Plasma Membrane Receptors Involved in the Binding and Response of Osteoclasts to Noncellular Components of the Bone
Abstract
1. Introduction
2. Osteoclasts: Specialized Bone-Resorbing Cells
3. The Role of Integrins in Regulating Osteoclast Activity
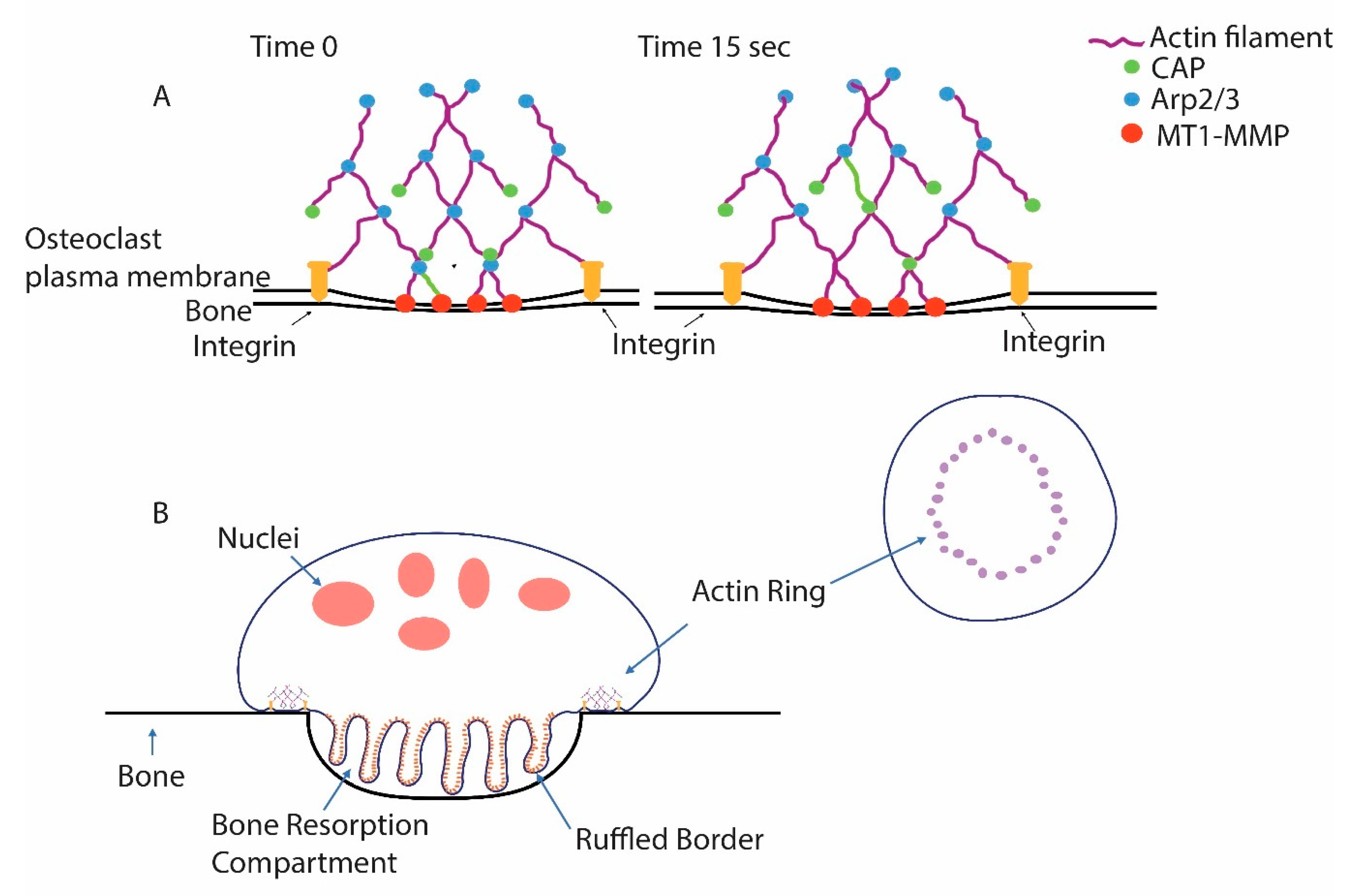
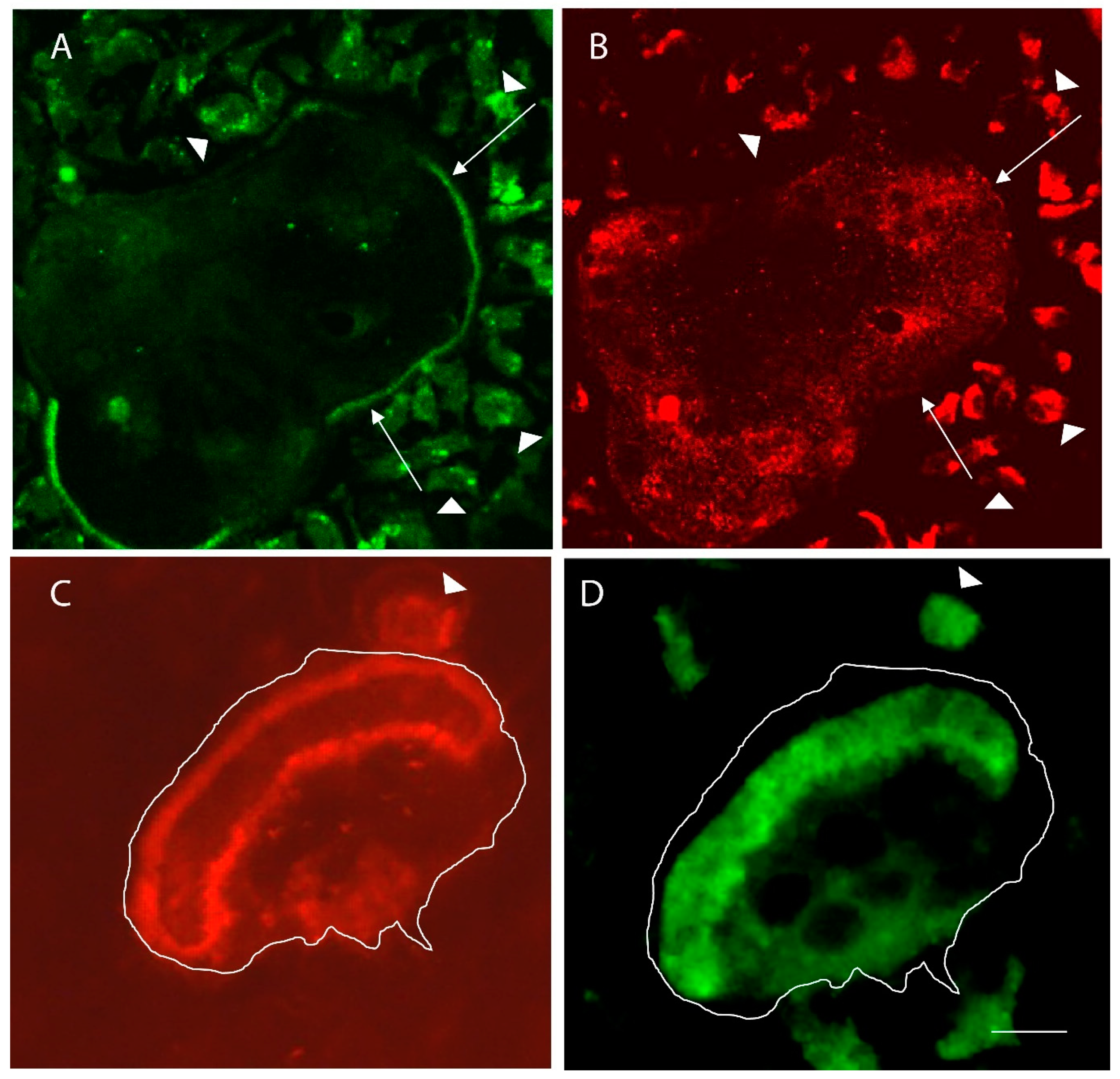
4. Identification of a Minimal Substrate Required to Stimulate Osteoclasts to Produce Actin Rings and Ruffled Borders
5. Other Membrane Receptors Involved in Regulating Osteoclast Activation
5.1. CD44 and IgSF11
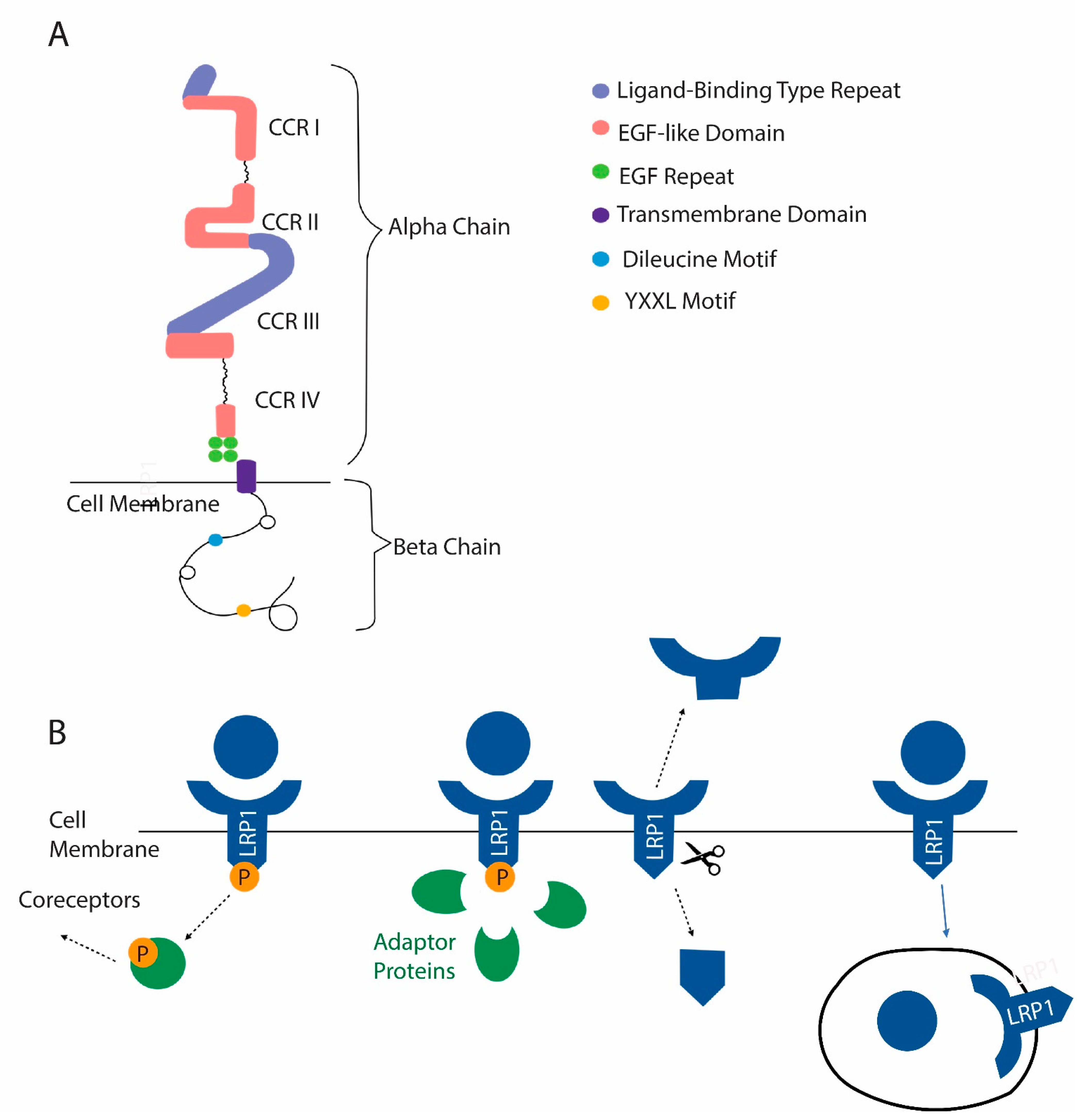
5.2. Low-Density-Lipoprotein-Related Protein 1 (LRP1, Also Known as CD91 and α-2-Macroglobulin Receptor)
6. Matrix Receptors in Extracellular Vesicles (EVs) Released by Osteoclasts
7. Calcium and Osteoclasts
8. Direct Response of Osteoclasts to Mechanical Stimulation
9. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| Arp2/3 | Actin-related 2/3 |
| V-ATPases | Vacuolar H+-ATPases |
| CLC-7 | Chloride voltage-gated channel 7 |
| OSTM1 | Osteopetrosis-associated transmembrane protein 1 |
| RGD | Arginine-glycine-aspartic acid |
| c-src | Cellular-proto-oncogene tyrosine-protein kinase Src |
| PILP | Polymer-induced liquid precursor |
| LRP1 | LDL-receptor-related protein 1 |
| TRPV4 | Transient receptor potential cation channel subfamily V member 4 |
| EV | Extracellular vesicle |
| RANK | Receptor activator of nuclear factor kappa B |
| RANKL | Receptor activator of nuclear factor kappa B-ligand |
| MT1-MMP | Membrane type 1-matrix metalloproteinase |
| CAP | Capping protein |
References
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Clarke, B. Normal Bone Anatomy and Physiology. Clin. J. Am. Soc. Nephrol. 2008, 3, S131–S139. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone metastases: An overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Ristow, O.; Gerngroß, C.; Schwaiger, M.; Hohlweg-Majert, B.; Kehl, V.; Jansen, H.; Hahnefeld, L.; Otto, S.; Pautke, C. Is Bone Turnover of Jawbone and Its Possible over Suppression by Bisphosphonates of Etiologic Importance in Pathogenesis of Bisphosphonate-Related Osteonecrosis? J. Oral Maxillofac. Surg. 2014, 72, 903–910. [Google Scholar] [CrossRef]
- DeLuca, H.F.; Vitamin, D. Historical Overview. Vitam. Horm. 2016, 100, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Levin, V.A.; Jiang, X.; Kagan, R. Estrogen therapy for osteoporosis in the modern era. Osteoporos. Int. 2018, 29, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Honma, M.; Ikebuchi, Y.; Suzuki, H. RANKL as a key figure in bridging between the bone and immune system: Its physiological functions and potential as a pharmacological target. Pharmacol. Ther. 2021, 218, 107682. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Xiong, J.; O’Brien, C.A. Osteocyte RANKL: New insights into the control of bone remodeling. J. Bone Miner. Res. 2012, 27, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Mochizuki, S.-I.; Tomoyasu, A.; Yano, K.; Goto, M.; Murakami, A.; et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 1998, 95, 3597–3602. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Boyle, W.J.; Penninger, J. Osteoprotegerin ligand: A common link between osteoclastogenesis, lymph node formation and lymphocyte development. Immunol. Cell Biol. 1999, 77, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.J.; Sims, N. RANKL/OPG; Critical role in bone physiology. Rev. Endocr. Metab. Disord. 2015, 16, 131–139. [Google Scholar] [CrossRef]
- Ikebuchi, Y.; Aoki, S.; Honma, M.; Hayashi, M.; Sugamori, Y.; Khan, M.; Kariya, Y.; Kato, G.; Tabata, Y.; Penninger, J.; et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nat. Cell Biol. 2018, 561, 195–200. [Google Scholar] [CrossRef]
- Sims, N.A.; Martin, T.J. Osteoclasts Provide Coupling Signals to Osteoblast Lineage Cells Through Multiple Mechanisms. Annu. Rev. Physiol. 2020, 82, 507–529. [Google Scholar] [CrossRef]
- Marie, P.J.; Cohen-Solal, M. The Expanding Life and Functions of Osteogenic Cells: From Simple Bone-Making Cells to Multifunctional Cells and Beyond. J. Bone Miner. Res. 2018, 33, 199–210. [Google Scholar] [CrossRef]
- De Vries, T.J.; Huesa, C. The Osteocyte as a Novel Key Player in Understanding Periodontitis Through its Expression of RANKL and Sclerostin: A Review. Curr. Osteoporos. Rep. 2019, 17, 116–121. [Google Scholar] [CrossRef]
- Robling, A.G.; Bonewald, L.F. The Osteocyte: New Insights. Annu. Rev. Physiol. 2020, 82, 485–506. [Google Scholar] [CrossRef]
- Holliday, L.S.; De Faria, L.P.; Rody, J.W.J. Actin and Actin-Associated Proteins in Extracellular Vesicles Shed by Osteoclasts. Int. J. Mol. Sci. 2020, 21, 158. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A.; Martin, T.J. Coupling Signals between the Osteoclast and Osteoblast: How are Messages Transmitted between These Temporary Visitors to the Bone Surface? Front. Endocrinol. 2015, 6, 41. [Google Scholar] [CrossRef]
- Miyamoto, T.; Ohneda, O.; Arai, F.; Iwamoto, K.; Okada, S.; Takagi, K.; Anderson, D.M.; Suda, T. Bifurcation of osteoclasts and dendritic cells from common progenitors. Blood 2001, 98, 2544–2554. [Google Scholar] [CrossRef] [PubMed]
- Väänänen, H.K.; Laitala-Leinonen, T. Osteoclast lineage and function. Arch. Biochem. Biophys. 2008, 473, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Zuo, J.; Holliday, L.S. Specialized Roles for Actin in Osteoclasts: Unanswered Questions and Therapeutic Opportunities. Biomolecules 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Destaing, O.; Petropoulos, C.; Albiges-Rizo, C. Coupling between acto-adhesive machinery and ECM degradation in invadosomes. Cell Adhes. Migr. 2014, 8, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Génot, E.; Gligorijevic, B. Invadosomes in their natural habitat. Eur. J. Cell Biol. 2014, 93, 367–379. [Google Scholar] [CrossRef]
- Seano, G.; Primo, L. Podosomes and invadopodia: Tools to breach vascular basement membrane. Cell Cycle 2015, 14, 1370–1374. [Google Scholar] [CrossRef][Green Version]
- Holtrop, M.E.; King, G.J. The ultrastructure of the osteoclast and its functional implications. Clin. Orthop. Relat. Res. 1977, 123, 177–196. [Google Scholar] [CrossRef]
- King, G.J.; Holtrop, M.E. Actin-like filaments in bone cells of cultured mouse calvaria as demonstrated by binding to heavy meromyosin. J. Cell Biol. 1975, 66, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Vaananen, H.; Zhao, H.; Mulari, M.; Halleen, J. The cell biology of osteoclast function. J. Cell Sci. 2000, 113, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Dresner-Pollak, R.; Rosenblatt, M. Blockade of osteoclast-mediated bone resorption through occupancy of the integrin receptor: A potential approach to the therapy of osteoporosis. J. Cell. Biochem. 1994, 56, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Wang, B.; Yang, X.; He, B.; Hao, D.; Yan, L. Integrin-associated molecules and signalling cross talking in osteoclast cytoskeleton regulation. J. Cell. Mol. Med. 2020, 24, 3271–3281. [Google Scholar] [CrossRef]
- Duong, L.T.; Rodan, G.A. Integrin-mediated signaling in the regulation of osteoclast adhesion and activation. Front Biosci. 1998, 3, 757–768. [Google Scholar] [CrossRef]
- Helfrich, M.; Nesbitt, S.; Lakkakorpi, P.; Barnes, M.; Bodary, S.; Shankar, G.; Mason, W.; Mendrick, D.; Väänänen, H.; Horton, M. β1 integrins and osteoclast function: Involvement in collagen recognition and bone resorption. Bone 1996, 19, 317–328. [Google Scholar] [CrossRef]
- Mulari, M.T.K.; Zhao, H.; Lakkakorpi, P.T.; Väänänen, H.K. Osteoclast Ruffled Border Has Distinct Subdomains for Secretion and Degraded Matrix Uptake. Traffic 2003, 4, 113–125. [Google Scholar] [CrossRef]
- Brömme, D.; Okamoto, K.; Wang, B.B.; Biroc, S. Human Cathepsin O2, a Matrix Protein-degrading Cysteine Protease Expressed in Osteoclasts. J. Biol. Chem. 1996, 271, 2126–2132. [Google Scholar] [CrossRef]
- Everts, V.; Korper, W.; Hoeben, K.A.; Jansen, I.D.; Brömme, D.; Cleutjens, K.B.; Heeneman, S.; Peters, C.; Reinheckel, T.; Saftig, P.; et al. Osteoclastic Bone Degradation and the Role of Different Cysteine Proteinases and Matrix Metalloproteinases: Differences Between Calvaria and Long Bone. J. Bone Miner. Res. 2006, 21, 1399–1408. [Google Scholar] [CrossRef]
- Blair, H.C.; Teitelbaum, S.; Ghiselli, R.; Gluck, S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science 1989, 245, 855–857. [Google Scholar] [CrossRef]
- Väänänen, H.K.; Karhukorpi, E.K.; Sundquist, K.; Wallmark, B.; Roininen, I.; Hentunen, T.; Tuukkanen, J.; Lakkakorpi, P. Evidence for the presence of a proton pump of the vacuolar H(+)-ATPase type in the ruffled borders of osteoclasts. J. Cell Biol. 1990, 111, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Kornak, U.; Kasper, D.; Bösl, M.R.; Kaiser, E.; Schweizer, M.; Schulz, A.; Friedrich, W.; Delling, G.; Jentsch, T.J. Loss of the ClC-7 Chloride Channel Leads to Osteopetrosis in Mice and Man. Cell 2001, 104, 205–215. [Google Scholar] [CrossRef]
- Lange, P.; Wartosch, L.; Jentsch, T.J.; Fuhrmann, J. ClC-7 requires Ostm1 as a β-subunit to support bone resorption and lysosomal function. Nat. Cell Biol. 2006, 440, 220–223. [Google Scholar] [CrossRef]
- Frattini, A.; Orchard, P.J.; Sobacchi, C.; Giliani, S.; Abinun, M.; Mattsson, J.P.; Keeling, D.J.; Andersson, A.-K.; Wallbrandt, P.; Zecca, L.; et al. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat. Genet. 2000, 25, 343–346. [Google Scholar] [CrossRef]
- Ramírez, A.; Faupel, J.; Goebel, I.; Stiller, A.; Beyer, S.; Stöckle, C.; Hasan, C.; Bode, U.; Kornak, U.; Kubisch, C. Identification of a novel mutation in the coding region of the grey-lethal geneOSTM1in human malignant infantile osteopetrosis. Hum. Mutat. 2004, 23, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Holliday, L.S. Vacuolar H+-ATPases (V-ATPases) as therapeutic targets: A brief review and recent developments. Biotarget 2017, 1, 18. [Google Scholar] [CrossRef]
- Gluck, S.L.; Lee, B.S.; Wang, S.P.; Underhill, D.; Nemoto, J.; Holliday, L.S. Plasma membrane V-ATPases in proton-transporting cells of the mammalian kidney and osteoclast. Acta Physiol. Scand. Suppl. 1998, 643, 203–212. [Google Scholar]
- Wang, R.; Long, T.; Hassan, A.; Wang, J.; Sun, Y.; Xie, X.-S.; Li, X. Cryo-EM structures of intact V-ATPase from bovine brain. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Abbas, Y.M.; Wu, D.; Bueler, S.A.; Robinson, C.V.; Rubinstein, J.L. Structure of V-ATPase from the mammalian brain. Science 2020, 367, 1240–1246. [Google Scholar] [CrossRef]
- Saltel, F.; Destaing, O.; Bard, F.; Eichert, D.; Jurdic, P. Apatite-mediated Actin Dynamics in Resorbing Osteoclasts. Mol. Biol. Cell 2004, 15, 5231–5241. [Google Scholar] [CrossRef]
- Destaing, O.; Saltel, F.; Géminard, J.-C.; Jurdic, P.; Bard, F. Podosomes Display Actin Turnover and Dynamic Self-Organization in Osteoclasts Expressing Actin-Green Fluorescent Protein. Mol. Biol. Cell 2003, 14, 407–416. [Google Scholar] [CrossRef]
- Lee, B.S.; Gluck, S.L.; Holliday, L.S. Interaction between Vacuolar H+-ATPase and Microfilaments during Osteoclast Activation. J. Biol. Chem. 1999, 274, 29164–29171. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Bubb, M.R.; Yarmola, E.G.; Zuo, J.; Jiang, J.; Lee, B.S.; Lu, M.; Gluck, S.L.; Hurst, I.R.; Holliday, L.S. Vacuolar H+-ATPase Binding to Microfilaments: Regulation in response to phosphatidylinositol 3-kinase activity and detailed characterization of the actin-binding site in subunit B. J. Biol. Chem. 2004, 279, 7988–7998. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.M.; Khoo, W.H.; Ng, P.Y.; Xiao, Y.; Zamerli, J.; Thatcher, P.; Kyaw, W.; Pathmanandavel, K.; Grootveld, A.K.; Moran, I.; et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell 2021, 184, 1330–1347.e13. [Google Scholar] [CrossRef] [PubMed]
- Lakkakorpi, P.T.; Väänänen, K.H. Kinetics of the osteoclast cytoskeleton during the resorption cycle in vitro. J. Bone Miner. Res. 1991, 6, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Chellaiah, M.; Hruška, K. Osteopontin stimulates gelsolin-associated phosphoinositide levels and phosphatidylinositol triphosphate-hydroxyl kinase. Mol. Biol. Cell 1996, 7, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, I.; Takahashi, N.; Sasaki, T.; Tanaka, S.; Udagawa, N.; Murakami, H.; Kimura, K.; Kabuyama, Y.; Kurokawa, T.; Suda, T.; et al. Wortmannin, a specific inhibitor of phosphatidylinositol-3 kinase, blocks osteoclastic bone resorption. FEBS Lett. 1995, 361, 79–84. [Google Scholar] [CrossRef]
- Zuo, J.; Jiang, J.; Chen, S.-H.; Vergara, S.; Gong, Y.; Xue, J.; Huang, H.; Kaku, M.; Holliday, L.S. Actin Binding Activity of Subunit B of Vacuolar H+-ATPase Is Involved in Its Targeting to Ruffled Membranes of Osteoclasts. J. Bone Miner. Res. 2006, 21, 714–721. [Google Scholar] [CrossRef]
- Kane, P.M. Regulation of V-ATPases by reversible disassembly. FEBS Lett. 2000, 469, 137–141. [Google Scholar] [CrossRef]
- Holliday, L.S.; Welgus, H.G.; Fliszar, C.J.; Veith, G.M.; Jeffrey, J.J.; Gluck, S.L. Initiation of Osteoclast Bone Resorption by Interstitial Collagenase. J. Biol. Chem. 1997, 272, 22053–22058. [Google Scholar] [CrossRef]
- Holliday, L.; Welgus, H.; Hanna, J.; Lee, B.; Lu, M.; Jeffrey, J.; Gluck, S. Interstitial Collagenase Activity Stimulates the Formation of Actin Rings and Ruffled Membranes in Mouse Marrow Osteoclasts. Calcif. Tissue Int. 2003, 72, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Dolce, C.; Vakani, A.; Archer, L.; Morris-Wiman, J.; Holliday, L. Effects of echistatin and an RGD peptide on orthodontic tooth movement. J. Dent. Res. 2003, 82, 682–686. [Google Scholar] [CrossRef]
- Holliday, L.; Vakani, A.; Archer, L.; Dolce, C. Effects of Matrix Metalloproteinase Inhibitors on Bone Resorption and Orthodontic Tooth Movement. J. Dent. Res. 2003, 82, 687–691. [Google Scholar] [CrossRef]
- Pirapaharan, D.C.; Olesen, J.B.; Andersen, T.L.; Christensen, S.B.; Kjærsgaard-Andersen, P.; Delaisse, J.-M.; Søe, K. Catabolic activity of osteoblast-lineage cells contributes to osteoclastic bone resorption in vitro. J. Cell Sci. 2019, 132, jcs.229351. [Google Scholar] [CrossRef]
- Huveneers, S.; Danen, E. Adhesion signaling-crosstalk between integrins, Src and Rho. J. Cell Sci. 2009, 122, 1059–1069. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Reed, J.C. Anchorage dependence, integrins, and apoptosis. Cell 1994, 77, 477–478. [Google Scholar] [CrossRef]
- Giancotti, F.G. Integrin Signaling. Science 1999, 285, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.C.; Varmus, H.E.; Bishop, J.M. Cellular homologue (c-src) of the transforming gene of Rous sarcoma virus: Isolation, mapping, and transcriptional analysis of c-src and flanking regions. Proc. Natl. Acad. Sci. USA 1981, 78, 5842–5846. [Google Scholar] [CrossRef]
- Guinebault, C.; Payrastre, B.; Racaud-Sultan, C.; Mazarguil, H.; Breton, M.; Mauco, G.; Plantavid, M.; Chap, H. Integrin-dependent translocation of phosphoinositide 3-kinase to the cytoskeleton of thrombin-activated platelets involves specific interactions of p85 alpha with actin filaments and focal adhesion kinase. J. Cell Biol. 1995, 129, 831–842. [Google Scholar] [CrossRef]
- Schlaepfer, D.D.; Hanks, S.K.; Hunter, T.; Van Der Geer, P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nat. Cell Biol. 1994, 372, 786–791. [Google Scholar] [CrossRef]
- Turner, C.; Miller, J. Primary sequence of paxillin contains putative SH2 and SH3 domain binding motifs and multiple LIM domains: Identification of a vinculin and pp125Fak-binding region. J. Cell Sci. 1994, 107, 1583–1591. [Google Scholar] [CrossRef]
- Malosio, M.L.; De Curtis, I.; De Curtis, I. Tyrosine phosphorylation induced by integrin-mediated adhesion of retinal neurons to laminin. Int. J. Dev. Neurosci. 1996, 14, 269–281. [Google Scholar] [CrossRef]
- Lowe, C.; Yoneda, T.; Boyce, B.F.; Chen, H.; Mundy, G.R.; Soriano, P. Osteopetrosis in Src-deficient mice is due to an autonomous defect of osteoclasts. Proc. Natl. Acad. Sci. USA 1993, 90, 4485–4489. [Google Scholar] [CrossRef]
- Schwartzberg, P.L.; Xing, L.; Hoffmann, O.; Lowell, C.A.; Garrett, L.; Boyce, B.F.; Varmus, H.E. Rescue of osteoclast function by transgenic expression of kinase-deficient Src in src-/- mutant mice. Genes Dev. 1997, 11, 2835–2844. [Google Scholar] [CrossRef]
- Soriano, P.; Montgomery, C.; Geske, R.; Bradley, A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 1991, 64, 693–702. [Google Scholar] [CrossRef]
- Miyauchi, A.; Alvarez, J.; Greenfield, E.M.; Teti, A.M.; Grano, M.; Colucci, S.; Zambonin-Zallone, A.; Ross, F.P.; Teitelbaum, S.; Cheresh, D.; et al. Binding of osteopontin to the osteoclast integrin alpha v beta 3. Osteoporos Int. 1993, 3 (Suppl. S1), 132–135. [Google Scholar] [CrossRef] [PubMed]
- Ross, F.; Chappel, J.; Alvarez, J.; Sander, D.; Butler, W.; Farach-Carson, M.; Mintz, K.; Robey, P.; Teitelbaum, S.; Cheresh, D. Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin alpha v beta 3 potentiate bone resorption. J. Biol. Chem. 1993, 268, 9901–9907. [Google Scholar] [CrossRef]
- Faccio, R.; Takeshita, S.; Zallone, A.; Ross, F.P.; Teitelbaum, S.L. c-Fms and the alphavbeta3 integrin collaborate during osteoclast differentiation. J. Clin. Investig. 2003, 111, 749–758. [Google Scholar] [CrossRef]
- Engleman, V.W.; Nickols, G.A.; Ross, F.P.; Horton, M.A.; Griggs, D.W.; Settle, S.L.; Ruminski, P.G.; Teitelbaum, S. A peptidomimetic antagonist of the alpha(v)beta3 integrin inhibits bone resorption in vitro and prevents osteoporosis in vivo. J. Clin. Investig. 1997, 99, 2284–2292. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, A.; Alvarez, J.; Greenfield, E.; Teti, A.; Grano, M.; Colucci, S.; Zambonin-Zallone, A.; Ross, F.; Teitelbaum, S.; Cheresh, D. Recognition of osteopontin and related peptides by an alpha v beta 3 integrin stimulates immediate cell signals in osteoclasts. J. Biol. Chem. 1991, 266, 20369–20374. [Google Scholar] [CrossRef]
- Nakamura, I.; Pilkington, M.; Lakkakorpi, P.; Lipfert, L.; Sims, S.; Dixon, S.J.; Rodan, G.; Duong, L. Role of alpha(v)beta(3) integrin in osteoclast migration and formation of the sealing zone. J. Cell Sci. 1999, 112, 3985–3993. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.P.; Hodivala-Dilke, K.; Zheng, M.-H.; Namba, N.; Lam, J.; Novack, D.; Feng, X.; Ross, F.P.; Hynes, R.O.; Teitelbaum, S.L. Mice lacking β3 integrins are osteosclerotic because of dysfunctional osteoclasts. J. Clin. Investig. 2000, 105, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Kitaura, H.; Sands, M.S.; Ross, F.P.; Teitelbaum, S.; Veis, D. Critical Role of β3 Integrin in Experimental Postmenopausal Osteoporosis. J. Bone Miner. Res. 2005, 20, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Novack, D.V.; Faccio, R.; Ory, D.S.; Aya, K.; Boyer, M.I.; McHugh, K.P.; Ross, F.P.; Teitelbaum, S.L. A Glanzmann’s mutation in β3 integrin specifically impairs osteoclast function. J. Clin. Investig. 2001, 107, 1137–1144. [Google Scholar] [CrossRef]
- George, J.N.; Caen, J.P.; Nurden, A.T. Glanzmann’s thrombasthenia: The spectrum of clinical disease. Blood 1990, 75, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Sago, K.; Teitelbaum, S.; Venstrom, K.; Reichardt, L.F.; Ross, F.P. The integrin alphavbeta5 is expressed on avian osteoclast precursors and regulated by retinoic acid. J. Bone Miner. Res. 1999, 14, 32–38. [Google Scholar] [CrossRef]
- Lane, N.E.; Yao, W.; Nakamura, M.C.; Humphrey, M.B.; Kimmel, D.; Huang, X.; Sheppard, D.; Ross, F.P.; Teitelbaum, S.L. Mice Lacking the Integrin β5 Subunit Have Accelerated Osteoclast Maturation and Increased Activity in the Estrogen-Deficient State. J. Bone Miner. Res. 2004, 20, 58–66. [Google Scholar] [CrossRef]
- Townsend, P.A.; Villanova, I.; Teti, A.M.; Horton, M.A. β1 Integrin antisense oligodeoxynucleotides: Utility in controlling osteoclast function. Eur. J. Cell Biol. 1999, 78, 485–496. [Google Scholar] [CrossRef]
- Nakayamada, S.; Okada, Y.; Saito, K.; Tamura, M.; Tanaka, Y. β1 Integrin/Focal Adhesion Kinase-mediated Signaling Induces Intercellular Adhesion Molecule 1 and Receptor Activator of Nuclear Factor κB Ligand on Osteoblasts and Osteoclast Maturation. J. Biol. Chem. 2003, 278, 45368–45374. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Yamada, K.M.; Brown, K.E. Integrin regulatory switching in development: Oscillation of beta 5 integrin mRNA expression during epithelial-mesenchymal interactions in tooth development. Int. J. Dev. Biol. 1994, 38, 553–556. [Google Scholar]
- Damsky, C.; Librach, C.; Lim, K.; Fitzgerald, M.; McMaster, M.; Janatpour, M.; Zhou, Y.; Logan, S.; Fisher, S. Integrin switching regulates normal trophoblast invasion. Development 1994, 120, 3657–3666. [Google Scholar] [CrossRef]
- Contractor, T.; Babiarz, B.; Kowalski, A.J.; Rittling, S.R.; Sørensen, E.S.; Denhardt, D.T. Osteoclasts resorb protein-free mineral (Osteologic discs) efficiently in the absence of osteopontin. In Vivo 2005, 19, 335–341. [Google Scholar]
- Sun-Wada, G.-H.; Wada, Y.; Futai, M. Vacuolar H+ pumping ATPases in luminal acidic organelles and extracellular compartments: Common rotational mechanism and diverse physiological roles. J. Bioenerg. Biomembr. 2003, 35, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Jee, S.-S.; Thula, T.T.; Gower, L.B. Development of bone-like composites via the polymer-induced liquid-precursor (PILP) process. Part 1: Influence of polymer molecular weight. Acta Biomater. 2010, 6, 3676–3686. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Douglas, E.P.; Gower, L.B. Patterning Inorganic (CaCO3) Thin Films via a Polymer-Induced Liquid-Precursor Process. Langmuir 2007, 23, 4862–4870. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.E.; Thula-Mata, T.; Toro, E.J.; Yeh, Y.-W.; Holt, C.; Holliday, L.S.; Gower, L.B. Multifunctional role of osteopontin in directing intrafibrillar mineralization of collagen and activation of osteoclasts. Acta Biomater. 2014, 10, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Thula, T.T.; Svedlund, F.; Rodriguez, D.E.; Podschun, J.; Pendi, L.; Gower, L.B. Mimicking the Nanostructure of Bone: Comparison of Polymeric Process-Directing Agents. Polymers 2010, 3, 10–35. [Google Scholar] [CrossRef]
- Gower, L.B. Biomimetic Model Systems for Investigating the Amorphous Precursor Pathway and Its Role in Biomineralization. Chem. Rev. 2008, 108, 4551–4627. [Google Scholar] [CrossRef]
- Boskey, A.L. Osteopontin and Related Phosphorylated Sialoproteins: Effects on Mineralization. Ann. N. Y. Acad. Sci. 1995, 760, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Wang, C.; Zhang, D.; Wang, B.; Hou, W.; Zhou, Y. Osteopontin in Bone Metabolism and Bone Diseases. Med. Sci. Monit. 2020, 26, e919159-1. [Google Scholar] [CrossRef]
- Rodan, G.A. Osteopontin Overview. Ann. N. Y. Acad. Sci. 1995, 760, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yokosaki, Y.; Matsuura, N.; Sasaki, T.; Murakami, I.; Schneider, H.; Higashiyama, S.; Saitoh, Y.; Yamakido, M.; Taooka, Y.; Sheppard, D. The Integrin α9β1 Binds to a Novel Recognition Sequence (SVVYGLR) in the Thrombin-cleaved Amino-terminal Fragment of Osteopontin. J. Biol. Chem. 1999, 274, 36328–36334. [Google Scholar] [CrossRef] [PubMed]
- Green, P.M.; Ludbrook, S.B.; Miller, D.D.; Horgan, C.M.; Barry, S.T. Structural elements of the osteopontin SVVYGLR motif important for the interaction with α4 integrins. FEBS Lett. 2001, 503, 75–79. [Google Scholar] [CrossRef]
- Rody, W.J., Jr.; Chamberlain, C.A.; Emory-Carter, A.K.; McHugh, K.P.; Wallet, S.M.; Spicer, V.; Krokhin, O.; Holliday, L.S. The proteome of extracellular vesicles released by clastic cells differs based on their substrate. PLoS ONE 2019, 14, e0219602. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.; Priestley, G.V.; Papayannopoulou, T. Deletion of α4 Integrins from Adult Hematopoietic Cells Reveals Roles in Homeostasis, Regeneration, and Homing. Mol. Cell. Biol. 2003, 23, 9349–9360. [Google Scholar] [CrossRef] [PubMed]
- Priestley, G.V.; Scott, L.M.; Ulyanova, T.; Papayannopoulou, T. Lack of α4 integrin expression in stem cells restricts competitive function and self-renewal activity. Blood 2006, 107, 2959–2967. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ponta, H.; Sherman, L.S.; Herrlich, P.A. CD44: From adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar] [CrossRef]
- Kania, J.R.; Kupfer, S.R.; Kehat-Stadler, T. CD44 Antibodies Inhibit Osteoclast Formation. J. Bone Miner. Res. 1997, 12, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Spessotto, P.; Rossi, F.M.; Degan, M.; Di Francia, R.; Perris, R.; Colombatti, A.; Gattei, V. Hyaluronan-CD44 interaction hampers migration of osteoclast-like cells by down-regulating MMP-9. J. Cell Biol. 2002, 158, 1133–1144. [Google Scholar] [CrossRef]
- Chellaiah, M.A.; Kizer, N.; Biswas, R.; Alvarez, U.; Strauss-Schoenberger, J.; Rifas, L.; Rittling, S.R.; Denhardt, D.T.; Hruska, K.A. Osteopontin Deficiency Produces Osteoclast Dysfunction Due to Reduced CD44 Surface Expression. Mol. Biol. Cell 2003, 14, 173–189. [Google Scholar] [CrossRef]
- de Vries, T.J.; Schoenmaker, T.; Beertsen, W.; van der Neut, R.; Everts, V. Effect of CD44 deficiency on in vitro and in vivo osteoclast formation. J. Cell. Biochem. 2005, 94, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Takegahara, N.; Walsh, M.C.; Choi, Y. CD44 Can Compensate for IgSF11 Deficiency by Associating with the Scaffold Protein PSD-95 during Osteoclast Differentiation. Int. J. Mol. Sci. 2020, 21, 2646. [Google Scholar] [CrossRef] [PubMed]
- Kohara, Y.; Haraguchi, R.; Kitazawa, R.; Kitazawa, S. Knockdown of Lrp1 in RAW264 cells inhibits osteoclast differentiation and osteoclast-osteoblast interactions in vitro. Biochem. Biophys. Res. Commun. 2020, 523, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Behler-Janbeck, F.; Beil, F.T.; Koehne, T.; Müller, B.; Schmidt, T.; Heine, M.; Ochs, L.; Yilmaz, T.; Dietrich, M.; et al. Lrp1 in osteoblasts controls osteoclast activity and protects against osteoporosis by limiting PDGF–RANKL signaling. Bone Res. 2018, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Li, J.; Liu, H.; Foxa, G.E.; Weaver, K.; Li, J.; Williams, B.; Yang, T. LRP1 Suppresses Bone Resorption in Mice by Inhibiting the RANKL-Stimulated NF-κB and p38 Pathways During Osteoclastogenesis. J. Bone Miner. Res. 2018, 33, 1773–1784. [Google Scholar] [CrossRef] [PubMed]
- Vi, L.; Baht, G.S.; Soderblom, E.J.; Whetstone, H.; Wei, Q.; Furman, B.; Puviindran, V.; Nadesan, P.; Foster, M.; Poon, R.; et al. Macrophage cells secrete factors including LRP1 that orchestrate the rejuvenation of bone repair in mice. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Van Gool, B.; Dedieu, S.; Emonard, H.; Roebroek, A.J.M. The Matricellular Receptor LRP1 Forms an Interface for Signaling and Endocytosis in Modulation of the Extracellular Tumor Environment. Front. Pharmacol. 2015, 6, 271. [Google Scholar] [CrossRef] [PubMed]
- Wujak, L.; Schnieder, J.; Schaefer, L.; Wygrecka, M. LRP1: A chameleon receptor of lung inflammation and repair. Matrix Biol. 2018, 68–69, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Holliday, L.S.; Patel, S.S. RANKL and RANK in extracellular vesicles: Surprising new players in bone remodeling. Extracell. Vesicles Circ. Nucleic Acids 2021, 2, 18–28. [Google Scholar] [CrossRef]
- Deng, L.; Wang, Y.; Peng, Y.; Wu, Y.; Ding, Y.; Jiang, Y.; Shen, Z.; Fu, Q. Osteoblast-derived microvesicles: A novel mechanism for communication between osteoblasts and osteoclasts. Bone 2015, 79, 37–42. [Google Scholar] [CrossRef]
- Cappariello, A.; Loftus, A.; Muraca, M.; Maurizi, A.; Rucci, N.; Teti, A. Osteoblast-Derived Extracellular Vesicles Are Biological Tools for the Delivery of Active Molecules to Bone. J. Bone Miner. Res. 2018, 33, 517–533. [Google Scholar] [CrossRef]
- Huynh, N.; VonMoss, L.; Smith, D.; Rahman, I.; Felemban, M.; Zuo, J.; Rody, J.W.; McHugh, K.; Holliday, L. Characterization of Regulatory Extracellular Vesicles from Osteoclasts. J. Dent. Res. 2016, 95, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, J.; Guo, B.; Liang, C.; Dang, L.; Lu, C.; He, X.; Cheung, H.Y.-S.; Xu, L.; Lu, C.; et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat. Commun. 2016, 7, 10872. [Google Scholar] [CrossRef]
- Sun, W.; Zhao, C.; Li, Y.; Wang, L.; Nie, G.; Peng, J.; Wang, A.; Zhang, P.; Tian, W.; Li, Q.; et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov. 2016, 2, 16015. [Google Scholar] [CrossRef]
- Wortzel, I.; Dror, S.; Kenific, C.M.; Lyden, D. Exosome-Mediated Metastasis: Communication from a Distance. Dev. Cell 2019, 49, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, S.N.; Meckes, D.G., Jr. Extracellular Vesicle Integrins Distinguish Unique Cancers. Proteomes 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lian, S.; Zhou, Y.; Li, B.; Lu, Y.; Yeung, I.; Jia, L. Tumor-derived exosomes can specifically prevent cancer metastatic organotropism. J. Control. Release 2021, 331, 404–415. [Google Scholar] [CrossRef]
- Sung, B.H.; Ketova, T.; Hoshino, D.; Zijlstra, A.; Weaver, A.M. Directional cell movement through tissues is controlled by exosome secretion. Nat. Commun. 2015, 6, 7164. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, D.; Kirkbride, K.C.; Costello, K.; Clark, E.S.; Sinha, S.; Grega-Larson, N.; Tyska, M.J.; Weaver, A.M. Exosome Secretion Is Enhanced by Invadopodia and Drives Invasive Behavior. Cell Rep. 2013, 5, 1159–1168. [Google Scholar] [CrossRef]
- Sung, B.H.; Weaver, A.M. Exosome secretion promotes chemotaxis of cancer cells. Cell Adhes. Migr. 2017, 11, 187–195. [Google Scholar] [CrossRef]
- Murray, J.B.; Mikhael, C.; Han, G.; de Faria, L.P.; Rody, W.J.; Holliday, L.S. Activation of (pro)renin by (pro)renin receptor in extracellular vesicles from osteoclasts. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Tzaridis, T.; Reiners, K.S.; Weller, J.; Bachurski, D.; Schäfer, N.; Schaub, C.; Hallek, M.; Scheffler, B.; Glas, M.; Herrlinger, U.; et al. Analysis of Serum miRNA in Glioblastoma Patients: CD44-Based Enrichment of Extracellular Vesicles Enhances Specificity for the Prognostic Signature. Int. J. Mol. Sci. 2020, 21, 7211. [Google Scholar] [CrossRef] [PubMed]
- Holliday, L.S.; Lu, M.; Lee, B.S.; Nelson, R.D.; Solivan, S.; Zhang, L.; Gluck, S.L. The Amino-terminal Domain of the B Subunit of Vacuolar H+-ATPase Contains a Filamentous Actin Binding Site. J. Biol. Chem. 2000, 275, 32331–32337. [Google Scholar] [CrossRef]
- Zhao, H.; Ito, Y.; Chappel, J.; Andrews, N.W.; Teitelbaum, S.L.; Ross, F.P. Synaptotagmin VII Regulates Bone Remodeling by Modulating Osteoclast and Osteoblast Secretion. Dev. Cell 2008, 14, 914–925. [Google Scholar] [CrossRef]
- MacDougall, D.D.; Lin, Z.; Chon, N.L.; Jackman, S.L.; Lin, H.; Knight, J.D.; Anantharam, A. The high-affinity calcium sensor synaptotagmin-7 serves multiple roles in regulated exocytosis. J. Gen. Physiol. 2018, 150, 783–807. [Google Scholar] [CrossRef]
- Volynski, K.E.; Krishnakumar, S.S. Synergistic control of neurotransmitter release by different members of the synaptotagmin family. Curr. Opin. Neurobiol. 2018, 51, 154–162. [Google Scholar] [CrossRef]
- Yadav, K.S.; Astudillo, H.V.M.; Colina-Tenorio, L.; Bouillenne, F.; Degand, H.; Morsomme, P.; González-Halphen, D.; Boekema, E.J.; Cardol, P. Atypical composition and structure of the mitochondrial dimeric ATP synthase from Euglena gracilis. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Calle, J.; Tu, X.; Pacheco-Costa, R.; McAndrews, K.; Edwards, R.; Pellegrini, G.G.; Kuhlenschmidt, K.; Olivos, N.; Robling, A.; Peacock, M.; et al. Control of Bone Anabolism in Response to Mechanical Loading and PTH by Distinct Mechanisms Downstream of the PTH Receptor. J. Bone Miner. Res. 2016, 32, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.A.; Almeida, E.A.; Hill, E.L.; Aguirre, J.I.; Rivera, M.F.; Nachbandi, I.; Wronski, T.J.; Van Der Meulen, M.C.H.; Globus, R.K. Role for β1 integrins in cortical osteocytes during acute musculoskeletal disuse. Matrix Biol. 2008, 27, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Litzenberger, J.B.; Kim, J.-B.; Tummala, P.; Jacobs, C.R. β1 Integrins Mediate Mechanosensitive Signaling Pathways in Osteocytes. Calcif. Tissue Int. 2010, 86, 325–332. [Google Scholar] [CrossRef]
- Mizoguchi, F.; Mizuno, A.; Hayata, T.; Nakashima, K.; Heller, S.; Ushida, T.; Sokabe, M.; Miyasaka, N.; Suzuki, M.; Ezura, Y.; et al. Transient receptor potential vanilloid 4 deficiency suppresses unloading-induced bone loss. J. Cell. Physiol. 2008, 216, 47–53. [Google Scholar] [CrossRef]
- Papachroni, K.K.; Karatzas, D.N.; Papavassiliou, K.A.; Basdra, E.K.; Papavassiliou, A.G. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol. Med. 2009, 15, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Z.; Xu, X.-Y.; Guo, C.; Yan, Y.-X.; Li, R.-X.; Song, M. Differential effects of mechanical strain on osteoclastogenesis and osteoclast-related gene expression in RAW264.7 cells. Mol. Med. Rep. 2012, 6, 409–415. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suzuki, N.; Yoshimura, Y.; Deyama, Y.; Suzuki, K.; Kitagawa, Y. Mechanical stress directly suppresses osteoclast differenti-ation in RAW264.7 cells. Int. J. Mol. Med. 2008, 21, 291–296. [Google Scholar] [PubMed]
- Kulkarni, R.N.; Voglewede, P.A.; Liu, D. Mechanical vibration inhibits osteoclast formation by reducing DC-STAMP receptor expression in osteoclast precursor cells. Bone 2013, 57, 493–498. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karanth, D.S.; Martin, M.L.; Holliday, L.S. Plasma Membrane Receptors Involved in the Binding and Response of Osteoclasts to Noncellular Components of the Bone. Int. J. Mol. Sci. 2021, 22, 10097. https://doi.org/10.3390/ijms221810097
Karanth DS, Martin ML, Holliday LS. Plasma Membrane Receptors Involved in the Binding and Response of Osteoclasts to Noncellular Components of the Bone. International Journal of Molecular Sciences. 2021; 22(18):10097. https://doi.org/10.3390/ijms221810097
Chicago/Turabian StyleKaranth, Divakar S., Macey L. Martin, and Lexie S. Holliday. 2021. "Plasma Membrane Receptors Involved in the Binding and Response of Osteoclasts to Noncellular Components of the Bone" International Journal of Molecular Sciences 22, no. 18: 10097. https://doi.org/10.3390/ijms221810097
APA StyleKaranth, D. S., Martin, M. L., & Holliday, L. S. (2021). Plasma Membrane Receptors Involved in the Binding and Response of Osteoclasts to Noncellular Components of the Bone. International Journal of Molecular Sciences, 22(18), 10097. https://doi.org/10.3390/ijms221810097





