Cell-Penetrating Delivery of Nitric Oxide by Biocompatible Dinitrosyl Iron Complex and Its Dermato-Physiological Implications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cellular Uptake of Dinitrosyl Iron Complex (DNIC) [Fe2(μ-SCH2CH2COOH)2(NO)4] (DNIC-2) and Intracellular Release of Nitric Oxide (NO)
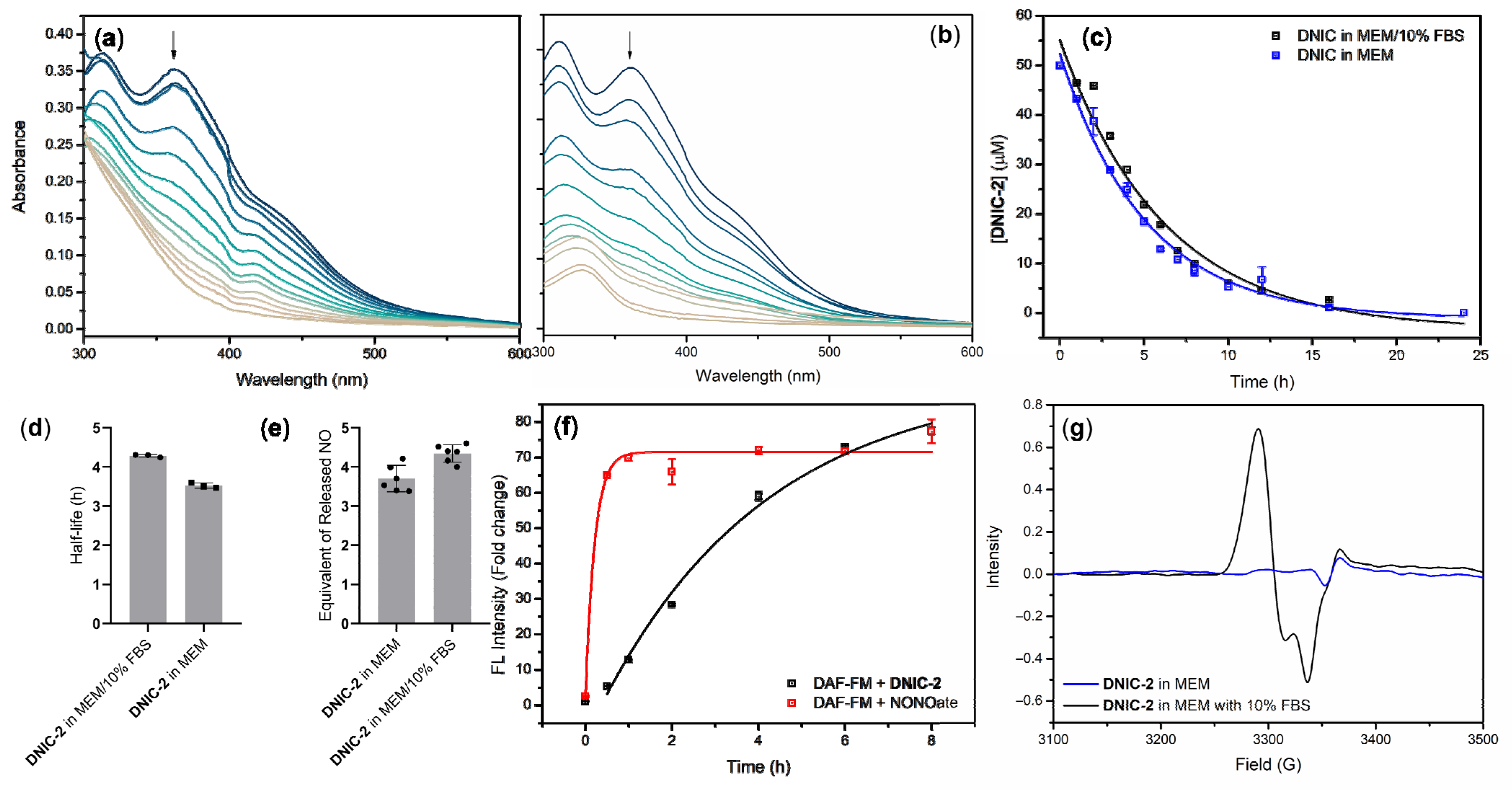
2.1.1. Kinetic Study of NO-Release Reactivity for DNIC-2
2.1.2. Cellular Uptake of DNIC-2 and Intracellular Release of NO in CCD-966SK Human Skin Fibroblast Cells
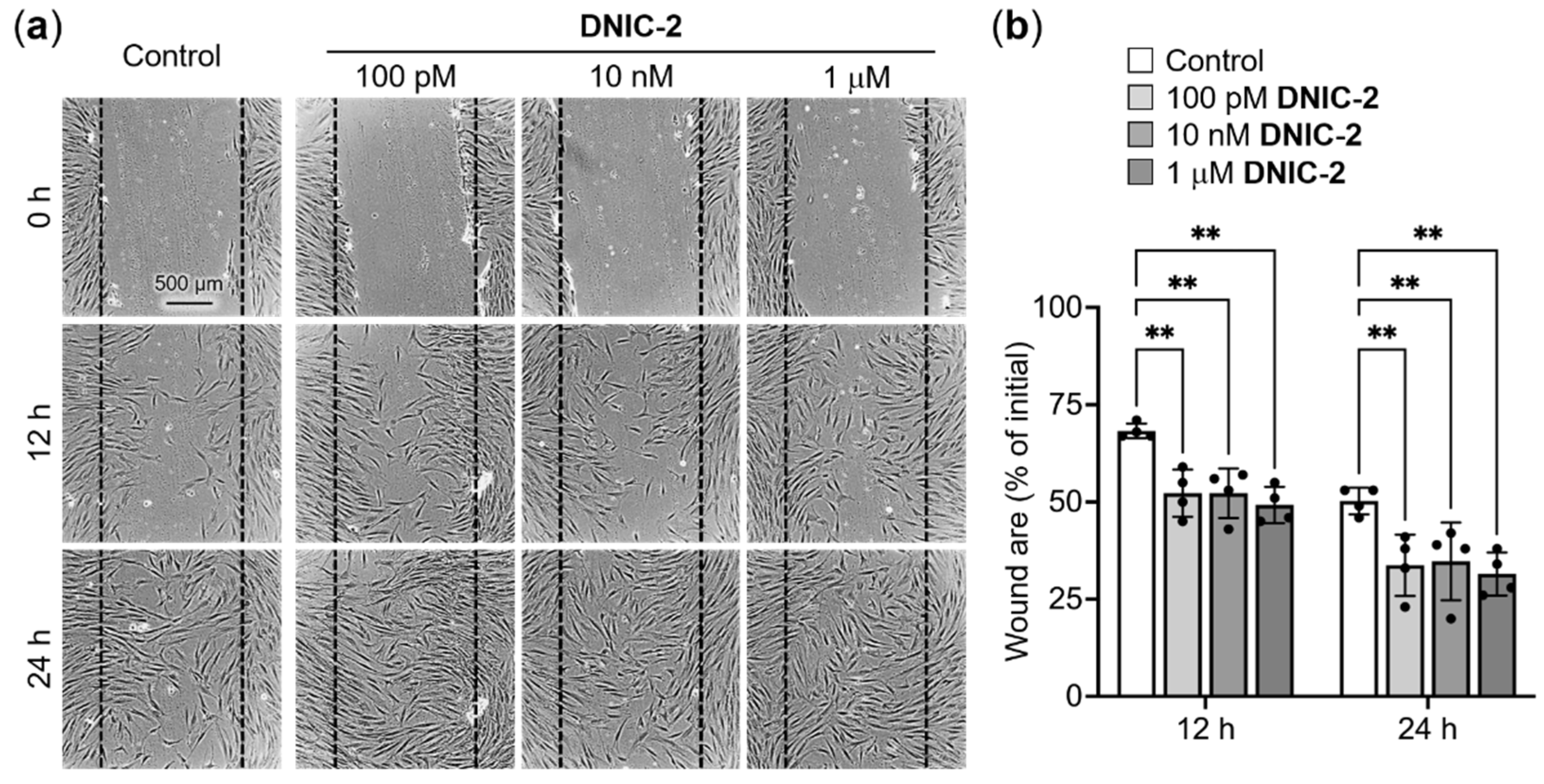
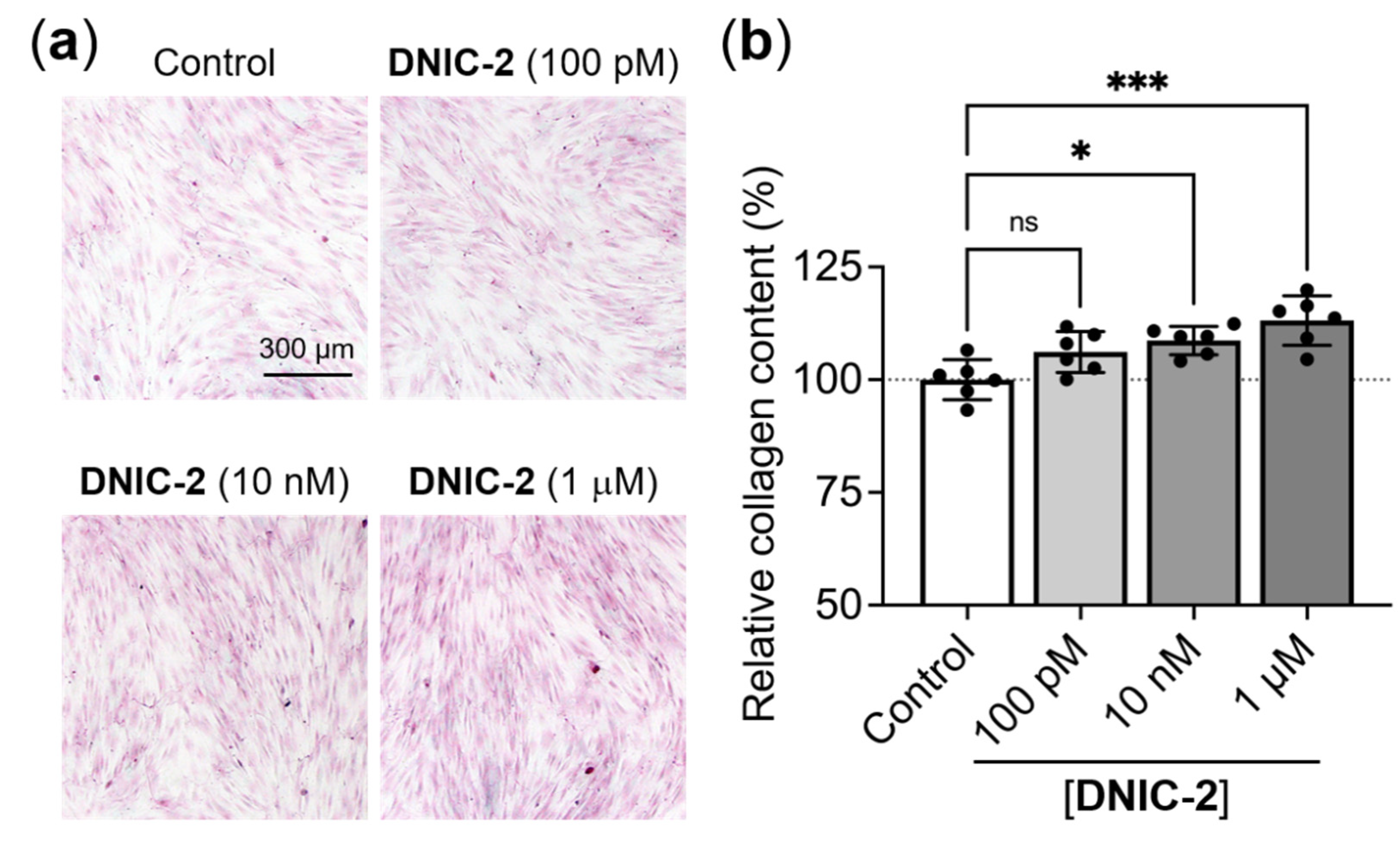
2.2. NO-Delivery DNIC-2 Promoted the Proliferation, Migration and Collagen Deposition of Skin Fibroblasts
2.3. NO-Delivery DNIC-2 Had No Effects on the Pigmentation of Melanocytes
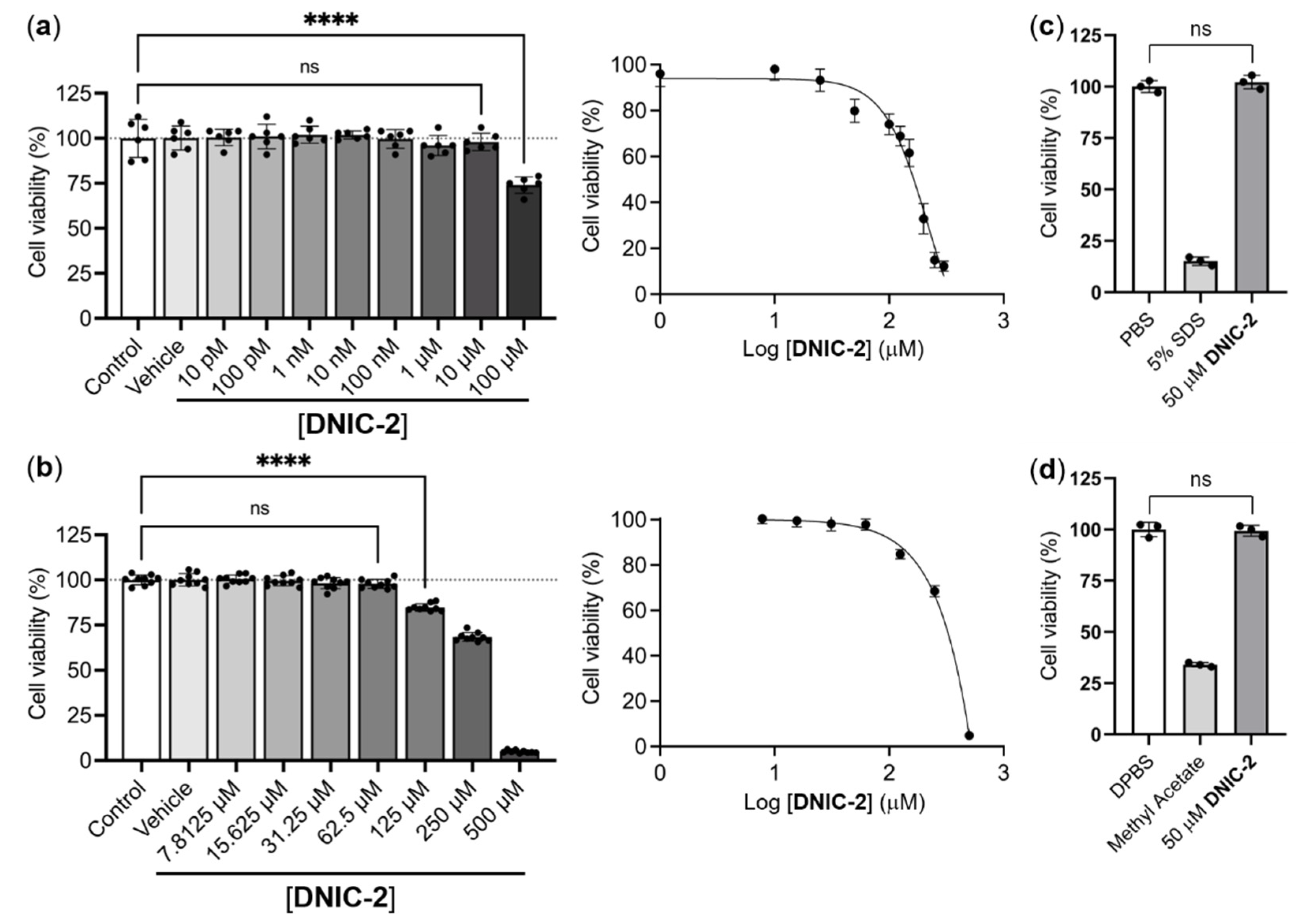
2.4. Biocompatibility Evaluation of DNIC-2
2.4.1. In Vitro Evaluation of Biocompatibility of DNIC-2
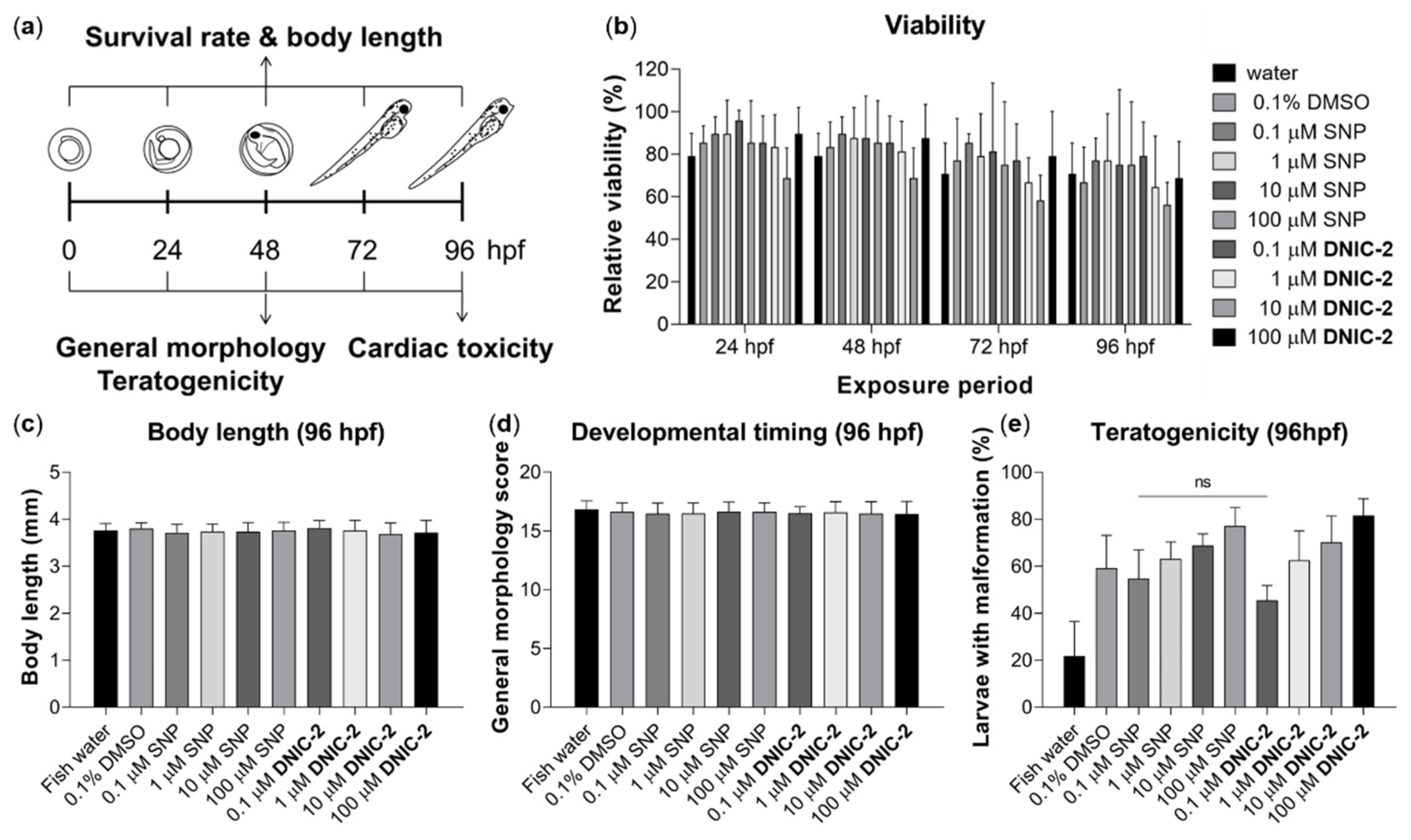
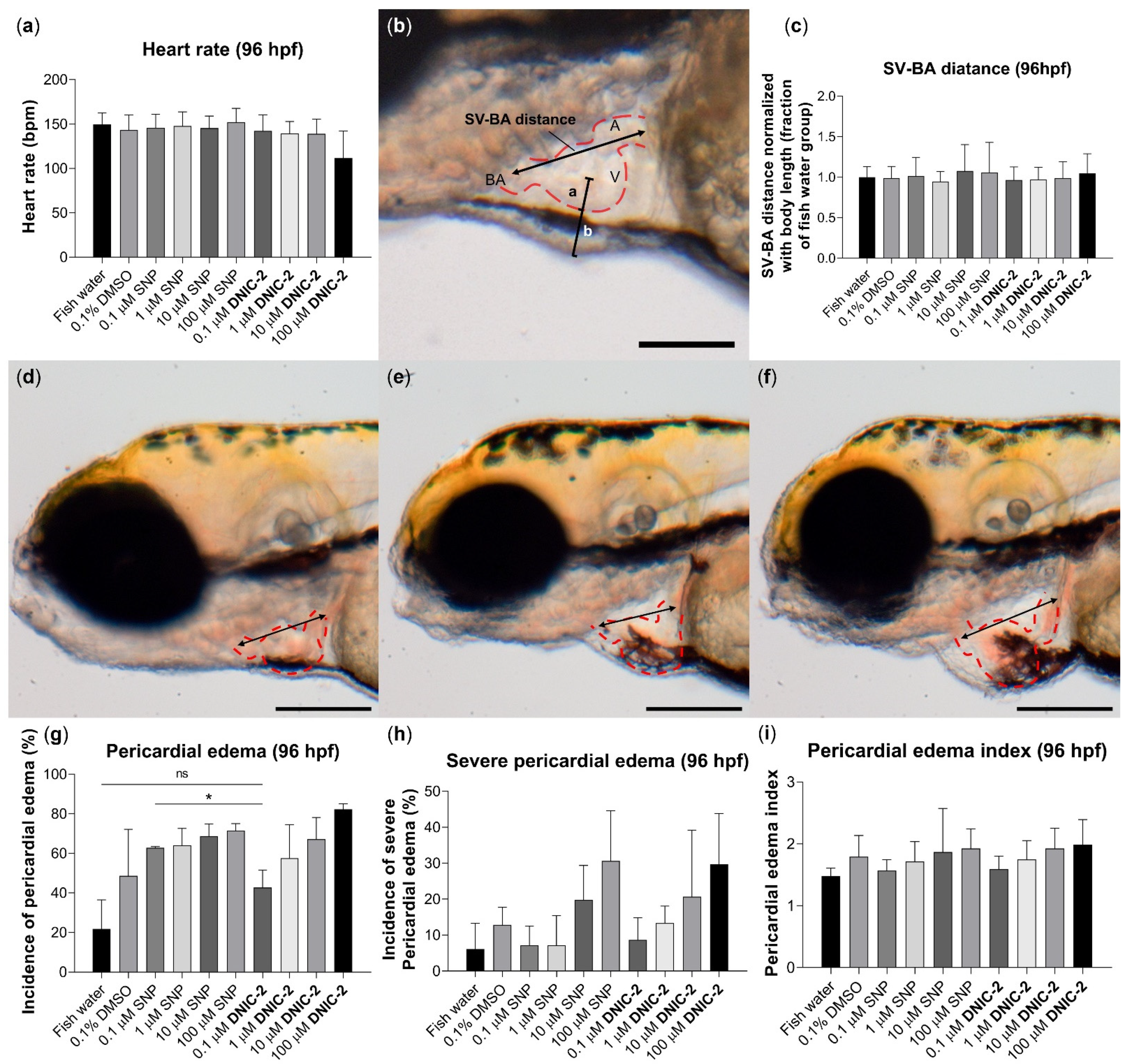
2.4.2. Biocompatibility Evaluation of DNIC-2 in Zebrafish Embryo
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Nalbandyan, R.M.; Vanin, A.F.; Blumenfeld, L.A. Free radicals in yeast cells. In Proceedings of the Meeting “Free Radicals Processes in Biological Systems”, Moscow, Russia; 1964; p. 18. [Google Scholar]
- Vanin, A.F.; Nalbandian, R.M. Free Radicals of a New Type in Yeast Cells. Biofizika 1965, 10, 167–168. [Google Scholar]
- Vanin, A.F.; Nalbandyan, R.M. Free radical species with unpaired electron localization on sulfur atom in yeast cells. Biofizika 1966, 11, 178–179. [Google Scholar] [PubMed]
- Vithayathil, A.J.; Ternberg, J.L.; Commoner, B. Changes in electron spin resonance signals of rat liver during chemical carcinogenesis. Nature 1965, 207, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Vanin, A.F.; Kubrina, L.N.; Kiladze, S.V.; Burbaev, D. Factors influencing formation of dinitrosyl complexes of non-heme iron in vitro preparations of mouse liver and yeasts. Biofizika 1977, 22, 646–650. [Google Scholar] [PubMed]
- Vanin, A.F.; Kiladze, S.V.; Kubrina, L.N. Factors influencing formation of dinitrosyl complexes of non-heme iron in the organs of animals in vivo. Biofizika 1977, 22, 850–855. [Google Scholar] [PubMed]
- Rogers, P.A.; Ding, H. L-cysteine-mediated destabilization of dinitrosyl iron complexes in proteins. J. Biol. Chem. 2001, 276, 30980–30986. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Rogers, P.A.; Ding, H. Repair of nitric oxide-modified ferredoxin [2Fe-2S] cluster by cysteine desulfurase (IscS). J. Biol. Chem. 2002, 277, 12868–12873. [Google Scholar] [CrossRef] [Green Version]
- Tinberg, C.E.; Tonzetich, Z.J.; Wang, H.; Do, L.H.; Yoda, Y.; Cramer, S.P.; Lippard, S.J. Characterization of iron dinitrosyl species formed in the reaction of nitric oxide with a biological Rieske center. J. Am. Chem. Soc. 2010, 132, 18168–18176. [Google Scholar] [CrossRef] [PubMed]
- Ekanger, L.A.; Oyala, P.H.; Moradian, A.; Sweredoski, M.J.; Barton, J.K. Nitric Oxide Modulates Endonuclease III Redox Activity by a 800 mV Negative Shift upon [Fe4S4] Cluster Nitrosylation. J. Am. Chem. Soc. 2018, 140, 11800–11810. [Google Scholar] [CrossRef] [Green Version]
- Bosworth, C.A.; Toledo, J.C., Jr.; Zmijewski, J.W.; Li, Q.; Lancaster, J.R., Jr. Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide. Proc. Natl. Acad. Sci. USA 2009, 106, 4671–4676. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.-L.; Chen, C.-C.; Hsu, I.-J.; Ke, S.-C.; Hsieh, C.-H.; Chiang, K.-A.; Lee, G.-H.; Wang, Y.; Chen, J.-M.; Lee, J.-F.; et al. Photochemistry of the dinitrosyl iron complex [S5Fe(NO)2]- leading to reversible formation of [S5Fe(μ-S)2FeS5]2-: Spectroscopic characterization of species relevant to the nitric oxide modification and repair of [2Fe-2S] ferredoxins. Inorg. Chem. 2004, 43, 5159–5167. [Google Scholar] [CrossRef]
- Harrop, T.C.; Song, D.; Lippard, S.J. Interaction of nitric oxide with tetrathiolato iron(II) complexes: Relevance to the reaction pathways of iron nitrosyls in sulfur-rich biological coordination environments. J. Am. Chem. Soc. 2006, 128, 3528–3529. [Google Scholar] [CrossRef]
- Tsai, M.-L.; Tsou, C.-C.; Liaw, W.-F. Dinitrosyl iron complexes (DNICs): From biomimetic synthesis and spectroscopic characterization toward unveiling the biological and catalytic roles of DNICs. Acc. Chem. Res. 2015, 48, 1184–1193. [Google Scholar] [CrossRef]
- Fitzpatrick, J.; Kim, E. Synthetic modeling chemistry of iron-sulfur clusters in nitric oxide signaling. Acc. Chem. Res. 2015, 48, 2453–2461. [Google Scholar] [CrossRef]
- Pereira, J.C.; Iretskii, A.V.; Han, R.M.; Ford, P.C. Dinitrosyl iron complexes with cysteine. Kinetics studies of the formation and reactions of DNICs in aqueous solution. J. Am. Chem. Soc. 2015, 137, 328–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speelman, A.L.; Zhang, B.; Silakovi, A.; Skodje, K.M.; Alp, E.E.; Zhao, J.; Hu, M.Y.; Kim, E.; Krebs, C.; Lehnert, N. Unusual Synthetic Pathway for an {Fe(NO)2}9 Dinitrosyl Iron Complex (DNIC) and Insight into DNIC Electronic Structure via Nuclear Resonance Vibrational Spectroscopy. Inorg. Chem. 2016, 55, 5485–5501. [Google Scholar] [CrossRef]
- Truzzi, D.R.; Medeiros, N.M.; Augusto, O.; Ford, P.C. Dinitrosyl Iron Complexes (DNICs). From Spontaneous Assembly to Biological Roles. Inorg. Chem. 2021, 60. in press. [Google Scholar]
- Arnold, W.P.; Mittal, C.K.; Katsuki, S.; Murad, F. Nitric oxide activates guanylate cyclase and increases guanosine 3’:5’-cyclic monophosphate levels in various tissue preparations. Proc. Natl. Acad. Sci. USA 1977, 74, 3203–3207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignarro, L.J.; Byrns, R.E.; Wood, K.S. Endothelium-dependent modulation of cGMP levels and intrinsic smooth muscle tone in isolated bovine intrapulmonary artery and vein. Circ. Res. 1987, 60, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Vanin, A.F. Dinitrosyl iron complexes with thiol-containing ligands as a “working form” of endogenous nitric oxide. Nitric Oxide 2016, 54, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Vanin, A.F. Dinitrosyl Iron Complexes As a “Working Form” of Nitric Oxide in Living Organisms; Cambridge Scholars Publishing: Newcastle, UK, 2019; p. 279. [Google Scholar]
- Lu, T.-T.; Wang, Y.-M.; Hung, C.-H.; Chiou, S.-J.; Liaw, W.-F. Bioinorganic Chemistry of the Natural [Fe(NO)2] Motif: Evolution of a Functional Model for NO-Related Biomedical Application and Revolutionary Development of a Translational Model. Inorg. Chem. 2018, 57, 12425–12443. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, H.-Y.; Chung, C.-W.; Santos, J.H.; Villaflores, O.B.; Lu, T.-T. Fe in biosynthesis, translocation, and signal transduction of NO: Toward bioinorganic engineering of dinitrosyl iron complexes into NO-delivery scaffolds for tissue engineering. Dalton Trans. 2019, 48, 9431–9453. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-W.; Lin, Y.-H.; Lin, M.-H.; Huang, Y.-R.; Chou, C.-H.; Hong, H.-C.; Wang, M.-R.; Tseng, Y.-T.; Liao, P.-C.; Chung, M.-C.; et al. Extension of C. elegans lifespan using the center dot NO-delivery dinitrosyl iron complexes. J. Biol. Inorg. Chem. 2018, 23, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Skodje, K.M.; Kwon, M.-Y.; Chung, S.W.; Kim, E. Coordination-triggered NO release from a dinitrosyl iron complex leads to anti-inflammatory activity. Chem. Sci. 2014, 5, 2374–2378. [Google Scholar] [CrossRef]
- Pectol, D.C.; DeLaney, C.R.; Zhu, J.; Mellott, D.M.; Katzfuss, A.; Taylor, Z.W.; Meek, T.D.; Darensbourg, M.Y. Dinitrosyl iron complexes (DNICs) as inhibitors of the SARS-CoV-2 main protease. Chem. Commun. 2021, 57, 8352–8355. [Google Scholar] [CrossRef]
- Wu, S.-C.; Lu, C.-Y.; Chen, Y.-L.; Lo, F.-C.; Wang, T.-Y.; Chen, Y.-J.; Yuan, S.-S.; Liaw, W.-F.; Wang, Y.-M. Water-Soluble Dinitrosyl Iron Complex (DNIC): A Nitric Oxide Vehicle Triggering Cancer Cell Death via Apoptosis. Inorg. Chem. 2016, 55, 9383–9392. [Google Scholar] [CrossRef]
- Sung, Y.-C.; Jin, P.-R.; Chu, L.-A.; Hsu, F.-F.; Wang, M.-R.; Chang, C.-C.; Chiou, S.-J.; Qiu, J.T.; Gao, D.-Y.; Lin, C.-C.; et al. Delivery of nitric oxide with a nanocarrier promotes tumour vessel normalization and potentiates anti-cancer therapies. Nat. Nanotechnol. 2019, 14, 1160–1169. [Google Scholar] [CrossRef]
- Chazov, E.I.; Rodnenkov, O.V.; Zorin, A.V.; Lakomkin, V.L.; Gramovich, V.V.; Vyborov, O.N.; Dragnev, A.G.; Timoshin, C.A.; Buryachkovskaya, L.I.; Abramov, A.A.; et al. Hypotensive effect of Oxacom® containing a dinitrosyl iron complex with glutathione: Animal studies and clinical trials on healthy volunteers. Nitric Oxide 2012, 26, 148–156. [Google Scholar] [CrossRef]
- Gosteev, A.; Zorin, A.V.; Rodnenkov, O.V.; Dragnev, A.G.; Chazov, E.I. Hemodynamic effects of the synthetic analogue of endogenous nitric oxide (II) donors a dinitrosyl iron complex in hypertensive patients with uncomplicated hypertensive crisis. Ter. Arkh. 2014, 86, 49–55. [Google Scholar] [PubMed]
- Chen, Y.-J.; Wu, S.-C.; Wang, H.-C.; Wu, T.-H.; Yuan, S.-F.; Lu, T.-T.; Liaw, W.-F.; Wang, Y.-M. Activation of Angiogenesis and Wound Healing in Diabetic Mice Using NO-Delivery Dinitrosyl Iron Complexes. Mol. Pharm. 2019, 16, 4241–4251. [Google Scholar] [CrossRef]
- Andreyev-Andriyevsky, A.A.; Mikoyan, V.D.; Serezhenkov, V.A.; Vanin, A.F. Penile erectile activity of dinitrosyl iron complexes with thiol-containing ligands. Nitric Oxide 2011, 24, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-R.; Huang, Y.-D.; Hong, Y.-H.; Liu, Y.-H.; Narwane, M.; Chang, Y.-H.; Dinh, T.K.; Hsieh, H.-T.; Hseuh, Y.-J.; Wu, P.-C.; et al. Endogenous Conjugation of Biomimetic Dinitrosyl Iron Complex with Protein Vehicles for Oral Delivery of Nitric Oxide to Brain and Activation of Hippocampal Neurogenesis. JACS Au 2021, 1, 998–1013. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-C.; Chang, F.-C.; Hong, Y.-H.; Li, J.-C.; Chen, P.-L.; Chen, C.-H.; Chiu, T.-W.; Lu, T.-T.; Wang, Y.-M.; Kao, C.-F. Assessing the cognitive status of Drosophila by the value-based feeding decision. NPJ Aging Mech. Disease 2021, 7, ASAP. [Google Scholar] [CrossRef]
- Cho, S.-L.; Liao, C.-J.; Lu, T.-T. Synthetic methodology for preparation of dinitrosyl iron complexes. J. Biol. Inorg. Chem. 2019, 24, 495–515. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Chiou, S.J. trans-Bis(μ-2-hydroxy-ethanethiol-ato-κ2S:S)bis-[dinitro-syliron(II)](Fe-Fe). Acta Cryst. E 2009, 65, m1600. [Google Scholar] [CrossRef] [Green Version]
- Shmatko, N.Y.; Korchagin, D.V.; Shilov, G.V.; Sanina, N.A.; Aldoshin, S.M. Molecular and Crystal Structure of a Cationic Dinitrosyl Iron Complex with 1,3-Dimethylthiourea. J. Struct. Chem. 2017, 58, 353–355. [Google Scholar] [CrossRef]
- Pulukkody, R.; Chupik, R.B.; Montalvo, S.K.; Khan, S.; Bhuvanesh, N.; Lim, S.M.; Darensbourg, M.Y. Toward biocompatible dinitrosyl iron complexes: Sugar-appended thiolates. Chem. Commun. 2017, 53, 1180–1183. [Google Scholar] [CrossRef]
- Pokidova, O.V.; Luzhkov, V.B.; Emel’yanova, N.S.; Krapivin, V.B.; Kotelnikov, A.I.; Sanina, N.A.; Aldoshin, S.M. Effect of albumin on the transformation of dinitrosyl iron complexes with thiourea ligands. Dalton Trans. 2020, 49, 12674–12685. [Google Scholar] [CrossRef]
- Pokidova, O.V.; Kormukhina, A.Y.; Kotelnikov, A.I.; Rudneva, T.N.; Lyssenko, K.A.; Sanina, N.A. Features of the decomposition of cationic nitrosyl iron complexes with N-ethylthiourea and penicillamine ligands in the presence of albumin. Inorg. Chim. Acta 2021, 524, 120453. [Google Scholar] [CrossRef]
- Pectol, D.C.; Khan, S.; Elsabahy, M.; Wooley, K.L.; Lim, S.M.; Darensbourg, M.Y. Effects of Glutathione and Histidine on NO Release from a Dimeric Dinitrosyl Iron Complex (DNIC). Inorg. Chem. 2020, 59, 16998–17008. [Google Scholar] [CrossRef]
- Pectol, D.C.; Khan, S.; Chupik, R.B.; Elsabahy, M.; Wooley, K.L.; Darensbourg, M.Y.; Lim, S.M. Toward the Optimization of Dinitrosyl Iron Complexes as Therapeutics for Smooth Muscle Cells. Mol. Pharm. 2019, 16, 3178–3187. [Google Scholar] [CrossRef]
- Watts, R.N.; Richardson, D.R. Nitrogen monoxide (no) and glucose: Unexpected links between energy metabolism and no-mediated iron mobilization from cells. J. Biol. Chem. 2001, 276, 4724–4732. [Google Scholar] [CrossRef] [Green Version]
- Watts, R.N.; Hawkins, C.; Ponka, P.; Richardson, D.R. Nitrogen monoxide (NO)-mediated iron release from cells is linked to NO-induced glutathione efflux via multidrug resistance-associated protein 1. Proc. Natl. Acad. Sci. USA 2006, 103, 7670–7675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lok, H.C.; Suryo Rahmanto, Y.; Hawkins, C.L.; Kalinowski, D.S.; Morrow, C.S.; Townsend, A.J.; Ponka, P.; Richardson, D.R. Nitric oxide storage and transport in cells are mediated by glutathione S-transferase P1-1 and multidrug resistance protein 1 via dinitrosyl iron complexes. J. Biol. Chem. 2012, 287, 607–618. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.D.; Chen, A.F. Nitric oxide: A newly discovered function on wound healing. Acta Pharmacol. Sin. 2005, 26, 259–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witte, M.B.; Thornton, F.J.; Efron, D.T.; Barbul, A. Enhancement of fibroblast collagen synthesis by nitric oxide. Nitric Oxide 2000, 4, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Schwentker, A.; Billiar, T.R. Inducible nitric oxide synthase: From cloning to therapeutic applications. World J. Surg. 2002, 26, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, Y.; Tang, Y.; Shen, H.; Li, J.; Yi, Z.; Ke, Q.; Xu, H. Copper-Based Metal-Organic Framework as a Controllable Nitric Oxide-Releasing Vehicle for Enhanced Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 18319–18331. [Google Scholar] [CrossRef]
- Tung, C.Y.; Tseng, Y.T.; Lu, T.T.; Liaw, W.F. Insight into the Electronic Structure of Biomimetic Dinitrosyliron Complexes (DNICs): Toward the Syntheses of Amido-Bridging Dinuclear DNICs. Inorg. Chem. 2021, 60. in press. [Google Scholar]
- Cesareo, E.; Parker, L.J.; Pedersen, J.Z.; Nuccetelli, M.; Mazzetti, A.P.; Pastore, A.; Federici, G.; Caccuri, A.M.; Ricci, G.; Adams, J.J.; et al. Nitrosylation of human glutathione transferase P1-1 with dinitrosyl diglutathionyl iron complex in vitro and in vivo. J. Biol. Chem. 2005, 280, 42172–42180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsou, C.-C.; Lu, T.-T.; Liaw, W.-F. EPR, UV-Vis, IR, and X-ray demonstration of the anionic dimeric dinitrosyl iron complex [(NO)2Fe(μ-StBu)2Fe(NO)2]-: Relevance to the products of nitrosylation of cytosolic and mitochondrial aconitases, and high-potential iron proteins. J. Am. Chem. Soc. 2007, 129, 12626–12627. [Google Scholar] [CrossRef] [PubMed]
- Boese, M.; Mordvintcev, P.I.; Vanin, A.F.; Busse, R.; Mulsch, A. S-nitrosation of serum albumin by dinitrosyl-iron complex. J. Biol. Chem. 1995, 270, 29244–29249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, G.; Nguyen, L.N.; Macherla, C.; Chi, Y.; Friedman, J.M.; Nosanchuk, J.D.; Martinez, L.R. Nitric oxide-releasing nanoparticles accelerate wound healing by promoting fibroblast migration and collagen deposition. Am. J. Pathol. 2012, 180, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Krischel, V.; Bruch-Gerharz, D.; Suschek, C.; Kroncke, K.D.; Ruzicka, T.; Kolb-Bachofen, V. Biphasic effect of exogenous nitric oxide on proliferation and differentiation in skin derived keratinocytes but not fibroblasts. J. Investig. Dermatol. 1998, 111, 286–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Frank, S.; Kampfer, H.; Wetzler, C.; Pfeilschifter, J. Nitric oxide drives skin repair: Novel functions of an established mediator. Kidney Int. 2002, 61, 882–888. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.J.; Uehara, Y.; Park, J.Y.; Chung, K.W.; Ha, Y.M.; Kim, J.M.; Song, Y.M.; Chun, P.; Park, J.W.; Moon, H.R.; et al. Suppression of melanogenesis by a newly synthesized compound, MHY966 via the nitric oxide/protein kinase G signaling pathway in murine skin. J. Dermatol. Sci. 2012, 68, 164–171. [Google Scholar] [CrossRef]
- Horikoshi, T.; Nakahara, M.; Kaminaga, H.; Sasaki, M.; Uchiwa, H.; Miyachi, Y. Involvement of nitric oxide in UVB-induced pigmentation in guinea pig skin. Pigment Cell Res. 2000, 13, 358–363. [Google Scholar] [CrossRef]
- Beekhuijzen, M.; de Koning, C.; Flores-Guillen, M.E.; de Vries-Buitenweg, S.; Tobor-Kaplon, M.; van de Waart, B.; Emmen, H. From cutting edge to guideline: A first step in harmonization of the zebrafish embryotoxicity test (ZET) by describing the most optimal test conditions and morphology scoring system. Reprod. Toxicol. 2015, 56, 64–76. [Google Scholar] [CrossRef]
- Li, J.; Jia, W.; Zhao, Q. Excessive nitrite affects zebrafish valvulogenesis through yielding too much NO signaling. PLoS ONE 2014, 9, e92728. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.W.; Lu, Y.J.; Lin, Y.J.; Huang, Y.T.; Hsieh, L.H.; Wu, B.H.; Lin, Y.C.; Chen, L.C.; Wang, H.W.; Chuang, J.C.; et al. Transplantation of 3D MSC/HUVEC spheroids with neuroprotective and proangiogenic potentials ameliorates ischemic stroke brain injury. Biomaterials 2021, 272, 120765. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-C.; Chen, S.-C.; Hsueh, Y.-J.; Shen, Y.-C.; Tsai, M.-Y.; Hsu, L.-W.; Yeh, C.-K.; Chen, H.-C.; Huang, C.-C. Gelatin scaffold with multifunctional curcumin-loaded lipid-PLGA hybrid microparticles for regenerating corneal endothelium. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111753. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Chu, Y.; Li, Y.; Han, Y.; Yu, D.; Liu, R.; Zheng, Z.; Song, L.; Fang, J.; Li, X.; et al. Adipose-derived mesenchymal stromal cells promote corneal wound healing by accelerating the clearance of neutrophils in cornea. Cell Death Dis. 2020, 11, 707. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.Y.; Ha, Y.; Park, A.H.; Kwon, O.W.; Kim, Y.J. Leathesia difformis Extract Inhibits alpha-MSH-Induced Melanogenesis in B16F10 Cells via Down-Regulation of CREB Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, T.I.; Jung, H.J.; Lee, Y.M.; Lee, S.; Kim, G.H.; Kan, S.Y.; Kang, H.; Oh, T.; Ko, H.M.; Kwak, K.C.; et al. Zerumbone, a Tropical Ginger Sesquiterpene of Zingiber officinale Roscoe, Attenuates alpha-MSH-Induced Melanogenesis in B16F10 Cells. Int. J. Mol. Sci. 2018, 19, 3149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, Y.C.; Ko, J.H.; Kang, H.K.; Kim, S.; Kang, C.I.; Lee, J.N.; Park, S.M.; Hyun, C.G. Antimelanogenic Effects of Polygonum tinctorium Flower Extract from Traditional Jeju Fermentation via Upregulation of Extracellular Signal-Regulated Kinase and Protein Kinase B Activation. Int. J. Mol. Sci. 2018, 19, 2895. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.E.; Fang, Y.Q.; Ho, C.T.; Assuncao, M.; Lin, S.J.; Wang, Y.C.; Blocki, A.; Huang, C.C. Bioactive Decellularized Extracellular Matrix Derived from 3D Stem Cell Spheroids under Macromolecular Crowding Serves as a Scaffold for Tissue Engineering. Adv. Healthc. Mater. 2021, 10, e2100024. [Google Scholar] [CrossRef]
- Chatatikun, M.; Yamauchi, T.; Yamasaki, K.; Aiba, S.; Chiabchalard, A. Anti melanogenic effect of Croton roxburghii and Croton sublyratus leaves in alpha-MSH stimulated B16F10cells. J. Tradit. Complement. Med. 2019, 9, 66–72. [Google Scholar] [CrossRef]
- OECD. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method; OECD Publishing: Paris, France, 2010. [Google Scholar] [CrossRef]
- OECD. Test No. 492: “Reconstructed Human Cornea-Like Epithelium (RhCE) Test Method for Identifying Chemicals Not Requiring Classification and Labeling for Eye Irritation or Serious Eye Damage”; OECD Publishing: Paris, France, 2019. [Google Scholar] [CrossRef] [Green Version]
- Lanham, K.A.; Prasch, A.L.; Weina, K.M.; Peterson, R.E.; Heideman, W. A dominant negative zebrafish Ahr2 partially protects developing zebrafish from dioxin toxicity. PLoS ONE 2011, 6, e28020. [Google Scholar] [CrossRef] [Green Version]
- Manjunatha, B.; Han, L.; Kundapur, R.R.; Liu, K.; Lee, S.J. Herbul black henna (hair dye) causes cardiovascular defects in zebrafish (Danio rerio) embryo model. Environ. Sci. Pollut. Res. Int. 2020, 27, 14150–14159. [Google Scholar] [CrossRef]
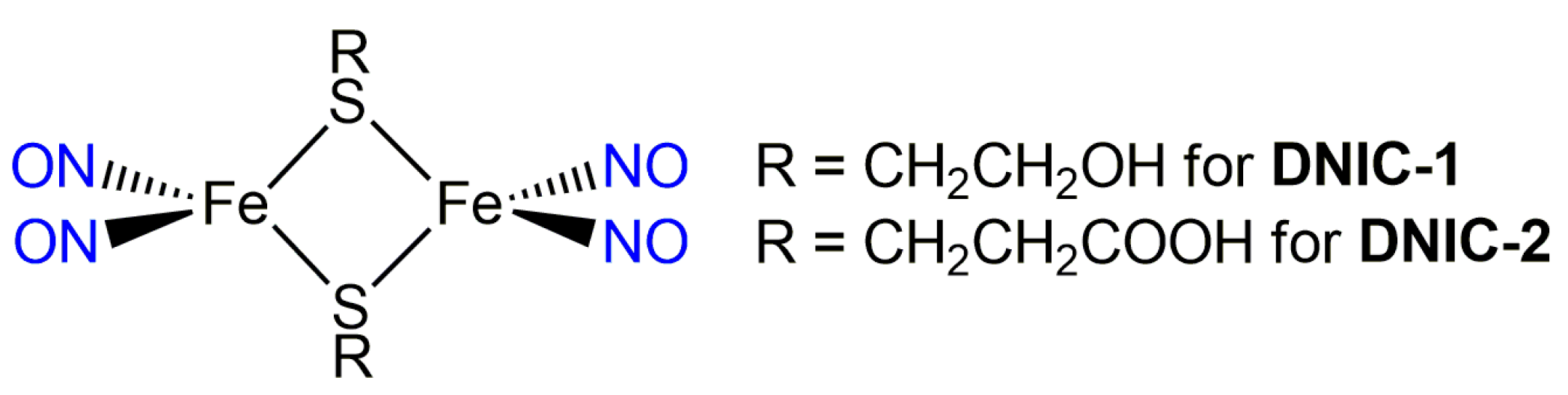



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Chen, Y.-H.; Chiu, H.; Ko, Y.-H.; Wang, R.-T.; Wang, W.-P.; Chuang, Y.-J.; Huang, C.-C.; Lu, T.-T. Cell-Penetrating Delivery of Nitric Oxide by Biocompatible Dinitrosyl Iron Complex and Its Dermato-Physiological Implications. Int. J. Mol. Sci. 2021, 22, 10101. https://doi.org/10.3390/ijms221810101
Chen Y-C, Chen Y-H, Chiu H, Ko Y-H, Wang R-T, Wang W-P, Chuang Y-J, Huang C-C, Lu T-T. Cell-Penetrating Delivery of Nitric Oxide by Biocompatible Dinitrosyl Iron Complex and Its Dermato-Physiological Implications. International Journal of Molecular Sciences. 2021; 22(18):10101. https://doi.org/10.3390/ijms221810101
Chicago/Turabian StyleChen, Yu-Chieh, Yi-Hong Chen, Han Chiu, Yi-Hsuan Ko, Ruei-Ting Wang, Wei-Ping Wang, Yung-Jen Chuang, Chieh-Cheng Huang, and Tsai-Te Lu. 2021. "Cell-Penetrating Delivery of Nitric Oxide by Biocompatible Dinitrosyl Iron Complex and Its Dermato-Physiological Implications" International Journal of Molecular Sciences 22, no. 18: 10101. https://doi.org/10.3390/ijms221810101
APA StyleChen, Y.-C., Chen, Y.-H., Chiu, H., Ko, Y.-H., Wang, R.-T., Wang, W.-P., Chuang, Y.-J., Huang, C.-C., & Lu, T.-T. (2021). Cell-Penetrating Delivery of Nitric Oxide by Biocompatible Dinitrosyl Iron Complex and Its Dermato-Physiological Implications. International Journal of Molecular Sciences, 22(18), 10101. https://doi.org/10.3390/ijms221810101






