Abstract
Sjögren’s syndrome (SS) is a chronic autoimmune disease characterized by dysfunction of salivary and lacrimal glands, resulting in xerostomia (dry mouth) and keratoconjunctivitis sicca (dry eyes). Autoantibodies, such as anti-SSA and anti-SSB antibodies, are hallmarks and important diagnostic factors for SS. In our previous study, we demonstrated that SS-like xerostomia was observed in SATB1 conditional knockout (SATB1cKO) mice, in which the floxed SATB1 gene was specifically deleted in hematopoietic cells as early as 4 weeks of age. In these mice, autoantibodies were not detected until 8 weeks of age in SATB1cKO mice, although exocrine gland function reached its lowest at this age. Therefore, other markers may be necessary for the diagnosis of SS in the early phase. Here, we found that mRNA expression of the interferon γ (IFN-γ) gene and the IFN-responsive indoleamine 2,3-dioxygenase (IDO) gene is upregulated in the salivary glands of SATB1cKO mice after 3 and 4 weeks of age, respectively. We detected l-kynurenine (l-KYN), an intermediate of l-tryptophan (l-Trp) metabolism mediated by IDO, in the serum of SATB1cKO mice after 4 weeks of age. In addition, the upregulation of IDO expression was significantly suppressed by the administration of IFN-γ neutralizing antibodies in SATB1cKO mice. These results suggest that the induction of IFN-dependent IDO expression is an initial event that occurs immediately after the onset of SS in SATB1cKO mice. These results also imply that serum l-KYN could be used as a marker for SS diagnosis in the early phases of the disease before autoantibodies are detectable.
1. Introduction
Sjögren’s syndrome (SS) is a systemic autoimmune disease in which the salivary and lacrimal glands are functionally impaired [1]. As a result, SS patients suffer from xerostomia (dry mouth) and keratoconjunctivitis sicca (dry eye) [2]. Since any damage to the exocrine glands is functionally and structurally irreversible, SS patients require moisturizing treatments of the mouth and eyes throughout their lives [3]. Therefore, early diagnosis of SS is important for appropriate treatment of the disease to ensure a better quality of life.
Special AT-rich sequence binding protein-1 (SATB1) is a chromatin organizer that positively and negatively regulates the expression of various genes [4,5]. SATB1 is expressed by the earliest hematopoietic stem cells (HSCs) and is necessary to maintain HSC stemness [6,7]. Although SATB1 is expressed in various hematopoietic cell types, such as dendritic cells [8,9], SATB1 expression in T-lineage cells has garnered interest. This is because SATB1 expression is higher in T lineage cells than in other types of hematopoietic cells [9,10], especially CD4/CD8 double-positive thymocytes, in which positive selection events occur [11,12]. Indeed, positive selection is impaired in both SATB1 null and hematopoietic cell-specific SATB1 conditional knockout (SATB1cKO) mice, which were used in this study [10,11]. In addition, negative selection events are impaired in the absence of SATB1 [11]. Furthermore, the development of regulatory T (Treg) cells in the thymus is blocked in SATB1cKO mice [11,13,14], suggesting that SATB1 is necessary to establish immune tolerance.
Disturbances in immune tolerance may lead to autoimmune reactions [15]. In a previous study, SATB1cKO mice begin to die after 24 weeks of age due to renal failure, most likely caused by lupus nephritis associated with systemic lupus erythematosus (SLE) [11]. Notably, salivary gland dysfunction is observed in SATB1cKO mice as early as 4 weeks of age, with the maximum severity observed at ~8 weeks after birth [16]. Immune cell infiltration and the destruction of the salivary gland structure were observed in SATB1cKO mice, which is also a characteristic feature observed in patients with SS [16]. Therefore, SATB1cKO mice were used as the mouse model for SS. Since individual variations in the onset and progression of SS disease have been observed in other SS model mice, SATB1cKO mice are useful tools for analyzing SS pathogenesis, especially in the early phase of the disease. We previously demonstrated that the levels of anti-SSA and anti-SSB antibodies, which are used in the clinical diagnosis of SS, are upregulated when saliva production reaches its lowest levels in SATB1cKO mice [16]. This finding implies that markers other than the presence of autoantibodies are necessary to properly diagnose SS in the early phase of the disease when the exocrine function is maintained (see Figure 1).
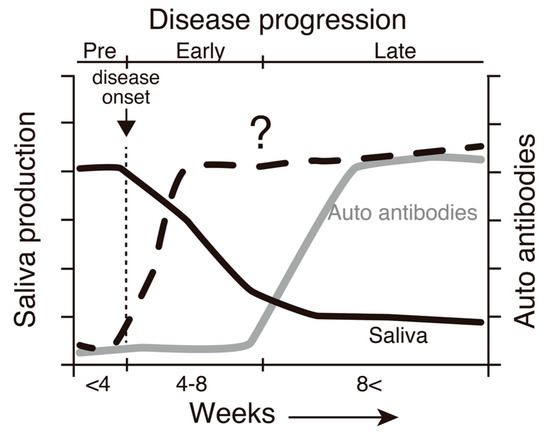
Figure 1.
A schematic diagram demonstrating a correlation between SS-like pathogenesis and the age of SATB1cKO mice based on our previous investigation [16]. A decrease in saliva production is initiated at 4 weeks of age (solid line). The production levels reach the basal level at ~8 weeks of age, when autoantibodies, such as anti-SSA and anti-SSB antibodies, are detected in the serum (dotted line). Therefore, we can distinguish the three distinct SS stages in SATB1cKO mice: pre (before 4 weeks of age), early (between 4 and 8 weeks old) and late (older than 8 weeks after birth). In the present study, we examined factors whose levels may be elevated in the early phase of the disease in SATB1cKO mice (gray line).
Here, we found that the levels of l-kynurenine (l-KYN), an intermediate in the breakdown of l-tryptophan (l-Trp) [17], were upregulated in the serum of SATB1cKO mice as early as 4 weeks of age. The expression of indoleamine 2,3-dioxygenase (IDO, encoded by the IDO1 gene) [18], which mediates the generation of l-KYN from l-Trp, was upregulated in the salivary glands of 4-week-old SATB1cKO mice, suggesting that the induction of IDO expression might be one of the earliest responses in exocrine glands injured by an autoimmune reaction. The presence of l-KYN in serum could be a noninvasive diagnostic marker for SS before the deterioration of the exocrine gland function.
2. Results
2.1. Expression of IFN-γ Is Upregulated in the Salivary Gland of SATB1cKO Mice upon Reduction in Saliva Production
We previously demonstrated that SATB1cKO mice are prone to autoimmune diseases and develop SS-like symptoms, such as the loss of saliva production and the infiltration of immune cells in the salivary glands as early as 4 weeks after birth [11,16]. In addition, lacrimal gland dysfunction was also observed in the SATBcKO mice after 4 weeks of age (data not shown). Although the pathophysiology is thought to vary across patients with SS, the involvement of interferon γ (IFN-γ) in SS pathogenesis has been suggested in some patients with SS and the SS mice model [19,20,21]. Therefore, we first quantified IFN-γ expression in the salivary glands of the SATB1cKO and wild type (WT) mice. The quantitative PCR analysis showed that IFN-γ expression was upregulated in the salivary glands of the SATB1cKO mice even at 3 weeks of age, a week earlier than the onset of SS, which led to the reduction in saliva production (Figure 2a). CD4+ helper T (Th) cells infiltrate the salivary glands of the SATB1cKO mice [16]. We examined the expression of signature cytokines produced by these Th cells. We observed that most Th cells in the salivary glands of the SATB1cKO mice were IFN-γ-producing Th1 cells (Figure 2b,c). The percentage of Th1 cells in the total CD4+ T cell population that infiltrated the salivary glands of the SATB1cKO mice was not significantly different but showed a minor reduction between 3 and 12 weeks after birth (Figure 2c). Distinct Th17 cells were also observed in addition to Th1 cells; however, very few Th2 cells were present in the SATB1cKO salivary glands (Figure 2b and 2c). In addition to IFN-γ, IL-6, a pro-inflammatory cytokine, was also expressed in SATB1cKO salivary glands after 4 weeks of age (Figure 2d), implying that inflammation is observed following prominent salivary gland destruction in the SATB1cKO mice.
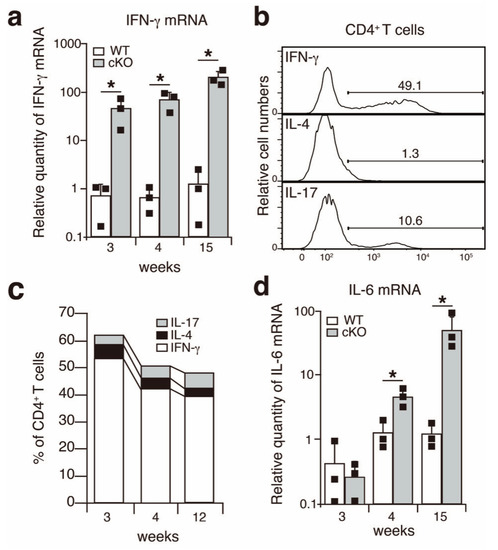
Figure 2.
IFN-γ is upregulated in the salivary glands of SATB1cKO mice. (a,d) Quantitative PCR analyses of the expression of IFN-γ (a) and IL-6 (d) in the salivary glands of WT (open bar) and SATB1cKO mice (filled bar). Each result was normalized to the expression level of HPRT. Dots in the graph indicate individual values (n = 3). The Student’s t-test was used for statistical analysis (* p < 0.05). (b) A representative FACS plot showing the expression of IFN-γ, IL-4 and IL-17 upon infiltration of CD4+ T cells in the salivary glands of SATB1cKO mice in the late SS phase (12 weeks old). (c) The percentage of CD4+ T cells expressing IFN-γ (open), IL-4 (filled with black) and IL-17 (filled with grey) that infiltrated the salivary gland of SATB1cKO mice at 3, 4 and 12 weeks after birth. The mean value (n = 3–5) is shown.
Although upregulation of the expression of IFN-γ and IL-6 was observed in the salivary glands in the early phase of SS in the SATB1cKO mice, these two cytokines were not detected at 3 weeks of age (pre-SS phase) or 5 weeks of age (early SS phase) in the serum of the SATB1cKO mice (Figure 3). In the late phase of SS, both IFN-γ (Figure 3a) and IL-6 (Figure 3b) were present in the serum of the SATB1cKO mice. Therefore, serum IFN-γ and IL-6 can be used as indicators of the late SS phase but not for the early phase of SS in the SATB1cKO mice, as is the case with anti-SSA and anti-SSB antibodies (see Figure 1).
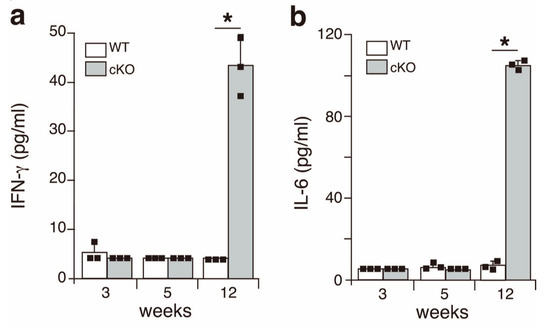
Figure 3.
Inflammatory cytokines are present in the serum of SATB1cKO mice in the late but not the early phase of SS. The levels of IFN-γ (a) and IL-6 (b) were quantified in the serum obtained from WT (open bar) and SATB1cKO mice (filled bar) at different ages indicated in the figures. Dots in the graph indicate individual values (n = 3). The Student’s t-test was used for statistical analysis (* p < 0.05).
2.2. l-Kynurenine Is Detected in the Serum of SATB1cKO Mice as Early as Four Weeks after Birth
Since IFN-γ demonstrates diverse effects, we hypothesized that the expression of IFN-responsive genes might be upregulated in the salivary glands of SATB1cKO mice. One of the IFN-responsive genes, IDO, was upregulated at and after 4 weeks of age in the SATB1cKO mice (Figure 4a). We next examined IDO protein expression in the salivary glands of the SATB1cKO mice. We stained sections of the salivary glands harvested from the SATB1cKO and WT mice at various ages with anti-IDO antibodies in conjunction with anti-CD4 and anti-B220 antibodies. In accordance with the results shown in Figure 4a, the IDO protein was not detected in the salivary glands of the WT mice at any age or in the 3-week-old SATB1cKO mice (Figure 4b). However, IDO expression was observed in the salivary glands of the 4- and 8-week-old SATB1cKO mice (Figure 4b). Exocrine gland dysfunction in SATB1cKO mice is initiated at 4 weeks after birth when infiltrated lymphocytes are sparsely present in the salivary gland (Figure 4b) [16]. Nevertheless, IDO expression was observed in most gland cells, not just only in the cells around the foci with infiltrated lymphocytes (Figure 4b), suggesting that IDO expression might indeed be induced by soluble factors, such as IFN-γ.
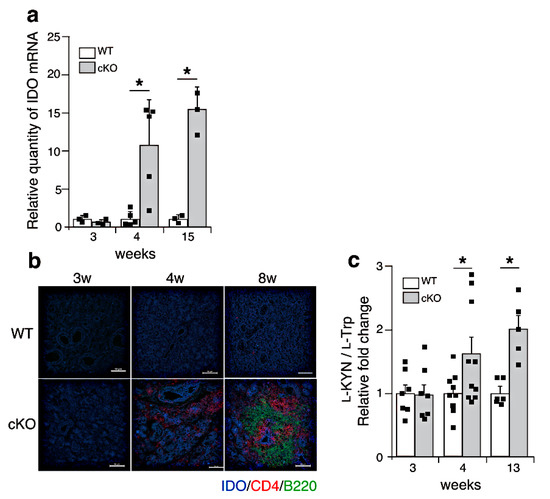
Figure 4.
l-Trp metabolism is upregulated in SATB1cKO mice after the onset of SS. (a) Quantitative PCR analyses of IDO expression in the salivary glands of WT (open bar) and SATB1cKO mice (filled bar). Each result was normalized to the expression level of HPRT. Dots in the graph indicate individual values (n = 3–5). The Student’s t-test was used for statistical analysis (* p < 0.05). (b) Sections of the salivary glands from WT and SATB1cKO mice were stained with fluorescein-labeled anti-mouse IDO (blue), anti-mouse CD4 (Red) and anti-mouse B220 (green) antibodies. Scale bars represent 100 μm. (c) Serum was collected from WT (open bar) and SATB1cKO mice (filled bar) at different ages and analyzed for l-Trp and l-KYN levels using LC-MS/MS. The ratio of l-KYN to l-Trp in the serum was calculated. Normalized values, which are indicated as dots, in SATB1cKO mice to WT mice are shown (n = 6–9). The Mann–Whitney U test was used for statistical analysis (* p < 0.05).
IDO is an enzyme that degrades l-Trp, an essential amino acid, into active metabolites such as l-KYN [22]. We next examined the presence of l-KYN in the serum of the WT and SATB1cKO mice using mass spectrometry. We found that l-KYN levels were increased in the serum of the SATB1cKO mice as early as 4 weeks of age (Figure 4c) when the dysfunction of salivary and lacrimal glands was observed. This finding suggests that l-KYN could be a serum biomarker for the early phase of SS.
2.3. IDO Expression Is Induced in SATB1cKO Mice in an IFN-γ-Dependent Manner
SATB1cKO mice develop SS and SLE. However, indicators of SLE, such as anti-dsDNA antibodies in the serum and deposition of immune complexes in the kidney, are observed after 12 weeks of age [11]. Therefore, it is plausible that IDO is expressed systemically after a certain age in SATB1cKO mice. To address this issue, we examined the IDO mRNA expression in various organs of the WT and SATB1cKO mice at 4 and 12 weeks of age, at the onset of symptoms of SS and SLE, respectively [11,16]. IDO expression was slightly increased in the spleen of the SATB1cKO mice 4 weeks after birth (Figure 5). In the 12-week-old SATB1cKO mice, a significantly higher IDO expression was observed in the liver and kidney of the SATB1cKO mice than that in the WT mice (Figure 5), implying that IDO expression may be induced in multiple organs when an autoimmune reaction activates systemic inflammatory responses.
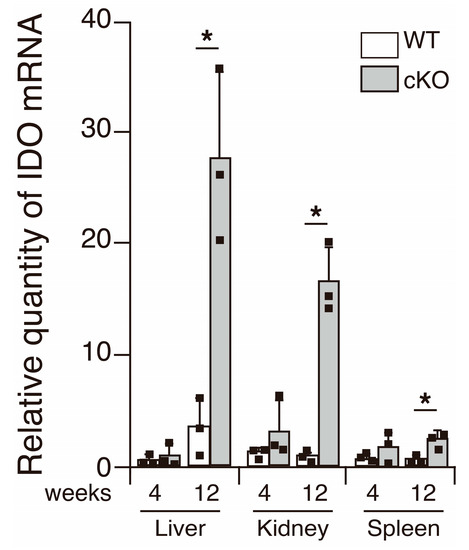
Figure 5.
IDO expression in various organs. IDO expression was examined using quantitative PCR analyses performed with total RNA purified from the liver, kidney and spleen of WT (open bar) and SATB1cKO mice (filled bar) at 4 and 12 weeks of age. Each result was normalized to the expression level of HPRT. Dots in the graph indicate individual values (n = 3). The Student’s t-test was used for statistical analysis (* p < 0.05).
Finally, to elucidate the causative relationship between IFN-γ and IDO expression in the salivary glands of the SATB1cKO mice, we intraperitoneally injected neutralizing IFN-γ antibodies into the 3-week-old SATB1cKO mice every 3 days, with a total of 20 injections, to inhibit IFN-γ function. No improvement in saliva production was observed in the SATB1cKO mice injected with anti-IFN-γ antibodies (Figure 6a), despite reports stating that IFN-γ is necessary for SS pathogenesis in some mouse models [21]. Nevertheless, IDO expression was significantly lower in the salivary glands of the anti-IFN-γ-treated SATB1cKO mice (Figure 6b). In addition, serum l-KYN levels decreased in the SATB1cKO mice following the anti-IFN-γ-antibody injection (Figure 6c). These results demonstrate that the induction of IDO expression in the salivary glands of the SATB1cKO mice is mediated by IFN-γ. Overall, the results of this study suggest that serum l-KYN can be a useful marker for the diagnosis of SS in the early phase before the generation of autoantibodies.
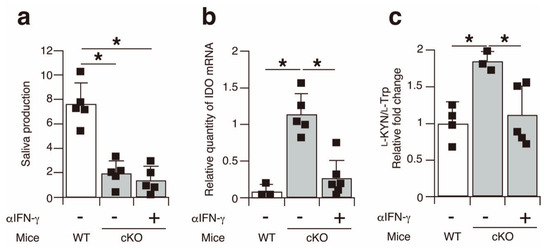
Figure 6.
IDO expression in the salivary glands of SATB1cKO mice is upregulated in an IFN-γ-dependent manner. (a) Saliva production was measured in WT mice (open bar) and SATB1cKO mice (filled bar) with or without injection of anti-IFN-γ antibody. (b) Quantitative PCR analyses of IDO expression in the salivary glands harvested from WT mice (open bar) and SATB1cKO mice (filled bar) with or without injection of anti-IFN-γ antibodies. Each result was normalized to the expression level of HPRT. (c) Serum was collected from WT mice (open bar) and SATB1cKO mice (filled bar) with or without injection of anti-IFN-γ antibodies and analyzed for l-Trp and l-KYN levels using LC-MS/MS. The ratio of l-KYN to l-Trp in the serum was calculated. Normalized values in SATB1cKO mice to WT mice are shown. Dots in the graphs indicate individual values (n = 3–6). The Student’s t-test was used for statistical analysis (* p < 0.05).
3. Discussion
Currently, there is no effective treatment for SS. Therefore, symptomatic treatment for dry mouth and dry eye is important for maintaining the quality of life of patients with SS [23]. Although autoimmune responses cause SS, treatment with immunosuppressive drugs has not successfully treated the disease [23]. One possible reason for this is that the exocrine function already deteriorates at the time of diagnosis, and exocrine dysfunction is irreversible. In this case, some immunosuppressive treatments, which have not been proven efficacious in previous clinical trials, might cease SS disease progression if administered early. Therefore, the identification of useful biomarkers for early SS diagnosis is a crucial first step in the development of novel treatments for SS. In this study, we demonstrated that an increase in serum l-KYN levels could be a useful marker for the diagnosis of SS.
IDO was identified as a type I IFN response gene that mediates the generation of l-KYN from l-Trp in the l-Trp metabolism pathway, while also demonstrating immunosuppressive functions [18,22]. Therefore, it seems that the upregulation of IDO in the salivary and lacrimal glands of SATB1cKO mice after the onset of SS symptoms is a negative feedback reaction against autoimmunity. IFN-γ (known as type II IFN) also mediates the induction of IDO expression in various cell types [24,25]. This expression is induced maybe because the promoter of the IDO gene possesses IFN-stimulated response elements (ISRE) and IFN-γ-activated sequences, which form sites for the binding of IRFs and Stats, respectively [26,27,28,29,30]. In addition, the administration of anti-IFN-γ neutralizing antibodies in the SATB1cKO mice significantly suppressed the upregulation of IDO expression in the salivary glands (Figure 6b), suggesting that the IFN-γ produced by Th1 cells might be an inducer of IDO expression in exocrine gland cells. Importantly, SS patients exhibit higher IFN-γ levels and enhanced Th1 activity in salivary glands compared to non-SS sicca patients [19,20], implying that the presence of l-KYN in the serum can be used as a marker to distinguish between autoimmune-mediated sicca (i.e., SS) and non-SS sicca. Notably, salivary gland dysfunction in the SATB1cKO mice was not improved by anti-IFN-γ antibody injections (Figure 6a). In this sense, it is worth knowing that no significant improvement in SS symptoms was observed in a phase II study examining the effects of an inhibitor of Jak1, an indispensable signaling molecule whose action is mediated via IFN-γ receptors [23]. Therefore, SS pathogenesis in SATB1cKO mice might be similar to that observed in human SS. Since lymphopenic RAG2-deficient mice develop SS after being injected with T cells derived from SATB1cKO mice [16], some T cell factors other than IFN-γ might be involved in SS pathogenesis in the SATB1cKO mice. Controversially, in another SS model of non-obese diabetic (NOD) mice, SS symptoms were not observed in the absence of IFN-γ or IFN-γ receptor genes [21]. In addition, Zhou et al. showed that the neutralization of IFN-γ improved salivary gland dysfunction in NOD mice [31]. Accordingly, the pathogenesis of SS may vary in different mouse models.
In SATB1cKO mice, l-KYN can be detected in the serum as early as 4 weeks after birth (Figure 4c), although diagnostic factors in several SS classification criteria [32,33,34], such as anti-SSA or anti-SSB antibodies, are only detected after 8 weeks of age [16]. IDO activity in SS patients is higher than that in healthy controls; however, IDO expression in each patient was not addressed [35]. Maria et al. demonstrated that IDO expression is upregulated in the peripheral blood of some SS patients [36]. However, it is not clear whether the upregulation in IDO expression is a result of an autoimmune reaction in the exocrine glands. In addition, it has not been elucidated whether IDO is upregulated in the exocrine glands during disease development in patients with SS. Therefore, further studies are necessary to gain an insight into the clinical importance of IDO activity in patients with SS.
4. Materials and Methods
4.1. Mice
SATB1fl/flVav-Cre+ mice were generated as previously described [16,37]. Vav-Cre mice [38] were purchased from Jackson Laboratory (Bar Harbor, ME, USA). RAG2-/- mice were maintained in the laboratory. C57BL/6 mice were obtained from CLEA Japan (Tokyo, Japan). All mice used in this study had a C57BL/6 background and were maintained under specific pathogen-free conditions at the Toho University School of Medicine animal facility. The Toho University Administrative Panel approved all experiments using mice for Animal Care (21-52-435) and recombinant DNA (21-52-440).
4.2. Flow Cytometry
Antibodies used for cell surface and intracellular staining were as follows: anti-CD4-PE-Cy7 (GK1.5), anti-CD8-APC-Cy7 (53-6.7), anti CD16/32 (93), anti-IL-17-PE (TC11-18H10.1) and anti-IFN-γ-FITC (XMG1.2) were obtained from BioLegend. Anti-TCRβ-APC (H57-597) was obtained from Tombo Bioscience. Salivary glands were enzymatically digested, and infiltrating cells were isolated as previously described [16]. For intracellular staining of IL-17 and IFN-γ, the BD Cytofix/CytopermTM Fixation/Permeabilization Kit (BD) was used according to the manufacturer’s protocol. Stained cells were analyzed using an LSRFortessaTM Flow Cytometer (BD), and the data were analyzed with FlowJo software (version 9.8.1; Tree Star, Ashland, OR, USA).
4.3. RNA Extraction and Real-Time PCR
Total RNA was extracted using Isogen II (Nippon Gene, Tokyo, Japan), and the RNA concentration was determined spectrophotometrically at 260 nm. First-strand synthesis was performed using a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA). Quantitative PCR was performed using the TaqMan gene expression assay kit, following the manufacturer’s instructions (Applied Biosystems) on an ABI 7500 fast system (Applied Biosystems). The following primers were used (Applied Biosystems): Hprt, Mm00446968_ml; Ido1, Mm00492590_ml; Ifng, Mm00801778_ml; and Il6, Mm00446190_ml. Expression measurements were determined using the delta–delta Ct methods and normalized to HPRT levels.
4.4. Cytokine Assay
The protein expression of inflammatory cytokines was measured using the HQPLEX Analyte Kit (Cellector, San Diego, CA, USA), according to the manufacturer’s instructions. The results were analyzed using BeadLogic software (Inivai Technologies, Mentone, Australia).
4.5. Histopathology and Immunohistochemistry
For microscopic immunofluorescent analysis, salivary glands were frozen in O.C.T. compound (Tissue-Tek, Sakura Finetek, Tokyo, Japan), sliced at a 6-micrometer thickness, and fixed with 4% PFA for 10 min at room temperature (RT), washed and blocked with phosphate-buffered saline (PBS) containing 5% BSA (Merck, Darmstadt, Germany) and 5% rat serum (ImmunoBioScience Corp., Mukilteo, WA, USA) for 1 h at RT, and then incubated with a rat anti-mouse IDO antibody (BioLegend, San Diego, CA, USA) at 4 °C overnight. The sections were further incubated with Alexa 647-conjugated anti-rat IgG (H+L) (Molecular Probes Eugene, OR, USA) for 1 h at RT. The sections were then stained with Alexa 594-conjugated anti-mouse CD4 (GK1.5) and Alexa 488-labeled anti-mouse B220 (RA3-6B2), purchased from BioLegend, for 2 h at 4 °C. Stained sections were mounted with Dako Fluorescent Mounting Medium (Dako, Santa Clara, CA, USA) and examined using an A1R confocal laser-scanning microscope (Nikon, Tokyo, Japan).
4.6. Detection of l-Trp and l-KYN in Serum Using Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
Mouse serum samples for l-Trp and l-KYN measurements were prepared according to our previous report [39,40] with minor modifications. Briefly, l-Trp and l-KYN concentrations were determined using LC-MS/MS after derivatization with (R)-DBD-PyNCS. The serum ratio of l-KYN to l-Trp concentrations ([l-KYN]/[l-Trp]) was calculated and compared between SATB1cKO and WT mice. The results shown in the figures are relative [l-KYN]/[l-Trp] ratios in SATB1cKO mice against the value in WT mice.
4.7. In Vivo Neutralization of IFN-γ Functions
To block IFN-γ in vivo, mice were injected intraperitoneally with 200 μg of anti-mouse IFN-γ antibody (InVivoMAb, Bio X Cell, Lebanon, NH, USA) every 3 days for 60 days, as described previously [41].
4.8. Measurement of Saliva Volume
The mice were anesthetized using an intraperitoneal (i.p.) injection of a mixture of 0.75 mg/Kg medetomidine (Nippon Zenyaku Kogyo, Koriyama, Japan), 4 mg/kg midazolam (SANDOZ, Yamagata, Japan) and 5 mg/kg butorphanol tartrate (Meiji Seika Pharma, Tokyo, Japan). To stimulate saliva secretion, 0.5 mg/kg pilocarpine hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) was intraperitoneally administered. Saliva was collected using a micropipette for 15 min. The volume of saliva was normalized to the bodyweights of the mice.
4.9. Statistical Analysis
Statistical analysis was performed using Student’s t-test to compare means, or the Mann–Whitney U test with the assumption of unequal variance and a confidence level of 95%.
Author Contributions
Investigation, Y.T., M.O. and T.M.; resources, T.K.-S.; supervision, T.F. and M.K.; visualization, Y.T.; writing—original draft, Y.T. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a MEXT/JSPS KAKENHI (JP24390121 to M.K. and Y.T., JP26670240 to M.K., 18K07075 to Y.T.), a Strategic Research Foundation Grant-aided Project for Private Schools at Heisei 26th (S1411015) from the Ministry of Education, Culture, Sports, Science and Technology to M.K., a Research Promotion Grant from Toho University Graduate School of Medicine (14-02 to M.K.); Project Research Grants from Toho University School of Medicine (23-4 and 26-22 to Y.T.), the Public Foundation of the Vaccination Research Center to M.K., Toho University Joint research Fund to M.K., Y.T., M.O. and K.F., Initiative for Realizing Diversity in the Research Environment from Japan Science and Technology Agency to Y.T. and M.O., an NIH grant (R37CA39681) to T.K.-S.; and a Grant-in Aid for Private University Research Branding Project from the MEXT and Toho University Grant for Research Initiative Program to M.K.
Institutional Review Board Statement
The Toho University Administrative Panel approved all experiments using mice for Animal Care (21-52-435, updated on 1 April 2021) and recombinant DNA (21-52-440, updated on 1 April 2021).
Acknowledgments
We would like to thank Hideto Kameda, Toshihiro Nanki, Yuichi Hori, Taku Naito, Taku Kuwabara, Michitsune Arita, Akiko Inoue and Marii Ise for discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fox, R.I. Sjogren’s syndrome. Lancet 2005, 366, 321–331. [Google Scholar] [CrossRef]
- Kassan, S.S.; Moutsopoulos, H.M. Clinical manifestations and early diagnosis of Sjogren syndrome. Arch. Intern. Med. 2004, 164, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zeron, P.; Bombardieri, S.; Bootsma, H.; De Vita, S.; Dorner, T.; Fisher, B.A.; Gottenberg, J.E.; Hernandez-Molina, G.; Kocher, A.; et al. EULAR recommendations for the management of Sjogren’s syndrome with topical and systemic therapies. Ann. Rheum. Dis. 2020, 79, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, L.A.; Joh, T.; Kohwi, Y.; Kohwi-Shigematsu, T. A tissue-specific MAR/SAR DNA-binding protein with unusual binding site recognition. Cell 1992, 70, 631–645. [Google Scholar] [CrossRef]
- Cai, S.; Lee, C.C.; Kohwi-Shigematsu, T. SATB1 packages densely looped, transcriptionally active chromatin for coordinated expression of cytokine genes. Nat. Genet. 2006, 38, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Will, B.; Vogler, T.O.; Bartholdy, B.; Garrett-Bakelman, F.; Mayer, J.; Barreyro, L.; Pandolfi, A.; Todorova, T.I.; Okoye-Okafor, U.C.; Stanley, R.F.; et al. Satb1 regulates the self-renewal of hematopoietic stem cells by promoting quiescence and repressing differentiation commitment. Nat. Immunol. 2013, 14, 437–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doi, Y.; Yokota, T.; Satoh, Y.; Okuzaki, D.; Tokunaga, M.; Ishibashi, T.; Sudo, T.; Ueda, T.; Shingai, Y.; Ichii, M.; et al. Variable SATB1 Levels Regulate Hematopoietic Stem Cell Heterogeneity with Distinct Lineage Fate. Cell Rep. 2018, 23, 3223–3235. [Google Scholar] [CrossRef]
- Tesone, A.J.; Rutkowski, M.R.; Brencicova, E.; Svoronos, N.; Perales-Puchalt, A.; Stephen, T.L.; Allegrezza, M.J.; Payne, K.K.; Nguyen, J.M.; Wickramasinghe, J.; et al. Satb1 Overexpression Drives Tumor-Promoting Activities in Cancer-Associated Dendritic Cells. Cell Rep. 2016, 14, 1774–1786. [Google Scholar] [CrossRef] [Green Version]
- Satoh, Y.; Yokota, T.; Sudo, T.; Kondo, M.; Lai, A.; Kincade, P.W.; Kouro, T.; Iida, R.; Kokame, K.; Miyata, T.; et al. The Satb1 protein directs hematopoietic stem cell differentiation toward lymphoid lineages. Immunity 2013, 38, 1105–1115. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, J.D.; Yasui, D.H.; Niida, H.; Joh, T.; Loh, D.Y.; Kohwi-Shigematsu, T. The MAR-binding protein SATB1 orchestrates temporal and spatial expression of multiple genes during T-cell development. Genes Dev. 2000, 14, 521–535. [Google Scholar]
- Kondo, M.; Tanaka, Y.; Kuwabara, T.; Naito, T.; Kohwi-Shigematsu, T.; Watanabe, A. SATB1 Plays a Critical Role in Establishment of Immune Tolerance. J. Immunol. 2016, 196, 563–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, L.; Kyewski, B.; Allen, P.M.; Hogquist, K.A. Positive and negative selection of the T cell repertoire: What thymocytes see (and don’t see). Nat. Rev. Immunol. 2014, 14, 377–391. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, Y.; Ohkura, N.; Kidani, Y.; Vandenbon, A.; Hirota, K.; Kawakami, R.; Yasuda, K.; Motooka, D.; Nakamura, S.; Kondo, M.; et al. Guidance of regulatory T cell development by Satb1-dependent super-enhancer establishment. Nat. Immunol. 2017, 18, 173–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakugawa, K.; Kojo, S.; Tanaka, H.; Seo, W.; Endo, T.A.; Kitagawa, Y.; Muroi, S.; Tenno, M.; Yasmin, N.; Kohwi, Y.; et al. Essential Roles of SATB1 in Specifying T Lymphocyte Subsets. Cell Rep. 2017, 19, 1176–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, R.H. Historical overview of immunological tolerance. Cold Spring Harb Perspect. Biol. 2012, 4, a006908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Sotome, T.; Inoue, A.; Mukozu, T.; Kuwabara, T.; Mikami, T.; Kowhi-Shigematsu, T.; Kondo, M. SATB1 Conditional Knockout Results in Sjogren’s Syndrome in Mice. J. Immunol. 2017, 199, 4016–4022. [Google Scholar] [CrossRef]
- Munn, D.H.; Mellor, A.L. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol. 2016, 37, 193–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, R.; Imanishi, J.; Oku, T.; Kishida, T.; Hayaishi, O. Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc. Natl. Acad. Sci. USA 1981, 78, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.H.; Lee, Y.J.; Hyon, J.Y.; Yun, P.Y.; Song, Y.W. Salivary cytokine profiles in primary Sjogren’s syndrome differ from those in non-Sjogren sicca in terms of TNF-alpha levels and Th-1/Th-2 ratios. Clin. Exp. Rheumatol. 2011, 29, 970–976. [Google Scholar]
- Van Woerkom, J.M.; Kruize, A.A.; Wenting-van Wijk, M.J.; Knol, E.; Bihari, I.C.; Jacobs, J.W.; Bijlsma, J.W.; Lafeber, F.P.; van Roon, J.A. Salivary gland and peripheral blood T helper 1 and 2 cell activity in Sjogren’s syndrome compared with non-Sjogren’s sicca syndrome. Ann. Rheum. Dis. 2005, 64, 1474–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, S.; Brayer, J.; Gao, J.; Brown, V.; Killedar, S.; Yasunari, U.; Peck, A.B. A dual role for interferon-gamma in the pathogenesis of Sjogren’s syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand. J. Immunol. 2004, 60, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Jaronen, M.; Quintana, F.J. Immunological Relevance of the Coevolution of IDO1 and AHR. Front. Immunol. 2014, 5, 521. [Google Scholar] [CrossRef] [Green Version]
- Parisis, D.; Chivasso, C.; Perret, J.; Soyfoo, M.S.; Delporte, C. Current State of Knowledge on Primary Sjogren’s Syndrome, an Autoimmune Exocrinopathy. J. Clin. Med. 2020, 9, 2299. [Google Scholar] [CrossRef] [PubMed]
- Byrne, G.I.; Lehmann, L.K.; Kirschbaum, J.G.; Borden, E.C.; Lee, C.M.; Brown, R.R. Induction of tryptophan degradation in vitro and in vivo: A gamma-interferon-stimulated activity. J. Interferon Res. 1986, 6, 389–396. [Google Scholar] [CrossRef]
- Mellor, A.L.; Munn, D.H. Tryptophan catabolism and T-cell tolerance: Immunosuppression by starvation? Immunol. Today 1999, 20, 469–473. [Google Scholar] [CrossRef]
- Dai, W.; Gupta, S.L. Regulation of indoleamine 2,3-dioxygenase gene expression in human fibroblasts by interferon-gamma. Upstream control region discriminates between interferon-gamma and interferon-alpha. J. Biol. Chem. 1990, 265, 19871–19877. [Google Scholar] [CrossRef]
- Kadoya, A.; Tone, S.; Maeda, H.; Minatogawa, Y.; Kido, R. Gene structure of human indoleamine 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 1992, 189, 530–536. [Google Scholar] [CrossRef]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef]
- Michalska, A.; Blaszczyk, K.; Wesoly, J.; Bluyssen, H.A.R. A Positive Feedback Amplifier Circuit That Regulates Interferon (IFN)-Stimulated Gene Expression and Controls Type I and Type II IFN Responses. Front. Immunol. 2018, 9, 1135. [Google Scholar] [CrossRef] [Green Version]
- Jiang, G.M.; Wang, H.S.; Du, J.; Ma, W.F.; Wang, H.; Qiu, Y.; Zhang, Q.G.; Xu, W.; Liu, H.F.; Liang, J.P. Bortezomib Relieves Immune Tolerance in Nasopharyngeal Carcinoma via STAT1 Suppression and Indoleamine 2,3-Dioxygenase Downregulation. Cancer Immunol. Res. 2017, 5, 42–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Jin, J.O.; Kawai, T.; Yu, Q. Endogenous programmed death ligand-1 restrains the development and onset of Sjgren’s syndrome in non-obese diabetic mice. Sci. Rep. 2016, 6, 39105. [Google Scholar] [CrossRef] [Green Version]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjogren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef] [Green Version]
- Shiboski, S.C.; Shiboski, C.H.; Criswell, L.; Baer, A.; Challacombe, S.; Lanfranchi, H.; Schiodt, M.; Umehara, H.; Vivino, F.; Zhao, Y.; et al. American College of Rheumatology classification criteria for Sjogren’s syndrome: A data-driven, expert consensus approach in the Sjogren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res. (Hoboken) 2012, 64, 475–487. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann. Rheum. Dis. 2017, 76, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Pertovaara, M.; Raitala, A.; Uusitalo, H.; Pukander, J.; Helin, H.; Oja, S.S.; Hurme, M. Mechanisms dependent on tryptophan catabolism regulate immune responses in primary Sjogren’s syndrome. Clin. Exp. Immunol. 2005, 142, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Maria, N.I.; van Helden-Meeuwsen, C.G.; Brkic, Z.; Paulissen, S.M.; Steenwijk, E.C.; Dalm, V.A.; van Daele, P.L.; Martin van Hagen, P.; Kroese, F.G.; van Roon, J.A.; et al. Association of Increased Treg Cell Levels With Elevated Indoleamine 2,3-Dioxygenase Activity and an Imbalanced Kynurenine Pathway in Interferon-Positive Primary Sjogren’s Syndrome. Arthritis Rheumatol. 2016, 68, 1688–1699. [Google Scholar] [CrossRef] [PubMed]
- Skowronska-Krawczyk, D.; Ma, Q.; Schwartz, M.; Scully, K.; Li, W.; Liu, Z.; Taylor, H.; Tollkuhn, J.; Ohgi, K.A.; Notani, D.; et al. Required enhancer-matrin-3 network interactions for a homeodomain transcription program. Nature 2014, 514, 257–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Boer, J.; Williams, A.; Skavdis, G.; Harker, N.; Coles, M.; Tolaini, M.; Norton, T.; Williams, K.; Roderick, K.; Potocnik, A.J.; et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur. J. Immunol. 2003, 33, 314–325. [Google Scholar] [CrossRef]
- Takahashi, S.; Iizuka, H.; Kuwabara, R.; Naito, Y.; Sakamoto, T.; Miyagi, A.; Onozato, M.; Ichiba, H.; Fukushima, T. Determination of l-tryptophan and l-kynurenine derivatized with (R)-4-(3-isothiocyanatopyrrolidin-1-yl)-7-(N,N-dimethylaminosulfonyl)-2,1,3-benzo xadiazole by LC-MS/MS on a triazole-bonded column and their quantification in human serum. Biomed. Chromatogr. 2016, 30, 1481–1486. [Google Scholar] [CrossRef]
- Iizuka, H.; Harashima, T.; Takahashi, S.; Kuwabara, R.; Naito, Y.; Sakamoto, T.; Onozato, M.; Ichiba, H.; Fukushima, T. Chromatographic profiles of tryptophan and kynurenine enantiomers derivatized with (S)-4-(3-isothiocyanatopyrrolidin-1-yl)-7-(N,N-dimethylaminosulfonyl)-2,1,3-benzo xadiazole using LC-MS/MS on a triazole-bonded column. Chirality 2017, 29, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Glasner, A.; Levi, A.; Enk, J.; Isaacson, B.; Viukov, S.; Orlanski, S.; Scope, A.; Neuman, T.; Enk, C.D.; Hanna, J.H.; et al. NKp46 Receptor-Mediated Interferon-gamma Production by Natural Killer Cells Increases Fibronectin 1 to Alter Tumor Architecture and Control Metastasis. Immunity 2018, 48, 396–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).