Crosstalk between Metabolic Disorders and Immune Cells
Abstract
:1. Chronic Inflammation and Obesity
2. Biology of Macrophages in Adipose Tissue
2.1. Adipose Tissue-Resident Macrophages
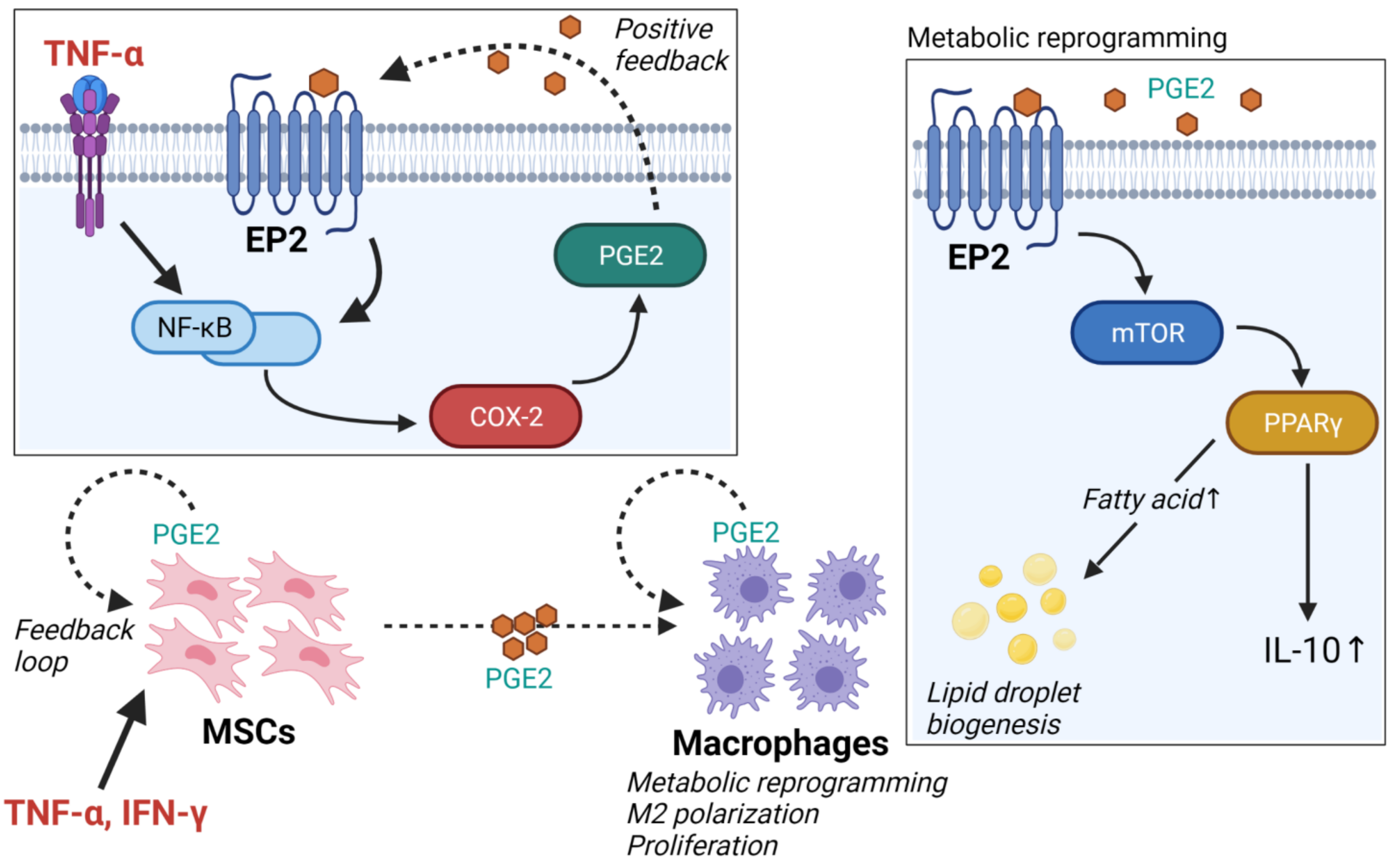
2.2. Adipose Tissue-Derived Mesenchymal Stem Cells
3. Adipose Tissue-Resident Lymphocytes
3.1. CD4+ T Cells
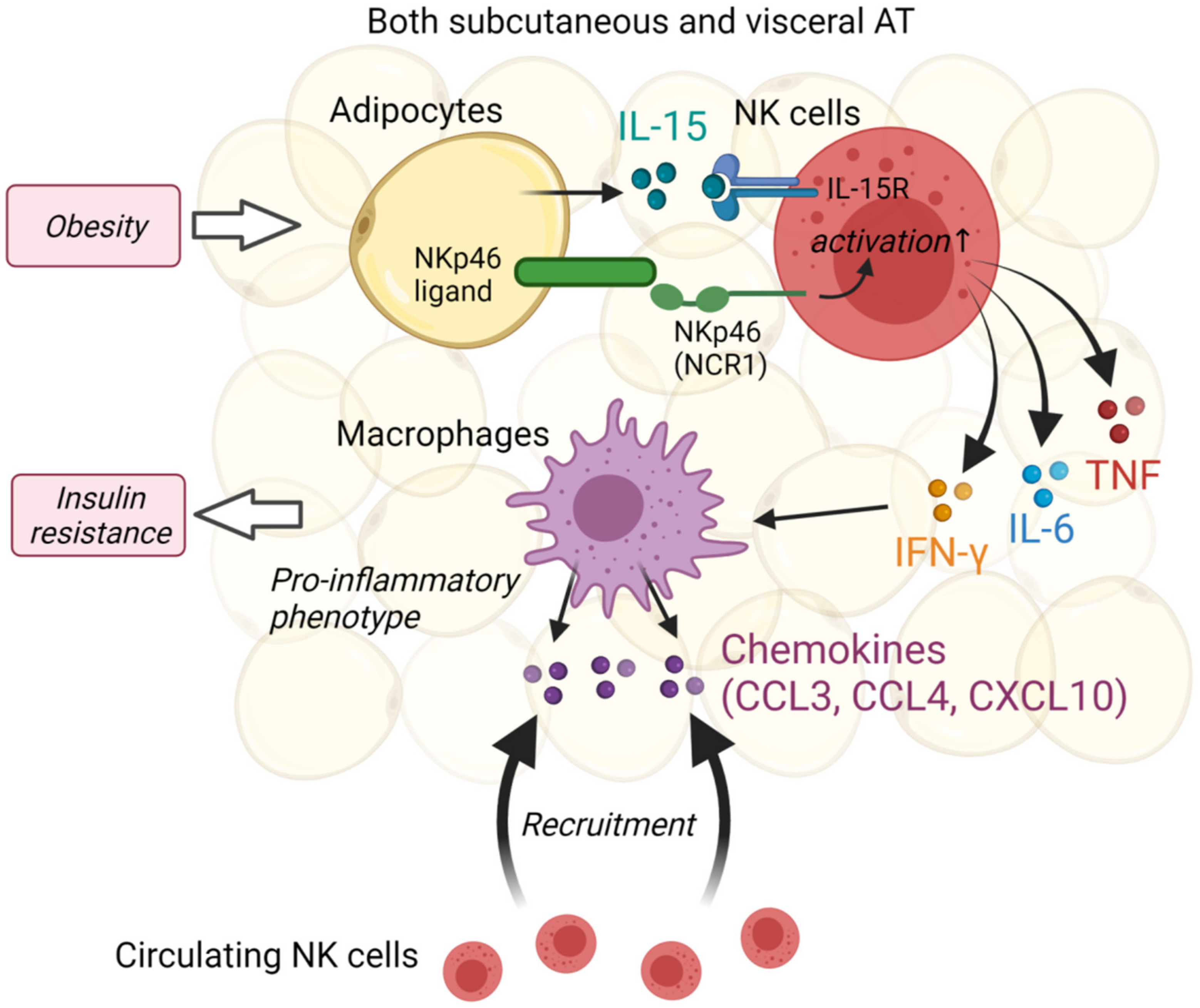
3.2. Natural Killer Cells
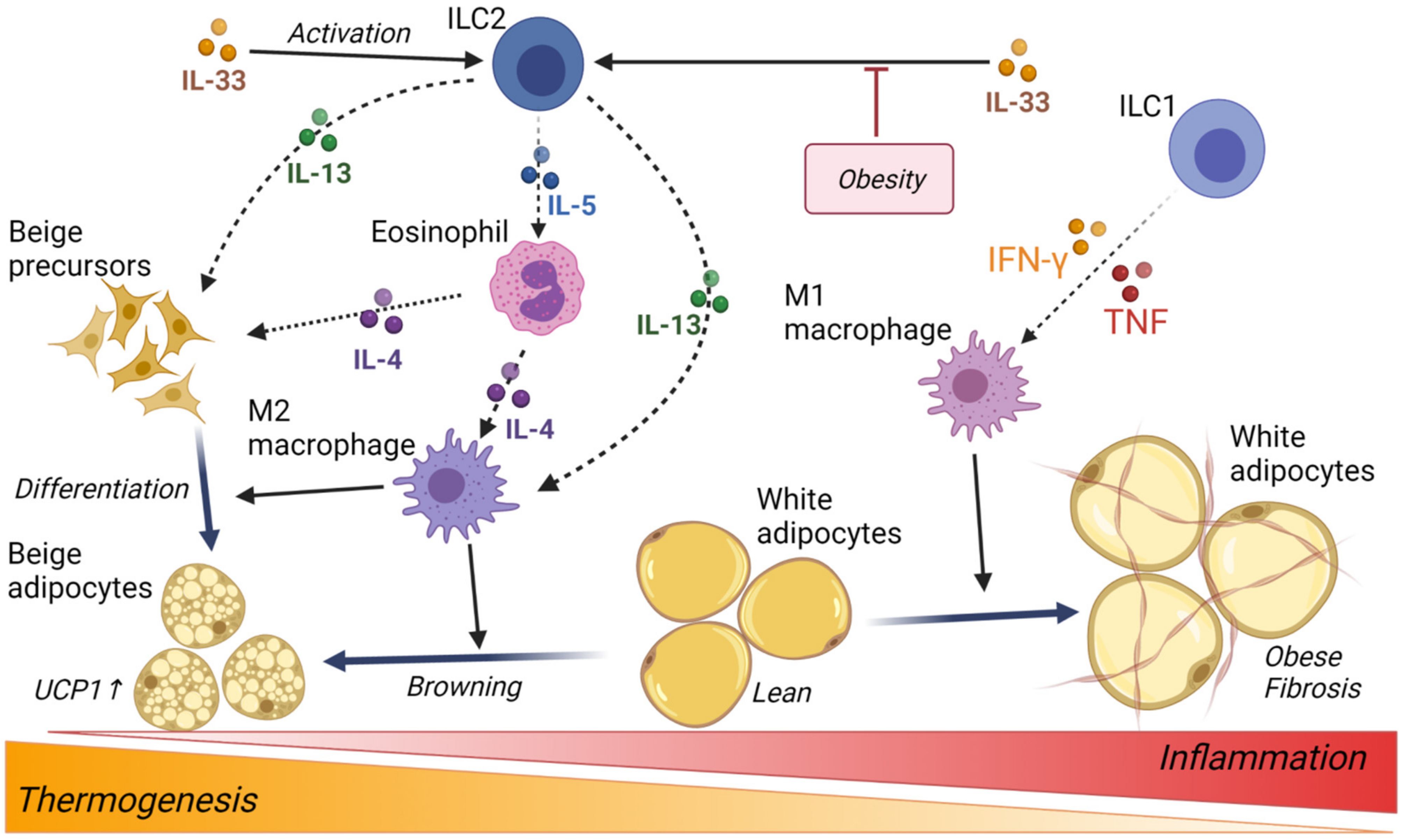
3.3. Innate Lymphoid Cells
4. Adipocyte Thermogenesis and Browning
5. Liver-Resident Natural Killer Cells
6. Links with Human Pathology
6.1. Metabolically Healthy Obesity
6.2. Non-Obese Metabolic Disorder
6.3. Cytokine for Type 2 Diabetes Treatments
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Hong, E.G.; Ko, H.J.; Cho, Y.R.; Kim, H.J.; Ma, Z.X.; Yu, T.Y.; Friedline, R.H.; Kurt-Jones, E.; Finberg, R.; Fischer, M.A.; et al. Interleukin-10 Prevents Diet-Induced Insulin Resistance by Attenuating Macrophage and Cytokine Response in Skeletal Muscle. Diabetes 2009, 58, 2525–2535. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.M.; Zhang, C.B.; Ma, Y.J.; Bu, L.; Yan, L.N.; Liu, D.X. Hydrodynamic Delivery of mIL10 Gene Protects Mice from High-fat Diet-induced Obesity and Glucose Intolerance. Mol. Ther. 2013, 21, 1852–1861. [Google Scholar] [CrossRef] [Green Version]
- Fischer-Posovszky, P.; Wang, Q.A.; Asterholm, I.W.; Rutkowski, J.M.; Scherer, P.E. Targeted Deletion of Adipocytes by Apoptosis Leads to Adipose Tissue Recruitment of Alternatively Activated M2 Macrophages. Endocrinology 2011, 152, 3074–3081. [Google Scholar] [CrossRef]
- Lee, Y.H.; Petkova, A.P.; Granneman, J.G. Identification of an Adipogenic Niche for Adipose Tissue Remodeling and Restoration. Cell Metab. 2013, 18, 355–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kambara, K.; Ohashi, W.; Tomita, K.; Takashina, M.; Fujisaka, S.; Hayashit, R.; Mori, H.; Tobe, K.; Hattori, Y. In Vivo Depletion of CD206(+) M2 Macrophages Exaggerates Lung Injury in Endotoxemic Mice. Am. J. Pathol. 2015, 185, 162–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawaz, A.; Aminuddin, A.; Kado, T.; Takikawa, A.; Yamamoto, S.; Tsuneyama, K.; Igarashi, Y.; Ikutani, M.; Nishida, Y.; Nagai, Y.; et al. CD206(+) M2-like macrophages regulate systemic glucose metabolism by inhibiting proliferation of adipocyte progenitors. Nat. Commun. 2017, 8, 286. [Google Scholar] [CrossRef]
- Soucie, E.L.; Weng, Z.M.; Geirsdottir, L.; Molawi, K.; Maurizio, J.; Fenouil, R.; Mossadegh-Keller, N.; Gimenez, G.; VanHille, L.; Beniazza, M.; et al. Lineage-specific enhancers activate self-renewal genes in macrophages and embryonic stem cells. Science 2016, 351, aad5510. [Google Scholar] [CrossRef] [Green Version]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langin, D.; Arner, P. Importance of TNF alpha and neutral lipases in human adipose tissue lipolysis. Trends Endocrinol. Metab. 2006, 17, 314–320. [Google Scholar] [CrossRef]
- Lancaster, G.I.; Langley, K.G.; Berglund, N.A.; Kammoun, H.L.; Reibe, S.; Estevez, E.; Weir, J.; Mellett, N.A.; Pernes, G.; Conway, J.R.W.; et al. Evidence that TLR4 Is Not a Receptor for Saturated Fatty Acids but Mediates Lipid-Induced Inflammation by Reprogramming Macrophage Metabolism. Cell Metab. 2018, 27, 1096–1110. [Google Scholar] [CrossRef] [Green Version]
- Korbecki, J.; Bajdak-Rusinek, K. The effect of palmitic acid on inflammatory response in macrophages: An overview of molecular mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.P.; Urso, C.J.; Jadeja, V. Saturated Fatty Acids in Obesity-Associated Inflammation. J. Inflamm. Res. 2020, 13, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charles-Messance, H.; Mitchelson, K.A.J.; Castro, E.D.; Sheedy, F.J.; Roche, H.M. Regulating metabolic inflammation by nutritional modulation. J. Allergy Clin. Immunol. 2020, 146, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, K.A.; Amici, S.A.; Webb, L.M.; Ruiz-Rosado, J.D.; Popovich, P.G.; Partida-Sanchez, S.; Guerau-de-Arellano, M. Novel Markers to Delineate Murine M1 and M2 Macrophages. PLoS ONE 2015, 10, e0145342. [Google Scholar] [CrossRef] [Green Version]
- Na, Y.R.; Jung, D.; Gu, G.J.; Seok, S.H. GM-CSF Grown Bone Marrow Derived Cells Are Composed of Phenotypically Different Dendritic Cells and Macrophages. Mol. Cells 2016, 39, 734–741. [Google Scholar] [CrossRef] [Green Version]
- Erlich, Z.; Shlomovitz, I.; Edry-Botzer, L.; Cohen, H.; Frank, D.; Wang, H.Q.; Lew, A.M.; Lawlor, K.E.; Zhan, Y.F.; Vince, J.E.; et al. Macrophages, rather than DCs, are responsible for inflammasome activity in the GM-CSF BMDC model. Nat. Immunol. 2019, 20, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; O’Neill, L.A. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur. J. Immunol. 2016, 46, 13–21. [Google Scholar] [CrossRef]
- Zhuang, H.D.; Lv, Q.; Zhong, C.; Cui, Y.R.; He, L.L.; Zhang, C.; Yu, J. Tiliroside Ameliorates Ulcerative Colitis by Restoring the M1/M2 Macrophage Balance via the HIF-1 alpha/glycolysis Pathway. Front. Immunol. 2021, 12, 951. [Google Scholar] [CrossRef]
- Odegaard, J.I.; Ricardo-Gonzalez, R.R.; Goforth, M.H.; Morel, C.R.; Subramanian, V.; Mukundan, L.; Eagle, A.R.; Vats, D.; Brombacher, F.; Ferrante, A.W.; et al. Macrophage-specific PPAR gamma controls alternative activation and improves insulin resistance. Nature 2007, 447, 1116–1120. [Google Scholar] [CrossRef] [Green Version]
- Kelly, B.; O’Neill, L.A.J. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef] [Green Version]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.Y.; Deng, Z.H.; Zhang, J.Y.; Yang, C.; Liu, J.J.; Han, W.D.; Ye, P.; Si, Y.L.; Chen, G.H. Mesenchymal stem cells promote type 2 macrophage polarization to ameliorate the myocardial injury caused by diabetic cardiomyopathy. J. Transl. Med. 2019, 17, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasandan, A.B.; Jahnavi, S.; Shashank, C.; Prasad, P.; Kumar, A.; Prasanna, J. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE(2)-dependent mechanism. Sci. Rep. 2016, 6, 38308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, D.; Xu, Y.; Liu, Q.L.; Zhang, Q. Mesenchymal Stem Cell-Macrophage Crosstalk and Maintenance of Inflammatory Microenvironment Homeostasis. Front. Cell Dev. Biol. 2021, 9, 1628. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Souza-Moreira, L.; Morell, M.; Caro, M.; O’Valle, F.; Gonzalez-Rey, E.; Delgado, M. Adipose-derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut 2013, 62, 1131–1141. [Google Scholar] [CrossRef]
- Souza-Moreira, L.; Soares, V.C.; Dias, S.D.G.; Bozza, P.T. Adipose-derived Mesenchymal Stromal Cells Modulate Lipid Metabolism and Lipid Droplet Biogenesis via AKT/mTOR -PPAR gamma Signalling in Macrophages. Sci. Rep. 2019, 9, 20304. [Google Scholar] [CrossRef] [Green Version]
- Aleshin, S.; Grabeklis, S.; Hanck, T.; Sergeeva, M.; Reiser, G. Peroxisome Proliferator-Activated Receptor (PPAR)-gamma Positively Controls and PPAR alpha Negatively Controls Cyclooxygenase-2 Expression in Rat Brain Astrocytes through a Convergence on PPAR beta/delta via Mutual Control of PPAR Expression Levels. Mol. Pharmacol. 2009, 76, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.B.; Araujo-Santos, T.; Lazaro-Souza, M.; Carneiro, A.B.; Ibraim, I.C.; Jesus-Santos, F.H.; Luz, N.F.; Pontes, S.D.; Entringer, P.F.; Descoteaux, A.; et al. Leishmania infantum lipophosphoglycan induced-Prostaglandin E-2 production in association with PPAR-gamma expression via activation of Toll like receptors-1 and 2. Sci. Rep. 2017, 7, 14321. [Google Scholar] [CrossRef]
- Markovic, T.; Jakopin, Z.; Dolenc, M.S.; Mlinaric-Rascan, I. Structural features of subtype-selective EP receptor modulators. Drug Discov. Today 2017, 22, 57–71. [Google Scholar] [CrossRef] [Green Version]
- Aoki, T.; Frosen, J.; Fukuda, M.; Bando, K.; Shioi, G.; Tsuji, K.; Ollikainen, E.; Nozaki, K.; Laakkonen, J.; Narumiya, S. Prostaglandin E-2-EP2-NF-kappa B signaling in macrophages as a potential therapeutic target for intracranial aneurysms. Sci. Signal. 2017, 10, eaah6037. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.M.F.; Cheng, Y.Y.; Tang, T.W.H.; Shih, C.; Chen, J.H.; Hsieh, P.C.H. Prostaglandin E-2 Receptor 2 Modulates Macrophage Activity for Cardiac Repair. J. Am. Heart Assoc. 2018, 7, e009216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minhas, P.S.; Latif-Hernandez, A.; McReynolds, M.R.; Durairaj, A.S.; Wang, Q.; Rubin, A.; Joshi, A.U.; He, J.Q.; Gauba, E.; Liu, L.; et al. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature 2021, 590, 122–128. [Google Scholar] [CrossRef]
- Johansson, J.U.; Woodling, N.S.; Wang, Q.; Panchal, M.; Hang, X.B.; Trueba-Saiz, A.; Brown, H.D.; Mhatre, S.D.; Loui, T.; Andreasson, K.I. Prostaglandin signaling suppresses beneficial microglial function in Alzheimer’s disease models. J. Clin. Investig. 2015, 125, 350–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Ftesi, S.; Chang, E.I.; Longaker, M.T.; Gurtner, G.C. Aging and Diabetes Impair the Neovascular Potential of Adipose-Derived Stromal Cells. Plast. Reconstr. Surg. 2009, 123, 475–485. [Google Scholar] [CrossRef] [Green Version]
- Ciprandi, G.; Murdaca, G.; Colombo, B.M.; De Amici, M.; Marseglia, G.L. Serum vascular endothelial growth factor in allergic rhinitis and systemic lupus erythematosus. Hum. Immunol. 2008, 69, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, R.A.; Nada, W.M.; Hadhoud, K.M.; El-Tarhony, S.A. The role of vascular endothelial growth factor in the progression of diabetic vascular complications. Eye 2010, 24, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.D.; Ren, J.; Bai, Y.; Pei, X.T.; Han, Y. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int. J. Biochem. Cell Biol. 2019, 109, 59–68. [Google Scholar] [CrossRef]
- Becker, M.; Levings, M.K.; Daniel, C. Adipose-tissue regulatory T cells: Critical players in adipose-immune crosstalk. Eur. J. Immunol. 2017, 47, 1867–1874. [Google Scholar] [CrossRef]
- Cipolletta, D.; Feuerer, M.; Li, A.; Kamei, N.; Lee, J.; Shoelson, S.E.; Benoist, C.; Mathis, D. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue T-reg cells. Nature 2012, 486, 549–553. [Google Scholar] [CrossRef]
- Cipolletta, D.; Cohen, P.; Spiegelman, B.M.; Benoist, C.; Mathis, D. Appearance and disappearance of the mRNA signature characteristic of T-reg cells in visceral adipose tissue: Age, diet, and PPAR gamma effects. Proc. Natl. Acad. Sci. USA 2015, 112, 482–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S.; et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009, 15, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Travers, R.L.; Motta, A.C.; Betts, J.A.; Bouloumie, A.; Thompson, D. The impact of adiposity on adipose tissue-resident lymphocyte activation in humans. Int. J. Obes. 2015, 39, 762–769. [Google Scholar] [CrossRef] [Green Version]
- Wouters, K.; Gaens, K.; Bijnen, M.; Verboven, K.; Jocken, J.; Wetzels, S.; Wijnands, E.; Hansen, D.; van Greevenbroek, M.; Duijvestijn, A.; et al. Circulating classical monocytes are associated with CD11c(+) macrophages in human visceral adipose tissue. Sci. Rep. 2017, 7, 42665. [Google Scholar] [CrossRef] [PubMed]
- Ivanova-Todorova, E.; Bochev, I.; Dimitrov, R.; Belemezova, K.; Mourdjeva, M.; Kyurkchiev, S.; Kinov, P.; Altankova, I.; Kyurkchiev, D. Conditioned Medium from Adipose Tissue-Derived Mesenchymal Stem Cells Induces CD4+FOXP3+Cells and Increases IL-10 Secretion. J. Biomed. Biotechnol. 2012, 2012, 295167. [Google Scholar] [CrossRef]
- Song, K.J.; Cai, H.H.; Zhang, D.M.; Huang, R.C.; Sun, D.H.; He, Y.L. Effects of human adipose-derived mesenchymal stem cells combined with estrogen on regulatory T cells in patients with premature ovarian insufficiency. Int. Immunopharmacol. 2018, 55, 257–262. [Google Scholar] [CrossRef]
- O’Rourke, R.W.; Gaston, G.D.; Meyer, K.A.; White, A.E.; Marks, D.L. Adipose tissue NK cells manifest an activated phenotype in human obesity. Metab. Clin. Exp. 2013, 62, 1557–1561. [Google Scholar] [CrossRef] [Green Version]
- Boulenouar, S.; Michelet, X.; Duquette, D.; Alvarez, D.; Hogan, A.E.; Dold, C.; O’Connor, D.; Stutte, S.; Tavakkoli, A.; Winters, D.; et al. Adipose Type One Innate Lymphoid Cells Regulate Macrophage Homeostasis through Targeted Cytotoxicity. Immunity 2017, 46, 273–286. [Google Scholar] [CrossRef] [Green Version]
- Pierce, J.R.; Maples, J.M.; Hickner, R.C. IL-15 concentrations in skeletal muscle and subcutaneous adipose tissue in lean and obese humans: Local effects of IL-15 on adipose tissue lipolysis. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1131–E1139. [Google Scholar] [CrossRef] [Green Version]
- Al-Attar, A.; Presnell, S.R.; Clasey, J.L.; Long, D.E.; Walton, R.G.; Sexton, M.; Starr, M.E.; Kern, P.A.; Peterson, C.A.; Lutz, C.T. Human Body Composition and Immunity: Visceral Adipose Tissue Produces IL-15 and Muscle Strength Inversely Correlates with NK Cell Function in Elderly Humans. Front. Immunol. 2018, 9, 440. [Google Scholar] [CrossRef] [Green Version]
- Costello, R.T.; Rey, J.; Fauriat, C.; Gastaut, J.A.; Olive, D. New approaches in the immunotherapy of haematological malignancies. Eur. J. Haematol. 2003, 70, 333–345. [Google Scholar] [CrossRef]
- Costa, P.; Rusconi, S.; Mavilio, D.; Fogli, M.; Murdaca, G.; Pende, D.; Mingari, M.C.; Galli, M.; Moretta, L.; De Maria, A. Differential disappearance of inhibitory natural killer cell receptors during HAART and possible impairment of HIV-1-specific CD8 cytotoxic T lymphocytes. Aids 2001, 15, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Wensveen, F.M.; Jelencic, V.; Valentic, S.; Sestan, M.; Wensveen, T.T.; Theurich, S.; Glasner, A.; Mendrila, D.; Stimac, D.; Wunderlich, F.T.; et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat. Immunol. 2015, 16, 376–385. [Google Scholar] [CrossRef]
- Lee, B.C.; Kim, M.S.; Pae, M.; Yamamoto, Y.; Eberle, D.; Shimada, T.; Kamei, N.; Park, H.S.; Sasorith, S.; Woo, J.R.; et al. Adipose Natural Killer Cells Regulate Adipose Tissue Macrophages to Promote Insulin Resistance in Obesity. Cell Metab. 2016, 23, 685–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, F.J.; Imani, S.; Tao, L.; Deng, Y.C.; Cai, Y. Natural Killer Cells: Friend or Foe in Metabolic Diseases? Front. Immunol. 2021, 12, 134. [Google Scholar] [CrossRef]
- Lee, H.; Da Silva, I.P.; Palendira, U.; Scolyer, R.A.; Long, G.A.V.; Wilmott, J.S. Targeting NK Cells to Enhance Melanoma Response to Immunotherapies. Cancers 2021, 13, 1363. [Google Scholar] [CrossRef]
- La Cava, A. Leptin in inflammation and autoimmunity. Cytokine 2017, 98, 51–58. [Google Scholar] [CrossRef]
- Song, J.F.; Deng, T. The Adipocyte and Adaptive Immunity. Front. Immunol. 2020, 11, 593058. [Google Scholar] [CrossRef] [PubMed]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, H.V.; Luu, N.K.; Son, H.A.; Hoan, N.V.; Hung, T.T.; Velavan, T.P.; Toan, N.L. Adiponectin and pro-inflammatory cytokines are modulated in Vietnamese patients with type 2 diabetes mellitus. J. Diabetes Investig. 2017, 8, 295–305. [Google Scholar] [CrossRef]
- Francisco, V.; Pino, J.; Campos-Cabaleiro, V.; Ruiz-Fernandez, C.; Mera, A.; Gonzalez-Gay, M.A.; Gomez, R.; Gualillo, O. Obesity, Fat Mass and Immune System: Role for Leptin. Front. Physiol. 2018, 9, 640. [Google Scholar] [CrossRef] [Green Version]
- Wrann, C.D.; Laue, T.; Hubner, L.; Kuhlmann, S.; Jacobs, R.; Goudeva, L.; Nave, H. Short-term and long-term leptin exposure differentially affect human natural killer cell immune functions. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E108–E116. [Google Scholar] [CrossRef] [Green Version]
- Jahn, J.; Spielau, M.; Brandsch, C.; Stangl, G.I.; Delank, K.S.; Bahr, I.; Berreis, T.; Wrann, C.D.; Kielstein, H. Decreased NK cell functions in obesity can be reactivated by fat mass reduction. Obesity 2015, 23, 2233–2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef]
- O’Sullivan, T.E.; Rapp, M.Y.; Fan, X.Y.; Weizman, O.E.; Bhardwaj, P.; Adams, N.M.; Walzer, T.; Dannenberg, A.J.; Sun, J.C. Adipose-Resident Group 1 Innate Lymphoid Cells Promote Obesity-Associated Insulin Resistance. Immunity 2016, 45, 428–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.D.; Shen, L.; Sun, X.T.; Liu, F.C.; Feng, W.H.; Jiang, C.P.; Chu, X.H.; Ye, X.; Jiang, C.; Wang, Y.; et al. Adipose group 1 innate lymphoid cells promote adipose tissue fibrosis and diabetes in obesity. Nat. Commun. 2019, 10, 3254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molofsky, A.B.; Nussbaum, J.C.; Liang, H.E.; Van Dyken, S.J.; Cheng, L.E.; Mohapatra, A.; Chawla, A.; Locksley, R.M. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 2013, 210, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Brestoff, J.R.; Kim, B.S.; Saenz, S.A.; Stine, R.R.; Monticelli, L.A.; Sonnenberg, G.F.; Thome, J.J.; Farber, D.L.; Lutfy, K.; Seale, P.; et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 2015, 519, 242–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.W.; Odegaard, J.I.; Mukundan, L.; Qiu, Y.F.; Molofsky, A.B.; Nussbaum, J.C.; Yun, K.R.; Locksley, R.M.; Chawla, A. Activated Type 2 Innate Lymphoid Cells Regulate Beige Fat Biogenesis. Cell 2015, 160, 74–87. [Google Scholar] [CrossRef] [Green Version]
- Everaere, L.; Yahia, S.A.; Boute, M.; Audousset, C.; Chenivesse, C.; Tsicopoulos, A. Innate lymphoid cells at the interface between obesity and asthma. Immunology 2018, 153, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, K.A.; Lidell, M.E.; Orava, J.; Heglind, M.; Westergren, R.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.J.; Enerback, S.; et al. Brief Report: Functional Brown Adipose Tissue in Healthy Adults. N. Engl. J. Med. 2009, 360, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Ezquerro, S.; Mendez-Gimenez, L.; Becerril, S.; Fruhbeck, G. Revisiting the adipocyte: A model for integration of cytokine signaling in the regulation of energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E691–E714. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.F.; Nguyen, K.D.; Odegaard, J.I.; Cui, X.J.; Tian, X.Y.; Locksley, R.M.; Palmiter, R.D.; Chawla, A. Eosinophils and Type 2 Cytokine Signaling in Macrophages Orchestrate Development of Functional Beige Fat. Cell 2014, 157, 1292–1308. [Google Scholar] [CrossRef] [Green Version]
- Fischer, K.; Ruiz, H.H.; Jhun, K.; Finan, B.; Oberlin, D.J.; van der Heide, V.; Kalinovich, A.V.; Petrovic, N.; Wolf, Y.; Clemmensen, C.; et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat. Med. 2017, 23, 623–630. [Google Scholar] [CrossRef]
- Rajbhandari, P.; Thomas, B.J.; Feng, A.C.; Hong, C.; Wang, J.X.; Vergnes, L.; Sallam, T.; Wang, B.; Sandhu, J.; Seldin, M.M.; et al. IL-10 Signaling Remodels Adipose Chromatin Architecture to Limit Thermogenesis and Energy Expenditure. Cell 2018, 172, 218–233.e17. [Google Scholar] [CrossRef] [Green Version]
- Villarroya, F.; Cereijo, R.; Gavalda-Navarro, A.; Villarroya, J.; Giralt, M. Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J. Int. Med. 2018, 284, 492–504. [Google Scholar] [CrossRef] [Green Version]
- Schulz, T.J.; Huang, P.; Huang, T.L.; Xue, R.D.; McDougall, L.E.; Townsend, K.L.; Cypess, A.M.; Mishina, Y.; Gussoni, E.; Tseng, Y.H. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 2013, 495, 379–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravussin, Y.; Xiao, C.Y.; Gavrilova, O.; Reitman, M.L. Effect of Intermittent Cold Exposure on Brown Fat Activation, Obesity, and Energy Homeostasis in Mice. PLoS ONE 2014, 9, e85876. [Google Scholar] [CrossRef]
- Keipert, S.; Lutter, D.; Schroeder, B.O.; Brandt, D.; Stahlman, M.; Schwarzmayr, T.; Graf, E.; Fuchs, H.; de Angelis, M.H.; Tschop, M.H.; et al. Endogenous FGF21-signaling controls paradoxical obesity resistance of UCP1-deficient mice. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, A.K.; Damgaard, A.; Mackey, A.L.; Schjerling, P.; Magnusson, P.; Olesen, A.T.; Kjaer, M.; Scheele, C. An anti-inflammatory phenotype in visceral adipose tissue of old lean mice, augmented by exercise. Sci. Rep. 2019, 9, 12069. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, A.; Krause, F.N.; Moran, A.; MacCannell, A.D.V.; Scragg, J.L.; McNally, B.D.; Boateng, E.; Murfitt, S.A.; Virtue, S.; Wright, J.; et al. Brown and beige adipose tissue regulate systemic metabolism through a metabolite interorgan signaling axis. Nat. Commun. 2021, 12, 1–21. [Google Scholar] [CrossRef]
- Scheele, C.; Nielsen, S. Metabolic regulation and the anti-obesity perspectives of human brown fat. Redox Biol. 2017, 12, 770–775. [Google Scholar] [CrossRef]
- Bettini, S.; Favaretto, F.; Compagnin, C.; Belligoli, A.; Sanna, M.; Fabris, R.; Serra, R.; Dal Pra, C.; Prevedello, L.; Foletto, M.; et al. Resting Energy Expenditure, Insulin Resistance and UCP1 Expression in Human Subcutaneous and Visceral Adipose Tissue of Patients with Obesity. Front. Endocrinol. 2019, 10, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.; Park, H.S.; Kim, J.; Jang, Y.J.; Kim, J.H.; Lee, Y.; Heo, Y. Depot-specific UCP1 expression in human white adipose tissue and its association with obesity-related markers. Int. J. Obes. 2020, 44, 697–706. [Google Scholar] [CrossRef]
- Wu, D.; Molofsky, A.B.; Liang, H.E.; Ricardo-Gonzalez, R.R.; Jouihan, H.A.; Bando, J.K.; Chawla, A.; Locksley, R.M. Eosinophils Sustain Adipose Alternatively Activated Macrophages Associated with Glucose Homeostasis. Science 2011, 332, 243–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wueest, S.; Rapold, R.A.; Schumann, D.M.; Rytka, J.M.; Schildknecht, A.; Nov, O.; Chervonsky, A.V.; Rudich, A.; Schoenle, E.J.; Donath, M.Y.; et al. Deletion of Fas in adipocytes relieves adipose tissue inflammation and hepatic manifestations of obesity in mice. J. Clin. Investig. 2010, 120, 191–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, E.W.; Lee, M.; Song, J.W.; Kim, K.; Lee, J.; Yang, J.; Lee, S.H.; Kim, I.Y.; Choi, J.H.; Seong, J.K. Fas mutation reduces obesity by increasing IL-4 and IL-10 expression and promoting white adipose tissue browning. Sci. Rep. 2020, 10, 12001. [Google Scholar] [CrossRef]
- Matsui, Y.; Tomaru, U.; Miyoshi, A.; Ito, T.; Fukaya, S.; Miyoshi, H.; Atsumi, T.; Ishizu, A. Overexpression of TNF-alpha converting enzyme promotes adipose tissue inflammation and fibrosis induced by high fat diet. Exp. Mol. Pathol. 2014, 97, 354–358. [Google Scholar] [CrossRef]
- Kern, L.; Mittenbuhler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNF alpha and IL-6 Signaling: The Missing Link between Obesity and Inflammation-Driven Liver and Colorectal Cancers. Cancers 2019, 11, 24. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.Y.; Tian, Z.G. Roles of Hepatic Innate and Innate-Like Lymphocytes in Nonalcoholic Steatohepatitis. Front. Immunol. 2020, 11, 1500. [Google Scholar] [CrossRef]
- Martinez-Chantar, M.L.; Delgado, T.C.; Beraza, N. Revisiting the Role of Natural Killer Cells in Non-Alcoholic Fatty Liver Disease. Front. Immunol. 2021, 12, 322. [Google Scholar] [CrossRef]
- Michelet, X.; Dyck, L.; Hogan, A.; Loftus, R.M.; Duquette, D.; Wei, K.; Beyaz, S.; Tavakkoli, A.; Foley, C.; Donnelly, R.; et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 2018, 19, 1330–1340. [Google Scholar] [CrossRef]
- Cuff, A.O.; Sillito, F.; Dertschnig, S.; Hall, A.; Luong, T.V.; Chakraverty, R.; Male, V. The Obese Liver Environment Mediates Conversion of NK Cells to a Less Cytotoxic ILC1-Like Phenotype. Front. Immunol. 2019, 10, 2180. [Google Scholar] [CrossRef] [Green Version]
- Luci, C.; Vieira, E.; Perchet, T.; Gual, P.; Golub, R. Natural Killer Cells and Type 1 Innate Lymphoid Cells Are New Actors in Non-alcoholic Fatty Liver Disease. Front. Immunol. 2019, 10, 1192. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Citro, V.; Balsano, C.; Capone, D. Age and Interleukin-15 Levels Are Independently Associated with Intima-Media Thickness in Obesity-Related NAFLD Patients. Front. Med. 2021, 8, 634962. [Google Scholar] [CrossRef]
- Racanelli, V.; Rehermann, B. The liver as an immunological organ. Hepatology 2006, 43, S54–S62. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Yoshida, O.; Dou, L.; Spadaro, A.V.; Isse, K.; Ross, M.A.; Stolz, D.B.; Kimura, S.; Du, Q.; Demetris, A.J.; et al. IRF-1 Promotes Liver Transplant Ischemia/Reperfusion Injury via Hepatocyte IL-15/IL-15R alpha Production. J. Immunol. 2015, 194, 6045–6056. [Google Scholar] [CrossRef] [Green Version]
- Cepero-Donates, Y.; Rakotoarivelo, V.; Mayhue, M.; Ma, A.; Chen, Y.G.; Ramanathan, S. Homeostasis of IL-15 dependent lymphocyte subsets in the liver. Cytokine 2016, 82, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Zahran, W.E.; El-Dien, K.A.S.; Kamel, P.G.; El-Sawaby, A.S. Efficacy of Tumor Necrosis Factor and Interleukin-10 Analysis in the Follow-up of Nonalcoholic Fatty Liver Disease Progression. Indian J. Clin. Biochem. 2013, 28, 141–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velho, S.; Paccaud, F.; Waeber, G.; Vollenweider, P.; Marques-Vidal, P. Metabolically healthy obesity: Different prevalences using different criteria. Eur. J. Clin. Nutr. 2010, 64, 1043–1051. [Google Scholar] [CrossRef]
- Smith, G.I.; Mittendorfer, B.; Klein, S. Metabolically healthy obesity: Facts and fantasies. J. Clin. Investig. 2019, 129, 3978–3989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, S.; Teixeira, L.; Aguilar, E.; Oliveira, M.; Savassi-Rocha, A.; Pelaez, J.N.; Capettini, L.; Diniz, M.T.; Ferreira, A.; Alvarez-Leite, J. Modulation of adipose tissue inflammation by FOXP3+ Treg cells, IL-10, and TGF-beta in metabolically healthy class III obese individuals. Nutrition 2014, 30, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Proto, J.D.; Doran, A.C.; Gusarova, G.; Yurdagul, A.; Sozen, E.; Subramanian, M.; Islam, M.N.; Rymond, C.C.; Du, J.; Hook, J.; et al. Regulatory T Cells Promote Macrophage Efferocytosis during Inflammation Resolution. Immunity 2018, 49, 666–667.e6. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Schlegel, M.P.; Afonso, M.S.; Brown, E.J.; Rahman, K.; Weinstock, A.; Sansbury, B.E.; Corr, E.M.; van Solingen, C.; Koelwyn, G.J.; et al. Regulatory T Cells License Macrophage Pro-Resolving Functions During Atherosclerosis Regression. Circ. Res. 2020, 127, 335–353. [Google Scholar] [CrossRef]
- Beppu, L.Y.; Mooli, R.G.R.; Qu, X.Y.; Marrero, G.J.; Finley, C.A.; Fooks, A.N.; Mullen, Z.P.; Frias, A.B.; Sipula, I.; Xie, B.X.; et al. Tregs facilitate obesity and insulin resistance via a Blimp-1/IL-10 axis. JCI Insight 2021, 6, e140644. [Google Scholar] [CrossRef] [PubMed]
- Brovkina, O.; Nikitin, A.; Khodyrev, D.; Shestakova, E.; Sklyanik, I.; Panevina, A.; Stafeev, I.; Menshikov, M.; Kobelyatskaya, A.; Yurasov, A.; et al. Role of MicroRNAs in the Regulation of Subcutaneous White Adipose Tissue in Individuals with Obesity and without Type 2 Diabetes. Front. Endocrinol. 2019, 10, 840. [Google Scholar] [CrossRef]
- Zou, M.L.; Chen, Z.H.; Teng, Y.Y.; Liu, S.Y.; Jia, Y.; Zhang, K.W.; Sun, Z.L.; Wu, J.J.; Yuan, Z.D.; Feng, Y.; et al. The Smad Dependent TGF-beta and BMP Signaling Pathway in Bone Remodeling and Therapies. Front. Mol. Biosci. 2021, 8, 389. [Google Scholar] [CrossRef]
- Menke, A.; Casagrande, S.; Geiss, L.; Cowie, C.C. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA 2015, 314, 1021–1029. [Google Scholar] [CrossRef] [Green Version]
- Takeno, K.; Tamura, Y.; Kawaguchi, M.; Kakehi, S.; Watanabe, T.; Funayama, T.; Furukawa, Y.; Kaga, H.; Yamamoto, R.; Kim, M.; et al. Relation Between Insulin Sensitivity and Metabolic Abnormalities in Japanese Men with BMI of 23-25 kg/m2. J. Clin. Endocrinol. Metab. 2016, 101, 3676–3684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadowaki, S.; Tamura, Y.; Someya, Y.; Takeno, K.; Kaga, H.; Sugimoto, D.; Kakehi, S.; Funayama, T.; Furukawa, Y.; Suzuki, R.; et al. Fatty Liver Has Stronger Association with Insulin Resistance Than Visceral Fat Accumulation in Nonobese Japanese Men. J. Endocr. Soc. 2019, 3, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.L.; Curb, J.D.; Davis, J.; Shintani, T.; Perez, M.H.; Apau-Ludlum, N.; Johnson, C.; Harrigan, R. Use of the Dietary Supplement 5-Aminiolevulinic Acid (5-ALA) and Its Relationship with Glucose Levels and Hemoglobin A1C among Individuals with Prediabetes. Clin. Transl. Sci. 2012, 5, 314–320. [Google Scholar] [CrossRef] [Green Version]
- Higashikawa, F.; Noda, M.; Awaya, T.; Tanaka, T.; Sugiyama, M. 5-aminolevulinic acid, a precursor of heme, reduces both fasting and postprandial glucose levels in mildly hyperglycemic subjects. Nutrition 2013, 29, 1030–1036. [Google Scholar] [CrossRef]
- Saitoh, S.; Okano, S.; Nohara, H.; Nakano, H.; Shirasawa, N.; Naito, A.; Yamamoto, M.; Kelly, V.P.; Takahashi, K.; Tanaka, T.; et al. 5-aminolevulinic acid (ALA) deficiency causes impaired glucose tolerance and insulin resistance coincident with an attenuation of mitochondrial function in aged mice. PLoS ONE 2018, 13, e0189593. [Google Scholar] [CrossRef] [Green Version]
- Van Wijk, K.; Akabane, T.; Kimura, T.; Saitoh, S.; Okano, S.; Kelly, V.P.; Takagi, M.; Kodama, K.; Takahashi, K.; Tanaka, T.; et al. Heterozygous disruption of ALAS1 in mice causes an accelerated age-dependent reduction in free heme, but not total heme, in skeletal muscle and liver. Arch. Biochem. Biophys. 2021, 697, 108721. [Google Scholar] [CrossRef] [PubMed]
- Okano, S.; Zhou, L.Y.; Kusaka, T.; Shibata, K.; Shimizu, K.; Gao, X.; Kikuchi, Y.; Togashi, Y.; Hosoya, T.; Takahashi, S.; et al. Indispensable function for embryogenesis, expression and regulation of the nonspecific form of the 5-aminolevulinate synthase gene in mouse. Genes Cells 2010, 15, 77–89. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Hiraiwa, Y.; Hagiya, Y.; Nakajima, M.; Tanaka, T.; Ogura, S. 5-Aminolevulinic acid regulates the immune response in LPS-stimulated RAW 264.7 macrophages. BMC Immunol. 2018, 19, 41. [Google Scholar] [CrossRef]
- Fujino, M.; Nishio, Y.; Ito, H.; Tanaka, T.; Li, X.K. 5-Aminolevulinic acid regulates the inflammatory response and alloimmune reaction. Int. Immunopharmacol. 2016, 37, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, M.; Kamimura, D.; Hirano, T. Pleiotropy and Specificity: Insights from the Interleukin 6 Family of Cytokines. Immunity 2019, 50, 812–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ettinger, M.P.; Littlejohn, T.W.; Schwartz, S.L.; Weiss, S.R.; McIlwain, H.H.; Heymsfield, S.B.; Bray, G.A.; Roberts, W.G.; Heyman, E.R.; Stambler, N.; et al. Recombinant variant of ciliary neurotrophic factor for weight loss in obese adults—A randomized, dose-ranging study. JAMA 2003, 289, 1826–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.J.; Turpin-Nolan, S.; Febbraio, M.A. IL-6 family cytokines as potential therapeutic strategies to treat metabolic diseases. Cytokine 2021, 144, 155549. [Google Scholar] [CrossRef] [PubMed]
- Kraakman, M.J.; Kammoun, H.L.; Allen, T.L.; Deswaerte, V.; Henstridge, D.C.; Estevez, E.; Matthews, V.B.; Neill, B.; White, D.A.; Murphy, A.J.; et al. Blocking IL-6 trans-Signaling Prevents High-Fat Diet-Induced Adipose Tissue Macrophage Recruitment but Does Not Improve Insulin Resistance. Cell Metab. 2015, 21, 403–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Findeisen, M.; Allen, T.L.; Henstridge, D.C.; Kammoun, H.; Brandon, A.E.; Baggio, L.L.; Watt, K.I.; Pal, M.; Cron, L.; Estevez, E.; et al. Treatment of type 2 diabetes with the designer cytokine IC7Fc. Nature 2019, 574, 63–68. [Google Scholar] [CrossRef]
- Lizcano, F. The Beige Adipocyte as a Therapy for Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 5058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurylowicz, A.; Puzianowska-Kuznicka, M. Induction of Adipose Tissue Browning as a Strategy to Combat Obesity. Int. J. Mol. Sci. 2020, 21, 6241. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saitoh, S.; Van Wijk, K.; Nakajima, O. Crosstalk between Metabolic Disorders and Immune Cells. Int. J. Mol. Sci. 2021, 22, 10017. https://doi.org/10.3390/ijms221810017
Saitoh S, Van Wijk K, Nakajima O. Crosstalk between Metabolic Disorders and Immune Cells. International Journal of Molecular Sciences. 2021; 22(18):10017. https://doi.org/10.3390/ijms221810017
Chicago/Turabian StyleSaitoh, Shinichi, Koen Van Wijk, and Osamu Nakajima. 2021. "Crosstalk between Metabolic Disorders and Immune Cells" International Journal of Molecular Sciences 22, no. 18: 10017. https://doi.org/10.3390/ijms221810017
APA StyleSaitoh, S., Van Wijk, K., & Nakajima, O. (2021). Crosstalk between Metabolic Disorders and Immune Cells. International Journal of Molecular Sciences, 22(18), 10017. https://doi.org/10.3390/ijms221810017





