Vitreoretinal Surgery in the Prevention and Treatment of Toxic Tumour Syndrome in Uveal Melanoma: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Search Methods
2.2. Selection Process
3. Results and Discussion
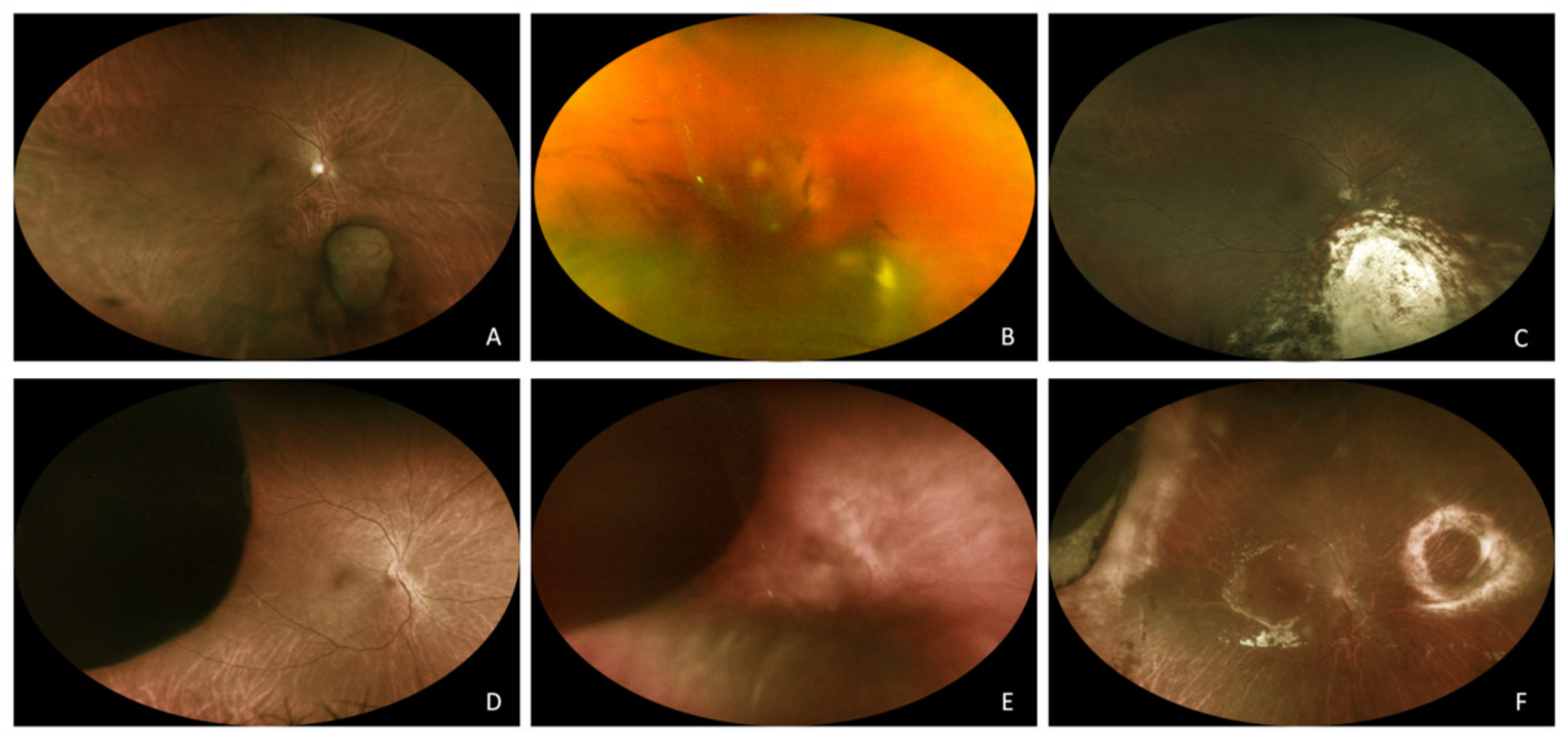
3.1. Endoresection
| Author(s), Reference | Number of Analysed Eyes | Inclusion Criteria | Type of RT | Time from RT to Surgery | LBD (mm) | Tumour Thickness (mm) | Follow-Up Duration (Months) | Globe Preservation Rate | NVG Rate | ERD Recurrence Rate | Preop BCVA | Final BCVA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bechrakis NE et al., Int Ophthalmol Clin. 2006 [14] | 58 | Postequatorial tumours ≥7 mm thickness | PBT | 9 days (4–21) | 15.6 (11.1–21.6) | 8.8 (7–14.5) | 18 (3–48) | 91.6% | 1.7% | 32.1% | 20/40 (20/20–20/400) | 20/200 (20/50-HM) |
| Kubicka-Trząska A, Arch Med Sci-Civiliz Dis. 2020 [12] | 13 | Postequatorial tumours LBD ≤ 15.0 mm, thickness ≥ 8.0 mm | PBT | 5.6 weeks (3–9) | 13.4 (10.6–15.0) | 7.6 (5.8–9.3) | 61.6 (34–84) | 100% | 0% | 23.1% | 6/50 (6/7.5–CF) | 30.1% ≥ 6/15 69.9% CF-NLP |
| Bechrakis NE et al., Klin Monbl Augenheilkd. 2009 [13] | 142 | Posterior tumours ≥ 7 mm thickness | PBT | 12 days (3–20) | N/A | N/A | 30 | 97% | 0% | N/A | N/A | N/A |
| Sinyavskiy OA, J Neurosurg. 2016 [17] | 21 | Equatorial or posterior LBD ≥ 10 mm | GKRS | 20 days (3–97) | 13.9 (10.0–18.2) | 8.9 (31.-15.5) | 23 (8–41) | 100% | 19% | 14.3% | ≥20/50: 33.3% 20/50–20/400: 28.6% ≤20/400: 38.1% | ≥20/50: 23.8% 20/50–20/400: 42.8% ≤20/400: 33.3% |
| Biewald E, Br J Ophthalmol. 2017 [15] | 200 | ≥ 8 mm thickness Absence of NVG | GKRS | 10 days | 12 (6.3–20) | 9.4 (6–14.8) | 32.3 | 89% | 0% | 30% | ≥20/50: 49.5% 20/50–20/400: 37.1% ≤20/400: 13.4% | ≥20/50: 13.4% 20/50–20/400: 33.6% ≤20/400: 53% |
| Schilling, Klin Monbl Augenheilkd. 2006 [16] | 43 | LBD < 15 mm >7 mm thickness >3 mm distance from foveola or optic disc | GRKS | 11.4 ± 2.1 | 9.5 ± 1.4 | 13.7 | 87% | 7% | 20/50 | 20/80 | ||
| Suesskind, JAMA Ophthalmol. 2013 [18] | 18 | >6 mm thickness, touching the optic disc | GKRS | 14.22 ± 3.28 | 8.31± 1.50 | 23.0 (0.1–81.1) | 87% | 0% | 20/50 (20/20–20/200) | median loss of Snellen lines–22 (−38 to 0) | ||
| McCannel TA, Eye (Lond). 2013 [19] | 5 | Mild signs of radiation retinopathy | I-125-brachytherapy | 26.8 months (13–62) | NA | 5.8 (2.03–8.9) | 62.4 (30–117) | 100% | 20% | 0% | 20/120 (20/400–20/25) | CF (NLP-20/70) |
3.2. Endodrainage and Other Non-Resecting Techniques
3.3. Endoresection Compared to Endodrainage and to No Adjuvant Surgical Treatment after PBT
3.4. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Damato, B. Progress in the management of patients with uveal melanoma. The 2012 Ashton Lecture. Eye 2012, 26, 1157–1172. [Google Scholar] [CrossRef] [Green Version]
- Groenewald, C.; Konstantinidis, L.; Damato, B. Effects of radiotherapy on uveal melanomas and adjacent tissues. Eye 2013, 27, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Damato, B. Vasculopathy after treatment of choroidal melanoma. In Retinal Vascular Disease; Joussen, A.M., Gardner, T.W., Kirchhof, B., Ryan, S.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 582–591. ISBN 9783540295419. [Google Scholar]
- Damato, B. Developments in the management of uveal melanoma. Clin. Experiment. Ophthalmol. 2004, 32, 639–647. [Google Scholar] [CrossRef]
- Conway, R.M.; Poothullil, A.M.; Daftari, I.K.; Weinberg, V.; Chung, J.E.; O’Brien, J.M. Estimates of ocular and visual retention following treatment of extra-large uveal melanomas by proton beam radiotherapy. Arch. Ophthalmol. 2006, 124, 838–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivak-Callcott, J.A.; O’Day, D.M.; Gass, J.D.; Tsai, J.C. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology 2001, 108, 1767–1776, quiz 1777, 1800. [Google Scholar] [CrossRef]
- Konstantinidis, L.; Groenewald, C.; Coupland, S.E.; Damato, B. Trans-scleral local resection of toxic choroidal melanoma after proton beam radiotherapy. Br. J. Ophthalmol. 2014, 98, 775–779. [Google Scholar] [CrossRef]
- Foss, A.J.; Whelehan, I.; Hungerford, J.L.; Anderson, D.F.; Errington, R.D.; Kacperek, A.; Restori, M.; Kongerud, J.; Sheen, M. Predictive factors for the development of rubeosis following proton beam radiotherapy for uveal melanoma. Br. J. Ophthalmol. 1997, 81, 748–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibran, S.K.; Kapoor, K.G. Management of exudative retinal detachment in choroidal melanoma. Clin. Experiment. Ophthalmol. 2009, 37, 654–659. [Google Scholar] [CrossRef]
- Char, D.H.; Bove, R.; Phillips, T.L. Laser and proton radiation to reduce uveal melanoma-associated exudative retinal detachments. Am. J. Ophthalmol. 2003, 136, 180–182. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubicka-Trząska, A.; Morawski, K.; Markiewicz, A.; Romanowska-Dixon, B. Prevention and treatment of the toxic tumour syndrome following primary proton beam therapy of choroidal melanomas. Arch. Med. Sci. Civiliz. Dis. 2020, 5, 22–28. [Google Scholar]
- Bechrakis, N.E.; Petousis, V.; Krause, L.; Wachtlin, J.; Willerding, G.; Foerster, M.H. Surgical treatment modalities in uveal melanomas. Klin. Mon. Augenheilkd. 2009, 226, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Bechrakis, N.E.; Foerster, M.H. Neoadjuvant proton beam radiotherapy combined with subsequent endoresection of choroidal melanomas. Int. Ophthalmol. Clin. 2006, 46, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Biewald, E.; Lautner, H.; Gök, M.; Horstmann, G.A.; Sauerwein, W.; Flühs, D.; Bornfeld, N. Endoresection of large uveal melanomas: Clinical results in a consecutive series of 200 cases. Br. J. Ophthalmol. 2017, 101, 204–208. [Google Scholar] [CrossRef]
- Schilling, H.; Bornfeld, N.; Talies, S.; Anastassiou, G.; Schüler, A.; Horstmann, G.A.; Jurklies, B. Endoresection of large uveal melanomas after pretreatment by single-dose stereotactic convergence irradiation with the leksell gamma knife—First experience on 46 cases. Klin. Mon. Augenheilkd. 2006, 223, 513–520. [Google Scholar] [CrossRef]
- Sinyavskiy, O.A.; Troyanovsky, R.L.; Ivanov, P.I.; Golovin, A.S.; Tibilov, A.V.; Solonina, S.N.; Astapenko, A.M.; Zubatkina, I.S. Microinvasive tumor endoresection in combination with ocular stereotactic radiosurgery. J. Neurosurg. 2016, 125, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Suesskind, D.; Scheiderbauer, J.; Buchgeister, M.; Partsch, M.; Budach, W.; Bartz-Schmidt, K.U.; Ritz, R.; Grisanti, S.; Paulsen, F. Retrospective evaluation of patients with uveal melanoma treated by stereotactic radiosurgery with and without tumor resection. JAMA Ophthalmol. 2013, 131, 630–637. [Google Scholar] [CrossRef] [Green Version]
- McCannel, T.A. Post-brachytherapy tumor endoresection for treatment of toxic maculopathy in choroidal melanoma. Eye 2013, 27, 984–988. [Google Scholar] [CrossRef] [Green Version]
- Oliver, S.C.N.; Leu, M.Y.; DeMarco, J.J.; Chow, P.E.; Lee, S.P.; McCannel, T.A. Attenuation of iodine 125 radiation with vitreous substitutes in the treatment of uveal melanoma. Arch. Ophthalmol. 2010, 128, 888–893. [Google Scholar] [CrossRef]
- Lyons, L.J.; Hinds, E.D.; Chexal, S.; Berger, B. Silicone Oil and Iodine-125 Brachytherapy for Uveal Melanoma in High-Risk Patients. Cureus 2019, 11, e5270. [Google Scholar] [CrossRef] [Green Version]
- McCannel, T.A.; McCannel, C.A. External Drainage for Primary Surgical Management of Uveal Melanoma Exudative Retinal Detachment. Retina 2017, 37, 1006–1007. [Google Scholar] [CrossRef] [PubMed]
- Seibel, I.; Cordini, D.; Willerding, G.; Riechardt, A.I.; Joussen, A.M. Endodrainage, Tumor Photocoagulation, and Silicone Oil Tamponade for Primary Exudative Retinal Detachment due to Choroidal Melanoma Persisting after Proton Beam Therapy. Ocul. Oncol. Pathol. 2014, 1, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Seibel, I.; Riechardt, A.I.; Heufelder, J.; Cordini, D.; Joussen, A.M. Adjuvant Ab Interno Tumor Treatment after Proton Beam Irradiation. Am. J. Ophthalmol. 2017, 178, 94–100. [Google Scholar] [CrossRef]
- Seibel, I.; Cordini, D.; Willerding-Beaucamp, G.; Heufelder, J.; Joussen, A. Minimally invasive therapy of exudative retinal detachment (ERD) due to choroidal or ciliary body melanoma after irradiation therapy compared to endoresection. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4243. [Google Scholar]
- Schönfeld, S.; Cordini, D.; Riechardt, A.I.; Seibel, I.; Willerding, G.; Bechrakis, N.E.; Moser, L.; Joussen, A.M. Proton beam therapy leads to excellent local control rates in choroidal melanoma in the intermediate fundus zone. Am. J. Ophthalmol. 2014, 158, 1184–1191. [Google Scholar] [CrossRef]
- Cassoux, N.; Cayette, S.; Plancher, C.; Lumbroso-Le Rouic, L.; Levy-Gabriel, C.; Asselain, B.; Sastre, X.; Couturier, J.; Arrufat, S.; Piperno-Neumann, S.; et al. Choroidal Melanoma: Does endoresection prevent neovascular glaucoma in patient treated with proton beam irradiation? Retina 2013, 33, 1441–1447. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Seibel, I.; Cordini, D.; Hager, A.; Tillner, J.; Riechardt, A.I.; Heufelder, J.; Davids, A.M.; Rehak, M.; Joussen, A.M. Predictive risk factors for radiation retinopathy and optic neuropathy after proton beam therapy for uveal melanoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 1787–1792. [Google Scholar] [CrossRef]
- Nagineni, C.N.; Kommineni, V.K.; William, A.; Detrick, B.; Hooks, J.J. Regulation of VEGF expression in human retinal cells by cytokines: Implications for the role of inflammation in age-related macular degeneration. J. Cell. Physiol. 2012, 227, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Gerhardinger, C.; Brown, L.F.; Roy, S.; Mizutani, M.; Zucker, C.L.; Lorenzi, M. Expression of vascular endothelial growth factor in the human retina and in nonproliferative diabetic retinopathy. Am. J. Pathol. 1998, 152, 1453–1462. [Google Scholar] [PubMed]
- Peddada, K.V.; Sangani, R.; Menon, H.; Verma, V. Complications and adverse events of plaque brachytherapy for ocular melanoma. J. Contemp. Brachytherapy 2019, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.-K.; Schalenbourg, A.; Bovey, E.; Zografos, L.; Wolfensberger, T.J. Role of vitreoretinal surgery in maximizing treatment outcome following complications after proton therapy for uveal melanoma. Retina 2013, 33, 1777–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, J.C.; Liebenberg, L.; Scholtz, R.P.; Torr, G. Fatal air embolism during endoresection of choroidal melanoma. Retin. Cases Br. Rep. 2014, 8, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Meraz Gutiérrez, M.P.; Camara Rodriguez, E.J.; Pando Cifuentes, A.; Ortiz-Ramirez, G.Y.; Soberón Ventura, V. Venous-air embolism during vitrectomy for endoresection of choroidal melanoma: Case report. Eur. J. Ophthalmol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
| Author(s), Reference | Number of Analysed Eyes | Type of Radiotherapy | Time from Radiotherapy to Surgery (Months) | LBD (mm) | Tumour Thickness (mm) | Follow-Up Duration (Months) | Globe Preservation Rate | NVG Rate | Preop BCVA (logMAR) | Final BCVA (logMAR) |
|---|---|---|---|---|---|---|---|---|---|---|
| Seibel I et al., Ocul Oncol Pathol. 2014 [23] | 20 | PBT | 4.5 (0.1–9.2) | 14.2 (7.7–18.5) | 6.2 (3.8–9.9) | 38.4 (12.0–122.0) | 95% | 5% | 1.1 (2.0–0.5) | 2 years post: 1.4 (2.0–0.4) 3 years post: 1.7 (2.2–0.7) 4 years post: 1.8 (2.2–1.0) |
| McCannel TA, Retina 2017 [22] | 20 PPV + SO + I-brachytherapy | I-125-brachytherapy | 16.4 (1.7) | 7.8 (2.7) | 19.4 (12.2–36.7) | 100% | 5% | 0.16 (0.21) | 0.83 (0.86) | |
| 20 I-brachytherapy | 17.5 (1.8) | 7.9 (2.1) | 22.5 (12.0–56.2) | 95% | 0% | 0.54 (0.59) | 2.06 (1.4) | |||
| Lyons LJ, Cureus. 2019 [21] | 5 | I-125-brachytherapy | 12.1 (9.7–14.5) | 6.2 (3.18–11.02) | 45 (12–56) | 80% | 20% | 0.32 | 0.28 |
| Seibel I et al., Am J Ophthalmol. 2017 [24] | Cassoux N, Retina 2013 [27] | Seibel I et al., Invest. Ophthalmol. Vis. Sci. 2013 [25] | Schönfeld S et al., Am J Ophthalmol. 2014 [26] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PBT +ER | PBT + EDR | PBT | PBT + ER | PBT | PBT + TTT | PBT + ER | PBT + EDR | PBT + ER | PBT | |
| Number of eyes | 445 | 242 | 216 | 63 | 57 | 51 | 183 | 28 | 44 | 18 |
| Inclusion criteria | ≥7 mm thickness ≥8 mm LBD Exudative RD | >6 mm thickness >10 mm LBD | ≥7 mm thickness ≥8 mm LBD Exudative RD | ≥6 mm Large size | ||||||
| Follow-up duration (months) | 53.9 (12–156.5) | 34.8 (12–102.7) | 60.6 (29–169.3) | 23 (20–26) | 112 (107–126) | 99 (89–124) | 45.4 (2.8–123.5) | 27.3 (4.2–95.1) | 64.4 | 77.2 |
| Tumour thickness (mm) | 10.0 (6.0–16.4) | 7.8 (5.9–11.2) | 8.1 (7–15.1) | 9 (6–12.2) | 8 (5.1–11.3) | 8 (5.3–11.8) | 6.49± 2.17 (1.91–10.2) | 9.1± 1.5 | 3.9 ± 2.0 | |
| LBD (mm) | 14.5 (8.0–21.5) | 14.7 (12.1–26.1) | 14.4 (8.0–21.9) | 14 (8–19) | 17.5 (10–23.3) | 17.4 (12.5–22.7) | 13.7 ± 3.2 | 10.6 ± 3.0 | ||
| Retinal detachment | 100% | 100% | 100% | 74.9% | 100% | 100% | ||||
| Distance to fovea | 2.7 (0–12.1) | 2.4 (0–19.0) | 2.1 (0–21.5) | 5.1 ± 2.2 | 3.7 ± 1.9 | |||||
| Distance to optic disc | 2.3 (0–13.2) | 2.4 (0–17.0) | 3.1 (0–18.7) | 5.3 ± 2.3 | 3.9 ± 1.9 | |||||
| T [28] | T1:1.6% T2: 19.7% T3: 56.6% T4: 22.0% | T1:3.3% T2: 16.5% T3: 42.6% T4: 37.6% | T1: 0 T2: 35.6% T3: 44.0% T4: 20.4% | T1 0% T2 19% T3 75% T4 6% | T1 0% T2 2% T3 51% T4 47% | T1 0% T2 0% T3 63% T4 37% | ||||
| AJCC prognostic staging [28] | I: 1.6% IIA:19.5% IIB: 51.5% IIIA: 20.7% IIIB: 6.3% IIIC: 0.4% | I: 2.1% IIA: 25.2% IIB: 33.5% IIIA: 26.0% IIIB: 11.1% IIIC: 2.1% | I: 0% IIA: 4.2% IIB: 28.7% IIIA:46.3% IIIB:18.0% IIIC: 2.8% | |||||||
| Enucleation rate | 19 (4.3%) | 10 (4.1%) | 21 (9.7%) | 3 (6%) | 14 (25%) | 5 (10%) | 1 (2.3%) | 1(5.5%) | ||
| NGV rate | 54 (12.1%) | 51 (21.1%) | 126 (58.3%) | 4 (7%) | 33 (58%) | 25 (49%) | 4 (18.6%) | 4 (23.5%) | ||
| Preoperatory BCVA | <1/100: 17% 1/100 to 20/50: 59% >20/50: 24% | <1/100: 18% 1/100 to 20/50: 55% >20/50: 27% | <1/100: 10% 1/100 to 20/50: 70% >20/50: 20% | 0.35 logMAR (20/45) | 0.5 logMAR (20/60) | 20/16–20/50 = 59.1% 20/63–20/160 = 31.8% <20/200 = 9.1% | 20/16–20/50 = 83.3% 20/63–20/160 = 11.1% <20/200 = 5.6% | |||
| Final BCVA | <1/100: 68% 1/100 to 20/50: 13% >20/50: 5% | <1/100: 67% 1/100 to 20/50: 28% >20/50: 5% | <1/100: 72% 1/100 to 20/50: 26% >20/50: 2% | 1.0 logMAR (20/200) | 0.8 logMAR (20/120) | 20/16–20/50 = 18.2% 20/63–20/160 = 20.4% <20/200 = 61.4% | 20/16–20/50 = 50.0% 20/63–20/160 = 16.7% <20/200 = 33.3% | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, M.R.; Catania, F.; Confalonieri, F.; Zollet, P.; Allegrini, D.; Sergenti, J.; Lanza, F.B.; Ferrara, M.; Angi, M. Vitreoretinal Surgery in the Prevention and Treatment of Toxic Tumour Syndrome in Uveal Melanoma: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 10066. https://doi.org/10.3390/ijms221810066
Romano MR, Catania F, Confalonieri F, Zollet P, Allegrini D, Sergenti J, Lanza FB, Ferrara M, Angi M. Vitreoretinal Surgery in the Prevention and Treatment of Toxic Tumour Syndrome in Uveal Melanoma: A Systematic Review. International Journal of Molecular Sciences. 2021; 22(18):10066. https://doi.org/10.3390/ijms221810066
Chicago/Turabian StyleRomano, Mario R., Fiammetta Catania, Filippo Confalonieri, Piero Zollet, Davide Allegrini, Jessica Sergenti, Francesco B. Lanza, Mariantonia Ferrara, and Martina Angi. 2021. "Vitreoretinal Surgery in the Prevention and Treatment of Toxic Tumour Syndrome in Uveal Melanoma: A Systematic Review" International Journal of Molecular Sciences 22, no. 18: 10066. https://doi.org/10.3390/ijms221810066
APA StyleRomano, M. R., Catania, F., Confalonieri, F., Zollet, P., Allegrini, D., Sergenti, J., Lanza, F. B., Ferrara, M., & Angi, M. (2021). Vitreoretinal Surgery in the Prevention and Treatment of Toxic Tumour Syndrome in Uveal Melanoma: A Systematic Review. International Journal of Molecular Sciences, 22(18), 10066. https://doi.org/10.3390/ijms221810066








