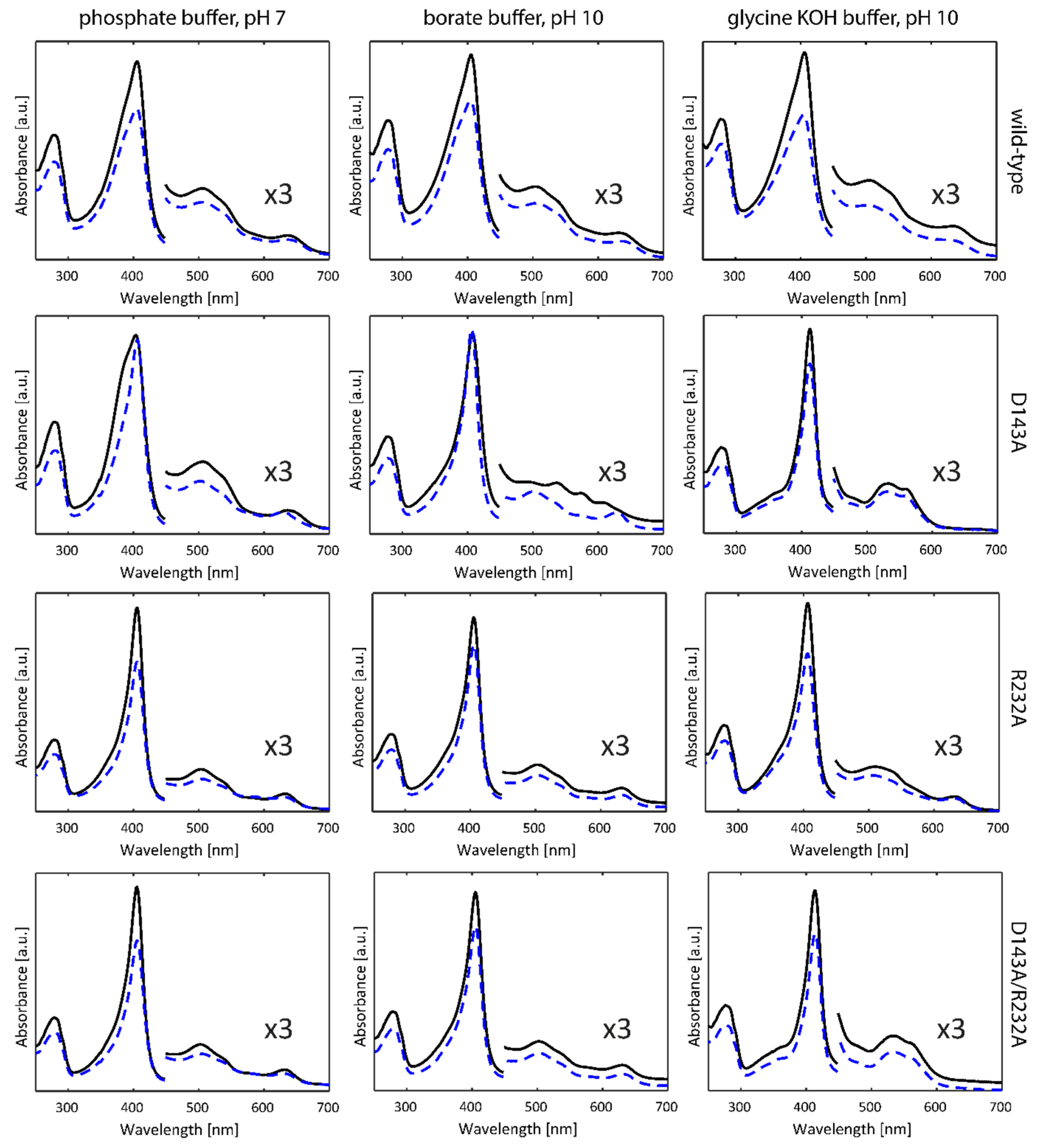
2.1. Optical Absorption Spectroscopy
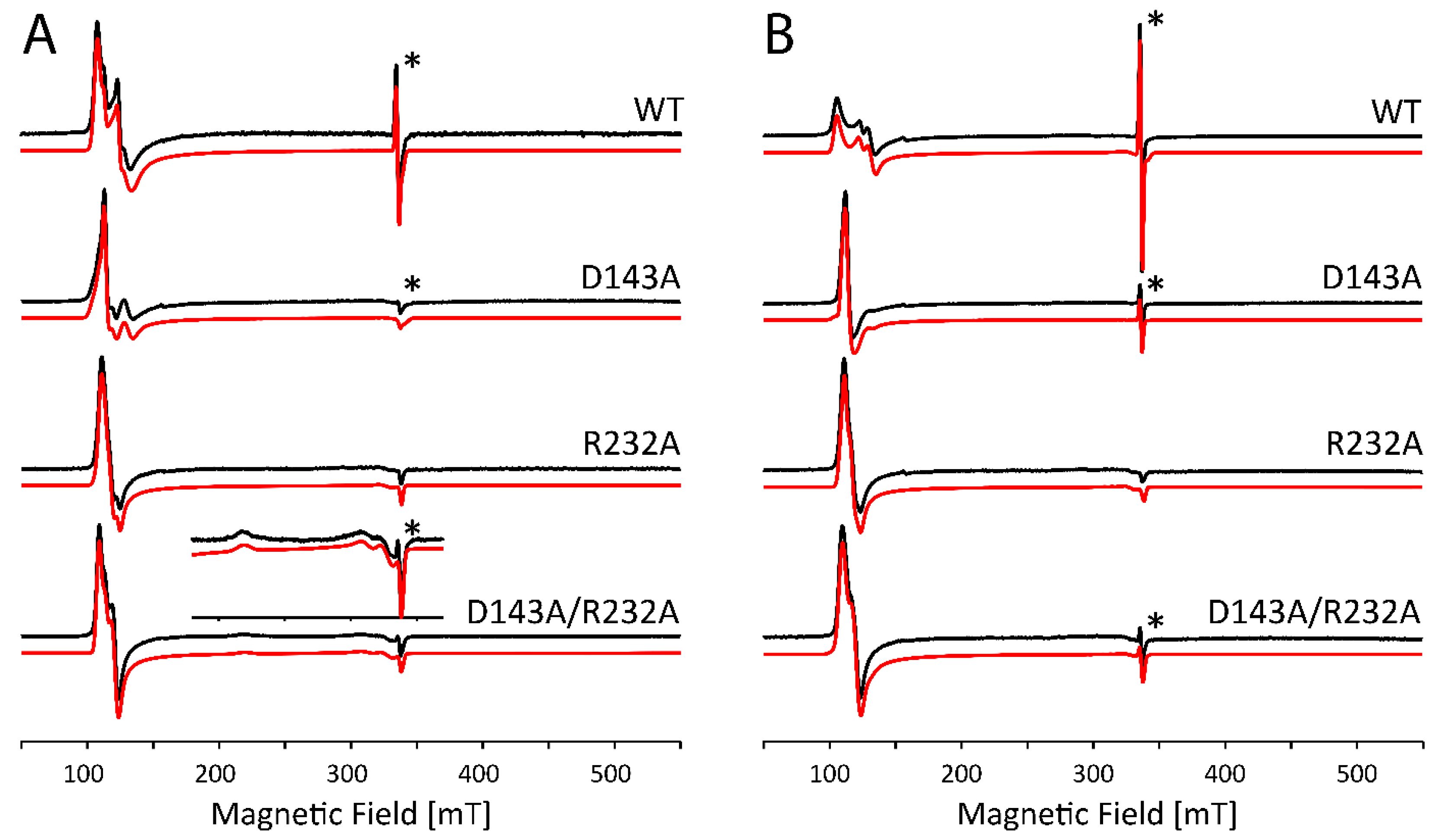
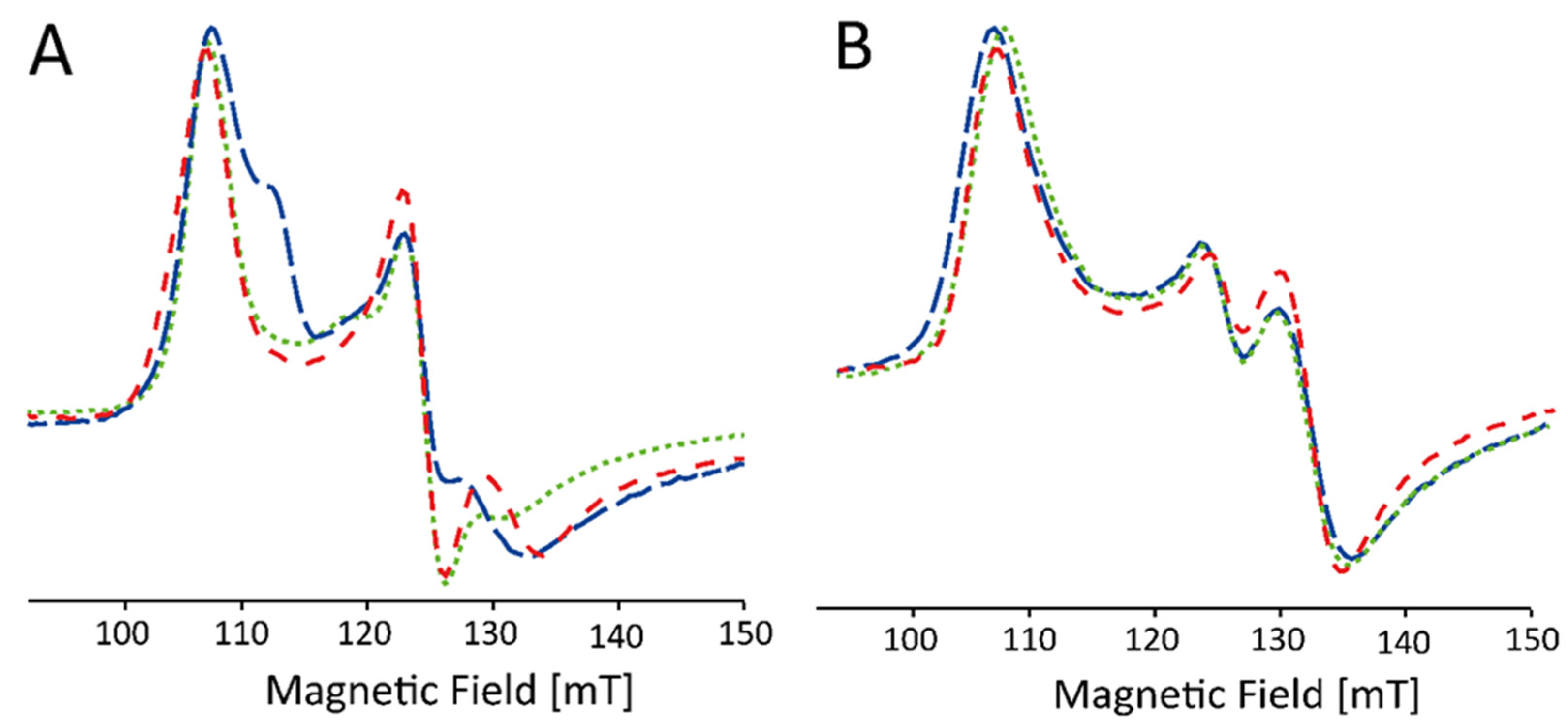
2.2. Influence of Buffer and Glycerol on CW EPR Spectra
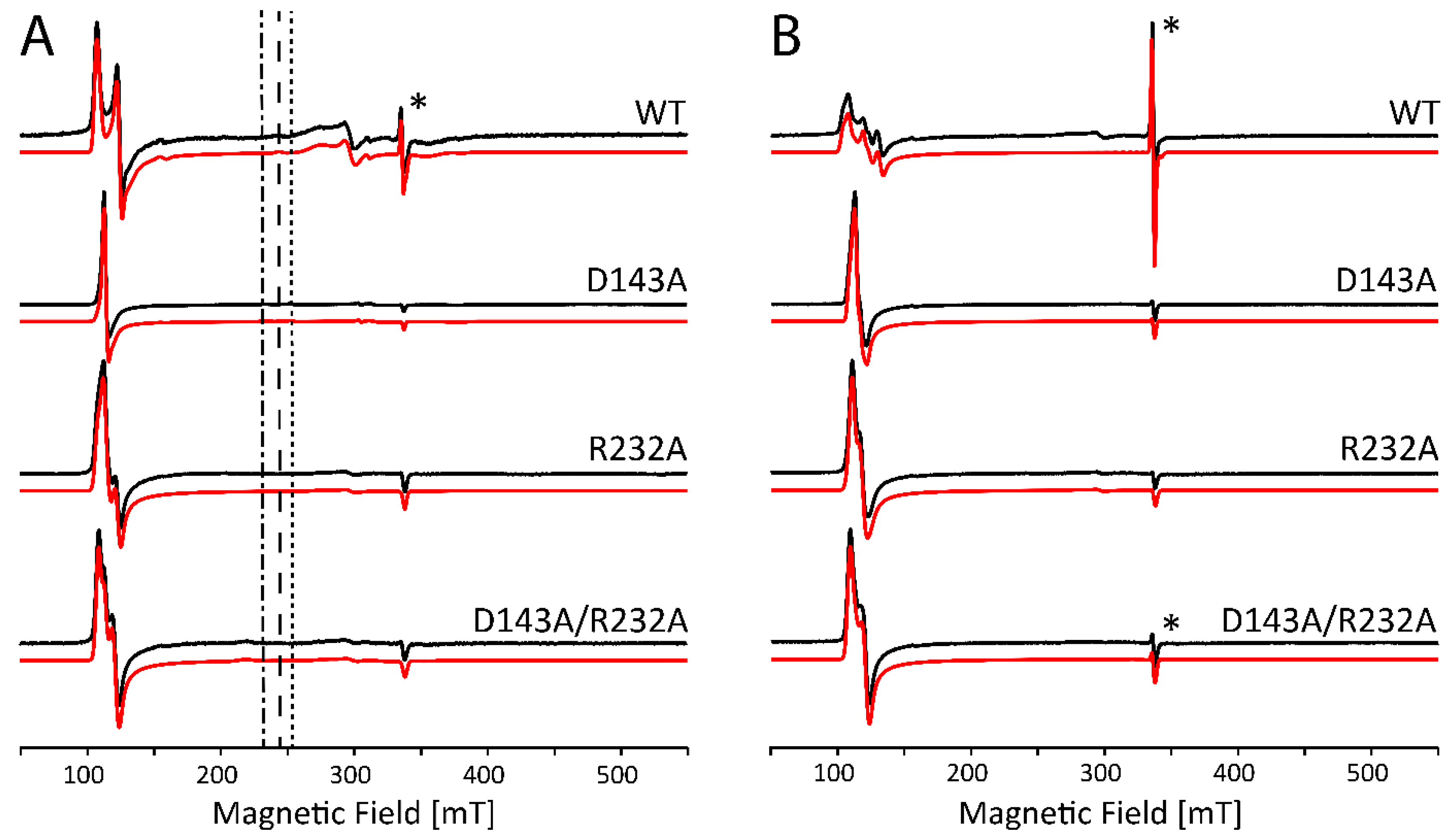
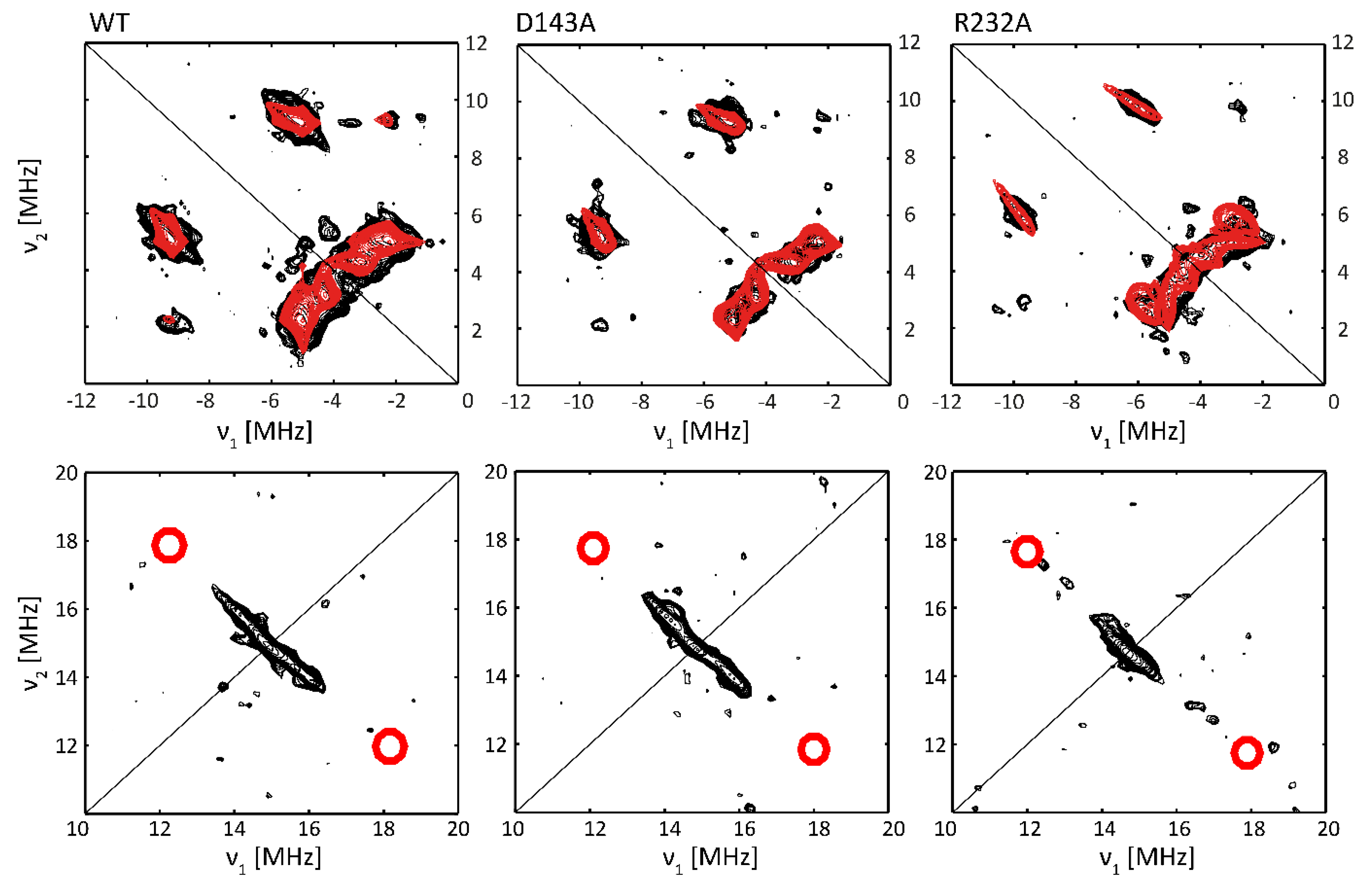
2.3. Subtle Changes in the Heme Pocket Revealed by Pulsed EPR
3. Discussion
3.1. Heme Cavity Heterogeneity and the Influence of Glycerol
3.2. The Non-Innocent Effect of Buffer Molecules
3.3. Alkaline Transition in KpDyP Variants
3.4. The Distal Heme Side in KpDyP
4. Materials and Methods
4.1. Protein Preparation
4.2. Optical Absorption Spectroscopy
4.3. EPR Spectroscopy
- X-band continuous-wave (CW) EPR experiments were performed on a Bruker ESP300E spectrometer (Bruker Biospin, Rheinstetten, Germany) operating at a microwave frequency of ca. 9.44 GHz equipped with a liquid-helium cryostat (Oxford Inc., Oxford, UK) to enable temperatures from 2.5 K up to room temperature. Calibration of the magnetic field was done using a Bruker ER035M NMR Gaussmeter. The EPR tubes were vacuum-pumped to 1 mbar prior to and during the experiments to remove excess of paramagnetic dioxygen.
- X-band HYSCORE (hyperfine sublevel correlation spectroscopy) experiments [57] were carried out on a Bruker E580 Elexsys spectrometer (Bruker Biospin, Rheinstetten, Germany) (microwave frequency ≈ 9.74 GHz) equipped with an Oxford Instruments gas-flow cryogenic system to obtain an operating temperature of 4 K. The pulse sequence π/2-τ- π/2-t1-π-t2- π/2-τ-echo was performed using tπ/2 = 16 ns, tπ = 32 ns and was repeated for four different τ-values (96, 104, 114 and 128 ns) at a magnetic field corresponding to gzeff. Matched HYSCORE [58] was performed at a magnetic field corresponding to gyeff, using tπ/2 = 16 ns, tπ = 32 ns, tHTA = 24 ns and was repeated for two different τ-values (144 and 164 ns). t1 and t2 were varied in time steps of 16 ns, starting from 96 ns to 4896 ns. The HYSCORE spectra are baseline corrected using a third-order polynomial, apodized with a Hamming window and zero-filled. After Fourier transformation, the absolute value spectrum was calculated. HYSCORE measurements recorded with different τ-values were added together as indicated in the figure captions.
- W-band (93.98 GHz) electron spin echo (ESE)-detected EPR were performed on a Bruker Elexsys E680 spectrometer (Bruker Biospin, Rheinstetten, Germany) equipped with a standard single-mode cylindrical resonator from Bruker and a continuous-flow cryostat and superconducting magnet from Oxford Instruments. Using a π/2-τ-π-τ-echo sequence, the low-field part (<1800 mT) was obtained using π/2 (π) pulse lengths of 60 (120) ns and τ = 200 ns. The high-field part, representative for the low-spin signals, required π/2 (π) pulse lengths of 80 (160) ns and τ = 400 ns.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mathews, C.K.; van Holde, K.E.; Ahern, K.G. Biochemistry, 3rd ed.; Benjamin Cummings: London, UK, 2000. [Google Scholar]
- Paoli, M.; Marles-Wright, J.; Smith, A. Structure-function relationships in heme-proteins. DNA Cell Biol. 2002, 21, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Walker, F.A. Magnetic spectroscopic (EPR, ESEEM, Mössbauer, MCD and NMR) studies of low-spin ferriheme centers and their corresponding heme proteins. Coord. Chem. Rev. 1999, 185–186, 471–534. [Google Scholar] [CrossRef]
- Van Doorslaer, S.; Cuypers, B. Electron paramagnetic resonance of globin proteins–a successful match between spectroscopic development and protein research. Mol. Phys. 2018, 116, 287–309. [Google Scholar] [CrossRef]
- Van Doorslaer, S. Probing the structure–function relationship of heme proteins using multifrequency pulse EPR techniques. In High Resolution EPR; Springer: Berlin/Heidelberg, Germany, 2009; pp. 397–417. [Google Scholar]
- Svistunenko, D.A.; Worrall, J.A.; Chugh, S.B.; Haigh, S.C.; Ghiladi, R.A.; Nicholls, P. Ferric haem forms of Mycobacterium tuberculosis catalase-peroxidase probed by EPR spectroscopy: Their stability and interplay with pH. Biochimie 2012, 94, 1274–1280. [Google Scholar] [CrossRef]
- Aasa, R.; Ellfolk, N.; Rönnberg, M.; Vänngård, T. Electron paramagnetic resonance studies of Pseudomonas cytochrome c peroxidase. Biochim. Biophys. Acta (BBA)-Protein Struct. 1981, 670, 170–175. [Google Scholar] [CrossRef]
- Hollenberg, P.; Hager, L.; Blumberg, W.; Peisach, J. An electron paramagnetic resonance study of the high and low spin forms of chloroperoxidase. J. Biol. Chem. 1980, 255, 4801–4807. [Google Scholar] [CrossRef]
- Yonetani, T.; Wilson, D.F.; Seamonds, B. Studies on cytochrome c peroxidase: VIII. The effect of temperature on light absorptions of the enzyme and its derivatives. J. Biol. Chem. 1966, 241, 5347–5352. [Google Scholar] [CrossRef]
- Yonetani, T.; Anni, H. Yeast cytochrome c peroxidase. Coordination and spin states of heme prosthetic group. J. Biol. Chem. 1987, 262, 9547–9554. [Google Scholar] [CrossRef]
- Yoshida, T.; Sugano, Y. A structural and functional perspective of DyP-type peroxidase family. Arch. Biochem. Biophys. 2015, 574, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Pfanzagl, V.; Nys, K.; Bellei, M.; Michlits, H.; Mlynek, G.; Battistuzzi, G.; Djinovic-Carugo, K.; Van Doorslaer, S.; Furtmüller, P.G.; Hofbauer, S. Roles of distal aspartate and arginine of B-class dye-decolorizing peroxidase in heterolytic hydrogen peroxide cleavage. J. Biol. Chem. 2018, 293, 14823–14838. [Google Scholar] [CrossRef]
- Pfanzagl, V.; Bellei, M.; Hofbauer, S.; Laurent, C.V.; Furtmüller, P.G.; Oostenbrink, C.; Battistuzzi, G.; Obinger, C. Redox thermodynamics of B-class dye-decolorizing peroxidases. J. Inorg. Biochem. 2019, 199, 110761. [Google Scholar] [CrossRef]
- Pfanzagl, V.; Beale, J.H.; Michlits, H.; Schmidt, D.; Gabler, T.; Obinger, C.; Djinović-Carugo, K.; Hofbauer, S. X-ray–induced photoreduction of heme metal centers rapidly induces active-site perturbations in a protein-independent manner. J. Biol. Chem. 2020, 295, 13488–13501. [Google Scholar] [CrossRef]
- Sugano, Y.; Yoshida, T. DyP-Type Peroxidases: Recent Advances and Perspectives. Int. J. Mol. Sci. 2021, 22, 5556. [Google Scholar] [CrossRef]
- Zámocký, M.; Hofbauer, S.; Schaffner, I.; Gasselhuber, B.; Nicolussi, A.; Soudi, M.; Pirker, K.F.; Furtmüller, P.G.; Obinger, C. Independent evolution of four heme peroxidase superfamilies. Arch. Biochem. Biophys. 2015, 574, 108–119. [Google Scholar] [CrossRef]
- Hofbauer, S.; Schaffner, I.; Furtmüller, P.G.; Obinger, C. Chlorite dismutases–a heme enzyme family for use in bioremediation and generation of molecular oxygen. Biotechnol. J. 2014, 9, 461–473. [Google Scholar] [CrossRef]
- Goblirsch, B.; Kurker, R.C.; Streit, B.R.; Wilmot, C.M.; DuBois, J.L. Chlorite dismutases, DyPs, and EfeB: 3 microbial heme enzyme families comprise the CDE structural superfamily. J. Mol. Biol. 2011, 408, 379–398. [Google Scholar] [CrossRef]
- Sündermann, A.; Reif, M.M.; Hofbauer, S.; Obinger, C.; Oostenbrink, C. Investigation of ion binding in chlorite dismutases by means of molecular dynamics simulations. Biochemistry 2014, 53, 4869–4879. [Google Scholar] [CrossRef]
- Hofbauer, S.; Bellei, M.; Sündermann, A.; Pirker, K.F.; Hagmüller, A.; Mlynek, G.; Kostan, J.; Daims, H.; Furtmüller, P.G.; Djinović-Carugo, K. Redox thermodynamics of high-spin and low-spin forms of chlorite dismutases with diverse subunit and oligomeric structures. Biochemistry 2012, 51, 9501–9512. [Google Scholar] [CrossRef]
- Hofbauer, S.; Pfanzagl, V.; Michlits, H.; Schmidt, D.; Obinger, C.; Furtmüller, P.G. Understanding molecular enzymology of porphyrin-binding α+ β barrel proteins-One fold, multiple functions. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2020, 1869, 140536. [Google Scholar] [CrossRef]
- Chovancova, E.; Pavelka, A.; Benes, P.; Strnad, O.; Brezovsky, J.; Kozlikova, B.; Gora, A.; Sustr, V.; Klvana, M.; Medek, P. CAVER 3.0: A tool for the analysis of transport pathways in dynamic protein structures. PLoS Comput. Biol. 2012, 8, e1002708. [Google Scholar] [CrossRef]
- Leigh, J.S., Jr.; Reed, G.H. Electron paramagnetic resonance studies in frozen aqueous solutions. Elimination of freezing artifacts. J. Phys. Chem. 1971, 75, 1202–1204. [Google Scholar] [CrossRef]
- Svistunenko, D.A.; Sharpe, M.A.; NichollsI, P.; Blenkinsop, C.; Davies, N.A.; Dunne, J.; Wilson, M.T.; Cooper, C.E. The pH dependence of naturally occurring low-spin forms of methaemoglobin and metmyoglobin: An EPR study. Biochem. J. 2000, 351, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Smulevich, G.; Feis, A.; Howes, B.D. Fifteen years of Raman spectroscopy of engineered heme containing peroxidases: What have we learned? Acc. Chem. Res. 2005, 38, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Maltempo, M.M. Magnetic state of an unusual bacterial heme protein. J. Chem. Phys. 1974, 61, 2540–2547. [Google Scholar] [CrossRef]
- George, S.J.; Kvaratskhelia, M.; Dilworth, M.J.; Thorneley, R.N. Reversible alkaline inactivation of lignin peroxidase involves the release of both the distal and proximal site calcium ions and bishistidine co-ordination of the haem. Biochem. J. 1999, 344, 237–244. [Google Scholar] [CrossRef]
- Howes, B.; Feis, A.; Indiani, C.; Marzocchi, M.; Smulevich, G. Formation of two types of low-spin heme in horseradish peroxidase isoenzyme A2 at low temperature. JBIC J. Biol. Inorg. Chem. 2000, 5, 227–235. [Google Scholar] [CrossRef]
- Kraus, D.W.; Wittenberg, J. Hemoglobins of the Lucina pectinata/bacteria symbiosis. I. Molecular properties, kinetics and equilibria of reactions with ligands. J. Biol. Chem. 1990, 265, 16043–16053. [Google Scholar] [CrossRef]
- Tamura, M.; Hori, H. Optical and magnetic measurements of horseradish peroxidase III. Electron paramagnetic resonance studies at liquid-hydrogen and-helium temperatures. Biochim. Biophys. Acta (BBA)-Enzymol. 1972, 284, 20–29. [Google Scholar] [CrossRef]
- Hori, H.; Yonetani, T. Powder and single-crystal electron paramagnetic resonance studies of yeast cytochrome c peroxidase and its peroxide and its peroxide compound, Compound ES. J. Biol. Chem. 1985, 260, 349–355. [Google Scholar] [CrossRef]
- Nicoletti, F.P.; Droghetti, E.; Howes, B.D.; Bustamante, J.P.; Bonamore, A.; Sciamanna, N.; Estrin, D.A.; Feis, A.; Boffi, A.; Smulevich, G. H-bonding networks of the distal residues and water molecules in the active site of Thermobifida fusca hemoglobin. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2013, 1834, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Tilleman, L.; Germani, F.; De Henau, S.; Helbo, S.; Desmet, F.; Berghmans, H.; Van Doorslaer, S.; Hoogewijs, D.; Schoofs, L.; Braeckman, B.P. A globin domain in a neuronal transmembrane receptor of caenorhabditis elegans and ascaris suum: Molecular modeling and functional properties. J. Biol. Chem. 2015, 290, 10336–10352. [Google Scholar] [CrossRef]
- Vinck, E.; Van Doorslaer, S.; Dewilde, S.; Moens, L. Structural change of the heme pocket due to disulfide bridge formation is significantly larger for neuroglobin than for cytoglobin. J. Am. Chem. Soc. 2004, 126, 4516–4517. [Google Scholar] [CrossRef]
- Salerno, J.C.; Martasek, P.; Roman, L.J.; Masters, B.S.S. Electron paramagnetic resonance spectroscopy of the heme domain of inducible nitric oxide synthase: Binding of ligands at the arginine site induces changes in the heme ligation geometry. Biochemistry 1996, 35, 7626–7630. [Google Scholar] [CrossRef] [PubMed]
- Serra, I.; García-Rubio, I.; Van Doorslaer, S. Pitfalls in sample preparation of metalloproteins for low-temperature EPR—The example of alkaline myoglobin. Appl. Magn. Reson. 2021. [Google Scholar] [CrossRef]
- Trandafir, F.; Heerdt, P.; Fittipaldi, M.; Vinck, E.; Dewilde, S.; Moens, L.; Van Doorslaer, S. Studying high-spin ferric heme proteins by pulsed EPR spectroscopy: Analysis of the ferric form of the E7Q mutant of human neuroglobin. Appl. Magn. Reson. 2007, 31, 553–572. [Google Scholar] [CrossRef]
- Smirnova, T.I.; Weber, R.T.; Davis, M.F.; Franzen, S. Substrate binding triggers a switch in the iron coordination in dehaloperoxidase from Amphitrite ornata: HYSCORE experiments. J. Am. Chem. Soc. 2008, 130, 2128–2129. [Google Scholar] [CrossRef]
- Fittipaldi, M.; García-Rubio, I.; Trandafir, F.; Gromov, I.; Schweiger, A.; Bouwen, A.; Van Doorslaer, S. A multi-frequency pulse EPR and ENDOR approach to study strongly coupled nuclei in frozen solutions of high-spin ferric heme proteins. J. Phys. Chem. B 2008, 112, 3859–3870. [Google Scholar] [CrossRef]
- García-Rubio, I.; Fittipaldi, M.; Trandafir, F.; Van Doorslaer, S. A multifrequency HYSCORE study of weakly coupled nuclei in frozen solutions of high-spin aquometmyoglobin. Inorg. Chem. 2008, 47, 11294–11304. [Google Scholar] [CrossRef] [PubMed]
- García-Rubio, I.; Braun, M.; Gromov, I.; Thöny-Meyer, L.; Schweiger, A. Axial coordination of heme in ferric CcmE chaperone characterized by EPR spectroscopy. Biophys. J. 2007, 92, 1361–1373. [Google Scholar] [CrossRef][Green Version]
- Maltempo, M.; Ohlsson, P.; Paul, K.; Petersson, L.; Ehrenberg, A. Electron paramagnetic resonance analyses of horseradish peroxidase in situ and after purification. Biochemistry 1979, 18, 2935–2941. [Google Scholar] [CrossRef]
- Peisach, J.; Blumberg, W.; Ogawa, S.; Rachmilewitz, E.; Oltzik, R. The effects of protein conformation on the heme symmetry in high spin ferric heme proteins as studied by electron paramagnetic resonance. J. Biol. Chem. 1971, 246, 3342–3355. [Google Scholar] [CrossRef]
- Blumberg, W.; Peisach, J.; Wittenberg, B.A.; Wittenberg, J.B. The electronic structure of protoheme proteins: I. An electron paramagnetic resonance and optical study of horseradish peroxidase and its derivatives. J. Biol. Chem. 1968, 243, 1854–1862. [Google Scholar] [CrossRef]
- Linde, D.; Pogni, R.; Canellas, M.; Lucas, F.; Guallar, V.; Baratto, M.C.; Sinicropi, A.; Sáez-Jiménez, V.; Coscolin, C.; Romero, A. Catalytic surface radical in dye-decolorizing peroxidase: A computational, spectroscopic and site-directed mutagenesis study. Biochem. J. 2015, 466, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Yonetani, T. 6 Cytochrome c peroxidase. In The Enzymes; Elsevier: Amsterdam, The Netherlands, 1976; Volume 13, pp. 345–361. [Google Scholar]
- Cao, E.; Chen, Y.; Cui, Z.; Foster, P.R. Effect of freezing and thawing rates on denaturation of proteins in aqueous solutions. Biotechnol. Bioeng. 2003, 82, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, A.R.; Cannistraro, S. Solvent modulation of the structural heterogeneity in FeIII myoglobin samples: A low temperature EPR investigation. Eur. Biophys. J. 1993, 22, 259–267. [Google Scholar] [CrossRef]
- Smulevich, G.; Mantini, A.R.; English, A.M.; Mauro, J.M. Effects of temperature and glycerol on the resonance Raman spectra of cytochrome c peroxidase and selected mutants. Biochemistry 1989, 28, 5058–5064. [Google Scholar] [CrossRef]
- Yatsunyk, L.A.; Dawson, A.; Carducci, M.D.; Nichol, G.S.; Walker, F.A. Models of the cytochromes: Crystal structures and EPR spectral characterization of low-spin bis-imidazole complexes of (OETPP) FeIII having intermediate ligand plane dihedral angles. Inorg. Chem. 2006, 45, 5417–5428. [Google Scholar] [CrossRef]
- Williams-Smith, D.L.; Bray, R.; Barber, M.; Tsopanakis, A.; Vincent, S. Changes in apparent pH on freezing aqueous buffer solutions and their relevance to biochemical electron-paramagnetic-resonance spectroscopy. Biochem. J. 1977, 167, 593–600. [Google Scholar] [CrossRef]
- Sitter, A.J.; Shifflett, J.; Terner, J. Resonance Raman spectroscopic evidence for heme iron-hydroxide ligation in peroxidase alkaline forms. J. Biol. Chem. 1988, 263, 13032–13038. [Google Scholar] [CrossRef]
- Neri, F.; Indiani, C.; Welinder, K.G.; Smulevich, G. Mutation of the distal arginine in Coprinus cinereus peroxidase: Structural implications. Eur. J. Biochem. 1998, 251, 830–838. [Google Scholar] [CrossRef]
- Howes, B.D.; Rodriguez-Lopez, J.N.; Smith, A.T.; Smulevich, G. Mutation of distal residues of horseradish peroxidase: Influence on substrate binding and cavity properties. Biochemistry 1997, 36, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, C.; Datta, A.; Butcher, R.J. Highly efficient one-pot, three-component Mannich reaction catalysed by boric acid and glycerol in water with major ‘syn’diastereoselectivity. Tetrahedron Lett. 2009, 50, 4246–4250. [Google Scholar] [CrossRef]
- Scholes, C.P.; Lapidot, A.; Mascarenhas, R.; Inubushi, T.; Isaacson, R.A.; Feher, G. Electron nuclear double resonance (ENDOR) from heme and histidine nitrogens in single crystals of aquometmyoglobin. J. Am. Chem. Soc. 1982, 104, 2724–2735. [Google Scholar] [CrossRef]
- Höfer, P.; Grupp, A.; Nebenführ, H.; Mehring, M. Hyperfine sublevel correlation (hyscore) spectroscopy: A 2D ESR investigation of the squaric acid radical. Chem. Phys. Lett. 1986, 132, 279–282. [Google Scholar] [CrossRef]
- Jeschke, G.; Rakhmatullin, R.; Schweiger, A. Sensitivity Enhancement by Matched Microwave Pulses in One- and Two-Dimensional Electron Spin Echo Envelope Modulation Spectroscopy. J. Magn. Reson. 1998, 131, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Taha, M.; Lee, M.-J. Interactions of TRIS [tris(hydroxymethyl)aminomethane] and related buffers with peptide backbone: Thermodynamic characterization. Phys. Chem. Chem. Phys. 2010, 12, 12840–12850. [Google Scholar] [CrossRef]
- Pielak, G.J. Buffers, Especially the Good Kind. Biochemistry 2021. [Google Scholar] [CrossRef]

| gz | gy | gx | Ref. | |
|---|---|---|---|---|
| WT KpDyP | ||||
| OH2 | 2.77 | 2.17 | 1.77 | this work |
| D143A KpDyP | ||||
| OH1′ | 2.89 | 2.12 | 1.78 | this work |
| OH2′ | 2.728 | 2.15 | 1.772 | this work |
| OH3′ | 2.67 | 2.214 | 1.82 | this work |
| HRP | 2.94 | 2.08 | 1.63 | [30] |
| CcP | 2.74 | 2.22 | 1.74 | [31] |
| swMb | 2.55 | 2.17 | 1.85 | [29] |
| CeGLB-33 | 2.62 2.845 | 2.20 2.12 | 1.815 1.69 | [33] |
| TfHb | 2.73 2.66 2.82 | 2.19 2.19 2.32 | 1.76 1.81 1.60 | [32] |
| |Azeff| [MHz] | |Qz| [MHz] | |
|---|---|---|
| KpDyP (this work) WT 14Nporf,1 | 6.90 | 0.25 |
| 14Nporf,2 | 6.70 | 0.28 |
| D143A 14Nporf,1 | 6.90 | 0.25 |
| 14Nporf,2 | 6.70 | 0.28 |
| R232A 14Nporf,1 | 7.07 | 0.32 |
| 14Nporf,2 | 6.95 | 0.28 |
| metMb [39] 14Nporf,1 | 7.42 | 0.33 |
| 14Nporf,2 | 7.10 | 0.23 |
| NGB [37] 14Nporf | 7.25 | 0.23 |
| Dehaloperoxidase [38] | ||
| 14Nporf | 7.50 | n.r. |
| CcmE chaperone [41] 14Nporf | 8.01 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nys, K.; Pfanzagl, V.; Roefs, J.; Obinger, C.; Van Doorslaer, S. In Vitro Heme Coordination of a Dye-Decolorizing Peroxidase—The Interplay of Key Amino Acids, pH, Buffer and Glycerol. Int. J. Mol. Sci. 2021, 22, 9849. https://doi.org/10.3390/ijms22189849
Nys K, Pfanzagl V, Roefs J, Obinger C, Van Doorslaer S. In Vitro Heme Coordination of a Dye-Decolorizing Peroxidase—The Interplay of Key Amino Acids, pH, Buffer and Glycerol. International Journal of Molecular Sciences. 2021; 22(18):9849. https://doi.org/10.3390/ijms22189849
Chicago/Turabian StyleNys, Kevin, Vera Pfanzagl, Jeroen Roefs, Christian Obinger, and Sabine Van Doorslaer. 2021. "In Vitro Heme Coordination of a Dye-Decolorizing Peroxidase—The Interplay of Key Amino Acids, pH, Buffer and Glycerol" International Journal of Molecular Sciences 22, no. 18: 9849. https://doi.org/10.3390/ijms22189849
APA StyleNys, K., Pfanzagl, V., Roefs, J., Obinger, C., & Van Doorslaer, S. (2021). In Vitro Heme Coordination of a Dye-Decolorizing Peroxidase—The Interplay of Key Amino Acids, pH, Buffer and Glycerol. International Journal of Molecular Sciences, 22(18), 9849. https://doi.org/10.3390/ijms22189849