Increased Autotaxin Levels in Severe COVID-19, Correlating with IL-6 Levels, Endothelial Dysfunction Biomarkers, and Impaired Functions of Dendritic Cells
Abstract
:1. Introduction
2. Results
2.1. Increased ENPP2 mRNA Levels in Nasopharyngeal Swab Samples from COVID-19 Patients
2.2. Increased Serum ATX Protein Levels in Severe COVID-19 Patients
2.3. Dex Therapeutic Effects in COVID-19 Include the Suppression of ATX Serum Levels
2.4. The ENPP2 Expression Landscape in COVID-19
2.5. A Role for ATX in the Homeostasis of Dendritic Cells?
3. Discussion
4. Materials and Methods
4.1. Human Patients and Samples
4.2. Enzyme-Linked Immunosorbent Assay (ELISA)
4.3. RNA Extraction and Q-RT-PCR
4.4. Bulk/Single Cell RNA-seq Data Analysis and Mining
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020, 19, 141–154. [Google Scholar] [CrossRef]
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 puzzle: Deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 2021, 9, 622–642. [Google Scholar] [CrossRef]
- Huertas, A.; Montani, D.; Savale, L.; Pichon, J.; Tu, L.; Parent, F.; Guignabert, C.; Humbert, M. Endothelial cell dysfunction: A major player in SARS-CoV-2 infection (COVID-19)? Eur. Respir. J. 2020, 56, 2001634. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers 2019, 5, 18. [Google Scholar] [CrossRef]
- Burnham, E.L.; Janssen, W.J.; Riches, D.W.; Moss, M.; Downey, G.P. The fibroproliferative response in acute respiratory distress syndrome: Mechanisms and clinical significance. Eur. Respir. J. 2014, 43, 276–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiese, A.; Manetti, A.C.; La Russa, R.; Di Paolo, M.; Turillazzi, E.; Frati, P.; Fineschi, V. Autopsy findings in COVID-19-related deaths: A literature review. Forensic. Sci. Med. Pathol. 2020, 17, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Jian, W.; Su, Z.; Chen, M.; Peng, H.; Peng, P.; Lei, C.; Chen, R.; Zhong, N.; Li, S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020, 55, 2001217. [Google Scholar] [CrossRef]
- Frija-Masson, J.; Debray, M.P.; Gilbert, M.; Lescure, F.X.; Travert, F.; Borie, R.; Khalil, A.; Crestani, B.; d’Ortho, M.P.; Bancal, C. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur. Respir. J. 2020, 56, 81. [Google Scholar] [CrossRef] [PubMed]
- Gassel, R.J.J.; Bels, J.L.M.; Raafs, A.; Bussel, B.C.T.; Poll, M.C.G.; Simons, S.O.; Meer, L.W.L.; Gietema, H.A.; Posthuma, R.; Santen, S. High Prevalence of Pulmonary Sequelae at 3 Months after Hospital Discharge in Mechanically Ventilated Survivors of COVID-19. Am. J. Respir. Crit. Care Med. 2021, 203, 371–374. [Google Scholar] [CrossRef]
- George, P.M.; Barratt, S.L.; Condliffe, R.; Desai, S.R.; Devaraj, A.; Forrest, I.; Gibbons, M.A.; Hart, N.; Jenkins, R.G.; McAuley, D.F.; et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax 2020, 75, 1009–1016. [Google Scholar] [CrossRef]
- Myall, K.J.; Mukherjee, B.; Castanheira, A.M.; Lam, J.L.; Benedetti, G.; Mak, S.M.; Preston, R.; Thillai, M.; Dewar, A.; Molyneaux, P.L.; et al. Persistent Post-COVID-19 Interstitial Lung Disease. An Observational Study of Corticosteroid Treatment. Ann. Am. Thorac. Soc. 2021, 18, 799–806. [Google Scholar] [CrossRef]
- Bharat, A.; Querrey, M.; Markov, N.S.; Kim, S.; Kurihara, C.; Garza-Castillon, R.; Manerikar, A.; Shilatifard, A.; Tomic, R.; Politanska, Y.; et al. Lung transplantation for patients with severe COVID-19. Sci. Transl. Med. 2020, 12, eabe4282. [Google Scholar] [CrossRef]
- Wu, M.; Chen, Y.; Xia, H.; Wang, C.; Tan, C.Y.; Cai, X.; Liu, Y.; Ji, F.; Xiong, P.; Liu, R.; et al. Transcriptional and proteomic insights into the host response in fatal COVID-19 cases. Proc. Natl. Acad. Sci. USA 2020, 117, 28336–28343. [Google Scholar] [CrossRef]
- McDonald, L.T. Healing after COVID-19: Are survivors at risk for pulmonary fibrosis? Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L257–l265. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.J.; Menon, A.A.; Ghosh, A.J.; Putman, R.K.; Fredenburgh, L.E.; El-Chemaly, S.Y.; Goldberg, H.J.; Baron, R.M.; Hunninghake, G.M.; Doyle, T.J. Increased Odds of Death for Patients with Interstitial Lung Disease and COVID-19: A Case-Control Study. Am. J. Respir. Crit. Care Med. 2020, 202, 1710–1713. [Google Scholar] [CrossRef]
- Drake, T.M.; Docherty, A.B.; Harrison, E.M.; Quint, J.K.; Adamali, H.; Agnew, S.; Babu, S.; Barber, C.M.; Barratt, S.; Bendstrup, E.; et al. Outcome of Hospitalization for COVID-19 in Patients with Interstitial Lung Disease. An International Multicenter Study. Am. J. Respir. Crit. Care Med. 2020, 202, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- Beltramo, G.; Cottenet, J.; Mariet, A.S.; Georges, M.; Piroth, L.; Tubert-Bitter, P.; Bonniaud, P.; Quantin, C. Chronic respiratory diseases are predictors of severe outcome in COVID-19 hospitalised patients: A nationwide study. Eur. Respir. J. 2021, 3, 74. [Google Scholar] [CrossRef]
- Magkrioti, C.; Galaris, A.; Kanellopoulou, P.; Stylianaki, E.A.; Kaffe, E.; Aidinis, V. Autotaxin and chronic inflammatory diseases. J. Autoimmun. 2019, 104, 102327. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, N.; Mouratis, M.A.; Tzouvelekis, A.; Kaffe, E.; Valavanis, C.; Vilaras, G.; Karameris, A.; Prestwich, G.D.; Bouros, D.; Aidinis, V. Pulmonary autotaxin expression contributes to the pathogenesis of pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2012, 47, 566–574. [Google Scholar] [CrossRef]
- Tager, A.M.; LaCamera, P.; Shea, B.S.; Campanella, G.S.; Selman, M.; Zhao, Z.; Polosukhin, V.; Wain, J.; Karimi-Shah, B.A.; Kim, N.D.; et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat. Med. 2008, 14, 45–54. [Google Scholar] [CrossRef]
- Ninou, I.; Magkrioti, C.; Aidinis, V. Autotaxin in Pathophysiology and Pulmonary Fibrosis. Front. Med. 2018, 5, 180. [Google Scholar] [CrossRef]
- Ninou, I.; Kaffe, E.; Muller, S.; Budd, D.C.; Stevenson, C.S.; Ullmer, C.; Aidinis, V. Pharmacologic targeting of the ATX/LPA axis attenuates bleomycin-induced pulmonary fibrosis. Pulm. Pharmacol. Ther. 2018, 52, 32–40. [Google Scholar] [CrossRef]
- Tager, A.M. Autotaxin emerges as a therapeutic target for idiopathic pulmonary fibrosis: Limiting fibrosis by limiting lysophosphatidic acid synthesis. Am. J. Respir. Cell Mol. Biol. 2012, 47, 563–565. [Google Scholar] [CrossRef] [Green Version]
- Maher, T.M.; Kreuter, M.; Lederer, D.J.; Brown, K.K.; Wuyts, W.; Verbruggen, N.; Stutvoet, S.; Fieuw, A.; Ford, P.; Abi-Saab, W.; et al. Rationale, design and objectives of two phase III, randomised, placebo-controlled studies of GLPG1690, a novel autotaxin inhibitor, in idiopathic pulmonary fibrosis (ISABELA 1 and 2). BMJ Open Respir. Res. 2019, 6, e000422. [Google Scholar] [CrossRef]
- Magkrioti, C.; Aidinis, V. ATX and LPA signalling in lung pathophysiology. World J. Respirol. 2013, 3, 77–103. [Google Scholar] [CrossRef]
- Kaffe, E.; Katsifa, A.; Xylourgidis, N.; Ninou, I.; Zannikou, M.; Harokopos, V.; Foka, P.; Dimitriadis, A.; Evangelou, K.; Moulas, A.N.; et al. Hepatocyte autotaxin expression promotes liver fibrosis and cancer. Hepatology 2017, 65, 1369–1383. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, M.J.; Humphreys, I.S.; Rudge, S.A.; Wilson, G.K.; Bhattacharya, B.; Ciaccia, M.; Hu, K.; Zhang, Q.; Mailly, L.; Reynolds, G.M.; et al. Autotaxin-lysophosphatidic acid receptor signalling regulates hepatitis C virus replication. J. Hepatol. 2017, 9, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.-L.; Hou, Y.-L.; Li, D.-T.; Li, F.-Z. Laboratory findings of COVID-19: A systematic review and meta-analysis. Scand. J. Clin. Lab. Investig. 2020, 80, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Ulhaq, Z.S.; Soraya, G.V. Interleukin-6 as a potential biomarker of COVID-19 progression. Méd. Mal. Infect. 2020, 50, 382–383. [Google Scholar] [CrossRef]
- Sun, S.; Wang, R.; Song, J.; Guan, M.; Li, N.; Zhang, X.; Zhao, Z.; Zhang, J. Blocking gp130 signaling suppresses autotaxin expression in adipocytes and improves insulin sensitivity in diet-induced obesity. J. Lipid Res. 2017, 58, 2102–2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castelino, F.V.; Bain, G.; Pace, V.A.; Black, K.E.; George, L.; Probst, C.K.; Goulet, L.; Lafyatis, R.; Tager, A.M. An Autotaxin/Lysophosphatidic Acid/Interleukin-6 Amplification Loop Drives Scleroderma Fibrosis. Arthritis Rheumatol. 2016, 68, 2964–2974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassiliou, A.G.; Keskinidou, C.; Jahaj, E.; Gallos, P.; Dimopoulou, I.; Kotanidou, A.; Orfanos, S.E. ICU Admission Levels of Endothelial Biomarkers as Predictors of Mortality in Critically Ill COVID-19 Patients. Cells 2021, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; Cavalcanti, A.B.; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [PubMed]
- Ota, M.; Nagafuchi, Y.; Hatano, H.; Ishigaki, K.; Terao, C.; Takeshima, Y.; Yanaoka, H.; Kobayashi, S.; Okubo, M.; Shirai, H.; et al. Dynamic landscape of immune cell-specific gene regulation in immune-mediated diseases. Cell 2021, 184, 3006–3021.e17. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.C.; Stoddard, N.C.; Chun, J. LPA receptor signaling: Pharmacology, physiology, and pathophysiology. J. Lipid Res. 2014, 55, 1192–1214. [Google Scholar] [CrossRef] [Green Version]
- Emo, J.; Meednu, N.; Chapman, T.J.; Rezaee, F.; Balys, M.; Randall, T.; Rangasamy, T.; Georas, S.N. Lpa2 Is a Negative Regulator of Both Dendritic Cell Activation and Murine Models of Allergic Lung Inflammation. J. Immunol. 2012, 188, 3784–3790. [Google Scholar] [CrossRef] [PubMed]
- Tazi, A.; Bouchonnet, F.; Grandsaigne, M.; Boumsell, L.; Hance, A.J.; Soler, P. Evidence that granulocyte macrophage-colony-stimulating factor regulates the distribution and differentiated state of dendritic cells/Langerhans cells in human lung and lung cancers. J. Clin. Investig. 1993, 91, 566–576. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Castaño, A.R.; Segelke, B.W.; Stura, E.A.; Peterson, P.A.; Wilson, I.A. Crystal structure of mouse CD1: An MHC-like fold with a large hydrophobic binding groove. Science 1997, 277, 339–345. [Google Scholar] [CrossRef]
- Bourgeois, E.A.; Subramaniam, S.; Cheng, T.Y.; De Jong, A.; Layre, E.; Ly, D.; Salimi, M.; Legaspi, A.; Modlin, R.L.; Salio, M.; et al. Bee venom processes human skin lipids for presentation by CD1a. J. Exp. Med. 2015, 212, 149–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condamine, T.; Le Texier, L.; Howie, D.; Lavault, A.; Hill, M.; Halary, F.; Cobbold, S.; Waldmann, H.; Cuturi, M.C.; Chiffoleau, E. Tmem176B and Tmem176A are associated with the immature state of dendritic cells. J. Leukoc. Biol. 2010, 88, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Picotto, G.; Morse, L.R.; Nguyen, N.; Saltzman, J.; Battaglino, R. TMEM176A and TMEM176B Are Candidate Regulators of Inhibition of Dendritic Cell Maturation and Function after Chronic Spinal Cord Injury. J. Neurotrauma 2020, 37, 528–533. [Google Scholar] [CrossRef]
- Kostadinova, L.; Shive, C.L.; Anthony, D.D. Elevated Autotaxin and LPA Levels During Chronic Viral Hepatitis and Hepatocellular Carcinoma Associate with Systemic Immune Activation. Cancers 2019, 11, 1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshita, S.; Ichikawa, Y.; Umemura, T.; Usami, Y.; Sugiura, A.; Shibata, S.; Yamazaki, T.; Fujimori, N.; Komatsu, M.; Matsumoto, A.; et al. Serum autotaxin is a useful liver fibrosis marker in patients with chronic hepatitis B virus infection. Hepatol. Res. 2018, 48, 275–285. [Google Scholar] [CrossRef]
- Barbayianni, E.; Kaffe, E.; Aidinis, V.; Kokotos, G. Autotaxin, a secreted lysophospholipase D, as a promising therapeutic target in chronic inflammation and cancer. Prog. Lipid Res. 2015, 58, 76–96. [Google Scholar] [CrossRef]
- Gao, L.; Li, X.; Wang, H.; Liao, Y.; Zhou, Y.; Wang, K.; Hu, J.; Cheng, M.; Zeng, Z.; Wang, T.; et al. Autotaxin levels in serum and bronchoalveolar lavage fluid are associated with inflammatory and fibrotic biomarkers and the clinical outcome in patients with acute respiratory distress syndrome. J. Intensive Care 2021, 9, 44. [Google Scholar] [CrossRef]
- Casari, I.; Manfredi, M.; Metharom, P.; Falasca, M. Dissecting lipid metabolism alterations in SARS-CoV-2. Prog. Lipid Res. 2021, 82, 101092. [Google Scholar] [CrossRef]
- Smyth, S.S.; Kraemer, M.; Yang, L.; Van Hoose, P.; Morris, A.J. Roles for lysophosphatidic acid signaling in vascular development and disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158734. [Google Scholar] [CrossRef]
- Dusaulcy, R.; Rancoule, C.; Gres, S.; Wanecq, E.; Colom, A.; Guigne, C.; van Meeteren, L.A.; Moolenaar, W.H.; Valet, P.; Saulnier-Blache, J.S. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J. Lipid Res. 2011, 52, 1247–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandon, J.A.; Kraemer, M.; Vandra, J.; Halder, S.; Ubele, M.; Morris, A.J.; Smyth, S.S. Adipose-derived autotaxin regulates inflammation and steatosis associated with diet-induced obesity. PLoS ONE 2019, 14, e0208099. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, K.; Nzirorera, C.; Cowie, A.M.; Varghese, G.P.; Trivedi, P.; Eichmann, T.O.; Biswas, D.; Touaibia, M.; Morris, A.J.; Aidinis, V.; et al. Autotaxin-LPA signaling contributes to obesity-induced insulin resistance in muscle and impairs mitochondrial metabolism. J. Lipid Res. 2018, 59, 1805–1817. [Google Scholar] [CrossRef] [Green Version]
- Reeves, V.L.; Trybula, J.S.; Wills, R.C.; Goodpaster, B.H.; Dube, J.J.; Kienesberger, P.C.; Kershaw, E.E. Serum Autotaxin/ENPP2 correlates with insulin resistance in older humans with obesity. Obesity 2015, 23, 2371–2376. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, K.; Paramel, G.V.; Kienesberger, P.C. Lysophosphatidic Acid Signaling in Obesity and Insulin Resistance. Nutrients 2018, 10, 399. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Pan, Y.; Yin, Y.; Chen, W.; Li, X. Association of dyslipidemia with the severity and mortality of coronavirus disease 2019 (COVID-19): A meta-analysis. Virol. J. 2021, 18, 157. [Google Scholar] [CrossRef]
- Adam, S.; Ho, J.H.; Bashir, B.; Iqbal, Z.; Ferdousi, M.; Syed, A.A.; Soran, H. The impact of atherosclerotic cardiovascular disease, dyslipidaemia and lipid lowering therapy on Coronavirus disease 2019 outcomes: An examination of the available evidence. Curr. Opin. Lipidol. 2021, 32, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Vitiello, A. The advantages of drug treatment with statins in patients with SARS-CoV-2 infection. Wien. Klin. Wochenschr. 2021, 16, 1–8. [Google Scholar] [CrossRef]
- Subir, R.; Jagat, J.M.; Kalyan, K.G. Pros and cons for use of statins in people with coronavirus disease-19 (COVID-19). Diabetes Metab. Syndr. 2020, 14, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Mitacchione, G.; Schiavone, M.; Curnis, A.; Arca, M.; Antinori, S.; Gasperetti, A.; Mascioli, G.; Severino, P.; Sabato, F.; Caracciolo, M.M.; et al. Impact of prior statin use on clinical outcomes in COVID-19 patients: Data from tertiary referral hospitals during COVID-19 pandemic in Italy. J. Clin. Lipidol. 2021, 15, 68–78. [Google Scholar] [CrossRef]
- Daniels, L.B.; Sitapati, A.M.; Zhang, J.; Zou, J.; Bui, Q.M.; Ren, J.; Longhurst, C.A.; Criqui, M.H.; Messer, K. Relation of Statin Use Prior to Admission to Severity and Recovery Among COVID-19 Inpatients. Am. J. Cardiol. 2020, 136, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Kusama, T.; Mukai, M.; Ayaki, M.; Imamura, F.; Tatsuta, M.; Matsumoto, Y.; Nakamura, H.; Inoue, M. Inhibition of lysophosphatidic acid-induced RhoA activation and tumor cell invasion by 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors. Int. J. Oncol. 2003, 23, 1173–1178. [Google Scholar] [CrossRef]
- Kaneyuki, U.; Ueda, S.; Yamagishi, S.; Kato, S.; Fujimura, T.; Shibata, R.; Hayashida, A.; Yoshimura, J.; Kojiro, M.; Oshima, K.; et al. Pitavastatin inhibits lysophosphatidic acid-induced proliferation and monocyte chemoattractant protein-1 expression in aortic smooth muscle cells by suppressing Rac-1-mediated reactive oxygen species generation. Vasc. Pharmacol. 2007, 46, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Marjot, T.; Webb, G.J.; Barritt, A.S.; Moon, A.M.; Stamataki, Z.; Wong, V.W.; Barnes, E. COVID-19 and liver disease: Mechanistic and clinical perspectives. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 348–364. [Google Scholar] [CrossRef]
- Trovato, F.M.; Zia, R.; Napoli, S.; Wolfer, K.; Huang, X.; Morgan, P.E.; Husbyn, H.; Elgosbi, M.; Lucangeli, M.; Miquel, R.; et al. Dysregulation of the LPC-ATX-LPA axis in ACLF is associated with mortality and systemic inflammation via LPA-dependent monocyte activation. Hepatology 2021, 3, 22. [Google Scholar] [CrossRef]
- Guirao, J.J.; Cabrera, C.M.; Jiménez, N.; Rincón, L.; Urra, J.M. High serum IL-6 values increase the risk of mortality and the severity of pneumonia in patients diagnosed with COVID-19. Mol. Immunol. 2020, 128, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Herold, T.; Jurinovic, V.; Arnreich, C.; Lipworth, B.J.; Hellmuth, J.C.; von Bergwelt-Baildon, M.; Klein, M.; Weinberger, T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020, 146, 128–136.e4. [Google Scholar] [CrossRef]
- Meng, G.; Tang, X.; Yang, Z.; Zhao, Y.; Curtis, J.M.; McMullen, T.P.W.; Brindley, D.N. Dexamethasone decreases the autotaxin-lysophosphatidate-inflammatory axis in adipose tissue: Implications for the metabolic syndrome and breast cancer. FASEB J. 2018, 13, 1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, G.; Wuest, M.; Tang, X.; Dufour, J.; McMullen, T.P.W.; Wuest, F.; Murray, D.; Brindley, D.N. Dexamethasone Attenuates X-Ray-Induced Activation of the Autotaxin-Lysophosphatidate-Inflammatory Cycle in Breast Tissue and Subsequent Breast Fibrosis. Cancers 2020, 12, 999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Okudaira, S.; Kishi, Y.; Ohkawa, R.; Iseki, S.; Ota, M.; Noji, S.; Yatomi, Y.; Aoki, J.; Arai, H. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J. Biol. Chem. 2006, 281, 25822–25830. [Google Scholar] [CrossRef] [Green Version]
- van Meeteren, L.A.; Ruurs, P.; Stortelers, C.; Bouwman, P.; van Rooijen, M.A.; Pradere, J.P.; Pettit, T.R.; Wakelam, M.J.; Saulnier-Blache, J.S.; Mummery, C.L.; et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol. Cell. Biol. 2006, 26, 5015–5022. [Google Scholar] [CrossRef] [Green Version]
- Fotopoulou, S.; Oikonomou, N.; Grigorieva, E.; Nikitopoulou, I.; Paparountas, T.; Thanassopoulou, A.; Zhao, Z.; Xu, Y.; Kontoyiannis, D.L.; Remboutsika, E.; et al. ATX expression and LPA signalling are vital for the development of the nervous system. Dev. Biol. 2010, 339, 451–464. [Google Scholar] [CrossRef] [Green Version]
- Yukiura, H.; Hama, K.; Nakanaga, K.; Tanaka, M.; Asaoka, Y.; Okudaira, S.; Arima, N.; Inoue, A.; Hashimoto, T.; Arai, H.; et al. Autotaxin regulates vascular development via multiple lysophosphatidic acid (LPA) receptors in zebrafish. J. Biol. Chem. 2011, 286, 43972–43983. [Google Scholar] [CrossRef] [Green Version]
- Gralinski, L.E.; Menachery, V.D.; Morgan, A.P.; Totura, A.L.; Beall, A.; Kocher, J.; Plante, J.; Harrison-Shostak, D.C.; Schafer, A.; Pardo-Manuel de Villena, F.; et al. Allelic Variation in the Toll-Like Receptor Adaptor Protein Ticam2 Contributes to SARS-Coronavirus Pathogenesis in Mice. G3 Bethesda 2017, 7, 1653–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gralinski, L.E.; Ferris, M.T.; Aylor, D.L.; Whitmore, A.C.; Green, R.; Frieman, M.B.; Deming, D.; Menachery, V.D.; Miller, D.R.; Buus, R.J.; et al. Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross. PLoS Genet. 2015, 11, e1005504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, H.; Newton, R.; Klein, R.; Morita, Y.; Gunn, M.D.; Rosen, S.D. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat. Immunol. 2008, 9, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Takeda, Y.; Matoba, K.; Kawanami, D.; Nagai, Y.; Akamine, T.; Ishizawa, S.; Kanazawa, Y.; Yokota, T.; Utsunomiya, K. ROCK2 Regulates Monocyte Migration and Cell to Cell Adhesion in Vascular Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizza, C.; Leitinger, N.; Yue, J.; Fischer, D.J.; Wang, D.A.; Shih, P.T.; Lee, H.; Tigyi, G.; Berliner, J.A. Lysophosphatidic acid as a regulator of endothelial/leukocyte interaction. Lab. Investig. 1999, 79, 1227–1235. [Google Scholar] [PubMed]
- Shlyonsky, V.; Naeije, R.; Mies, F. Possible role of lysophosphatidic acid in rat model of hypoxic pulmonary vascular remodeling. Pulm. Circ. 2014, 4, 471–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, R.; Rai, V. Lysophosphatidic acid converts monocytes into macrophages in both mice and humans. Blood 2017, 129, 1177–1183. [Google Scholar] [CrossRef] [Green Version]
- Ninou, I.; Sevastou, I.; Magkrioti, C.; Kaffe, E.; Stamatakis, G.; Thivaios, S.; Panayotou, G.; Aoki, J.; Kollias, G.; Aidinis, V. Genetic deletion of Autotaxin from CD11b+ cells decreases the severity of experimental autoimmune encephalomyelitis. PLoS ONE 2020, 15, e0226050. [Google Scholar] [CrossRef] [Green Version]
- Santos-Nogueira, E.; Lopez-Serrano, C.; Hernandez, J.; Lago, N.; Astudillo, A.M.; Balsinde, J.; Estivill-Torrus, G.; de Fonseca, F.R.; Chun, J.; Lopez-Vales, R. Activation of Lysophosphatidic Acid Receptor Type 1 Contributes to Pathophysiology of Spinal Cord Injury. J. Neurosci. 2015, 35, 10224–10235. [Google Scholar] [CrossRef] [Green Version]
- Martino, A.; Volpe, E.; Baldini, P.M. The influence of lysophosphatidic acid on the immunophenotypic differentiation of human monocytes into dendritic cells. Haematologica 2006, 91, 1273–1274. [Google Scholar]
- Chen, R.; Roman, J.; Guo, J.; West, E.; McDyer, J.; Williams, M.A.; Georas, S.N. Lysophosphatidic acid modulates the activation of human monocyte-derived dendritic cells. Stem Cells Dev. 2006, 15, 797–804. [Google Scholar] [CrossRef]
- Siegal, F.P.; Kadowaki, N.; Shodell, M.; Fitzgerald-Bocarsly, P.A.; Shah, K.; Ho, S.; Antonenko, S.; Liu, Y.J. The nature of the principal type 1 interferon-producing cells in human blood. Science 1999, 284, 1835–1837. [Google Scholar] [CrossRef] [Green Version]
- Saichi, M.; Ladjemi, M.Z.; Korniotis, S.; Rousseau, C.; Ait Hamou, Z.; Massenet-Regad, L.; Amblard, E.; Noel, F.; Marie, Y.; Bouteiller, D.; et al. Single-cell RNA sequencing of blood antigen-presenting cells in severe COVID-19 reveals multi-process defects in antiviral immunity. Nat. Cell Biol. 2021, 23, 538–551. [Google Scholar] [CrossRef]
- Zhou, R.; To, K.K.; Wong, Y.C.; Liu, L.; Zhou, B.; Li, X.; Huang, H.; Mo, Y.; Luk, T.Y.; Lau, T.T.; et al. Acute SARS-CoV-2 Infection Impairs Dendritic Cell and T Cell Responses. Immunity 2020, 53, 864–877.e5. [Google Scholar] [CrossRef]
- Sánchez-Cerrillo, I.; Landete, P.; Aldave, B.; Sánchez-Alonso, S.; Sánchez-Azofra, A.; Marcos-Jiménez, A.; Ávalos, E.; Alcaraz-Serna, A.; de Los Santos, I.; Mateu-Albero, T.; et al. COVID-19 severity associates with pulmonary redistribution of CD1c+ DCs and inflammatory transitional and nonclassical monocytes. J. Clin. Investig. 2020, 130, 6290–6300. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Cisse, B.; Caton, M.L.; Lehner, M.; Maeda, T.; Scheu, S.; Locksley, R.; Holmberg, D.; Zweier, C.; den Hollander, N.S.; Kant, S.G.; et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell 2008, 135, 37–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; He, P.; No, Y.R.; Yun, C.C. Krüppel-like factor 5 incorporates into the β-catenin/TCF complex in response to LPA in colon cancer cells. Cell. Signal. 2015, 27, 961–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spagnolo, P.; Balestro, E.; Aliberti, S.; Cocconcelli, E.; Biondini, D.; Casa, G.D.; Sverzellati, N.; Maher, T.M. Pulmonary fibrosis secondary to COVID-19: A call to arms? Lancet Respir. Med. 2020, 8, 750–752. [Google Scholar] [CrossRef]
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020, 6, 3022. [Google Scholar] [CrossRef]
- Vasarmidi, E.; Tsitoura, E.; Spandidos, D.A.; Tzanakis, N.; Antoniou, K.M. Pulmonary fibrosis in the aftermath of the COVID-19 era (Review). Exp. Ther. Med. 2020, 20, 2557–2560. [Google Scholar] [CrossRef]
- Stuart, T.; Butler, A.; Hoffman, P.; Hafemeister, C.; Papalexi, E.; Mauck, W.M., III; Hao, Y.; Stoeckius, M.; Smibert, P.; Satija, R. Comprehensive Integration of Single-Cell Data. Cell 2019, 177, 1888–1902.e21. [Google Scholar] [CrossRef]
- Travaglini, K.J.; Nabhan, A.N.; Penland, L.; Sinha, R.; Gillich, A.; Sit, R.V.; Chang, S.; Conley, S.D.; Mori, Y.; Seita, J.; et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 2020, 587, 619–625. [Google Scholar] [CrossRef]
- Fanidis, D.; Moulos, P. Integrative, normalization-insusceptible statistical analysis of RNA-Seq data, with improved differential expression and unbiased downstream functional analysis. Brief. Bioinform. 2020, 22, 156. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- McCarthy, D.J.; Chen, Y.; Smyth, G.K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012, 40, 4288–4297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Rosenstiel, P.C.; Schulenburg, H. ABSSeq: A new RNA-Seq analysis method based on modelling absolute expression differences. BMC Genom. 2016, 17, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
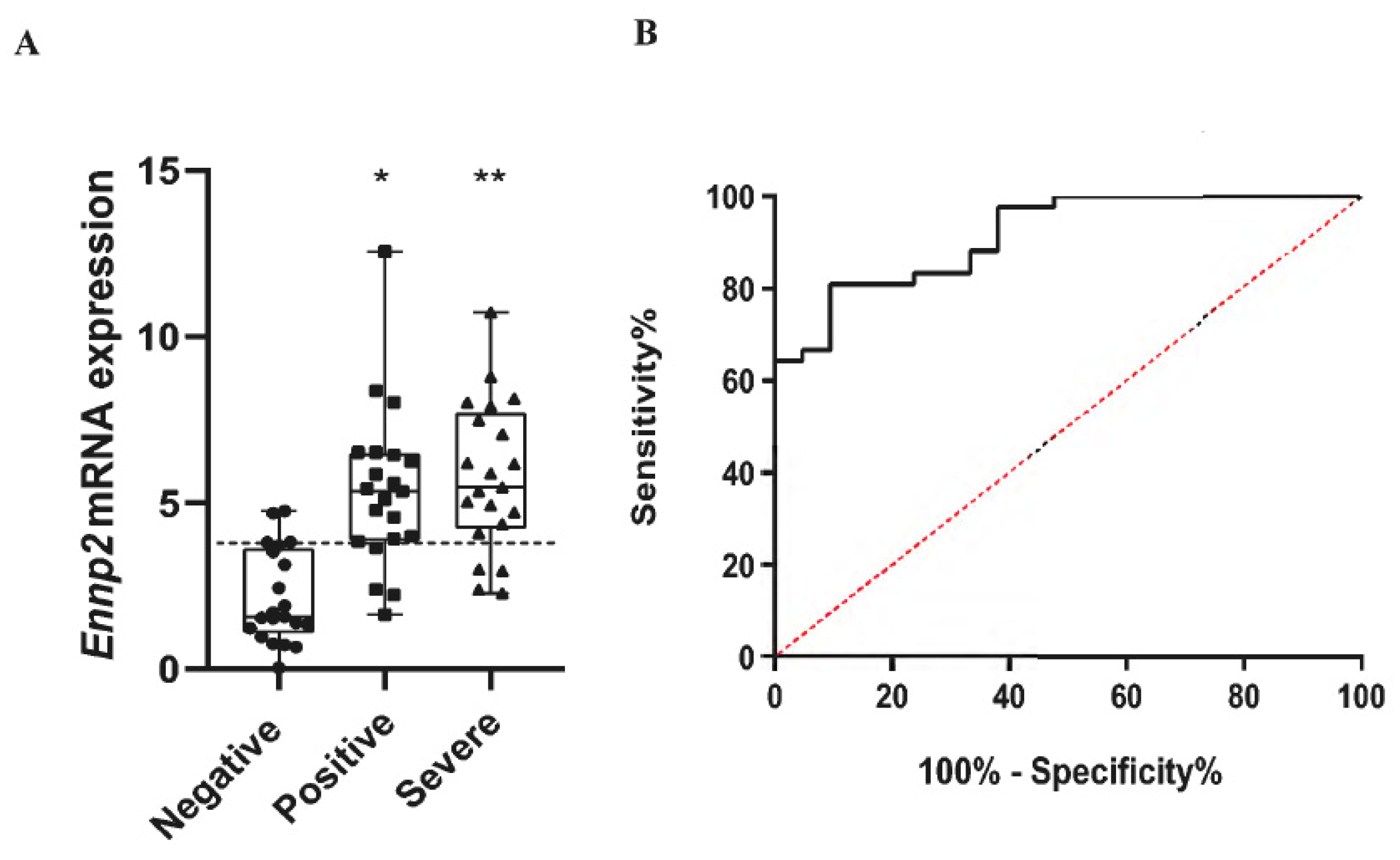


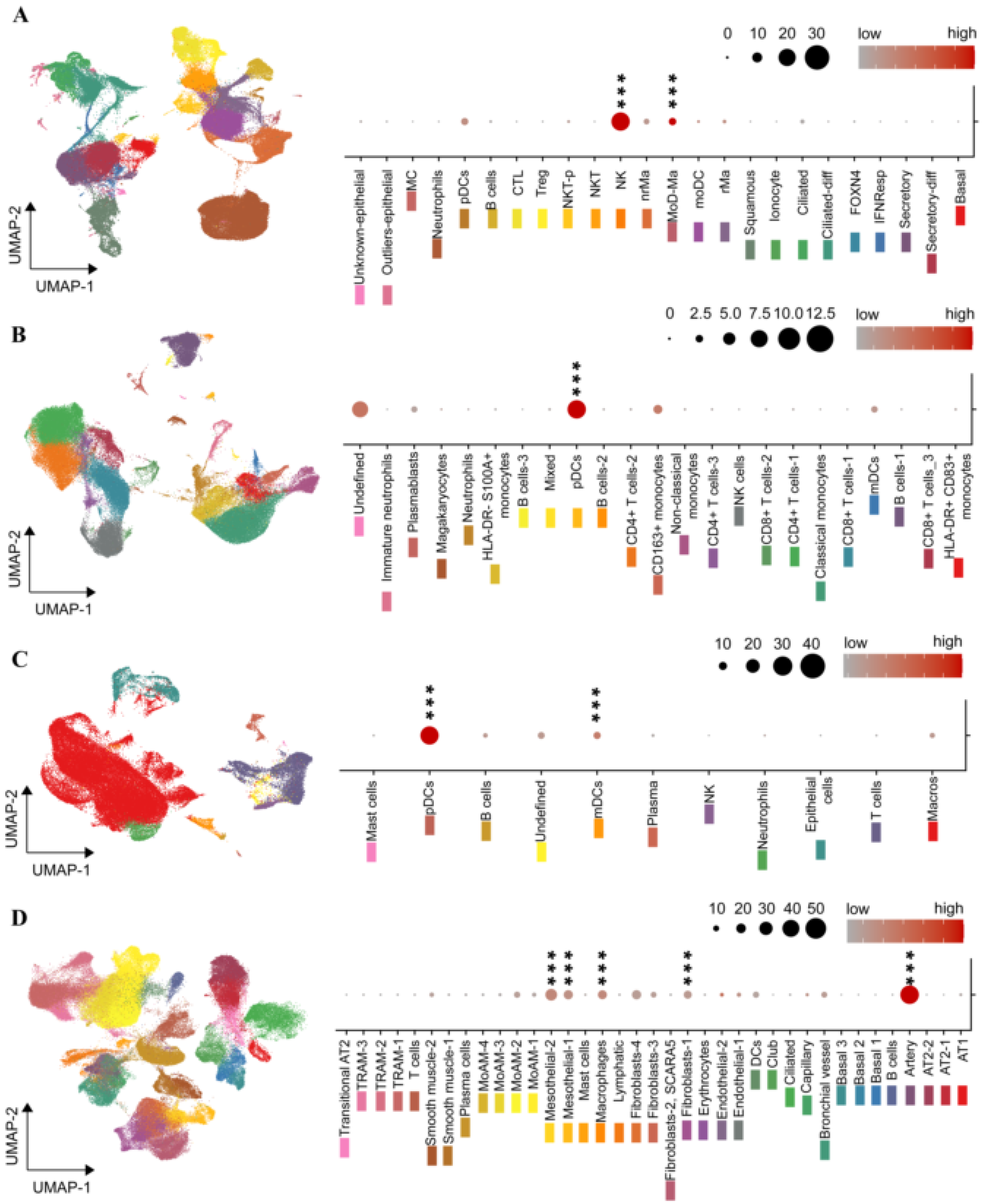
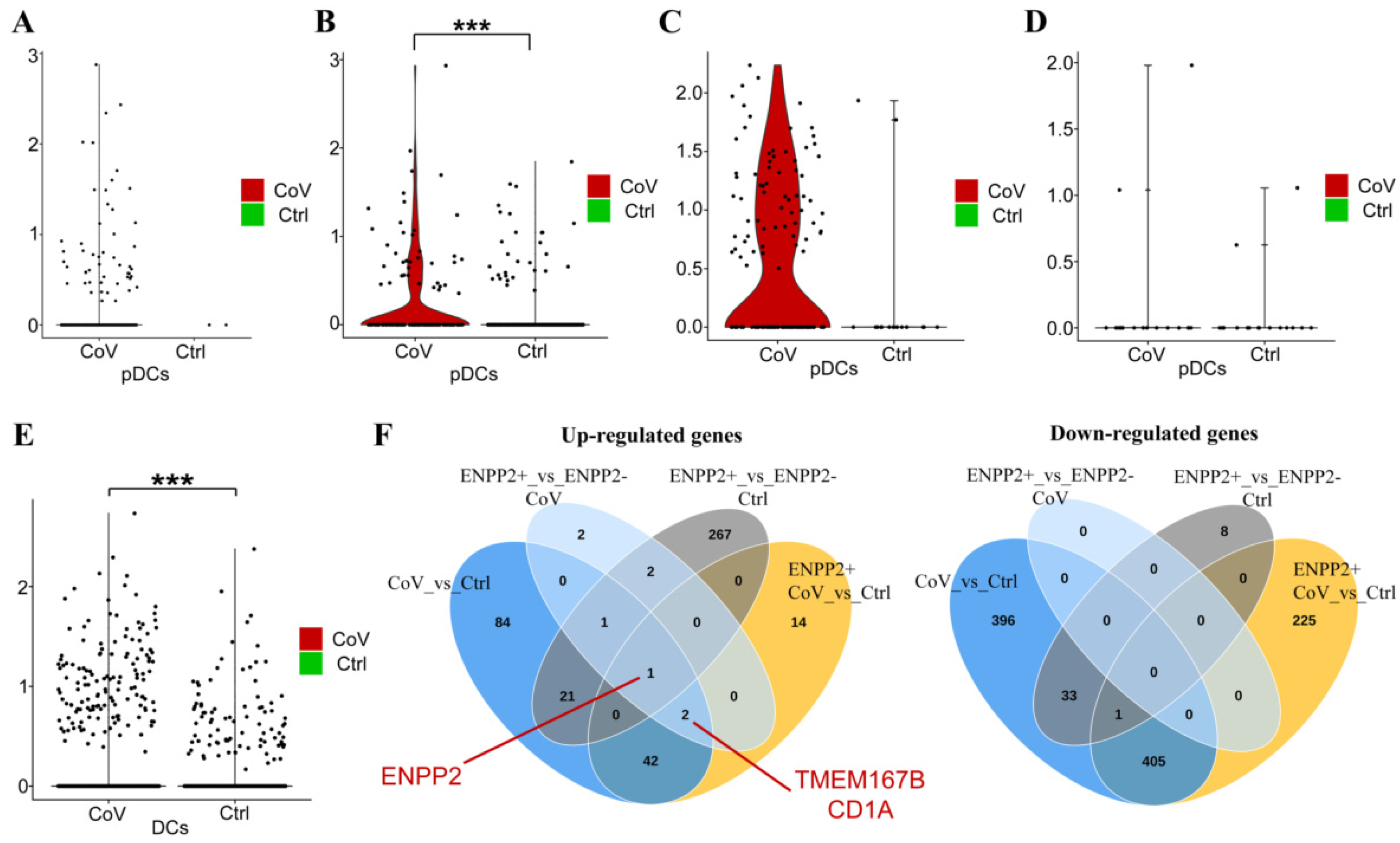
| Negative Healthy | Positive Mild | Positive Severe/Critical | |
|---|---|---|---|
| Number of patients (n) | 21 | 21 | 21 |
| ATX (2-ΔΔCt, mean ± SD) | 2.15 ± 1.37 | 5.38 ± 2.34 **** | 5.76 ± 2.18 **** |
| Sex | |||
| Male Female Not recorded | 8 (38.09%) 13 (61.9%) 0 | 10 (47.6%) 9 (42.8%) 2 (9.52%) | 17 (80.95%) 4 (19.04%) 0 |
| Age (years, mean ± SD) | 50.15 ± 20.86 | 37.78 ± 11.89 | 63.38 ± 17.23 |
| WARD No Dex | ICU No Dex | ICU + Dex | |
|---|---|---|---|
| Number of patients (n) | 47 | 37 | 32 |
| ATX (ng/mL, mean ± SD) | 310.32 ± 98.85 * | 443 ± 172.90 | 246.15 ± 73.74 * |
| Sex | |||
| Male Female | 33 (70.21%) 14 (29.78%) | 31 (83.78%) 6 (16.21%) | 22 (62.5%) 10 (31.25%) |
| Age (years, mean ± SD) | 54.63 ± 15.46 | 63.54 ± 10.89 | 65.5 ± 10.7 |
| Comorbidities n (%) | |||
| Hypertension Diabetes Coronary artery disease COPD Asthma Hyperlipidemia Hepatitis | 13 (27.65%) 4 (8.51%) 8 (17.02%) 1 (2.12%) 2 (4.25%) 9 (19.14%) 0 (0%) | 17 (45.94%) 5 (13.51%) 4 (10.81%) 1 (2.7%) 1 (2.7%) 9 (24.32%) 1 (2.7%) | 12 (37.5%) 5 (15.62%) 4 (12.5%) 2 (6.25%) 1 (3.12%) 8 (25%) 0 (0%) |
| COVID-19 treatment Azithromycin/chloroquine/lopinavir/ritonavir Azithromycin/chloroquine Lopinavir/ritonavir/chloroquine Chloroquine Plasma | 0 6 0 0 0 | 11 7 2 3 1 | |
| Clinical measurements | |||
| Mean arterial pressure (mmHg) | 83.19 ± 8.86 | 82.83 ± 16.52 | 77.55 ± 8.54 |
| PaO2/FiO2 (mmHg) | 301.5 ± 79.81 * | 194.86 ± 86.64 | 85.94 ± 15.97 * |
| Glucose (mg/dL) | 133.5 ± 113.3 | 164.53 ± 77.73 | 164.06 ± 75.40 |
| Creatinine (mg/dL) | 0.9 ± 0.33 | 1.02 ± 0.32 | 0.95 ± 0.72 |
| CRP (mg/dL) | 6.8 ± 8.96 | 14 ± 10.17 | 13.83 ± 9.6 |
| Total bilirubin (mg/dL) | 0.5 ± 0.33 | 0.73 ± 0.5 | 0.61 ± 0.29 |
| White blood cell count (per μL) | 6995 ± 3468 | 10,125 ± 4633 | 11,705 ± 10,372 |
| Neutrophils (%) | 69.34 ± 13.51 | 81.34 ± 6.64 | 83.12 ± 12.2 |
| Lymphocytes (%) | 24.03 ± 10.89 * | 12.63 ± 5.63 | 11.12 ± 11.23 |
| Platelets (per μL) | 240,297 ± 110,028 | 237,783 ± 101,338 | 257,000 ± 79,581 |
| INR (median IQR) | 1.06 ± 0.09 | 2.07 ± 5.73 | 1.26 ± 0.65 |
| D-dimer (pg/mL) | 1.19 ± 1.72 | 0.47 ± 0.26 | 1.39 ± 0.93 |
| AST (IU/L) | 36.65 ± 30.65 | 54.18 ± 39.95 | 121.4 ± 329.9 |
| ALT (IU/L) | 33.15 ± 23.58 | 45.9 ± 28.08 | 60.8 ± 72.4 |
| LDH (U/L) | 286.36 ± 122.08 * | 498.48 ± 242.34 | 591.23 ± 490.84 |
| Fibrinogen (mg/dL) | 514.06 ± 176.18 | 638.18 ± 158.76 | 630.3 ± 172.2 |
| Ferritin (pg/mL) | 513.48 ± 815.55 | 2786 ± 694.48 | 912.47 ± 826.91 |
| APACHE II score | 5.25 ± 2.94 | 14.27 ± 5.08 | 15.4 ± 3.89 |
| SOFA score | 2 ± 1 | 6.83 ± 3.08 | 5.4 ± 1.81 |
| ICU No Dex | ICU + Dex | |
|---|---|---|
| Number of patients (n) | 12 | 23 |
| ATX (ng/mL, mean ±SD) | 624.36 ± 203.5 | 404.16 ± 145.5 ** |
| Sex | ||
| Male Female | 9 (75%) 3 (25%) | 18 (78.26%) 5 (21.73%) |
| Age (years, mean ±SD) | 66.75 ± 13.31 | 59.43 ± 15.42 |
| Comorbidities n (%) | ||
| Hypertension Diabetes Coronary artery disease COPD Asthma Hyperlipidemia Hepatitis | 5 (41.6%) 0 (0%) 2 (16.6%) 0 (0%) 0 (0%) 3 (25%) (%) | 10 (43.47%) 4 (17.39%) 1 (4.34%) 2 (8.69%) 0 (0%) 6 (26.08%) (%) |
| COVID 19 treatment | ||
| Azithromycin/chloroquine/lopinavir/ritonavir Azithromycin/chloroquine Lopinavir/ritonavir/chloroquine Chloroquine Plasma | 4 (33.33%) 6 (50%) 1 (8.33%) 0 (%) 0 (%) | 0 (0%) 5 (21.73%) 0 (0%) 0 (%) 0 (%) |
| Clinical measurements | ||
| Glucose (mg/dL) | 120.5 ± 24.57 | 154.04 ± 47.98 * |
| Creatinine (mg/dL) | 0.95 ± 0.42 | 0.93 ± 0.38 |
| CRP (mg/dL) | 8.53 ± 5.51 | 15.55 ± 12.68 |
| Total bilirubin (mg/dL) | 0.67 ± 0.27 | 0.7 ± 0.36 |
| Lymphocytes (absolute number) | 0.62 ± 0.35 | 0.83 ± 0.5 |
| INR (median IQR) | 1.09 ± 0.13 | 1.07 ± 0.12 |
| D-dimer (pg/mL) | 2.21 ± 2.14 | 1.45 ± 1.72 |
| LDH (U/L) | 370 ± 129 | 455.08 ± 188.56 |
| Fibrinogen (mg/dL) | 630.55 ± 168.1 | 545 ± 182.32 |
| Ferritin (pg/mL) | 950 ± 382.64 | 1131.09 ± 1223.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikitopoulou, I.; Fanidis, D.; Ntatsoulis, K.; Moulos, P.; Mpekoulis, G.; Evangelidou, M.; Vassiliou, A.G.; Dimakopoulou, V.; Jahaj, E.; Tsipilis, S.; et al. Increased Autotaxin Levels in Severe COVID-19, Correlating with IL-6 Levels, Endothelial Dysfunction Biomarkers, and Impaired Functions of Dendritic Cells. Int. J. Mol. Sci. 2021, 22, 10006. https://doi.org/10.3390/ijms221810006
Nikitopoulou I, Fanidis D, Ntatsoulis K, Moulos P, Mpekoulis G, Evangelidou M, Vassiliou AG, Dimakopoulou V, Jahaj E, Tsipilis S, et al. Increased Autotaxin Levels in Severe COVID-19, Correlating with IL-6 Levels, Endothelial Dysfunction Biomarkers, and Impaired Functions of Dendritic Cells. International Journal of Molecular Sciences. 2021; 22(18):10006. https://doi.org/10.3390/ijms221810006
Chicago/Turabian StyleNikitopoulou, Ioanna, Dionysios Fanidis, Konstantinos Ntatsoulis, Panagiotis Moulos, George Mpekoulis, Maria Evangelidou, Alice G. Vassiliou, Vasiliki Dimakopoulou, Edison Jahaj, Stamatios Tsipilis, and et al. 2021. "Increased Autotaxin Levels in Severe COVID-19, Correlating with IL-6 Levels, Endothelial Dysfunction Biomarkers, and Impaired Functions of Dendritic Cells" International Journal of Molecular Sciences 22, no. 18: 10006. https://doi.org/10.3390/ijms221810006
APA StyleNikitopoulou, I., Fanidis, D., Ntatsoulis, K., Moulos, P., Mpekoulis, G., Evangelidou, M., Vassiliou, A. G., Dimakopoulou, V., Jahaj, E., Tsipilis, S., Orfanos, S. E., Dimopoulou, I., Angelakis, E., Akinosoglou, K., Vassilaki, N., Tzouvelekis, A., Kotanidou, A., & Aidinis, V. (2021). Increased Autotaxin Levels in Severe COVID-19, Correlating with IL-6 Levels, Endothelial Dysfunction Biomarkers, and Impaired Functions of Dendritic Cells. International Journal of Molecular Sciences, 22(18), 10006. https://doi.org/10.3390/ijms221810006









