Tissue-Specific Proteome and Subcellular Microscopic Analyses Reveal the Effect of High Salt Concentration on Actin Cytoskeleton and Vacuolization in Aleurone Cells during Early Germination of Barley
Abstract
:1. Introduction
2. Results
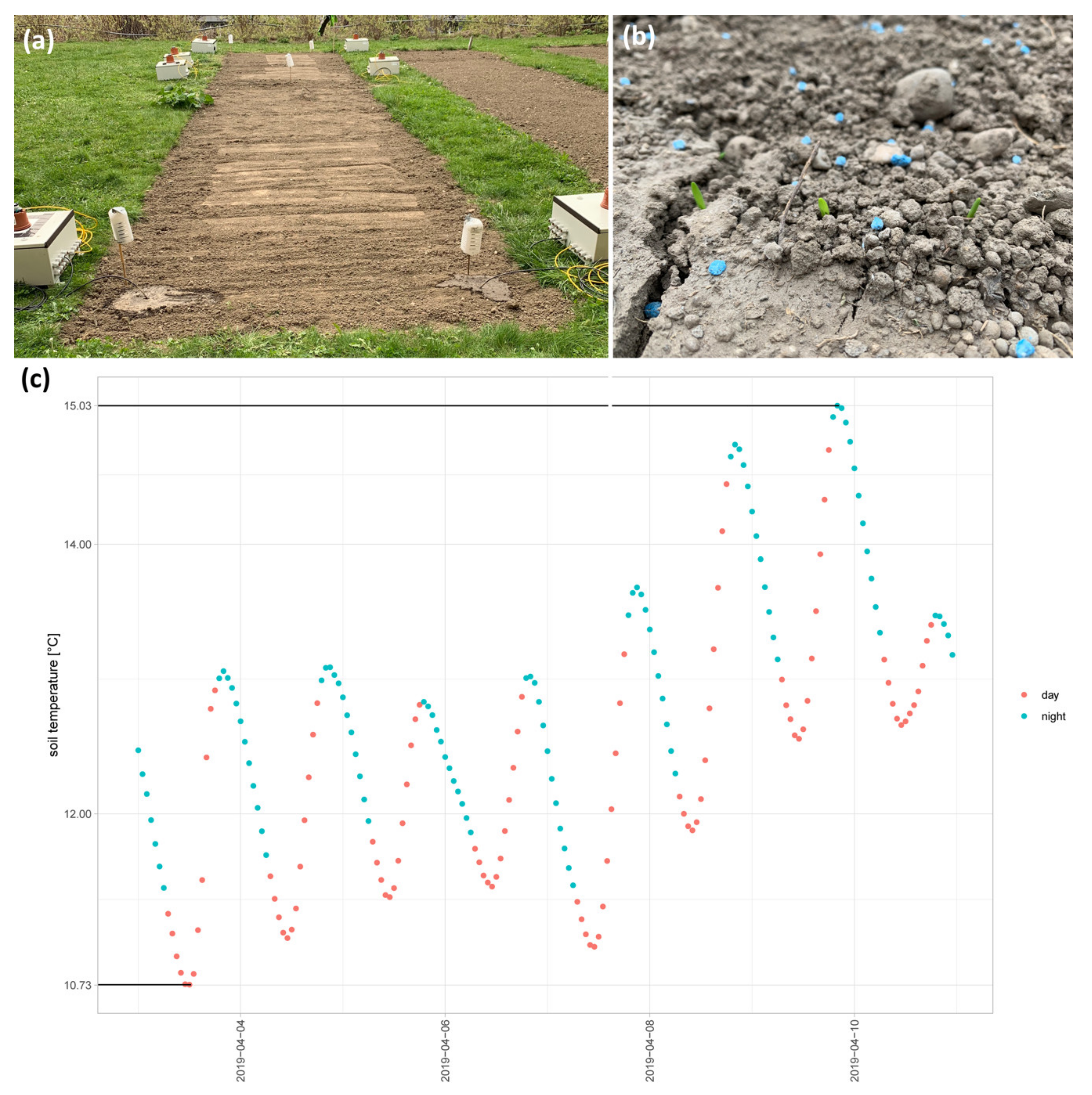
2.1. Application of Natural Conditions to the Lab: First Steps to #Asnearaspossibletonature
2.2. The Germination Rate of GP Is Reduced under High Salt Conditions (EC30) Compared to Tap Water (H2O)
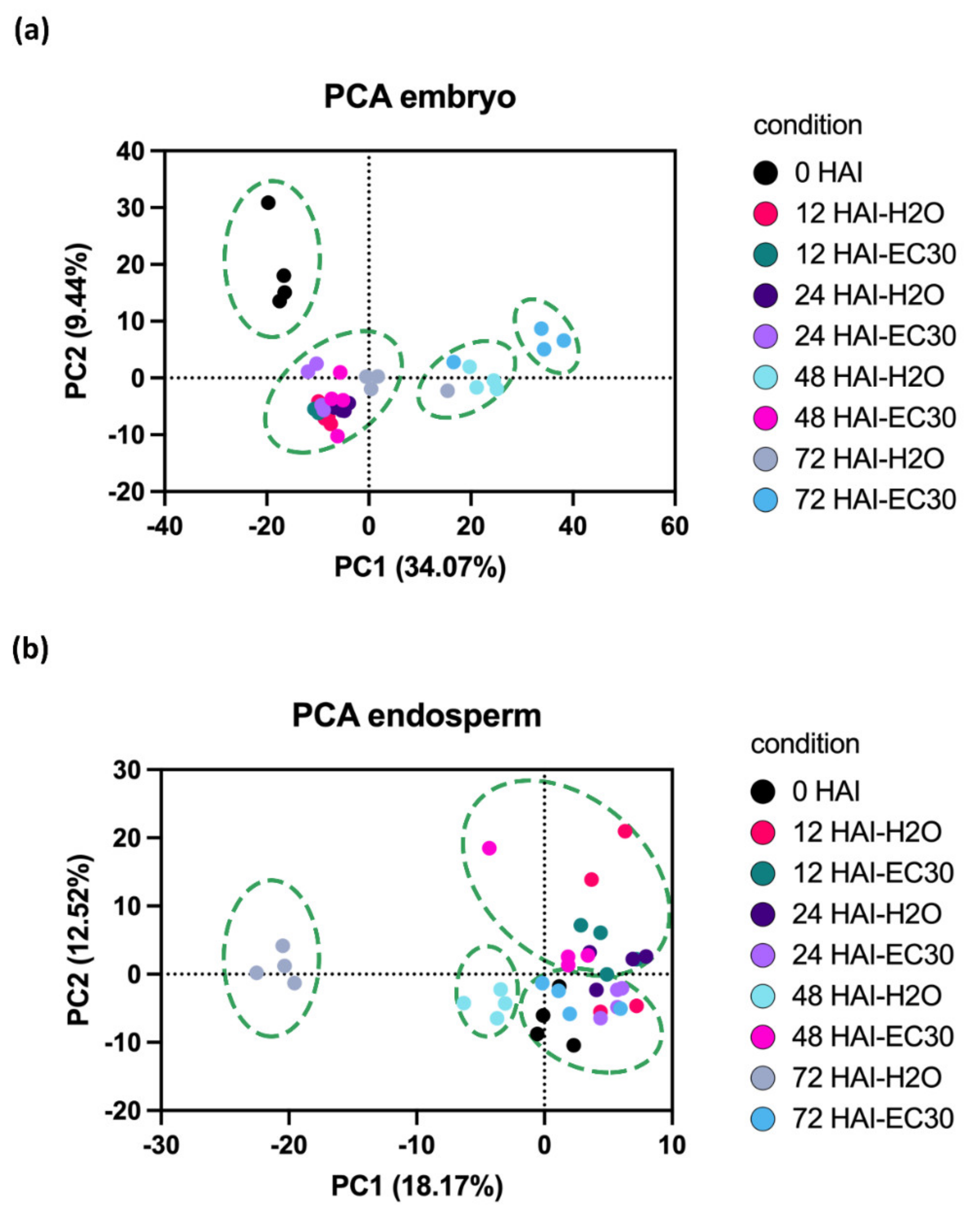
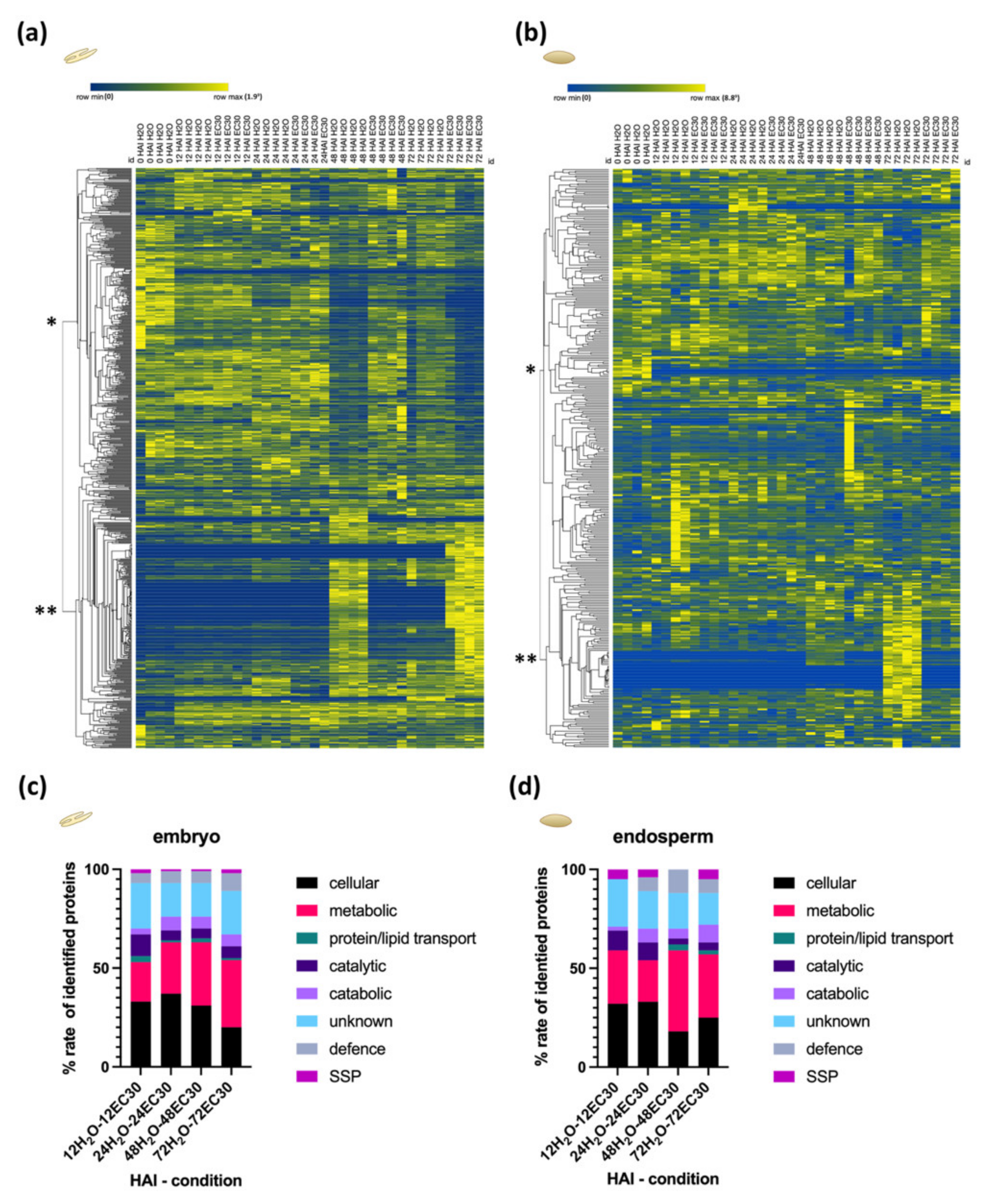
2.3. Characterization of the Proteome of Early Germinated Barley Grains in H2O Versus EC30 Treatments
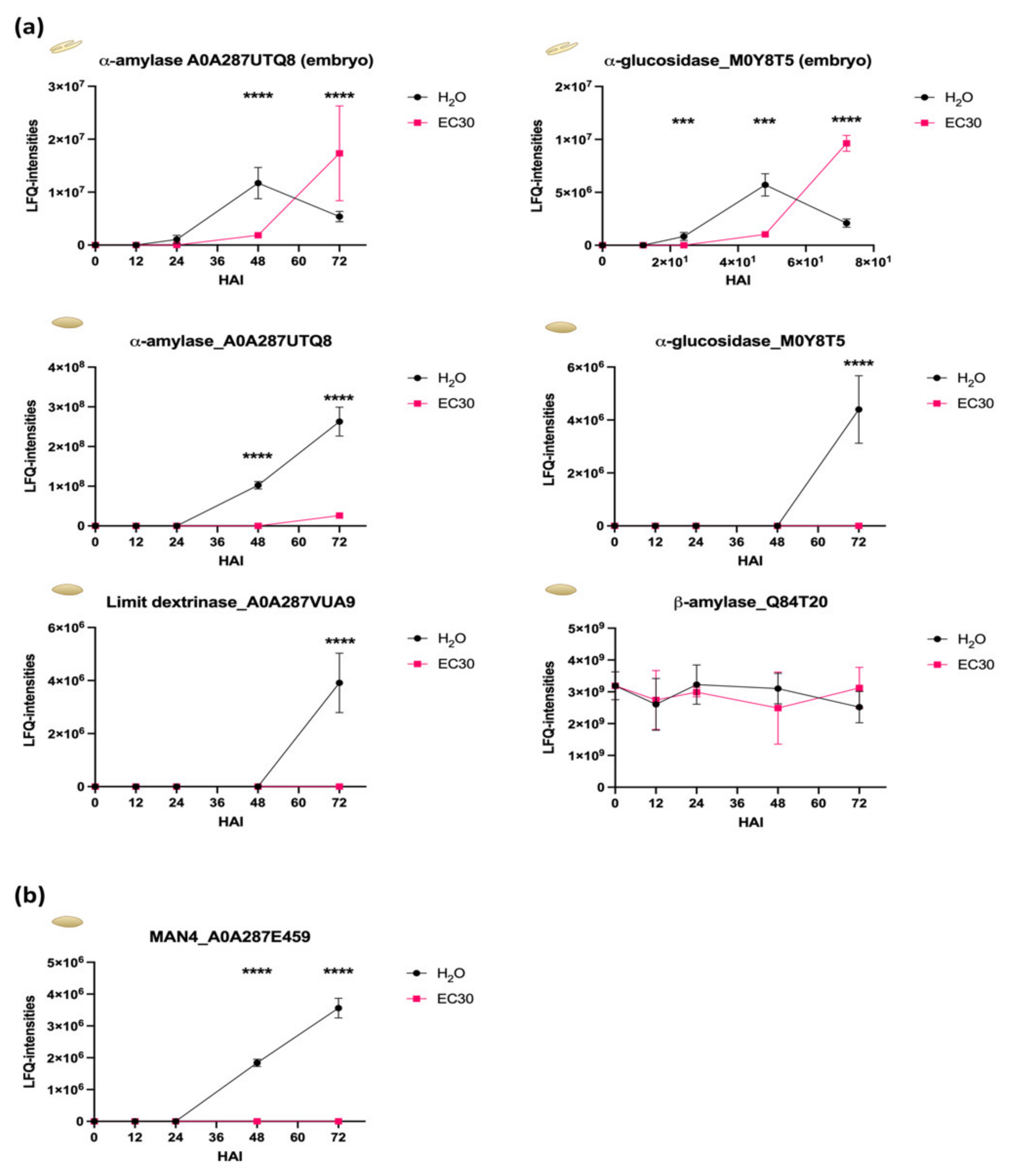
2.4. The Energy Resource Mobilization during the Germination Process
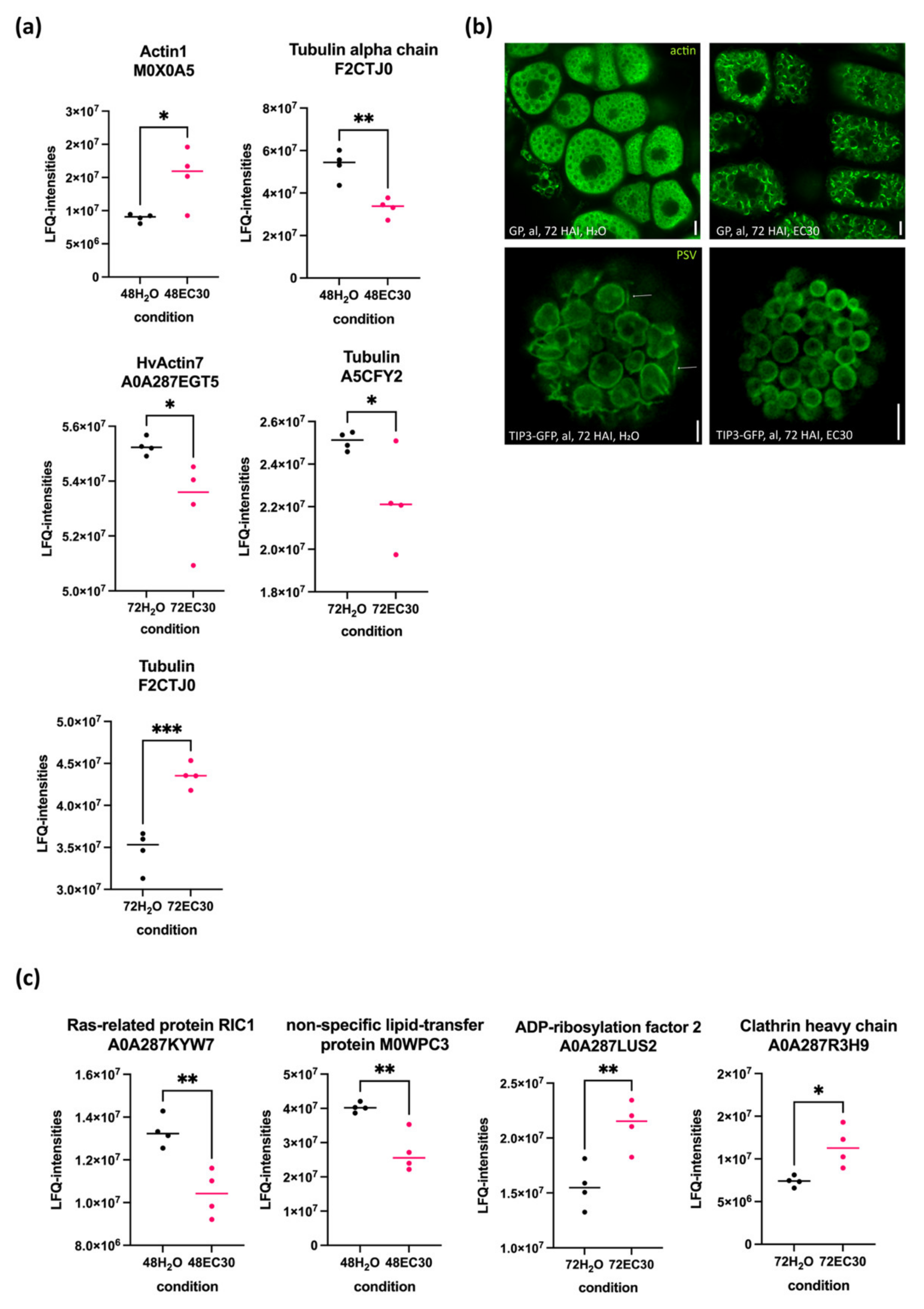
2.5. The Actin and Tubulin Cytoskeletons and the Rearrangement of Vacuoles Are Affected by EC30 in the Endosperm
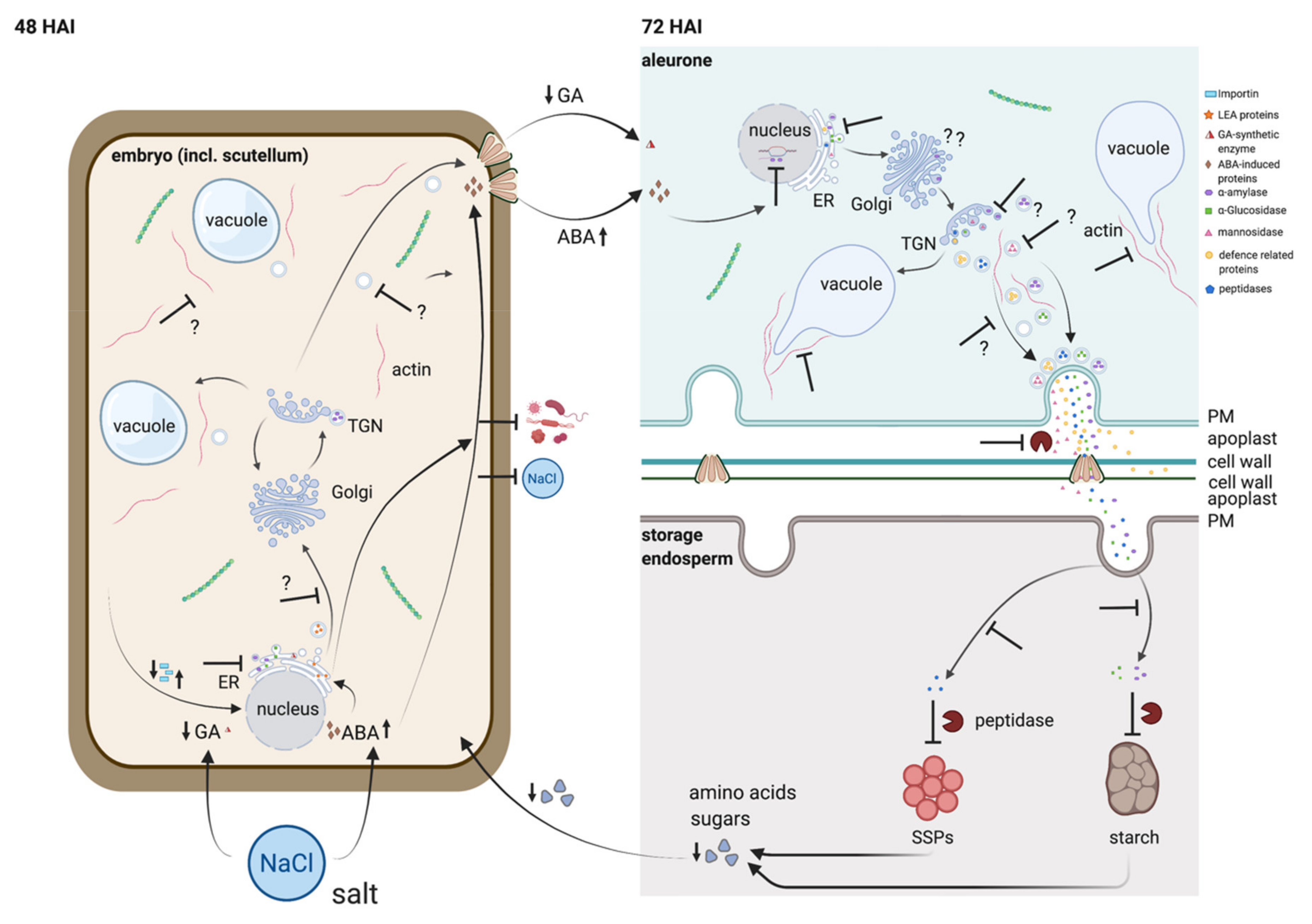
3. Discussion
3.1. Getting Away from Petri Dishes to More Natural but Controlled Settings in the Lab: #Asnearaspossibletonature
3.2. 48 HAI Could Be a Key Point in Time for the Germination under EC30-Like Conditions
3.3. The Antagonistic Regulation of GA3 and ABA Is Putatively Affected by Salt
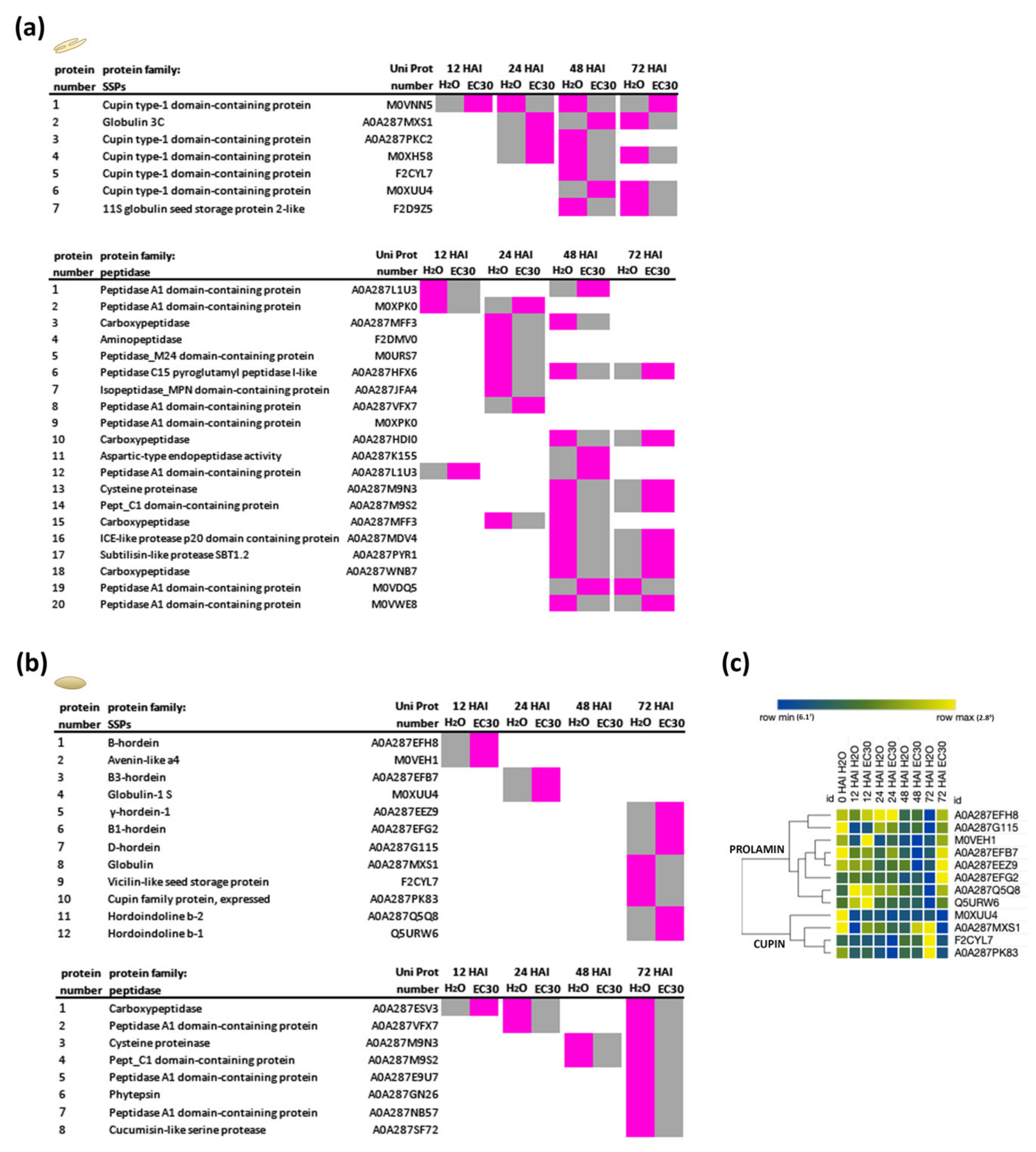
3.4. Seed Storage Proteins in the Endosperm Are Less Digested under EC30 Conditions
3.5. The Cytoskeleton Is Affected by the EC30 Treatment during Germination
3.6. Vacuolization Is Inhibited by EC30
3.7. Actin Is a Putative Key Player Not Only for Vacuolization but for the Transport of Secretory Proteins from the Aleurone to the Endosperm
4. Materials and Methods
4.1. Soil Temperature Data
4.2. Germination and Viability Assay
4.3. Measurement of Weight, Wet, and Dry Weight of GP Grains during Germination
4.4. Protein Extraction and Mass Spectrometry-Analysis
4.5. Data Processing and Protein Identification
4.6. Data Visualization and Statistical Analyses
4.7. Microscopic Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CASP | Cyclase-associated protein (CASP) |
| EC | Electric conductivity |
| GP | Golden Promise |
| HAI | Hours after imbibition |
| LFQ | Label-free quantification |
| PCA | Principial component analysis |
| PSV | Protein storage vacuole |
| SSP | Seed storage protein |
References
- Tuan, P.A.; Sun, M.; Nguyen, T.-N.; Park, S.; Ayele, B.T. Molecular Mechanisms of Seed Germination. In Sprouted Grains; Feng, H., Nemzer, B., DeVries, J.W., Eds.; AACC International Press: Washington, DC, USA, 2019; pp. 1–24. [Google Scholar] [CrossRef]
- Muntz, K.; Belozersky, M.A.; Dunaevsky, Y.E.; Schlereth, A.; Tiedemann, J. Stored proteinases and the initiation of storage protein mobilization in seeds during germination and seedling growth. J. Exp. Bot. 2001, 52, 1741–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan-Wilson, A.L.; Wilson, K.A. Mobilization of seed protein reserves. Physiol. Plant 2012, 145, 140–153. [Google Scholar] [CrossRef]
- Weitbrecht, K.; Muller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, O.A. Endosperm Development: Cellularization and Cell Fate Specification. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 233–267. [Google Scholar] [CrossRef] [Green Version]
- Olsen, O.A. Nuclear endosperm development in cereals and Arabidopsis thaliana. Plant Cell 2004, 16, S214–S227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, T.E.; Gallie, D.R. Programmed cell death during endosperm development. Plant Mol. Biol. 2000, 44, 283–301. [Google Scholar] [CrossRef]
- Moore, K.L.; Tosi, P.; Palmer, R.; Hawkesford, M.J.; Grovenor, C.R.; Shewry, P.R. The Dynamics of Protein Body Formation in Developing Wheat Grain. Plant Biotechnol. J. 2016, 14, 1876–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shewry, P.; Jenkins, J.A.; Beaudoin, F.; Mills, E.N. The Classification, Functions and Evolutionary Relationships of Plant Proteins in Relation to Food Allergies. In Plant Food Allergens; Blackwell Publishing Ltd.: Oxford, UK, 2007. [Google Scholar] [CrossRef]
- Shutov, A.D.; Vaintraub, I.A. Degradation of storage proteins in germinating seeds. Phytochemistry 1987, 26, 1557–1566. [Google Scholar] [CrossRef]
- Hammerton, R.W.; Ho, T.H. Hormonal regulation of the development of protease and carboxypeptidase activities in barley aleurone layers. Plant Physiol. 1986, 80, 692–697. [Google Scholar] [CrossRef] [Green Version]
- Ranki, H.; Sopanen, T. Secretion of alpha-amylase by the aleurone layer and the scutellum of germinating barley grain. Plant Physiol. 1984, 75, 710–715. [Google Scholar] [CrossRef] [Green Version]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef]
- Intergovernmental Technical Panel on Soils (ITPS); Food and Agriculture Organization of the United Nations. Status of the World’s Soil Resources: Main Report; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; Available online: http://www.fao.org/documents/card/en/c/c6814873-efc3-41db-b7d3-2081a10ede50/ (accessed on 10 August 2021).
- Stanners, I.D.; Boureau, P. Europe’s Environment: The Dobris Assessment; Office for Official Publications of the European Communities: Luxembourg, 1995. [Google Scholar]
- Zhang, H.; Irving, L.J.; McGill, C.; Matthew, C.; Zhou, D.; Kemp, P. The effects of salinity and osmotic stress on barley germination rate: Sodium as an osmotic regulator. Ann. Bot. 2010, 106, 1027–1035. [Google Scholar] [CrossRef]
- Isayenkov, S.V. Genetic sources for the development of salt tolerance in crops. Plant Growth Regul. 2019, 89, 1–17. [Google Scholar] [CrossRef]
- Colmer, T.D.; Flowers, T.J.; Munns, R. Use of wild relatives to improve salt tolerance in wheat. J. Exp. Bot. 2006, 57, 1059–1078. [Google Scholar] [CrossRef] [Green Version]
- Bonsager, B.C.; Finnie, C.; Roepstorff, P.; Svensson, B. Spatio-temporal changes in germination and radical elongation of barley seeds tracked by proteome analysis of dissected embryo, aleurone layer, and endosperm tissues. Proteomics 2007, 7, 4528–4540. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Usadel, B.; Winter, A.; Radchuk, V.; Scholz, U.; Stein, N.; Weschke, W.; Strickert, M.; Close, T.J.; Stitt, M.; et al. Barley grain maturation and germination: Metabolic pathway and regulatory network commonalities and differences highlighted by new MapMan/PageMan profiling tools. Plant Physiol. 2008, 146, 1738–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mock, H.P.; Finnie, C.; Witzel, K.; Svensson, B. The Barley Genome; Springer International Publishing: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Liew, L.C.; Narsai, R.; Wang, Y.; Berkowitz, O.; Whelan, J.; Lewsey, M.G. Temporal tissue-specific regulation of transcriptomes during barley (Hordeum vulgare) seed germination. Plant J. 2020, 101, 700–715. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Rupasinghe, T.; Callahan, D.L.; Natera, S.H.A.; Smith, P.M.C.; Hill, C.B.; Roessner, U.; Boughton, B.A. Spatio-Temporal Metabolite and Elemental Profiling of Salt Stressed Barley Seeds During Initial Stages of Germination by MALDI-MSI and micro-XRF Spectrometry. Front. Plant Sci. 2019, 10, 1139. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.G.; Shin, H.T.; Ha, J.K.; Lai, H.L.; Cheng, K.J.; Lee, J.H. Effect of germination temperature on characteristics of phytase production from barley. Bioresour. Technol. 2005, 96, 1297–1303. [Google Scholar] [CrossRef]
- Nonogaki, H.; Chen, F.; Bradford, K.J. Mechanisms and Genes Involved in Germination Sensu Stricto. In Annual Plant Reviews Volume 27: Seed Development, Dormancy and Germination; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 264–304. [Google Scholar] [CrossRef]
- Ma, Z.; Bykovac, N.V.; Igamberdieva, A.U. Cell signaling mechanisms and metabolic regulation of germination and dormancy in barley seeds. Crop J. 2017, 5, 459–477. [Google Scholar] [CrossRef]
- Hara-Nishimura, I.; Nishimura, M.; Daussant, J. Conversion of free β-amylase to bound β-amylase on starch granules in the barley endosperm during desiccation phase of seed development. Protoplasma 1986, 134, 149–153. [Google Scholar] [CrossRef]
- Finnie, C.; Andersen, B.; Shahpiri, A.; Svensson, B. Proteomes of the barley aleurone layer: A model system for plant signalling and protein secretion. Proteomics 2011, 11, 1595–1605. [Google Scholar] [CrossRef]
- Iglesias-Fernandez, R.; Pastor-Mora, E.; Vicente-Carbajosa, J.; Carbonero, P. A Possible Role of the Aleurone Expressed Gene HvMAN1 in the Hydrolysis of the Cell Wall Mannans of the Starchy Endosperm in Germinating Hordeum vulgare L. Seeds. Front. Plant Sci. 2019, 10, 1706. [Google Scholar] [CrossRef]
- Ibl, V.; Stoger, E. Live Cell Imaging During Germination Reveals Dynamic Tubular Structures Derived from Protein Storage Vacuoles of Barley Aleurone Cells. Plants 2014, 3, 442–457. [Google Scholar] [CrossRef]
- Šamaj, J.; Müller, J.; Beck, M.; Böhm, N.; Menzel, D. Vesicular trafficking, cytoskeleton and signalling in root hairs and pollen tubes. Trends Plant Sci. 2006, 11, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Zhang, D.; Wang, J.; Wang, J.; Ren, P.; Yao, L.; Si, E.; Kong, Y.; Wang, H. Integrative Transcriptomic and Proteomic Analyses of Molecular Mechanism Responding to Salt Stress during Seed Germination in Hulless Barley. Int. J. Mol. Sci. 2020, 21, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poorter, H.; Fiorani, F.; Pieruschka, R.; Wojciechowski, T.; van der Putten, W.H.; Kleyer, M.; Schurr, U.; Postma, J. Pampered inside, pestered outside? Differences and similarities between plants growing in controlled conditions and in the field. N. Phytol. 2016, 212, 838–855. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.; GÛmez-Cadenas, A.; Zentella, R.; Casaretto, J. Crosstalk Between Gibberellin and Abscisic Acid in Cereal Aleurone. J. Plant Growth Regul. 2003, 22, 185–194. [Google Scholar] [CrossRef]
- Gómez-Cadenas, A.; Zentella, R.; Walker-Simmons, M.K.; Ho, T.H. Gibberellin/abscisic acid antagonism in barley aleurone cells: Site of action of the protein kinase PKABA1 in relation to gibberellin signaling molecules. Plant Cell 2001, 13, 667–679. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.; Wang, Y.; Zhang, S.; Zhang, J. Salt-stress-induced ABA accumulation is more sensitively triggered in roots than in shoots. J. Exp. Bot. 2002, 53, 2201–2206. [Google Scholar] [CrossRef] [Green Version]
- Graether, S.P.; Boddington, K.F. Disorder and function: A review of the dehydrin protein family. Front. Plant Sci. 2014, 5, 576. [Google Scholar] [CrossRef] [Green Version]
- Shewry, P.R.; Napier, J.A.; Tatham, A.S. Seed storage proteins: Structures and biosynthesis. Plant Cell 1995, 7, 945–956. [Google Scholar] [CrossRef] [Green Version]
- Shewry, P.R.; Halford, N.G. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shewry, P.R.; Tatham, A.S. The prolamin storage proteins of cereal seeds: Structure and evolution. Biochem. J. 1990, 267, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shewry, P.R. The synthesis, processing, and deposition of gluten proteins in the developing wheat grain. Cereal Food World 1999, 44, 587–589. [Google Scholar]
- Dubreil, L.; Compoint, J.-P.; Marion, D. Interaction of Puroindolines with Wheat Flour Polar Lipids Determines Their Foaming Properties. J. Agr. Food Chem. 1997, 45, 108–116. [Google Scholar] [CrossRef]
- Lesage, V.S.; Bouchet, B.; Rhazi, L.; Elmorjani, K.; Branlard, G.; Marion, D. New insight into puroindoline function inferred from their subcellullar localization in developing hard and soft near-isogenic endosperm and their relationship with polymer size of storage proteins. J. Cereal Sci. 2011, 53, 231–238. [Google Scholar] [CrossRef]
- Ibl, V.; Stoger, E. The formation, function and fate of protein storage compartments in seeds. Protoplasma 2012, 249, 379–392. [Google Scholar] [CrossRef]
- Ibl, V.; Kapusi, E.; Arcalis, E.; Kawagoe, Y.; Stoger, E. Fusion, rupture, and degeneration: The fate of in vivo-labelled PSVs in developing barley endosperm. J. Exp. Bot. 2014, 65, 3249–3261. [Google Scholar] [CrossRef] [Green Version]
- Roustan, V.; Hilscher, J.; Weidinger, M.; Reipert, S.; Shabrangy, A.; Gebert, C.; Dietrich, B.; Dermendjiev, G.; Schnurer, M.; Roustan, P.J.; et al. Protein sorting into protein bodies during barley endosperm development is putatively regulated by cytoskeleton members, MVBs and the HvSNF7s. Sci. Rep. 2020, 10, 1864. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Jones, B.L. Characterization of germinated barley endoproteolytic enzymes by two dimensional gel electrophoresis. J. Cereal Sci. 1995, 21, 145–153. [Google Scholar] [CrossRef]
- Diaz-Mendoza, M.; Diaz, I.; Martinez, M. Insights on the Proteases Involved in Barley and Wheat Grain Germination. Int. J. Mol. Sci. 2019, 20, 2087. [Google Scholar] [CrossRef] [Green Version]
- Sreenivasulu, N.; Radchuk, V.; Strickert, M.; Miersch, O.; Weschke, W.; Wobus, U. Gene expression patterns reveal tissue-specific signaling networks controlling programmed cell death and ABA-regulated maturation in developing barley seeds. Plant J. 2006, 47, 310–327. [Google Scholar] [CrossRef]
- Jacobsen, J.V.; Beach, L.R. Control of transcription of α-amylase and rRNA genes in barley aleurone protoplasts by gibberellin and abscisic acid. Nature 1985, 316, 275–277. [Google Scholar] [CrossRef]
- Jones, R.L. Gibberellic acid and the fine structure of barley aleurone cells: II. Changes during the synthesis and secretion of alpha-amylase. Planta 1969, 88, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Poulle, M.; Jones, B.L. A Proteinase from Germinating Barley: I. Purification and Some Physical Properties of a 30 kD Cysteine Endoproteinase from Green Malt. Plant Physiol. 1988, 88, 1454–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koehler, S.M.; Ho, T.H. A major gibberellic acid-induced barley aleurone cysteine proteinase which digests hordein: Purification and characterization. Plant Physiol. 1990, 94, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Koehler, S.; Ho, T.H. Purification and characterization of gibberellic acid-induced cysteine endoproteases in barley aleurone layers. Plant Physiol. 1988, 87, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Mikkonen, A.; Porali, I.; Cercos, M.; Ho, T.H. A major cysteine proteinase, EPB, in germinating barley seeds: Structure of two intronless genes and regulation of expression. Plant Mol. Biol. 1996, 31, 239–254. [Google Scholar] [CrossRef]
- Rogers, J.C.; Dean, D.; Heck, G.R. Aleurain: A barley thiol protease closely related to mammalian cathepsin H. Proc. Natl. Acad. Sci. USA 1985, 82, 6512–6516. [Google Scholar] [CrossRef] [Green Version]
- Rogers, J.C. Two barley alpha-amylase gene families are regulated differently in aleurone cells. J. Biol. Chem. 1985, 260, 3731–3738. [Google Scholar] [CrossRef]
- Martinez, M.; Rubio-Somoza, I.; Carbonero, P.; Diaz, I. A cathepsin B-like cysteine protease gene from Hordeum vulgare (gene CatB) induced by GA in aleurone cells is under circadian control in leaves. J. Exp. Bot. 2003, 54, 951–959. [Google Scholar] [CrossRef] [Green Version]
- Gubler, F.; Ashford, A.E.; Jacobsen, J.V. The release of alpha-amylase through gibberellin-treated barley aleurone cell walls: An immunocytochemical study with Lowicryl K4M. Planta 1987, 172, 155–161. [Google Scholar] [CrossRef]
- Han, C.; He, D.; Li, M.; Yang, P. In-depth proteomic analysis of rice embryo reveals its important roles in seed germination. Plant Cell Physiol. 2014, 55, 1826–1847. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhang, L.J.; Huang, R.D. Cytoskeleton and plant salt stress tolerance. Plant Signal. Behav. 2011, 6, 29–31. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhang, L.; Yuan, M.; Ge, Y.; Liu, Y.; Fan, J.; Ruan, Y.; Cui, Z.; Tong, S.; Zhang, S. The microfilament cytoskeleton plays a vital role in salt and osmotic stress tolerance in Arabidopsis. Plant. Biol. 2010, 12, 70–78. [Google Scholar] [CrossRef]
- Ono, S. The role of cyclase-associated protein in regulating actin filament dynamics—More than a monomer-sequestration factor. J. Cell Sci. 2013, 126, 3249–3258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeks, M.J.; Rodrigues, C.; Dimmock, S.; Ketelaar, T.; Maciver, S.K.; Malhó, R.; Hussey, P.J. Arabidopsis CAP1—A key regulator of actin organisation and development. J. Cell Sci. 2007, 120, 2609–2618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheuring, D.; Lofke, C.; Kruger, F.; Kittelmann, M.; Eisa, A.; Hughes, L.; Smith, R.S.; Hawes, C.; Schumacher, K.; Kleine-Vehn, J. Actin-dependent vacuolar occupancy of the cell determines auxin-induced growth repression. Proc. Natl. Acad. Sci. USA 2016, 113, 452–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, J.K.; Lee, S.J. Straying off the highway: Trafficking of secreted plant proteins and complexity in the plant cell wall proteome. Plant Physiol. 2010, 153, 433–436. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.J.; Ho, T.H. An abscisic acid-induced protein, HVA22, inhibits gibberellin-mediated programmed cell death in cereal aleurone cells. Plant Physiol. 2008, 147, 1710–1722. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ding, B.; Guo, Y.; Li, M.; Chen, S.; Huang, G.; Xie, X. Overexpression of a wheat phospholipase D gene, TaPLDalpha, enhances tolerance to drought and osmotic stress in Arabidopsis thaliana. Planta 2014, 240, 103–115. [Google Scholar] [CrossRef]
- Shen, P.; Wang, R.; Jing, W.; Zhang, W. Rice phospholipase Dalpha is involved in salt tolerance by the mediation of H(+)-ATPase activity and transcription. J. Integr. Plant Biol. 2011, 53, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cadenas, A.; Verhey, S.D.; Holappa, L.D.; Shen, Q.; Ho, T.H.; Walker-Simmons, M.K. An abscisic acid-induced protein kinase, PKABA1, mediates abscisic acid-suppressed gene expression in barley aleurone layers. Proc. Natl. Acad. Sci. USA 1999, 96, 1767–1772. [Google Scholar] [CrossRef] [Green Version]
- Shen, Q.; Gomez-Cadenas, A.; Zhang, P.; Walker-Simmons, M.K.; Sheen, J.; Ho, T.H. Dissection of abscisic acid signal transduction pathways in barley aleurone layers. Plant Mol. Biol. 2001, 47, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A. Seeds: Physiology of Development and Germination. J. Derek Bewley, Michael Black. Q. Rev. Biol. 1995, 70, 223–224. [Google Scholar] [CrossRef]
- Cox, J.; Matic, I.; Hilger, M.; Nagaraj, N.; Selbach, M.; Olsen, J.V.; Mann, M. A practical guide to the MaxQuant computational platform for SILAC-based quantitative proteomics. Nat. Protoc. 2009, 4, 698–705. [Google Scholar] [CrossRef]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Carlson, A.; Sinitcyn, P.; Mann, M.; Cox, J. Visualization of LC-MS/MS proteomics data in MaxQuant. Proteomics 2015, 15, 1453–1456. [Google Scholar] [CrossRef] [Green Version]
- Shabrangy, A.; Roustan, V.; Reipert, S.; Weidinger, M.; Roustan, P.-J.; Stoger, E.; Weckwerth, W.; Ibl, V. Using RT-qPCR, Proteomics, and Microscopy to Unravel the Spatio-Temporal Expression and Subcellular Localization of Hordoindolines Across Development in Barley Endosperm. Front. Plant Sci. 2018, 9, 775. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Bielow, C.; Mastrobuoni, G.; Kempa, S. Proteomics Quality Control: Quality Control Software for MaxQuant Results. J. Proteome Res. 2016, 15, 777–787. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dermendjiev, G.; Schnurer, M.; Weiszmann, J.; Wilfinger, S.; Ott, E.; Gebert, C.; Weckwerth, W.; Ibl, V. Tissue-Specific Proteome and Subcellular Microscopic Analyses Reveal the Effect of High Salt Concentration on Actin Cytoskeleton and Vacuolization in Aleurone Cells during Early Germination of Barley. Int. J. Mol. Sci. 2021, 22, 9642. https://doi.org/10.3390/ijms22179642
Dermendjiev G, Schnurer M, Weiszmann J, Wilfinger S, Ott E, Gebert C, Weckwerth W, Ibl V. Tissue-Specific Proteome and Subcellular Microscopic Analyses Reveal the Effect of High Salt Concentration on Actin Cytoskeleton and Vacuolization in Aleurone Cells during Early Germination of Barley. International Journal of Molecular Sciences. 2021; 22(17):9642. https://doi.org/10.3390/ijms22179642
Chicago/Turabian StyleDermendjiev, Georgi, Madeleine Schnurer, Jakob Weiszmann, Sarah Wilfinger, Emanuel Ott, Claudia Gebert, Wolfram Weckwerth, and Verena Ibl. 2021. "Tissue-Specific Proteome and Subcellular Microscopic Analyses Reveal the Effect of High Salt Concentration on Actin Cytoskeleton and Vacuolization in Aleurone Cells during Early Germination of Barley" International Journal of Molecular Sciences 22, no. 17: 9642. https://doi.org/10.3390/ijms22179642
APA StyleDermendjiev, G., Schnurer, M., Weiszmann, J., Wilfinger, S., Ott, E., Gebert, C., Weckwerth, W., & Ibl, V. (2021). Tissue-Specific Proteome and Subcellular Microscopic Analyses Reveal the Effect of High Salt Concentration on Actin Cytoskeleton and Vacuolization in Aleurone Cells during Early Germination of Barley. International Journal of Molecular Sciences, 22(17), 9642. https://doi.org/10.3390/ijms22179642





