Mass Spectrometric Evaluation of β-Cyclodextrins as Potential Hosts for Titanocene Dichloride
Abstract
1. Introduction
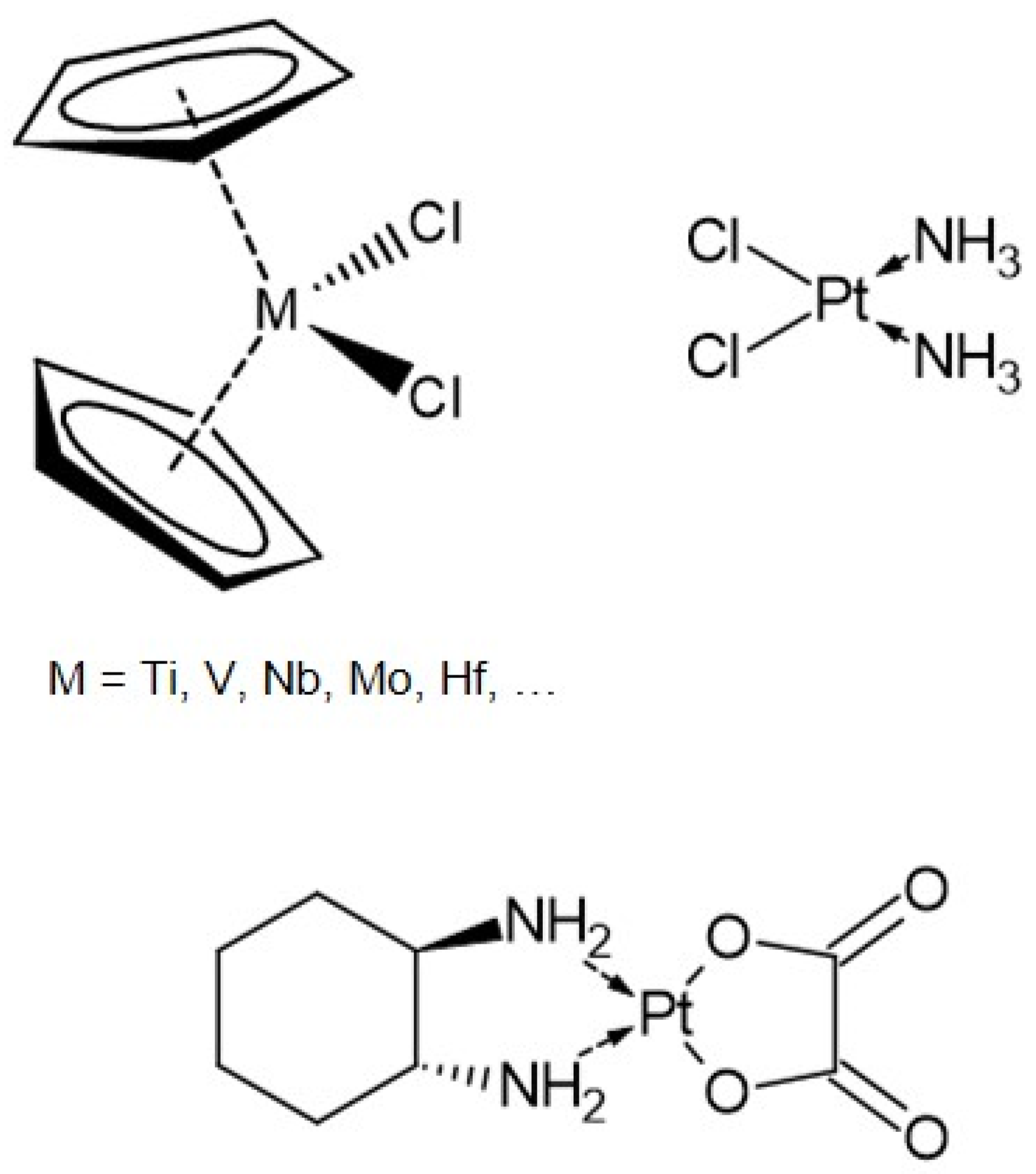
1.1. Bent Metallocenes
1.2. Cyclodextrins as Host Molecules
1.3. Investigation of Complexes
1.4. Mass Spectrometry
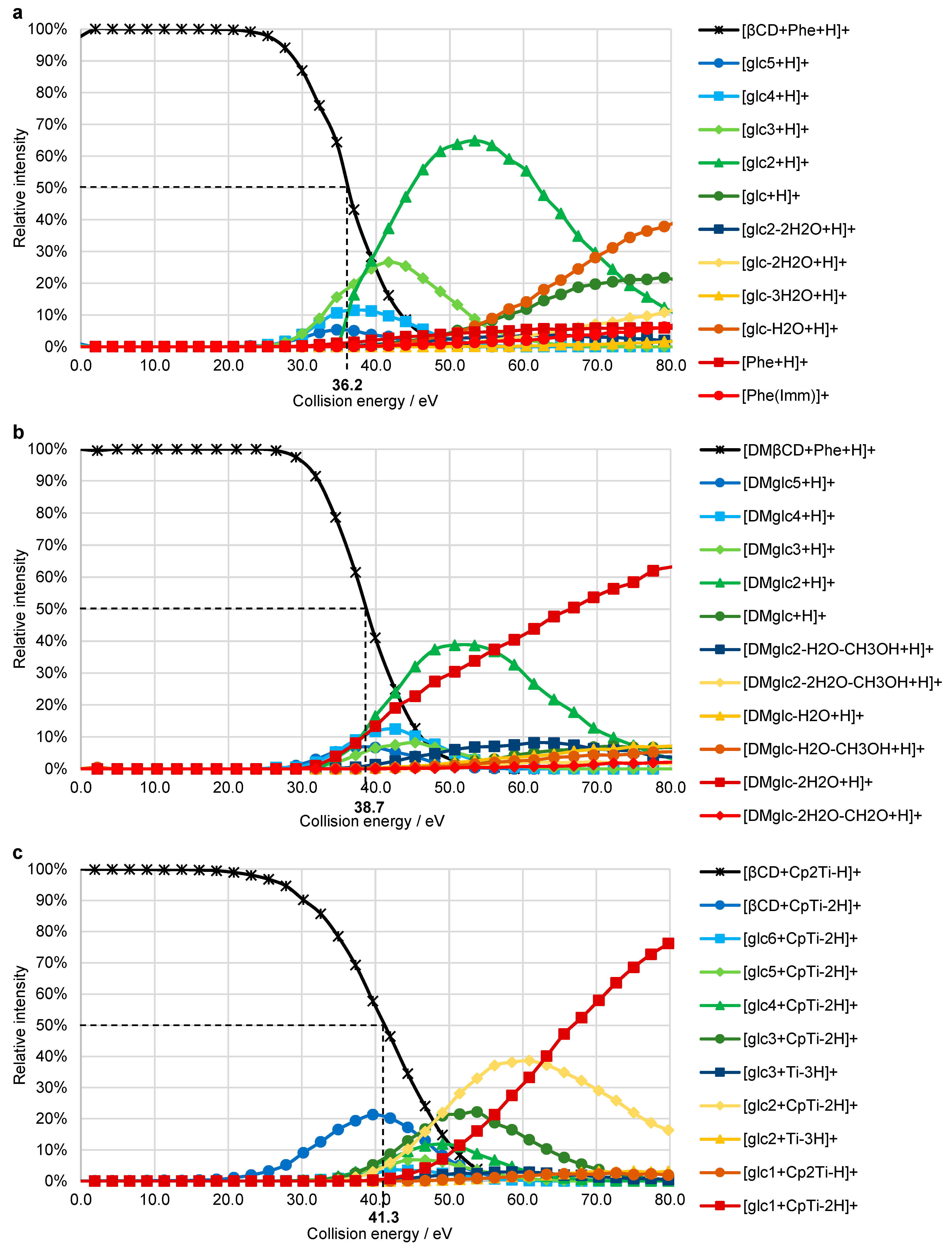
2. Results
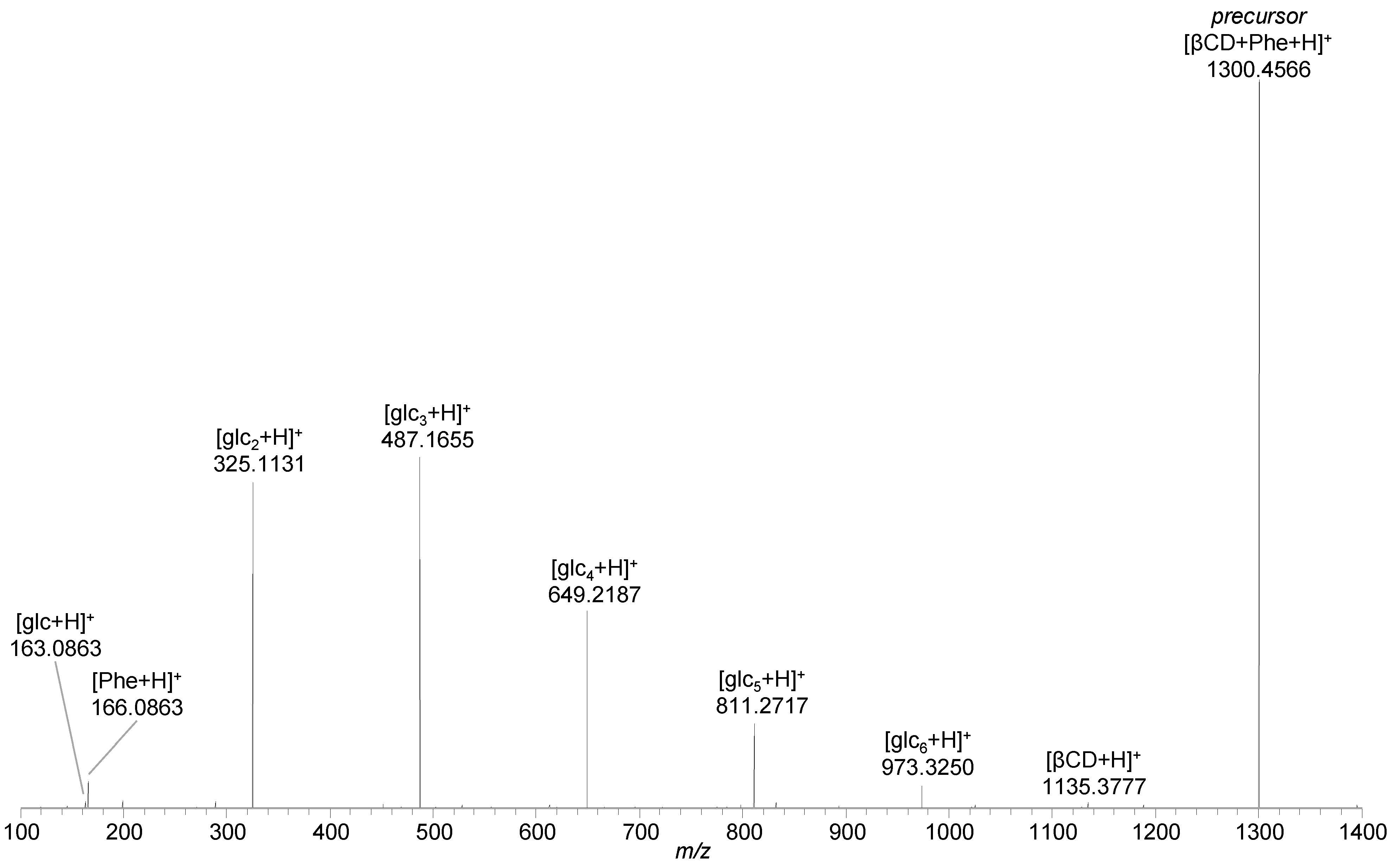
2.1. Phenylalanine and Oxaliplatin Complexes
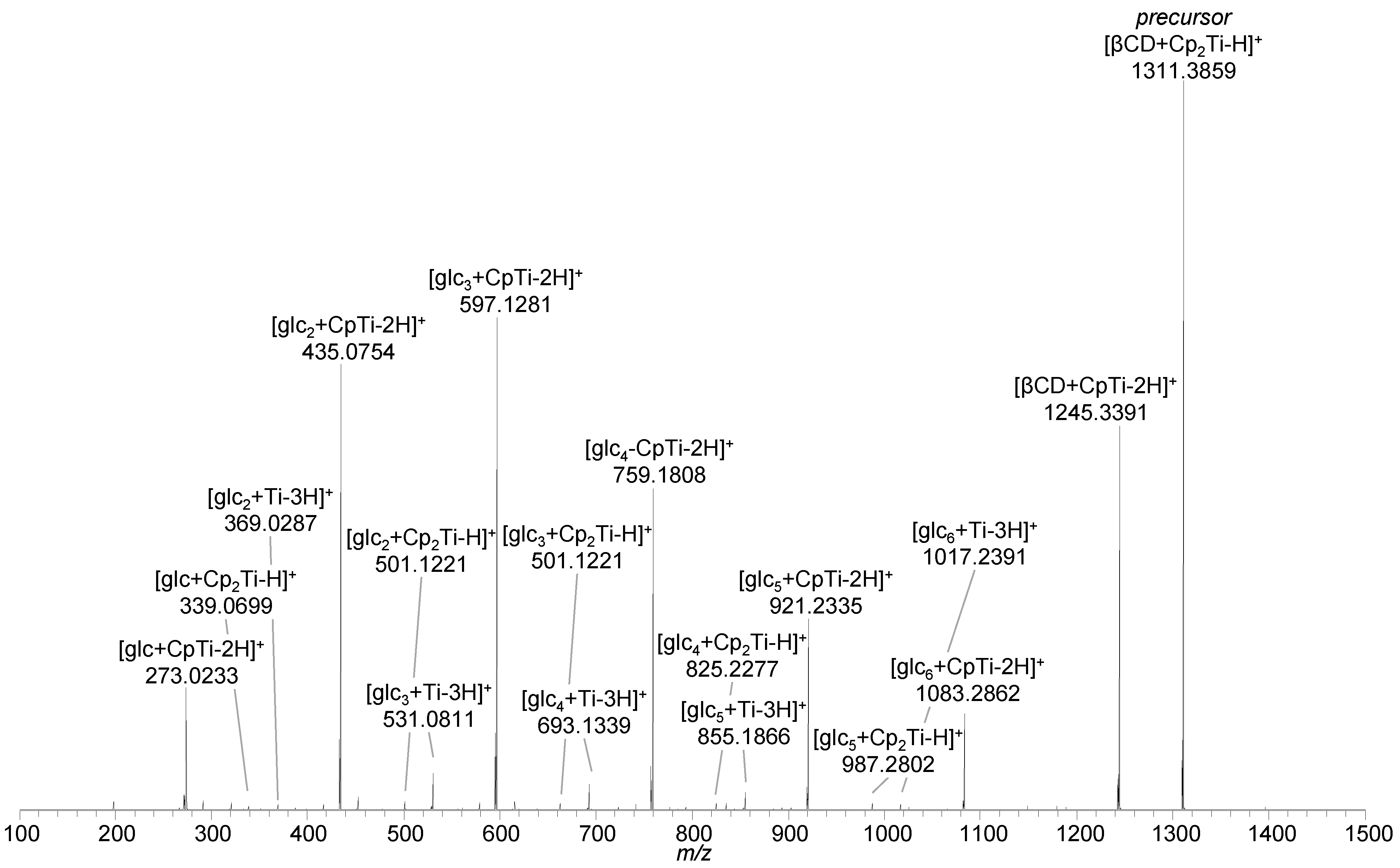
2.2. Titanocene Complex
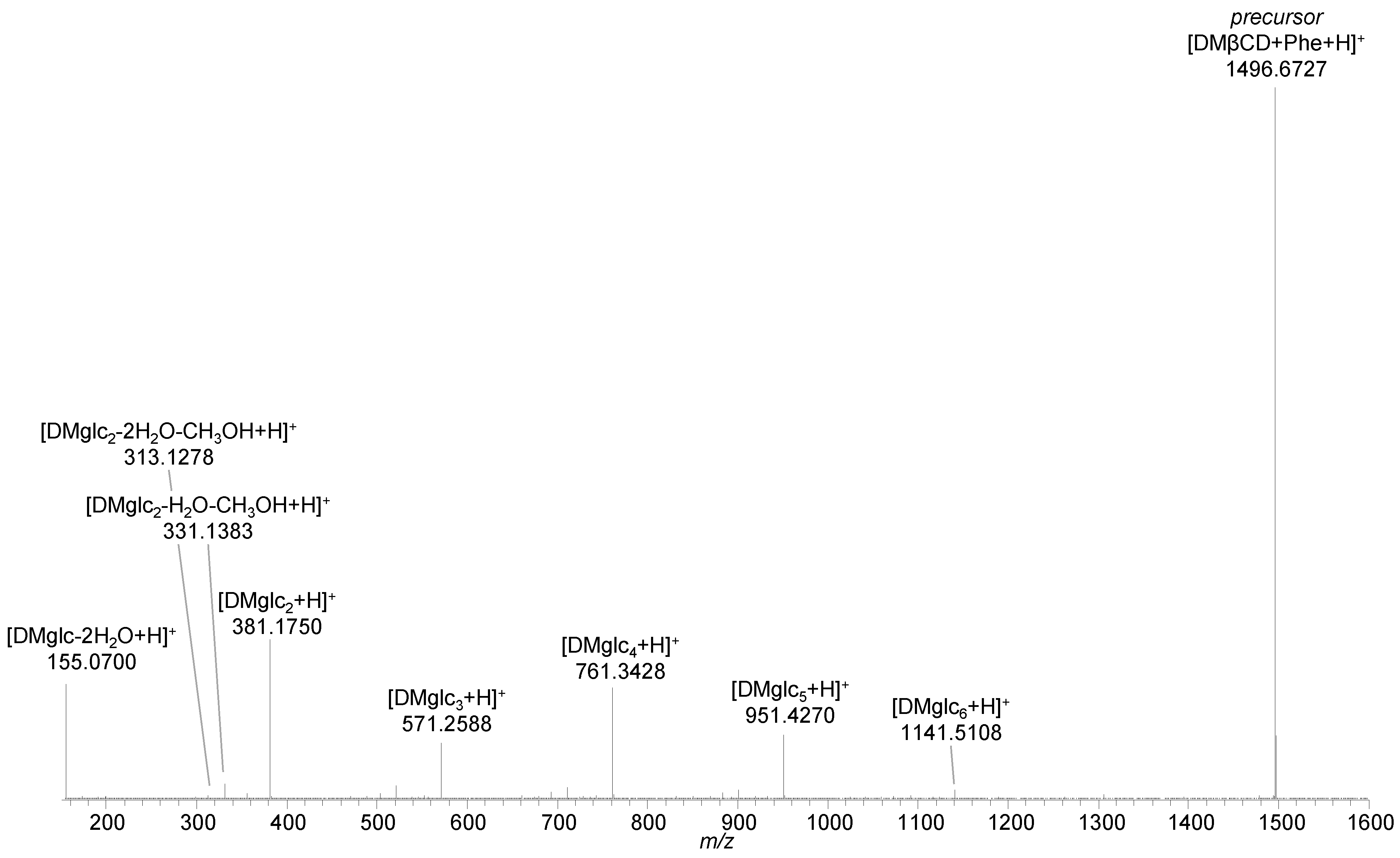
2.3. Methylated β-Cyclodextrins
2.4. Breakdown Curves
3. Discussion
4. Materials and Methods
Mass Spectrometry
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. Biomed. Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef]
- di Cagno, M. The Potential of Cyclodextrins as Novel Active Pharmaceutical Ingredients: A Short Overview. Molecules 2017, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Waern, J.B.; Harding, M.M. Bioorganometallic Chemistry of Molybdocene Dichloride. J. Organomet. Chem. 2004, 689, 4655–4668. [Google Scholar] [CrossRef]
- Anconi, C.P.A.; Da Silva Delgado, L.; Alves Dos Reis, J.B.; De Almeida, W.B.; Costa, L.A.S.; Dos Santos, H.F. Inclusion Complexes of α-Cyclodextrin and the Cisplatin Analogues Oxaliplatin, Carboplatin and Nedaplatin: A Theoretical Approach. Chem. Phys. Lett. 2011, 515, 127–131. [Google Scholar] [CrossRef]
- Causey, P.W.; Baird, M.C.; Cole, S.P.C. Synthesis, Characterization, and Assessment of Cytotoxic Properties of a Series of Titanocene Dichloride Derivatives. Organometallics 2004, 23, 4486–4494. [Google Scholar] [CrossRef]
- Harding, M.M.; Mokdsi, G. Antitumour Metallocenes: Structure-Activity Studies and Interactions with Biomolecules. Curr. Med. Chem. 2000, 7, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Abeysinghe, P.M.; Harding, M.M. Antitumour Bis(Cyclopentadienyl) Metal Complexes: Titanocene and Molybdocene Dichloride and Derivatives. Dalt. Trans. 2007, 3474–3482. [Google Scholar] [CrossRef]
- Lümmen, G.; Sperling, H.; Luboldt, H.; Otto, T.; Rübben, H. Phase II Trial of Titanocene Dichloride in Advanced Renal-Cell Carcinoma. Cancer Chemother. Pharmacol. 1998, 42, 415–417. [Google Scholar] [CrossRef]
- Kröger, N.; Kleeberg, U.R.; Mross, K.; Edler, L.; Hossfeld, D.K. Phase II Clinical Trial of Titanocene Dichloride in Patients with Metastatic Breast Cancer. Onkologie 2000, 23, 60–62. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, L. The Hydrolysis Chemistry of Anticancer Drug Titanocene Dichloride: An Insight from Theoretical Study. J. Mol. Struct. Theochem 2010, 940, 45–49. [Google Scholar] [CrossRef]
- Toney, J.H.; Marks, T.J. Hydrolysis Chemistry of the Metallocene Dichlorides M(H5-C5H5)2Cl2, M = Titanium, Vanadium, or Zirconium. Aqueous Kinetics, Equilibria, and Mechanistic Implications for a New Class of Antitumor Agents. J. Am. Chem. Soc. 1985, 107, 947–953. [Google Scholar] [CrossRef]
- Morales, A.; Struppe, J.; Meléndez, E. Host-Guest Interactions between Niobocene Dichloride and α-, β-, and γ-Cyclodextrins: Preparation and Characterization. J. Incl. Phenom. Macrocycl. Chem. 2008, 60, 263–270. [Google Scholar] [CrossRef]
- Braga, S.S.; Marques, M.P.M.; Sousa, J.B.; Pillinger, M.; Teixeira-Dias, J.J.C.; Gonçalves, I.S. Inclusion of Molybdenocene Dichloride (Cp2MoCl2) in 2-Hydroxypropyl- and Trimethyl-β-Cyclodextrin: Structural and Biological Properties. J. Organomet. Chem. 2005, 690, 2905–2912. [Google Scholar] [CrossRef]
- Meléndez, E. Metallocenes as Target Specific Drugs for Cancer Treatment. Inorganica Chim. Acta 2012, 393, 36–52. [Google Scholar] [CrossRef][Green Version]
- Meléndez, E. Bioorganometallic Chemistry of Molybdenocene Dichloride and Its Derivatives. J. Organomet. Chem. 2012, 706–707, 4–12. [Google Scholar] [CrossRef]
- Erxleben, A.; Claffey, J.; Tacke, M. Binding and Hydrolysis Studies of Antitumoural Titanocene Dichloride and Titanocene Y with Phosphate Diesters. J. Inorg. Biochem. 2010, 104, 390–396. [Google Scholar] [CrossRef]
- Vessières, A.; Plamont, M.-A.; Cabestaing, C.; Claffey, J.; Dieckmann, S.; Hogan, M.; Müller-Bunz, H.; Strohfeldt, K.; Tacke, M. Proliferative and Anti-Proliferative Effects of Titanium- and Iron-Based Metallocene Anti-Cancer Drugs. J. Organomet. Chem. 2009, 694, 874–879. [Google Scholar] [CrossRef]
- Cini, M.; Bradshaw, T.D.; Woodward, S. Using Titanium Complexes to Defeat Cancer: The View from the Shoulders of Titans. Chem. Soc. Rev. 2017, 46, 1040–1051. [Google Scholar] [CrossRef]
- Riviş, A.; Hǎdǎrugǎ, N.G.; Gârban, Z.; Hǎdǎrugǎ, D.I. Titanocene/Cyclodextrin Supramolecular Systems: A Theoretical Approach. Chem. Cent. J. 2012, 6, 129–139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in Pharmaceutical Formulations II: Solubilization, Binding Constant, and Complexation Efficiency. Drug Discov. Today 2016, 21, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Plumb, J.A.; Venugopal, B.; Oun, R.; Gomez-Roman, N.; Kawazoe, Y.; Venkataramanan, N.S.; Wheate, N.J. Cucurbit[7]Uril Encapsulated Cisplatin Overcomes Cisplatin Resistance via a Pharmacokinetic Effect. Metallomics 2012, 4, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in Pharmaceutical Formulations I: Structure and Physicochemical Properties, Formation of Complexes, and Types of Complex. Drug Discov. Today 2016, 21, 356–362. [Google Scholar] [CrossRef]
- Senthilnathan, D.; Solomon, R.V.; Kiruthika, S.; Venuvanalingam, P.; Sundararajan, M. Are Cucurbiturils Better Drug Carriers for Bent Metallocenes? Insights from Theory. J. Biol. Inorg. Chem. 2018, 23, 413–423. [Google Scholar] [CrossRef]
- Ramanathan, R.; Prokai, L. Electrospray Ionization Mass Spectrometric Study of Encapsulation of Amino Acids by Cyclodextrins. J. Am. Soc. Mass Spectrom. 1995, 6, 866–871. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, Physicochemical Properties and Pharmaceutical Applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-Based Pharmaceutics: Past, Present and Future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Loftsson, T.; Saokham, P.; Sá Couto, A.R. Self-Association of Cyclodextrins and Cyclodextrin Complexes in Aqueous Solutions. Int. J. Pharm. 2019, 560, 228–234. [Google Scholar] [CrossRef]
- Hapiot, F.; Tilloy, S.; Monflier, E. Cyclodextrins as Supramolecular Hosts for Organometallic Complexes. Chem. Rev. 2006, 106, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.U.; Lee, S.S.; Lee, S.; Oh, H.B. Noncovalent Complexes of Cyclodextrin with Small Organic Molecules: Applications and Insights into Host–Guest Interactions in the Gas Phase and Condensed Phase. Molecules 2020, 25, 4048. [Google Scholar] [CrossRef]
- Rudolph, S.; Riedel, E.; Henle, T. Studies on the Interaction of the Aromatic Amino Acids Tryptophan, Tyrosine and Phenylalanine as Well as Tryptophan-Containing Dipeptides with Cyclodextrins. Eur. Food Res. Technol. 2018, 244, 1511–1519. [Google Scholar] [CrossRef]
- Silion, M.; Fifere, A.; Lungoci, A.L.; Marangoci, N.L.; Ibanescu, S.A.; Zonda, R.; Rotaru, A.; Pinteală, M. Mass Spectrometry as a Complementary Approach for Noncovalently Bound Complexes Based on Cyclodextrins. In Advances in Experimental Medicine and Biology; Woods, A.G., Darie, C.C., Eds.; NLM (Medline); Springer: Berlin/Heidelberg, Germany, 2019; Volume 1140, pp. 685–701. [Google Scholar] [CrossRef]
- Braga, S.S.; Gonçalves, I.S.; Pillinger, M.; Ribeiro-Claro, P.; Teixeira-Dias, J.J. Experimental and Theoretical Study of the Interaction of Molybdenocene Dichloride (Cp2MoCl2) with β-Cyclodextrin. J. Organomet. Chem. 2001, 632, 11–16. [Google Scholar] [CrossRef]
- Guo, M.; Song, F.; Liu, Z.; Liu, S. Characterization of Non-Covalent Complexes of Rutin with Cyclodextrins by Electrospray Ionization Tandem Mass Spectrometry. J. Mass Spectrom. 2004, 39, 594–599. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Jiang, K.; Li, K.; Cong, Y.; Pu, S.; Jin, Y.; Lin, J. Preparation, Characterisation and Antitumour Activity of β-, γ- and HP-β-Cyclodextrin Inclusion Complexes of Oxaliplatin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 152, 501–508. [Google Scholar] [CrossRef]
- Morales, A.; Weber, R.T.; Melendez, E. Spectroscopic and Thermal Characterization of the Host–Guest Interactions between A-, Β- and Γ-cyclodextrins and Vanadocene Dichloride. Appl. Organomet. Chem. 2008, 22, 440–450. [Google Scholar] [CrossRef]
- Morales, A.; Santana, A.; Althoff, G.; Melendez, E. Host–Guest Interactions between Calixarenes and Cp2NbCl2. J. Organomet. Chem. 2011, 696, 2519–2527. [Google Scholar] [CrossRef][Green Version]
- Turel, I.; Demšar, A.; Košmrlj, J. The Interactions of Titanocene Dihalides with α-, β- and γ-Cyclodextrin Host Molecules. J. Incl. Phenom. Macrocycl. Chem. 1999, 35, 595–604. [Google Scholar] [CrossRef]
- Bakhtiar, R.; Kaifer, A.E. Mass Spectrometry Studies on the Complexation of Several Organometallic Complexes by α- and β-Cyclodextrins. Rapid Commun. Mass Spectrom. 1998, 12, 111–114. [Google Scholar] [CrossRef]
- Lebrilla, C.B. The Gas-Phase Chemistry of Cyclodextrin Inclusion Complexes. Acc. Chem. Res. 2001, 34, 653–661. [Google Scholar] [CrossRef]
- Barylyuk, K.; Balabin, R.M.; Grünstein, D.; Kikkeri, R.; Frankevich, V.; Seeberger, P.H.; Zenobi, R. What Happens to Hydrophobic Interactions during Transfer from the Solution to the Gas Phase? The Case of Electrospray-Based Soft Ionization Methods. J. Am. Soc. Mass Spectrom. 2011, 22, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Bruni, P.S.; Schürch, S. Fragmentation Mechanisms of Protonated Cyclodextrins in Tandem Mass Spectrometry. Carbohydr. Res. 2021, 504, 108316. [Google Scholar] [CrossRef] [PubMed]
- Memboeuf, A.; Jullien, L.; Lartia, R.; Brasme, B.; Gimbert, Y. Tandem Mass Spectrometric Analysis of a Mixture of Isobars Using the Survival Yield Technique. J. Am. Soc. Mass Spectrom. 2011, 22, 1744–1752. [Google Scholar] [CrossRef]
- Dossmann, H.; Fontaine, L.; Weisgerber, T.; Bonnet, V.; Monflier, E.; Ponchel, A.; Przybylski, C. First Steps to Rationalize Host-Guest Interaction between α-, β-, and γ-Cyclodextrin and Divalent First-Row Transition and Post-Transition Metals (Subgroups VIIB, VIIIB, and IIB). Inorg. Chem. 2021, 60, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Przybylski, C.; Bonnet, V. Discrimination of Cyclic and Linear Oligosaccharides by Tandem Mass Spectrometry Using Collision-Induced Dissociation (CID), Pulsed-Q-Dissociation (PQD) and the Higherenergy C-Trap Dissociation Modes. Rapid Commun. Mass Spectrom. 2013, 27, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Szabó, D.; Schlosser, G.; Vékey, K.; Drahos, L.; Révész, Á. Collision Energies on QTof and Orbitrap Instruments: How to Make Proteomics Measurements Comparable? J. Mass Spectrom. 2021, 56, e4693. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruni, P.S.; Schürch, S. Mass Spectrometric Evaluation of β-Cyclodextrins as Potential Hosts for Titanocene Dichloride. Int. J. Mol. Sci. 2021, 22, 9789. https://doi.org/10.3390/ijms22189789
Bruni PS, Schürch S. Mass Spectrometric Evaluation of β-Cyclodextrins as Potential Hosts for Titanocene Dichloride. International Journal of Molecular Sciences. 2021; 22(18):9789. https://doi.org/10.3390/ijms22189789
Chicago/Turabian StyleBruni, Pia S., and Stefan Schürch. 2021. "Mass Spectrometric Evaluation of β-Cyclodextrins as Potential Hosts for Titanocene Dichloride" International Journal of Molecular Sciences 22, no. 18: 9789. https://doi.org/10.3390/ijms22189789
APA StyleBruni, P. S., & Schürch, S. (2021). Mass Spectrometric Evaluation of β-Cyclodextrins as Potential Hosts for Titanocene Dichloride. International Journal of Molecular Sciences, 22(18), 9789. https://doi.org/10.3390/ijms22189789





