Association of miR-499 Polymorphism and Its Regulatory Networks with Hashimoto Thyroiditis Susceptibility: A Population-Based Case-Control Study
Abstract
:1. Introduction
2. Results
2.1. Genotypes, Allele Frequencies, and Their Association with HT Risk
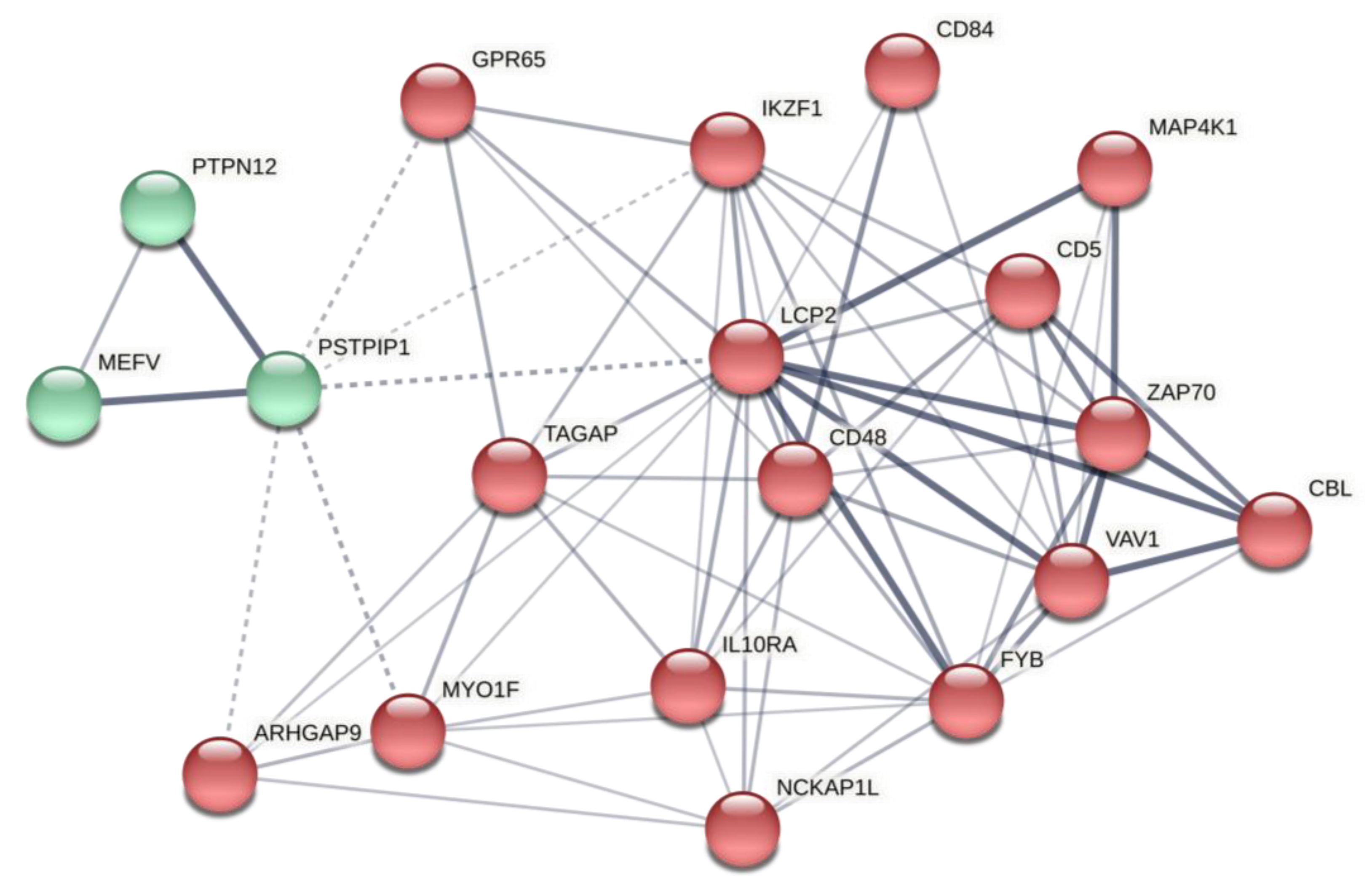
2.2. Predicted Gene Networks of miR-499
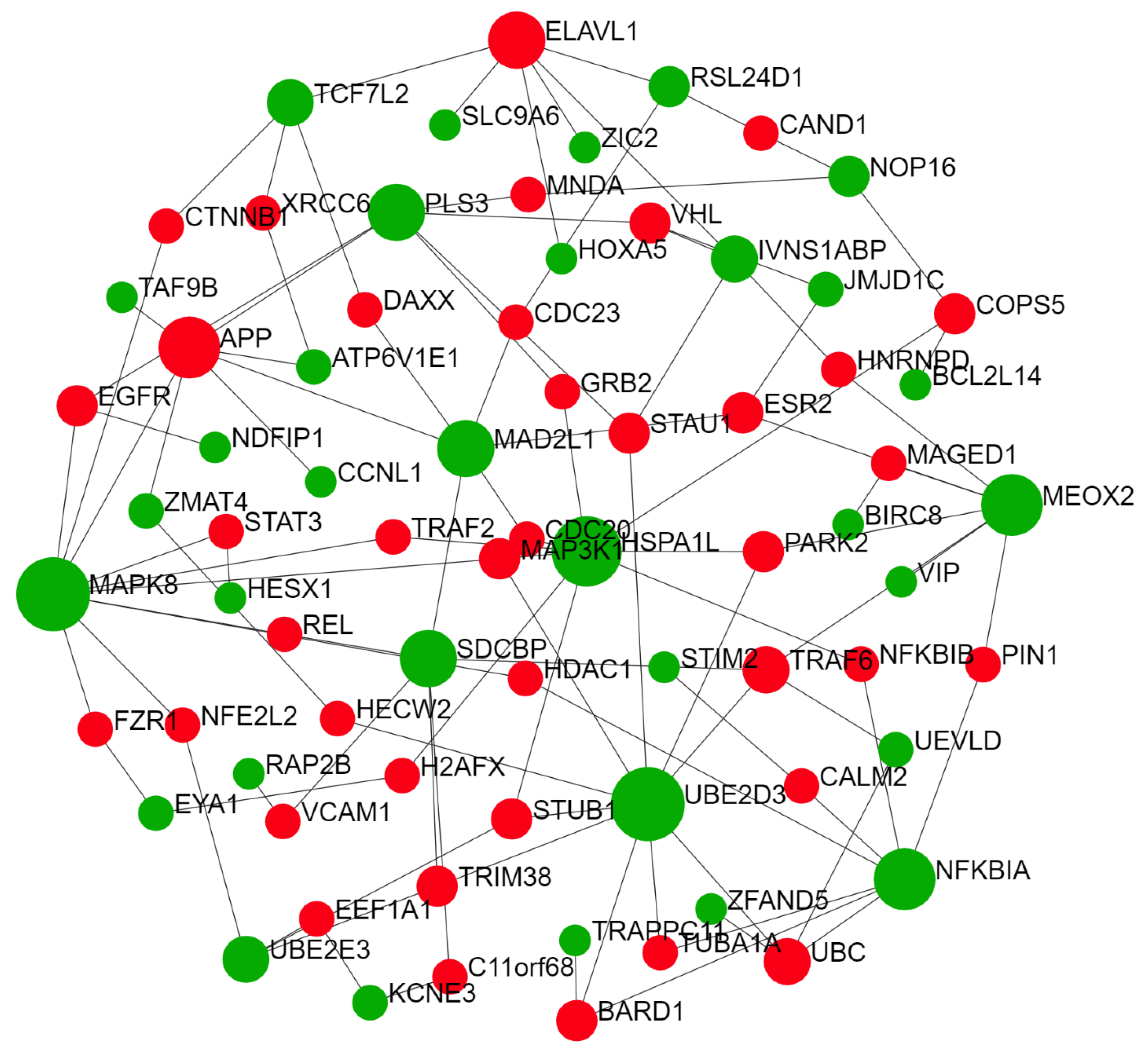
2.2.1. Thyroid-Specific PPIN
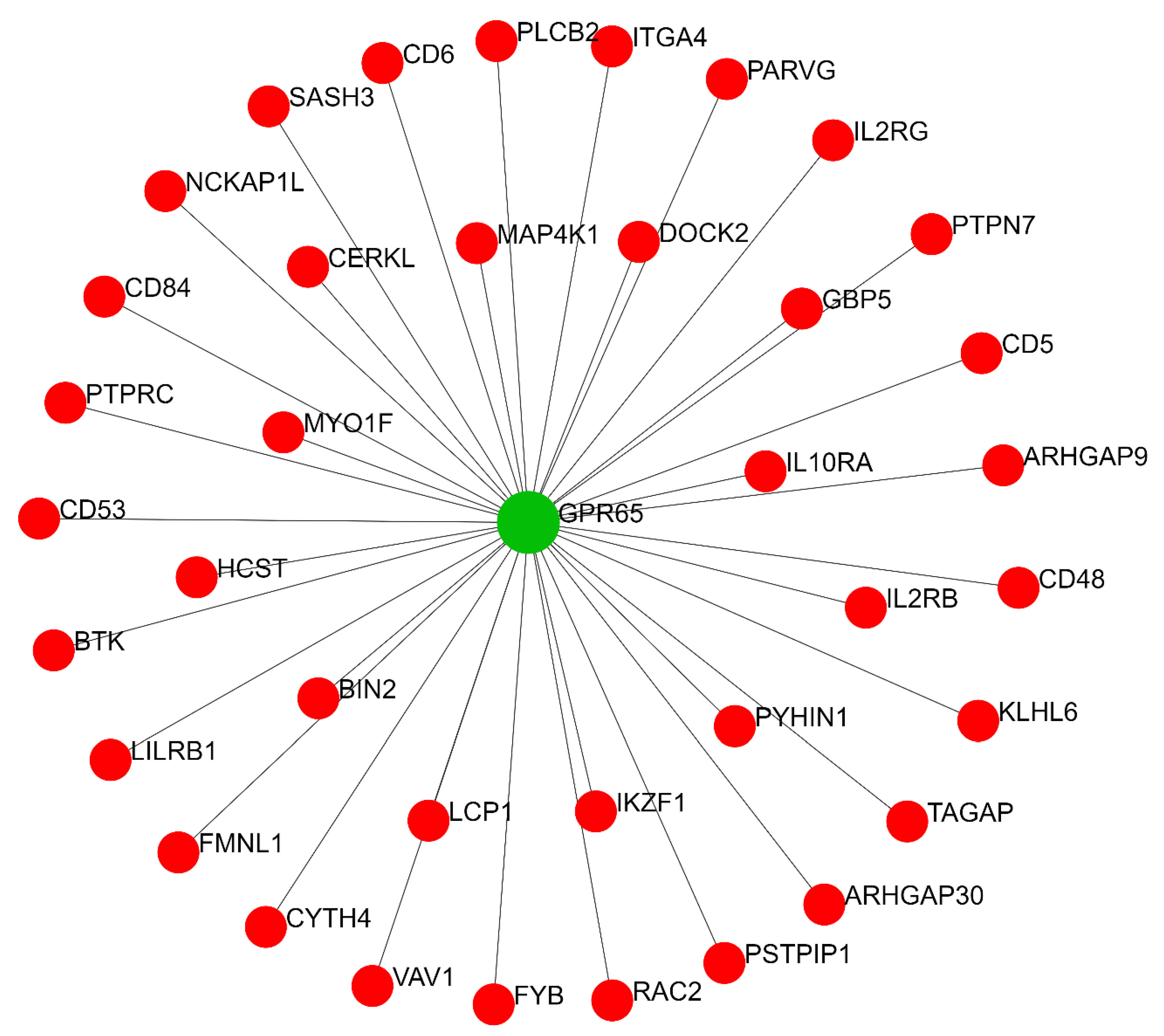
2.2.2. Thyroid-Specific Co-Expression Genes Network
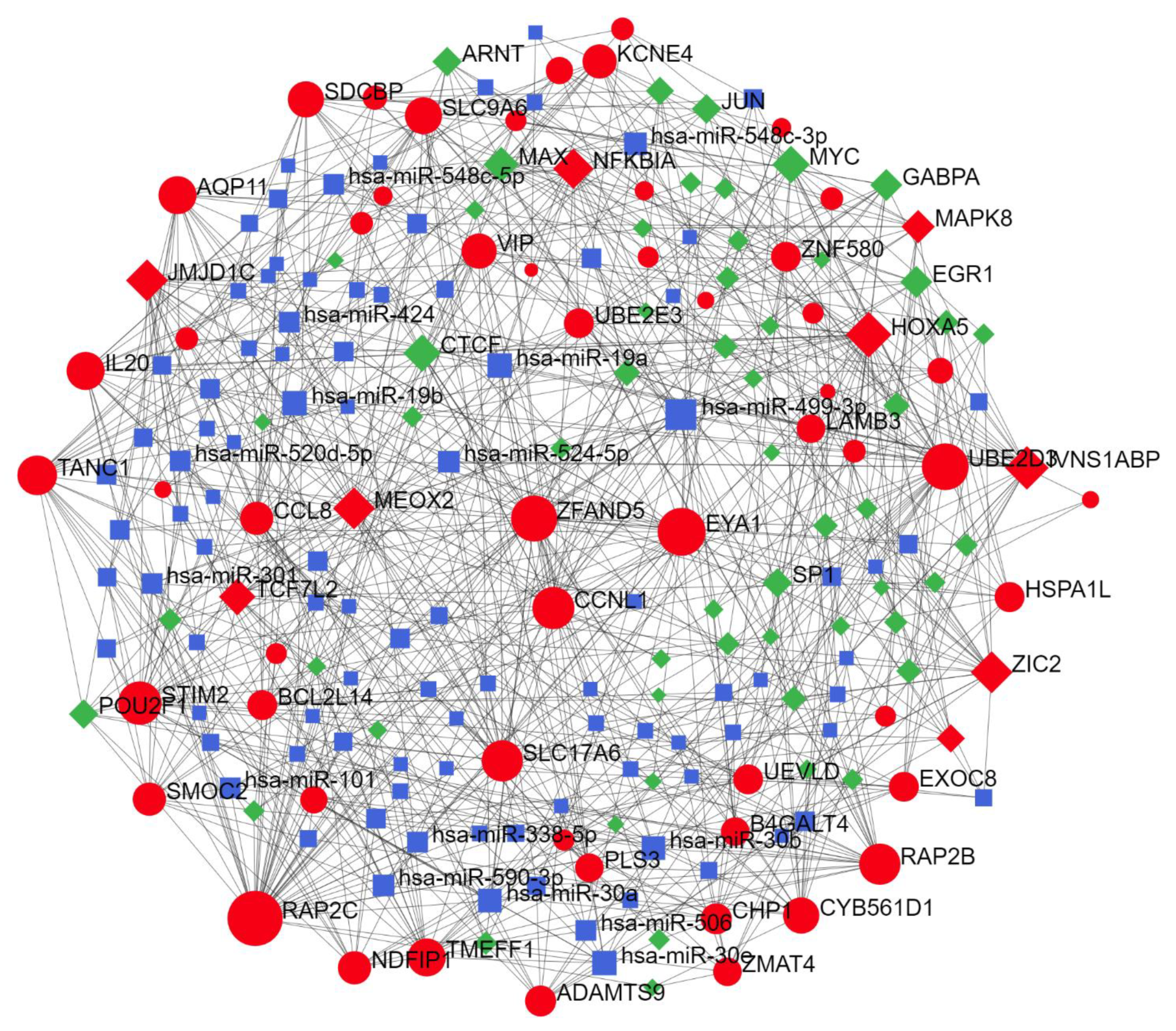
2.2.3. Thyroid-Specific miR-TF Network
3. Discussion
4. Materials and Methods
4.1. Study Population
4.1.1. Sample Size Calculation
4.1.2. Patients and Controls
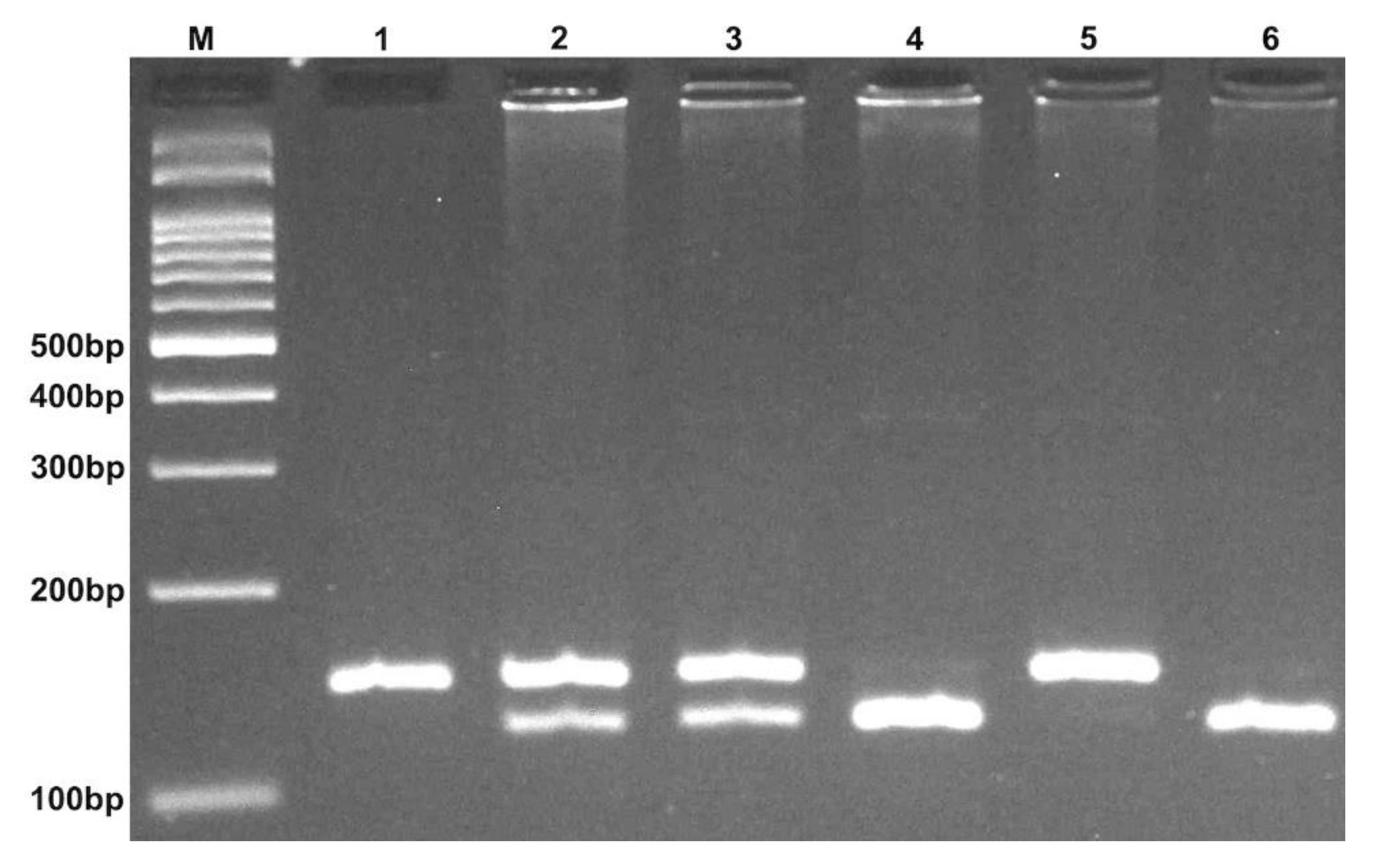
4.2. DNA Extraction and Genotyping
4.3. Prediction of Tissue-Specific, Co-Expression, and miR-TF Gene Networks
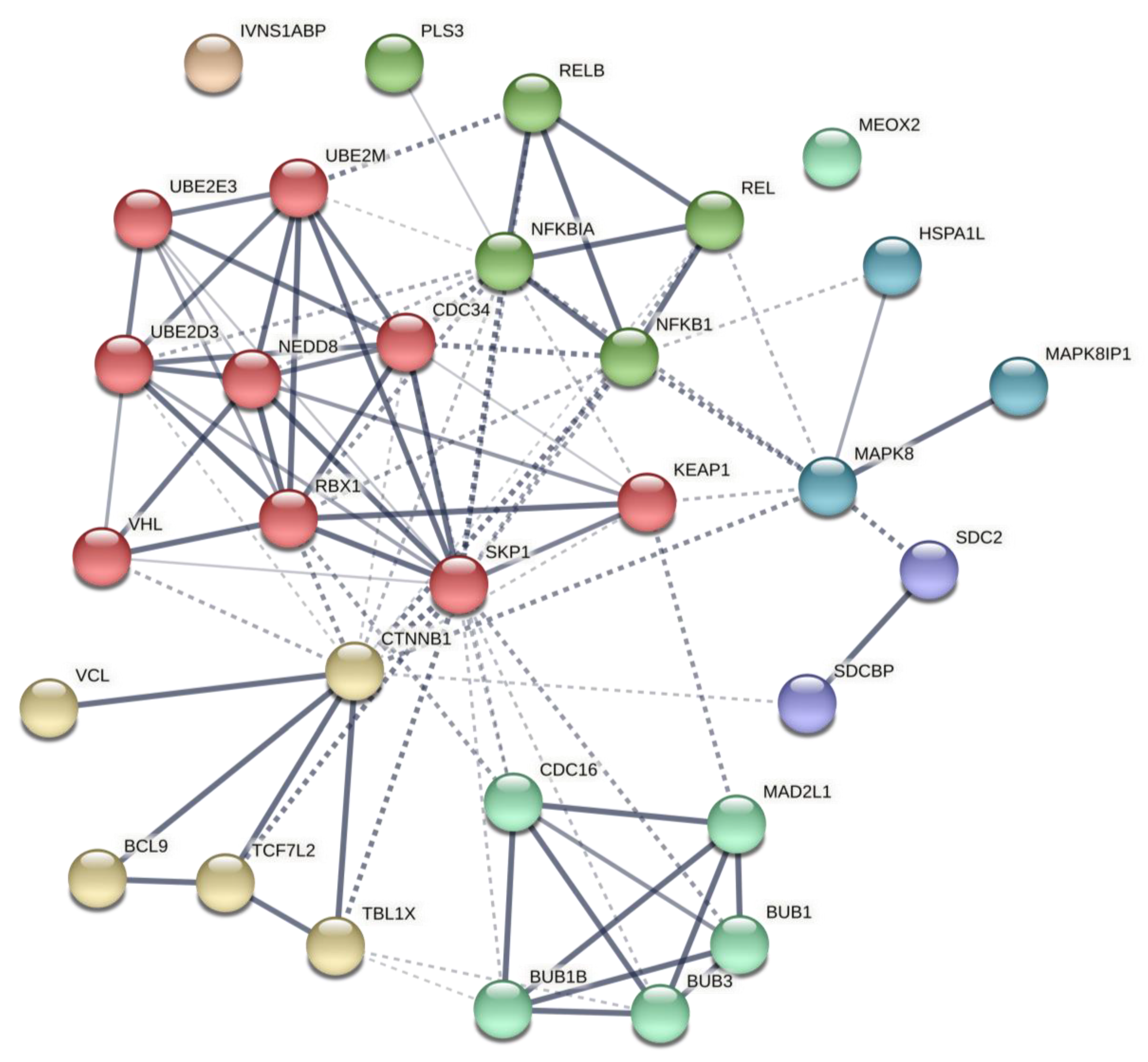
4.4. Post-Analysis of Predicted Networks
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Yuan, J.; Zhu, Y.F.; Yang, X.J.; Wang, Q.; Xu, J.; He, S.T.; Zhang, J.A. Imbalance of Th17/treg in different subtypes of autoimmune thyroid diseases. Cell. Physiol. Biochem. 2016, 40, 245–252. [Google Scholar] [CrossRef]
- Sakr, M.F. Hashimoto’s disease. In Thyroid Disease: Challenges and Debates; Springer International Publishing: Cham, Switzerland, 2020; pp. 71–132. [Google Scholar]
- Braverman, L.E.; Cooper, D. Werner & Ingbar’s the Thyroid: A Fundamental and Clinical Text; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Guarneri, F.; Benvenga, S. Environmental factors and genetic background that interact to cause autoimmune thyroid disease. Curr. Opin. Endocrinol. Diabetes Obes. 2007, 14, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Hasham, A.; Tomer, Y. Genetic and epigenetic mechanisms in thyroid autoimmunity. Immunol. Res. 2012, 54, 204–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Cheng, W.; Ma, Y.; Zhu, J. Vitamin D receptor gene FokI but not TaqI, ApaI, BsmI polymorphism is associated with Hashimoto’s thyroiditis: A meta-analysis. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, K.; Jiang, L.; Zhang, L.; Shi, H.; Cui, D. Associations between three CTLA-4 polymorphisms and Hashimoto’s thyroiditis risk: An updated meta-analysis with trial sequential analysis. Genet. Test. Mol. Biomark. 2018, 22, 224–236. [Google Scholar] [CrossRef]
- Almeida, M.I.; Reis, R.M.; Calin, G.A. MicroRNA history: Discovery, recent applications, and next frontiers. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2011, 717, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and function. Thromb. Haemost. 2012, 107, 605–610. [Google Scholar] [CrossRef] [Green Version]
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Pauley, K.M.; Cha, S.; Chan, E.K.L. MicroRNA in autoimmunity and autoimmune diseases. J. Autoimmun. 2009, 32, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Bernecker, C.; Lenz, L.; Ostapczuk, M.S.; Schinner, S.; Willenberg, H.; Ehlers, M.; Vordenbäumen, S.; Feldkamp, J.; Schott, M. MicroRNAs miR-146a1, miR-155_2, and miR-200a1 are regulated in autoimmune thyroid diseases. Thyroid 2012, 22, 1294–1295. [Google Scholar] [CrossRef]
- Venugopal, P.; Lavu, V.; Rao, S.R.; Venkatesan, V. Association of microRNA-125a and microRNA-499a polymorphisms in chronic periodontitis in a sample south Indian population: A hospital-based genetic association study. Gene 2017, 631, 10–15. [Google Scholar] [CrossRef]
- Kiselev, I.; Bashinskaya, V.; Kulakova, O.; Baulina, N.; Popova, E.; Boyko, A.; Favorova, O. Variants of MicroRNA genes: Gender-specific associations with multiple sclerosis risk and severity. Int. J. Mol. Sci. 2015, 16, 20067–20081. [Google Scholar] [CrossRef] [Green Version]
- Duan, R.; Pak, C.; Jin, P. Single nucleotide polymorphism associated with mature miR-125a alters the processing of pri-miRNA. Hum. Mol. Genet. 2007, 16, 1124–1131. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Li, M.; Sun, T.; Han, D.; Guo, X.; Chen, X.; Wan, Q.; Zhang, X.; Wang, J. A polymorphism rs3746444 within the pre-miR-499 alters the maturation of miR-499-5p and its antiapoptotic function. J. Cell. Mol. Med. 2018, 22, 5418–5428. [Google Scholar] [CrossRef] [Green Version]
- Cai, T.; Li, J.; An, X.; Yan, N.; Li, D.; Jiang, Y.; Wang, W.; Shi, L.; Qin, Q.; Song, R.; et al. Polymorphisms in MIR499A and MIR125A gene are associated with autoimmune thyroid diseases. Mol. Cell. Endocrinol. 2017, 440, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Kim, P.M.; Sprecher, E.; Trifonov, V.; Gerstein, M. The importance of bottlenecks in protein networks: Correlation with gene essentiality and expression dynamics. PLoS Comput. Biol. 2007, 3, e59. [Google Scholar] [CrossRef] [PubMed]
- Zaletel, K.; Gaberscek, S. Hashimoto’s thyroiditis: From genes to the disease. Curr. Genom. 2011, 12, 576–588. [Google Scholar] [CrossRef] [Green Version]
- Douville, J.M.; Cheung, D.Y.; Herbert, K.L.; Moffatt, T.; Wigle, J.T. Mechanisms of MEOX1 and MEOX2 regulation of the cyclin dependent kinase inhibitors p21 CIP1/WAF1 and p16 INK4a in vascular endothelial cells. PLoS ONE 2011, 6, e29099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Rabson, A.B.; Gorski, D.H. MEOX2 regulates nuclear factor-kappaB activity in vascular endothelial cells through interactions with p65 and IkappaBbeta. Cardiovasc. Res. 2010, 87, 723–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.; Leal, A.D.; Gorski, D.H. The homeobox gene Gax inhibits angiogenesis through inhibition of nuclear factor-kappaB-dependent endothelial cell gene expression. Cancer Res. 2005, 65, 1414–1424. [Google Scholar] [CrossRef] [Green Version]
- Kegelman, T.P.; Das, S.K.; Emdad, L.; Hu, B.; Menezes, M.E.; Bhoopathi, P.; Wang, X.-Y.; Pellecchia, M.; Sarkar, D.; Fisher, P.B. Targeting tumor invasion: The roles of MDA-9/Syntenin. Expert Opin. Ther. Targets 2015, 19, 97–112. [Google Scholar] [CrossRef] [Green Version]
- Gimferrer, I.; Ibáñez, A.; Farnós, M.; Sarrias, M.-R.; Fenutría, R.; Roselló, S.; Zimmermann, P.; David, G.; Vives, J.; Serra-Pagès, C.; et al. The lymphocyte receptor CD6 interacts with syntenin-1, a scaffolding protein containing PDZ domains. J. Immunol. 2005, 175, 1406. [Google Scholar] [CrossRef] [Green Version]
- Sala-Valdés, M.; Gordón-Alonso, M.; Tejera, E.; Ibáñez, A.; Cabrero, J.R.; Ursa, A.; Mittelbrunn, M.; Lozano, F.; Sánchez-Madrid, F.; Yáñez-Mó, M. Association of syntenin-1 with M-RIP polarizes Rac-1 activation during chemotaxis and immune interactions. J. Cell Sci. 2012, 125, 1235–1246. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Larrea, J.; Merlos-Suárez, A.; Ureña, J.M.; Baselga, J.; Arribas, J. A role for a PDZ protein in the early secretory pathway for the targeting of proTGF-α to the cell surface. Mol. Cell 1999, 3, 423–433. [Google Scholar] [CrossRef]
- Geijsen, N.; Uings, I.J.; Pals, C.; Armstrong, J.; McKinnon, M.; Raaijmakers, J.A.; Lammers, J.-W.J.; Koenderman, L.; Coffer, P.J. Cytokine-specific transcriptional regulation through an IL-5Rα interacting protein. Science 2001, 293, 1136–1138. [Google Scholar] [CrossRef] [PubMed]
- Beekman, J.M.; Verhagen, L.P.; Geijsen, N.; Coffer, P.J. Regulation of myelopoiesis through syntenin-mediated modulation of IL-5 receptor output. Blood J. Am. Soc. Hematol. 2009, 114, 3917–3927. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Du, Y.; Zhang, Z.; Chen, G.; Zhang, M.; Shu, H.-B.; Zhai, Z.; Chen, D. Syntenin negatively regulates TRAF6-mediated IL-1R/TLR4 signaling. Cell. Signal. 2008, 20, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Majer, O.; Liu, B.; Kreuk, L.S.; Krogan, N.; Barton, G.M. UNC93B1 recruits syntenin-1 to dampen TLR7 signalling and prevent autoimmunity. Nature 2019, 575, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Rajendra, R.; Malegaonkar, D.; Pungaliya, P.; Marshall, H.; Rasheed, Z.; Brownell, J.; Liu, L.F.; Lutzker, S.; Saleem, A.; Rubin, E.H. Topors functions as an E3 ubiquitin ligase with specific E2 enzymes and ubiquitinates p53. J. Biol. Chem. 2004, 279, 36440–36444. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Chen, R.; Luo, X.; Zhang, W.-D.; Qin, J.-J. The E2 ubiquitin-conjugating enzyme UbcH5c: An emerging target in cancer and immune disorders. Drug Discov. Today 2020, 25, 1988–1997. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Rincón, M.; Pedraza-Alva, G. JNK and p38 MAP kinases in CD4+ and CD8+ T cells. Immunol. Rev. 2003, 192, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Castro-Alcaraz, S.; Miskolci, V.; Kalasapudi, B.; Davidson, D.; Vancurova, I. NF-κB regulation in human neutrophils by nuclear IκBα: Correlation to apoptosis. J. Immunol. 2002, 169, 3947–3953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.-L.; Zou, Y.-F.; Feng, X.-L.; Shi, H.-J.; Du, X.-F.; Shao, M.-H.; Gu, Y.; Zhou, Q. Association of the NFKBIA gene polymorphisms with susceptibility to autoimmune and inflammatory diseases: A meta-analysis. Inflamm. Res. 2011, 60, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Karin, M. Nuclear factor-κB—A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Kurylowicz, A.; Miśkiewicz, P.; Bar-Andziak, E.; Nauman, J.; Bednarczuk, T. Association of polymorphism in genes encoding kappaB inhibitors (IkappaB) with susceptibility to and phenotype of Graves’ disease: A case-control study. Thyroid Res. 2009, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Suryawanshi, A.; Tadagavadi, R.K.; Swafford, D.; Manicassamy, S. Modulation of inflammatory responses by Wnt/β-catenin signaling in dendritic cells: A novel immunotherapy target for autoimmunity and cancer. Front. Immunol. 2016, 7, 460. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Chi, S.; Xue, J.; Yang, J.; Li, F.; Liu, X. Emerging role and therapeutic implication of Wnt signaling pathways in autoimmune diseases. J. Immunol. Res. 2016, 2016, 9392132. [Google Scholar] [CrossRef] [Green Version]
- Staal, F.J.T.; Luis, T.C.; Tiemessen, M.M. WNT signalling in the immune system: WNT is spreading its wings. Nat. Rev. Immunol. 2008, 8, 581–593. [Google Scholar] [CrossRef]
- Kuryłowicz, A.; Nauman, J. The role of nuclear factor-kappaB in the development of autoimmune diseases: A link between genes and environment. Acta Biochim. Pol. 2008, 55, 629–647. [Google Scholar] [CrossRef]
- Ishii, S.; Kihara, Y.; Shimizu, T. Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J. Biol. Chem. 2005, 280, 9083–9087. [Google Scholar] [CrossRef] [Green Version]
- Tosa, N.; Murakami, M.; Jia, W.Y.; Yokoyama, M.; Masunaga, T.; Iwabuchi, C.; Inobe, M.; Iwabuchi, K.; Miyazaki, T.; Onoe, K. Critical function of T cell death-associated gene 8 in glucocorticoid-induced thymocyte apoptosis. Int. Immunol. 2003, 15, 741–749. [Google Scholar] [CrossRef]
- Ashwell, J.D.; Lu, F.W.; Vacchio, M.S. Glucocorticoids in T cell development and function. Annu. Rev. Immunol. 2000, 18, 309–345. [Google Scholar] [CrossRef]
- Radu, C.G.; Nijagal, A.; McLaughlin, J.; Wang, L.; Witte, O.N. Differential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cells. Proc. Natl. Acad. Sci. USA 2005, 102, 1632–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saoudi, A.; Kassem, S.; Dejean, A.; Gaud, G. Rho-GTPases as key regulators of T lymphocyte biology. Small GTPases 2014, 5, e983862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyre, S.; Hinks, A.; Bowes, J.; Flynn, E.; Martin, P.; Wilson, A.G.; Morgan, A.W.; Emery, P.; Steer, S.; Hocking, L.J.; et al. Overlapping genetic susceptibility variants between three autoimmune disorders: Rheumatoid arthritis, type 1 diabetes and coeliac disease. Arthritis Res. Ther. 2010, 12, R175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berge, T.; Leikfoss, I.S.; Brorson, I.S.; Bos, S.D.; Page, C.M.; Gustavsen, M.W.; Bjølgerud, A.; Holmøy, T.; Celius, E.G.; Damoiseaux, J.; et al. The multiple sclerosis susceptibility genes TAGAP and IL2RA are regulated by vitamin D in CD4+ T cells. Genes Immun. 2016, 17, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Krendel, M.; Mooseker, M.S. Myosins: Tails (and heads) of functional diversity. Physiology 2005, 20, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Bement, W.M.; Hasson, T.; Wirth, J.A.; Cheney, R.E.; Mooseker, M.S. Identification and overlapping expression of multiple unconventional myosin genes in vertebrate cell types. Proc. Natl. Acad. Sci. USA 1994, 91, 11767. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.V.; Mehal, W.Z.; Dong, X.; Heinrich, V.; Pypaert, M.; Mellman, I.; Dembo, M.; Mooseker, M.S.; Wu, D.; Flavell, R.A. Modulation of cell adhesion and motility in the immune system by Myo1f. Science 2006, 314, 136. [Google Scholar] [CrossRef] [Green Version]
- Ling, P.; Meyer, C.F.; Redmond, L.P.; Shui, J.W.; Davis, B.; Rich, R.R.; Hu, M.C.; Wange, R.L.; Tan, T.H. Involvement of hematopoietic progenitor kinase 1 in T cell receptor signaling. J. Biol. Chem. 2001, 276, 18908–18914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, J.; Kiefer, F.; Dang, A.; Hashimoto, A.; Cobb, M.H.; Kurosaki, T.; Weiss, A. HPK1 is activated by lymphocyte antigen receptors and negatively regulates AP-1. Immunity 2000, 12, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, S.; Okamoto, M.; Yamada, K.; Okamoto, N.; Goitsuka, R.; Arnold, R.; Kiefer, F.; Kitamura, D. B cell adaptor containing src homology 2 domain (BASH) links B cell receptor signaling to the activation of hematopoietic progenitor kinase 1. J. Exp. Med. 2001, 194, 529–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Zhou, G.; Hu, M.C.; Yao, Z.; Tan, T.H. Activation of the hematopoietic progenitor kinase-1 (HPK1)-dependent, stress-activated c-Jun N-terminal kinase (JNK) pathway by transforming growth factor beta (TGF-beta)-activated kinase (TAK1), a kinase mediator of TGF beta signal transduction. J. Biol. Chem. 1997, 272, 22771–22775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, D.; Brechmann, M.; Röhling, S.; Tapernoux, M.; Mock, T.; Winter, D.; Lehmann, W.D.; Kiefer, F.; Thome, M.; Krammer, P.H.; et al. Phosphorylation of CARMA1 by HPK1 is critical for NF-kappaB activation in T cells. Proc. Natl. Acad. Sci. USA 2009, 106, 14508–14513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, D.; Golks, A.; Kiefer, F.; Krammer, P.H.; Arnold, R. Activation or suppression of NFkappaB by HPK1 determines sensitivity to activation-induced cell death. EMBO J. 2005, 24, 4279–4290. [Google Scholar] [CrossRef]
- De Velde, H.V.; von Hoegen, I.; Luo, W.; Parnes, J.R.; Thielemans, K. The B-cell surface protein CD72/Lyb-2 is the ligand for CDS. Nature 1991, 351, 662–665. [Google Scholar] [CrossRef]
- Azzam, H.S.; DeJarnette, J.B.; Huang, K.; Emmons, R.; Park, C.-S.; Sommers, C.L.; El-Khoury, D.; Shores, E.W.; Love, P.E. Fine tuning of TCR signaling by CD5. J. Immunol. 2001, 166, 5464–5472. [Google Scholar] [CrossRef]
- Perez-Villar, J.J.; Whitney, G.S.; Bowen, M.A.; Hewgill, D.H.; Aruffo, A.A.; Kanner, S.B. CD5 negatively regulates the T-cell antigen receptor signal transduction pathway: Involvement of SH2-containing phosphotyrosine phosphatase SHP-1. Mol. Cell. Biol. 1999, 19, 2903–2912. [Google Scholar] [CrossRef] [Green Version]
- Domingues, R.G.; Lago-Baldaia, I.; Pereira-Castro, I.; Fachini, J.M.; Oliveira, L.; Drpic, D.; Lopes, N.; Henriques, T.; Neilson, J.R.; Carmo, A.M.; et al. CD5 expression is regulated during human T-cell activation by alternative polyadenylation, PTBP1, and miR-204. Eur. J. Immunol. 2016, 46, 1490–1503. [Google Scholar] [CrossRef] [Green Version]
- Yi, W.; Wang, J.; Yao, Z.; Kong, Q.; Zhang, N.; Mo, W.; Xu, L.; Li, X. The expression status of ZIC2 as a prognostic marker for nasopharyngeal carcinoma. Int. J. Clin. Exp. Pathol. 2018, 11, 4446. [Google Scholar]
- Sun, L.; Lin, Y.; Wang, G.; Zhang, L.; Hu, L.; Lu, Z. Correlation of zinc finger protein 2, a prognostic biomarker, with immune infiltrates in liver cancer. Biosci. Rep. 2021, 41, BSR20203115. [Google Scholar] [CrossRef]
- Taira, K.; Umikawa, M.; Takei, K.; Myagmar, B.-E.; Shinzato, M.; Machida, N.; Uezato, H.; Nonaka, S.; Kariya, K.-I. The Traf2-and Nck-interacting kinase as a putative effector of Rap2 to regulate actin cytoskeleton. J. Biol. Chem. 2004, 279, 49488–49496. [Google Scholar] [CrossRef] [Green Version]
- Machida, N.; Umikawa, M.; Takei, K.; Sakima, N.; Myagmar, B.-E.; Taira, K.; Uezato, H.; Ogawa, Y.; Kariya, K.-I. Mitogen-activated protein kinase kinase kinase kinase 4 as a putative effector of Rap2 to activate the c-Jun N-terminal kinase. J. Biol. Chem. 2004, 279, 15711–15714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Qiu, J.; Zhang, T.; Yang, Y.; Guo, S.; Li, T.; Jiang, K.; Zahoor, A.; Deng, G.; Qiu, C. MicroRNA-188-5p promotes apoptosis and inhibits cell proliferation of breast cancer cells via the MAPK signaling pathway by targeting Rap2c. J. Cell. Physiol. 2020, 235, 2389–2402. [Google Scholar] [CrossRef]
- Huang, H.-P.; Liu, W.-J.; Guo, Q.-L.; Bai, Y.-Q. Effect of silencing HOXA5 gene expression using RNA interference on cell cycle and apoptosis in Jurkat cells. Int. J. Mol. Med. 2016, 37, 669–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, J.F.; McAdara, J.; Yaron, Y.; Sakaguchi, M.; Fraser, J.K.; Gasson, J.C. Characterization of HOX gene expression during myelopoiesis: Role of HOX A5 in lineage commitment and maturation. Blood J. Am. Soc. Hematol. 1999, 93, 3391–3400. [Google Scholar] [CrossRef]
- Wu, J.; Richards, M.H.; Huang, J.; Al-Harthi, L.; Xu, X.; Lin, R.; Xie, F.; Gibson, A.W.; Edberg, J.C.; Kimberly, R.P. Human FasL gene is a target of β-catenin/T-cell factor pathway and complex FasL haplotypes alter promoter functions. PLoS ONE 2011, 6, e26143. [Google Scholar] [CrossRef] [PubMed]
- Tsedensodnom, O.; Koga, H.; Rosenberg, S.A.; Nambotin, S.B.; Carroll, J.J.; Wands, J.R.; Kim, M. Identification of T-cell factor-4 isoforms that contribute to the malignant phenotype of hepatocellular carcinoma cells. Exp. Cell Res. 2011, 317, 920–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, H.; Tsedensodnom, O.; Tomimaru, Y.; Walker, E.J.; Lee, H.C.; Kim, K.M.; Yano, H.; Wands, J.R.; Kim, M. Loss of the SxxSS motif in a human T-cell factor-4 isoform confers hypoxia resistance to liver cancer: An oncogenic switch in Wnt signaling. PLoS ONE 2012, 7, e39981. [Google Scholar] [CrossRef] [Green Version]
- Szepietowska, B.; Moczulski, D.; Wawrusiewicz-Kurylonek, N.; Grzeszczak, W.; Gorska, M.; Szelachowska, M. Transcription factor 7-like 2-gene polymorphism is related to fasting C peptide in latent autoimmune diabetes in adults (LADA). Acta Diabetol. 2010, 47, 83–86. [Google Scholar] [CrossRef]
- Ceribelli, A.; Nahid, M.A.; Satoh, M.; Chan, E.K. MicroRNAs in rheumatoid arthritis. FEBS Lett. 2011, 585, 3667–3674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baltimore, D.; Boldin, M.P.; O’connell, R.M.; Rao, D.S.; Taganov, K.D. MicroRNAs: New regulators of immune cell development and function. Nat. Immunol. 2008, 9, 839. [Google Scholar] [CrossRef]
- Park, R.; Lee, W.J.; Ji, J.D. Association between the three functional miR-146a single-nucleotide polymorphisms, rs2910164, rs57095329, and rs2431697, and autoimmune disease susceptibility: A meta-analysis. Autoimmunity 2016, 49, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Eskandari-Nasab, E.; Zakeri, Z.; Atabaki, M.; Bahari, G.; Jahantigh, M.; Taheri, M.; Ghavami, S. Association of pre-miRNA-146a rs2910164 and pre-miRNA-499 rs3746444 polymorphisms and susceptibility to rheumatoid arthritis. Mol. Med. Rep. 2013, 7, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Dorn, G.W.; Matkovich, S.J.; Eschenbacher, W.H.; Zhang, Y. A human 3′ miR-499 mutation alters cardiac mRNA targeting and function. Circ. Res. 2012, 110, 958–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-H.; He, K.; Shi, G. Effects of MicroRNA-499 on the inflammatory damage of endothelial cells during coronary artery disease via the targeting of PDCD4 through the NF-Κβ/TNF-α signaling pathway. Cell. Physiol. Biochem. 2017, 44, 110–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elessawi, D.F.; Radwan, N.K.; Nosseir, N.M.; Tawfik, M.S. Micro RNA 499 gene expression and interleukin 17 in Egyptian patients with Behçet’s disease. Egypt. Rheumatol. 2019, 42, 135–139. [Google Scholar] [CrossRef]
- Van der Helm-Van, A.H.; Huizinga, T.W. Advances in the genetics of rheumatoid arthritis point to subclassification into distinct disease subsets. Arthritis Res. Ther. 2008, 10, 205. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-X.; Jiao, J.-Q.; Li, Q.; Long, B.; Wang, K.; Liu, J.-P.; Li, Y.-R.; Li, P.-F. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat. Med. 2011, 17, 71–78. [Google Scholar] [CrossRef]
- Alemán-Ávila, I.; Jiménez-Morales, M.; Beltrán-Ramírez, O.; Barbosa-Cobos, R.E.; Jiménez-Morales, S.; Sánchez-Muñoz, F.; Valencia-Pacheco, G.; Amezcua-Guerra, L.M.; Juárez-Vicuña, Y.; Hernández, D.M.R.-B. Functional polymorphisms in pre-miR146a and pre-miR499 are associated with systemic lupus erythematosus but not with rheumatoid arthritis or Graves’ disease in Mexican patients. Oncotarget 2017, 8, 91876. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, W.; Ding, X.; Xu, W.; Liu, D.; Su, B.; Sun, Y. Variations within 3′-UTR of MDM4 gene contribute to clinical outcomes of advanced non-small cell lung cancer patients following platinum-based chemotherapy. Oncotarget 2017, 8, 16313. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Zhang, J.L.; Shi, Y.Y.; Li, D.D.; Chen, J.; Huang, Z.C.; Cai, B.; Song, X.B.; Li, L.X.; Ying, B.W. Association study of single nucleotide polymorphisms in pre-miRNA and rheumatoid arthritis in a Han Chinese population. Mol. Biol. Rep. 2011, 38, 4913–4919. [Google Scholar] [CrossRef]
- Lu, L.; Tu, Y.; Liu, L.; Qi, J.; He, L. MicroRNA-499 rs3746444 polymorphism and autoimmune diseases risk: A meta-analysis. Mol. Diagn. Ther. 2014, 18, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, H.; Chen, L.; Wang, Y.; Yao, X.; Jiang, X. Association of microRNAs genes polymorphisms with arthritis: A systematic review and meta-analysis. Biosci. Rep. 2019, 39, BSR20190298. [Google Scholar] [CrossRef]
- Fu, L.; Jin, L.; Yan, L.; Shi, J.; Wang, H.; Zhou, B.; Wu, X. Comprehensive review of genetic association studies and meta-analysis on miRNA polymorphisms and rheumatoid arthritis and systemic lupus erythematosus susceptibility. Hum. Immunol. 2016, 77, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, K.; Zhou, R. Meta-analysis of pre-miRNA polymorphisms association with susceptibility to autoimmune diseases. Immunol. Investig. 2014, 43, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pu, J.; Wang, X.; Shen, L.; Zhao, G.; Zhuang, C.; Liu, R. IRAK1 rs3027898 C/A polymorphism is associated with risk of rheumatoid arthritis. Rheumatol. Int. 2013, 33, 369–375. [Google Scholar] [CrossRef]
- Yang, X.-K.; Li, P.; Zhang, C.; Leng, R.-X.; Li, S.; Liu, J.; Li, B.-Z.; Pan, H.-F.; Ye, D.-Q. Association between IRAK1 rs3027898 and miRNA-499 rs3746444 polymorphisms and rheumatoid arthritis. Z. Rheumatol. 2017, 76, 622–629. [Google Scholar] [CrossRef]
- Schaefer, M.H.; Lopes, T.J.; Mah, N.; Shoemaker, J.E.; Matsuoka, Y.; Fontaine, J.-F.; Louis-Jeune, C.; Eisfeld, A.J.; Neumann, G.; Perez-Iratxeta, C. Adding protein context to the human protein-protein interaction network to reveal meaningful interactions. PLoS Comput. Biol. 2013, 9, e1002860. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, C.; Bucci, I.; Napolitano, G. The role of the transcription factor nuclear factor-kappa B in thyroid autoimmunity and cancer. Front. Endocrinol. 2018, 9, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aminorroaya, A.; Meamar, R.; Amini, M.; Feizi, A.; Tabatabae, A.; Faghih Imani, E. Incidence of thyroid dysfunction in an Iranian adult population: The predictor role of thyroid autoantibodies: Results from a prospective population-based cohort study. Eur. J. Med. Res. 2017, 22, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemi, M.; Moradi, N.; Ziaee, S.A.; Narouie, B.; Soltani, M.H.; Rezaei, M.; Shahkar, G.; Taheri, M. Association between single nucleotide polymorphism in miR-499, miR-196a2, miR-146a and miR-149 and prostate cancer risk in a sample of Iranian population. J. Adv. Res. 2016, 7, 491–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basha, O.; Shpringer, R.; Argov, C.M.; Yeger-Lotem, E. The DifferentialNet database of differential protein–protein interactions in human tissues. Nucleic Acids Res. 2018, 46, D522–D526. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Zhang, C.; Arif, M.; Liu, Z.; Benfeitas, R.; Bidkhori, G.; Deshmukh, S.; Al Shobky, M.; Lovric, A.; Boren, J. TCSBN: A database of tissue and cancer specific biological networks. Nucleic Acids Res. 2018, 46, D595–D600. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.-P.; Wu, C.; Miao, H.; Wu, H. RegNetwork: An integrated database of transcriptional and post-transcriptional regulatory networks in human and mouse. Database 2015, 2015, bav095. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. elife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Zhou, G.; Soufan, O.; Ewald, J.; Hancock, R.E.; Basu, N.; Xia, J. NetworkAnalyst 3.0: A visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019, 47, W234–W241. [Google Scholar] [CrossRef] [Green Version]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R. The reactome pathway knowledgebase. Nucleic Acids Res. 2020, 48, D498–D503. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2018, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Demographic | HT, n = 150 | Control, n = 152 | p-Value | |
|---|---|---|---|---|
| Age, year (mean ± SD) | 37.96 ± 12.06 | 36.22 ± 12.86 | 0.226 | |
| Sex | Male | 17 | 24 | 0.314 |
| Female | 133 | 128 | ||
| Polymorphisms | Gentic Model | Genotype | HT, n (%) | Controls, n (%) | OR (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| rs3746444 (miR-499) | Codominant | TT | 37 (24.7) | 78 (51.3) | 1.00 | - |
| CT | 93 (62.0) | 59 (38.8) | 3.32 (2.00–5.53) | <0.001 | ||
| CC | 20 (13.3) | 15 (9.9) | 2.81 (1.30–6.10) | 0.014 | ||
| Dominant | TT | 37 (24.7) | 78 (51.3) | 1.00 | - | |
| CT + CC | 113 (75.3) | 74 (48.7) | 3.22 (1.97–5.25) | <0.001 | ||
| Recessive | TT + CT | 130 (86.7) | 137 (90.1) | 1.00 | - | |
| CC | 20 (13.3) | 15 (9.9) | 1.41 (0.69–2.86) | 0.447 | ||
| Overdominant | CC + TT | 57 (38) | 93 (61.2) | 1.0 | - | |
| CT | 93 (62.0) | 59 (38.8) | 2.57 (1.62–4.09) | <0.001 | ||
| Allele | T | 167 (55.7) | 215 (70.7) | 1.00 | - | |
| C | 133 (44.3) | 89 (29.3) | 1.92 (1.37–2.69) | 0.001 |
| Networks | Label | Degree | Betweenness |
|---|---|---|---|
| Tissue specific PPIN | MEOX2 | 174 | 105,725.28 |
| SDCBP | 83 | 62,693.4 | |
| UBE2D3 | 71 | 58,615.64 | |
| MAPK8 | 71 | 52,068.96 | |
| NFKBIA | 47 | 29,486.37 | |
| Co-expression gene network | GPR65 | 37 | 666 |
| TAGAP | 1 | 0 | |
| MYO1F | 1 | 0 | |
| MAP4K1 | 1 | 0 | |
| CD5 | 1 | 0 | |
| miR-TF network | ZIC2 | 292 | 220,452.09 |
| NFKBIA | 102 | 71,452.21 | |
| RAP2C | 76 | 29,506.18 | |
| HOXA5 | 72 | 33,168.41 | |
| TCF7L2 | 58 | 33,137.3 |
| Pathway Enriched by Tissue-Specific Protein–Protein Interaction Network | |||||
|---|---|---|---|---|---|
| Pathway | Total | Expected | Hits | p-Value | FDR * |
| Antigen processing: ubiquitination and proteasome degradation | 224 | 10.4 | 50 | 9.93 × 10−22 | 1.39 × 10−18 |
| Immune system | 1140 | 52.7 | 120 | 6.66 × 10−21 | 4.67 × 10−18 |
| Class I MHC mediated antigen processing and presentation | 267 | 12.4 | 53 | 1.66 × 10−20 | 7.77 × 10−18 |
| Adaptive immune system | 654 | 30.3 | 83 | 9.72 × 10−19 | 3.41 × 10−16 |
| TRIF-mediated TLR3/TLR4 signaling | 87 | 4.03 | 23 | 3.91 × 10−12 | 1.02 × 10−09 |
| MyD88-independent cascade | 88 | 4.07 | 23 | 5.07 × 10−12 | 1.02 × 10−9 |
| TLR3 cascade | 88 | 4.07 | 23 | 5.07 × 10−12 | 1.02 × 10−9 |
| Activated TLR4 signalling | 100 | 4.63 | 24 | 1.26 × 10−11 | 2.21 × 10−9 |
| TLR4 cascade | 103 | 4.77 | 24 | 2.49 × 10−11 | 3.87 × 10−9 |
| RIG-I/MDA5 mediated induction of IFN-alpha/beta pathways | 67 | 3.1 | 19 | 8.12 × 10−11 | 1.14 × 10−8 |
| Pathway Enriched by Gene Co-Expression Network | |||||
| Rho GTPase cycle | 123 | 0.368 | 5 | 2.43 × 10−5 | 1.70 × 10−2 |
| Signaling by Rho GTPases | 123 | 0.368 | 5 | 2.43 × 10−5 | 1.70 × 10−2 |
| Immune system | 1140 | 3.41 | 11 | 1.26 × 10−4 | 0.0589 |
| Interleukin−3, 5 and GM-CSF signaling | 51 | 0.153 | 3 | 4.37 × 10−4 | 0.153 |
| Immunoregulatory interactions between a lymphoid and a non-lymphoid cell | 80 | 0.24 | 3 | 1.63 × 10−3 | 0.457 |
| Adaptive immune system | 654 | 1.96 | 7 | 2.08 × 10−3 | 0.479 |
| Hemostasis | 511 | 1.53 | 6 | 2.99 × 10−3 | 0.479 |
| Cell surface interactions at the vascular wall | 99 | 0.297 | 3 | 0.003 | 0.479 |
| Interleukin receptor SHC signaling | 28 | 0.0839 | 2 | 0.00308 | 0.479 |
| Antigen activates B-cell receptor leading to generation of second messengers | 32 | 0.0959 | 2 | 0.00401 | 0.506 |
| Pathway Enriched by miR-TF Network | |||||
| TRIF-mediated TLR3/TLR4 signaling | 87 | 3.15 | 25 | 1.65 × 10−16 | 1.04 × 10−13 |
| MyD88-independent cascade | 88 | 3.19 | 25 | 2.23 × 10−16 | 1.04 × 10−13 |
| TLR3 cascade | 88 | 3.19 | 25 | 2.23 × 10−16 | 1.04 × 10−13 |
| Activated TLR4 signalling | 100 | 3.62 | 26 | 5.98 × 10−16 | 2.09 × 10−13 |
| TLR4 cascade | 103 | 3.73 | 26 | 1.31 × 10−15 | 3.68 × 10−13 |
| TRAF6 mediated induction of proinflammatory cytokines | 62 | 2.25 | 20 | 1.66 × 10−14 | 3.89 × 10−12 |
| TLR10 cascade | 74 | 2.68 | 21 | 6.41 × 10−14 | 9.99 × 10−12 |
| TLR5 cascade | 74 | 2.68 | 21 | 6.41 × 10−14 | 9.99 × 10−12 |
| MyD88 cascade initiated on plasma membrane | 74 | 2.68 | 21 | 6.41 × 10−14 | 9.99 × 10−12 |
| TRAF6 mediated induction of NFkB and MAP kinases upon TLR7/8 or 9 activation | 76 | 2.75 | 21 | 1.15 × 10−13 | 1.62 × 10−11 |
| Description | Nodes | Edges | Expected Number of Edges | Avg. Node Degree | Avg. local Clustering Coefficient | Inflation Parameter (MCL) | Enrichment p-Value |
|---|---|---|---|---|---|---|---|
| Network 1 (based on initial PPIN) | 31 | 96 | 46 | 6.19 | 0.627 | 4 | 1.24 × 10−10 |
| Network 2 (based on initial miR-TF) | 20 | 45 | 23 | 4.5 | 0.612 | 4 | 3.16 × 10−5 |
| Network 3 (based on initial co-expression) | 19 | 68 | 8 | 7.16 | 0.718 | 4 | <1.0 × 10−16 |
| Polymorphisms | Primer Sequence (5′→3′) | Restriction Enzyme | Fragment (bp) |
|---|---|---|---|
| rs3746444 T > C (miR-499) | F: CAAAGTCTTCACTTCCCTGCCA R: GATGTTTAACTCCTCTCCACGTGATC | BclI | CC: 146 CT: 146 + 122 + 24 TT: 122 + 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabasi, F.; Hasanpour, V.; Sarhadi, S.; Kaykhaei, M.A.; Pourzand, P.; Heravi, M.; Langari, A.A.; Bahari, G.; Taheri, M.; Hashemi, M.; et al. Association of miR-499 Polymorphism and Its Regulatory Networks with Hashimoto Thyroiditis Susceptibility: A Population-Based Case-Control Study. Int. J. Mol. Sci. 2021, 22, 10094. https://doi.org/10.3390/ijms221810094
Tabasi F, Hasanpour V, Sarhadi S, Kaykhaei MA, Pourzand P, Heravi M, Langari AA, Bahari G, Taheri M, Hashemi M, et al. Association of miR-499 Polymorphism and Its Regulatory Networks with Hashimoto Thyroiditis Susceptibility: A Population-Based Case-Control Study. International Journal of Molecular Sciences. 2021; 22(18):10094. https://doi.org/10.3390/ijms221810094
Chicago/Turabian StyleTabasi, Farhad, Vahed Hasanpour, Shamim Sarhadi, Mahmoud Ali Kaykhaei, Pouria Pourzand, Mehrdad Heravi, Ahmad Alinaghi Langari, Gholamreza Bahari, Mohsen Taheri, Mohammad Hashemi, and et al. 2021. "Association of miR-499 Polymorphism and Its Regulatory Networks with Hashimoto Thyroiditis Susceptibility: A Population-Based Case-Control Study" International Journal of Molecular Sciences 22, no. 18: 10094. https://doi.org/10.3390/ijms221810094
APA StyleTabasi, F., Hasanpour, V., Sarhadi, S., Kaykhaei, M. A., Pourzand, P., Heravi, M., Langari, A. A., Bahari, G., Taheri, M., Hashemi, M., & Ghavami, S. (2021). Association of miR-499 Polymorphism and Its Regulatory Networks with Hashimoto Thyroiditis Susceptibility: A Population-Based Case-Control Study. International Journal of Molecular Sciences, 22(18), 10094. https://doi.org/10.3390/ijms221810094







