Abstract
Macro-autophagy (autophagy) is a highly conserved eukaryotic intracellular process of self-digestion caused by lysosomes on demand, which is upregulated as a survival strategy upon exposure to various stressors, such as metabolic insults, cytotoxic drugs, and alcohol abuse. Paradoxically, autophagy dysfunction also contributes to cancer and aging. It is well known that regulating autophagy by targeting specific regulatory molecules in its machinery can modulate multiple disease processes. Therefore, autophagy represents a significant pharmacological target for drug development and therapeutic interventions in various diseases, including cancers. According to the framework of autophagy, the suppression or induction of autophagy can exert therapeutic properties through the promotion of cell death or cell survival, which are the two main events targeted by cancer therapies. Remarkably, natural products have attracted attention in the anticancer drug discovery field, because they are biologically friendly and have potential therapeutic effects. In this review, we summarize the up-to-date knowledge regarding natural products that can modulate autophagy in various cancers. These findings will provide a new position to exploit more natural compounds as potential novel anticancer drugs and will lead to a better understanding of molecular pathways by targeting the various autophagy stages of upcoming cancer therapeutics.
1. Introduction
Cellular homoeostasis requires a stable balance between biosynthetic renewal and catabolic processes. Macro-autophagy, hereafter referred to as autophagy, and the ubiquitin-proteasome system (UPS) are two primarily distinct proteolytic systems in eukaryotic cells that have wide-scale degradation [1]. Given that highly selective UPS can generally only recognize short-lived protein substrates in the cell renovation system, autophagy has been well-appreciated and has followed a complex model of execution [2]. Autophagy is a self-digestive process that facilitates eukaryotic intracellular nutrient recycling via the lysosomal degradation of long-living, unwanted cellular proteins as well as damaged or defective organelles, including the mitochondria, the endoplasmic reticulum (ER), the Golgi apparatus and peroxisomes [3,4]. Autophagy has an established role in cell metabolism and energy homeostasis through the catabolism of proteins, lipids (lipophagy), carbohydrates (glycophagy) and iron (ferritinophagy), which fuels energy and nutrient stores [5]. In response to a wide variety of cellular stressors, including metabolic stress, autophagy is typically activated as a pro-survival mechanism in normal and cancerous cells [6,7]. However, in recent years, accumulated evidence has concentrated on the importance of autophagy and the variety of roles it plays in a variety of human diseases, including cancers. For instance, alterations in autophagy and inherited mutations in autophagy-related genes (ATGs) that regulate autophagy have been implicated in human cancer [4,8,9]. Autophagy has complex and context-dependent actions in cancers, and interventions to activate and suppress it have been planned as cancer therapies [4,10]. Thus, this review on natural products may reveal new therapeutic strategies that can regulate the progression of autophagy-mediated disorders, particularly cancers.
Today, although many chemotherapeutic agents have been developed to treat cancer, the effectiveness of many cancer medications remains limited or unsatisfactory. Therefore, the development of effective and non-toxic anticancer drugs or strategies is highly urgent and desirable. In the last few decades, a series of natural products with the ability to regulate physiological functions have been isolated and exploited from plants, animals and microorganisms, with most of them revealing obvious anticancer activity [11,12,13,14]. Well-tolerated and less toxic natural products will help patients to achieve better therapeutic results and will improve quality of life. Many chemotherapeutic agents have been identified by investigating potential compounds from plants, animals, and microorganisms, including marine organisms, which have been found to exert anti-cancer effects against a variety of tumors [11,12,13,14]. As a result, over 49% of approved and pre-DPA applications are natural products or agents derived from natural products, with the exception of antibodies and vaccines [15]. With respect to antitumor agents, more than 53% of anti-cancer agents applied in medicine are unaltered natural products, botanical drugs (defined mixture) and derivatives of natural products [16]. To develop an effective autophagy-targeting therapy, it is essential to identify key targets in the autophagy pathway in order to develop novel therapeutics. As discussed earlier, autophagy can play a protective or destructive role in the state of a disease, and thus it would be valuable to identify and develop pharmacologic agents that can exactly induce or inhibit this cellular process. A wide variety of potentially “druggable” targets in different stages of autophagy have been identified [4,9,17], and several natural products are capable of inducing and/or inhibiting autophagy, as discussed in this paper.
2. Molecular Mechanisms and Morphological Features of Autophagy
The following specific stages of autophagy are involved in the execution of the final degradation stage of recycling and energy production (Figure 1). The ultrastructural features of autophagy are shown in Figure 2.
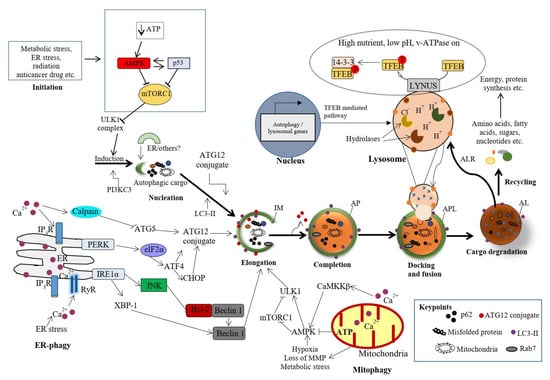
Figure 1.
Molecular mechanisms of various stages of autophagy. Autophagy is activated in response to various cellular stresses and is triggered by a decrease in rapamycin complex 1 (mTORC1) activity due to the activation of AMP-activated protein kinase (AMPK) or p53 signaling. mTORC1 suppresses the activity of Unc-51-like autophagy activating kinase 1 (ULK1) complex. Therefore, inhibition of mTORC1 causes the initialization of the ULK1-mediated formation of the isolation (autophagosomal) membrane (IM) in association with the class III phosphatidylinositide 3-kinase (PI3K) complex (PI3KC3). The IM expands into an autophagosome (AP) with a double-layer membrane, which can engulf any cellular component, including proteins, damaged organelles and lipid droplets. The AP merges with the lysosome (via LAMP-1, 2), forming autophagolysosome (APL) or autolysosome (AL), and resulting in the degradation of the cargo by cathepsins and the autophagic lysosome reformation (ALR). The nucleation, elongation and maturation of the IM are dependent on two ubiquitin-like conjugation systems (ATG12 and ATG8), which involve multiple autophagy proteins, including Beclin1, ATG5, ATG16 and MT-associated protein 1 light chain 3 (LC3). The AL provides an acidic milieu for hydrolytic enzymes to digest the engulfed components. Nuclear localization of transcription factor EB (TFEB) is critical to the formation of lysosomes and to the enhanced expression of autophagy proteins. Importantly, autophagy could be selective of mitochondria (mitophagy) or ER (ER-phagy) [7]. However, the detailed mechanisms of this selected autophagy are beyond the scope of this study.
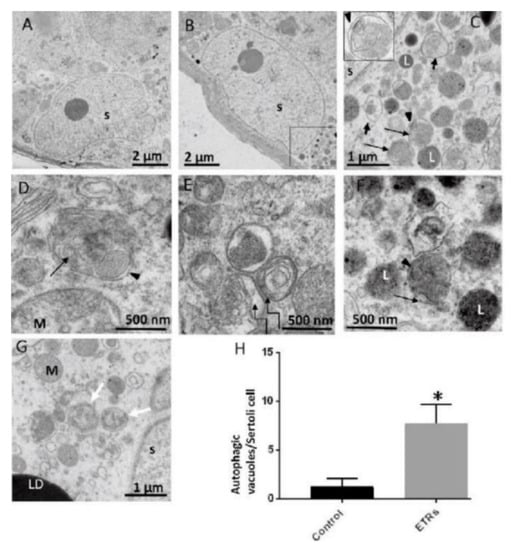
Figure 2.
Ultrastructural features of upregulated autophagy in ethanol-treated Sertoli cells (ETR SCs). TEM of the control (A) and ETRs (B–G). The histogram (H) shows a significant increase in the number of autophagic vacuoles (AVs) in ETR SCs. The long black arrows indicate autophagosomes with a double limiting membrane (arrow heads) (magnified in the inset in (C)). The short arrows indicate autolysosomes. The broken arrows in (E) show multilamellar bodies, while the white arrows in (G) show autophagosomes containing fragmented mitochondria. Note the characteristic perinuclear localization of AVs. S: SC nucleus; L: lysosome; M: mitochondria; LD: lipid droplet. AVs include autophagosomes and autolysosomes. * p < 0.05. This was reprinted from Reference [3].
2.1. Autophagy Initiation
Autophagy is initiated by a network of complex molecular machineries that centrally involve a set of ATG proteins that modulate IM (originally termed as the phagophore) formation and autophagosome maturation. Under cellular stress, the serine/threonine protein kinase ULK1 and class III PI3K complexes are the two major protein structures involved in the initiation of autophagy in mammalian cells. It has been reported that mTORC1 inhibits the ULK1 complex, while AMPK acts as a positive regulator of ULK1 by suppressing mTORC1 in nutrient-sensing pathways (Figure 1) [18,19]. The origin of the membrane for the formation of autophagosome has been studied thoroughly, but remains an enigma in the field of autophagy [20]. While electron microscopic static images provide detailed morphological data, they do not completely explain the dynamics of autophagy, especially the sequential completion of autophagosomes and the fusion with lysosomes. Live-cell imaging techniques suggest that effective vesicular trafficking from existing compartments and membranes involves IM formation [10,21,22]. Several organelles, such as the ER [20], the Golgi apparatus, the mitochondrial outer membrane (MOM), endosomes, the plasma membrane and the nuclear envelope [23], may donate membranes for IM formation. It was also found that the specialized ER regions called ER exit sites (ERES) are important mediators for IM formation [20]. Moreover, several inter-organelle-contact sites have emerged as dynamic spots for the lipid transition between the membranes and the growth of nascent IM. ER–mitochondria contact sites, also known as mitochondria-associated ER membranes (MAMs), are claimed to be the source of IM [24,25]. MAMs spatially overlap with specialized membrane compartments, called omegasomes (omega-shaped structures), and these serve as a cradle of IM formation and autophagosomal vesicles close to the ER.
2.2. Cargo Nucleation, Elongation and Enclosure of IM
During the initiation of autophagy, the localization of the ULK1 complex in the IM site regulates the vesicle nucleation machinery. The nucleation machinery of the initial IM is dependent on local phosphatidylinositol 3-phosphate (PI3P) production, which is marked by its binding protein, i.e., the double FYVE domain-containing protein 1 (DFCP1) by the class III PI3K complex. Thus, vesicle nucleation involves the ULK1 complex mechanistic link core complex, which consists of class III PI3K (PIK3C3), Beclin1, p150, ATG14L and the activating molecule in Beclin1-regulated autophagy protein 1 (Ambra1) [26,27]. The elongation step of the IM is controlled by two evolutionarily conserved ubiquitin-like (UBL) conjugation systems called the ATG12–ATG5–ATG16 complex (also known as the ATG12 conjugation system) and the LC3-phosphatidylethanolamine (LC3-PE) conjugation system [28,29] (Figure 2).
In response to various stressors, such as starvation, oxidative stress and hypoxia, specific cargos are sequestrated with autophagy receptors in IM. The autophagy receptors bind specifically to ubiquitinated cargos that are tagged with degradation signals of the autophagy machinery through their LC3 interacting regions (LIR) [30,31]. In general, the LIR motifs interact with autophagy regulatory proteins of the LC3/GABARAP family [32]. In mammalian cells, more than twenty autophagy receptors have been identified, and sequestosome-1 (p62) is one of the most common autophagy receptors [33]. LC3-II is the major autophagy marker, and its upregulation is an indicator of autophagy activation and the formation of dual-membrane autophagosomes. Using immunofluorescence and immunohistochemistry (IHC) methods, the autophagosomes appear as dots or puncta, indicating the expression of LC3-II, which could be detected as shown in Figure 3 [3,7]. An autophagic flux involves the formation of autophagosomes, their administration to lysosomes, and the subsequent degradation and release of degraded macromolecules into cytosol, which are then recycled. This is called productive or completed autophagy. Thus, the increases in the level of LC3-II, as evidenced by the accumulation of autophagosomes, are not measurements of the autophagic flux per se, but may reflect the induction of autophagy sequestration and/or the inhibition of autophagosome clearance, which results from the fusion failure with lysosomes or lysosomal dysfunction. This incomplete or impaired autophagic flux may result in cell death, such as apoptosis or cell death with autophagic features (sometimes called autophagic cell death). Autophagic flux can be monitored through the use of inhibitors such as choloroquine (CQ), bafilomycin A1 (Baf A1) or lysosomal protease inhibitors. This can be determined by measuring the levels of LC3-II in both the presence and absence of saturating levels of inhibitors; if flux is occurring, the amount of LC3-II will be higher in the presence of the inhibitor [3,7,8,9,10].
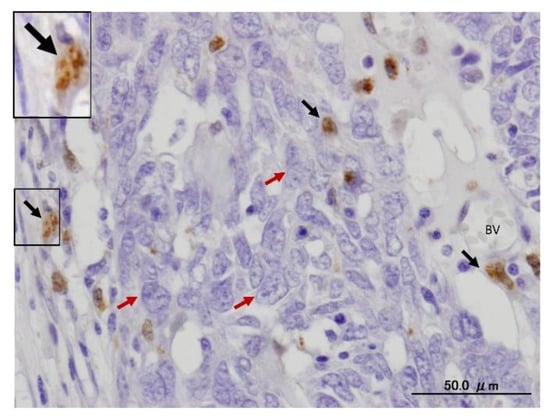
Figure 3.
Expression of LC3 in stromal cells of serious human ovarian carcinoma using IHC. The black arrows indicate LC3-II puncta in stromal cells, whereas the red arrows mark ovarian cancer cells. The framed area is magnified in the inset. BV, blood vessel. The avidin biotin complex (ABC) IHC method is performed. The diaminobenzidine (DAB) is used as a chromogen (brown reaction), whereas the hematoxylin (blue) is applied for nuclear counterstaining.
2.3. Transport of Autophagosomes
Autophagosomes (double membrane vesicles) are randomly formed throughout the cytosol, concentrating and continuously moving during the maturation process. In general, microtubules (MTs) serve as an interconnected network of intracellular movement powered by specific motor proteins, including the kinesin and dynein protein families [34]. Kinesins normally transport the cargoes to the peripheral plus-ends of MTs, whereas dyneins, a class of AAA+-ATPase-associated motors, are involved in the delivery of autophagosomes towards the minus-end [35]. The coordinated transport of lysosomes and autophagosomes in the perinuclear zone is necessary for the adequate fusion of these two organelles [36,37], as shown in Figure 2. Under various stress conditions, the intracellular pH increases, resulting in the relocation of lysosomes to the perinuclear region, where mature autophagosomes are transported to the same region by the MTs [3,36]. Consequently, the lysosomes in the perinuclear areas merge with mature autophagosomes in order to acidify them [3,38]. This is shown in Figure 2.
2.4. Autolysosome Formation, Vesicle Degradation and ALR Cycle
As the mature autophagosome docks to the lysosome, a single-membrane autolysosome is formed [39]. Merging autophagosomes with late endosomes to form amphisomes, prior to fusion with the lysosome, is also reported to increase cargo delivery and reduce the pH of autolysosomes [40]. Upon fusion with mature lysosomes, the intralysosomal contents degrade with the release of end products into the cytosol, producing local nutrient availability as a source of cellular energy. This process results in the reactivation of mTOR, a key regulator of autophagy, and the regeneration of mature lysosomes from autolysosomes, which is a process called ALR [41,42]. While there is significant insight into each of these stages of autophagy, the molecular mechanisms controlling the biogenesis of autophagosomes, autolysosomes and lysosomes are complex. In response to intracellular and environmental stressors, autophagy is primarily regulated by two critical signaling pathways, i.e., the mTOR-dependent and mTOR-independent signaling pathways, which include Ca2+, inositol 1,4,5-trisphosphate (IP3) receptor (IP3R), AMPK, stress activated enzyme c-Jun N-terminal kinase (JNK), and B-cell lymphoma 2 (Bcl-2) homology (BH) domain 3 (BH3)-only proteins [42]. It has been reported that nuclear translocation of TFEB positively regulates the formation of lysosomes and enhances the expression of autophagy proteins, specifically, under starvation, oxidative and nitrative stress (Figure 4). Using various methods, we recently provided evidence of the enhanced nuclear translocation of TFEB in the SCs of ETRs, which is correlated with the upregulation of autophagy and mitophagy proteins [43], as shown in Figure 4.
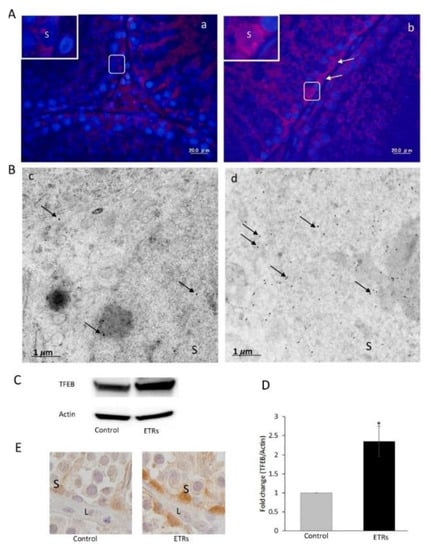
Figure 4.
Elevated expression and nuclear translocation of TFEB in ETR SCs. (A) The IF of TFEB expressions in the control (a) and ETRs (b). The insets present higher magnifications of the framed areas. Note the overexpression of TFEB (white arrows) in the SC nuclei of ETRs. (B) Immunogold labeling of TFEB (black arrows, 15 nm gold particles) in the control (c) and ETR SCs (d). (C) Western blot of TFEB in the control and ETR tests (n = 3). (D) Histogram showing a significant increase in TFEB expression in the ETR tests. * p < 0.05 (t-test). (E) IHC showing TFEB nuclear translocation in ETR SCs (part of a seminiferous tubule), confirming the IF and IEM results (A,B). S: SC nucleus; L: Leydig cell. This was reprinted from Reference [43].
3. Natural Products as Inhibitors of Autophagy in Cancer
Autophagy is mandatory to maintain cell homeostasis. In healthy cells, this homeostatic activity provides a strong barrier against oncogenesis. As a result, many oncoproteins inhibit and several oncosuppressor proteins promote autophagy. In addition, autophagy might contribute to oncogene-induced cell death or oncogene-induced senescence, which are two fundamental oncosuppressive mechanisms. Additionally, autophagy is necessary for optimal anticancer immunosurveillance. However, autophagy enhances the progression of established cancers via multiple mechanisms, and pharmacological inhibitors of autophagy may have robust antineoplastic effects, at least in certain contexts. Enhanced autophagy in the stromal compartment of pancreatic cancers supports tumor growth via autophagy-mediated secretion of the nonessential amino acid alanine, thus fueling the mitochondrial metabolism of cancer cells and allowing them to thrive in an austere microenvironment [8,9,10]. Therefore, as shown in Figure 3, the enhanced expression of LC3-II in the stromal cells of ovarian cancer may fuel the growth of cancer cells. Natural products have historically been regarded as different doctrines of traditional medicine or folk medicine for the management and treatment of a variety of human diseases, including cancer, by inhibiting autophagy pathways [4,44,45]. Several studies have shown that the inhibition of autophagy using natural compounds can effectively enhance the cancer cell death induced by diverse anticancer drugs. Importantly, the use of natural products in the suppression of autophagy can be carried out by the specific targeting of various structures in the autophagy pathway (isolation membranes, autophagosomes, autolysosomes and lysosomes), as shown in Figure 1.
3.1. Class III-PI3K Complex Inhibitors
Class III PI3K mediates the production of PI3P, a key lipid-signaling molecule that is known to be required for autophagosome formation via the recruitment of autophagy machinery at the IM (Figure 1). Evidence has confirmed that class III-PI3K inhibitors interfere with the formation of autophagosomes. The main inhibitors for the early stages of autophagy, including the mechanism of action (M/A), are given in Table 1.

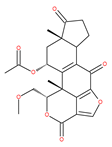
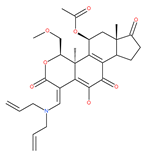
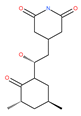
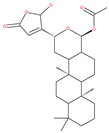
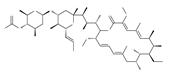
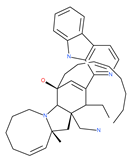
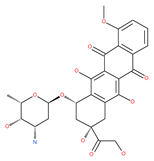
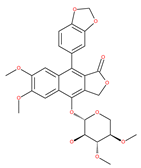
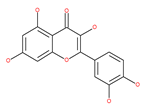
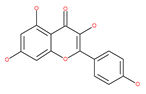
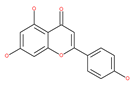
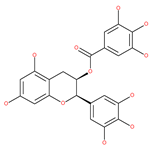
Table 1.
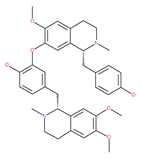
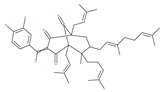
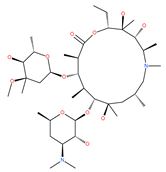
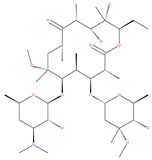
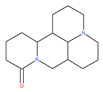
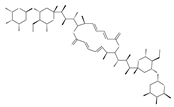
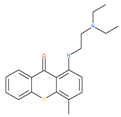
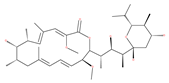
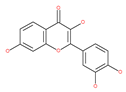
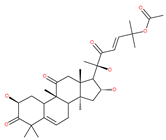
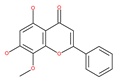
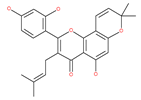
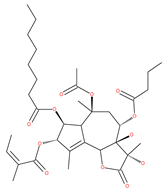
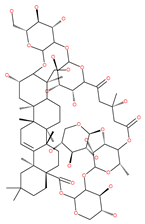
Examples of natural materials that modulate autophagy, including chemical structure.
Based on their blocking effect on class III PI3K activity, the natural product wortmannin [46,47] and its concurrent synthetic compounds, 3-methyladenine (3-MA), KU55933 and LY294002, are well recognized as early stage autophagy inhibitors in the literature [190]. Wortmannin was originally identified as a potent inhibitor of the neutrophil respiratory burst and smooth muscle myosin light chain kinase (MLCK) [48]. Later on, it has become clear that wortmannin is a more potent inhibitor of the PI3K superfamily than of the MLCK. More recently, wortmannin has also been reported to suppress polo-like kinase 1 (PLK1) [191], mTOR [49], DNA-dependent protein kinase (DNA-PK) [192] and ataxia–telangiectasia mutated (ATM) [193] at micromolar concentrations [194]. Unlike 3-MA, wortmannin is a more practical and feasible autophagy inhibitor due to more persistent suppression on class III PI3K, regardless of the nutritional status. While 3-MA, wortmannin, and LY294002 have been useful in many contexts to inhibit autophagy and to eventually sensitize tumor cells to death, they also target class I PI3Ks. As non-specific inhibitors of PI3K, these compounds affect several cell processes and are toxic after long-term exposure [195]. Moreover, these compounds have been shown to play a role in many other cellular processes, including fluid-phase endocytosis and cell migration [196]. Thus, these compounds have been unsuitable for clinical applications due to their inherent toxicity, poor solubility and low stability [33]. However, nanoparticle (NP)-based drug delivery of wortmannin reduces its toxicity and increases its solubility [197], and thus NP-wortmannin acted as a potent radiosensitizer in vitro and in vivo in a mouse xenograft model of cancer. This indicates that the administration of wortmannin nanoparticle medications may have clinical applications [197]. Interestingly, a synthetic derivative of wortmannin, PX-866 (sonolisib), is a potent pan-isoform inhibitor of PI3K that blocks temozolomide-induced autophagy and promotes apoptosis in glioblastoma cells [50]. Treatment with PX-866, in combination with docetaxel, is well tolerated without evidence of cumulative toxicity and controls disease progression in patients with advanced solid tumors. Furthermore, PX-866 was identified as completing several clinical trial evaluations (Identifier: NCT01331083, NCT01204099, NCT01252628, and NCT01259869) for the treatment of patients with recurrent or metastatic cancer [51,52,53]. Several reports have shown that cycloheximide is a fast and effective inhibitor of the early stages of autophagy [54,55]. However, cycloheximide is generally used only for autophagy inhibition in vitro because it is not suitable for human use due to significant toxic side effects, including DNA damage and teratogenesis [55].
Petrosaspongiolide M (PSM) exerts inhibitory effects on autophagy in the human histiocytic lymphoma (U937) cells by downregulating Beclin1 levels. As an immunoproteasomal inhibitor, PSM binds the active sites in the inner core of proteasome in U937 cells and accumulates ubiquitinated proteins, as well as p53, which is a regulator of the cell-cycle and cell death [56]. In this regard, PSM represents an interesting molecule for the modulation of intracellular proteolysis through the dual inhibition of proteasome and autophagy [57]. Epirubicin (EPI) is an anthracycline drug that has been widely used to treat bladder cancer. As a topoisomerase-II inhibitor, EPI causes apoptosis in cancer cells by inducing DNA damages. However, resistance to EPI becomes a great challenge in treating bladder cancer because it induces cytoprotective autophagy in bladder cancer cell lines T24 and BIU87 via the activation of JNK-mediated phosphorylation of Bcl-2 and disruption of the Bcl-2/Beclin1 complex [198]. The green tea derivative tea polyphenol (TP) displays strong biological effects, including anticancer properties. TP inhibits EPI-induced autophagy and promotes EPI-induced apoptosis in human bladder cancer cells [198]. Harmine analogues have long been considered to be anticancer agents due to their reported anti-proliferative activity. While harmines seem to cause DNA damage and inhibits cellular enzymes, the precise mechanism of action of harmines remains elusive. It has been found that N2-benzyl and N9-arylated alkyl, which are analogues of harmine, strongly inhibit the growth of cancer cells that originate from the breast, lung, bone and pancreas, but not that of normal fibroblasts via the induction of apoptosis and inhibition of autophagy by reducing the conversion efficiency of LC3-I to LC3-II [58].
3.2. IM Elongation and Enclosure Stage Inhibitors
Since MTs have a major role in autophagy pathways in non-mitotic cells, MTs may be effective targets in cancer cell death. The MT targeting agents (MTAs) of natural drugs have shown potential therapeutic benefits in cancers [59]. Many natural agents and/or their MTA analogues may bind to the tubulin and alter the assembly properties used in tumors by inhibiting mitosis. Based on the role of the MT network in autophagy, pharmacological MTAs are classified into two main groups. The first group is microtubule-destabilizing agents (or antipolymerization drugs), such as the vinca alkaloids (vinblastine, vincristine, vinorelbine, vindesine and vinflunine), colchicine, cryptophycins, halichondrins, estramustine and combretastatins, which are used clinically or are under clinical investigation for the treatment of cancer [59]. The second group is MT-stabilizing agents, which stimulate MT polymerization, with examples including paclitaxel (Taxol), docetaxel (Taxotere), the epothilones, and discodermolide.
Vinca alkaloids are considered “wonder drugs” for fighting cancer [13,60]. For example, they are used in the treatment of childhood hematologic malignancies (leukemia). Vinca alkaloids vinblastine or vincristine are able to depolymerize the whole MT network (both acetylated and non-acetylated forms) and reduce the conversion efficiency of LC3-I to LC3-II. These alkaloids contribute to their anticancer activity by preventing autophagosome formation and maturation [60]. At higher concentrations, vinblastin reduces the autophagic marker p62 and completely inhibits the merging of autophagosomes with lysosomes [60].
Colchicine was approved by the Food and Drug Administration (FDA) in 2009 for the treatment of gout attacks and familial Mediterranean fever. Previous studies have shown that colchicine induces autophagy and senescence in lung cancer cells at a clinically acceptable level. However, extensive research studies suggest that it prevents autophagosome formation by inhibiting MT polymerization as a tubulin binder and thus acts as mitotic spindle poison in cells. Combretastatins exhibit cytotoxic properties and inhibit tubulin polymerization in cancer cells in vitro [60]. Combretastatin A-4 (CA-4, also known as fosbretabulin), the most potent member of this family, has a great effect in antitumor therapy and has entered several clinical trials for solid tumors. CA-4 phosphate (CA-4P), a water-soluble prodrug of CA-4, has also progressed into clinical trials for the treatment of solid tumors (Table 2). Since CA-4 has a high affinity to tubulin and destabilizes the tubulin polymers of the cytoskeleton, it is also used as an angiogenesis inhibitor and a vascular disrupting agent [60,61]. Some reports have shown that CA-4 induces prosurvival autophagy in both human osteosarcoma [62] and adenocarcinoma cells [63]. However, evidence has indicated that the suppression of autophagy has been suggested to potentially enhance the therapeutic efficacy of CA-4 [63,64].

Table 2.
Ongoing and completed clinical trials with natural products for the treatment of cancer (the structures of these products are shown in Table 1).
As an antioxidant agent, N-acetyl cysteine (NAC) effectively abolishes oxidative stress markers, such as intracellular reactive oxygen species (ROS) [65], and alters the cellular redox status. NAC therefore plays an important role in triggering apoptosis and inhibiting the autophagy induced by starvation, trehalose and recombinant human arginase (rhArg) in COS-7 and HeLa cells. NAC also induces apoptosis in colon carcinoma cells by increasing the pro-apoptotic Bax levels and by increasing susceptibility to the chemotherapeutic agent 5-fluorouracil (5-FU) [66].
The natural valosin inhibitor containing protein (VCP or p97) xanthohumol (XN) has been considered for its potentially beneficial effects in HeLa cells, including the inhibition of diacylglycerol acyltransferase, induction of apoptosis, as well as the inhibition of autophagy via the upregulation of p62 and LC3-II [67].
To date, over 10 different salvianolic acids have been identified and referred to: salvianolic acid A, B, C, D, E, F, G, etc. Salvianolic acid A (Sal A) and salvianolic acid B (Sal B) are the most abundant compounds among salvianolic acids. Both in vitro and in vivo, most of the salvianolic acids showed anti-inflammatory and antioxidative effects [68]. Some studies predict that Sal A and Sal B will have therapeutic effects on breast cancer, lung cancer and liver cancer. Sal A reverses the resistance of circulating cancer cells (CCC) in breast cancer MCF-7/PTX cells to paclitaxel [69,70]. Sal A potentially reduces A549 lung cancer cell growth, and promotes apoptosis by enhancing the expression of phosphatase and the tensin homolog deleted on chromosome 10 (PTEN) and localized on the cytoplasmic membrane, which in turn inhibits PI3K signaling. Sal A inhibits the growth of mouse lung cancer cells by inhibiting the expression of c-myc and JNK [68]. Sal B, combined with other compounds, inhibits migration, invasion and the epithelial–mesenchymal transition (EMT) process of A549 cells by PTEN/PI3K/protein kinase B (PKB or AKT) [71]. Additionally, Sal B inhibits the proliferation of breast cancer cells and promotes their apoptosis [68]. Sal B reduces the incidence of squamous cell carcinoma (SCC) by inhibiting angiogenesis and decreasing the expression of hypoxia-inducible factor 1α (HIF-1α) and vascular endothelial growth factor (VEGF) [68]. Sal B exerts some inhibitive effects on lung cancer [72] in vivo. Sal B could activate apoptosis in human hepatocellular carcinoma (HCC) through the mitochondrial pathway [73]. Interestingly, Sal B induces autophagy in both hepatoma cells and colorectal cancer (CRC) cell lines. The Sal B-induced autophagy, which is mediated by the AKT/mTOR signaling pathway, can play a pro-apoptotic role in cancer cells [73]. It is also reported that Sal B inhibits the early stages of autophagy and interferes with the development of autophagosome via the inhibition of LC3 lipidation and by blocking the elongation of IM [68,73].
Deguelin effectively inhibits autophagy in several types of cancer, including pancreatic cancer, by blocking LC3 lipidation. Deguelin induces incomplete autophagy in pancreatic cancer cells by inhibiting autophagy flux, as evidenced by the impairment of autophagosome maturation and the subsequent accumulation of LC3-II and p62 in dose- and time-dependent manners [74]. Doxorubicin-induced autophagy (Dox) plays a pro-survival role in pancreatic cancer cells; thus, the pharmacological inhibition of autophagy by QC or the silence of ATG5 enhances Dox-induced cancer cell death. Similarly, deguelin’s inhibition of autophagy has also chemosensitized pancreatic cancer cell lines to Dox [74]. However, a previous study showed that deguelin may induce both apoptosis and autophagy in cultured head and neck SCCs. This is mediated by the inhibition of AKT signaling, the downregulation of survivin and cycline-dependent kinase 4 (Cdk4) expression, as well as the disruption of their association with heat shock protein 90 (Hsp-90) [75].
3.3. Docking and Fusion Stage Inhibitors
Polyether ionophores, such as monensin and nigericin, are produced by Streptomyces species and are potent antimicrobial and anticancer agents that belong to a large class of naturally occurring polyketides [76]. A number of studies have revealed that monensin and nigericin have been linked to autophagy and cell death via the interference from the fusion of autophagosome and lysosome, and thus blocks the maturation of the autophagic process. These two compounds block autophagic flux, resulting in the accumulation of autophagy flux markers LC3-II and p62, along with the cleavage of caspase-3, caspase-9 and poly(ADP-ribose) polymerase 1 (PARP-1), which is a hallmark of caspase-dependent apoptosis [77,78]. In addition, another polyether antibiotic, salinomycin, is reported to inhibit autophagic flux in several cancer cell lines [199].
Asparagine-rich foods include dairy products, whey, beef, poultry, eggs, fish, seafood, asparagus, potatoes, legumes, nuts, seeds, soy, and whole grains. Low asparagine-containing foods mostly include fruits and vegetables, which can slow down breast cancer metastasis [79]. Mice harboring primary 4T1 mammary tumors treated with L-asparaginase or fed with a low-asparagine diet experienced a decrease in metastases with no effect on primary tumor growth. This can be attributed to blockage of the fusion of autophagosomes with lysosomes and the suppression of lysosomal functions [79]. Additionally, L-asparaginase has been reported to be an important drug for the treatment of acute lymphoblastic leukemia (ALL) cells in the last few decades [80]. Contradictory reports indicate that L-asparaginase catalyzes the conversion of L-asparagine to aspartic acid and ammonia, resulting in the deprivation of circulating asparagine. This leads to metabolic stress, as evidenced by the inhibition of both glycolysis and oxidative phosphorylation, and the activation of autophagy in ALL cells [81].
Liensinine suppresses autophagic degradation by blocking autophagosome–lysosome fusion and the subsequent accumulation of autophagosomes in breast cancer cells. While the inhibitory effect on autophagy is similar to CQ and Baf A1, liensinine’s blockage of autophagosome–lysosome fusion differs from these inhibitors. CQ and Baf A1 cause alkalinized lysosomal pH, suppress the fusion process, and impair the action of lysosomal hydrolases, whereas lysosomal pH is unaffected in response to liensinine treatment. Thus, lysosomal pH may not be necessary to inhibit autophagosome–lysosome fusion by liensinine [200]. Interestingly, liensinine interferes with the recruitment of the small binding protein GTP RAB7A in lysosomes, but not in autophagosomes, and suppresses the transport of endocytic cathepsins to lysosomes and finally stops autophagosome–lysosome fusion. Additionally, the combination of liensinine and Dox causes the synergistic inhibition of the viability and induction of death in breast cancer cells due to altering autophagy/mitophagy by liensinine [200]. A recent and contradictory study found that the bisbenzylisoquinoline alkaloids neferine, liensinine and isoliensinine inhibit cell growth and exhibit significant anti-migration activities in prostate cancer cells. They induce apoptosis and autophagy by activating cleaved caspase-9, cleaved PARP, Bax, and LC3-II, but reduced the expression of Bcl-2 and PARP proteins in LNCaP cells in short-term treatments (24 h) [201].
Oblongifoline C (OC) and guttiferone K (GUTK) are the major active components of the Garcinia yunnanensis Hu fruit with anticancer activities, but they act through various mechanisms [82]. OC promotes apoptosis and inhibits autophagy and cancer metastasis [83,84]. Similarly, GUTK activates apoptosis, arrests the cell-cycle, and promotes autophagy [85,86]. However, OC and GUTK show synergistic inhibition on colorectal cancer (CRC) HCT116 cells. Moreover, the combination of OC and GUTK markedly increases the cleavage of casapse-3, enhances cellular ROS production and upregulates JNK protein phosphorylation, resulting in autophagy initiation. OC acts as a powerful autophagic flux inhibitor by blocking autophagosome–lysosome fusion and increases the pH in acid compartments. In addition, OC inhibits lysosomal proteolytic activity and downregulates lysosomal cathepsins. Importantly, OC efficiently sensitizes nutrient-deprived cancer cells to caspase 3-dependent apoptosis in vitro [82].
3.4. Late-Stage Disruptors
Currently, most of the natural compounds are currently used to inhibit autophagy at a late phase of autophagy. For instance, lysosomotropic agents (QC), vacuolar-type ATPase inhibitors (V-ATPase) (Baf A1) and lysosomal-type protease inhibitors (pepstatin A) all interfere with the final steps of the autophagy pathway. These compounds inhibit the degradation of autolysosome by lysosomal enzymes, leading to cytoplasmic accumulation, which may be toxic to cells. Since lysosomes are involved in many biological processes besides autophagy (e.g., endocytosis), these molecules have multiple off-target effects.
3.4.1. Acidification Stage Inhibitors
The quinine extracted from the Cinchona bark, and its synthetic analogue CQ (orginallyt used as antimalarial agents), are repurposed for cancer treatment. Thus, these drugs, including the antimalarial quinine, CQ, and its derivatives, block the acidification processes of autolysosome and lysosomes in cancer cells, resulting in a reduction in autophagic flux and cell death [202].
As autophagy plays an essential role in effective cellular response in host defense against mycobacterial NTM infections, the use of autophagy blockers, such as azithromycin in patients with chronic inflammatory lung diseases, may result in highly pathogenic and fatal infections [87]. Therapeutic doses of azithromycin suppress the clearance of autophagosomes by altering lysosomal acidification and autophagic degradation in macrophages, resulting in a failure to kill intracellular mycobacteria and the persistence of lung infections [87]. Clarithromycin strongly attenuates the late stages of the autophagy process in myeloma cells by halting the fusion of autophagosomes with lysosomes and altering lysosomal acidification, causing the induction of cell death [88].
Matrin shows inhibitory effects on proliferation and metastasis, and causes apoptosis in a variety of malignant cells, e.g., C6 glioma cells. As an autophagy inhibitor, matrine elevates pH values in endosomes/lysosomes, which in turn inhibits trafficking and lysosomal proteases in human gastric cancer cells [89]. Recent contradictory evidence has shown that matrine induces autophagy in human hepatoma cells with inactive p53 by the induction of the AMPK signaling pathway [90]. Martine-derived MASM, a potent derivative of matrine, possesses potency against cancer cells by inducing autophagy and apoptosis through ROS-mediated PI3K/AKT/mTOR and the extracellular signal-regulated kinase1/2 (ERK1/2)/p38 signaling pathway in epithelial cancer cell lines [91]. Thus, the action of matrine is cell-specific and signaling pathway-dependent.
Elaiophylin acts as an autophagy inhibitor because it disrupts lysosomal degradation. It blocks autophagic flux in the late stages of autophagy in ovarian carcinoma cells. Elaiophylin usage could promote a substantial accumulation of autophagosomes [92]. Additionally, elaiophylin abrogates the maturation of cathepsins B and D and induces subsequent lysosomal membrane permeabilization (LMP). Elaiophylin decreases cell viability and induces cell death via the inhibition of autophagy and sensitizes the antitumor effect to cisplatin in vitro. Administration of elaiophylin (dose 2 mg/kg) displays a significant antitumor effect without toxicity [92]. Another report suggests that elaiophylin exerts anti-myeloma activity by blocking autophagy flux, inducing apoptosis and arresting proliferation in multiple myeloma cells [93].
An anti-schistosome agent, lucanthone, impairs autophagy by inducing the accumulation of p62 and disrupting lysosomal functioning. In addition, lucanthone stimulates apoptosis via cathepsin D accumulation and potentiates the histone deacetylase inhibitor and vorinostat-mediated cell death [94]. Lucanthone, in association with chemotherapeutic agents, has already reached clinical trials (Table 2) and is currently in phase II clinical trials for glioblastoma multiforme.
3.4.2. Vacuolar-Type H+-ATPase (V-ATPase) Inhibitors
V-ATPase is found in the membranes of many organelles, including lysosomes, endosomes and secretory vesicles, where they play a variety of vital roles in many cellular processes, and its dysregulation leads to the maintenance of the acidic milieu, thus causing several diseases, such as osteoporosis and cancer [203].
Macrolide antibiotics bafilomycins A1, B1, D, F, G, H, I and J are potent autophagy inhibitors that function via the induction of autophagosomes accumulation. Baf A1 is considered to be a selective prototypical V-ATPase inhibitor at low nanomolar concentrations and is therefore often used (Baf A1 clamp assay) to block late autophagic flux [95]. Baf A1 may also target early stages of autophagy by activating mTOR signaling, thus disassociating the Beclin1-class III complex and inhibiting autolysosomal formation [96]. Thus, Baf A1 prevents activation of lysosomal enzymes via blocking its acidification process [97]. In addition, Baf A1 induces the binding of Beclin1 to Bcl-2, which further inhibits autophagy and promotes apoptotic cell death [96]. Baf A1 also targets mitochondria and induces caspase-independent apoptosis by inducing the translocation of apoptosis-inducing factors from mitochondria to the nucleus [96]. Another selective V-ATPase inhibitor, concanamycin-A, also increases the accumulation of autophagosomes [98]. Concanamycin A shows significant global toxicity due to the inhibition of the V-ATPase in several tissues. It has been reported that manzamine A has shown anticancer activity against pancreatic cancer cells through the inhibition of V-ATPase and autophagy through the accumulation of lysosomes/autolysosomes [99]. For example, manzamine A is active against AsPC-1 pancreatic adenocarcinoma cells by reducing cell dissociation, abrogating cell migration, and sensitizing cells to apoptosis [100].
Dox delivers antitumoral activity in two basic ways: through interference with DNA synthesis and through the induction of its damage [101]. Dox also blocks autophagic flux by impairing lysosome acidification through the suppression of V-ATPase activity and lysosomal function. Despite its highly beneficial effects against cancer, its clinical uses are limited by its severe side effects, such as life-threatening cardiotoxicity, particularly in children with cancer. Since intracellular Ca2+ signaling has been reported to play an important role in the regulation of autophagy, Dox causes the significant abnormal accumulation of cytosolic Ca2+ in human cardiac progenitor/stem cells and causes cardiotoxicity [102]. Another natural V-ATPase inhibitor, cleistanthin A, has shown cytotoxicity in several tumor cell lines. Archazolid, another well-investigated V-ATPase inhibitor, reduces protease activity, such as B-cathepsin in vitro and in vivo [103].
3.4.3. Lysosomal Hydrolytic Enzyme Inhibitors
Leupeptin impairs amphisome–lysosome fusion and suppresses cathepsin B, H and L [104]. Leupeptin also inhibits reversible trypsin-like serine proteases and most cysteine proteases (including trypsin, papain, cathepsin B and calpain) [103]. Pepstatin A is an inhibitor of acid proteases (aspartyl peptidases). It forms a 1:1 complex with proteases such as pepsin, renin, cathepsin D, bovine chymosin, and protease B [105].
4. Natural Products as Inducers of Autophagy in Cancer
In the following section, the main natural products are found to activate autophagy (Table 1) and consequently modulate cancer cells in various models. The molecular targets (where known) for these mammalian cells are shown in Figure 1.
4.1. Initiation Stage Activators: mTOR Inhibitors
Caloric restriction (CR) or fasting is one natural and effective phenomenon that induces autophagy, as it activates multiple regulatory pathways. For example, CR results in the inhibition of mTORC1 and the activation of AMPK, which in turn activates the autophagy-promoting ULK1 complex (Figure 1) [106]. Furthermore, CR stimulates sirtuin 1 (SIRT1), which deacetylates and thereby activates essential autophagic proteins [106].
While the inhibitor mTOR rapamycin (sirolimus) is known to be a powerful autophagy inducer that extends the lifespan of various organisms, from flies to mammals [106,107,108], it has serious adverse effects, including insulin resistance [106]. Most of these side effects have been attributed to the chronic inhibition of mTORC2 [204]. Consequently, considerable effort has been devoted to discovering specific mTORC1 inhibitors, such as semi-synthetic analogues of rapamycin (known as rapalogs), including temsirolimus, everolimus, deforolimus, zotarolimus, biolimus, WYE-592 and ILS-920 [205], which have fewer side effects. These rapalogs are allosteric selective inhibitors of mTORC1 that affect downstream targets, including the activation of autophagy [206]. However, their efficacy in inhibiting tumor growth is limited due to the lack of inhibition of mTORC2 and other compensatory signaling pathways that promote cell survival [206]. Mechanistically, rapamycin is an allosteric inhibitor of mTOR and only suppresses part of the mTORC1 function, whereas both PP242 and Torin1 are catalytic inhibitors that are able to completely suppress both mTORC1 and mTORC2 via binding to ATP-binding sites [206].
4.2. Polyphenolic Compounds
Polyphenolic compounds are the most common bioactive secondary plant metabolites that are present in fruits, vegetables, seeds, and others, and they have a wide range of activities in the prevention and treatment of various diseases, including cancers [207]. Several of the beneficial effects of polyphenols have been attributed to their antioxidant activity [208]. Additionally, polyphenols affect numerous cellular targets that can modulate autophagy, which interferes with the symptoms and putative causes of cancers [208]. Today, it is widely accepted that dietary flavonoids, including the commonly occurring flavonols quercetin and kaempferol, the flavones apigenin and luteolin, green tea catechins, and the isoflavone genistein, have strong anticancer potentials, thus exerting antiproliferative, cytotoxic, proapoptotic, anti-inflammatory, antiangiogenic, antimetastatic, and antiinvasive activities. The strong potential of these compounds in the fight against cancer has been proven in numerous experimental studies, in both in vitro cell cultures as well as in animal (rodent) models [208,209,210].
4.2.1. Quercetin
Quercetin is a well-known antioxidant flavonoid that has antitumor effects [109]. Quercetin induces autophagy in different cancer cells through the modulation of the AKT-mTOR signal pathway [110]. Quercetin triggers autophagy by AMPK activation and the accumulation of HIF-1α, which represses mTOR signaling and induces the expression of Bcl-2/adenovirus E1B 19 kDa protein-interacting protein 3/ligand (BNIP3/BNIP3L) to disrupt the Beclin1/Bcl-2 (Bcl-xL) complex [110]. Poly(DL-lactide-co-glycolide) quercetin nanoparticles stimulate autophagy and cell death through the suppression of the AKT/mTOR signaling pathway in human neuroglioma cells [110]. Quercetin induces ER stress, activates protective autophagy and apoptosis, and simultaneously stimulates signal transduction and the activation of the transcription axis 3 (p-STAT3)/Bcl-2 in ovarian cancer. As a chemopreventive agent, quercetin plays an important role in modulating chemotherapeutic drug sensitivity [110]. Quercetin also regulates apoptosis and autophagy-related pathways and facilitates gemcitabine (an analog of deoxycytidine for DNA synthesis inhibition) chemosensitivity through the receptor involved in advanced glycation end products (RAGE)/PI3K/AKT/mTOR axis in human pancreatic cancer cells. Quercetin also suppresses multidrug resistance protein 1 (MDR1) expression, blocks drug efflux via P-glycoprotein (P-gp) transport proteins, and increases the activity of anti-cancer drugs in uterine sarcoma MES-SA cells [110]. Quercetin-induced initial autophagy in gastric cancer protects cancer cells from late apoptosis [211]. Rutin, also called rutoside, quercetin-3-O-rutinoside, and sophorin isolated from Toona sinensis Roem (Meliaceae), has clinically relevant functions as an anti-inflammatory and antioxidant agent [212]. Similar to quercetin, luteoloside, isolated from the medicinal plant Gentiana macrophylla, has oncosuppressive effects in humans [213].
4.2.2. Magnolol
Magnolol, a plant that is widely used in traditional Japanese and Chinese medicines, is isolated from the root of magnolia officinalis. It is well known that magnolol has anti-inflammatory, anti-diabetic, anti-microbial, anti-neurodegenerative and anti-depressant properties. Recently, in vivo and in vitro studies have shown that the treatment of neuroblastoma cancer cells with magnolol can induce autophagy/mitophagy and apoptosis in treated cells. Importantly, blocking autophagy/mitophagy significantly enhances the anti-cancer effectiveness of magnolol, suggesting that targeting autophagy/mitophagy can be a promising strategy to overcome chemoresistance and to improve cancer therapy [214].
4.2.3. Kaempferol
Kaempferol, a polyphenol flavonoid, is found in different fruits (e.g., grapes) and vegetables (e.g., tomatoes). Kaempferol modulates autophagy in noncancerous cells in order to protect cells against malfunction, and it induces cell death by enhancing autophagy via the elevation of the p-AMP-activated kinase protein, LC3-II, and Beclin1 in gastric cancer cells [111].
4.2.4. Apigenin
Apigenin, a bioflavonoid, is widely present in fruits and vegetables, such as parsley, orange, tea, chamomile and seasonings. Apigenin has been shown to possess significant anti-inflammatory, antioxidant and oncosuppressive properties. In cancer cells, apigenin inhibits growth and proliferation through its preventive effects via the modulation of apoptosis and autophagy [112]. The mechanism underlying the anti-tumor effects of apigenin in hepatocellular carcinoma HepG2 cells is related to the induction of apoptosis and autophagy through the inhibition of the PI3K/AKT/mTOR pathway [113]. However, apigenin inhibits autophagy flux in the primary human epidermal keratinocytes (HEKs) and the cutaneous squamous cell carcinoma cell line COLO-16. Moreover, apigenin can enhance the effect of chemotherapeutic agents and reduce chemoresistance by inhibiting drug efflux [112]. Gao et al. [215] investigated the possibility of a chemo-sensitization effect of apigenin in a Dox-resistant HCC cell line (BEL-7402/ADM). Apigenin treatment enhances Dox sensitivity, induces microRNA-520b (miR-520b) expression and inhibits ATG7-dependent autophagy in these cells. ATG 7 acts as a potential target of miR-520b [215]. Moreover, combined with N-(4-hydroxyphenyl) retinamide, apigenin may suppress starvation-induced autophagy and promote apoptosis in human malignant neuroblastoma cells [216]. There is also evidence that suggests that apigenin can induce autophagic cell death in human papillary thyroid carcinoma cells [217]. Vitexin (apigenin-8-C-glucoside, c-glycosylated flavone) is found in various medicinal plants [218]. The biochemical properties of vitexin, such as its anticancer and antioxidant effects, are well-documented [112]. Vitexin has been reported to inhibit autophagy in a multi-drug-resistant (MDR) line of human colon cancer cells (HCT-116DR). Mechanistically, vitexin could reduce the level of autophagy in cancer cells (via the suppression of ATG 5 and Beclin1 expression levels) and simultaneously increase the apoptotic response through the enhancement of the cleavage of caspase-3 and -9 [219].
4.2.5. Coffee and Tea: (−)-epigallocatechin-3-gallate (EGCG), Catechin and Epicatechin
Coffee and tea are the most consumed beverages worldwide, and have an impressive impact on the economies of the countries that produce them. Coffee is prepared from the seeds of coffee plants, genus Coffea, which include different species. Tea made from the leaves of the plant Camellia sinensis is a popular beverage [220]. An elegant study showed that caffeine, the main constituent of coffee beans and tea leaves, is a potent stimulator of hepatic autophagic flux; caffeine-induced autophagy involves the down-regulation of mTOR signaling and alterations in hepatic amino acids and sphingolipid levels. Caffeine also promotes AMPK-dependent autophagy through calcium-mediated pathways in skeletal muscle cells [221]. It can be found in quantities of up to 70–350 mg per cup of coffee and has been linked to numerous health benefits, such as a reduced risk of some forms of cancers, including breast cancer [222]. However, coffee consumption has been associated with a risk of the development of various forms of cancers, including CRC [223] and bladder cancer [224]. Via the inhibition of enzymatic activity of mTORC1, Pietrocola el al. [225] has shown that consumption of both natural and decaffeinated brands of coffee by mice increases autophagic flux (1-4 h after intake) in the liver, muscles and hearts of the treated animals. However, they concluded that caffeine is not only responsible for increased autophagy, but also polyphenols, such as chlorogenic acid (CGA), EGCG, (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG), catechin and (−)-epicatechin within coffee, and tea may have an even stronger effect on autophagy activation and the reduction in protein acetylation. CGAs can activate AMPK-dependent autophagy pathways. These products show the effect of ameliorating a variety of human diseases, such as cancers [114]. The cosmetic industry has shown a growing interest in these polyphenols, since they are able to extend longevity significantly under several stress conditions by reducing skin aging and age-related diseases [207]. EGCG upregulates AMPK activity in a dose-dependent manner, while the mTOR pathway is inhibited in hepatoma cells [115]. An increasing amount of evidence has shown that the dietary intake of proanthocyanidins plays an essential role in the chemoprevention or chemotherapy of tumors [226]. In vitro and in vivo toxicity experiments have demonstrated that proanthocyanidins have anticancer effects on various human cancers, such as CRC, pancreatic cancer, HCC, non-small cell lung cancer (NSCLC), squamous cell carcinoma (SCC), as well as head and neck squamous cancer. Grape seed proanthocyanidins, formed by the polymerization of catechins and/or epicatechins, induce autophagy by inducing the phosphorylation of the mitogen-activated protein kinase (MAPK) pathway and by reducing the expression of survivin, which is a member of the inhibitor of apoptosis (IAP) gene family in HepG2 cells [227].
4.2.6. Genistein
Genistein, a natural isoflavone polyphenol, has been reported to exhibit multiple beneficial effects on human health, including anticancer properties that target multiple cancer cells, such as ovarian cancer and human breast MCF-7 cells [116] through several mechanisms, which include the induction of autophagic cell death [117]. Several studies found that genistein can potentiate the antitumor effects of chemotherapeutic agents (e.g., 5-FU, gemcitabine, cisplatin and oxaliplatin) by modulating the autophagic-apoptotic pathway. For instance, the combination of 5-FU and genistein can induce autophagic cell death in cancer cells by significantly altering the expression of two important molecules, Bcl-2 and Beclin1, which regulate autophagy [117]. The oncosuppressive effect of genistein is associated with the inhibition of PI3K-AKT signaling activation [118]. Ali et al. [228] also reported that genistein inhibits nuclear receptor co-repressor (N-CoR) misfolding, which is an important component in the activation of the oncogenic survival pathway in NSCLC, and was found to be associated with heat shock cognate 70 kDa protein (HSC70), which is a molecular chaperone in autophagy. Surprisingly, genistein induces the overexpression of TFEB, which is a master regulator of lysosomal biogenesis and an enhancer of autophagy protein expression [229]. Moreover, genistein-mediated suppression of mTOR increases dephosphorylation and the subsequent nuclear translocation of TFEB, which is associated with a significant increase in lysosomal content and activity in treated cancer and non-cancerous cells. Thus, genistein appears to be a potentially beneficial agent in the treatment of lysosomal storage diseases and cancers [230,231].
4.2.7. Curcumin Derivatives
Curcumin has been used as a food colorant in dietary supplements and herbal medicines in Asian populations [119]. Curcumin has numerous pharmacological activities, including antioxidant and anticancer activities [112,120]. Curcumin has been reported to induce autophagy in chronic myeloid leukemia, malignant glioma, esophageal cancer, colon cancer, uterine leiomyosarcoma, ovarian cancer and lung adenocarcinoma via mechanisms related to the reduction in cell viability, proliferation, migration and invasion [119]. Curcumin also induces autophagy-associated apoptosis in mesothelioma and chronic myelogenous leukemia cells by modulating PI3K/AKT/mTOR and the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathways [112]. Curcumin inhibits the growth of malignant gliomas, lung adenocarcinoma and melanoma cells in vitro and in vivo by downregulating the PI3K/AKT/mTOR signaling pathway and activating the AMPK pathway, and finally by promoting autophagy [112]. In addition, curcumin induces apoptosis in human malignant mesothelioma, which is an aggressive malignancy and is inherently chemo-resistant [112,121]. Curcumin-induced cell death is highly correlated with the enhancement of apoptosis or autophagy, mitochondrial membrane potential (MMP) and the activation of caspase-3. In addition, curcumin can reduce the expression of Bcl-2 proteins in K562 cells [122,123]. Curcumin has been shown to activate mitochondrial-mediated apoptosis and autophagy in adriamycin-induced human hepatoma G2 (HepG2) [123], due to the reduced proportion of Bcl-2/Bax protein and caspase-3 activation. Moreover, curcumin treatment can result in the mitochondrial fission of HepG2 cells, the reduction in MMP and autophagy activation [123]. It has been proposed that curcumin reverses cisplatin chemoresistance via the regulation of oxidative stress and autophagy flux in the MDR cell line A549/cDDP [112]. Curcumin also sensitizes MDR breast cancer cells to cisplatin treatnment and activates autophagy by suppressing the PI3K/AKT/mTOR pathway [112]. Interestingly, curcumin also regulates prosurvival autophagy in HCT116 cells that are mediated by the overexpression and nuclear translocation of TFEB and the inhibition of mTOR [121,232]. The monocarbonyl analog of curcumin, B19 or curcumin bis-dehydroxy, induces autophagy and apoptosis via the ER-stress route in ovarian and colon cancer cells [118]. The curcumin analogue, hydrazinobenzoylcurcumin, can also induce autophagic cell death in human non-small lung epithelial carcinoma (A549) cells [121]. Tetrahydrocurcumin, a major metabolite of curcumin, significantly reduces the activity of the PI3K/AKT/mTOR and MAPK signaling pathways and induces autophagic cell death in human leukemia HL-60 cells [233,234]. Another curcumin analogue, the 3,5-bis (2-hydroxybenzylidine) tetrahydro-4H-pyran-4-1 glutathione conjugate (EF25-[GSH]2), inhibits the growth of hepatocellular carcinoma in vitro and in vivo by modulating the autophagic pathway and enhancing apoptosis [235]. Besides activating autophagy, curcumin also exhibits time- or concentration-dependent inhibition of cell proliferation, autophagy and apoptosis in K562 cells, SKN and SK-UT-1 uterine leiomyosarcoma cells [236]. Curcumin therapy has been reported to mitigate autophagy and to reverse drug-resistance through the potent activation of Keap1 transcription, which is crucial for the erythroid 2 like 2 (Nrf2) signaling pathway [112,237].
4.2.8. Resveratrol
Resveratrol has the potential to slow down the progression of many age-related diseases (ARDs), including different types of cancer. Resveratrol has potentially beneficial effects, including improving mitochondrial quality control and glucose tolerance through AMPK activation [118]. Several studies have suggested the growth inhibitory efficacy of resveratrol in several types of cancer cell, such as HCC, breast cancer, gastric cancer and leukemia [124]. Interestingly, resveratrol significantly inhibits breast cancer stem cell proliferation by inducing autophagy through the suppression of the Wnt/β-catenin signaling pathway. Resveratrol treatment in cancer cells results in autophagic cell death via multiple pathways, including JNK-mediated p62 expression, AMPK activation and the Beclin1-independent pathway [125]. Resveratrol has been reported to reduce AKT phosphorylation and mTOR signaling by p70S6K, which is a direct mTOR substrate. Resveratrol treatment decreases ER Ca2+ storage and store-operated calcium entry (SOCE), which induces ER stress, thereby activating AMPK and inhibiting the AKT/mTOR pathway [126]. Moreover, some studies have also suggested that resveratrol may potentially be useful in cancer chemotherapy for HCC and leukemia, when used in combination with other drugs, mainly due to its effect on apoptosis [126]. Rapamycin, in combination with resveratrol, significantly inhibits the growth of estrogen receptor-positive and estrogen receptor-negative breast cancer cells by preventing the activation of the AKT pathway, autophagy, and stimulating apoptosis [121]. In another study, resveratrol, in combination with the carfilzomib proteasome inhibitor (at low concentrations), synergistically increases apoptosis in myeloma cells through the simultaneous induction of autophagy [238]. This compound can also increase the susceptibility of melanoma, prostate and NSCLC cancers to chemotherapy [112]. In another study, resveratrol was reported to attenuate autophagy in cigarette smoke-induced cytotoxic stress responses in lung cells via the activation of SIRT1, which is a potent inducer of autophagy [121]. Pterostilbene (trans-3,5-dimethoxy-4-hydroxystilbene), a resveratrol analogue, triggers autophagy-induced apoptosis in cisplatin-resistant human oral cancer cells via the triggering of SIRT1 [239].
4.2.9. Propolis Extract: Chrysin
Propolis is a complex resinous mixture produced by honeybees and has multiple pharmacological properties, including anticancer activity. Brazilian green propolis extract, which contains the active ingredients cinnamic acid derivative artepillin C, is an attractive agent for cancer treatments [127]. In addition, the ethanol extracts of Chinese and Brazilian green propolis have been reported to induce autophagy in prostate cancer CWR22Rv1 cells via the upregulation of LC3-II [127]. The apoptosis induced by artepillin C is exacerbated by cotreatment with autophagy inhibitors, such as CQ [127]. A number of studies have confirmed the biological properties of chrysin, including its anti-inflammatory and anti-tumor activity [240]. Chrysin is an effective component in sensitizing human glioblastoma cells (GBM8901) to temozolomide (TMZ). It inhibits TMZ-induced autophagy by reducing the expression levels of LC3-II, ATG7 and Beclin1, and by suppressing the expression of O6-methylguanine-methyltransferase (MGMT) DNA, which may be involved in chemoresistance to TMZ [112].
4.2.10. Fisetin
Fisetin, a flavonoid polyphenol, is known to exhibit multiple pharmacological activities, including anti-inflammatory and anticancer activities in various cell types, such as prostate, colon, breast, and leiomyoma cells [128]. Fisetin induces autophagy and apoptosis in various cancer cells, such as pancreatic cancers and human melanoma, via ER stress-and mitochondrial stress-dependent pathways [128]. Treatment of prostate cancer cells with fisetin suppresses mTOR activity and downregulates the subunits Raptor, Rictor, PRAS40 and GβL, in addition to activating the mTOR repressor tuberous sclerosis complex 2 (TSC2). Fisetin has been shown to be a dual inhibitor of PI3K/AKT and mTOR in prostate cancer cells and in human NSCLC cells, as well as an inducer of autophagy in pancreatic cancer cells via ER stress- and mitochondrial stress-dependent pathways [129,130]. Fisetin has been reported to induce autophagic-programmed cell death rather than cytoprotective autophagy in human NSCLC, liver cancer, prostate cancer, laryngeal cancer, and uterine leiomyomas, all through apoptosis signaling pathways [129]. Fisetin’s effects on autophagy are cell-type-dependent, since this compound inhibits autophagy in HepG2 cells via the PI3K/AKT/mTOR and AMPK pathways [241].
4.2.11. Rottlerin
Rottlerin, a traditional Indian subcontinent medicine, displays antioxidant properties and anticancer potential against different cancer cells, e.g., breast cancer, with various mechanisms, including the induction of autophagy and apoptosis [118]. Singh et al. [137] reported that 2 µM rottlerin (24 h treatment) activates autophagy in pancreatic cancer stem cells by inhibiting mTOR signaling. In prostate cancer stem cells, it represses mTOR, which is accompanied by an increase in the expression of ATG proteins, including ATG5, ATG7, ATG12 and Beclin1 [138,139]. In addition, it is a protein kinase C δ (PKC-δ)-selective inhibitor, which in turn leads to the suppression of NF-κB signaling and the consequent activation of autophagy in breast, pancreatic and colon cancer cells. [139]. Rottlerin inhibits NF-κB and activates AMPK in breast and colon cancerous cells, resulting in a significant reduction in cellular ATP levels and autophagy induction. Rottlerin-induced autophagy leads to apoptotic cell death by multiple signaling pathways, such as PKCδ/transglutaminase 2 (TG2)-dependent and -independent pathways in pancreatic cancer cells, PKCδ-independent mechanism in HT1080 human fibrosarcoma cells, and inhibition of PI3K/AKT/mTORC1 pathways in prostate cancer stem cells (CSCs) [138], breast CSCs [242] and human pancreatic CSCs [137].
In addition, a variety of polyphenolic natural compounds or nutraceuticals isolated from fruits, vegetables, spices, nuts, legumes, herbs, etc., also regulate autophagy signaling pathways and exhibit potent anticancer activities. For example, cucurbitacin B enhances the anticancer effects of clinical chemotherapeutic drugs, including cisplatin, gemcitabine, methotrexate, docetaxel, and gemcitabine. It induces autophagy and DNA damage, as evidenced by the increasing ROS formation and autophagic protein expression in MCF-7 breast cancer cells [131,132]. Wogonin exerts inhibitory growth effects on the SW48 CRC cells by inducing autophagic and apoptotic cell death via modulating the PI3K/AKT signaling pathways. Wogonin upregulates autophagic proteins such as LC3II and Beclin1, in addition to apoptotic proteins, such as caspase 3, 8 and 9 and Bax [125,133,134]. Morusin has been highlighted for its versatile potential against human pathologies, including cancer and immune dysfunctions. Morusin treatment leads to mTOR1 inhibition and the subsequent activation of AMPK, resulting in ULK1-mediated autophagy activation [135,136]. Naringin inhibits human gastric carcinoma, via the induction of autophagy, by activating Beclin1 and LC3-II via the activation of MAPKs pathways [243]. 6-C-(E-phenylethenyl) naringenin (6-CEPN) has been shown to suppress colon cancer cell proliferation via the induction of necrotic cell death and autophagy by the inhibition of c-Raf/MAPK (MEK)/ERK and PI3K/AKT/mTOR signaling pathways [244].
4.3. Terpenoids
Paclitaxel and its semisynthetic analogue docetaxel have been widely prescribed antineoplastic agents (approved by the FDA in 1992) over the past several decades for a broad range of malignancies, such as ovarian cancer, breast cancer, and NSCLC, either as a monotherapy or in combination with cisplatin. The anticancer activity of this drug is attributed to its unique mechanism of action, i.e., causing mitotic arrest in cancer cells, which leads to apoptosis through the inhibition of microtubule depolymerization [60]. However, resistance to paclitaxel has become a major limitation of clinical success [140]. While the molecule or key mechanism associated with paclitaxel resistance in cancers remains uncertain, paclitaxel’s regulation of autophagy is one reason. It has been reported that paclitaxel promotes autophagy in ovarian cancer, cervical cancer SiHa cells, lung cancer cells, gastric cancer BGC823 cells and bladder urothelial carcinoma (BUC) cells [60]. Paclitaxel treatment has also been found to induce autophagy in A549 cells, U87 glioma cells, human PC-3 prostate cancer and colon HT-29 cancer cells via the increasing expression levels of LC3-II, ATG5 and Beclin1 in a dose-dependent manner. Additionally, paclitaxel-induced autophagy has been reported to play a critical role in mediating caspase independent cell death. Paclitaxel also inhibits autophagy in breast cancer cells and cervical cancer cells [60,141], suggesting that paclitaxel has different effects on autophagy in various cancer cells. As paclitaxel may generate unacceptable levels of toxicity in normal cells in clinical settings, more experiments need to be focused on how to enhance its effectiveness as well as to reduce its toxicity.
4.3.1. γ-Tocotrienol
The use of tocotrienols, such as α-tocotrienol, β-tocotrienol, γ-tocotrienol and δ-tocotrienol, as dietary supplements in Asian populations is considerably higher than in developed countries. Most obviously, tocotrienols, members of the vitamin E superfamily, are characterized by their antioxidant, anti-inflammatory and anticancer activity [121]. Tiwari et al. [142] found that γ-tocotrienol treatment in breast cancer cells could induce ER stress and concurrent autophagy-mediated cell death. Oridonin (7,20-epoxy-ent-kauranes), a diterpenoid isolated from the medicinal herb Rabdosia rubescens, has been shown to display potent anticancer activity against a wide ranges of cancer cell types, such as breast cancer cells, melanoma and cervical carcinoma cells by inducing autophagy-mediated apoptosis [142]. In combination with oridonin, γ-tocotrienol has been shown to synergistically induce autophagic and apoptotic effects in mouse breast cancer cells [142]. This combination significantly enhanced the expression of autophagy markers, including LC3B-II, Beclin1, ATG3, ATG7, ATG5-ATG12 and cathepsin D [142]. γ-tocotrienol treatment promotes apoptosis and autophagy in human prostate cancer PC-3 and LNCaP cells [245]. Tocomin, which is a mixture of naturally occurring tocotrienols (T3s), inhibits proliferation and induces apoptosis in breast cancer cells [246].
4.3.2. Ursolic Acid
Ursolic acid exhibits antitumoral activity by inhibiting proliferation, suppressing DNA replication, inducing the release of Ca2+, and activating caspases in several cancers, including breast carcinoma, melanoma, leukemia, hepatoma and prostate cancer [121]. In vivo, ursolic acid inhibits the growth of HCT15 cells by modulating autophagy involving the JNK pathway [121]. The pro-autophagic effects of ursolic acid in the suppression of TC-1 cervical cancer cells and NSCLC cells have been reported to be mediated by LC3-II and ATG5, depending on the concentration. In addition, ursolic acid triggers autophagy in MCF7 breast cancer cells through ER stress [121,144]. In another study, ursolic acid induced autophagy and apoptosis in glioblastoma U87MG cells by three different mechanisms, including phosphorylated extracellular signal-regulated kinase (PERK)/eukaryotic initiation factor 2α (eIF2α)/C/EBP homologous protein (CHOP), calmodulin-dependent kinase protein kinase (CaMMK)/AMPK/mTOR, and inositol-requiring enzyme 1α (IRE1α)/JNK signaling [121]. Ursolic acid-induced autophagy in PC3 prostate cancer cells is mediated by the Beclin1 and AKT/mTOR pathways [121,144].
4.3.3. β-Elemene
β-elemene inhibits the activity of the PI3K/AKT/mTOR/p70S6K1 pathway, thus triggering autophagy and apoptosis in human NSCLC A549 cells and human renal-cell carcinoma 786-0 cells [121]. In the treated cells, induction of autophagy is protective, since the inhibition of autophagy with CQ significantly enhances the antitumor effect of β-elemene [121]. β-elemene has been shown to have the potential to reverse chemotherapeutic drug resistance. For example, β-elemene increases the sensitivity of 5-fluorouracil in p53 wild-type CRC cells [145] and reverses the resistance to gefitinib in NSCLC [146].
4.3.4. (−)-Guaiol
(−)-Guaiol is well known for its antibacterial activity [147]. (−)-Guaiol inhibits the proliferation of NSCLC cells by inducing autophagy via specifically targeting mTOR phosphorylation at serine 2481 signaling pathways [147].
4.3.5. Sesquiterpene Lactones: F1012-2
Sesquiterpene lactones (SLs), such as F1012-2, thapsigargin, parthenolide, and isoalentolactone, are plant-derived constituents that have a variety of biological activities in inhibiting proliferation, migration, invasion and inducing apoptosis in different types of cancer cells, such as lung cancer, breast cancer, leukemia, and CRC [148]. F1012-2 isolated from a perennial herbaceous plant (Eupatorium lindleyanum DC) inhibits the cell growth of triple negative breast cancer (TNBC) (MDA-MB-231 and MDA-MB-468) [148]. The cell growth inhibitory mechanisms of F1012-2 in TNBC cells are demonstrated by inducing apoptosis in a caspase-dependent manner, as well as the activation of autophagy. Simultaneously, F1012-2-induced apoptosis is enhanced by the inhibition of autophagy [148]. Similarly, ergolide [247], anthecotulide [248], CLE-10 [249], elephantopinolide A-P [250], and bigelovin [251] activate apoptotic and autophagic pathways in malignant melanoma, breast cancer (MDA-MB-231), HCC and liver cancer cells, respectively. Calcium ions (Ca2+) are an essential factor for the regulation of autophagy, because it has been shown that Ca2+ release helps to drive membrane fusion to a particular area from the ER, in the vicinity of autophagosomes and lysosomes by binding with IP3R [42]. Thapsigargin (TG) causes the transient elevation of cytosolic Ca2+ release from ER stores and the depletion of intracellular Ca2+ stores in several cells types due to the potent and specific inhibition of intracellular Ca2+ ATPases (sarcoplasmic-/endoplasmic reticulum sarco/ER Ca2+ ATPase, SERCA) [252]. TG therapy also leads to necrotic cell death, which results from excessive damage to the mitochondrial pool and activation of autophagy independent mTOR pathways [252]. The role of TG in autophagy is debated, with earlier reports claiming both inductive and inhibiting effects [148]. TG inhibits autophagy by specifically blocking autophagosome–lysosome fusion, as well as autophagic flux by interfering with Rab GTPases and Rab7 function [148].
4.4. Saponin Compounds
4.4.1. Tubeimoside-1
Tubeimoside-1 (TBMS1) has been proven to have potent anticancer activities in human prostate, lung, liver, cervical, and gastric cancer cells [149]. TBMS1 is identified as a potent activator of autophagy in human breast and liver cancer cells via LC3-II accumulation [150] and AMPK activation [151]. Inhibition of cytoprotective autophagy can enhance the cytocidal effect of TBMS1 in breast cancer cells by promoting apoptotic cell death [150]. TBMS1 inhibits cell proliferation in melanoma cells in vitro and tumorigenecity in vivo. Interestingly, TBMS1 inhibits cell proliferation by the activation of the MEK1/2-ERK1/2 pathway on the one hand, and it triggers cytoprotective autophagy in melanoma cells on the other hand. The strength of the two opposing forces determines the fate of the cells. TBMS1 also interacts with protein-tyrosine phosphatase 1B (PTP1B), which further hyperactivates MEK1/2-ERK1/2 cascades, leading to the inhibition of cell proliferation and the partial distortion of prosurvival autophagy [253]. Another interesting report suggests that TBMS1 exerts anticancer effects in lung cancer cells via blocking of the late-stage of autophagy flux via the impairment of lysosomal acidification through v-ATPase inhibition and the induction of apoptosis by lysosomal-dependent pathways. TBMS1 promotes mitochondrial fission and the dynamin-related protein (DRP1), which is a small GTPase-mediated fragmentation, and thereby leads to ROS accumulation. Impairment of lysosomal acidification blocks the removal of dysfunctional mitochondria and results in ROS accumulation; this causes further damage to the lysosomal membrane and leads to cathepsin B leakage from lysosomes. This leakage upregulates the Bax-mediated MOM potential (MOMP), and subsequently, cytosolic cytochrome c-mediated caspase-dependent apoptosis [254].
4.4.2. Paris Polyphylla
The Paris polyphylla extract has been reported to inhibit cell growth, EMT and invasion in breast cancer, ovarian carcinoma and lung cancer cells [152]. Moreover, pennogenin 3-O-beta-chacotrioside and polyphyllin VI are active components of the ethanolic extract from P. polyphylla (EEPP), inducing cell death in DLD-1 human CRC via the upregulation of autophagy markers LC3-II and Beclin1. In addition, EEPP therapy, in combination with Dox, improves cytotoxicity in these malignant cells [152]. Diosgenin-enriched P. polyphylla rhizome extract (DPPE) shows cytotoxicity and anti-cancer activities in breast cancer cells [255].
4.4.3. Ophiopogonin B
Ophiopogonin B has been verified to inhibit cell proliferation in numerous NSCLC cells. Ophiopogonin B induces autophagy in H157 and H460 cells and adenocarcinoma A549 by upregulating the conversion of LC3-I to LC3-II and increasing the expression of ATG3 and ATG5-ATG12 in treated cells [153].
4.4.4. Betulinic Acid
Betulinic acid (BA) exhibits a variety of biological activities, including anticancer effects. The anticancer activity has been linked to its ability to directly trigger autophagy-mediated apoptosis via the mitochondrial pathway, such as MMP [154]. BA inhibits cell proliferation and induces apoptosis in CRC cells, HepG2 and SMMC-7721 HCC [155]. BA treatment induces autophagy via the inhibition of the AKT/mTOR signaling pathway. Blockage of autophagy enhances BA-induced proliferation inhibition and apoptosis in CRC cells [156]. BA inhibits breast cancer metastases by targeting glucose-regulated protein 78 (GRP78), a major chaperone in ER that is frequently strongly expressed in most solid tumors. This GRP78 chaperone contributes to the acquisition of metastatic phenotypes, including apoptosis resistance and drug resistance [256]. The reduced congener of BA, botulin, also has several pharmacologic effects, including anti-cancer effects. Betulin exhibits inhibitory effects on colorectal metastasis by inducing cell-cycle arrest and autophagy in metastatic CRC cells via AMPK and PI3K/AKT/mTOR signaling pathways. In addition, betulin induces caspase-dependent apoptosis via decreasing the phosphorylation of the MAPK signaling pathway in metastatic CRC cells [257].
4.5. Alkaloids
4.5.1. Camptothecin
Camptothecin (CPT), a potent topoisomerase I inhibitor, displays oncosuppressive activities against leukemia, human colon cancer and a variety of solid tumor systems [157]. Interestingly, the combination of miR-15a and miR-16, which are two potent inducers of autophagy, enhances the chemotherapeutic efficacy of CPT against cancer cells [157]. CPT enhances c-Myc-mediated ER stress and ROS generation, leading to autophagy activation via the induction of Ca2-mediated AMPK and the JNK/activator protein 1 (AP-1) pathway [158]. CPT also generates ROS to modulate the AMPK/mTOR/ULK1 axis in order to finally promote protective autophagy in esophageal cancer [159]. The combination of CPT and pulsatilla saponin D, a powerful autophagy inhibitor, has a synergistic anti-breast cancer effect by interrupting autophagic-lysosomal function and promoting ubiquitous p62 mediated protein aggregation [258]. CPT derivatives, such as belotecan and methylenebis, also induce autophagy, but with different mechanisms. Belotecan induces autophagy by decreasing the level of p62, whereas methylenedes do not affect the level of p62, but upregulate the level of LC3-II. Interestingly, methylenebis and belotecan enhance the synergic antitumoral efficacy by inducing the apoptosis of tumor cells [259].
4.5.2. Berberine
Berberine (BBR) has diverse pharmacological properties, including cancer modulation [160,161]. BBR induces autophagic cell death and apoptosis in human gastric cancer, glioblastoma multiforme (GBM), breast cancer, and hepatoma cells by the activation of Beclin1, as well as the inhibition of the mTOR signaling pathway [160,161]. BBR-associated photodynamic therapy induces autophagy and apoptosis in renal carcinoma cells [162]. In addition, BBR has been shown to induce autophagic cell death by increasing the binding capacity of GRP78 levels in cancer cells [160,161]. BBR sensitizes human HCC to ionizing radiation by blocking autophagy and cell-cycle arrest, which results in senescence [260].
4.5.3. Tetrandrine
Tetrandrine (TET) possesses multiple pharmacological properties against a wide variety of cancers [163]. The antitumor effects of TET are associated with the induction of apoptosis and autophagy, the reversal of MDR, and the enhancement of radiation sensitization [163,164]. TET has been proven to be a potent broad-spectrum autophagy agonist with effects on a variety of cell lines, including triple-negative breast cancer cells, human HCC, nasopharyngeal carcinoma by inhibiting PI3K/AKT/mTOR signaling [164,165]. TET inhibits human PML-RARα-positive acute promyelocytic leukemia (APL) cell proliferation and induces autophagy by activating ROS generation and Notch1 signaling [261]. TET induces autophagy and apoptosis in a dose-dependent manner in pituitary adenoma (PA) cells. TET induces autophagy in these cells by down-regulating the MAPK/STAT3 signal at low concentrations (1.25 μM). However, at a higher dose (5.0 μM) of TET, pituitary adenoma (PA) cells partially die from caspase-dependent apoptosis [262]. Unlike an autophagy enhancer, Qiu et al. presented TET as a powerful lysosomal inhibitor, blocking the autophagic flux at the lysosomal degradation step in tumor cells [263]. TET increases the sensitivity of different cancer cells to gefitinib [264], cisplatin [265] and tamoxifen [266].
4.5.4. Protopine
Protopine exhibits a number of pharmacological properties, including anticancer activities. Protopine suppresses the cell proliferation of colon cancer, inhibits cell adhesion in breast cancer and induces apoptotic cell death in prostate cancer [166]. Protopine is capable of activating p53-mediated transcriptional activity and promotes the stabilization of the p53 protein. Protopine induces autophagy by enhancing LC3-II turnover, along with reducing the levels of p62 in human colon cancer cells [166].
4.5.5. Neferine
Neferine possesses antitumor activity via the suppression of cell proliferation and the inhibition of cell growth in different human cancers [167]. Neferine can induce autophagy in various cancer cells, such as human ovarian cancer via the inactivation of mTOR and the activation of p38 MAPK/JNK signaling pathways [168]. Neferine induces autophagic cell death in a panel of cancer cells, including apoptosis-defective and -resistant cancer cells or isogenic cancer cells, via Ca2+ mobilization through the activation of ryanodine receptor and ULK1/PERK and AMPK/mTOR signaling cascades [169]. Neferine also enhances the tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-mediated autophagic cell death in human prostate cancer cells via the JNK pathway [267]. Furthermore, neferine provokes autophagy and apoptosis in human neuroblastoma cells by reducing the levels of focal adhesion kinase (FAK) and 70 kDa ribosomal S6 kinase 1 (S6K1) [268]. Neferine induces ROS-dependent mitochondrial mediated apoptosis by increasing the expression of proapoptotic proteins Bax, cytochrome c, cleaved caspase-3 and caspase-9. It also activates autophagy via increasing Beclin1, ATG4, ATG5 and ATG12, LC3-II expression levels in cervical cancer cells [269]. Additionally, neferine enhances cisplatin-induced autophagic cell death in human lung adenocarcinoma via downregulation of the PI3K/AKT/mTOR signaling pathway [270]. Further evidence suggests that the neferine anti-angiogenesis mechanism in high-grade serious ovarian carcinoma occurs by inducing autophagy through the inhibition of the mTOR/p70S6K pathway and the suppression of the polarization of M2 tumor-associated macrophages [271].
4.5.6. Graveoline
Graveoline triggers autophagic cell death in skin melanomas via the elevation ROS generation [170].
4.6. Quinonoids
Quinonoid compounds participate in multiple biological oxidative systems by serving as prime links in electron transport chains of the metabolic pathways. This redox characteristic accounts for the inherent cytotoxicity of quinonoids.
4.6.1. Thymoquinone
Thymoquinone (TQ) has been revealed to exert outstanding pharmacological potential, including anticancer effects in both in vitro and in vivo models. TQ exhibits a high anticancer efficacy against various cancers cells, such as breast, lung, colon, ovary, larynx cervical, and prostate cancer, as well as multiple myeloma, myeloblastic leukemia, glioblastoma and osteosarcoma [171]. While the impact of TQ has been studied in many types of cancer, the molecular mechanisms underlying its action are complex and paradoxical due to the multiple targets (e.g., carcinogen metabolizing enzymes, transcription factors, etc.) involved in tumorigenesis or development of drug resistance [172]. TQ inhibits the metastasis of renal cell cancer cells [173], breast cancer cells [174] and human renal carcinoma cells [272] by inducing autophagy via the generation of ROS and the activation of the AMPK/mTOR signaling pathway. By inducing the chemomodulatory potential, TQ synergizes gemcitabine anti-breast cancer activity against human breast adenocarcinoma and ductal carcinoma cells via modulating its apoptotic and autophagic activities [174]. A combination of TQ with cisplatin diminishes the resistance fraction of cisplatin and improves its anticancer activity against head and neck cancer cells [273]. Similarly, TQ, in combination with TMZ, which is currently part of the standard treatment for glioblastoma (GBM), potently inhibits the growth of human GBM cell lines by transcriptional impairment of autophagy and the activation of apoptosis [274]. TQ has been shown to induce caspase-independent autophagic cell death in colon cancer cells via mitochondrial dysfunction (by induction of MOMP and activation of JNK and p38) [121,275].
4.6.2. Celastrol
Celastrol is known for its potent anticancer activities. It has been demonstrated that celastrol inhibits the proliferation of various cancer cells and the growth of tumors in preclinical mouse models [121]. Celastrol can induce paraptosis, autophagy and apoptosis in different cancer cells, including HCC and osteosarcoma cells by modulating multiple pathways such as ER stress [121,175,176,177]. Celastrol has been reported to induce autophagy and apoptosis in different tumor cells, such as glioma and gastric cancer via the ROS/JNK and AKT/mTOR signaling pathways [276,277]. Furthermore, co-treatment of NSCLC cells with celastrol and erastin, which are ferroptosis inducers, initiates ATG5/ATG7-dependent autophagy and PINK1/Parkin-dependent mitophagy via the generation of ROS, the disruption of MMP and the promotion of mitochondrial fission [278]. Celastrol also stimulates Ca2+-mediated autophagic cell death by inhibiting both SERCA and P-gp in MDR tumor cells [279]. In combination with afatinib, celastrol induces paraptosis and subsequent cell death in NSCLC via ER stress, ROS accumulation and mitochondrial Ca2+ overload [280]. Celastrol also induces lipophagy via the activation of the liver-X receptors α (LXRα)/ATP-binding cassette transporter A1 (ABCA1) pathway in clear cell renal carcinoma [281]. Celastrol promotes Nur77 translocation from the nucleus to the mitochondria, where it interacts with TNF receptor-associated factor 2 (TRAF2), a scaffold protein and E3 ubiquitin ligase that is important in inflammatory signaling [282]. In human prostate cancer cells, celastrol induces autophagy by targeting the androgen receptor (AR)/miR-101. Celastrol-induced autophagy inversely correlates with AR expression levels, which in turn suppresses miR-101 expression, and thereby augments prostate cancer cell death [283]. Celastrol acts as a sensitizing agent to TRAIL-initiated lung cancer cell death via ROS generation and a decrease in MMP [284].
4.6.3. Pristimerin
Pristimerin has been reported to provide a variety of anticancer activities via triggering autophagy-mediated cell death through ROS production and the activation of the JNK signaling pathway [178,179].
4.6.4. Plumbagin
Plumbagin has been shown to exert a wide spectrum of pharmacological effects, including anticarcinogenic action against a variety of human cancer cells [180]. Accumulating evidence shows that the anticancer effects of plumbagin are mainly attributed to the induction of autophagy and apoptosis through intracellular ROS generation, activation of the AMPK pathway and inhibition of the PI3K/AKT/mTOR pathway [181,182,183].
4.7. Omega-3 Polyunsaturated Fatty Acids (ω-3-PUFA)
The ω3-PUFAs mainly consist of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). The anticancer properties and mechanism of action of ω3-PUFAs have been demonstrated in several cancers; however, autophagic and apoptotic cell death are the main mechanisms of DHA-induced cytotoxicity in these tumor cells. DHA induces autophagy and apoptosis in human cancer cells harboring wild-type p53 [187] and in prostate cancer cells expressing mutant p53 [188] through p53/AMPK/mTOR signaling [189]. ω3-PUFAs, including high-dose DHA, cause apoptotic and autophagic cell death in GBM cell lines by upregulating the expression of p62 [285], inducing PARP cleavage and activating the AMPK/mTOR pathway [286]. Increasing evidence shows that ω3-PUFAs exhibit anti-melanoma activity. Treatment of pulmonary melanoma with DHA-rich algal oil induces autophagy by inactivating mTOR and activating the JNK pathway, resulting in significant suppression of cell outgrowth. [287]. DHA promotes immunogenic apoptosis by inhibiting the STAT3 pathway in human multiple myeloma cells with no toxicity in peripheral blood mononuclear cells (PBMCs) and dendritic cells (DCs). It also activates autophagy in PBMCs and DCs, which potentially act as immune boosts [288]. There is evidence that EPA and DHA also induce anticancer effects by means of their conversion to their corresponding ethanolamine derivatives in breast carcinomas. This is done by binding and activating different receptors and distinct signaling pathways [289]. For instance, DHA and EPA-dopamine conjugates, such as DHA-dopamine (DHADA) and EPA-dopamine (EPADA), inhibit cell growth and trigger autophagy and apoptosis in breast cancer cells via the peroxisome proliferator-activated receptor γ (PPARγ) [290]. Cotreatment of retinoic acid and ω-3 PUFA activates autophagy in breast cancer cells by activating the p38 MAPK signaling pathways [291]. DHA enhances the anticancer drug oxaliplatin-induced autophagic cell death via activating Sestrin 2 and increasing ER stress in CRC cells [292]. Co-treatment with DHA and vitamin E delta-tocotrienol (Delta-T3) reduces lipid droplet biogenesis and potentiates lipophagy in TNBC MDA-MB-231 cells, resulting in the mitigation of breast cancer malignancy [293].
4.8. Miscellaneous
4.8.1. Trichostatin A
Several histone deacetylase inhibitors (HDACIs), such as trichostatin A (TSA), SAHA (also known as vorinostat) and depsipeptide, have been widely studied as cancer therapeutic agents. TSA-induced apoptosis is associated with multiple mechanisms, with the most likely being the modulation of autophagy. The molecular mechanisms underlying TSA-mediated autophagy are still not clear, and autophagic cell death remains controversial and most likely context-dependent. TSA is able to induce autophagy in human cancer cells through the inhibition of the mTOR pathway and enhancing forkhead box protein1 (FOXO1)-dependent pathways [184]. Histone deacetylase inhibitors, valproic acid and TSA induce apoptosis and autophagy in pancreatic cancer cells by increasing ROS production and triggering mitochondrial membrane depolarization, cytochrome c release and caspase 3 activation [185]. In cervical cancer cells, TSA (1 μM) induces autophagy by significantly suppressing protein arginine methyltransferase 5 (PRMT5) and transient receptor potential cation channel, subfamily V, member 6 (TRPV6) levels and enhancing stanniocalcin 1 (STC1) and JNK levels [186]. TSA suppresses cervical cancer cell proliferation and induces autophagic cell death through the regulation of the PRMT5/STC1/TRPV6/JNK axis. In NSCLC patients, TSA treatment enhances autophagy and reverses the chemoresistance of docetaxel or paclitaxel associated with insulin-like growth factor (IGF) binding protein-2 (IGF-BP2) expression, which has been shown to promote tumorigenesis, metastasis, and cancer stem cell expansion [294]. TSA reduces cell viability in cisplatin-resistant human ovarian cancer cells by inhibiting the volume-sensitive organic anion channel (VSOAC) via the induction of taurine transporter (TauT) activity and inducing autophagic cell death [295]. In combination with the autophagy inhibitor CQ, TSA synergistically exerts anti-tumor activity in H-ras transformed breast epithelial cells by blocking the mTOR-signaling pathway [296]. Co-treatment with the PI3K/mTOR dual inhibitor, BEZ235 and TSA significantly enhances autophagic cell death and induces anti-tumor activities in esophageal squamous cell carcinoma [297] and breast cancer [298] by depressing the PI3K/AKT/mTOR signaling pathway and upregulating the expression of LC3-II and Beclin1.
4.8.2. 6-Shogaol
6-shogaol, an active constituent of dietary ginger, exerts anti-inflammatory and anticancer properties. Treatment with 6-shogaol inhibits autophagy flux by increasing p62 and LC3 II levels in liver cancer cells. However, 6-shogaol, in combination with TRAIL, induces apoptosis via triggering ROS, upregulating p53 expression and altering the mitochondrial transmembrane potential (MTP) of these cells [299]. The antitumor activity of 6-shogaol in HCC indicates that it enhances autophagy by activating ROS and ER stress-associated proteins, and induces apoptosis by activating caspase-3 in cancer cells [300]. 6-shogaol inhibits cell survival and stimulates autophagy by suppressing the AKT/mTOR pathway in human non-small cell lung cancer A549 cells [301]. 6-shogaol induces both autophagic and apoptotic cell death in colorectal adenocarcinoma HT-29 cells [302]. 6-shogaol shows anti-tumor effect in cervical carcinoma by triggering the mitochondrial pathway of apoptosis and downregulating the PI3K/AKT/mTOR pathway [303]. 6-shogaol treatment in breast cancer cells results in the suppression of proliferation by the significant induction of apoptosis and the inhibition of autophagy by the regulation of the Notch signaling pathway (Hes1 and CyclinD1 genes). Here, the inhibition of autophagy by 6-shogaol leads to the enhancement of breast cancer cell apoptosis [304]. However, 6-shogaol induces autophagic cell death in breast cancer cells and CSC-like spheroids via γ-secretase mediated down-regulation of Notch signaling (reduction in the expression levels of cleaved Notch1 and its target proteins Hes1 and Cyclin D1) [305]. In addition, 6-shogaol potentiates the anticancer efficacy of 5-FU, oxaliplatin, and irinotecan by activating apoptosis and autophagy in CRC cells in hypoxic/aglycemic (glucose starvation) conditions [306].
4.8.3. 7-Trehalose
Trehalose is a disaccharide that is present in a wide variety of organisms, including bacteria, yeasts, fungi, insects, invertebrates and higher plants, where it may serve as a source of energy and carbon. Trehalose is a powerful autophagy inducer used for the clearance of misfolded proteins in cells and animal models of neurodegenerative diseases. Recently, it has been reported that trehalose induces cytoprotective autophagy and mitophagy (via enhancing autophagic flux) in prostatic cancer cells that show resistance to chemotherapy [307].
5. Concluding Remarks and Future Directions
Since the defects of autophagy have been linked to many human diseases, modulation of autophagy can prevent or treat various human cancers. It has been reported that autophagy plays a dual role in cancer, showing both anti-tumor and tumor promotion effects, depending on the carcinogenic stage, tissues involved, and microenvironment. Autophagy can prevent cancer through the clearance of oncogenic factors; however, it can promote cancer progression and metastasis in well-established tumors. The inhibition of autophagy in chemoresistant cancer cells can cause cell death, while triggering excessive and uncontrolled formation, and the accumulation of autophagosomes and auyolysosomes can stimulate apoptotic or cell death with autophagic features. The identification of natural products as modulators has already provided a significant understanding of the molecular mechanisms of autophagy. Recent advances in technology, such as high-throughput, image-based screens and high-powered scanning microscopy, have been developed by different labs to identify natural modulators, as well as dynamics of autophagy for quantitative analysis of autophagic machinery. As discussed throughout this review, the anticancer effects of the enormous range of natural products are related to their autophagy-modulating actions, either the suppression or induction of autophagy. However, several obstacles with experimental bases and clinical trials have hindered the direct execution of autophagy modulators in the clinic. It is likely that many of these obstacles can be circumvented upon the development of more selective autophagy modulators, more precise biomarkers of the autophagy machinery and more animal models of autophagy deficiency in vivo.
Practically, the take home action of autophagy in cancers depends on the type of tumor, stage of tumorigenesis, tumor niches, as well as the genetic, epigenetic and metabolic contexts. Discovery of the precise role of autophagy in tumor development and progression is vital for the development of novel anticancer therapies that target cancer eradication. In cancer therapy, numerous unresolved problems exist. On the one hand, conventional chemotherapy and radiation therapy leads to increased toxicity in normal cells and tumor cells, limiting their use for cancer treatment. Thus, the development new and effective therapeutic agents with minimal toxicity to normal cells is of paramount urgency. Natural products with anticancer action have expanded their consideration due to their favorable safety and efficacy profiles in clinical trials. On the other hand, tumor resistance is increasingly incorporated into chemotherapy, radiotherapy or targeted therapy. One of the reasons for this augmented resistance is the failure of apoptosis in cancer cells. Autophagy-mediated cell death may be an alternative solution for this problem. Natural products that have the capability to manipulate both autophagy and apoptosis may assist cancer cell death. In addition, natural products, in combination with conventional therapeutic tactics, may offer greater effectiveness against malignancy. Furthermore, natural products, such as magnolol [214], which manipulate both autophagy and mitophagy in cancer cells (dual mechanism) may be important therapeutic targets [308,309]. In addition, natural products that clear lipid droplets via lipophagy-dependent or -independent mechanisms may prevent cancer progression, as these organelles are formed in various cancers, including breast, prostate, and clear renal cell carcinoma, and play a role in cancer progression [310,311].
A recent article reported the successful application of computer-assisted methods in screening a unique and diverse collection of an inhouse library consisting of about 1000 individual natural products such as alkaloids, terpenoids, Diels–Alder-type adducts, isoflavones, chalcones, and cannabinoids. However, the compounds derived from these natural products have anticancer and antimicrobial properties, which could be controlled by autophagic machinery [312]. Interestingly, there is increasing evidence that autophagy proteins activate extracellular vesicles’ (EVs) biogenesis. The pathways between autophagy and EVs occur not only in mammalian cells, but also in plants. This can be significant in the context of cancer treatment through the interaction between natural products derived from EVs, which are loaded with oncosuppressive cargoes on mammalian cancer cells [313].
Author Contributions
M.A.A.A.-B. designed, edited, revised and proof-read the manuscript, Y.I., S.A., N.R. and H.S.A. revised the final draft of the manuscript, and N.E. provided images, wrote, critically revised and approved the final draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Koji Kumagai for providing a human ovarian cancer sample (from Takatsuki Red Cross Hospital, Takatsuki, Osaka, Japan) used for IHC with LC3.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| UPS | ubiquitin-proteasome system |
| ER | endoplasmic reticulum |
| ATG | Autophagy-related genes |
| FDA | Food and Drug Administration |
| IM | isolation membrane |
| ULK1 | Unc-51-like autophagy activating kinase 1 |
| PI3K | Phosphatidylinositide 3-kinase |
| mTORC1 | mechanistic target of rapamycin complex 1 |
| AMPK | AMP-activated protein kinase |
| MOM | Mitochondrial outer membrane |
| MOMP | MOM potential |
| ERES | ER exit sites |
| MAMs | mitochondria-associated ER membranes |
| PI3P | Phosphatidylinositol 3-phosphate |
| DFCP1 | double FYVE domain–containing protein 1 |
| Ubl | ubiquitin-like |
| LC3 | MT-associated protein 1 light chain 3 |
| LC3-PE | LC3-phosphatidylethanolamine |
| LIR | LC3 interacting regions |
| Ambra1 | Activating Molecule In Beclin1-Regulated Autophagy Protein 1 |
| p62/ SQSTM1 | Sequestosome-1 |
| MTs | Microtubules |
| ALR | autophagic lysosome regeneration |
| IP3R | inositol 1,4,5-trisphosphate (IP3) receptor |
| JNK | c-Jun N-terminal kinase |
| BH3 | Bcl-2 homology (BH) domain 3 |
| 3-MA | 3-methyladenine |
| MLCK | myosin light chain kinase |
| PLK1 | Polo-like kinase 1 |
| DNA-PK | DNA-dependent protein kinase |
| ATM | ataxia–telangiectasia mutated |
| NP | Nanoparticle |
| NCT | National Clinical Trial |
| PSM | Petrosaspongiolide M |
| EPI | Epirubicin |
| Bcl-2 | B-cell lymphoma 2 |
| TP | tea polyphenol |
| MTAs | MT-targeting agents |
| CA-4 | Combretastatin A-4 |
| CA-4P | CA-4 phosphate |
| NAC | N-acetyl cysteine |
| ROS | reactive oxygen species |
| rhArg | recombinant human arginase |
| 5-FU | 5-fluorouracil |
| VCP | Valosin Containing Protein or p97 |
| XN | xanthohumol |
| Sal A | salvianolic acid A |
| Sal B | salvianolic acid B |
| SCC | squamous cell carcinoma |
| CCC | circulating cancer cells |
| PTEN | phosphatase and tensin homolog deleted on chromosome 10 |
| EMT | epithelial-mesenchymal transition |
| AKT | protein kinase B (PKB) |
| HIF-1α | hypoxia-inducible factor 1α |
| VEGF | Vascular endothelial growth factor |
| HCC | hepatocellular carcinoma |
| CRC | colorectal cancer |
| Dox | Doxorubicin |
| Cdk4 | cyclin-dependent kinase 4 |
| Hsp-90 | heat shock protein-90 |
| PARP-1 | poly (ADP-ribose) polymerase 1 |
| ALL | acute lymphoblastic leukaemia |
| CQ | Chloroquine |
| Baf A1 | Bafilomycin A1 |
| OC | Oblongifolin C |
| GUTK | guttiferone K |
| v-ATPase | vacuolar-type ATPase |
| LMP | lysosomal membrane permeabilization |
| SAR | structure-activity relationship |
| CR | Caloric restriction |
| SIRT1 | Sirtuin 1 |
| BNIP3/BNIP3L | BCL2/adenovirus E1B 19 kDa protein-interacting protein 3/ligand |
| STAT3 | Signal transducer and activator of transcription 3 |
| RAGE | receptor for advanced glycation end products |
| MDR1 | multidrug resistance protein 1 |
| P-gp | P-glycoprotein |
| NLRP3 | NLR family pyrin domain containing 3 |
| EGCG | (−)-epigallocatechin-3-gallate |
| CGA | chlorogenic acid |
| EGC | (−)-epigallocatechin |
| ECG | (−)-epicatechin-3-gallate |
| NSCLC | non-small cell lung cancer |
| MAPK | mitogen-activated protein kinase |
| N-CoR | nuclear receptor co-repressor |
| HSC70 | Heat shock cognate 71 kDa protein |
| TFEB | transcription factor EB |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| MMP | mitochondrial membrane potential |
| Nrf2 | Nuclear Factor, Erythroid 2 Like 2 |
| ARDs | age-related diseases |
| CRM | caloric restriction mimetic |
| p70S6K | phosphorylation of p70 ribosomal protein S6 kinase |
| SOCE | store operated calcium entry |
| TMZ | Temozolomide |
| MGMT | O6-methylguanine-DNA methyltransferase |
| TSC2 | Tuberous Sclerosis Complex 2 |
| PKC-δ | protein kinase C δ |
| TG2 | Transglutaminase |
| CSCs | cancer stem cells |
| 6-CEPN | 6-C-(E-phenylethenyl)naringenin |
| BUC | bladder urothelial carcinoma |
| PERK | phosphorylated extracellular signal-regulated kinase |
| eIF2α | Eukaryotic initiation factor 2α |
| CHOP | C/EBP homologous protein |
| CaMMK | Calmodulin- dependent kinase protein kinase |
| IRE1α | inositol-requiring enzyme 1α |
| TNBC | triple-negative breast cancer |
| MPT | mitochondrial permeability transition |
| SERCA | Sarcoplasmic-/ endoplasmic reticulum sarco/ER Ca2+ ATPase |
| TG | Thapsigargin |
| TBMS1 | Tubeimoside-1 |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| PTP1B | protein-tyrosine phosphatase 1B |
| DRP1 | dynamin-related protein |
| EEPP | ethanolic extract from P. polyphylla |
| DPPE | Diosgenin enriched P. polyphylla rhizome extract |
| BA | Betulinic acid |
| GRP78 | glucose-regulated protein 78 |
| CPT | Camptothecin |
| AP-1 | Activator protein 1 |
| miR-15a | microRNA-15a |
| BBR | Berberine |
| PDT | photodynamic therapy |
| TET | Tetrandrine |
| APL | acute promyelocytic leukemia |
| PA | pituitary adenoma |
| TRAIL | Tumor necrosis factor (TNF)-related apoptosis-inducing ligand |
| TRAF2 | TNF receptor-associated factor 2 |
| FAK | focal adhesion kinase |
| S6K1 | 70-kDa ribosomal S6 kinase 1 |
| TQ | Thymoquinone |
| GBM | glioblastoma multiforme |
| LXRα | liver-X receptors α |
| ABCA1 | ATP-binding cassette transporter A1 |
| ccRCC | clear cell renal cell carcinoma |
| AR | androgen receptor |
| ω3-PUFAs | omega-3 polyunsaturated fatty acids |
| EPA | eicosapentaenoic acid |
| EPADA | EPA-dopamine |
| DHA | docosahexaenoic acid |
| DHADA | DHA-dopamine |
| MM | multiple myeloma |
| PBMCs | peripheral blood mononuclear cells |
| DCs | dendritic cells |
| PPARγ | peroxisome proliferator-activated receptor γ |
| Delta-T3 | vitamin E Delta-tocotrienol |
| HDACIs | histone deacetylase inhibitors |
| TSA | trichostatin A |
| SAHA | suberoylanilide hydroxamic acid |
| FOXO1 | forkhead box protein1 |
| PRMT5 | protein arginine methyltransferase 5 |
| TRPV6 | transient receptor potential cation channel, subfamily V, member 6 |
| STC1 | stanniocalcin 1 |
| IGFBP2 | insulin-like growth factor (IGF) binding protein-2 |
| VSOAC | volume-sensitive organic anion channel |
| TauT | taurine transporter |
| MTP | mitochondrial transmembrane potential |
| CLL | Chronic Lymphocytic Leukaemia |
| ANT | Active, not recruiting |
| T | terminated |
| C | Completed |
| S | Suspended |
| R | Recruitment |
| NR | Not yet recruiting |
| U | Unknown |
| ATRA | all-trans retinoic acid |
| CyP | Cyclophosphamide |
| Cape | Capecitabine |
| PCOS | Polycystic ovarian syndrome |
| FME | familial Mediterranean fever |
| HEKs | human epidermal keratinocytes |
| CSCC | cutaneous squamous cell carcinoma cell |
| NTM | nontuberculous mycobacteria |
| LYNUS | lysosomal nutrient sensing machinery |
References
- Pohl, C.; Dikic, I. Cellular quality control by the ubiquitin-proteasome system and autophagy. Science 2019, 366, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Saftig, P.; Klumperman, J. Lysosome biogenesis and lysosomal membrane proteins: Trafficking meets function. Nat. Rev. Mol. Cell Biol. 2009, 10, 623–635. [Google Scholar] [CrossRef]
- Horibe, A.; Eid, N.; Ito, Y.; Hamaoka, H.; Tanaka, Y.; Kondo, Y. Upregulated autophagy in Sertoli cells of ethanol-treated rats is associated with induction of inducible nitric oxide synthase (iNOS), androgen receptor suppression and germ cell apoptosis. Int. J. Mol. Sci. 2017, 18, 1061. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Debnath, J. Autophagy at the crossroads of catabolism and anabolism. Nat. Rev. Mol. Cell Biol. 2015, 16, 461–472. [Google Scholar] [CrossRef]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Pietrocola, F.; Bravo-San Pedro, J.M.; Amaravadi, R.K.; Baehrecke, E.H.; Cecconi, F.; Codogno, P.; Debnath, J.; Gewirtz, D.A.; Karantza, V.; et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015, 34, 856–880. [Google Scholar] [CrossRef]
- Amaravadi, R.K.; Kimmelman, A.C.; Debnath, J. Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discov. 2019, 9, 1167–1181. [Google Scholar] [CrossRef]
- Jung, S.; Jeong, H.; Yu, S.W. Autophagy as a decisive process for cell death. Exp. Mol. Med. 2020, 52, 921–930. [Google Scholar] [CrossRef]
- Deng, L.J.; Qi, M.; Li, N.; Lei, Y.H.; Zhang, D.M.; Chen, J.X. Natural products and their derivatives: Promising modulators of tumor immunotherapy. J. Leukoc. Biol. 2020, 108, 493–508. [Google Scholar] [CrossRef]
- Yin, B.; Fang, D.M.; Zhou, X.L.; Gao, F. Natural products as important tyrosine kinase inhibitors. Eur. J. Med. Chem. 2019, 182, 111664. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, K. Anticancer Activity of Natural Compounds from Plant and Marine Environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Morel, E.; Mehrpour, M.; Botti, J.; Dupont, N.; Hamai, A.; Nascimbeni, A.C.; Codogno, P. Autophagy: A Druggable Process. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 375–398. [Google Scholar] [CrossRef]
- Abada, A.; Elazar, Z. Getting ready for building: Signaling and autophagosome biogenesis. EMBO Rep. 2014, 15, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Sanchez-Wandelmer, J.; Ktistakis, N.T.; Reggiori, F. ERES: Sites for autophagosome biogenesis and maturation? J. Cell. Sci. 2015, 128, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Klionsky, D.J. Direct quantification of autophagic flux by a single molecule-based probe. Autophagy 2017, 13, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Morishita, H.; Kaizuka, T.; Hama, Y.; Mizushima, N. A new probe to measure autophagic flux in vitro and in vivo. Autophagy 2017, 13, 757–758. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, S.T.; Yoshimori, T. A current perspective of autophagosome biogenesis. Cell Res. 2014, 24, 58–68. [Google Scholar] [CrossRef]
- Hamasaki, M.; Furuta, N.; Matsuda, A.; Nezu, A.; Yamamoto, A.; Fujita, N.; Oomori, H.; Noda, T.; Haraguchi, T.; Hiraoka, Y.; et al. Autophagosomes form at ER-mitochondria contact sites. Nature 2013, 495, 389–393. [Google Scholar] [CrossRef]
- Krols, M.; Bultynck, G.; Janssens, S. ER-Mitochondria contact sites: A new regulator of cellular calcium flux comes into play. J. Cell Biol. 2016, 214, 367–370. [Google Scholar] [CrossRef]
- Polson, H.E.; de Lartigue, J.; Rigden, D.J.; Reedijk, M.; Urbe, S.; Clague, M.J.; Tooze, S.A. Mammalian Atg18 (WIPI2) localizes to omegasome-anchored phagophores and positively regulates LC3 lipidation. Autophagy 2010, 6, 506–522. [Google Scholar] [CrossRef]
- Dooley, H.C.; Razi, M.; Polson, H.E.; Girardin, S.E.; Wilson, M.I.; Tooze, S.A. WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol. Cell 2014, 55, 238–252. [Google Scholar] [CrossRef]
- Xie, Z.; Klionsky, D.J. Autophagosome formation: Core machinery and adaptations. Nat. Cell Biol. 2007, 9, 1102–1109. [Google Scholar] [CrossRef]
- Nedelsky, N.B.; Todd, P.K.; Taylor, J.P. Autophagy and the ubiquitin-proteasome system: Collaborators in neuroprotection. Biochim. Biophys. Acta 2008, 1782, 691–699. [Google Scholar] [CrossRef]
- Zientara-Rytter, K.; Subramani, S. AIM/LIR-based fluorescent sensors-new tools to monitor mAtg8 functions. Autophagy 2018, 14, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Jun, Y.W.; Choi, H.E.; Huh, Y.H.; Kaang, B.K.; Jang, D.J.; Lee, J.A. Development of LC3/GABARAP sensors containing a LIR and a hydrophobic domain to monitor autophagy. EMBO J. 2017, 36, 1100–1116. [Google Scholar] [CrossRef] [PubMed]
- Fracchiolla, D.; Sawa-Makarska, J.; Martens, S. Beyond Atg8 binding: The role of AIM/LIR motifs in autophagy. Autophagy 2017, 13, 978–979. [Google Scholar] [CrossRef]
- Deng, Z.; Lim, J.; Wang, Q.; Purtell, K.; Wu, S.; Palomo, G.M.; Tan, H.; Manfredi, G.; Zhao, Y.; Peng, J.; et al. ALS-FTLD-linked mutations of SQSTM1/p62 disrupt selective autophagy and NFE2L2/NRF2 anti-oxidative stress pathway. Autophagy 2020, 16, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Kast, D.J.; Dominguez, R. The Cytoskeleton-Autophagy Connection. Curr. Biol. 2017, 27, R318–R326. [Google Scholar] [CrossRef] [PubMed]
- Gross, S.P.; Vershinin, M.; Shubeita, G.T. Cargo transport: Two motors are sometimes better than one. Curr. Biol. 2007, 17, R478–R486. [Google Scholar] [CrossRef]
- Korolchuk, V.I.; Saiki, S.; Lichtenberg, M.; Siddiqi, F.H.; Roberts, E.A.; Imarisio, S.; Jahreiss, L.; Sarkar, S.; Futter, M.; Menzies, F.M.; et al. Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell. Biol. 2011, 13, 453–460. [Google Scholar] [CrossRef]
- Pous, C.; Codogno, P. Lysosome positioning coordinates mTORC1 activity and autophagy. Nat. Cell. Biol. 2011, 13, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Guardia, C.M.; Keren-Kaplan, T.; Bonifacino, J.S. Mechanisms and functions of lysosome positioning. J. Cell Sci. 2016, 129, 4329–4339. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Lian, S.; Khan, A.; Llop, J.R.; Samuelson, A.V.; Chen, W.; Klionsky, D.J.; Kishi, S. Autolysosome biogenesis and developmental senescence are regulated by both Spns1 and v-ATPase. Autophagy 2017, 13, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Yoshimori, T. New insights into autophagosome-lysosome fusion. J. Cell Sci. 2017, 130, 1209–1216. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, L. Development of Research into Autophagic Lysosome Reformation. Mol. Cells 2018, 41, 45–49. [Google Scholar] [PubMed]
- Al-Bari, M.A.A.; Xu, P. Molecular regulation of autophagy machinery by mTOR-dependent and -independent pathways. Ann. N. Y. Acad. Sci. 2020, 1467, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Eid, N.; Ito, Y.; Horibe, A.; Otsuki, Y.; Kondo, Y. Ethanol-Induced Mitochondrial Damage in Sertoli Cells is Associated with Parkin Overexpression and Activation of Mitophagy. Cells 2019, 25, 283. [Google Scholar] [CrossRef] [PubMed]
- Tisi, R.; Gaponenko, V.; Vanoni, M.; Sacco, E. Natural Products Attenuating Biosynthesis, Processing, and Activity of Ras Oncoproteins: State of the Art and Future Perspectives. Biomolecules 2020, 10, 1535. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Hannan, M.A.; Dash, R.; Rahman, M.H.; Islam, R.; Uddin, M.J.; Sohag, A.A.M.; Rahman, M.H.; Rhim, H. Phytochemicals as a Complement to Cancer Chemotherapy: Pharmacological Modulation of the Autophagy-Apoptosis Pathway. Front. Pharmacol. 2021, 12, 639628. [Google Scholar] [CrossRef]
- Dischler, N.M.; Xu, L.; Li, Y.; Nichols, C.B.; Alspaugh, J.A.; Bills, G.F.; Gloer, J.B. Wortmannin and Wortmannine Analogues from an Undescribed Niesslia sp. J. Nat. Prod. 2019, 82, 532–538. [Google Scholar] [CrossRef]
- Petiot, A.; Ogier-Denis, E.; Blommaart, E.F.; Meijer, A.J.; Codogno, P. Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J. Biol. Chem. 2000, 275, 992–998. [Google Scholar] [CrossRef]
- Nakanishi, S.; Kakita, S.; Takahashi, I.; Kawahara, K.; Tsukuda, E.; Sano, T.; Yamada, K.; Yoshida, M.; Kase, H.; Matsuda, Y.; et al. Wortmannin, a microbial product inhibitor of myosin light chain kinase. J. Biol. Chem. 1992, 267, 2157–2163. [Google Scholar] [CrossRef]
- Brunn, G.J.; Williams, J.; Sabers, C.; Wiederrecht, G.; Lawrence, J.C., Jr.; Abraham, R.T. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996, 15, 5256–5267. [Google Scholar] [CrossRef]
- Harder, B.G.; Peng, S.; Sereduk, C.P.; Sodoma, A.M.; Kitange, G.J.; Loftus, J.C.; Sarkaria, J.N.; Tran, N.L. Inhibition of phosphatidylinositol 3-kinase by PX-866 suppresses temozolomide-induced autophagy and promotes apoptosis in glioblastoma cells. Mol. Med. 2019, 25, 49. [Google Scholar] [CrossRef]
- Hotte, S.J.; Chi, K.N.; Joshua, A.M.; Tu, D.; Macfarlane, R.J.; Gregg, R.W.; Ruether, J.D.; Basappa, N.S.; Finch, D.; Salim, M.; et al. A Phase II Study of PX-866 in Patients With Recurrent or Metastatic Castration-resistant Prostate Cancer: Canadian Cancer Trials Group Study IND205. Clin. Genitourin. Cancer 2019, 17, 201–208.e1. [Google Scholar] [CrossRef]
- Yam, C.; Xu, X.; Davies, M.A.; Gimotty, P.A.; Morrissette, J.J.D.; Tetzlaff, M.T.; Wani, K.M.; Liu, S.; Deng, W.; Buckley, M.; et al. A Multicenter Phase I Study Evaluating Dual PI3K and BRAF Inhibition with PX-866 and Vemurafenib in Patients with Advanced BRAF V600-Mutant Solid Tumors. Clin. Cancer Res. 2018, 24, 22–32. [Google Scholar] [CrossRef]
- Pitz, M.W.; Eisenhauer, E.A.; MacNeil, M.V.; Thiessen, B.; Easaw, J.C.; Macdonald, D.R.; Eisenstat, D.D.; Kakumanu, A.S.; Salim, M.; Chalchal, H.; et al. Phase II study of PX-866 in recurrent glioblastoma. Neuro-Oncol. 2015, 17, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Watanabe-Asano, T.; Kuma, A.; Mizushima, N. Cycloheximide inhibits starvation-induced autophagy through mTORC1 activation. Biochem. Biophys. Res. Commun. 2014, 445, 334–339. [Google Scholar] [CrossRef]
- Blommaart, E.F.; Luiken, J.J.; Blommaart, P.J.; van Woerkom, G.M.; Meijer, A.J. Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J. Biol. Chem. 1995, 270, 2320–2326. [Google Scholar] [CrossRef] [PubMed]
- Della Sala, G.; Agriesti, F.; Mazzoccoli, C.; Tataranni, T.; Costantino, V.; Piccoli, C. Clogging the Ubiquitin-Proteasome Machinery with Marine Natural Products: Last Decade Update. Mar. Drugs 2018, 16, 467. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.C.; Margarucci, L.; Riccio, R.; Bonfili, L.; Mozzicafreddo, M.; Eleuteri, A.M.; Casapullo, A. Mechanistic insights on petrosaspongiolide M inhibitory effects on immunoproteasome and autophagy. Biochim. Biophys. Acta 2014, 1844, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Ren, Y.; Wang, F.; Tian, D.; Yao, X.; Zhang, Y.; Tang, J. Harmines inhibit cancer cell growth through coordinated activation of apoptosis and inhibition of autophagy. Biochem. Biophys. Res. Commun. 2018, 498, 99–104. [Google Scholar] [CrossRef]
- Kaul, R.; Risinger, A.L.; Mooberry, S.L. Microtubule-Targeting Drugs: More than Antimitotics. J. Nat. Prod. 2019, 82, 680–685. [Google Scholar] [CrossRef]
- Mukhtar, E.; Adhami, V.M.; Mukhtar, H. Targeting microtubules by natural agents for cancer therapy. Mol. Cancer Ther. 2014, 13, 275–284. [Google Scholar] [CrossRef]
- Karatoprak, G.S.; Kupeli Akkol, E.; Genc, Y.; Bardakci, H.; Yucel, C.; Sobarzo-Sanchez, E. Combretastatins: An Overview of Structure, Probable Mechanisms of Action and Potential Applications. Molecules 2020, 25, 2560. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, W.; Xu, J.; Zhang, T.; Zuo, D.; Zhou, Z.; Lin, B.; Wang, G.; Wang, Z.; Sun, W.; et al. NDRG1 inhibition sensitizes osteosarcoma cells to combretastatin A-4 through targeting autophagy. Cell Death Dis. 2017, 8, e3048. [Google Scholar] [CrossRef]
- Greene, L.M.; O’Boyle, N.M.; Nolan, D.P.; Meegan, M.J.; Zisterer, D.M. The vascular targeting agent Combretastatin-A4 directly induces autophagy in adenocarcinoma-derived colon cancer cells. Biochem. Pharmacol. 2012, 84, 612–624. [Google Scholar] [CrossRef]
- Li, Y.; Luo, P.; Wang, J.; Dai, J.; Yang, X.; Wu, H.; Yang, B.; He, Q. Autophagy blockade sensitizes the anticancer activity of CA-4 via JNK-Bcl-2 pathway. Toxicol. Appl. Pharmacol. 2014, 274, 319–327. [Google Scholar] [CrossRef]
- Zafarullah, M.; Li, W.Q.; Sylvester, J.; Ahmad, M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol. Life Sci. 2003, 60, 6–20. [Google Scholar] [CrossRef]
- Wu, M.S.; Lien, G.S.; Shen, S.C.; Yang, L.Y.; Chen, Y.C. N-acetyl-L-cysteine enhances fisetin-induced cytotoxicity via induction of ROS-independent apoptosis in human colonic cancer cells. Mol. Carcinog. 2014, 53 (Suppl. S1), E119–E129. [Google Scholar] [CrossRef]
- Sasazawa, Y.; Kanagaki, S.; Tashiro, E.; Nogawa, T.; Muroi, M.; Kondoh, Y.; Osada, H.; Imoto, M. Xanthohumol impairs autophagosome maturation through direct inhibition of valosin-containing protein. ACS Chem. Biol. 2012, 7, 892–900. [Google Scholar] [CrossRef]
- Ma, L.; Tang, L.; Yi, Q. Salvianolic Acids: Potential Source of Natural Drugs for the Treatment of Fibrosis Disease and Cancer. Front. Pharmacol. 2019, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, S.; Zhang, W.; Zheng, X.; Hu, S.; Pang, C.; Lu, J.; Xing, J.; Dong, Y. Salvianolic acid A reverses paclitaxel resistance in human breast cancer MCF-7 cells via targeting the expression of transgelin 2 and attenuating PI3 K/Akt pathway. Phytomedicine 2014, 21, 1725–1732. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, S.; Yang, Q.; Cai, J.; Zhang, W.; You, H.; Xing, J.; Dong, Y. Salvianolic acid A reverses the paclitaxel resistance and inhibits the migration and invasion abilities of human breast cancer cells by inactivating transgelin 2. Cancer Biol. Ther. 2015, 16, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiu, S.; Qian, L.; Tian, Y.; Chen, Y.; Bi, L.; Chen, W. OCF can repress tumor metastasis by inhibiting epithelial-mesenchymal transition involved in PTEN/PI3K/AKT pathway in lung cancer cells. PLoS ONE 2017, 12, e0174021. [Google Scholar] [CrossRef]
- Tao, L.; Wang, S.; Zhao, Y.; Sheng, X.; Wang, A.; Zheng, S.; Lu, Y. Phenolcarboxylic acids from medicinal herbs exert anticancer effects through disruption of COX-2 activity. Phytomedicine 2014, 21, 1473–1482. [Google Scholar] [CrossRef]
- Gong, L.; Di, C.; Xia, X.; Wang, J.; Chen, G.; Shi, J.; Chen, P.; Xu, H.; Zhang, W. AKT/mTOR signaling pathway is involved in salvianolic acid B-induced autophagy and apoptosis in hepatocellular carcinoma cells. Int. J. Oncol. 2016, 49, 2538–2548. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.D.; Zhao, Y.; Zhang, M.; He, R.Z.; Shi, X.H.; Guo, X.J.; Shi, C.J.; Peng, F.; Wang, M.; Shen, M.; et al. Inhibition of Autophagy by Deguelin Sensitizes Pancreatic Cancer Cells to Doxorubicin. Int. J. Mol. Sci. 2017, 18, 370. [Google Scholar] [CrossRef]
- Yang, Y.L.; Ji, C.; Bi, Z.G.; Lu, C.C.; Wang, R.; Gu, B.; Cheng, L. Deguelin induces both apoptosis and autophagy in cultured head and neck squamous cell carcinoma cells. PLoS ONE 2013, 8, e54736. [Google Scholar] [CrossRef]
- Khan, N.; Yilmaz, S.; Aksoy, S.; Uzel, A.; Tosun, C.; Kirmizibayrak, P.B.; Bedir, E. Polyethers isolated from the marine actinobacterium Streptomyces cacaoi inhibit autophagy and induce apoptosis in cancer cells. Chem. Biol. Interact. 2019, 307, 167–178. [Google Scholar] [CrossRef]
- Gao, G.; Liu, F.; Xu, Z.; Wan, D.; Han, Y.; Kuang, Y.; Wang, Q.; Zhi, Q. Evidence of nigericin as a potential therapeutic candidate for cancers: A review. Biomed. Pharmacother. 2021, 137, 111262. [Google Scholar] [CrossRef]
- Choi, H.S.; Jeong, E.H.; Lee, T.G.; Kim, S.Y.; Kim, H.R.; Kim, C.H. Autophagy Inhibition with Monensin Enhances Cell Cycle Arrest and Apoptosis Induced by mTOR or Epidermal Growth Factor Receptor Inhibitors in Lung Cancer Cells. Tuberc. Respir. Dis. 2013, 75, 9–17. [Google Scholar] [CrossRef][Green Version]
- Knott, S.R.V.; Wagenblast, E.; Khan, S.; Kim, S.Y.; Soto, M.; Wagner, M.; Turgeon, M.O.; Fish, L.; Erard, N.; Gable, A.L.; et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 2018, 554, 378–381. [Google Scholar] [CrossRef]
- Heo, Y.A.; Syed, Y.Y.; Keam, S.J. Pegaspargase: A Review in Acute Lymphoblastic Leukaemia. Drugs 2019, 79, 767–777. [Google Scholar] [CrossRef]
- Takahashi, H.; Inoue, J.; Sakaguchi, K.; Takagi, M.; Mizutani, S.; Inazawa, J. Autophagy is required for cell survival under L-asparaginase-induced metabolic stress in acute lymphoblastic leukemia cells. Oncogene 2017, 36, 4267–4276. [Google Scholar] [CrossRef]
- Li, H.; Meng, X.X.; Zhang, L.; Zhang, B.J.; Liu, X.Y.; Fu, W.W.; Tan, H.S.; Lao, Y.Z.; Xu, H.X. Oblongifolin C and guttiferone K extracted from Garcinia yunnanensis fruit synergistically induce apoptosis in human colorectal cancer cells in vitro. Acta Pharmacol. Sin. 2017, 38, 252–263. [Google Scholar] [CrossRef]
- Feng, C.; Zhou, L.Y.; Yu, T.; Xu, G.; Tian, H.L.; Xu, J.J.; Xu, H.X.; Luo, K.Q. A new anticancer compound, oblongifolin C, inhibits tumor growth and promotes apoptosis in HeLa cells through Bax activation. Int. J. Cancer 2012, 131, 1445–1454. [Google Scholar] [CrossRef]
- Lao, Y.; Wan, G.; Liu, Z.; Wang, X.; Ruan, P.; Xu, W.; Xu, D.; Xie, W.; Zhang, Y.; Xu, H.; et al. The natural compound oblongifolin C inhibits autophagic flux and enhances antitumor efficacy of nutrient deprivation. Autophagy 2014, 10, 736–749. [Google Scholar] [CrossRef]
- Wu, M.; Lao, Y.; Xu, N.; Wang, X.; Tan, H.; Fu, W.; Lin, Z.; Xu, H. Guttiferone K induces autophagy and sensitizes cancer cells to nutrient stress-induced cell death. Phytomedicine 2015, 22, 902–910. [Google Scholar] [CrossRef]
- Kan, W.L.; Yin, C.; Xu, H.X.; Xu, G.; To, K.K.; Cho, C.H.; Rudd, J.A.; Lin, G. Antitumor effects of novel compound, guttiferone K, on colon cancer by p21Waf1/Cip1-mediated G(0)/G(1) cell cycle arrest and apoptosis. Int. J. Cancer 2013, 132, 707–716. [Google Scholar] [CrossRef]
- Renna, M.; Schaffner, C.; Brown, K.; Shang, S.; Tamayo, M.H.; Hegyi, K.; Grimsey, N.J.; Cusens, D.; Coulter, S.; Cooper, J.; et al. Azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection. J. Clin. Investig. 2011, 121, 3554–3563. [Google Scholar] [CrossRef]
- Nakamura, M.; Kikukawa, Y.; Takeya, M.; Mitsuya, H.; Hata, H. Clarithromycin attenuates autophagy in myeloma cells. Int. J. Oncol. 2010, 37, 815–820. [Google Scholar]
- Wang, Z.; Zhang, J.; Wang, Y.; Xing, R.; Yi, C.; Zhu, H.; Chen, X.; Guo, J.; Guo, W.; Li, W.; et al. Matrine, a novel autophagy inhibitor, blocks trafficking and the proteolytic activation of lysosomal proteases. Carcinogenesis 2013, 34, 128–138. [Google Scholar] [CrossRef]
- Xie, S.B.; He, X.X.; Yao, S.K. Matrine-induced autophagy regulated by p53 through AMP-activated protein kinase in human hepatoma cells. Int. J. Oncol. 2015, 47, 517–526. [Google Scholar] [CrossRef]
- Zou, Y.; Sarem, M.; Xiang, S.; Hu, H.; Xu, W.; Shastri, V.P. Autophagy inhibition enhances Matrine derivative MASM induced apoptosis in cancer cells via a mechanism involving reactive oxygen species-mediated PI3K/Akt/mTOR and Erk/p38 signaling. BMC Cancer 2019, 19, 949. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Fang, Y.; Yang, Y.; Qin, Y.; Wu, P.; Wang, T.; Lai, H.; Meng, L.; Wang, D.; Zheng, Z.; et al. Elaiophylin, a novel autophagy inhibitor, exerts antitumor activity as a single agent in ovarian cancer cells. Autophagy 2015, 11, 1849–1863. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, P.; Chen, X.; Zhao, L.; Tan, J.; Yang, Y.; Fang, Y.; Zhou, J. The novel autophagy inhibitor elaiophylin exerts antitumor activity against multiple myeloma with mutant TP53 in part through endoplasmic reticulum stress-induced apoptosis. Cancer Biol. Ther. 2017, 18, 584–595. [Google Scholar] [CrossRef]
- Pinto, M.M.M.; Palmeira, A.; Fernandes, C.; Resende, D.; Sousa, E.; Cidade, H.; Tiritan, M.E.; Correia-da-Silva, M.; Cravo, S. From Natural Products to New Synthetic Small Molecules: A Journey through the World of Xanthones. Molecules 2021, 26, 431. [Google Scholar] [CrossRef]
- Mattsson, J.P.; Vaananen, K.; Wallmark, B.; Lorentzon, P. Omeprazole and bafilomycin, two proton pump inhibitors: Differentiation of their effects on gastric, kidney and bone H+-translocating ATPases. Biochim. Biophys. Acta 1991, 1065, 261–268. [Google Scholar] [CrossRef]
- Yuan, N.; Song, L.; Zhang, S.; Lin, W.; Cao, Y.; Xu, F.; Fang, Y.; Wang, Z.; Zhang, H.; Li, X.; et al. Bafilomycin A1 targets both autophagy and apoptosis pathways in pediatric B-cell acute lymphoblastic leukemia. Haematologica 2015, 100, 345–356. [Google Scholar] [CrossRef]
- Yamamoto, A.; Tagawa, Y.; Yoshimori, T.; Moriyama, Y.; Masaki, R.; Tashiro, Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell. Struct. Funct. 1998, 23, 33–42. [Google Scholar] [CrossRef]
- Huss, M.; Ingenhorst, G.; Konig, S.; Gassel, M.; Drose, S.; Zeeck, A.; Altendorf, K.; Wieczorek, H. Concanamycin A, the specific inhibitor of V-ATPases, binds to the V(o) subunit c. J. Biol. Chem. 2002, 277, 40544–40548. [Google Scholar] [CrossRef]
- Kallifatidis, G.; Hoepfner, D.; Jaeg, T.; Guzman, E.A.; Wright, A.E. The marine natural product manzamine A targets vacuolar ATPases and inhibits autophagy in pancreatic cancer cells. Mar. Drugs 2013, 11, 3500–3516. [Google Scholar] [CrossRef]
- Guzman, E.A.; Johnson, J.D.; Linley, P.A.; Gunasekera, S.E.; Wright, A.E. A novel activity from an old compound: Manzamine A reduces the metastatic potential of AsPC-1 pancreatic cancer cells and sensitizes them to TRAIL-induced apoptosis. Investig. New Drugs 2011, 29, 777–785. [Google Scholar] [CrossRef]
- Chen, C.; Lu, L.; Yan, S.; Yi, H.; Yao, H.; Wu, D.; He, G.; Tao, X.; Deng, X. Autophagy and doxorubicin resistance in cancer. Anticancer Drugs 2018, 29, 1–9. [Google Scholar] [CrossRef]
- Shabalala, S.; Muller, C.J.F.; Louw, J.; Johnson, R. Polyphenols, autophagy and doxorubicin-induced cardiotoxicity. Life Sci. 2017, 180, 160–170. [Google Scholar] [CrossRef]
- Piao, S.; Amaravadi, R.K. Targeting the lysosome in cancer. Ann. N. Y. Acad. Sci. 2016, 1371, 45–54. [Google Scholar] [CrossRef]
- Haspel, J.; Shaik, R.S.; Ifedigbo, E.; Nakahira, K.; Dolinay, T.; Englert, J.A.; Choi, A.M. Characterization of macroautophagic flux in vivo using a leupeptin-based assay. Autophagy 2011, 7, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.T.; Sousa, D.; Paiva, A.M.; Palmeira, A.; Barbosa, J.; Pedro, M.; Pinto, M.M.; Sousa, E.; Vasconcelos, M.H. Modulation of Autophagy by a Thioxanthone Decreases the Viability of Melanoma Cells. Molecules 2016, 21, 1343. [Google Scholar] [CrossRef]
- Madeo, F.; Zimmermann, A.; Maiuri, M.C.; Kroemer, G. Essential role for autophagy in life span extension. J. Clin. Investig. 2015, 125, 85–93. [Google Scholar] [CrossRef]
- Li, J.; Kim, S.G.; Blenis, J. Rapamycin: One drug, many effects. Cell. Metab. 2014, 19, 373–379. [Google Scholar] [CrossRef]
- Lamming, D.W.; Ye, L.; Sabatini, D.M.; Baur, J.A. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J. Clin. Investig. 2013, 123, 980–989. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.L.; Xu, Q.Q.; Cheng, D.; Liu, K.D.; Sun, Z.Q. Quercetin inhibits invasion and angiogenesis of esophageal cancer cells. Pathol. Res. Pract. 2021, 222, 153455. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Alsahli, M.A.; Almatroudi, A.; Verma, A.K.; Aloliqi, A.; Allemailem, K.S.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic Targets of Quercetin, a Plant Flavonol, and Its Role in the Therapy of Various Types of Cancer through the Modulation of Various Cell Signaling Pathways. Molecules 2021, 26, 1315. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Tavakol, S.; Ahmadi, Z.; Roomiani, S.; Mohammadinejad, R.; Samarghandian, S. Therapeutic effects of kaempferol affecting autophagy and endoplasmic reticulum stress. Phytother. Res. 2020, 34, 911–923. [Google Scholar] [CrossRef]
- Sameiyan, E.; Hayes, A.W.; Karimi, G. The effect of medicinal plants on multiple drug resistance through autophagy: A review of in vitro studies. Eur. J. Pharmacol. 2019, 852, 244–253. [Google Scholar] [CrossRef]
- Yang, J.; Pi, C.; Wang, G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed. Pharmacother. 2018, 103, 699–707. [Google Scholar] [CrossRef]
- Fujiki, H.; Watanabe, T.; Sueoka, E.; Rawangkan, A.; Suganuma, M. Cancer Prevention with Green Tea and Its Principal Constituent, EGCG: From Early Investigations to Current Focus on Human Cancer Stem Cells. Mol. Cells 2018, 41, 73–82. [Google Scholar]
- Holczer, M.; Besze, B.; Zambo, V.; Csala, M.; Banhegyi, G.; Kapuy, O. Epigallocatechin-3-Gallate (EGCG) Promotes Autophagy-Dependent Survival via Influencing the Balance of mTOR-AMPK Pathways upon Endoplasmic Reticulum Stress. Oxid. Med. Cell Longev. 2018, 2018, 6721530. [Google Scholar] [CrossRef] [PubMed]
- Prietsch, R.F.; Monte, L.G.; da Silva, F.A.; Beira, F.T.; Del Pino, F.A.; Campos, V.F.; Collares, T.; Pinto, L.S.; Spanevello, R.M.; Gamaro, G.D.; et al. Genistein induces apoptosis and autophagy in human breast MCF-7 cells by modulating the expression of proapoptotic factors and oxidative stress enzymes. Mol. Cell. Biochem. 2014, 390, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Kang, Y.; Li, X.; Roife, D.; Zhang, R.; Fleming, J.B. Genistein potentiates the antitumor effect of 5-Fluorouracil by inducing apoptosis and autophagy in human pancreatic cancer cells. Anticancer Res. 2014, 34, 4685–4692. [Google Scholar]
- Wedel, S.; Manola, M.; Cavinato, M.; Trougakos, I.P.; Jansen-Durr, P. Targeting Protein Quality Control Mechanisms by Natural Products to Promote Healthy Ageing. Molecules 2018, 23, 1219. [Google Scholar] [CrossRef]
- Hsiao, Y.T.; Kuo, C.L.; Chueh, F.S.; Liu, K.C.; Bau, D.T.; Chung, J.G. Curcuminoids Induce Reactive Oxygen Species and Autophagy to Enhance Apoptosis in Human Oral Cancer Cells. Am. J. Chin. Med. 2018, 46, 1145–1168. [Google Scholar] [CrossRef] [PubMed]
- Hashemzaei, M.; Entezari Heravi, R.; Rezaee, R.; Roohbakhsh, A.; Karimi, G. Regulation of autophagy by some natural products as a potential therapeutic strategy for cardiovascular disorders. Eur. J. Pharmacol. 2017, 802, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Shanmugam, M.K.; Kumar, A.P.; Yap, C.T.; Sethi, G.; Bishayee, A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer 2019, 125, 1228–1246. [Google Scholar] [CrossRef]
- Xiaokaiti, Y.; Li, X. Natural Product Regulates Autophagy in Cancer. Adv. Exp. Med. Biol. 2020, 1207, 709–724. [Google Scholar]
- Zhang, S.F.; Wang, X.L.; Yang, X.Q.; Chen, N. Autophagy-associated targeting pathways of natural products during cancer treatment. Asian Pac. J. Cancer Prev. 2014, 15, 10557–10563. [Google Scholar] [CrossRef]
- Huang, X.M.; Yang, Z.J.; Xie, Q.; Zhang, Z.K.; Zhang, H.; Ma, J.Y. Natural products for treating colorectal cancer: A mechanistic review. Biomed. Pharmacother. 2019, 117, 109142. [Google Scholar] [CrossRef]
- Wang, S.F.; Wu, M.Y.; Cai, C.Z.; Li, M.; Lu, J.H. Autophagy modulators from traditional Chinese medicine: Mechanisms and therapeutic potentials for cancer and neurodegenerative diseases. J. Ethnopharmacol. 2016, 194, 861–876. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.X.; Ouyang, L.; Cheng, Y.; Liu, B. Plant natural compounds: Targeting pathways of autophagy as anti-cancer therapeutic agents. Cell Prolif. 2012, 45, 466–476. [Google Scholar] [CrossRef]
- Endo, S.; Hoshi, M.; Matsunaga, T.; Inoue, T.; Ichihara, K.; Ikari, A. Autophagy inhibition enhances anticancer efficacy of artepillin C, a cinnamic acid derivative in Brazilian green propolis. Biochem. Biophys. Res. Commun. 2018, 497, 437–443. [Google Scholar] [CrossRef]
- Park, B.S.; Choi, N.E.; Lee, J.H.; Kang, H.M.; Yu, S.B.; Kim, H.J.; Kang, H.K.; Kim, I.R. Crosstalk between Fisetin-induced Apoptosis and Autophagy in Human Oral Squamous Cell Carcinoma. J. Cancer 2019, 10, 138–146. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, H.J.; Kim, E.J.; Hwang, W.Y.; Jung, M.H.; Kim, K.S. Fisetin induces apoptosis in uterine leiomyomas through multiple pathways. Sci. Rep. 2020, 10, 7993. [Google Scholar] [CrossRef]
- Jia, S.; Xu, X.; Zhou, S.; Chen, Y.; Ding, G.; Cao, L. Fisetin induces autophagy in pancreatic cancer cells via endoplasmic reticulum stress- and mitochondrial stress-dependent pathways. Cell Death Dis. 2019, 10, 142. [Google Scholar] [CrossRef]
- Liu, X.; Duan, C.; Ji, J.; Zhang, T.; Yuan, X.; Zhang, Y.; Ma, W.; Yang, J.; Yang, L.; Jiang, Z.; et al. Cucurbitacin B induces autophagy and apoptosis by suppressing CIP2A/PP2A/mTORC1 signaling axis in human cisplatin resistant gastric cancer cells. Oncol. Rep. 2017, 38, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Sha, T.; Guo, J.; Li, W.; Lu, J.; Chen, X. Cucurbitacin B induces DNA damage and autophagy mediated by reactive oxygen species (ROS) in MCF-7 breast cancer cells. J. Nat. Med. 2015, 69, 522–530. [Google Scholar] [CrossRef]
- Tan, H.; Li, X.; Yang, W.H.; Kang, Y. A flavone, Wogonin from Scutellaria baicalensis inhibits the proliferation of human colorectal cancer cells by inducing of autophagy, apoptosis and G2/M cell cycle arrest via modulating the PI3K/AKT and STAT3 signalling pathways. J. BUON 2019, 24, 1143–1149. [Google Scholar]
- Feng, Q.; Wang, H.; Pang, J.; Ji, L.; Han, J.; Wang, Y.; Qi, X.; Liu, Z.; Lu, L. Prevention of Wogonin on Colorectal Cancer Tumorigenesis by Regulating p53 Nuclear Translocation. Front. Pharmacol. 2018, 9, 1356. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Park, S.H. Induction of cytoprotective autophagy by morusin via AMP-activated protein kinase activation in human non-small cell lung cancer cells. Nutr. Res. Pract. 2020, 14, 478–489. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zheng, H.; Liu, Q.; Zhang, H.; Wang, X.; Shen, T.; Wang, S.; Ren, D. Morusin induces apoptosis and autophagy via JNK, ERK and PI3K/Akt signaling in human lung carcinoma cells. Chem. Biol. Interact. 2020, 331, 109279. [Google Scholar] [CrossRef]
- Singh, B.N.; Kumar, D.; Shankar, S.; Srivastava, R.K. Rottlerin induces autophagy which leads to apoptotic cell death through inhibition of PI3K/Akt/mTOR pathway in human pancreatic cancer stem cells. Biochem. Pharmacol. 2012, 84, 1154–1163. [Google Scholar] [CrossRef]
- Kumar, D.; Shankar, S.; Srivastava, R.K. Rottlerin induces autophagy and apoptosis in prostate cancer stem cells via PI3K/Akt/mTOR signaling pathway. Cancer Lett. 2014, 343, 179–189. [Google Scholar] [CrossRef]
- Maioli, E.; Daveri, E.; Maellaro, E.; Ietta, F.; Cresti, L.; Valacchi, G. Non-conventional rottlerin anticancer properties. Arch. Biochem. Biophys. 2018, 645, 50–53. [Google Scholar] [CrossRef]
- Mosca, L.; Ilari, A.; Fazi, F.; Assaraf, Y.G.; Colotti, G. Taxanes in cancer treatment: Activity, chemoresistance and its overcoming. Drug Resist. Update 2021, 54, 100742. [Google Scholar] [CrossRef]
- Zou, S.H.; Du, X.; Lin, H.; Wang, P.C.; Li, M. Paclitaxel inhibits the progression of cervical cancer by inhibiting autophagy via lncRNARP11-381N20.2. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3010–3017. [Google Scholar] [PubMed]
- Tiwari, R.V.; Parajuli, P.; Sylvester, P.W. Synergistic anticancer effects of combined gamma-tocotrienol and oridonin treatment is associated with the induction of autophagy. Mol. Cell Biochem. 2015, 408, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, H.; Wu, R.; Chen, Z.Y.; Hu, Q.; Zhang, Y.F.; Gao, S.H.; Zhou, G.B. Autophagy inhibition enhances the inhibitory effects of ursolic acid on lung cancer cells. Int. J. Mol. Med. 2020, 46, 1816–1826. [Google Scholar] [CrossRef]
- Lin, C.W.; Chin, H.K.; Lee, S.L.; Chiu, C.F.; Chung, J.G.; Lin, Z.Y.; Wu, C.Y.; Liu, Y.C.; Hsiao, Y.T.; Feng, C.H.; et al. Ursolic acid induces apoptosis and autophagy in oral cancer cells. Environ. Toxicol. 2019, 34, 983–991. [Google Scholar] [CrossRef]
- Zhang, R.; Pan, T.; Xiang, Y.; Zhang, M.; Feng, J.; Liu, S.; Duan, T.; Chen, P.; Zhai, B.; Chen, X.; et al. beta-Elemene Reverses the Resistance of p53-Deficient Colorectal Cancer Cells to 5-Fluorouracil by Inducing Pro-death Autophagy and Cyclin D3-Dependent Cycle Arrest. Front. Bioeng. Biotechnol. 2020, 8, 378. [Google Scholar] [CrossRef]
- Liu, S.; Li, Q.; Li, G.; Zhang, Q.; Zhuo, L.; Han, X.; Zhang, M.; Chen, X.; Pan, T.; Yan, L.; et al. The mechanism of m(6)A methyltransferase METTL3-mediated autophagy in reversing gefitinib resistance in NSCLC cells by beta-elemene. Cell Death Dis. 2020, 11, 969. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, J.; Wu, J.; Huang, N.; Cui, Z.; Luo, Y.; Sun, F.; Pan, Q.; Li, Y.; Yang, Q. (−)-Guaiol regulates autophagic cell death depending on mTOR signaling in NSCLC. Cancer Biol. Ther. 2018, 19, 706–714. [Google Scholar] [CrossRef]
- Tian, S.; Chen, Y.; Yang, B.; Lou, C.; Zhu, R.; Zhao, Y.; Zhao, H. F1012-2 inhibits the growth of triple negative breast cancer through induction of cell cycle arrest, apoptosis, and autophagy. Phytother. Res. 2018, 32, 908–922. [Google Scholar] [CrossRef]
- Jiang, S.L.; Guan, Y.D.; Chen, X.S.; Ge, P.; Wang, X.L.; Lao, Y.Z.; Xiao, S.S.; Zhang, Y.; Yang, J.M.; Xu, X.J.; et al. Tubeimoside-1, a triterpenoid saponin, induces cytoprotective autophagy in human breast cancer cells in vitro via Akt-mediated pathway. Acta Pharmacol. Sin. 2019, 40, 919–928. [Google Scholar] [CrossRef]
- Islam, M.S.; Wang, C.; Zheng, J.; Paudyal, N.; Zhu, Y.; Sun, H. The potential role of tubeimosides in cancer prevention and treatment. Eur. J. Med. Chem. 2019, 162, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; You, L.; Qiu, Y.; Cui, X.; Wu, D. Tubeimoside I induces autophagy in HepG2 cells by activating the AMP-activated protein kinase signaling pathway. Oncol. Lett. 2020, 20, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.T.; Uen, W.C.; Choong, C.Y.; Shi, Y.C.; Lee, B.H.; Tai, C.J.; Tai, C.J. Paris Polyphylla Inhibits Colorectal Cancer Cells via Inducing Autophagy and Enhancing the Efficacy of Chemotherapeutic Drug Doxorubicin. Molecules 2019, 24, 2102. [Google Scholar] [CrossRef]
- Chen, M.; Guo, Y.; Zhao, R.; Wang, X.; Jiang, M.; Fu, H.; Zhang, X. Ophiopogonin B induces apoptosis, mitotic catastrophe and autophagy in A549 cells. Int. J. Oncol. 2016, 49, 316–324. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.; Chen, Y. Betulinic acid and the pharmacological effects of tumor suppression (Review). Mol. Med. Rep. 2016, 14, 4489–4495. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, S.; Qu, Z.; Luo, Y.; Chen, R.; Wei, S.; Yang, X.; Wang, Q. Betulinic acid induces autophagy-mediated apoptosis through suppression of the PI3K/AKT/mTOR signaling pathway and inhibits hepatocellular carcinoma. Am. J. Transl. Res. 2019, 11, 6952–6964. [Google Scholar] [PubMed]
- Wang, S.; Wang, K.; Zhang, C.; Zhang, W.; Xu, Q.; Wang, Y.; Zhang, Y.; Li, Y.; Zhang, Y.; Zhu, H.; et al. Overaccumulation of p53-mediated autophagy protects against betulinic acid-induced apoptotic cell death in colorectal cancer cells. Cell Death Dis. 2017, 8, e3087. [Google Scholar] [CrossRef]
- Hua, F.; Shang, S.; Hu, Z.W. Seeking new anti-cancer agents from autophagy-regulating natural products. J. Asian Nat. Prod. Res. 2017, 19, 305–313. [Google Scholar] [CrossRef]
- Jayasooriya, R.; Dilshara, M.G.; Karunarathne, W.; Molagoda, I.M.N.; Choi, Y.H.; Kim, G.Y. Camptothecin enhances c-Myc-mediated endoplasmic reticulum stress and leads to autophagy by activating Ca2+-mediated AMPK. Food Chem. Toxicol. 2018, 121, 648–656. [Google Scholar] [CrossRef]
- Heng, Y.; Liang, Y.; Zhang, J.; Li, L.; Zhang, W.; Jiang, Y.; Wang, S.; Jia, L. Camptothecin Inhibits Neddylation to Activate the Protective Autophagy Through NF-kappaB/AMPK/mTOR/ULK1 Axis in Human Esophageal Cancer Cells. Front. Oncol. 2021, 11, 671180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, X.; Cao, S.; Sun, Y.; He, X.; Jiang, B.; Yu, Y.; Duan, J.; Qiu, F.; Kang, N. Berberine represses human gastric cancer cell growth in vitro and in vivo by inducing cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and Akt signaling pathways. Biomed. Pharmacother. 2020, 128, 110245. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Du, X.; Ma, H.; Yao, J. The Anti-Cancer Mechanisms of Berberine: A Review. Cancer Manag. Res. 2020, 12, 695–702. [Google Scholar] [CrossRef]
- Lopes, T.Z.; de Moraes, F.R.; Tedesco, A.C.; Arni, R.K.; Rahal, P.; Calmon, M.F. Berberine associated photodynamic therapy promotes autophagy and apoptosis via ROS generation in renal carcinoma cells. Biomed. Pharmacother. 2020, 123, 109794. [Google Scholar] [CrossRef]
- Luan, F.; He, X.; Zeng, N. Tetrandrine: A review of its anticancer potentials, clinical settings, pharmacokinetics and drug delivery systems. J. Pharm. Pharmacol. 2020, 72, 1491–1512. [Google Scholar] [CrossRef]
- Wang, J.; Yao, Z.; Lai, X.; Bao, H.; Li, Y.; Li, S.; Chang, L.; Zhang, G. Tetrandrine sensitizes nasopharyngeal carcinoma cells to irradiation by inducing autophagy and inhibiting MEK/ERK pathway. Cancer Med. 2020, 9, 7268–7278. [Google Scholar] [CrossRef]
- Guo, Y.; Pei, X. Tetrandrine-Induced Autophagy in MDA-MB-231 Triple-Negative Breast Cancer Cell through the Inhibition of PI3K/AKT/mTOR Signaling. Evid. Based Complement. Alternat. Med. 2019, 2019, 7517431. [Google Scholar] [CrossRef]
- Son, Y.; An, Y.; Jung, J.; Shin, S.; Park, I.; Gwak, J.; Ju, B.G.; Chung, Y.H.; Na, M.; Oh, S. Protopine isolated from Nandina domestica induces apoptosis and autophagy in colon cancer cells by stabilizing p53. Phytother Res. 2019, 33, 1689–1696. [Google Scholar] [CrossRef]
- Marthandam Asokan, S.; Mariappan, R.; Muthusamy, S.; Velmurugan, B.K. Pharmacological benefits of neferine—A comprehensive review. Life Sci. 2018, 199, 60–70. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Li, Y.; Lu, S.; Lu, S.; Li, J.; Wang, Y.; Tian, X.; Wei, J.J.; Shao, C.; et al. Neferine induces autophagy of human ovarian cancer cells via p38 MAPK/ JNK activation. Tumour Biol. 2016, 37, 8721–8729. [Google Scholar] [CrossRef] [PubMed]
- Law, B.Y.K.; Michelangeli, F.; Qu, Y.Q.; Xu, S.W.; Han, Y.; Mok, S.W.F.; Dias, I.; Javed, M.U.; Chan, W.K.; Xue, W.W.; et al. Neferine induces autophagy-dependent cell death in apoptosis-resistant cancers via ryanodine receptor and Ca2+-dependent mechanism. Sci. Rep. 2019, 9, 20034. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bishayee, K.; Khuda-Bukhsh, A.R. Graveoline isolated from ethanolic extract of Ruta graveolens triggers apoptosis and autophagy in skin melanoma cells: A novel apoptosis-independent autophagic signaling pathway. Phytother Res. 2014, 28, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, A.G.M.; Hossain, M.K.; Basak, D.; Bin Sayeed, M.S. Thymoquinone as a Potential Adjuvant Therapy for Cancer Treatment: Evidence from Preclinical Studies. Front. Pharmacol. 2017, 8, 295. [Google Scholar] [CrossRef]
- Almajali, B.; Al-Jamal, H.A.N.; Taib, W.R.W.; Ismail, I.; Johan, M.F.; Doolaanea, A.A.; Ibrahim, W.N. Thymoquinone, as a Novel Therapeutic Candidate of Cancers. Pharmaceuticals 2021, 14, 369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, Y.; Huang, S.; Wang, G.; Han, R.; Lei, F.; Luo, A.; Jing, X.; Zhao, L.; Gu, S.; et al. Thymoquinone inhibits the metastasis of renal cell cancer cells by inducing autophagy via AMPK/mTOR signaling pathway. Cancer Sci. 2018, 109, 3865–3873. [Google Scholar] [CrossRef]
- Bashmail, H.A.; Alamoudi, A.A.; Noorwali, A.; Hegazy, G.; Ajabnoor, G.; Choudhry, H.; Al-Abd, A.M. Thymoquinone synergizes gemcitabine anti-breast cancer activity via modulating its apoptotic and autophagic activities. Sci. Rep. 2018, 8, 11674. [Google Scholar] [CrossRef]
- Ren, B.; Liu, H.; Gao, H.; Liu, S.; Zhang, Z.; Fribley, A.M.; Callaghan, M.U.; Xu, Z.; Zeng, Q.; Li, Y. Celastrol induces apoptosis in hepatocellular carcinoma cells via targeting ER-stress/UPR. Oncotarget 2017, 8, 93039–93050. [Google Scholar] [CrossRef]
- Chen, Y.; Ou, Y.; Tao, Y.; Liu, H.; Yin, H.; Zhong, S.; Yu, H.; Zhao, Z.; He, B. Effect and mechanisms of celastrol on the apoptosis of HOS osteosarcoma cells. Oncol. Rep. 2018, 40, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Zhang, J.; Sun, L.L.; Li, B.H.; Gao, H.L.; Xie, T.; Zhang, N.; Ye, Z.M. Celastrol induces apoptosis and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells: An in vitro and in vivo study. Cell Death Dis. 2015, 6, e1604. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, Y.; Zhong, J.; Bi, Y.; Liu, Y.; Ren, Z.; Li, X.; Jia, J.; Yu, M.; Yu, X. Pristimerin induces apoptosis and autophagy via activation of ROS/ASK1/JNK pathway in human breast cancer in vitro and in vivo. Cell Death Discov. 2019, 5, 125. [Google Scholar] [CrossRef]
- Li, J.J.; Yan, Y.Y.; Sun, H.M.; Liu, Y.; Su, C.Y.; Chen, H.B.; Zhang, J.Y. Anti-Cancer Effects of Pristimerin and the Mechanisms: A Critical Review. Front. Pharmacol. 2019, 10, 746. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, J.; Chen, L.; Guo, Q.; Yang, B.; Zhang, W.; Kang, W. Anticancer Effects and Mechanisms of Action of Plumbagin: Review of Research Advances. Biomed. Res. Int. 2020, 2020, 6940953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kan, H.; Liu, Y.; Ding, W. Plumbagin induces Ishikawa cell cycle arrest, autophagy, and apoptosis via the PI3K/Akt signaling pathway in endometrial cancer. Food Chem. Toxicol. 2021, 148, 111957. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, Y.; Wang, S.; Ma, J.; Peng, Y.; Yuan, X.; Lv, B.; Chen, W.; Wei, Y. Plumbagin induces autophagy and apoptosis of SMMC-7721 cells in vitro and in vivo. J. Cell Biochem. 2019, 120, 9820–9830. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Pan, S.T.; Zhou, X.; Ye, F.; Zhou, Q.; Shi, F.; He, F.; Yu, H.; Qiu, J. Plumbagin Enhances the Anticancer Efficacy of Cisplatin by Increasing Intracellular ROS in Human Tongue Squamous Cell Carcinoma. Oxid. Med. Cell. Longev. 2020, 2020, 5649174. [Google Scholar] [CrossRef]
- Zhang, J.; Ng, S.; Wang, J.; Zhou, J.; Tan, S.H.; Yang, N.; Lin, Q.; Xia, D.; Shen, H.M. Histone deacetylase inhibitors induce autophagy through FOXO1-dependent pathways. Autophagy 2015, 11, 629–642. [Google Scholar] [CrossRef]
- Gilardini Montani, M.S.; Granato, M.; Santoni, C.; Del Porto, P.; Merendino, N.; D’Orazi, G.; Faggioni, A.; Cirone, M. Histone deacetylase inhibitors VPA and TSA induce apoptosis and autophagy in pancreatic cancer cells. Cell Oncol. 2017, 40, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Cao, Y.M.; Rong, Z.P.; Ding, J.; Pan, X. Trichostatin A Induces Autophagy in Cervical Cancer Cells by Regulating the PRMT5-STC1-TRPV6-JNK Pathway. Pharmacology 2021, 106, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Jing, K.; Song, K.S.; Shin, S.; Kim, N.; Jeong, S.; Oh, H.R.; Park, J.H.; Seo, K.S.; Heo, J.Y.; Han, J.; et al. Docosahexaenoic acid induces autophagy through p53/AMPK/mTOR signaling and promotes apoptosis in human cancer cells harboring wild-type p53. Autophagy 2011, 7, 1348–1358. [Google Scholar] [CrossRef]
- Shin, S.; Jing, K.; Jeong, S.; Kim, N.; Song, K.S.; Heo, J.Y.; Park, J.H.; Seo, K.S.; Han, J.; Park, J.I.; et al. The omega-3 polyunsaturated fatty acid DHA induces simultaneous apoptosis and autophagy via mitochondrial ROS-mediated Akt-mTOR signaling in prostate cancer cells expressing mutant p53. Biomed. Res. Int. 2013, 2013, 568671. [Google Scholar] [CrossRef]
- Kim, N.; Jeong, S.; Jing, K.; Shin, S.; Kim, S.; Heo, J.Y.; Kweon, G.R.; Park, S.K.; Wu, T.; Park, J.I.; et al. Docosahexaenoic Acid Induces Cell Death in Human Non-Small Cell Lung Cancer Cells by Repressing mTOR via AMPK Activation and PI3K/Akt Inhibition. Biomed. Res. Int. 2015, 2015, 239764. [Google Scholar] [CrossRef]
- Farkas, T.; Daugaard, M.; Jaattela, M. Identification of small molecule inhibitors of phosphatidylinositol 3-kinase and autophagy. J. Biol. Chem. 2011, 286, 38904–38912. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, N.; Wu, J.; Dai, W.; Rosenblum, J.S. Polo-like kinases inhibited by wortmannin. Labeling site and downstream effects. J. Biol. Chem. 2007, 282, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Rao, S.; Tokuno, O.; Yamamoto, K.; Takata, M.; Takeda, S.; Utsumi, H. DNA-PK: The major target for wortmannin-mediated radiosensitization by the inhibition of DSB repair via NHEJ pathway. J. Radiat. Res. 2003, 44, 151–159. [Google Scholar] [CrossRef][Green Version]
- Sarkaria, J.N.; Tibbetts, R.S.; Busby, E.C.; Kennedy, A.P.; Hill, D.E.; Abraham, R.T. Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res. 1998, 58, 4375–4382. [Google Scholar] [PubMed]
- Bain, J.; Plater, L.; Elliott, M.; Shpiro, N.; Hastie, C.J.; McLauchlan, H.; Klevernic, I.; Arthur, J.S.; Alessi, D.R.; Cohen, P. The selectivity of protein kinase inhibitors: A further update. Biochem. J. 2007, 408, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Tan, H.L.; Shui, G.; Bauvy, C.; Huang, Q.; Wenk, M.R.; Ong, C.N.; Codogno, P.; Shen, H.M. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J. Biol. Chem. 2010, 285, 10850–10861. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Cuervo, A.M.; Seglen, P.O. Methods for monitoring autophagy from yeast to human. Autophagy 2007, 3, 181–206. [Google Scholar] [CrossRef]
- Karve, S.; Werner, M.E.; Sukumar, R.; Cummings, N.D.; Copp, J.A.; Wang, E.C.; Li, C.; Sethi, M.; Chen, R.C.; Pacold, M.E.; et al. Revival of the abandoned therapeutic wortmannin by nanoparticle drug delivery. Proc. Natl. Acad. Sci. USA 2012, 109, 8230–8235. [Google Scholar] [CrossRef]
- Gu, W.; Lin, Y.; Gou, X.; He, W. Tea Polyphenol inhibits autophagy to sensitize Epirubicin-induced apoptosis in human bladder cancer cells. Neoplasma 2017, 64, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, H.; Qaed, E.; Zhang, J.; Song, Y.; Wu, R.; Bu, X.; Wang, Q.; Tang, Z. Salinomycin, as an autophagy modulator—A new avenue to anticancer: A review. J. Exp. Clin. Cancer Res. 2018, 37, 26. [Google Scholar] [CrossRef]
- Zhou, J.; Li, G.; Zheng, Y.; Shen, H.M.; Hu, X.; Ming, Q.L.; Huang, C.; Li, P.; Gao, N. A novel autophagy/mitophagy inhibitor liensinine sensitizes breast cancer cells to chemotherapy through DNM1L-mediated mitochondrial fission. Autophagy 2015, 11, 1259–1279. [Google Scholar] [CrossRef]
- Liu, C.M.; Wu, Z.; Pan, B.; An, L.; Zhu, C.; Zhou, J.; Jiang, Y. The antiandrogenic effect of neferine, liensinine, and isoliensinine by inhibiting 5-alpha-reductase and androgen receptor expression via PI3K/AKT signaling pathway in prostate cancer. Pharmazie 2021, 76, 225–231. [Google Scholar] [PubMed]
- Al-Bari, M.A. Chloroquine analogues in drug discovery: New directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J. Antimicrob. Chemother. 2015, 70, 1608–1621. [Google Scholar] [CrossRef]
- Collins, M.P.; Forgac, M. Regulation and function of V-ATPases in physiology and disease. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183341. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.K.; Pennypacker, J.K. Drugs that modulate aging: The promising yet difficult path ahead. Transl. Res. 2014, 163, 456–465. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Gao, Q.; Yang, J.; Yan, X.; Zhao, H.; Su, L.; Yang, M.; Gao, C.; Yao, Y.; et al. Rapamycin directly activates lysosomal mucolipin TRP channels independent of mTOR. PLoS Biol. 2019, 17, e3000252. [Google Scholar] [CrossRef]
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef]
- Yessenkyzy, A.; Saliev, T.; Zhanaliyeva, M.; Masoud, A.R.; Umbayev, B.; Sergazy, S.; Krivykh, E.; Gulyayev, A.; Nurgozhin, T. Polyphenols as Caloric-Restriction Mimetics and Autophagy Inducers in Aging Research. Nutrients 2020, 12, 1344. [Google Scholar] [CrossRef]
- Messeha, S.S.; Zarmouh, N.O.; Soliman, K.F.A. Polyphenols Modulating Effects of PD-L1/PD-1 Checkpoint and EMT-Mediated PD-L1 Overexpression in Breast Cancer. Nutrients 2021, 13, 1718. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.; Xu, S.; Nabavi, S.M.; Amirkhani, M.A.; Sureda, A.; Tejada, S.; Lorigooini, Z. Epigenetic targeting of cancer stem cells by polyphenols (cancer stem cells targeting). Phytother. Res. 2021, 35, 3649–3664. [Google Scholar] [CrossRef]
- Musial, C.; Siedlecka-Kroplewska, K.; Kmiec, Z.; Gorska-Ponikowska, M. Modulation of Autophagy in Cancer Cells by Dietary Polyphenols. Antioxidants 2021, 10, 123. [Google Scholar] [CrossRef]
- Wang, K.; Liu, R.; Li, J.; Mao, J.; Lei, Y.; Wu, J.; Zeng, J.; Zhang, T.; Wu, H.; Chen, L.; et al. Quercetin induces protective autophagy in gastric cancer cells: Involvement of Akt-mTOR- and hypoxia-induced factor 1alpha-mediated signaling. Autophagy 2011, 7, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef]
- Shao, J.; Wang, C.; Li, L.; Liang, H.; Dai, J.; Ling, X.; Tang, H. Luteoloside Inhibits Proliferation and Promotes Intrinsic and Extrinsic Pathway-Mediated Apoptosis Involving MAPK and mTOR Signaling Pathways in Human Cervical Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1664. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, L.; Yi, T.; Xu, J.; Wang, J.; Qin, J.-J.; Chen, Q.; Yip, K.-M.; Pan, Y.H.; Hong, P.; et al. Synergistic effects of autophagy/mitophagy inhibitors and magnolol promote apoptosis and antitumor efficacy. Acta Pharm. Sin. B 2021, in press. [Google Scholar] [CrossRef]
- Gao, A.M.; Zhang, X.Y.; Hu, J.N.; Ke, Z.P. Apigenin sensitizes hepatocellular carcinoma cells to doxorubic through regulating miR-520b/ATG7 axis. Chem. Biol. Interact. 2018, 280, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Banik, N.L.; Ray, S.K. Combination of N-(4-hydroxyphenyl) retinamide and apigenin suppressed starvation-induced autophagy and promoted apoptosis in malignant neuroblastoma cells. Neurosci. Lett. 2011, 502, 24–29. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Cheng, X.; Gao, Y.; Zheng, J.; Xu, Q.; Sun, Y.; Guan, H.; Yu, H.; Sun, Z. Apigenin induces autophagic cell death in human papillary thyroid carcinoma BCPAP cells. Food. Funct. 2015, 6, 3464–3472. [Google Scholar] [CrossRef]
- He, M.; Min, J.W.; Kong, W.L.; He, X.H.; Li, J.X.; Peng, B.W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Cho, H.J.; Paul, S.; Jakhar, R.; Khan, I.; Lee, S.J.; Kim, B.Y.; Krishnan, M.; Khaket, T.P.; Lee, H.G.; et al. Vitexin induces apoptosis by suppressing autophagy in multi-drug resistant colorectal cancer cells. Oncotarget 2018, 9, 3278–3291. [Google Scholar] [CrossRef]
- Landais, E.; Moskal, A.; Mullee, A.; Nicolas, G.; Gunter, M.J.; Huybrechts, I.; Overvad, K.; Roswall, N.; Affret, A.; Fagherazzi, G.; et al. Coffee and Tea Consumption and the Contribution of Their Added Ingredients to Total Energy and Nutrient Intakes in 10 European Countries: Benchmark Data from the Late 1990s. Nutrients 2018, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Song, J.; Zhou, Y.; Cao, L.; Gong, Y.; Wei, Y.; Yang, H.; Tang, L. Methylxanthine derivatives promote autophagy in gastric cancer cells targeting PTEN. Anticancer Drugs 2019, 30, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Naveed, M.; BiBi, J.; Ali Kamboh, A.; Phil, L.; Chao, S. Potential nutraceutical and food additive properties and risks of coffee: A comprehensive overview. Crit. Rev. Food Sci. Nutr. 2019, 59, 3293–3319. [Google Scholar] [CrossRef]
- Buldak, R.J.; Hejmo, T.; Osowski, M.; Buldak, L.; Kukla, M.; Polaniak, R.; Birkner, E. The Impact of Coffee and Its Selected Bioactive Compounds on the Development and Progression of Colorectal Cancer In Vivo and In Vitro. Molecules 2018, 23, 3309. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Freedman, N.D.; Haiman, C.A.; Le Marchand, L.; Wilkens, L.R.; Setiawan, V.W. Prospective Study of Coffee Consumption and Cancer Incidence in Non-White Populations. Cancer Epidemiol. Biomark. Prev. 2018, 27, 928–935. [Google Scholar] [CrossRef]
- Pietrocola, F.; Malik, S.A.; Marino, G.; Vacchelli, E.; Senovilla, L.; Chaba, K.; Niso-Santano, M.; Maiuri, M.C.; Madeo, F.; Kroemer, G. Coffee induces autophagy in vivo. Cell Cycle 2014, 13, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.K.; Xu, S.; Li, S.; Zhang, Y. Proanthocyanidins Should Be a Candidate in the Treatment of Cancer, Cardiovascular Diseases and Lipid Metabolic Disorder. Molecules 2020, 25, 5971. [Google Scholar] [CrossRef]
- Wang, L.; Huang, W.; Zhan, J. Grape Seed Proanthocyanidins Induce Autophagy and Modulate Survivin in HepG2 Cells and Inhibit Xenograft Tumor Growth in Vivo. Nutrients 2019, 11, 2983. [Google Scholar] [CrossRef]
- Ali, A.B.; Nin, D.S.; Tam, J.; Khan, M. Role of chaperone mediated autophagy (CMA) in the degradation of misfolded N-CoR protein in non-small cell lung cancer (NSCLC) cells. PLoS ONE 2011, 6, e25268. [Google Scholar] [CrossRef]
- Moskot, M.; Montefusco, S.; Jakobkiewicz-Banecka, J.; Mozolewski, P.; Wegrzyn, A.; Di Bernardo, D.; Wegrzyn, G.; Medina, D.L.; Ballabio, A.; Gabig-Ciminska, M. The phytoestrogen genistein modulates lysosomal metabolism and transcription factor EB (TFEB) activation. J. Biol. Chem. 2014, 289, 17054–17069. [Google Scholar] [CrossRef]
- Argüello, G.; Balboa, E.; Tapia, P.J.; Castro, J.; Yañez, M.J.; Mattar, P.; Pulgar, R.; Zanlungo, S. Genistein Activates Transcription Factor EB and Corrects Niemann-Pick C Phenotype. Int. J. Mol. Sci. 2021, 22, 4220. [Google Scholar] [CrossRef]
- Astanina, E.; Bussolino, F.; Doronzo, G. Multifaceted activities of transcription factor EB in cancer onset and progression. Mol. Oncol. 2021, 15, 327–346. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Xu, J.; Lu, Y.; Jiang, J.; Wang, L.; Shen, H.M.; Xia, D. Curcumin targets the TFEB-lysosome pathway for induction of autophagy. Oncotarget 2016, 7, 75659–75671. [Google Scholar] [CrossRef]
- Qu, W.; Xiao, J.; Zhang, H.; Chen, Q.; Wang, Z.; Shi, H.; Gong, L.; Chen, J.; Liu, Y.; Cao, R.; et al. B19, a novel monocarbonyl analogue of curcumin, induces human ovarian cancer cell apoptosis via activation of endoplasmic reticulum stress and the autophagy signaling pathway. Int. J. Biol. Sci. 2013, 9, 766–777. [Google Scholar] [CrossRef]
- Wu, J.C.; Lai, C.S.; Badmaev, V.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Tetrahydrocurcumin, a major metabolite of curcumin, induced autophagic cell death through coordinative modulation of PI3K/Akt-mTOR and MAPK signaling pathways in human leukemia HL-60 cells. Mol. Nutr. Food Res. 2011, 55, 1646–1654. [Google Scholar] [CrossRef]
- Zhou, T.; Ye, L.; Bai, Y.; Sun, A.; Cox, B.; Liu, D.; Li, Y.; Liotta, D.; Snyder, J.P.; Fu, H.; et al. Autophagy and apoptosis in hepatocellular carcinoma induced by EF25-(GSH)2: A novel curcumin analog. PLoS ONE 2014, 9, e107876. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Takeda, T.; Tsuiji, K.; Wong, T.F.; Tadakawa, M.; Kondo, A.; Nagase, S.; Yaegashi, N. Curcumin induces cross-regulation between autophagy and apoptosis in uterine leiomyosarcoma cells. Int. J. Gynecol. Cancer 2013, 23, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Jiang, T.; Long, M.; Chen, J.; Ren, D.M.; Wong, P.K.; Chapman, E.; Zhou, B.; Zhang, D.D. A Curcumin Derivative That Inhibits Vinyl Carbamate-Induced Lung Carcinogenesis via Activation of the Nrf2 Protective Response. Antioxid. Redox Signal. 2015, 23, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yue, Y.; Chen, L.; Xu, C.; Wang, Y.; Du, L.; Xue, X.; Liu, Q.; Wang, Y.; Fan, F. Resveratrol Sensitizes Carfilzomib-Induced Apoptosis via Promoting Oxidative Stress in Multiple Myeloma Cells. Front. Pharmacol. 2018, 9, 334. [Google Scholar] [CrossRef]
- Cuanalo-Contreras, K.; Moreno-Gonzalez, I. Natural Products as Modulators of the Proteostasis Machinery: Implications in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 4666. [Google Scholar] [CrossRef]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Sundarraj, K.; Raghunath, A.; Panneerselvam, L.; Perumal, E. Fisetin Inhibits Autophagy in HepG2 Cells via PI3K/Akt/mTOR and AMPK Pathway. Nutr. Cancer 2020, 1–13. [Google Scholar] [CrossRef]
- Kumar, D.; Shankar, S.; Srivastava, R.K. Rottlerin-induced autophagy leads to the apoptosis in breast cancer stem cells: Molecular mechanisms. Mol. Cancer 2013, 12, 171. [Google Scholar] [CrossRef]
- Raha, S.; Kim, S.M.; Lee, H.J.; Yumnam, S.; Saralamma, V.V.; Ha, S.E.; Lee, W.S.; Kim, G.S. Naringin Induces Lysosomal Permeabilization and Autophagy Cell Death in AGS Gastric Cancer Cells. Am. J. Chin. Med. 2020, 48, 679–702. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, D.; Ru, B.; Cheng, K.W.; Hu, S.; Zhang, J.; Li, E.T.; Wang, M. 6-C-(E-phenylethenyl)naringenin induces cell growth inhibition and cytoprotective autophagy in colon cancer cells. Eur. J. Cancer 2016, 68, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Rao, X.; Jiang, Q. Gamma-tocotrienol profoundly alters sphingolipids in cancer cells by inhibition of dihydroceramide desaturase and possibly activation of sphingolipid hydrolysis during prolonged treatment. J. Nutr. Biochem. 2017, 46, 49–56. [Google Scholar] [CrossRef]
- Tran, A.T.; Ramalinga, M.; Kedir, H.; Clarke, R.; Kumar, D. Autophagy inhibitor 3-methyladenine potentiates apoptosis induced by dietary tocotrienols in breast cancer cells. Eur. J. Nutr. 2015, 54, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Yami, A.; Hamzeloo-Moghadam, M.; Darbandi, A.; Karami, A.; Mashati, P.; Takhviji, V.; Gharehbaghian, A. Ergolide, a potent sesquiterpene lactone induces cell cycle arrest along with ROS-dependent apoptosis and potentiates vincristine cytotoxicity in ALL cell lines. J. Ethnopharmacol. 2020, 253, 112504. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Han, X. Anthecotulide Sesquiterpene Lactone Exhibits Selective Anticancer Effects in Human Malignant Melanoma Cells by Activating Apoptotic and Autophagic Pathways, S-Phase Cell Cycle Arrest, Caspase Activation, and Inhibition of NF-kappaB Signalling Pathway. Med. Sci. Monit. 2019, 25, 2852–2858. [Google Scholar] [CrossRef]
- Tian, L.; Cheng, F.; Wang, L.; Qin, W.; Zou, K.; Chen, J. CLE-10 from Carpesium abrotanoides L. Suppresses the Growth of Human Breast Cancer Cells (MDA-MB-231) In Vitro by Inducing Apoptosis and Pro-Death Autophagy Via the PI3K/Akt/mTOR Signaling Pathway. Molecules 2019, 24, 1091. [Google Scholar] [CrossRef]
- Bai, M.; Chen, J.J.; Xu, W.; Dong, S.H.; Liu, Q.B.; Lin, B.; Huang, X.X.; Yao, G.D.; Song, S.J. Elephantopinolide A-P, germacrane-type sesquiterpene lactones from Elephantopus scaber induce apoptosis, autophagy and G2/M phase arrest in hepatocellular carcinoma cells. Eur. J. Med. Chem. 2020, 198, 112362. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, T.Y.; Nie, C.H.; Wan, D.L.; Zheng, S.S. Bigelovin, a sesquiterpene lactone, suppresses tumor growth through inducing apoptosis and autophagy via the inhibition of mTOR pathway regulated by ROS generation in liver cancer. Biochem. Biophys. Res. Commun. 2018, 499, 156–163. [Google Scholar] [CrossRef]
- Lindner, P.; Christensen, S.B.; Nissen, P.; Moller, J.V.; Engedal, N. Cell death induced by the ER stressor thapsigargin involves death receptor 5, a non-autophagic function of MAP1LC3B, and distinct contributions from unfolded protein response components. Cell. Commun. Signal. 2020, 18, 12. [Google Scholar] [CrossRef]
- Du, J.; Dong, Z.; Tan, L.; Tan, M.; Zhang, F.; Zhang, K.; Pan, G.; Li, C.; Shi, S.; Zhang, Y.; et al. Tubeimoside I Inhibits Cell Proliferation and Induces a Partly Disrupted and Cytoprotective Autophagy Through Rapidly Hyperactivation of MEK1/2-ERK1/2 Cascade via Promoting PTP1B in Melanoma. Front. Cell Dev. Biol. 2020, 8, 607757. [Google Scholar] [CrossRef]
- Wang, K.; Zhan, Y.; Chen, B.; Lu, Y.; Yin, T.; Zhou, S.; Zhang, W.; Liu, X.; Du, B.; Wei, X.; et al. Tubeimoside I-induced lung cancer cell death and the underlying crosstalk between lysosomes and mitochondria. Cell Death Dis. 2020, 11, 708. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.D.; Mishra, S.; Verma, S.S.; Shekher, A.; Rai, V.; Awasthee, N.; Das, T.J.; Paul, D.; Das, S.K.; Tag, H.; et al. Evaluation of antioxidant, anti-inflammatory and anticancer activities of diosgenin enriched Paris polyphylla rhizome extract of Indian Himalayan landraces. J. Ethnopharmacol. 2021, 270, 113842. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, P.; Wang, N.; Wang, S.; Yang, B.; Li, M.; Chen, J.; Situ, H.; Xie, M.; Lin, Y.; et al. Betulinic Acid Suppresses Breast Cancer Metastasis by Targeting GRP78-Mediated Glycolysis and ER Stress Apoptotic Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 8781690. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Mun, J.G.; Jeon, H.D.; Kee, J.Y.; Hong, S.H. Betulin Inhibits Lung Metastasis by Inducing Cell Cycle Arrest, Autophagy, and Apoptosis of Metastatic Colorectal Cancer Cells. Nutrients 2019, 12, 66. [Google Scholar] [CrossRef]
- Wang, K.; Tu, Y.; Wan, J.B.; Chen, M.; He, C. Synergistic anti-breast cancer effect of pulsatilla saponin D and camptothecin through interrupting autophagic-lysosomal function and promoting p62-mediated ubiquitinated protein aggregation. Carcinogenesis 2020, 41, 804–816. [Google Scholar] [CrossRef]
- Jang, M.; Kim, H.; Park, R.; Jo, D.; Lee, E.J.; Oh, W.K.; Park, J. 2,2′-Methylenebis (6-tert-butyl 4-methylphenol) enhances the antitumor efficacy of belotecan, a derivative of camptothecin, by inducing autophagy. Oncotarget 2017, 8, 115068–115078. [Google Scholar] [CrossRef]
- Ramesh, G.; Das, S.; Bola Sadashiva, S.R. Berberine, a natural alkaloid sensitizes human hepatocarcinoma to ionizing radiation by blocking autophagy and cell cycle arrest resulting in senescence. J. Pharm. Pharmacol. 2020, 72, 1893–1908. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Men, Q.; Wu, G.; Yu, C.; Huang, Z.; Liu, X.; Li, W. Tetrandrine induces autophagy and differentiation by activating ROS and Notch1 signaling in leukemia cells. Oncotarget 2015, 6, 7992–8006. [Google Scholar] [CrossRef]
- Lyu, L.; Hu, Y.; Yin, S.; Wang, L.; Ye, F.; Wang, M.; Zhou, Y.; Ma, W.; Chen, C.; Jiang, Y.; et al. Autophagy inhibition enhances anti-pituitary adenoma effect of tetrandrine. Phytother Res. 2021, 35, 4007–4021. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Su, M.; Xie, F.; Ai, J.; Ren, Y.; Zhang, J.; Guan, R.; He, W.; Gong, Y.; Guo, Y. Tetrandrine blocks autophagic flux and induces apoptosis via energetic impairment in cancer cells. Cell Death Dis. 2014, 5, e1123. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Ohta, S.; Kawakami, K.; Ikeda, M.; Takahashi, T.; Kobayashi, S.; Nomura, M. Tetrandrine Increases the Sensitivity of Human Lung Adenocarcinoma PC14 Cells to Gefitinib by Lysosomal Inhibition. Anticancer Res. 2019, 39, 6585–6593. [Google Scholar] [CrossRef]
- Ye, L.Y.; Hu, S.; Xu, H.E.; Xu, R.R.; Kong, H.; Zeng, X.N.; Xie, W.P.; Wang, H. The effect of tetrandrine combined with cisplatin on proliferation and apoptosis of A549/DDP cells and A549 cells. Cancer Cell Int. 2017, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yue, W.; Lang, H.; Ding, X.; Chen, X.; Chen, H. Resuming Sensitivity of Tamoxifen-Resistant Breast Cancer Cells to Tamoxifen by Tetrandrine. Integr. Cancer Ther. 2021, 20, 1534735421996822. [Google Scholar] [CrossRef] [PubMed]
- Nazim, U.M.; Yin, H.; Park, S.Y. Neferine treatment enhances the TRAILinduced apoptosis of human prostate cancer cells via autophagic flux and the JNK pathway. Int. J. Oncol. 2020, 56, 1152–1161. [Google Scholar]
- Pham, D.C.; Chang, Y.C.; Lin, S.R.; Fuh, Y.M.; Tsai, M.J.; Weng, C.F. FAK and S6K1 Inhibitor, Neferine, Dually Induces Autophagy and Apoptosis in Human Neuroblastoma Cells. Molecules 2018, 23, 3110. [Google Scholar] [CrossRef]
- Dasari, S.; Bakthavachalam, V.; Chinnapaka, S.; Venkatesan, R.; Samy, A.; Munirathinam, G. Neferine, an alkaloid from lotus seed embryo targets HeLa and SiHa cervical cancer cells via pro-oxidant anticancer mechanism. Phytother Res. 2020, 34, 2366–2384. [Google Scholar] [CrossRef] [PubMed]
- Kalai Selvi, S.; Vinoth, A.; Varadharajan, T.; Weng, C.F.; Vijaya Padma, V. Neferine augments therapeutic efficacy of cisplatin through ROS- mediated non-canonical autophagy in human lung adenocarcinoma (A549 cells). Food Chem. Toxicol. 2017, 103, 28–40. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Miao, C.; Wang, Y.; Xu, Y.; Dong, R.; Zhang, Z.; Griffin, B.B.; Yuan, C.; Yan, S.; et al. Anti-angiogenesis effect of Neferine via regulating autophagy and polarization of tumor-associated macrophages in high-grade serous ovarian carcinoma. Cancer Lett. 2018, 432, 144–155. [Google Scholar] [CrossRef]
- Liou, Y.F.; Chen, P.N.; Chu, S.C.; Kao, S.H.; Chang, Y.Z.; Hsieh, Y.S.; Chang, H.R. Thymoquinone suppresses the proliferation of renal cell carcinoma cells via reactive oxygen species-induced apoptosis and reduces cell stemness. Environ. Toxicol. 2019, 34, 1208–1220. [Google Scholar] [CrossRef]
- Alaufi, O.M.; Noorwali, A.; Zahran, F.; Al-Abd, A.M.; Al-Attas, S. Cytotoxicity of thymoquinone alone or in combination with cisplatin (CDDP) against oral squamous cell carcinoma in vitro. Sci. Rep. 2017, 7, 13131. [Google Scholar] [CrossRef]
- Pazhouhi, M.; Sariri, R.; Rabzia, A.; Khazaei, M. Thymoquinone synergistically potentiates temozolomide cytotoxicity through the inhibition of autophagy in U87MG cell line. Iran. J. Basic. Med. Sci. 2016, 19, 890–898. [Google Scholar]
- Chen, M.C.; Lee, N.H.; Hsu, H.H.; Ho, T.J.; Tu, C.C.; Hsieh, D.J.; Lin, Y.M.; Chen, L.M.; Kuo, W.W.; Huang, C.Y. Thymoquinone induces caspase-independent, autophagic cell death in CPT-11-resistant lovo colon cancer via mitochondrial dysfunction and activation of JNK and p38. J. Agric. Food Chem. 2015, 63, 1540–1546. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, P.; Wang, X.; Wang, L.; Zhu, Y.; Song, Y.; Gao, W. Celastrol mediates autophagy and apoptosis via the ROS/JNK and Akt/mTOR signaling pathways in glioma cells. J. Exp. Clin. Cancer Res. 2019, 38, 184. [Google Scholar] [CrossRef]
- Lee, H.W.; Jang, K.S.; Choi, H.J.; Jo, A.; Cheong, J.H.; Chun, K.H. Celastrol inhibits gastric cancer growth by induction of apoptosis and autophagy. BMB Rep. 2014, 47, 697–702. [Google Scholar] [CrossRef]
- Liu, M.; Fan, Y.; Li, D.; Han, B.; Meng, Y.; Chen, F.; Liu, T.; Song, Z.; Han, Y.; Huang, L.; et al. Ferroptosis inducer erastin sensitizes NSCLC cells to celastrol through activation of the ROS-mitochondrial fission-mitophagy axis. Mol. Oncol. 2021, 15, 2084–2105. [Google Scholar] [CrossRef]
- Xu, S.W.; Law, B.Y.K.; Qu, S.L.Q.; Hamdoun, S.; Chen, J.; Zhang, W.; Guo, J.R.; Wu, A.G.; Mok, S.W.F.; Zhang, D.W.; et al. SERCA and P-glycoprotein inhibition and ATP depletion are necessary for celastrol-induced autophagic cell death and collateral sensitivity in multidrug-resistant tumor cells. Pharmacol. Res. 2020, 153, 104660. [Google Scholar] [CrossRef]
- Dai, C.H.; Zhu, L.R.; Wang, Y.; Tang, X.P.; Du, Y.J.; Chen, Y.C.; Li, J. Celastrol acts synergistically with afatinib to suppress non-small cell lung cancer cell proliferation by inducing paraptosis. J. Cell Physiol. 2021, 236, 4538–4554. [Google Scholar] [CrossRef]
- Zhang, C.J.; Zhu, N.; Long, J.; Wu, H.T.; Wang, Y.X.; Liu, B.Y.; Liao, D.F.; Qin, L. Celastrol induces lipophagy via the LXRalpha/ABCA1 pathway in clear cell renal cell carcinoma. Acta Pharmacol. Sin. 2020, 42, 1472–1485. [Google Scholar] [CrossRef]
- Hu, M.; Luo, Q.; Alitongbieke, G.; Chong, S.; Xu, C.; Xie, L.; Chen, X.; Zhang, D.; Zhou, Y.; Wang, Z.; et al. Celastrol-Induced Nur77 Interaction with TRAF2 Alleviates Inflammation by Promoting Mitochondrial Ubiquitination and Autophagy. Mol. Cell 2017, 66, 141–153.e6. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, X.; Wang, H.; Yang, H. Celastrol Induces Autophagy by Targeting AR/miR-101 in Prostate Cancer Cells. PLoS ONE 2015, 10, e0140745. [Google Scholar] [CrossRef]
- Nazim, U.M.; Yin, H.; Park, S.Y. Autophagy flux inhibition mediated by celastrol sensitized lung cancer cells to TRAILinduced apoptosis via regulation of mitochondrial transmembrane potential and reactive oxygen species. Mol. Med. Rep. 2019, 19, 984–993. [Google Scholar] [PubMed]
- Tan, X.; Zou, L.; Qin, J.; Xia, D.; Zhou, Y.; Jin, G.; Jiang, Z.; Li, H. SQSTM1/p62 is involved in docosahexaenoic acid-induced cellular autophagy in glioblastoma cell lines. In Vitro Cell Dev. Biol. Anim. 2019, 55, 703–712. [Google Scholar] [CrossRef]
- Kim, S.; Jing, K.; Shin, S.; Jeong, S.; Han, S.H.; Oh, H.; Yoo, Y.S.; Han, J.; Jeon, Y.J.; Heo, J.Y.; et al. Omega3-polyunsaturated fatty acids induce cell death through apoptosis and autophagy in glioblastoma cells: In vitro and in vivo. Oncol. Rep. 2018, 39, 239–246. [Google Scholar]
- Tan, R.H.; Wang, F.; Fan, C.L.; Zhang, X.H.; Zhao, J.S.; Zhang, J.J.; Yang, Y.; Xi, Y.; Zou, Z.Q.; Bu, S.Z. Algal oil rich in n-3 polyunsaturated fatty acids suppresses B16F10 melanoma lung metastasis by autophagy induction. Food Funct. 2018, 9, 6179–6186. [Google Scholar] [CrossRef]
- D’Eliseo, D.; Di Renzo, L.; Santoni, A.; Velotti, F. Docosahexaenoic acid (DHA) promotes immunogenic apoptosis in human multiple myeloma cells, induces autophagy and inhibits STAT3 in both tumor and dendritic cells. Genes Cancer 2017, 8, 426–437. [Google Scholar] [CrossRef]
- Giordano, C.; Plastina, P.; Barone, I.; Catalano, S.; Bonofiglio, D. n-3 Polyunsaturated Fatty Acid Amides: New Avenues in the Prevention and Treatment of Breast Cancer. Int. J. Mol. Sci. 2020, 21, 2279. [Google Scholar] [CrossRef]
- Rovito, D.; Giordano, C.; Plastina, P.; Barone, I.; De Amicis, F.; Mauro, L.; Rizza, P.; Lanzino, M.; Catalano, S.; Bonofiglio, D.; et al. Omega-3 DHA- and EPA-dopamine conjugates induce PPARgamma-dependent breast cancer cell death through autophagy and apoptosis. Biochim. Biophys. Acta 2015, 1850, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Lin, G.; Song, C.; Wu, Y.; Feng, N.; Chen, W.; He, Z.; Chen, Y.Q. RA and omega-3 PUFA co-treatment activates autophagy in cancer cells. Oncotarget 2017, 8, 109135–109150. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, D.Y.; Kang, S.H.; Yun, H.K.; Kim, J.L.; Kim, B.R.; Park, S.H.; Na, Y.J.; Jo, M.J.; Jeong, Y.A.; et al. Docosahexaenoic Acid Enhances Oxaliplatin-Induced Autophagic Cell Death via the ER Stress/Sesn2 Pathway in Colorectal Cancer. Cancers 2019, 11, 982. [Google Scholar] [CrossRef] [PubMed]
- Pizato, N.; Kiffer, L.; Luzete, B.C.; Assumpcao, J.A.F.; Correa, L.H.; Melo, H.A.B.; Sant’Ana, L.P.; Ito, M.K.; Magalhaes, K.G. Omega 3-DHA and Delta-Tocotrienol Modulate Lipid Droplet Biogenesis and Lipophagy in Breast Cancer Cells: The Impact in Cancer Aggressiveness. Nutrients 2019, 11, 1199. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Yao, R.; Zhao, D.; Zhou, L.; Wu, Y.; Yang, Y.; Sun, Y.; Lu, L.; Gao, W. Trichostatin A reverses the chemoresistance of lung cancer with high IGFBP2 expression through enhancing autophagy. Sci. Rep. 2018, 8, 3917. [Google Scholar] [CrossRef] [PubMed]
- Lambert, I.H.; Nielsen, D.; Sturup, S. Impact of the histone deacetylase inhibitor trichostatin A on active uptake, volume-sensitive release of taurine, and cell fate in human ovarian cancer cells. Am. J. Physiol. Cell Physiol. 2020, 318, C581–C597. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Sun, X.; Zhang, Q.; Chen, X.; Zhao, T.; Lu, L.; Zhang, J.; Hong, Y. Histone deacetylase inhibitor trichostatin A and autophagy inhibitor chloroquine synergistically exert anti-tumor activity in H-ras transformed breast epithelial cells. Mol. Med. Rep. 2018, 17, 4345–4350. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zhu, Y.; Xu, X.; Zhu, Y.; Song, Y.; Pang, L.; Chen, Z. The anti-tumor effects of dual PI3K/mTOR inhibitor BEZ235 and histone deacetylase inhibitor Trichostatin A on inducing autophagy in esophageal squamous cell carcinoma. J. Cancer 2018, 9, 987–997. [Google Scholar] [CrossRef]
- Chen, L.; Jin, T.; Zhu, K.; Piao, Y.; Quan, T.; Quan, C.; Lin, Z. PI3K/mTOR dual inhibitor BEZ235 and histone deacetylase inhibitor Trichostatin A synergistically exert anti-tumor activity in breast cancer. Oncotarget 2017, 8, 11937–11949. [Google Scholar] [CrossRef]
- Nazim, U.M.; Park, S.Y. Attenuation of autophagy flux by 6-shogaol sensitizes human liver cancer cells to TRAIL-induced apoptosis via p53 and ROS. Int. J. Mol. Med. 2019, 43, 701–708. [Google Scholar] [CrossRef]
- Wu, J.J.; Omar, H.A.; Lee, Y.R.; Teng, Y.N.; Chen, P.S.; Chen, Y.C.; Huang, H.S.; Lee, K.H.; Hung, J.H. 6-Shogaol induces cell cycle arrest and apoptosis in human hepatoma cells through pleiotropic mechanisms. Eur. J. Pharmacol. 2015, 762, 449–458. [Google Scholar] [CrossRef]
- Hung, J.Y.; Hsu, Y.L.; Li, C.T.; Ko, Y.C.; Ni, W.C.; Huang, M.S.; Kuo, P.L. 6-Shogaol, an active constituent of dietary ginger, induces autophagy by inhibiting the AKT/mTOR pathway in human non-small cell lung cancer A549 cells. J. Agric. Food Chem. 2009, 57, 9809–9816. [Google Scholar] [CrossRef]
- Li, T.Y.; Chiang, B.H. 6-shogaol induces autophagic cell death then triggered apoptosis in colorectal adenocarcinoma HT-29 cells. Biomed. Pharmacother. 2017, 93, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.D.; He, Z.L.; Yao, H.L.; Xiao, J.S.; Li, L.; Gu, J.Z.; Shi, P.Z.; Wang, J.H.; Jiang, L.H. 6-Shogaol from ginger shows anti-tumor effect in cervical carcinoma via PI3K/Akt/mTOR pathway. Eur. J. Nutr. 2021. [Google Scholar] [CrossRef]
- Bawadood, A.S.; Al-Abbasi, F.A.; Anwar, F.; El-Halawany, A.M.; Al-Abd, A.M. 6-Shogaol suppresses the growth of breast cancer cells by inducing apoptosis and suppressing autophagy via targeting notch signaling pathway. Biomed. Pharmacother. 2020, 128, 110302. [Google Scholar] [CrossRef]
- Ray, A.; Vasudevan, S.; Sengupta, S. 6-Shogaol Inhibits Breast Cancer Cells and Stem Cell-Like Spheroids by Modulation of Notch Signaling Pathway and Induction of Autophagic Cell Death. PLoS ONE 2015, 10, e0137614. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, M.; Makuch, S.; Winograd, K.; Wisniewski, J.; Ziolkowski, P.; Agrawal, S. 6-Shogaol enhances the anticancer effect of 5-fluorouracil, oxaliplatin, and irinotecan via increase of apoptosis and autophagy in colon cancer cells in hypoxic/aglycemic conditions. BMC Complement. Med. Ther. 2020, 20, 141. [Google Scholar] [CrossRef]
- Cristofani, R.; Montagnani Marelli, M.; Cicardi, M.E.; Fontana, F.; Marzagalli, M.; Limonta, P.; Poletti, A.; Moretti, R.M. Dual role of autophagy on docetaxel-sensitivity in prostate cancer cells. Cell Death Dis. 2018, 9, 889. [Google Scholar] [CrossRef]
- Eid, N.; Kondo, Y. Parkin in cancer: Mitophagy-related/unrelated tasks. World J. Hepatol. 2017, 9, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Eid, N.; Ito, Y.; Otsuki, Y. Triggering of Parkin Mitochondrial Translocation in Mitophagy: Implications for Liver Diseases. Front. Pharmacol. 2016, 7, 100. [Google Scholar] [CrossRef]
- Eid, N.; Ito, Y.; Otsuki, Y. The autophagic response to alcohol toxicity: The missing layer. J. Hepatol. 2013, 59, 398. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Geng, F.; Cheng, X.; Guo, Q.; Zhong, Y.; Cloughesy, T.F.; Yong, W.H.; Chakravarti, A.; Guo, D. Lipid Droplets Maintain Energy Homeostasis and Glioblastoma Growth via Autophagic Release of Stored Fatty Acids. iScience 2020, 23, 101569. [Google Scholar] [CrossRef]
- Ghirga, F.; Quaglio, D.; Mori, M.; Cammarone, S.; Lazzetti, A.; Goggiamani, A.; Ingallina, C.; Botta, B.; Calcaterra, A. A unique high-diversity natural product collection as a reservoir of new therapeutic leads. Org. Chem. Front. 2021, 8, 996–1025. [Google Scholar] [CrossRef]
- Ito, Y.; Taniguchi, K.; Kuranaga, Y.; Eid, N.; Inomata, Y.; Lee, S.W.; Uchiyama, K. Uptake of MicroRNAs from Exosome-Like Nanovesicles of Edible Plant Juice by Rat Enterocytes. Int. J. Mol. Sci. 2021, 22, 3749. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).