Fetal Brain Elicits Sexually Conflicting Transcriptional Response to the Ablation of Uterine Forkhead Box A2 (Foxa2) in Mice
Abstract
:1. Introduction
2. Results
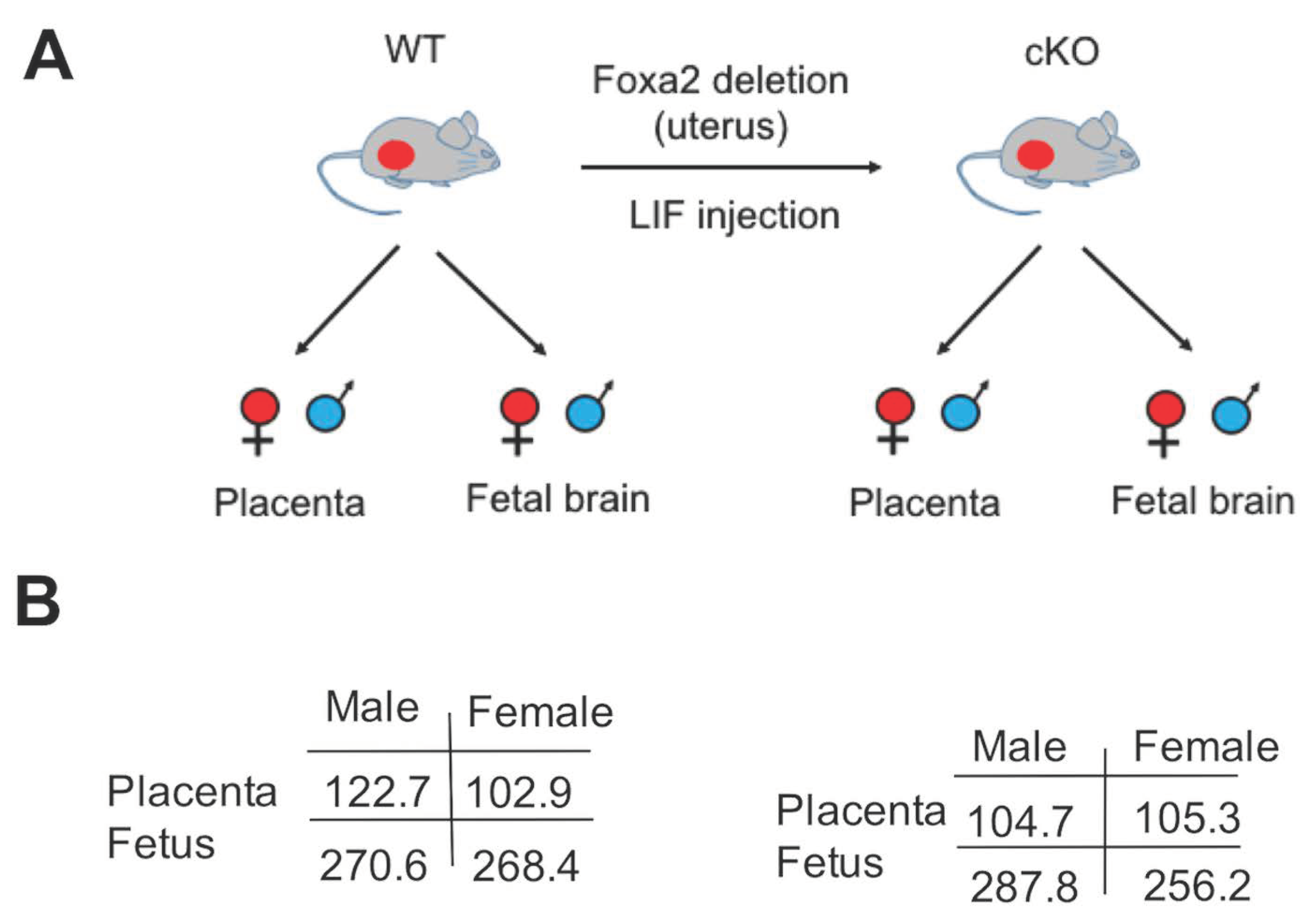
2.1. Lack of Uterine Foxa2 Altered Fetal and Placental Weight in a Sex-Biased Manner
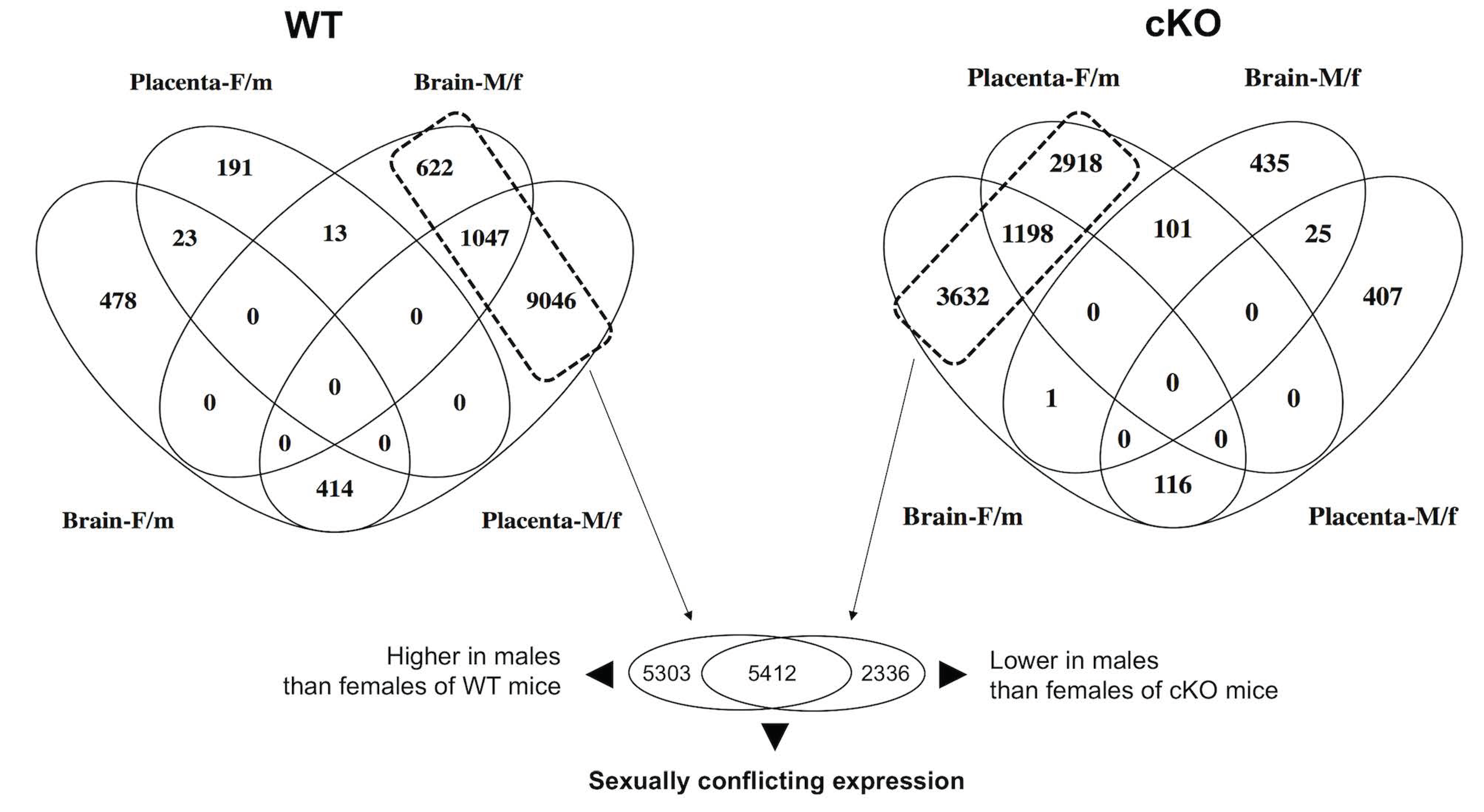
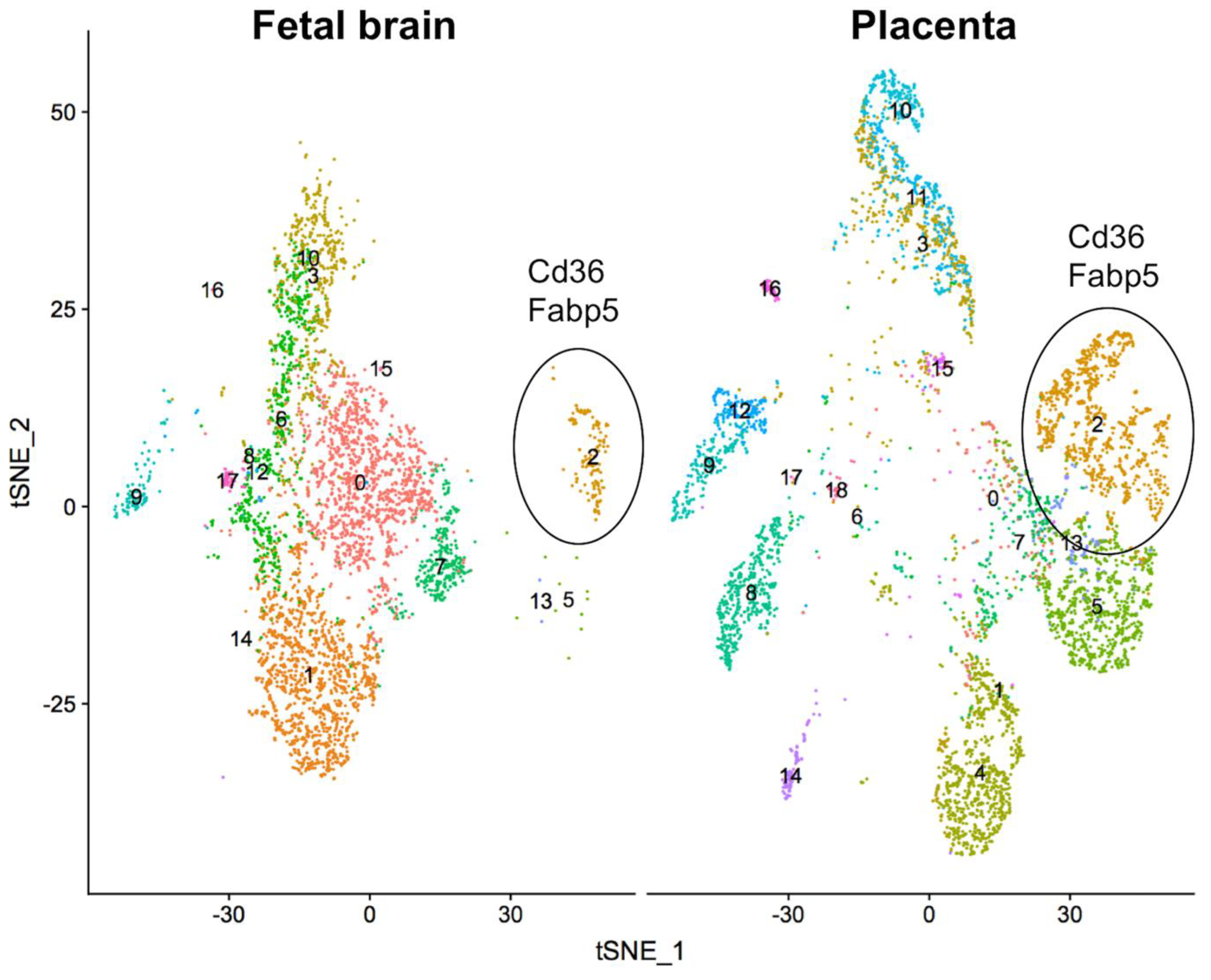
2.2. Global Alteration in Gene Expression of Fetal Brain and Placenta in Response to the Ablation of Uterine Foxa2
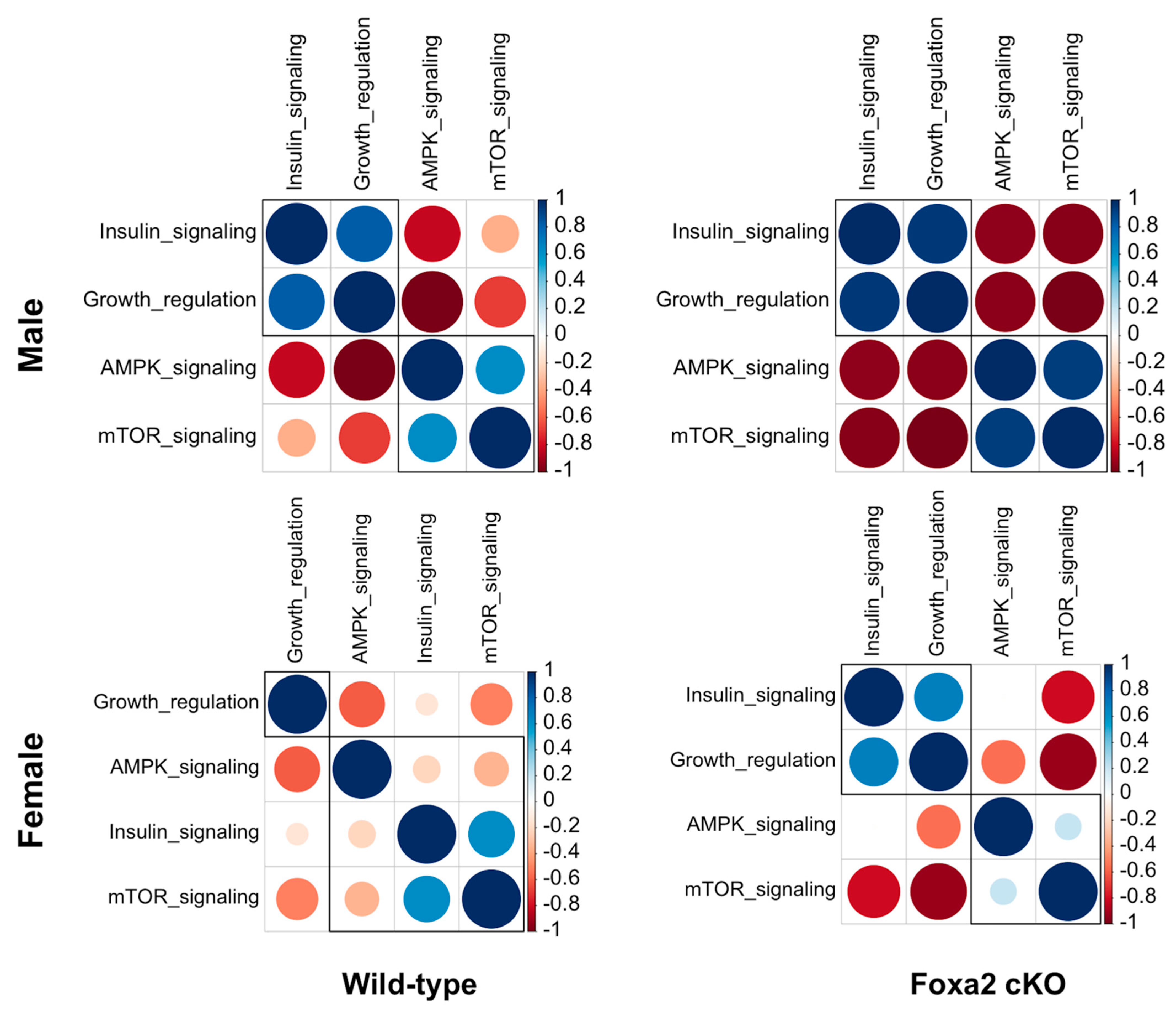
2.3. Lack of Uterine Foxa2 Altered Growth and Nutrient Signaling Genes in the Placenta and Fetal Brain
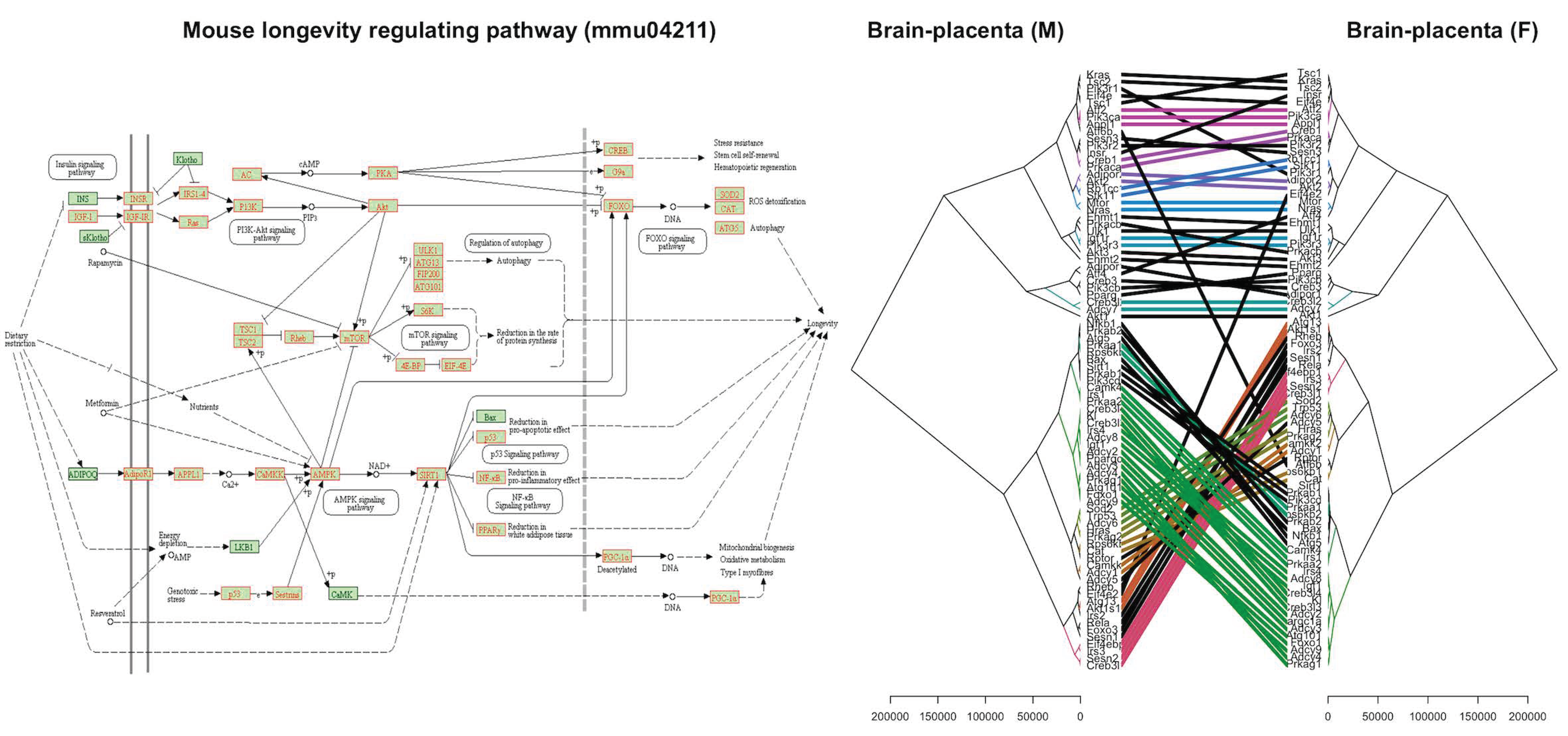
2.4. Differential Expression of Longevity Genes in the Placenta and Fetal Brain in Response to the Lack of Uterine Foxa2
2.5. Angiogenesis Genes Were Affected in the Fetal Brain and Placenta due to the Lack of Uterine Foxa2
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golson, M.L.; Kaestner, K.H. Fox transcription factors: From development to disease. Development 2016, 143, 4558–4570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burtscher, I.; Lickert, H. Foxa2 regulates polarity and epithelialization in the endoderm germ layer of the mouse embryo. Development 2009, 136, 1029–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, A.A.; Khazanov-Zisman, S.; Netser, Y.; Klar, A.; Ben-Arie, N. Nato3 plays an integral role in dorsoventral patterning of the spinal cord by segregating floor plate/p3 fates via Nkx2.2 suppression and Foxa2 maintenance. Development 2014, 141, 574–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, A.A.; Nissim-Eliraz, E.; Zisman, S.; Golan-Lev, T.; Schatz, O.; Klar, A.; Ben-Arie, N. Foxa2 regulates the expression of Nato3 in the floor plate by a novel evolutionarily conserved promoter. Mol. Cell. Neurosci. 2011, 46, 187–199. [Google Scholar] [CrossRef]
- Harrelson, Z.; Kaestner, K.H.; Evans, S.M. Foxa2 mediates critical functions of prechordal plate in patterning and morphogenesis and is cell autonomously required for early ventral endoderm morphogenesis. Biol. Open 2012, 1, 173–181. [Google Scholar] [CrossRef]
- Kelleher, A.; DeMayo, F.J.; Spencer, T.E. Uterine Glands: Developmental Biology and Functional Roles in Pregnancy. Endocr. Rev. 2019, 40, 1424–1445. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-W.; Kwak, I.; Lee, K.; Kim, T.H.; Large, M.; Stewart, C.L.; Kaestner, K.H.; Lydon, J.P.; DeMayo, F. Foxa2 Is Essential for Mouse Endometrial Gland Development and Fertility1. Biol. Reprod. 2010, 83, 396–403. [Google Scholar] [CrossRef] [Green Version]
- Kelleher, A.; Peng, W.; Pru, J.K.; Pru, C.A.; DeMayo, F.; Spencer, T.E. Forkhead box a2 (FOXA2) is essential for uterine function and fertility. Proc. Natl. Acad. Sci. USA 2017, 114, E1018–E1026. [Google Scholar] [CrossRef] [Green Version]
- Stewart, C.L.; Kaspar, P.; Brunet, L.J.; Bhatt, H.; Gadi, I.; Köntgen, F.; Abbondanzo, S.J. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 1992, 359, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Behura, S.K.; Kelleher, A.M.; Spencer, T.E. Evidence for functional interactions between the placenta and brain in pregnant mice. FASEB J. 2018, 33, 4261–4272. [Google Scholar] [CrossRef]
- Behura, S.K.; Dhakal, P.; Kelleher, A.M.; Balboula, A.; Patterson, A.; Spencer, T.E. The brain-placental axis: Therapeutic and pharmacological relevancy to pregnancy. Pharmacol. Res. 2019, 149, 104468. [Google Scholar] [CrossRef] [PubMed]
- Kratimenos, P.; Penn, A.A. Placental programming of neuropsychiatric disease. Pediatr. Res. 2019, 86, 157–164. [Google Scholar] [CrossRef]
- Kalisch-Smith, J.I.; Simmons, D.G.; Pantaleon, M.; Moritz, K.M. Sex differences in rat placental development: From pre-implantation to late gestation. Biol. Sex Differ. 2017, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, C.S. Sex-Specific Placental Responses in Fetal Development. Endocrinology 2015, 156, 3422–3434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenhouse, C.; Hogg, C.O.; Ashworth, C.J. Associations between fetal size, sex and placental angiogenesis in the pig. Biol. Reprod. 2018, 100, 239–252. [Google Scholar] [CrossRef] [Green Version]
- Tekola-Ayele, F.; Workalemahu, T.; Gorfu, G.; Shrestha, D.; Tycko, B.; Wapner, R.; Zhang, C.; Louis, G.M.B. Sex differences in the associations of placental epigenetic aging with fetal growth. Aging 2019, 11, 5412–5432. [Google Scholar] [CrossRef]
- Denisova, E.I.; Kozhevnikova, V.V.; Bazhan, N.M.; Makarova, E.N. Sex-specific effects of leptin administration to pregnant mice on the placentae and the metabolic phenotypes of offspring. FEBS Open Bio 2019, 10, 96–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saoi, M.; Kennedy, K.; Gohir, W.; Sloboda, D.M.; Britz-McKibbin, P. Placental Metabolomics for Assessment of Sex-specific Differences in Fetal Development During Normal Gestation. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shook, L.L.; Kislal, S.; Edlow, A.G. Fetal brain and placental programming in maternal obesity: A review of human and animal model studies. Prenat. Diagn. 2020, 40, 1126–1137. [Google Scholar] [CrossRef]
- Dhakal, P.; Kelleher, A.M.; Behura, S.K.; Spencer, T.E. Sexually dimorphic effects of forkhead box a2 (FOXA2) and uterine glands on decidualization and fetoplacental development. Proc. Natl. Acad. Sci. USA 2020, 117, 23952–23959. [Google Scholar] [CrossRef]
- Templeman, N.M.; Murphy, C.T. Regulation of reproduction and longevity by nutrient-sensing pathways. J. Cell Biol. 2017, 217, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Hart, B.; Morgan, E.; Alejandro, E.U. Nutrient sensor signaling pathways and cellular stress in fetal growth restriction. J. Mol. Endocrinol. 2019, 62, R155–R165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz, P.; Powell, T.L.; Jansson, T. The Role of Placental Nutrient Sensing in Maternal-Fetal Resource Allocation1. Biol. Reprod. 2014, 91, 82. [Google Scholar] [CrossRef] [Green Version]
- Efeyan, A.; Comb, W.C.; Sabatini, D.M. Nutrient-sensing mechanisms and pathways. Nature 2015, 517, 302–310. [Google Scholar] [CrossRef] [Green Version]
- Jansson, T.; Powell, T. Role of Placental Nutrient Sensing in Developmental Programming. Clin. Obstet. Gynecol. 2013, 56, 591–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacutu, R.; Thornton, D.; Johnson, E.; Budovsky, A.; Barardo, D.; Craig, T.; Diana, E.; Lehmann, G.; Toren, D.; Wang, J.; et al. Human Ageing Genomic Resources: New and updated databases. Nucleic Acids Res. 2017, 46, D1083–D1090. [Google Scholar] [CrossRef]
- Kanfi, Y.; Naiman, S.; Amir, G.; Peshti, V.; Zinman, G.; Nahum, L.; Bar-Joseph, Z.; Cohen, H.Y. The sirtuin SIRT6 regulates lifespan in male mice. Nature 2012, 483, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, R.; Zhou, Y.; Fei, L.; Sun, H.; Lai, S.; Saadatpour, A.; Zhou, Z.; Chen, H.; Ye, F.; et al. Mapping the Mouse Cell Atlas by Microwell-Seq. Cell 2018, 172, 1091–1107.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, L.; Perez-Garcia, V.; Hemberger, M. Regulation of Placental Development and Its Impact on Fetal Growth—New Insights From Mouse Models. Front. Endocrinol. 2018, 9, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, H.; Seki, R.; Yokonishi, S.; Yamauchi, T.; Yokoyama, K. Relationship between fetal weight, placental growth and litter size in mice from mid- to late-gestation. Reprod. Toxicol. 2006, 21, 267–270. [Google Scholar] [CrossRef]
- Garland, T., Jr. Trade-Offs. Curr. Biol. 2014, 24, R60–R61. [Google Scholar] [CrossRef] [Green Version]
- Roff Trade-offs between growth and reproduction: An analysis of the quantitative genetic evidence. J. Evol. Biol. 2000, 13, 434–445. [CrossRef]
- Ingleby, F.C.; Flis, I.; Morrow, E. Sex-Biased Gene Expression and Sexual Conflict throughout Development: Table 1. Cold Spring Harb. Perspect. Biol. 2014, 7, a017632. [Google Scholar] [CrossRef]
- Chen, D.-B.; Zheng, J. Regulation of Placental Angiogenesis. Microcirculation 2013, 21, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boeldt, D.S.; Bird, I.M. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J. Endocrinol. 2017, 232, R27–R44. [Google Scholar] [CrossRef] [Green Version]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [Green Version]
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef] [Green Version]
- Bonnin, A.; Goeden, N.; Chen, K.; Wilson, M.; King, J.; Shih, J.C.; Blakely, R.D.; Deneris, E.S.; Levitt, P. A transient placental source of serotonin for the fetal forebrain. Nature 2011, 472, 347–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, Q.; Han, W.; Yang, X. O-GlcNAc as an Integrator of Signaling Pathways. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef]
- Cork, G.K.; Thompson, J.; Slawson, C. Real Talk: The Inter-play Between the mTOR, AMPK, and Hexosamine Biosynthetic Pathways in Cell Signaling. Front. Endocrinol. 2018, 9, 522. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.K. Life history trade-offs and the partitioning of maternal investment. Evol. Med. Public Health 2018, 2018, 153–166. [Google Scholar] [CrossRef]
- Sund, N.J.; Ang, S.-L.; Sackett, S.D.; Shen, W.; Daigle, N.; Magnuson, M.; Kaestner, K.H. Hepatocyte Nuclear Factor 3β (Foxa2) Is Dispensable for Maintaining the Differentiated State of the Adult Hepatocyte. Mol. Cell. Biol. 2000, 20, 5175–5183. [Google Scholar] [CrossRef] [Green Version]
- Daikoku, T.; Ogawa, Y.; Terakawa, J.; Ogawa, A.; DeFalco, T.; Dey, S.K. Lactoferrin-iCre: A New Mouse Line to Study Uterine Epithelial Gene Function. Endocrinology 2014, 155, 2718–2724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhakal, P.; Soares, M.J. Single-step PCR-based genetic sex determination of rat tissues and cells. BioTechniques 2017, 62, 232–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behura, S.K.; Kelleher, A.; Spencer, T.E. Regulation of uterine genes during the peri-implantation period, and its relationship to the maternal brain in gestating mice. Mol. Reprod. Dev. 2020, 87, 482–492. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2013, 30, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.; Smyth, G. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2009, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Galili, T. dendextend: An R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef] [Green Version]
- E Meyer, P.; Lafitte, F.; Bontempi, G. minet: A R/Bioconductor Package for Inferring Large Transcriptional Networks Using Mutual Information. BMC Bioinform. 2008, 9, 1–10. [Google Scholar] [CrossRef]
- Butler, A.; Hoffman, P.; Smibert, P.; Papalexi, E.; Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018, 36, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Becht, E.; McInnes, L.; Healy, J.; Dutertre, C.-A.; Kwok, I.W.H.; Ng, L.G.; Ginhoux, F.; Newell, E.W. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 2018, 37, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Franzén, O.; Gan, L.-M.; Björkegren, J.L.M. PanglaoDB: A web server for exploration of mouse and human single-cell RNA sequencing data. Database 2019, 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhakal, P.; Strawn, M.; Samal, A.; Behura, S.K. Fetal Brain Elicits Sexually Conflicting Transcriptional Response to the Ablation of Uterine Forkhead Box A2 (Foxa2) in Mice. Int. J. Mol. Sci. 2021, 22, 9693. https://doi.org/10.3390/ijms22189693
Dhakal P, Strawn M, Samal A, Behura SK. Fetal Brain Elicits Sexually Conflicting Transcriptional Response to the Ablation of Uterine Forkhead Box A2 (Foxa2) in Mice. International Journal of Molecular Sciences. 2021; 22(18):9693. https://doi.org/10.3390/ijms22189693
Chicago/Turabian StyleDhakal, Pramod, Monica Strawn, Ananya Samal, and Susanta K. Behura. 2021. "Fetal Brain Elicits Sexually Conflicting Transcriptional Response to the Ablation of Uterine Forkhead Box A2 (Foxa2) in Mice" International Journal of Molecular Sciences 22, no. 18: 9693. https://doi.org/10.3390/ijms22189693
APA StyleDhakal, P., Strawn, M., Samal, A., & Behura, S. K. (2021). Fetal Brain Elicits Sexually Conflicting Transcriptional Response to the Ablation of Uterine Forkhead Box A2 (Foxa2) in Mice. International Journal of Molecular Sciences, 22(18), 9693. https://doi.org/10.3390/ijms22189693






