Mesenchymal Stem/Stromal Cell Therapy in Blood–Brain Barrier Preservation Following Ischemia: Molecular Mechanisms and Prospects
Abstract
1. Introduction
2. Structure of Blood–Brain Barrier
3. Blood–Brain Barrier Changes Following Ischemia-Induced Brain Injuries
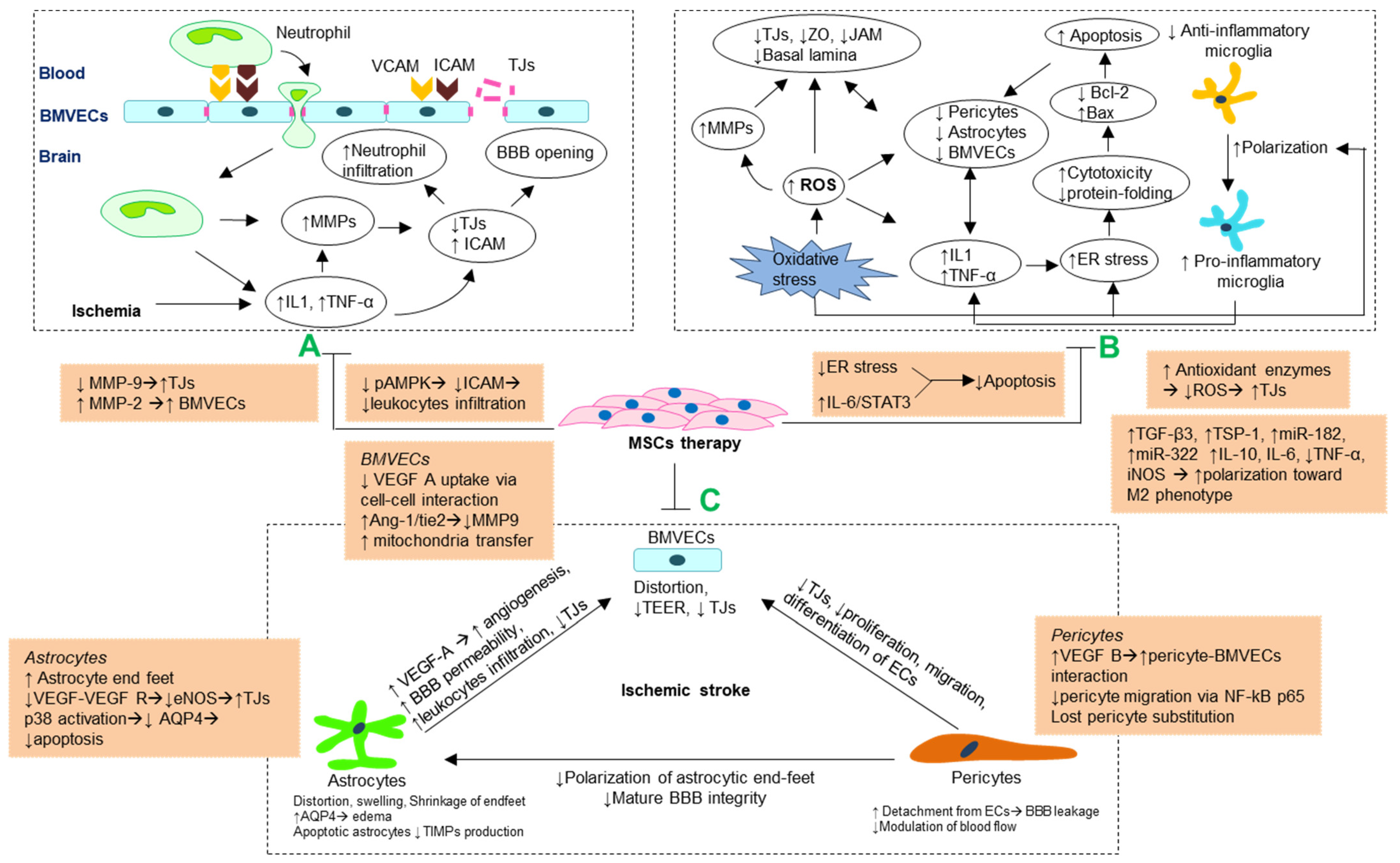
3.1. Tight Junction Disruption and Blood–Brain Barrier Opening
3.2. Morphological Changes and Impaired Interactions of Cellular Components of Blood–Brain Barrier
3.3. Increase of Blood–Brain Barrier Permeability Following Vascular Remodeling
4. Potential Mechanisms of Blood–Brain Barrier Preservation by MSCs Following Ischemia
4.1. MMP Regulation and Attenuating Leukocytes Infiltrations
4.2. Antioxidant and Anti-Inflammatory Mechanism
4.3. Stabilizing Morphology and Crosstalk of Cellular Components Blood–Brain Barrier
4.3.1. Brain Microvascular Endothelial Cells
4.3.2. Astrocytes
4.3.3. Pericytes
| Reference | Signaling Pathway | Component of BBB | Molecular Mechanism | Model | Number of Cells and Sources | Route | Time Treatment/Passage |
|---|---|---|---|---|---|---|---|
| [68] | ICAM/AMPK | MMPs, ICAM-1 | ↓ICAM-1 ↓neutrophil infiltration, ↓MMP-9 | tMCAO | 2 × 105 BMMSC | ICV | 15 min/3 |
| [146] | P38 | AQP-4 astrocytes | ↓AQP-4, ↓neuroinflammatory, ↓apoptotic astrocytes | tMCAO | 2 × 105 BMMSC | ICV | 20 min/3 |
| [27] | VEGF/eNOS | Astrocytes endfeet | ↑density of astrocytic endfeet, ↑VEGF/eNOS-dependent TJs | LPS | 1 × 106 BMMSC | IV | 4 h/6 |
| [115] | IL-6/STAT3 | Astrocytes | ↑IL-6 ↑anti-apoptosis of astrocytes | HIBD | 2 × 105 BMMSC | ICV | 5 days/3–5 |
| [35] | VEGF/Flk1 Ang1/Tie2 | Astrocytes BMVECs | ↑Ang1/Tie2 → ↑occludins and VEGF/Flk1 expression ↑vascular maturation | tMCAO | 3 × 106 BMMSC | IV | 24 h/- |
| [108] | PRDX4 | BMVECs | ↑PRDX4-mediated antioxidant ↓ ROS | tMCAO | 2 × 106 BMMSC | IV | 24 h/5–10 |
| [95] | - | BMVECs | ↓Neutrophil infiltration ↓Endothelial damage | GCI | 1 × 106 ADMSC | IV | Immediately/2 |
| [125] | ANXA1-FPR | BMVECs | ↓endothelial resistance | UCO OGD | BMMSC-EVs 2doses ~2 × 107 | IV | 1,4 days/- |
| [134] | Mitochondrial TNTs | BMVECs | Transfer mitochondrial to BMVECs via TNTs→↓oxidative stress | tMCAO | 5 × 105 BMMSC | IA | 24 h/3–5 |
| [133] | Mitochondrial TNTs | hUVECs | Transfer mitochondrial to hUVECs via TNTs→↓oxidative stress | OGD RO | - | - | 4 h/3–5 |
| [142] | TGF-β Smad2/3 | BMVECs | ↑VEGF, ↑Ang-1 | pMCAO | 2 × 106 BMMSC | IV | 3 h/3 |
| [36] | VEGF | Gap junctionBMVECs | ↑gap junction-mediated cell-cell interaction ↓glucose, ↓VEGF uptake in ECs | pMCAO | 5 × 105 BMMSC | IV | 24 h/9 |
| [37] | NF-kB p65 | Pericytes | ↓NF-kB p65→↓pericyte migration | SCI | BMMSC-EVs 1 dose ~2 × 106 | IV | 30 min/3–5 |
| [110] | ER stress | ER Astrocytes | ↓ER stress-induced apoptosis ↓inflammation | tMCAO | 2 × 106 3 doses ADMSC | IV | 0, 12, 24 h/2 |
| [94] | - | - | ↓pro-inflammatory, ↓polarize towards M1-phenotype | pMCAO | 4 × 106 cells/kg hAMSC | IV | 24 h/- |
5. Prospective of MSC-Based Strategies
5.1. Mesenchymal Stem Cell Therapy
5.2. MSC-Derived Extracellular Vesicle Therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Johnson, C.O.; Nguyen, M.; Roth, G.A.; Nichols, E.; Alam, T.; Abate, D.; Abd-Allah, F.; Abdelalim, A.; Abraha, H.N.; Abu-Rmeileh, N.M.; et al. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef]
- Candelario-Jalil, E. Injury and repair mechanisms in ischemic stroke: Considerations for the development of novel neurotherapeutics. Curr. Opin. Investig. Drugs (Lond. Engl. 2000) 2009, 10, 644–654. [Google Scholar]
- Neuwelt, E.A. Mechanisms of disease: The blood-brain barrier. Neurosurgery 2004, 54, 131–142. [Google Scholar] [CrossRef]
- Persidsky, Y.; Ramirez, S.; Haorah, J.; Kanmogne, G.D. Blood–brain Barrier: Structural Components and Function Under Physiologic and Pathologic Conditions. J. Neuroimmune Pharmacol. 2006, 1, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Saunders, N.R.; Liddelow, S.; Dziegielewska, K.M. Barrier Mechanisms in the Developing Brain. Front. Pharmacol. 2012, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, G.W.; Betz, A.L. The blood-brain barrier. Sci. Am. 1986, 255, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Schoknecht, K.; Shalev, H. Blood-brain barrier dysfunction in brain diseases: Clinical experience. Epilepsia 2012, 53, 7–13. [Google Scholar] [CrossRef]
- Yang, Y.; Rosenberg, G.A. Blood-Brain Barrier Breakdown in Acute and Chronic Cerebrovascular Disease. Stroke 2011, 42, 3323–3328. [Google Scholar] [CrossRef]
- Qin, W.; Li, J.; Zhu, R.; Gao, S.; Fan, J.; Xia, M.; Zhao, R.C.; Zhang, J. Melatonin protects blood-brain barrier integrity and permeability by inhibiting matrix metalloproteinase-9 via the NOTCH3/NF-κB pathway. Aging 2019, 11, 11391–11415. [Google Scholar] [CrossRef]
- Malemud, C.J. Matrix metalloproteinases (MMPs) in health and disease: An overview. Front. Biosci. 2006, 11, 1696–1701. [Google Scholar] [CrossRef]
- Yang, Y.; Estrada, E.Y.; Thompson, J.F.; Liu, W.; Rosenberg, G.A. Matrix Metalloproteinase-Mediated Disruption of Tight Junction Proteins in Cerebral Vessels is Reversed by Synthetic Matrix Metalloproteinase Inhibitor in Focal Ischemia in Rat. Br. J. Pharmacol. 2006, 27, 697–709. [Google Scholar] [CrossRef]
- Rosell, A.; Lo, E.H. Multiphasic roles for matrix metalloproteinases after stroke. Curr. Opin. Pharmacol. 2008, 8, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Chelluboina, B.; Nalamolu, K.R.; Mendez, G.G.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Veeravalli, K.K. Mesenchymal Stem Cell Treatment Prevents Post-Stroke Dysregulation of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases. Cell. Physiol. Biochem. 2017, 44, 1360–1369. [Google Scholar] [CrossRef]
- Paolinelli, R.; Corada, M.; Orsenigo, F.; Dejana, E. The molecular basis of the blood brain barrier differentiation and maintenance. Is it still a mystery? Pharmacol. Res. 2011, 63, 165–171. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Larrue, V.; von Kummer, R.; Müller, A.; Bluhmki, E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: A secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke 2001, 32, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Warach, S.; Latour, L.L. Evidence of Reperfusion Injury, Exacerbated by Thrombolytic Therapy, in Human Focal Brain Ischemia Using a Novel Imaging Marker of Early Blood–Brain Barrier Disruption. Stroke 2004, 35, 2659–2661. [Google Scholar] [CrossRef]
- Nagpal, A.; Choy, F.C.; Howell, S.; Hillier, S.; Chan, F.; Hamilton-Bruce, M.A.; Koblar, S.A. Safety and effectiveness of stem cell therapies in early-phase clinical trials in stroke: A systematic review and meta-analysis. Stem Cell Res. Ther. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Lees, J.S.; Sena, E.S.; Egan, K.; Antonic-Baker, A.; Koblar, S.; Howells, D.; MacLeod, M.R. Stem Cell-Based Therapy for Experimental Stroke: A Systematic Review and Meta-Analysis. Int. J. Stroke 2012, 7, 582–588. [Google Scholar] [CrossRef]
- Wagenaar, N.; Nijboer, C.H.; Van Bel, F. Repair of neonatal brain injury: Bringing stem cell-based therapy into clinical practice. Dev. Med. Child Neurol. 2017, 59, 997–1003. [Google Scholar] [CrossRef]
- Yong, K.W.; Choi, J.R.; Mohammadi, M.; Mitha, A.P.; Sanati-Nezhad, A.; Sen, A. Mesenchymal Stem Cell Therapy for Ischemic Tissues. Stem Cells Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Zhang, P.; Li, J.; Liu, Y.; Chen, X.; Kang, Q. Transplanted human embryonic neural stem cells survive, migrate, differentiate and increase endogenous nestin expression in adult rat cortical peri-infarction zone. Neuropathology 2009, 29, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Tae-Hoon, L.; Yoon-Seok, L. Transplantation of mouse embryonic stem cell after middle cerebral artery occlusion. Acta Cir. Bras. 2012, 27, 333–339. [Google Scholar] [CrossRef]
- Huang, L.; Wong, S.; Snyder, E.Y.; Hamblin, M.H.; Lee, J.-P. Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage ischemic-reperfusion cerebral injury. Stem Cell Res. Ther. 2014, 5, 1–16. [Google Scholar] [CrossRef]
- Leu, S.; Lin, Y.-C.; Yuen, C.-M.; Yen, C.-H.; Kao, Y.-H.; Sun, C.-K.; Yip, H.-K. Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J. Transl. Med. 2010, 8, 63. [Google Scholar] [CrossRef]
- Park, H.J.; Shin, J.Y.; Na Kim, H.; Oh, S.H.; Song, S.K.; Lee, P.H. Mesenchymal stem cells stabilize the blood–brain barrier through regulation of astrocytes. Stem Cell Res. Ther. 2015, 6, 187. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Shen, F.; Frenzel, T.; Zhu, W.; Ye, J.; Liu, J.; Chen, Y.; Su, H.; Young, W.L.; Yang, G.-Y. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann. Neurol. 2009, 67, 488–497. [Google Scholar] [CrossRef]
- Chau, M.J.; Deveau, T.C.; Song, M.; Gu, X.; Chen, N.; Wei, L. iPSC Transplantation Increases Regeneration and Functional Recovery After Ischemic Stroke in Neonatal Rats. Stem Cells 2014, 32, 3075–3087. [Google Scholar] [CrossRef] [PubMed]
- Honmou, O.; Onodera, R.; Sasaki, M.; Waxman, S.G.; Kocsis, J.D. Mesenchymal stem cells: Therapeutic outlook for stroke. Trends Mol. Med. 2012, 18, 292–297. [Google Scholar] [CrossRef]
- Klingemann, H.; Matzilevich, D.; Marchand, J. Mesenchymal Stem Cells—Sources and Clinical Applications. Transfus. Med. Hemotherapy 2008, 35, 2. [Google Scholar] [CrossRef]
- Lalu, M.M.; Montroy, J.; Dowlatshahi, D.; Hutton, B.; Juneau, P.; Wesch, N.; Zhang, S.Y.; McGinn, R.; Corbett, D.; Stewart, D.J.; et al. From the Lab to Patients: A Systematic Review and Meta-Analysis of Mesenchymal Stem Cell Therapy for Stroke. Transl. Stroke Res. 2019, 11, 345–364. [Google Scholar] [CrossRef]
- Satani, N.; Cai, C.; Giridhar, K.; McGhiey, D.; George, S.; Parsha, K.; Nghiem, D.M.; Valenzuela, K.S.; Riecke, J.; Vahidy, F.; et al. World-Wide Efficacy of Bone Marrow Derived Mesenchymal Stromal Cells in Preclinical Ischemic Stroke Models: Systematic Review and Meta-Analysis. Front. Neurol. 2019, 10, 405. [Google Scholar] [CrossRef]
- Li, Z.; Dong, X.; Tian, M.; Liu, C.; Wang, K.; Li, L.; Liu, Z.; Liu, J. Stem cell-based therapies for ischemic stroke: A systematic review and meta-analysis of clinical trials. Stem Cell Res. Ther. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Zacharek, A.; Chen, J.; Cui, X.; Li, A.; Li, Y.; Roberts, C.; Feng, Y.; Gao, Q.; Chopp, M. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J. Cereb. Blood Flow Metab. 2007, 27, 1684–1691. [Google Scholar] [CrossRef]
- Kikuchi-Taura, A.; Okinaka, Y.; Saino, O.; Takeuchi, Y.; Ogawa, Y.; Kimura, T.; Gul, S.; Claussen, C.; Boltze, J.; Taguchi, A. Gap junction-mediated cell-cell interaction between transplanted mesenchymal stem cells and vascular endothelium in stroke. Stem Cells 2021, 39, 904–912. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, Y.; Zhang, R.; Wen, L.; Wu, K.; Li, Y.; Yao, Y.; Duan, R.; Jia, Y. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Recovery Following Spinal Cord Injury via Improvement of the Integrity of the Blood-Spinal Cord Barrier. Front. Neurosci. 2019, 13, 209. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Andreone, B.J.; Chow, B.W.; Tata, A.; Lacoste, B.; Ben-Zvi, A.; Bullock, K.; Deik, A.A.; Ginty, D.D.; Clish, C.; Gu, C. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron 2017, 94, 581–594. [Google Scholar] [CrossRef]
- Engelhardt, B.; Sorokin, L. The blood–brain and the blood–cerebrospinal fluid barriers: Function and dysfunction. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Bauer, H.; Traweger, A. Tight Junctions of the Blood-Brain Barrier—A Molecular Gatekeeper. CNS Neurol. Disord. Drug Targets 2016, 15, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Haseloff, R.F.; Dithmer, S.; Winkler, L.; Wolburg, H.; Blasig, I.E. Transmembrane proteins of the tight junctions at the blood–brain barrier: Structural and functional aspects. Semin. Cell Dev. Biol. 2015, 38, 16–25. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Y.; Wang, D.; Zhang, Z. Shenmai injection maintains blood-brain barrier integrity following focal cerebral ischemia via modulating the expression and trafficking of occludin in lipid rafts. J. Ethnopharmacol. 2019, 237, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, C.; Wu, Y.; Chen, B.; Zhang, W.; Zhang, J. Autophagy Protects the Blood-Brain Barrier Through Regulating the Dynamic of Claudin-5 in Short-Term Starvation. Front. Physiol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Lampugnani, M.-G.; Moons, L.; Breviario, F.; Compernolle, V.; Bono, F.; Balconi, G.; Spagnuolo, R.; Oosthuyse, B.; Dewerchin, M.; et al. Targeted Deficiency or Cytosolic Truncation of the VE-cadherin Gene in Mice Impairs VEGF-Mediated Endothelial Survival and Angiogenesis. Cell 1999, 98, 147–157. [Google Scholar] [CrossRef]
- Kumar, N.M.; Gilula, N.B. The Gap Junction Communication Channel. Cell 1996, 84, 381–388. [Google Scholar] [CrossRef]
- Schulz, R.; Görge, P.M.; Görbe, A.; Ferdinandy, P.; Lampe, P.D.; Leybaert, L. Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection. Pharmacol. Ther. 2015, 153, 90–106. [Google Scholar] [CrossRef]
- Siracusa, R.; Fusco, R.; Cuzzocrea, S. Astrocytes: Role and Functions in Brain Pathologies. Front. Pharmacol. 2019, 10, 1114. [Google Scholar] [CrossRef]
- Argaw, A.T.; Asp, L.; Zhang, J.; Navrazhina, K.; Pham, T.; Mariani, J.N.; Mahase, S.; Dutta, D.; Seto, J.; Kramer, E.G.; et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Investig. 2012, 122, 2454–2468. [Google Scholar] [CrossRef]
- Abbott, N.J. Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 2002, 200, 523–534. [Google Scholar] [CrossRef]
- Gomez, D.E.; Alson, D.F.; Yoshiji, H.; Torgeirsson, U.P. Tissue inhibitors of metalloproteinases: Structure, regulation and biological functions. Eur. J. Cell Biol. 1997, 74, 111–122. [Google Scholar]
- Dore-Duffy, P. Pericytes: Pluripotent Cells of the Blood Brain Barrier. Curr. Pharm. Des. 2008, 14, 1581–1593. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems, and Promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef]
- Liu, S.; Agalliu, D.; Yu, C.; Fisher, M. The Role of Pericytes in Blood-Brain Barrier Function and Stroke. Curr. Pharm. Des. 2012, 18, 3653–3662. [Google Scholar] [CrossRef]
- Winkler, E.A.; Bell, R.D.; Zlokovic, B.V. Central nervous system pericytes in health and disease. Nat. Neurosci. 2011, 14, 1398–1405. [Google Scholar] [CrossRef]
- Allt, G.; Lawrenson, J. Pericytes: Cell biology and pathology. Cells Tissues Organs 2001, 169, 1–11. [Google Scholar] [CrossRef]
- Daneman, R.; Zhou, L.; Kebede, A.A.; Barres, B.A. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nat. Cell Biol. 2010, 468, 562–566. [Google Scholar] [CrossRef]
- Fujimoto, K. Pericyte-endothelial gap junctions in developing rat cerebral capillaries: A fine structural study. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1995, 242, 562–565. [Google Scholar] [CrossRef]
- Keaney, J.; Campbell, M. The dynamic blood-brain barrier. FEBS J. 2015, 282, 4067–4079. [Google Scholar] [CrossRef]
- Lehner, C.; Gehwolf, R.; Tempfer, H.; Krizbai, I.; Hennig, B.; Bauer, H.-C.; Bauer, H. Oxidative Stress and Blood–Brain Barrier Dysfunction Under Particular Consideration of Matrix Metalloproteinases. Antioxid. Redox Signal. 2011, 15, 1305–1323. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2010, 41, 271–290. [Google Scholar] [CrossRef]
- Liu, L.; Eckert, M.A.; Riazifar, H.; Kang, D.-K.; Agalliu, D.; Zhao, W. From Blood to the Brain: Can Systemically Transplanted Mesenchymal Stem Cells Cross the Blood-Brain Barrier? Stem Cells Int. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Segel, G.B.; Halterman, M.W.; Lichtman, M.A. The paradox of the neutrophil’s role in tissue injury. J. Leukoc. Biol. 2010, 89, 359–372. [Google Scholar] [CrossRef]
- Wang, C.; Börger, V.; Sardari, M.; Murke, F.; Skuljec, J.; Pul, R.; Hagemann, N.; Dzyubenko, E.; Dittrich, R.; Gregorius, J.; et al. Mesenchymal Stromal Cell–Derived Small Extracellular Vesicles Induce Ischemic Neuroprotection by Modulating Leukocytes and Specifically Neutrophils. Stroke 2020, 51, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wang, L.; Qu, M.; Liang, H.; Li, W.; Li, Y.; Deng, L.; Zhang, Z.; Yang, G.-Y. Mesenchymal stem cells attenuate blood-brain barrier leakage after cerebral ischemia in mice. J. Neuroinflamm. 2018, 15, 1–11. [Google Scholar] [CrossRef]
- Yang, Y.; Rosenberg, G.A. Matrix metalloproteinases as therapeutic targets for stroke. Brain Res. 2015, 1623, 30–38. [Google Scholar] [CrossRef] [PubMed]
- van den Steen, P.; Dubois, B.; Nelissen, I.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Biochemistry and Molecular Biology of Gelatinase B or Matrix Metalloproteinase-9 (MMP-9). Crit. Rev. Biochem. Mol. Biol. 2002, 37, 375–536. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, S.M.; Razak, K.A.; Ethell, I.M. A delicate balance: Role of MMP-9 in brain development and pathophysiology of neurodevelopmental disorders. Front. Cell. Neurosci. 2015, 9, 280. [Google Scholar] [CrossRef]
- Ahn, J.H.; Chen, B.H.; Park, J.H.; Na Shin, B.; Lee, T.-K.; Cho, J.H.; Lee, J.C.; Park, J.-R.; Yang, S.-R.; Ryoo, S.; et al. Early IV-injected human dermis-derived mesenchymal stem cells after transient global cerebral ischemia do not pass through damaged blood-brain barrier. J. Tissue Eng. Regen. Med. 2018, 12, 1646–1657. [Google Scholar] [CrossRef]
- Ridet, J.; Privat, A.; Malhotra, S.; Gage, F. Reactive astrocytes: Cellular and molecular cues to biological function. Trends Neurosci. 1997, 20, 570–577. [Google Scholar] [CrossRef]
- Higashida, T.; Kreipke, C.W.; Rafols, J.A.; Peng, C.; Schafer, S.; Schafer, P.; Ding, J.Y.; Dornbos, D.; Li, X.; Guthikonda, M.; et al. The role of hypoxia-inducible factor-1α, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury. J. Neurosurg. 2011, 114, 92–101. [Google Scholar] [CrossRef]
- da Fonseca, A.C.C.; Matias, D.; Garcia, C.; Amaral, R.; Henrique Geraldo, L.; Freitas, C.; Lima, F.R.S. The impact of microglial activation on blood-brain barrier in brain diseases. Front. Cell. Neurosci. 2014, 8, 362. [Google Scholar] [CrossRef]
- Vaccari, J.P.D.R.; Dietrich, W.D.; Keane, R.W. Activation and Regulation of Cellular Inflammasomes: Gaps in Our Knowledge for Central Nervous System Injury. Br. J. Pharmacol. 2014, 34, 369–375. [Google Scholar] [CrossRef]
- Fu, X.; Li, Q.; Feng, Z.; Mu, D. The roles of aquaporin-4 in brain edema following neonatal hypoxia ischemia and reoxygenation in a cultured rat astrocyte model. Glia 2007, 55, 935–941. [Google Scholar] [CrossRef]
- Argaw, A.T.; Gurfein, B.T.; Zhang, Y.; Zameer, A.; John, G.R. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. USA 2009, 106, 1977–1982. [Google Scholar] [CrossRef]
- Farina, C.; Aloisi, F.; Meinl, E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007, 28, 138–145. [Google Scholar] [CrossRef]
- Muir, E.; Adcock, K.; Morgenstern, D.; Clayton, R.; von Stillfried, N.; Rhodes, K.; Ellis, C.; Fawcett, J.; Rogers, J. Matrix metalloproteases and their inhibitors are produced by overlapping populations of activated astrocytes. Mol. Brain Res. 2002, 100, 103–117. [Google Scholar] [CrossRef]
- Duz, B.; Oztas, E.; Erginay, T.; Erdogan, E.; Gonul, E. The effect of moderate hypothermia in acute ischemic stroke on pericyte migration: An ultrastructural study. Cryobiology 2007, 55, 279–284. [Google Scholar] [CrossRef]
- Darland, D.; Massingham, L.; Smith, S.; Piek, E.; Saint-Geniez, M.; D’Amore, P. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev. Biol. 2003, 264, 275–288. [Google Scholar] [CrossRef]
- Winkler, E.A.; Bell, R.D.; Zlokovic, B.V. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol. Neurodegener. 2010, 5, 32. [Google Scholar] [CrossRef]
- Ramsauer, M.; Krause, D.; Dermietzel, R. Angiogenesis of the blood-brain barrier in vitro and the function of cerebral pericytes. FASEB J. 2002, 16, 1274–1276. [Google Scholar] [CrossRef]
- Al Ahmad, A.; Taboada, C.B.; Gassmann, M.; Ogunshola, O.O. Astrocytes and pericytes differentially modulate blood-brain barrier characteristics during development and hypoxic insult. J. Cereb. Blood Flow Metab. 2011, 31, 693–705. [Google Scholar] [CrossRef]
- Beck, H.; Plate, K.H. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009, 117, 481–496. [Google Scholar] [CrossRef]
- Marti, H.J.; Bernaudin, M.; Bellail, A.; Schoch, H.; Euler, M.; Petit, E.; Risau, W. Hypoxia-Induced Vascular Endothelial Growth Factor Expression Precedes Neovascularization after Cerebral Ischemia. Am. J. Pathol. 2000, 156, 965–976. [Google Scholar] [CrossRef]
- Lee, H.S.; Han, J.; Bai, H.-J.; Kim, K.-W. Brain angiogenesis in developmental and pathological processes: Regulation, molecular and cellular communication at the neurovascular interface. FEBS J. 2009, 276, 4622–4635. [Google Scholar] [CrossRef]
- Nam, H.S.; Kwon, I.; Lee, B.H.; Kim, H.; Kim, J.; An, S.; Lee, O.-H.; Lee, P.H.; Kim, H.O.; Namgoong, H.; et al. Effects of Mesenchymal Stem Cell Treatment on the Expression of Matrix Metalloproteinases and Angiogenesis during Ischemic Stroke Recovery. PLoS ONE 2015, 10, e0144218. [Google Scholar] [CrossRef]
- Valable, S.; Montaner, J.; Bellail, A.; Berezowski, V.; Brillault, J.; Cecchelli, R.; Divoux, D.; MacKenzie, E.T.; Bernaudin, M.; Roussel, S.; et al. VEGF-Induced BBB Permeability is Associated with an MMP-9 Activity Increase in Cerebral ischemia: Both Effects Decreased by ANG-1. Br. J. Pharmacol. 2005, 25, 1491–1504. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nagaoka, T.; Nair, G.; Ohno, K.; Duong, T.Q. Arterial spin labeling and dynamic susceptibility contrast CBF MRI in postischemic hyperperfusion, hypercapnia, and after mannitol injection. Br. J. Pharmacol. 2010, 31, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Candelario-Jalil, E.; Estrada, E.Y.; Rosenberg, G.A. Spatiotemporal Correlations between Blood-Brain Barrier Permeability and Apparent Diffusion Coefficient in a Rat Model of Ischemic Stroke. PLoS ONE 2009, 4, e6597. [Google Scholar] [CrossRef]
- Yang, Y.; Torbey, M.T. Angiogenesis and blood-brain barrier permeability in vascular remodeling after stroke. Curr. Neuropharmacol. 2020, 18, 1250–1265. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Takagi, T.; Kuramoto, Y.; Tatebayashi, K.; Shirakawa, M.; Yamahara, K.; Doe, N.; Yoshimura, S. Intravenous Administration of Human Amniotic Mesenchymal Stem Cells in the Subacute Phase of Cerebral Infarction in a Mouse Model Ameliorates Neurological Disturbance by Suppressing Blood Brain Barrier Disruption and Apoptosis via Immunomodulation. Cell Transplant. 2021, 30, 09636897211024183. [Google Scholar] [CrossRef]
- Chung, T.N.; Kim, J.H.; Choi, B.Y.; Chung, S.P.; Kwon, S.W.; Suh, S.W. Adipose-derived mesenchymal stem cells reduce neuronal death after transient global cerebral ischemia through prevention of blood-brain barrier disruption and endothelial damage. Stem Cells Transl. Med. 2014, 4, 178–185. [Google Scholar] [CrossRef]
- Yang, L.; Froio, R.M.; Sciuto, T.E.; Dvorak, A.M.; Alon, R.; Luscinskas, F.W. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-α-activated vascular endothelium under flow. Blood 2005, 106, 584–592. [Google Scholar] [CrossRef]
- Hardie, D.G.; Carling, D.; Carlson, M. The amp-activated/snf1 protein kinase subfamily: Metabolic Sensors of the Eukaryotic Cell? Annu. Rev. Biochem. 1998, 67, 821–855. [Google Scholar] [CrossRef]
- Ren, G.; Zhao, X.; Zhang, L.; Zhang, J.; L’Huillier, A.; Ling, W.; Roberts, A.I.; Le, A.D.; Shi, S.; Shao, C.; et al. Inflammatory Cytokine-Induced Intercellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule-1 in Mesenchymal Stem Cells Are Critical for Immunosuppression. J. Immunol. 2010, 184, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-Y.; Liang, Y.-H.; Wu, J.-C.; Wang, H.-S. Interleukin-1β Enhances Umbilical Cord Mesenchymal Stem Cell Adhesion Ability on Human Umbilical Vein Endothelial Cells via LFA-1/ICAM-1 Interaction. Stem Cells Int. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Rosenberg, G.A.; Estrada, E.Y.; Dencoff, J.E. Matrix Metalloproteinases and TIMPs Are Associated With Blood-Brain Barrier Opening After Reperfusion in Rat Brain. Stroke 1998, 29, 2189–2195. [Google Scholar] [CrossRef]
- Fujimoto, M.; Takagi, Y.; Aoki, T.; Hayase, M.; Marumo, T.; Gomi, M.; Nishimura, M.; Kataoka, H.; Hashimoto, N.; Nozaki, K. Tissue Inhibitor of Metalloproteinases Protect Blood—Brain Barrier Disruption in Focal Cerebral Ischemia. Br. J. Pharmacol. 2008, 28, 1674–1685. [Google Scholar] [CrossRef]
- Tejima, E.; Guo, S.; Murata, Y.; Arai, K.; Lok, J.; van Leyen, K.; Rosell, A.; Wang, X.; Lo, E.H. Neuroprotective effects of overexpressing tissue inhibitor of metalloproteinase TIMP-1. J. Neurotrauma 2009, 26, 1935–1941. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.; Ghorpade, A. Tissue inhibitor of metalloproteinase (TIMP)-1: The TIMPed balance of matrix metalloproteinases in the central nervous system. J. Neurosci. Res. 2003, 74, 801–806. [Google Scholar] [CrossRef]
- Menge, T.; Zhao, Y.; Zhao, J.; Wataha, K.; Gerber, M.; Zhang, J.; Letourneau, P.; Redell, J.; Shen, L.; Wang, J.; et al. Mesenchymal Stem Cells Regulate Blood-Brain Barrier Integrity Through TIMP3 Release After Traumatic Brain Injury. Sci. Transl. Med. 2012, 4, 161ra150. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Kirchgessner, A.; Tepper, D.; Leonard, A. Matrix Metalloproteinases and Blood-Brain Barrier Disruption in Acute Ischemic Stroke. Front. Neurol. 2013, 4, 32. [Google Scholar] [CrossRef]
- Huang, W.; Wang, T.; Zhang, D.; Zhao, T.; Dai, B.; Ashraf, A.; Wang, X.; Xu, M.; Millard, R.W.; Fan, G.-C.; et al. Mesenchymal Stem Cells Overexpressing CXCR4 Attenuate Remodeling of Postmyocardial Infarction by Releasing Matrix Metalloproteinase-9. Stem Cells Dev. 2012, 21, 778–789. [Google Scholar] [CrossRef]
- Song, K.; Li, Y.; Zhang, H.; An, N.; Wei, Y.; Wang, L.; Tian, C.; Yuan, M.; Sun, Y.; Xing, Y.; et al. Oxidative Stress-Mediated Blood-Brain Barrier (BBB) Disruption in Neurological Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 1–27. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Cai, J.; Qiu, Y.; Zheng, H.; Lai, X.; Sui, X.; Wang, Y.; Lu, Q.; Zhang, Y.; et al. Targeted homing of CCR2-overexpressing mesenchymal stromal cells to ischemic brain enhances post-stroke recovery partially through PRDX4-mediated blood-brain barrier preservation. Theranostics 2018, 8, 5929–5944. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liang, H.; Xi, Z.; Yang, Y.; Shan, H.; Wang, B.; Zhong, Z.; Xu, C.; Yang, G.-Y.; Sun, Q.; et al. BM-MSC Transplantation Alleviates Intracerebral Hemorrhage-Induced Brain Injury, Promotes Astrocytes Vimentin Expression, and Enhances Astrocytes Antioxidation via the Cx43/Nrf2/HO-1 Axis. Front. Cell Dev. Biol. 2020, 8, 302. [Google Scholar] [CrossRef]
- Chi, L.; Huang, Y.; Mao, Y.; Wu, K.; Zhang, L.; Nan, G. Tail Vein Infusion of Adipose-Derived Mesenchymal Stem Cell Alleviated Inflammatory Response and Improved Blood Brain Barrier Condition by Suppressing Endoplasmic Reticulum Stress in a Middle Cerebral Artery Occlusion Rat Model. Med. Sci. Monit. 2018, 24, 3946–3957. [Google Scholar] [CrossRef]
- Evans, M.A.; Broughton, B.R.S.; Drummond, G.R.; Ma, H.; Phan, T.G.; Wallace, E.M.; Lim, R.; Sobey, C.G. Amnion epithelial cells–a novel therapy for ischemic stroke? Neural Regen. Res. 2018, 13, 1346. [Google Scholar]
- Chen, X.; Yang, B.; Tian, J.; Hong, H.; Du, Y.; Li, K.; Li, X.; Wang, N.; Yu, X.; Wei, X. Dental Follicle Stem Cells Ameliorate Lipopolysaccharide-Induced Inflammation by Secreting TGF-β3 and TSP-1 to Elicit Macrophage M2 Polarization. Cell. Physiol. Biochem. 2018, 51, 2290–2308. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Hu, J.; Chen, F.; Qiao, S.; Sun, X.; Gao, L.; Xie, J.; Xu, B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc. Res. 2019, 115, 1205–1216. [Google Scholar] [CrossRef]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef]
- Gu, Y.; He, M.; Zhou, X.; Liu, J.; Hou, N.; Bin, T.; Zhang, Y.; Li, T.; Chen, J. Endogenous IL-6 of mesenchymal stem cell improves behavioral outcome of hypoxic-ischemic brain damage neonatal rats by supressing apoptosis in astrocyte. Sci. Rep. 2016, 6, srep18587. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Zhang, Y.; Yu, S.; Tuazon, J.P.; Lee, J.-Y.; Corey, S.; Kvederis, L.; Kingsbury, C.; Kaneko, Y. Neuroprotective effects of human bone marrow mesenchymal stem cells against cerebral ischemia are mediated in part by an anti-apoptotic mechanism. Neural Regen. Res. 2019, 14, 597–604. [Google Scholar] [CrossRef]
- Kaufman, R.J. Orchestrating the unfolded protein response in health and disease. J. Clin. Investig. 2002, 110, 1389–1398. [Google Scholar] [CrossRef]
- Szegezdi, E.; Logue, S.; Gorman, A.; Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a Major Cytokine in the Central Nervous System. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef]
- McArthur, S.; Loiola, R.A.; Maggioli, E.; Errede, M.; Virgintino, D.; Solito, E. The restorative role of annexin A1 at the blood–brain barrier. Fluids Barriers CNS 2016, 13, 17. [Google Scholar] [CrossRef]
- Luo, Z.Z.; Gao, Y.; Sun, N.; Zhao, Y.; Wang, J.; Tian, B.; Shi, J. Enhancing the interaction between annexin-1 and formyl peptide receptors regulates microglial activation to protect neurons from ischemia-like injury. J. Neuroimmunol. 2014, 276, 24–36. [Google Scholar] [CrossRef]
- Chen, K.; Bao, Z.; Gong, W.; Tang, P.; Yoshimura, T.; Wang, J.M. Regulation of inflammation by members of the formyl-peptide receptor family. J. Autoimmun. 2017, 85, 64–77. [Google Scholar] [CrossRef] [PubMed]
- He, H.-Q.; Ye, R.D. The Formyl Peptide Receptors: Diversity of Ligands and Mechanism for Recognition. Molecules 2017, 22, 455. [Google Scholar] [CrossRef]
- Maia, L.; De Moraes, C.N.; Dias, M.C.; Martinez, J.B.; Caballol, A.O.; Testoni, G.; De Queiroz, C.M.; Peña, R.D.; Landim-Alvarenga, F.; De Oliveira, E. A proteomic study of mesenchymal stem cells from equine umbilical cord. Theriogenology 2017, 100, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gussenhoven, R.; Klein, L.; Ophelders, D.R.M.G.; Habets, D.H.J.; Giebel, B.; Kramer, B.W.; Schurgers, L.J.; Reutelingsperger, C.P.M.; Wolfs, T.G.A.M. Annexin A1 as Neuroprotective Determinant for Blood-Brain Barrier Integrity in Neonatal Hypoxic-Ischemic Encephalopathy. J. Clin. Med. 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Cristante, E.; McArthur, S.; Mauro, C.; Maggioli, E.; Romero, I.A.; Wylezinska-Arridge, M.; Couraud, P.O.; Lopez-Tremoleda, J.; Christian, H.C.; Weksler, B.B.; et al. Identification of an essential endogenous regulator of blood-brain barrier integrity, and its pathological and therapeutic implications. Proc. Natl. Acad. Sci. USA 2013, 110, 832–841. [Google Scholar] [CrossRef]
- Sheikh, M.H.; Solito, E. Annexin A1: Uncovering the Many Talents of an Old Protein. Int. J. Mol. Sci. 2018, 19, 1045. [Google Scholar] [CrossRef]
- McArthur, S.; Cristante, E.; Paterno, M.; Christian, H.; Roncaroli, F.; Gillies, G.E.; Solito, E. Annexin A1: A Central Player in the Anti-Inflammatory and Neuroprotective Role of Microglia. J. Immunol. 2010, 185, 6317–6328. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.M.; Cheresh, D.A. Pathophysiological consequences of VEGF-induced vascular permeability. Nat. Cell Biol. 2005, 437, 497–504. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Zhang, P.; Gao, Y.; Li, C.-L.; Wang, H.-J.; Chen, L.-C.; Feng, Y.; Li, R.-Y.; Li, Y.-L.; Jiang, C.-L. Early VEGF inhibition attenuates blood-brain barrier disruption in ischemic rat brains by regulating the expression of MMPs. Mol. Med. Rep. 2017, 15, 57–64. [Google Scholar] [CrossRef]
- Kluge, M.A.; Fetterman, J.L.; Vita, J.A. Mitochondria and Endothelial Function. Circ. Res. 2013, 112, 1171–1188. [Google Scholar] [CrossRef]
- Nawaz, M.; Fatima, F. Extracellular Vesicles, Tunneling Nanotubes, and Cellular Interplay: Synergies and Missing Links. Front. Mol. Biosci. 2017, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Ji, K.; Guo, L.; Wu, W.; Lu, H.; Shan, P.; Yan, C. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia–reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc. Res. 2014, 92, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Guo, L.; Zhou, Z.; Pan, M.; Yan, C. Mesenchymal stem cells transfer mitochondria into cerebral microvasculature and promote recovery from ischemic stroke. Microvasc. Res. 2019, 123, 74–80. [Google Scholar] [CrossRef]
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial transfer from bone-marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef]
- Rodriguez, A.-M.; Nakhle, J.; Griessinger, E.; Vignais, M.L. Intercellular mitochondria trafficking highlighting the dual role of mesenchymal stem cells as both sensors and rescuers of tissue injury. Cell Cycle 2018, 17, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-L.; Fraser, J.L.; Yu, S.P.; Zhu, J.; Jiang, Y.-J.; Wei, L. The role of VEGF/VEGFR2 signaling in peripheral stimulation-induced cerebral neurovascular regeneration after ischemic stroke in mice. Exp. Brain Res. 2011, 214, 503–513. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, L.; Shu, B.; Tang, J.; Zhang, L.; Xie, J.; Qi, S.; Xu, Y. Granulocyte/Macrophage Colony-Stimulating Factor Influences Angiogenesis by Regulating the Coordinated Expression of VEGF and the Ang/Tie System. PLoS ONE 2014, 9, e92691. [Google Scholar] [CrossRef]
- Thurston, G.; Rudge, J.S.; Ioffe, E.; Zhou, H.; Ross, L.; Croll, S.; Glazer, N.; Holash, J.; McDonald, D.M.; Yancopoulos, G.D. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat. Med. 2000, 6, 460–463. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Zhang, L.; Croll, S.D.; Chopp, M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience 2002, 113, 683–687. [Google Scholar] [CrossRef]
- Xiang, J.; Hu, J.; Shen, T.; Liu, B.; Hua, F.; Zan, K.; Zu, J.; Cui, G.; Ye, X. Bone marrow mesenchymal stem cells-conditioned medium enhances vascular remodeling after stroke in type 2 diabetic rats. Neurosci. Lett. 2017, 644, 62–66. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, M.; Wu, X.; Li, Z.; Liu, B.; Gao, W.; Yue, J.; Liu, T. Promoting therapeutic angiogenesis of focal cerebral ischemia using thrombospondin-4 (TSP4) gene-modified bone marrow stromal cells (BMSCs) in a rat model. J. Transl. Med. 2019, 17, 1–13. [Google Scholar] [CrossRef]
- Herx, L.M.; Yong, V.W. Interleukin-1β is Required for the Early Evolution of Reactive Astrogliosis Following CNS Lesion. J. Neuropathol. Exp. Neurol. 2001, 60, 961–971. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Park, H.-J.; Lee, G.; Bang, O.Y.; Ahn, Y.H.; Joe, E.; Kim, H.O.; Lee, P.H. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia 2009, 57, 13–23. [Google Scholar] [CrossRef]
- Manley, G.T.; Fujimura, M.; Ma, T.; Noshita, N.; Filiz, F.; Bollen, A.W.; Chan, P.; Verkman, A. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000, 6, 159–163. [Google Scholar] [CrossRef]
- Tang, G.; Liu, Y.; Zhang, Z.; Lu, Y.; Wang, Y.; Huang, J.; Li, Y.; Chen, X.; Gu, X.; Wang, Y.; et al. Mesenchymal Stem Cells Maintain Blood-Brain Barrier Integrity by Inhibiting Aquaporin-4 Upregulation After Cerebral Ischemia. Stem Cells 2014, 32, 3150–3162. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, N.J.; Guruswamy, R.; ElAli, A. Vascular Endothelial Growth Factor Isoform-B Stimulates Neurovascular Repair After Ischemic Stroke by Promoting the Function of Pericytes via Vascular Endothelial Growth Factor Receptor-1. Mol. Neurobiol. 2018, 55, 3611–3626. [Google Scholar] [CrossRef]
- Tian, X.; Brookes, O.; Battaglia, G. Pericytes from Mesenchymal Stem Cells as a model for the blood-brain barrier. Sci. Rep. 2017, 7, 39676. [Google Scholar] [CrossRef] [PubMed]
- da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Z.G.; Li, Y.; Wang, L.; Xu, Y.X.; Gautam, S.C.; Lu, M.; Zhu, Z.; Chopp, M. Intravenous Administration of Human Bone Marrow Stromal Cells Induces Angiogenesis in the Ischemic Boundary Zone After Stroke in Rats. Circ. Res. 2003, 92, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Woodbury, D.; Schwarz, E.J.; Prockop, D.J.; Black, I.B. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 2000, 61, 364–370. [Google Scholar] [CrossRef]
- Ma, K.; Fox, L.; Shi, G.; Shen, J.; Liu, Q.; Pappas, J.D.; Cheng, J.; Qu, T. Generation of neural stem cell-like cells from bone marrow-derived human mesenchymal stem cells. Neurol. Res. 2011, 33, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhu, Q.; Xie, Q.; Liu, Z.; Gao, Y.; He, Y.; Tan, X.; Xu, Y. Low intensity ultrasound targeted microbubble destruction assists MSCs delivery and improves neural function in brain ischaemic rats. J. Drug Target. 2019, 28, 320–329. [Google Scholar] [CrossRef]
- Gonzales-Portillo, G.S.; Sanberg, P.R.; Franzblau, M.; Gonzales-Portillo, C.; Diamandis, T.; Staples, M.; Sanberg, C.D.; Borlongan, C.V. Mannitol-Enhanced Delivery of Stem Cells and Their Growth Factors across the Blood–Brain Barrier. Cell Transplant. 2014, 23, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-H.; Yu, F.-B.; Lei, T.; Gao, H.-J.; Li, P.-W.; Sun, Y.-X.; Huang, H.-Y.; Mu, Q.-C. Bone marrow mesenchymal stem cell therapy in ischemic stroke: Mechanisms of action and treatment optimization strategies. Neural Regen. Res. 2016, 11, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Chandan, R.; Mehta, S.M.; Banerjee, R. Ultrasound-Responsive Carriers for Therapeutic Applications. ACS Biomater. Sci. Eng. 2020, 6, 4731–4747. [Google Scholar] [CrossRef]
- Burgess, A.; Shah, K.; Hough, O.; Hynynen, K. Focused ultrasound-mediated drug delivery through the blood–brain barrier. Expert Rev. Neurother. 2015, 15, 477–491. [Google Scholar] [CrossRef]
- Gong, Z.; Ran, H.; Wu, S.; Zhu, J.; Zheng, J. Ultrasound-Microbubble Transplantation of Bone Marrow Stromal Cells Improves Neurological Function after Forebrain Ischemia in Adult Mice. Cell Biophys. 2014, 70, 499–504. [Google Scholar] [CrossRef]
- Toccaceli, G.; Barbagallo, G.; Peschillo, S. Low-intensity focused ultrasound for the treatment of brain diseases: Safety and feasibility. Theranostics 2019, 9, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.; Ayala-Grosso, C.A.; Ganguly, M.; Jordão, J.F.; Aubert, I.; Hynynen, K. Targeted Delivery of Neural Stem Cells to the Brain Using MRI-Guided Focused Ultrasound to Disrupt the Blood-Brain Barrier. PLoS ONE 2011, 6, e27877. [Google Scholar] [CrossRef]
- Kinoshita, M.; McDannold, N.; Jolesz, F.A.; Hynynen, K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc. Natl. Acad. Sci. USA 2006, 103, 11719–11723. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, C.; Wang, J.; Lin, X.; Zhang, L.; Yang, Y.; Wang, Y.; Zhang, Z.; Bulte, J.W.M.; Yang, G.-Y. MRI/SPECT/Fluorescent Tri-Modal Probe for Evaluating the Homing and Therapeutic Efficacy of Transplanted Mesenchymal Stem Cells in a Rat Ischemic Stroke Model. Adv. Funct. Mater. 2015, 25, 1024–1034. [Google Scholar] [CrossRef]
- Chu, C.; Jablonska, A.; Lesniak, W.G.; Thomas, A.M.; Lan, X.; Linville, R.M.; Li, S.; Searson, P.C.; Liu, G.; Pearl, M.; et al. Optimization of osmotic blood-brain barrier opening to enable intravital microscopy studies on drug delivery in mouse cortex. J. Control. Release 2020, 317, 312–321. [Google Scholar] [CrossRef]
- Rapoport, S.I. Osmotic Opening of the Blood–Brain Barrier: Principles, Mechanism, and Therapeutic Applications. Cell. Mol. Neurobiol. 2000, 20, 217–230. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Hadman, M.; Sanberg, C.D.; Sanberg, P.R. Central Nervous System Entry of Peripherally Injected Umbilical Cord Blood Cells Is Not Required for Neuroprotection in Stroke. Stroke 2004, 35, 2385–2389. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kang, H.-Y.; Kim, J.-H.; Park, D.-H. Mannitol Augments the Effects of Systemical Stem Cell Transplantation without Increasing Cell Migration in a Stroke Animal Model. Tissue Eng. Regen. Med. 2020, 17, 1–10. [Google Scholar] [CrossRef]
- Kurtz, A. Mesenchymal Stem Cell Delivery Routes and Fate. Int. J. Stem Cells 2008, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Guo, L.; Wang, S.; Zhang, Y.; Cai, T.; Zhao, R.C.H.; Wu, Y. The Size of Mesenchymal Stem Cells is a Significant Cause of Vascular Obstructions and Stroke. Stem Cell Rev. Rep. 2014, 10, 295–303. [Google Scholar] [CrossRef]
- Joshi, S.; Ergin, A.; Wang, M.; Reif, R.; Zhang, J.; Bruce, J.N.; Bigio, I. Inconsistent blood brain barrier disruption by intraarterial mannitol in rabbits: Implications for chemotherapy. J. Neuro-Oncol. 2011, 104, 11–19. [Google Scholar] [CrossRef]
- Cuccione, E.; Padovano, G.; Versace, A.; Ferrarese, C.; Beretta, S. Cerebral collateral circulation in experimental ischemic stroke. Exp. Transl. Stroke Med. 2016, 8, 1–9. [Google Scholar] [CrossRef][Green Version]
- Chu, C.; Liu, G.; Janowski, M.; Bulte, J.W.M.; Li, S.; Pearl, M.; Walczak, P. Real-Time MRI Guidance for Reproducible Hyperosmolar Opening of the Blood-Brain Barrier in Mice. Front. Neurol. 2018, 9, 921. [Google Scholar] [CrossRef]
- Walczak, P.; Wojtkiewicz, J.; Nowakowski, A.; Habich, A.; Holak, P.; Xu, J.; Adamiak, Z.; Chehade, M.; Pearl, M.S.; Gailloud, P.; et al. Real-time MRI for precise and predictable intra-arterial stem cell delivery to the central nervous system. Br. J. Pharmacol. 2016, 37, 2346–2358. [Google Scholar] [CrossRef]
- Linville, R.M.; DeStefano, J.G.; Sklar, M.B.; Xu, Z.; Farrell, A.M.; Bogorad, M.I.; Chu, C.; Walczak, P.; Cheng, L.; Mahairaki, V.; et al. Human iPSC-derived blood-brain barrier microvessels: Validation of barrier function and endothelial cell behavior. Biomaterials 2019, 190, 24–37. [Google Scholar] [CrossRef]
- Shahror, R.A.; Ali, A.; Wu, C.-C.; Chiang, Y.-H.; Chen, K.-Y. Enhanced Homing of Mesenchymal Stem Cells Overexpressing Fibroblast Growth Factor 21 to Injury Site in a Mouse Model of Traumatic Brain Injury. Int. J. Mol. Sci. 2019, 20, 2624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, W.; Chen, X.; Lian, Y.; Wang, J.; Cai, C.; Huang, L.; Wang, T.; Ren, J.; Xiang, A.P. CXCR5-Overexpressing Mesenchymal Stromal Cells Exhibit Enhanced Homing and Can Decrease Contact Hypersensitivity. Mol. Ther. 2017, 25, 1434–1447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, Y.; Lai, W.; Xu, Y.; Li, L.; Chen, Z.; Wu, W. Exogenous and endogenous therapeutic effects of combination Sodium Ferulate and bone marrow stromal cells (BMSCs) treatment enhance neurogenesis after rat focal cerebral ischemia. Metab. Brain Dis. 2013, 28, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.-K.; Wang, Z.; Munasinghe, J.; Leng, Y.; Leeds, P.; Chuang, D.-M. Mesenchymal Stem Cells Primed with Valproate and Lithium Robustly Migrate to Infarcted Regions and Facilitate Recovery in a Stroke Model. Stroke 2011, 42, 2932–2939. [Google Scholar] [CrossRef]
- Wei, N.; Yu, S.P.; Gu, X.; Taylor, T.M.; Song, D.; Liu, X.-F.; Wei, L. Delayed Intranasal Delivery of Hypoxic-Preconditioned Bone Marrow Mesenchymal Stem Cells Enhanced Cell Homing and Therapeutic Benefits after Ischemic Stroke in Mice. Cell Transplant. 2013, 22, 977–991. [Google Scholar] [CrossRef]
- Thom, S.R.; Bhopale, V.M.; Velazquez, O.C.; Goldstein, L.J.; Thom, L.H.; Buerk, D.J. Stem cell mobilization by hyperbaric oxygen. Am. J. Physiol.-Heart Circ. Physiol. 2006, 290, H1378–H1386. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Chio, C.-C.; Chang, C.-P.; Wang, L.-C.; Chiang, P.-M.; Niu, K.-C.; Tsai, K.-J. Long Course Hyperbaric Oxygen Stimulates Neurogenesis and Attenuates Inflammation after Ischemic Stroke. Mediat. Inflamm. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Song, M.; Mohamad, O.; Gu, X.; Wei, L.; Yu, S.P. Restoration of Intracortical and Thalamocortical Circuits after Transplantation of Bone Marrow Mesenchymal Stem Cells into the Ischemic Brain of Mice. Cell Transplant. 2013, 22, 2001–2015. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Promote Functional Recovery and Neurovascular Plasticity After Stroke in Rats. J. Cereb. Blood Flow Metab. 2013, 33, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Stewart, T.; Banks, W.A.; Zhang, J. The Transport Mechanism of Extracellular Vesicles at the Blood-Brain Barrier. Curr. Pharm. Des. 2018, 23, 6206–6214. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Savitz, S.; Satani, N. Mesenchymal Stem Cell Derived Extracellular Vesicles for Repairing the Neurovascular Unit after Ischemic Stroke. Cells 2021, 10, 767. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, T.J.; Kang, L.; Kim, Y.-J.; Kang, M.K.; Kim, J.; Ryu, J.H.; Hyeon, T.; Yoon, B.-W.; Ko, S.-B.; et al. Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials 2020, 243, 119942. [Google Scholar] [CrossRef]
- Rufino-Ramos, D.; Albuquerque, P.; Carmona, V.; Perfeito, R.; Nobre, R.J.; de Almeida, L.P. Extracellular vesicles: Novel promising delivery systems for therapy of brain diseases. J. Control. Release 2017, 262, 247–258. [Google Scholar] [CrossRef]
- Jo, W.; Kim, J.; Yoon, J.; Jeong, D.; Cho, S.; Jeong, H.; Yoon, Y.J.; Kim, S.C.; Gho, Y.S.; Park, J. Large-scale generation of cell-derived nanovesicles. Nanoscale 2014, 6, 12056–12064. [Google Scholar] [CrossRef]
- Pei, X.; Li, Y.; Zhu, L.; Zhou, Z. Astrocyte-derived exosomes suppress autophagy and ameliorate neuronal damage in experimental ischemic stroke. Exp. Cell Res. 2019, 382, 111474. [Google Scholar] [CrossRef]
- Bu, X.; Li, D.; Wang, F.; Sun, Q.; Zhang, Z. Protective Role of Astrocyte-Derived Exosomal microRNA-361 in Cerebral Ischemic-Reperfusion Injury by Regulating the AMPK/mTOR Signaling Pathway and Targeting CTSB. Neuropsychiatr. Dis. Treat. 2020, 16, 1863–1877. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Yang, C.; Xu, Z.; Huang, J.; Lin, J. Astrocyte-derived exosome-transported microRNA-34c is neuroprotective against cerebral ischemia/reperfusion injury via TLR7 and the NF-κB/MAPK pathways. Brain Res. Bull. 2020, 163, 84–94. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, Q.; Wang, P.; Jing, Y.; Yao, H.; Tang, Y.; Li, Z.; Zhang, H.; Xiu, R. Exosomes Derived from Pericytes Improve Microcirculation and Protect Blood–Spinal Cord Barrier After Spinal Cord Injury in Mice. Front. Neurosci. 2019, 13, 319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, Y.; Chopp, M.; Li, C.; Kemper, A.; Liu, X.; Wang, X.; Zhang, L.; Zhang, Z.G. Ischemic Cerebral Endothelial Cell–Derived Exosomes Promote Axonal Growth. Stroke 2020, 51, 3701–3712. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Zhang, W.; Chen, Y.; Bihl, J.C. Exosomes from miRNA-126-modified endothelial progenitor cells alleviate brain injury and promote functional recovery after stroke. CNS Neurosci. Ther. 2020, 26, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Maguire, C.A.; Balaj, L.; Sivaraman, S.; Crommentuijn, M.H.; Ericsson, M.; Mincheva-Nilsson, L.; Baranov, V.; Gianni, D.; Tannous, B.A.; Sena-Esteves, M.; et al. Microvesicle-associated AAV Vector as a Novel Gene Delivery System. Mol. Ther. 2012, 20, 960–971. [Google Scholar] [CrossRef]
- Lee, J.; Bayarsaikhan, D.; Arivazhagan, R.; Park, H.; Lim, B.; Gwak, P.; Jeong, G.-B.; Lee, J.; Byun, K.; Lee, B. CRISPR/Cas9 edited sRAGE-MSCs protect neuronal death in Parkinson’s disease model. Int. J. Stem Cells 2019, 12, 114. [Google Scholar] [CrossRef]
- Hudry, E.; Martin, C.; Gandhi, S.; György, B.; Scheffer, D.I.; Mu, D.; Merkel, S.F.; Mingozzi, F.; Fitzpatrick, Z.; Dimant, H.; et al. Exosome-associated AAV vector as a robust and convenient neuroscience tool. Gene Ther. 2016, 23, 380–392. [Google Scholar] [CrossRef]
- Votteler, J.; Ogohara, C.; Yi, S.; Hsia, Y.; Nattermann, U.; Belnap, D.M.; King, N.P.; Sundquist, W.I. Designed proteins induce the formation of nanocage-containing extracellular vesicles. Nat. Cell Biol. 2016, 540, 292–295. [Google Scholar] [CrossRef]
- Meca-Cortés, O.; Guerra-Rebollo, M.; Garrido, C.; Borrós, S.; Rubio, N.; Blanco, J. CRISPR/Cas9-Mediated Knockin Application in Cell Therapy: A Non-viral Procedure for Bystander Treatment of Glioma in Mice. Mol. Ther. Nucleic Acids 2017, 8, 395–403. [Google Scholar] [CrossRef]
- Schary, Y.; Brzezinski, R.; Teper-Shaihov, O.; Naftali-Shani, N.; Leor, J. CRISPR-Cas9-based gene editing of human mesenchymal stromal cells to improve the outcome of cell therapy. Eur. Heart J. 2020, 41, ehaa946.3658. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, P.T.; Wu, C.-C.; Chiang, Y.-H.; Hu, C.-J.; Chen, K.-Y. Mesenchymal Stem/Stromal Cell Therapy in Blood–Brain Barrier Preservation Following Ischemia: Molecular Mechanisms and Prospects. Int. J. Mol. Sci. 2021, 22, 10045. https://doi.org/10.3390/ijms221810045
Do PT, Wu C-C, Chiang Y-H, Hu C-J, Chen K-Y. Mesenchymal Stem/Stromal Cell Therapy in Blood–Brain Barrier Preservation Following Ischemia: Molecular Mechanisms and Prospects. International Journal of Molecular Sciences. 2021; 22(18):10045. https://doi.org/10.3390/ijms221810045
Chicago/Turabian StyleDo, Phuong Thao, Chung-Che Wu, Yung-Hsiao Chiang, Chaur-Jong Hu, and Kai-Yun Chen. 2021. "Mesenchymal Stem/Stromal Cell Therapy in Blood–Brain Barrier Preservation Following Ischemia: Molecular Mechanisms and Prospects" International Journal of Molecular Sciences 22, no. 18: 10045. https://doi.org/10.3390/ijms221810045
APA StyleDo, P. T., Wu, C.-C., Chiang, Y.-H., Hu, C.-J., & Chen, K.-Y. (2021). Mesenchymal Stem/Stromal Cell Therapy in Blood–Brain Barrier Preservation Following Ischemia: Molecular Mechanisms and Prospects. International Journal of Molecular Sciences, 22(18), 10045. https://doi.org/10.3390/ijms221810045






