Multiplicity of Glycosphingolipid-Enriched Microdomain-Driven Immune Signaling
Abstract
:1. Introduction
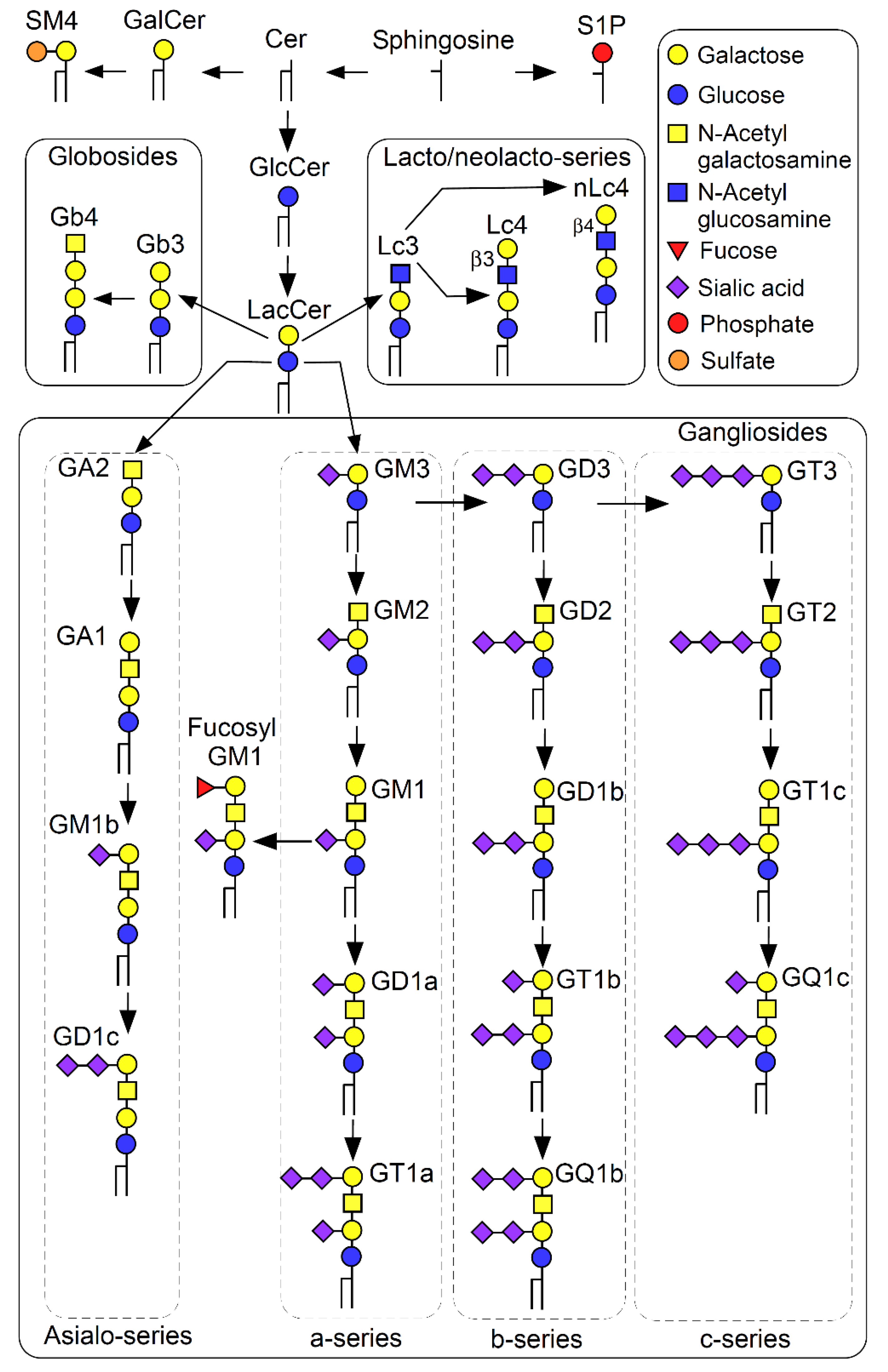
2. Physicochemical Properties of GSL-Enriched Microdomains
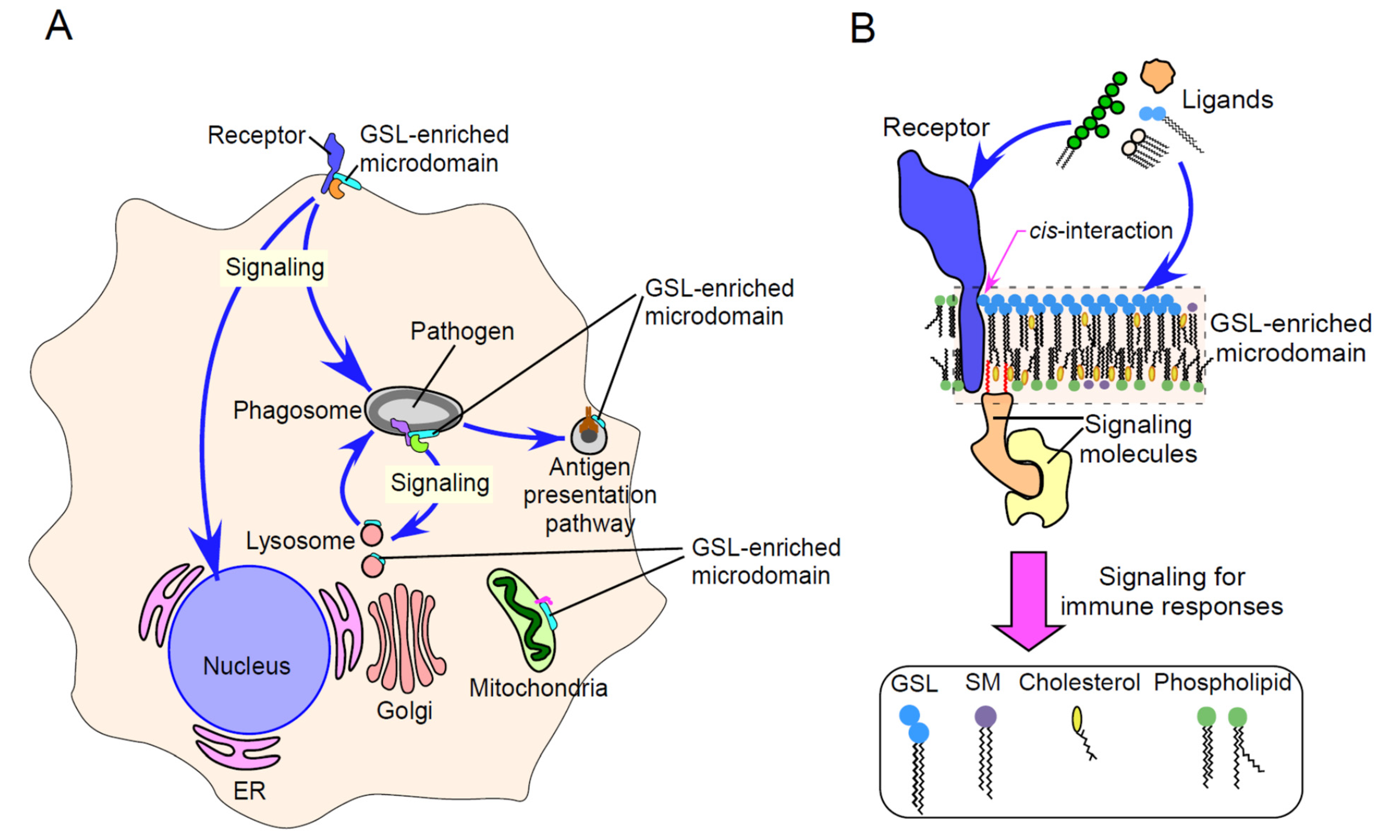
3. GSL-Enriched Microdomains as Regulators of Immune Receptor Signaling
4. GSL-Enriched Microdomains in Immune Functions
5. GSLs and Their Antibodies
6. GSL-Enriched Microdomain-Mediated Apoptosis and Autophagy
7. GSLs as Immunomodulators
8. GSL-Enriched Microdomains as Entry Sites for Pathogens and Toxins
9. Intracellular Interactions between GSL-Enriched Microdomains and Pathogens
10. Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Pike, L.J. Rafts defined: A report on the Keystone Symposium on Lipid Rafts and Cell Function. J. Lipid Res. 2006, 47, 1597–1598. [Google Scholar] [CrossRef] [Green Version]
- Murate, M.; Abe, M.; Kasahara, K.; Iwabuchi, K.; Umeda, M.; Kobayashi, T. Transbilayer distribution of lipids at nano scale. J. Cell Sci. 2015, 128, 1627–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaga, N.; Kazuno, S.; Taka, H.; Iwabuchi, K.; Murayama, K. Isolation and mass spectrometry characterization of molecular species of lactosylceramides using liquid chromatography-electrospray ion trap mass spectrometry. Anal. Biochem. 2005, 337, 316–324. [Google Scholar] [CrossRef]
- Hakomori, S. Structure, organization, and function of glycosphingolipids in membrane. Curr. Opin. Hematol. 2003, 10, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Held, W.; Mariuzza, R.A. Cis-trans interactions of cell surface receptors: Biological roles and structural basis. Cell Mol. Life Sci. 2011, 68, 3469–3478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakomori, S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu. Rev. Biochem. 1981, 50, 733–764. [Google Scholar] [CrossRef]
- Mukherjee, S.; Maxfield, F.R. Membrane domains. Annu. Rev. Cell Dev. Biol. 2004, 20, 839–866. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; London, E. Structure of detergent-resistant membrane domains: Does phase separation occur in biological membranes? Biochem. Biophys. Res. Commun. 1997, 240, 1–7. [Google Scholar] [CrossRef]
- Iwabuchi, K.; Handa, K.; Hakomori, S. Separation of “glycosphingolipid signaling domain” from caveolin-containing membrane fraction in mouse melanoma B16 cells and its role in cell adhesion coupled with signaling. J. Biol Chem. 1998, 273, 33766–33773. [Google Scholar] [CrossRef] [Green Version]
- Iwabuchi, K.; Yamamura, S.; Prinetti, A.; Handa, K.; Hakomori, S. GM3-enriched microdomain involved in cell adhesion and signal transduction through carbohydrate-carbohydrate interaction in mouse melanoma B16 cells. J. Biol. Chem. 1998, 273, 9130–9138. [Google Scholar] [CrossRef] [Green Version]
- Sonnino, S.; Prinetti, A.; Mauri, L.; Chigorno, V.; Tettamanti, G. Dynamic and structural properties of sphingolipids as driving forces for the formation of membrane domains. Chem. Rev. 2006, 106, 2111–2125. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.; Sheets, E.D.; Simson, R. Revisiting the fluid mosaic model of membranes. Science 1995, 268, 1441–1442. [Google Scholar] [CrossRef]
- Hakomori, S.; Handa, K.; Iwabuchi, K.; Yamamura, S.; Prinetti, A. New insights in glycosphingolipid function: “glycosignaling domain”, a cell surface assembly of glycosphingolipids with signal transducer molecules, involved in cell adhesion coupled with signaling. Glycobiology 1998, 8, xi–xix. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.D.; Puri, V.; Valiyaveettil, J.T.; Marks, D.L.; Bittman, R.; Pagano, R.E. Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol. Biol. Cell 2003, 14, 3254–3265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komura, N.; Suzuki, K.G.; Ando, H.; Konishi, M.; Koikeda, M.; Imamura, A.; Chadda, R.; Fujiwara, T.K.; Tsuboi, H.; Sheng, R.; et al. Raft-based interactions of gangliosides with a GPI-anchored receptor. Nat. Chem. Biol. 2016, 12, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Kusumi, A.; Fujiwara, T.K.; Tsunoyama, T.A.; Kasai, R.S.; Liu, A.A.; Hirosawa, K.M.; Kinoshita, M.; Matsumori, N.; Komura, N.; Ando, H.; et al. Defining raft domains in the plasma membrane. Traffic 2020, 21, 106–137. [Google Scholar] [CrossRef]
- Kinoshita, M.; Suzuki, K.G.; Matsumori, N.; Takada, M.; Ano, H.; Morigaki, K.; Abe, M.; Makino, A.; Kobayashi, T.; Hirosawa, K.M.; et al. Raft-based sphingomyelin interactions revealed by new fluorescent sphingomyelin analogs. J. Cell Biol. 2017, 216, 1183–1204. [Google Scholar] [CrossRef] [Green Version]
- Iwabuchi, K.; Prinetti, A.; Sonnino, S.; Mauri, L.; Kobayashi, T.; Ishii, K.; Kaga, N.; Murayama, K.; Kurihara, H.; Nakayama, H.; et al. Involvement of very long fatty acid-containing lactosylceramide in lactosylceramide-mediated superoxide generation and migration in neutrophils. Glycoconj. J. 2008, 25, 357–374. [Google Scholar] [CrossRef]
- Fujita, A.; Cheng, J.; Fujimoto, T. Segregation of GM1 and GM3 clusters in the cell membrane depends on the intact actin cytoskeleton. Biochim. Biophys. Acta 2009, 1791, 388–396. [Google Scholar] [CrossRef]
- Nakayama, H.; Kurihara, H.; Morita, Y.S.; Kinoshita, T.; Mauri, L.; Prinetti, A.; Sonnino, S.; Yokoyama, N.; Ogawa, H.; Takamori, K.; et al. Lipoarabinomannan binding to lactosylceramide in lipid rafts is essential for the phagocytosis of mycobacteria by human neutrophils. Sci. Signal. 2016, 9, ra101. [Google Scholar] [CrossRef]
- Pralle, A.; Keller, P.; Florin, E.L.; Simons, K.; Horber, J.K. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol. 2000, 148, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.G.; Fujiwara, T.K.; Sanematsu, F.; Iino, R.; Edidin, M.; Kusumi, A. GPI-anchored receptor clusters transiently recruit Lyn and G alpha for temporary cluster immobilization and Lyn activation: Single-molecule tracking study 1. J. Cell Biol. 2007, 177, 717–730. [Google Scholar] [CrossRef] [Green Version]
- Saxena, K.; Zimmermann, P.; Schmidt, R.R.; Shipley, G.G. Bilayer properties of totally synthetic C16:0-lactosyl-ceramide. Biophys. J. 2000, 78, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Ferraretto, A.; Pitto, M.; Palestini, P.; Masserini, M. Lipid domains in the membrane: Thermotropic properties of sphingomyelin vesicles containing GM1 ganglioside and cholesterol. Biochemistry 1997, 36, 9232–9236. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, K.; Nagaoka, I. Lactosylceramide-enriched glycosphingolipid signaling domain mediates superoxide generation from human neutrophils. Blood 2002, 100, 1454–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Róg, T.; Orłowski, A.; Llorente, A.; Skotland, T.; Sylvänne, T.; Kauhanen, D.; Ekroos, K.; Sandvig, K.; Vattulainen, I. Interdigitation of long-chain sphingomyelin induces coupling of membrane leaflets in a cholesterol dependent manner. Biochim. Biophys. Acta 2016, 1858, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Sandvig, K. The role of PS 18:0/18:1 in membrane function. Nat. Commun. 2019, 10, 2752. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Schmieder, S.; Pezeshkian, W.; Becken, U.; Wunder, C.; Chinnapen, D.; Ipsen, J.H.; Kenworthy, A.K.; Lencer, W.; Mayor, S.; et al. Ceramide structure dictates glycosphingolipid nanodomain assembly and function. Nat. Commun. 2021, 12, 3675. [Google Scholar] [CrossRef]
- Kabayama, K.; Sato, T.; Saito, K.; Loberto, N.; Prinetti, A.; Sonnino, S.; Kinjo, M.; Igarashi, Y.; Inokuchi, J. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc. Natl. Acad. Sci. USA 2007, 104, 13678–13683. [Google Scholar] [CrossRef] [Green Version]
- Coskun, U.; Grzybek, M.; Drechsel, D.; Simons, K. Regulation of human EGF receptor by lipids. Proc. Natl. Acad. Sci. USA 2011, 108, 9044–9048. [Google Scholar] [CrossRef] [Green Version]
- Yamakawa, D.; Katoh, D.; Kasahara, K.; Shiromizu, T.; Matsuyama, M.; Matsuda, C.; Maeno, Y.; Watanabe, M.; Nishimura, Y.; Inagaki, M. Primary cilia-dependent lipid raft/caveolin dynamics regulate adipogenesis. Cell Rep. 2021, 34, 108817. [Google Scholar] [CrossRef]
- Ansell, T.B.; Song, W.; Sansom, M.S.P. The Glycosphingolipid GM3 Modulates Conformational Dynamics of the Glucagon Receptor. Biophys. J. 2020, 119, 300–313. [Google Scholar] [CrossRef]
- Dam, D.H.M.; Wang, X.Q.; Sheu, S.; Vijay, M.; Shipp, D.; Miller, L.; Paller, A.S. Ganglioside GM3 Mediates Glucose-Induced Suppression of IGF-1 Receptor-Rac1 Activation to Inhibit Keratinocyte Motility. J. Investig. Dermatol. 2017, 137, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Mutoh, T.; Tokuda, A.; Miyadai, T.; Hamaguchi, M.; Fujiki, N. Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc. Natl. Acad. Sci. USA 1995, 92, 5087–5091. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, N.; Iwabuchi, K.; Kurihara, H.; Ishii, K.; Kobayashi, T.; Sasaki, T.; Hattori, N.; Mizuno, Y.; Hozumi, K.; Yamada, Y.; et al. Binding of laminin-1 to monosialoganglioside GM1 in lipid rafts is crucial for neurite outgrowth. J. Cell Sci. 2009, 122, 289–299. [Google Scholar] [CrossRef] [Green Version]
- Chiricozzi, E.; Biase, E.D.; Maggioni, M.; Lunghi, G.; Fazzari, M.; Pome, D.Y.; Casellato, R.; Loberto, N.; Mauri, L.; Sonnino, S. GM1 promotes TrkA-mediated neuroblastoma cell differentiation by occupying a plasma membrane domain different from TrkA. J. Neurochem. 2019, 149, 231–241. [Google Scholar] [CrossRef]
- Prasanna, X.; Jafurulla, M.; Sengupta, D.; Chattopadhyay, A. The ganglioside GM1 interacts with the serotonin1A receptor via the sphingolipid binding domain. Biochim. Biophys. Acta. 2016, 1858, 2818–2826. [Google Scholar] [CrossRef] [PubMed]
- Schnaar, R.L. Gangliosides of the Vertebrate Nervous System. J. Mol. Biol. 2016, 428, 3325–3336. [Google Scholar] [CrossRef] [Green Version]
- Chai, Q.; Arndt, J.W.; Dong, M.; Tepp, W.H.; Johnson, E.A.; Chapman, E.R.; Stevens, R.C. Structural basis of cell surface receptor recognition by botulinum neurotoxin B. Nature 2006, 444, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.; Ramirez-Franco, J.; Desplantes, R.; Debreux, K.; Ferracci, G.; Wernert, F.; Blanchard, M.P.; Maulet, Y.; Youssouf, F.; Sangiardi, M.; et al. Gangliosides interact with synaptotagmin to form the high-affinity receptor complex for botulinum neurotoxin B. Proc. Natl. Acad. Sci. USA 2019, 116, 18098–18108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate immune pattern recognition: A cell biological perspective. Annu. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef] [Green Version]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997, 388, 394–397. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef] [PubMed]
- Triantafilou, M.; Miyake, K.; Golenbock, D.T.; Triantafilou, K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J. Cell Sci. 2002, 115, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Ruysschaert, J.M.; Lonez, C. Role of lipid microdomains in TLR-mediated signalling. Biochim. Biophys. Acta 2015, 1848, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.G.; Zaslona, Z.; Galvan-Pena, S.; Koppe, E.L.; Sevin, D.C.; Angiari, S.; Triantafilou, M.; Triantafilou, K.; Modis, L.K.; O’Neill, L.A. An unexpected link between fatty acid synthase and cholesterol synthesis in proinflammatory macrophage activation. J. Biol. Chem. 2018, 293, 5509–5521. [Google Scholar] [CrossRef] [Green Version]
- Mobarak, E.; Haversen, L.; Manna, M.; Rutberg, M.; Levin, M.; Perkins, R.; Rog, T.; Vattulainen, I.; Boren, J. Glucosylceramide modifies the LPS-induced inflammatory response in macrophages and the orientation of the LPS/TLR4 complex in silico. Sci. Rep. 2018, 8, 13600. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, Y.; Nakajima, K.; Mutoh, T. Neuroprotection by Neurotropin through Crosstalk of Neurotrophic and Innate Immune Receptors in PC12 Cells. Int. J. Mol. Sci. 2020, 21, 6456. [Google Scholar] [CrossRef]
- McNamara, N.; Gallup, M.; Sucher, A.; Maltseva, I.; McKemy, D.; Basbaum, C. AsialoGM1 and TLR5 cooperate in flagellin-induced nucleotide signaling to activate Erk1/2. Am. J. Respir. Cell Mol. Biol. 2006, 34, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Wang, M.; Tapping, R.I.; Stepensky, V.; Nawar, H.F.; Triantafilou, M.; Triantafilou, K.; Connell, T.D.; Hajishengallis, G. Ganglioside GD1a is an essential coreceptor for Toll-like receptor 2 signaling in response to the B subunit of type IIb enterotoxin. J. Biol. Chem. 2007, 282, 7532–7542. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, H.; Yoshizaki, F.; Prinetti, A.; Sonnino, S.; Mauri, L.; Takamori, K.; Ogawa, H.; Iwabuchi, K. Lyn-coupled LacCer-enriched lipid rafts are required for CD11b/CD18-mediated neutrophil phagocytosis of nonopsonized microorganisms. J. Leukoc. Biol. 2008, 83, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Brandel, A.; Aigal, S.; Lagies, S.; Schlimpert, M.; Melendez, A.V.; Xu, M.; Lehmann, A.; Hummel, D.; Fisch, D.; Madl, J.; et al. The Gb3-enriched CD59/flotillin plasma membrane domain regulates host cell invasion by Pseudomonas aeruginosa. Cell. Mol. Life Sci. 2021, 78, 3637–3656. [Google Scholar] [CrossRef]
- Jongsma, M.L.M.; de Waard, A.A.; Raaben, M.; Zhang, T.; Cabukusta, B.; Platzer, R.; Blomen, V.A.; Xagara, A.; Verkerk, T.; Bliss, S.; et al. The SPPL3-Defined Glycosphingolipid Repertoire Orchestrates HLA Class I-Mediated Immune Responses. Immunity 2021, 54, 132–150.e139. [Google Scholar] [CrossRef] [PubMed]
- Barbat, C.; Trucy, M.; Sorice, M.; Garofalo, T.; Manganelli, V.; Fischer, A.; Mazerolles, F. p56lck, LFA-1 and PI3K but not SHP-2 interact with GM1- or GM3-enriched microdomains in a CD4-p56lck association-dependent manner. Biochem. J. 2007, 402, 471–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagafuku, M.; Okuyama, K.; Onimaru, Y.; Suzuki, A.; Odagiri, Y.; Yamashita, T.; Iwasaki, K.; Fujiwara, M.; Takayanagi, M.; Ohno, I.; et al. CD4 and CD8 T cells require different membrane gangliosides for activation. Proc. Natl. Acad. Sci. USA 2012, 109, E336–E342. [Google Scholar] [CrossRef] [Green Version]
- Minguet, S.; Klasener, K.; Schaffer, A.M.; Fiala, G.J.; Osteso-Ibanez, T.; Raute, K.; Navarro-Lerida, I.; Hartl, F.A.; Seidl, M.; Reth, M.; et al. Caveolin-1-dependent nanoscale organization of the BCR regulates B cell tolerance. Nat. Immunol. 2017, 10, 1150–1159. [Google Scholar] [CrossRef] [Green Version]
- Garofalo, T.; Misasi, R.; Mattei, V.; Giammarioli, A.M.; Malorni, W.; Pontieri, G.M.; Pavan, A.; Sorice, M. Association of the death-inducing signaling complex with microdomains after triggering through CD95/Fas. Evidence for caspase-8-ganglioside interaction in T cells. J. Biol. Chem. 2003, 278, 8309–8315. [Google Scholar] [CrossRef] [Green Version]
- Lamers, C.; Pluss, C.J.; Ricklin, D. The Promiscuous Profile of Complement Receptor 3 in Ligand Binding, Immune Modulation, and Pathophysiology. Front. Immunol. 2021, 12, 662164. [Google Scholar] [CrossRef] [PubMed]
- Rabb, H.; Michishita, M.; Sharma, C.P.; Brown, D.; Arnaout, M.A. Cytoplasmic tails of human complement receptor type 3 (CR3, CD11b/CD18) regulate ligand avidity and the internalization of occupied receptors. J. Immunol. 1993, 151, 990–1002. [Google Scholar]
- Sato, T.; Iwabuchi, K.; Nagaoka, I.; Adachi, Y.; Ohno, N.; Tamura, H.; Seyama, K.; Fukuchi, Y.; Nakayama, H.; Yoshizaki, F.; et al. Induction of human neutrophil chemotaxis by Candida albicans-derived beta-1,6-long glycoside side-chain-branched beta-glucan. J. Leukoc. Biol. 2006, 80, 204–211. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Ciampa, M.G.; Brasile, G.; Compostella, F.; Prinetti, A.; Nakayama, H.; Ekyalongo, R.C.; Iwabuchi, K.; Sonnino, S.; Mauri, L. Direct interaction, instrumental for signaling processes, between LacCer and Lyn in the lipid rafts of neutrophil-like cells. J. Lipid Res. 2015, 56, 129–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, T.; Boyd, B.; Price, M.; Lingwood, C.; Maloney, M. MHC class II proteins contain a potential binding site for the verotoxin receptor glycolipid CD77. Cell. Mol. Biol. 2001, 47, 1179–1185. [Google Scholar] [PubMed]
- Jongsma, M.L.M.; Neefjes, J.; Spaapen, R.M. Playing hide and seek: Tumor cells in control of MHC class I antigen presentation. Mol. Immunol. 2021, 136, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Nagafuku, M.; Suzuki, A.; Iwabuchi, K.; Inokuchi, J.I. The regulatory roles of glycosphingolipid-enriched lipid rafts in immune systems. FEBS Lett. 2018, 592, 3921–3942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciofani, M.; Zuniga-Pflucker, J.C. The thymus as an inductive site for T lymphopoiesis. Annu. Rev. Cell Dev. Biol. 2007, 23, 463–493. [Google Scholar] [CrossRef] [Green Version]
- Heuss, S.F.; Tarantino, N.; Fantini, J.; Ndiaye-Lobry, D.; Moretti, J.; Israel, A.; Logeat, F. A glycosphingolipid binding domain controls trafficking and activity of the mammalian notch ligand delta-like 1. PLoS ONE 2013, 8, e74392. [Google Scholar] [CrossRef]
- Sharabi, A.; Tsokos, G.C. T cell metabolism: New insights in systemic lupus erythematosus pathogenesis and therapy. Nat. Rev. Rheumatol. 2020, 16, 100–112. [Google Scholar] [CrossRef]
- McDonald, G.; Deepak, S.; Miguel, L.; Hall, C.J.; Isenberg, D.A.; Magee, A.I.; Butters, T.; Jury, E.C. Normalizing glycosphingolipids restores function in CD4+ T cells from lupus patients. J. Clin. Investig. 2014, 124, 712–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimata, H. GM1, a ganglioside that specifically enhances immunoglobulin production and proliferation in human plasma cells. Eur. J. Immunol. 1994, 24, 2910–2913. [Google Scholar] [CrossRef]
- Klasener, K.; Maity, P.C.; Hobeika, E.; Yang, J.; Reth, M. B cell activation involves nanoscale receptor reorganizations and inside-out signaling by Syk. eLife 2014, 3, e02069. [Google Scholar] [CrossRef]
- Shrestha, D.; Exley, M.A.; Vereb, G.; Szollosi, J.; Jenei, A. CD1d favors MHC neighborhood, GM1 ganglioside proximity and low detergent sensitive membrane regions on the surface of B lymphocytes. Biochim. Biophys. Acta. 2014, 1840, 667–680. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.; Smith, D.F.; Cummings, R.D.; Boligan, K.F.; Hamilton, R.G.; Bochner, B.S.; Miescher, S.; Simon, H.U.; Pashov, A.; Vassilev, T.; et al. The human IgG anti-carbohydrate repertoire exhibits a universal architecture and contains specificity for microbial attachment sites. Sci. Transl. Med. 2015, 7, 269ra1. [Google Scholar] [CrossRef] [Green Version]
- Okuda, T. Application of the Antibody-Inducing Activity of Glycosphingolipids to Human Diseases. Int. J. Mol. Sci. 2021, 22, 3776. [Google Scholar] [CrossRef] [PubMed]
- Cutillo, G.; Saariaho, A.H.; Meri, S. Physiology of gangliosides and the role of antiganglioside antibodies in human diseases. Cell. Mol. Immunol. 2020, 17, 313–322. [Google Scholar] [CrossRef]
- Ariga, T.; Yu, R.K. Antiglycolipid antibodies in Guillain-Barre syndrome and related diseases: Review of clinical features and antibody specificities. J. Neurosci. Res. 2005, 80, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, K.; Masuda, H.; Kaga, N.; Nakayama, H.; Matsumoto, R.; Iwahara, C.; Yoshizaki, F.; Tamaki, Y.; Kobayashi, T.; Hayakawa, T.; et al. Properties and functions of lactosylceramide from mouse neutrophils. Glycobiology 2015, 25, 655–668. [Google Scholar] [CrossRef]
- Okuda, T.; Fukui, A. Generation of anti-oligosaccharide antibodies that recognize mammalian glycoproteins by immunization with a novel artificial glycosphingolipid. Biochem. Biophys. Res. Commun. 2018, 497, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Shimizu, K.; Hasaba, S.; Date, M. Induction of specific adaptive immune responses by immunization with newly designed artificial glycosphingolipids. Sci. Rep. 2019, 9, 18803. [Google Scholar] [CrossRef]
- Shima, S.; Kawamura, N.; Ishikawa, T.; Masuda, H.; Iwahara, C.; Niimi, Y.; Ueda, A.; Iwabuchi, K.; Mutoh, T. Anti-neutral glycolipid antibodies in encephalomyeloradiculoneuropathy. Neurology. 2014, 82, 114–118. [Google Scholar] [CrossRef]
- Gajate, C.; Del Canto-Jañez, E.; Acuña, A.U.; Amat-Guerri, F.; Geijo, E.; Santos-Beneit, A.M.; Veldman, R.J.; Mollinedo, F. Intracellular triggering of Fas aggregation and recruitment of apoptotic molecules into Fas-enriched rafts in selective tumor cell apoptosis. J. Exp. Med. 2004, 200, 353–365. [Google Scholar] [CrossRef] [Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Gajate, C.; Gonzalez-Camacho, F.; Mollinedo, F. Involvement of raft aggregates enriched in Fas/CD95 death-inducing signaling complex in the antileukemic action of edelfosine in Jurkat cells. PLoS ONE 2009, 4, e5044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta 2013, 1833, 3448–3459. [Google Scholar] [CrossRef] [Green Version]
- Mollinedo, F.; Gajate, C. Lipid rafts as signaling hubs in cancer cell survival/death and invasion: Implications in tumor progression and therapy: Thematic Review Series: Biology of Lipid Rafts. J. Lipid Res. 2020, 61, 611–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollinedo, F.; Gajate, C. Lipid rafts, death receptors and CASMERs: New insights for cancer therapy. Future Oncol. 2010, 6, 491–494. [Google Scholar] [CrossRef] [Green Version]
- Mollinedo, F.; Gajate, C. Lipid rafts and clusters of apoptotic signaling molecule-enriched rafts in cancer therapy. Future Oncol. 2010, 6, 811–821. [Google Scholar] [CrossRef]
- Kiguchi, K.; Henning-Chubb, C.B.; Huberman, E. Glycosphingolipid patterns of peripheral blood lymphocytes, monocytes, and granulocytes are cell specific. J. Biochem. 1990, 107, 8–14. [Google Scholar] [CrossRef]
- Malorni, W.; Giammarioli, A.M.; Garofalo, T.; Sorice, M. Dynamics of lipid raft components during lymphocyte apoptosis: The paradigmatic role of GD3. Apoptosis 2007, 12, 941–949. [Google Scholar] [CrossRef]
- Malisan, F.; Testi, R. GD3 ganglioside and apoptosis. Biochim. Biophys. Acta 2002, 1585, 179–187. [Google Scholar] [CrossRef]
- Scorrano, L.; Petronilli, V.; Di Lisa, F.; Bernardi, P. Commitment to apoptosis by GD3 ganglioside depends on opening of the mitochondrial permeability transition pore. J. Biol. Chem. 1999, 274, 22581–22585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rippo, M.R.; Malisan, F.; Ravagnan, L.; Tomassini, B.; Condo, I.; Costantini, P.; Susin, S.A.; Rufini, A.; Todaro, M.; Kroemer, G.; et al. GD3 ganglioside directly targets mitochondria in a bcl-2-controlled fashion. FASEB J. 2000, 14, 2047–2054. [Google Scholar] [CrossRef]
- Garcia-Ruiz, C.; Colell, A.; Morales, A.; Calvo, M.; Enrich, C.; Fernandez-Checa, J.C. Trafficking of ganglioside GD3 to mitochondria by tumor necrosis factor-alpha. J. Biol. Chem. 2002, 277, 36443–36448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garofalo, T.; Giammarioli, A.M.; Misasi, R.; Tinari, A.; Manganelli, V.; Gambardella, L.; Pavan, A.; Malorni, W.; Sorice, M. Lipid microdomains contribute to apoptosis-associated modifications of mitochondria in T cells. Cell Death Differ. 2005, 12, 1378–1389. [Google Scholar] [CrossRef]
- Takeuchi, R.; Kambe, M.; Miyata, M.; Jeyadevan, U.; Tajima, O.; Furukawa, K.; Furukawa, K. TNFα-signal and cAMP-mediated signals oppositely regulate melanoma- associated ganglioside GD3 synthase gene in human melanocytes. Sci. Rep. 2019, 9, 14740. [Google Scholar] [CrossRef] [PubMed]
- Kina, K.; Masuda, H.; Nakayama, H.; Nagatsuka, Y.; Nabetani, T.; Hirabayashi, Y.; Takahashi, Y.; Shimada, K.; Daida, H.; Ogawa, H.; et al. The novel neutrophil differentiation marker phosphatidylglucoside mediates neutrophil apoptosis. J. Immunol. 2011, 186, 5323–5332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murate, M.; Hayakawa, T.; Ishii, K.; Inadome, H.; Greimel, P.; Watanabe, M.; Nagatsuka, Y.; Ito, K.; Ito, Y.; Takahashi, H.; et al. Phosphatidylglucoside forms specific lipid domains on the outer leaflet of the plasma membrane. Biochemistry 2010, 49, 4732–4739. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Nagatsuka, Y.; Kikuchi, J.; Yokote, T.; Hirabayashi, Y.; Hanafusa, T.; Ozawa, K.; Muroi, K. Preferential expression of phosphatidylglucoside along neutrophil differentiation pathway. Leuk. Lymphoma 2009, 50, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Nagatsuka, Y.; Hara-Yokoyama, M.; Kasama, T.; Takekoshi, M.; Maeda, F.; Ihara, S.; Fujiwara, S.; Ohshima, E.; Ishii, K.; Kobayashi, T.; et al. Carbohydrate-dependent signaling from the phosphatidylglucoside-based microdomain induces granulocytic differentiation of HL60 cells. Proc. Natl. Acad. Sci. USA 2003, 100, 7454–7459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, L.A.; Tavallai, S.; Hamed, H.A.; Cruickshanks, N.; Dent, P. The role of cell signalling in the crosstalk between autophagy and apoptosis. Cell. Signal. 2014, 26, 549–555. [Google Scholar] [CrossRef] [Green Version]
- Delgado, M.E.; Dyck, L.; Laussmann, M.A.; Rehm, M. Modulation of apoptosis sensitivity through the interplay with autophagic and proteasomal degradation pathways. Cell Death Dis. 2014, 5, e1011. [Google Scholar] [CrossRef] [Green Version]
- Cuervo, A.M. Autophagy: In sickness and in health. Trends Cell Biol. 2004, 14, 70–77. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Matarrese, P.; Garofalo, T.; Manganelli, V.; Gambardella, L.; Marconi, M.; Grasso, M.; Tinari, A.; Misasi, R.; Malorni, W.; Sorice, M. Evidence for the involvement of GD3 ganglioside in autophagosome formation and maturation. Autophagy 2014, 10, 750–765. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, M.; Furuta, N.; Matsuda, A.; Nezu, A.; Yamamoto, A.; Fujita, N.; Oomori, H.; Noda, T.; Haraguchi, T.; Hiraoka, Y.; et al. Autophagosomes form at ER-mitochondria contact sites. Nature 2013, 495, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, T.; Matarrese, P.; Manganelli, V.; Marconi, M.; Tinari, A.; Gambardella, L.; Faggioni, A.; Misasi, R.; Sorice, M.; Malorni, W. Evidence for the involvement of lipid rafts localized at the ER-mitochondria associated membranes in autophagosome formation. Autophagy 2016, 12, 917–935. [Google Scholar] [CrossRef] [Green Version]
- Molino, D.; Nascimbeni, A.C.; Giordano, F.; Codogno, P.; Morel, E. ER-driven membrane contact sites: Evolutionary conserved machineries for stress response and autophagy regulation? Commun. Integr. Biol. 2017, 10, e1401699. [Google Scholar] [CrossRef]
- Nascimbeni, A.C.; Giordano, F.; Dupont, N.; Grasso, D.; Vaccaro, M.I.; Codogno, P.; Morel, E. ER-plasma membrane contact sites contribute to autophagosome biogenesis by regulation of local PI3P synthesis. EMBO J. 2017, 36, 2018–2033. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Eissa, N.T. Autophagy in innate and adaptive immunity. Proc. Am. Thorac. Soc. 2010, 7, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Stone, K.; Jales, A.; Leitenberg, D.; Ladisch, S. Inhibition of TLR activation and up-regulation of IL-1R-associated kinase-M expression by exogenous gangliosides. J. Immunol. 2008, 180, 4425–4432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolaeva, S.; Bayunova, L.; Sokolova, T.; Vlasova, Y.; Bachteeva, V.; Avrova, N.; Parnova, R. GM1 and GD1a gangliosides modulate toxic and inflammatory effects of E. coli lipopolysaccharide by preventing TLR4 translocation into lipid rafts. Biochim. Biophys. Acta 2015, 1851, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Han, M.; Park, I.H.; Park, C.H.; Kwak, M.S.; Shin, J.S. Sulfatide Inhibits HMGB1 Secretion by Hindering Toll-Like Receptor 4 Localization Within Lipid Rafts. Front. Immunol. 2020, 11, 1305. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Ikeda, K.; Tokuda, N.; Nishitani, C.; Ohto, U.; Akashi-Takamura, S.; Ito, Y.; Uchikawa, M.; Kuroki, Y.; Taguchi, R.; et al. TLR4-MD-2 complex is negatively regulated by an endogenous ligand, globotetraosylceramide. Proc. Natl. Acad. Sci. USA 2013, 110, 4714–4719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitta, T.; Kanoh, H.; Inamori, K.I.; Suzuki, A.; Takahashi, T.; Inokuchi, J.I. Globo-series glycosphingolipids enhance Toll-like receptor 4-mediated inflammation and play a pathophysiological role in diabetic nephropathy. Glycobiology 2019, 29, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, H.; Nitta, T.; Go, S.; Inamori, K.I.; Veillon, L.; Nihei, W.; Fujii, M.; Kabayama, K.; Shimoyama, A.; Fukase, K.; et al. Homeostatic and pathogenic roles of GM3 ganglioside molecular species in TLR4 signaling in obesity. EMBO J. 2020, 39, e101732. [Google Scholar] [CrossRef]
- Su, L.; Athamna, M.; Wang, Y.; Wang, J.; Freudenberg, M.; Yue, T.; Wang, J.; Moresco, E.M.Y.; He, H.; Zor, T.; et al. Sulfatides are endogenous ligands for the TLR4-MD-2 complex. Proc. Natl. Acad. Sci. USA 2021, 118, e2105316118. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Izumi, Y.; Ishikawa, E.; Kiyotake, R.; Doi, R.; Iwai, S.; Omahdi, Z.; Yamaji, T.; Miyamoto, T.; Bamba, T.; et al. Intracellular metabolite beta-glucosylceramide is an endogenous Mincle ligand possessing immunostimulatory activity. Proc. Natl. Acad. Sci. USA 2017, 114, E3285–E3294. [Google Scholar] [CrossRef] [Green Version]
- Okubo, K.; Brenner, M.D.; Cullere, X.; Saggu, G.; Patchen, M.L.; Bose, N.; Mihori, S.; Yuan, Z.; Lowell, C.A.; Zhu, C.; et al. Inhibitory affinity modulation of FcgammaRIIA ligand binding by glycosphingolipids by inside-out signaling. Cell Rep. 2021, 35, 109142. [Google Scholar] [CrossRef]
- Jales, A.; Falahati, R.; Mari, E.; Stemmy, E.J.; Shen, W.; Southammakosane, C.; Herzog, D.; Ladisch, S.; Leitenberg, D. Ganglioside-exposed dendritic cells inhibit T-cell effector function by promoting regulatory cell activity. Immunology 2011, 132, 134–143. [Google Scholar] [CrossRef]
- Lee, H.C.; Wondimu, A.; Liu, Y.; Ma, J.S.; Radoja, S.; Ladisch, S. Ganglioside inhibition of CD8+ T cell cytotoxicity: Interference with lytic granule trafficking and exocytosis. J. Immunol. 2012, 189, 3521–3527. [Google Scholar] [CrossRef] [Green Version]
- Dillinger, B.; Ahmadi-Erber, S.; Lau, M.; Hoelzl, M.A.; Erhart, F.; Juergens, B.; Fuchs, D.; Heitger, A.; Ladisch, S.; Dohnal, A.M. IFN-gamma and tumor gangliosides: Implications for the tumor microenvironment. Cell. Immunol. 2018, 325, 33–40. [Google Scholar] [CrossRef]
- Kanda, N.; Tamaki, K. Ganglioside GQ1b enhances Ig production by human PBMCs. J. Allergy Clin. Immunol. 1998, 102, 813–820. [Google Scholar] [CrossRef]
- Chatterjee, S.; Balram, A.; Li, W. Convergence: Lactosylceramide-Centric Signaling Pathways Induce Inflammation, Oxidative Stress, and Other Phenotypic Outcomes. Int. J. Mol. Sci. 2021, 22, 1816. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S. Sphingolipids in atherosclerosis and vascular biology. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1523–1533. [Google Scholar] [CrossRef] [Green Version]
- Arai, T.; Bhunia, A.K.; Chatterjee, S.; Bulkley, G.B. Lactosylceramide stimulates human neutrophils to upregulate Mac-1, adhere to endothelium, and generate reactive oxygen metabolites in vitro. Circ. Res. 1998, 82, 540–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulain, D.; Jouault, T. Candida albicans cell wall glycans, host receptors and responses: Elements for a decisive crosstalk. Curr. Opin. Microbiol. 2004, 7, 342–349. [Google Scholar] [CrossRef]
- Kumar, A.; Suryadevara, N.; Hill, T.M.; Bezbradica, J.S.; Van Kaer, L.; Joyce, S. Natural Killer T Cells: An Ecological Evolutionary Developmental Biology Perspective. Front. Immunol. 2017, 8, 1858. [Google Scholar] [CrossRef] [Green Version]
- Kawano, T.; Cui, J.; Koezuka, Y.; Toura, I.; Kaneko, Y.; Sato, H.; Kondo, E.; Harada, M.; Koseki, H.; Nakayama, T.; et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc. Natl. Acad. Sci. USA 1998, 95, 5690–5693. [Google Scholar] [CrossRef] [Green Version]
- Rossjohn, J.; Pellicci, D.G.; Patel, O.; Gapin, L.; Godfrey, D.I. Recognition of CD1d-restricted antigens by natural killer T cells. Nat. Rev. Immunol. 2012, 12, 845–857. [Google Scholar] [CrossRef] [Green Version]
- Kain, L.; Webb, B.; Anderson, B.L.; Deng, S.; Holt, M.; Costanzo, A.; Zhao, M.; Self, K.; Teyton, A.; Everett, C.; et al. The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian alpha-linked glycosylceramides. Immunity 2014, 41, 543–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, B.; Gilbert, J.M.; Stehle, T.; Lencer, W.; Benjamin, T.L.; Rapoport, T.A. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 2003, 22, 4346–4355. [Google Scholar] [CrossRef] [Green Version]
- Fantini, J.; Di Scala, C.; Chahinian, H.; Yahi, N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents 2020, 55, 105960. [Google Scholar] [CrossRef]
- Fantini, J.; Chahinian, H.; Yahi, N. Leveraging coronavirus binding to gangliosides for innovative vaccine and therapeutic strategies against COVID-19. Biochem. Biophys. Res. Commun. 2021, 538, 132–136. [Google Scholar]
- Sorice, M.; Misasi, R.; Riitano, G.; Manganelli, V.; Martellucci, S.; Longo, A.; Garofalo, T.; Mattei, V. Targeting Lipid Rafts as a Strategy Against Coronavirus. Front. Cell Dev. Biol. 2020, 8, 618296. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, C.A. Role of verotoxin receptors in pathogenesis. Trends Microbiol. 1996, 4, 147–153. [Google Scholar] [CrossRef]
- Louise, C.B.; Kaye, S.A.; Boyd, B.; Lingwood, C.A.; Obrig, T.G. Shiga toxin-associated hemolytic uremic syndrome: Effect of sodium butyrate on sensitivity of human umbilical vein endothelial cells to Shiga toxin. Infect. Immun. 1995, 63, 2766–2769. [Google Scholar] [CrossRef] [Green Version]
- Takenouchi, H.; Kiyokawa, N.; Taguchi, T.; Matsui, J.; Katagiri, Y.U.; Okita, H.; Okuda, K.; Fujimoto, J. Shiga toxin binding to globotriaosyl ceramide induces intracellular signals that mediate cytoskeleton remodeling in human renal carcinoma-derived cells. J. Cell Sci. 2004, 117, 3911–3922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyrrell, G.J.; Ramotar, K.; Toye, B.; Boyd, B.; Lingwood, C.A.; Brunton, J.L. Alteration of the carbohydrate binding specificity of verotoxins from Gal alpha 1-4Gal to GalNAc beta 1-3Gal alpha 1-4Gal and vice versa by site-directed mutagenesis of the binding subunit. Proc. Natl. Acad. Sci. USA 1992, 89, 524–528. [Google Scholar] [CrossRef] [Green Version]
- Legros, N.; Dusny, S.; Humpf, H.U.; Pohlentz, G.; Karch, H.; Muthing, J. Shiga toxin glycosphingolipid receptors and their lipid membrane ensemble in primary human blood-brain barrier endothelial cells. Glycobiology 2017, 27, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Legros, N.; Pohlentz, G.; Steil, D.; Muthing, J. Shiga toxin-glycosphingolipid interaction: Status quo of research with focus on primary human brain and kidney endothelial cells. Int. J. Med. Microbiol. 2018, 308, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Detzner, J.; Krojnewski, E.; Pohlentz, G.; Steil, D.; Humpf, H.U.; Mellmann, A.; Karch, H.; Müthing, J. Shiga Toxin (Stx)-Binding Glycosphingolipids of Primary Human Renal Cortical Epithelial Cells (pHRCEpiCs) and Stx-Mediated Cytotoxicity. Toxins 2021, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Koo, S.; Jeong, D.G.; Tesh, V.L. Shiga Toxins as Multi-Functional Proteins: Induction of Host Cellular Stress Responses, Role in Pathogenesis and Therapeutic Applications. Toxins 2016, 8, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.S.; Tesh, V.L. Roles of Shiga Toxins in Immunopathology. Toxins 2019, 11, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bieri, J.; Leisi, R.; Bircher, C.; Ros, C. Human parvovirus B19 interacts with globoside under acidic conditions as an essential step in endocytic trafficking. PLoS Pathog. 2021, 17, e1009434. [Google Scholar] [CrossRef] [PubMed]
- Madar Johansson, M.; Belurier, E.; Papageorgiou, A.C.; Sundin, A.P.; Rahkila, J.; Kallonen, T.; Nilsson, U.J.; Maatsola, S.; Nyholm, T.K.M.; Kapyla, J.; et al. The binding mechanism of the virulence factor Streptococcus suis adhesin P subtype to globotetraosylceramide is associated with systemic disease. J. Biol. Chem. 2020, 295, 14305–14324. [Google Scholar] [CrossRef]
- Mukai, T.; Kaneko, S.; Matsumoto, M.; Ohori, H. Binding of Bifidobacterium bifidum and Lactobacillus reuteri to the carbohydrate moieties of intestinal glycolipids recognized by peanut agglutinin. Int. J. Food Microbiol. 2004, 90, 357–362. [Google Scholar] [CrossRef]
- De Bentzmann, S.; Roger, P.; Dupuit, F.; Bajolet-Laudinat, O.; Fuchey, C.; Plotkowski, M.C.; Puchelle, E. Asialo GM1 is a receptor for Pseudomonas aeruginosa adherence to regenerating respiratory epithelial cells. Infect. Immun. 1996, 64, 1582–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naroeni, A.; Porte, F. Role of cholesterol and the ganglioside GM(1) in entry and short-term survival of Brucella suis in murine macrophages. Infect. Immun. 2002, 70, 1640–1644. [Google Scholar] [CrossRef] [Green Version]
- Cuatrecasas, P. Vibrio cholerae choleragenoid. Mechanism of inhibition of cholera toxin action. Biochemistry 1973, 12, 3577–3581. [Google Scholar] [CrossRef]
- Cuatrecasas, P. Gangliosides and membrane receptors for cholera toxin. Biochemistry 1973, 12, 3558–3566. [Google Scholar] [CrossRef]
- Hyun, C.S.; Kimmich, G.A. Interaction of cholera toxin and Escherichia coli enterotoxin with isolated intestinal epithelial cells. Am. J. Physiol. 1984, 247, G623–G631. [Google Scholar] [CrossRef]
- Ewers, H.; Römer, W.; Smith, A.E.; Bacia, K.; Dmitrieff, S.; Chai, W.; Mancini, R.; Kartenbeck, J.; Chambon, V.; Berland, L.; et al. GM1 structure determines SV40-induced membrane invagination and infection. Nat. Cell Biol. 2010, 1, 11–18. [Google Scholar] [CrossRef]
- Saslowsky, D.E.; Te Welscher, Y.M.; Chinnapen, D.J.; Wagner, J.S.; Wan, J.; Kern, E.; Lencer, W.I. Ganglioside GM1-mediated transcytosis of cholera toxin bypasses the retrograde pathway and depends on the structure of the ceramide domain. J. Biol. Chem. 2013, 288, 25804–25809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabbani, A.M.; Raghunathan, K.; Lencer, W.I.; Kenworthy, A.K.; Kelly, C.V. Structured clustering of the glycosphingolipid GM1 is required for membrane curvature induced by cholera toxin. Proc. Natl. Acad. Sci. USA 2020, 117, 14978–14986. [Google Scholar] [CrossRef]
- Kuziemko, G.M.; Stroh, M.; Stevens, R.C. Cholera toxin binding affinity and specificity for gangliosides determined by surface plasmon resonance. Biochemistry 1996, 35, 6375–6384. [Google Scholar] [CrossRef]
- Masserini, M.; Freire, E.; Palestini, P.; Calappi, E.; Tettamanti, G. Fuc-GM1 ganglioside mimics the receptor function of GM1 for cholera toxin. Biochemistry 1992, 31, 2422–2426. [Google Scholar] [CrossRef]
- Suzuki, T.; Sometani, A.; Yamazaki, Y.; Horiike, G.; Mizutani, Y.; Masuda, H.; Yamada, M.; Tahara, H.; Xu, G.; Miyamoto, D.; et al. Sulphatide binds to human and animal influenza A viruses, and inhibits the viral infection. Biochem. J. 1996, 318, 389–393. [Google Scholar] [CrossRef] [Green Version]
- Bally, M.; Rydell, G.E.; Zahn, R.; Nasir, W.; Eggeling, C.; Breimer, M.E.; Svensson, L.; Hook, F.; Larson, G. Norovirus GII.4 virus-like particles recognize galactosylceramides in domains of planar supported lipid bilayers. Angew. Chem. Int. Ed. Engl. 2012, 51, 12020–12024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckmann, N.; Becker, K.A. Ceramide and Related Molecules in Viral Infections. Int. J. Mol. Sci. 2021, 22, 5676. [Google Scholar]
- Fantini, J.; Hammache, D.; Pieroni, G.; Yahi, N. Role of glycosphingolipid microdomains in CD4-dependent HIV-1 fusion. Glycoconj. J. 2000, 17, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Popik, W.; Alce, T.M.; Au, W.C. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4(+) T cells. J. Virol. 2002, 76, 4709–4722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamiyama, H.; Yoshii, H.; Tanaka, Y.; Sato, H.; Yamamoto, N.; Kubo, Y. Raft localization of CXCR4 is primarily required for X4-tropic human immunodeficiency virus type 1 infection. Virology 2009, 386, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanafusa, K.; Hotta, T.; Iwabuchi, K. Glycolipids: Linchpins in the Organization and Function of Membrane Microdomains. Front. Cell Dev. Biol. 2020, 8, 589799. [Google Scholar] [CrossRef]
- Oishi, K.; Morise, M.; Vo, L.K.; Tran, N.T.; Sahashi, D.; Ueda-Wakamatsu, R.; Nishimura, W.; Komatsu, M.; Shiozaki, K. Host lactosylceramide enhances Edwardsiella tarda infection. Cell. Microbiol. 2021, 9, e13365. [Google Scholar]
- Newburg, D.S.; Chaturvedi, P. Neutral glycolipids of human and bovine milk. Lipids 1992, 27, 923–927. [Google Scholar] [CrossRef]
- Ohno, N.; Uchiyama, M.; Tsuzuki, A.; Tokunaka, K.; Miura, N.N.; Adachi, Y.; Aizawa, M.W.; Tamura, H.; Tanaka, S.; Yadomae, T. Solubilization of yeast cell-wall beta-(1-->3)-D-glucan by sodium hypochlorite oxidation and dimethyl sulfoxide extraction. Carbohydr. Res. 1999, 316, 161–172. [Google Scholar] [CrossRef]
- Zimmerman, J.W.; Lindermuth, J.; Fish, P.A.; Palace, G.P.; Stevenson, T.T.; DeMong, D.E. A novel carbohydrate-glycosphingolipid interaction between a beta-(1-3)-glucan immunomodulator, PGG-glucan, and lactosylceramide of human leukocytes. J. Biol. Chem. 1998, 273, 22014–22020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.D.; Gordon, S. Immune recognition. A new receptor for beta-glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef]
- Briken, V.; Porcelli, S.A.; Besra, G.S.; Kremer, L. Mycobacterial lipoarabinomannan and related lipoglycans: From biogenesis to modulation of the immune response. Mol. Microbiol. 2004, 53, 391–403. [Google Scholar] [CrossRef]
- Mishra, A.K.; Driessen, N.N.; Appelmelk, B.J.; Besra, G.S. Lipoarabinomannan and related glycoconjugates: Structure, biogenesis and role in Mycobacterium tuberculosis physiology and host-pathogen interaction. FEMS Microbiol. Rev. 2011, 35, 1126–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, D.; Obregon-Henao, A.; Pham, H.; Chatterjee, D.; Brennan, P.J.; Jackson, M. Lipoarabinomannan of Mycobacterium: Mannose capping by a multifunctional terminal mannosyltransferase. Proc. Natl. Acad. Sci. USA 2008, 105, 17973–17977. [Google Scholar] [CrossRef] [Green Version]
- Belotserkovsky, I.; Brunner, K.; Pinaud, L.; Rouvinski, A.; Dellarole, M.; Baron, B.; Dubey, G.; Samassa, F.; Parsot, C.; Sansonetti, P.; et al. Glycan-Glycan Interaction Determines Shigella Tropism toward Human T Lymphocytes. mBio 2018, 9, e02309-17. [Google Scholar] [CrossRef] [Green Version]
- Haney, M.S.; Bohlen, C.J.; Morgens, D.W.; Ousey, J.A.; Barkal, A.A.; Tsui, C.K.; Ego, B.K.; Levin, R.; Kamber, R.A.; Collins, H.; et al. Identification of phagocytosis regulators using magnetic genome-wide CRISPR screens. Nat. Genet. 2018, 50, 1716–1727. [Google Scholar] [CrossRef] [PubMed]
- Niekamp, P.; Guzman, G.; Leier, H.C.; Rashidfarrokhi, A.; Richina, V.; Pott, F.; Barisch, C.; Holthuis, J.C.M.; Tafesse, F.G. Sphingomyelin Biosynthesis Is Essential for Phagocytic Signaling during Mycobacterium tuberculosis Host Cell Entry. mBio 2021, 12, e03141-20. [Google Scholar] [CrossRef] [PubMed]
- Pathak, D.; Mehendale, N.; Singh, S.; Mallik, R.; Kamat, S.S. Lipidomics Suggests a New Role for Ceramide Synthase in Phagocytosis. ACS Chem. Biol. 2018, 13, 2280–2287. [Google Scholar] [CrossRef] [Green Version]
- Ellison, C.J.; Kukulski, W.; Boyle, K.B.; Munro, S.; Randow, F. Transbilayer Movement of Sphingomyelin Precedes Catastrophic Breakage of Enterobacteria-Containing Vacuoles. Curr. Biol. 2020, 30, 2974–2983.e2976. [Google Scholar] [CrossRef] [PubMed]
- Thurston, T.L.; Wandel, M.P.; von Muhlinen, N.; Foeglein, A.; Randow, F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 2012, 482, 414–418. [Google Scholar] [CrossRef]
- Niekamp, P.; Sokoya, T.; Vittadello, L.; Deng, Y.; Kim, Y.; Hilderink, A.; Imlau, M.; Clarke, C.J.; Burd, C.G.; Holthuis, J.C.M. Ca2+-activated sphingomyelin scrambling and turnover mediate ESCRT-independent lysosomal repair. bioRxiv 2021. [Google Scholar] [CrossRef]
- Bussi, C.; Gutierrez, M.G. Mycobacterium tuberculosis infection of host cells in space and time. FEMS Microbiol. Rev. 2019, 43, 341–361. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Wang, J.; Sun, T.; Bian, G.; Pan, W.; Feng, T.; Wang, P.; Li, Y.; Dai, J. Glycosphingolipid GM3 is Indispensable for Dengue Virus Genome Replication. Int. J. Biol. Sci. 2016, 12, 872–883. [Google Scholar] [CrossRef] [Green Version]
- Yager, E.J.; Konan, K.V. Sphingolipids as Potential Therapeutic Targets against Enveloped Human RNA Viruses. Viruses 2019, 11, 912. [Google Scholar] [CrossRef] [Green Version]
- Dirlikov, E.; Torres, J.V.; Martines, R.B.; Reagan-Steiner, S.; Perez, G.V.; Rivera, A.; Major, C.; Matos, D.; Munoz-Jordan, J.; Shieh, W.J.; et al. Postmortem Findings in Patient with Guillain-Barre Syndrome and Zika Virus Infection. Emerg. Infect. Dis. 2018, 24, 114–117. [Google Scholar] [CrossRef] [Green Version]
- Cao-Lormeau, V.-M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Correa, J.; de Siqueira, I.C.; Mota, S.; do Rosario, M.S.; Pereira de Jesus, P.A.; Alcantara, L.C.J.; Ernst, J.D.; Rodriguez, A. Anti-ganglioside antibodies in patients with Zika virus infection-associated Guillain-Barre Syndrome in Brazil. PLoS Negl. Trop. Dis. 2019, 13, e0007695. [Google Scholar] [CrossRef] [Green Version]
- Leier, H.C.; Weinstein, J.B.; Kyle, J.E.; Lee, J.Y.; Bramer, L.M.; Stratton, K.G.; Kempthorne, D.; Navratil, A.R.; Tafesse, E.G.; Hornemann, T.; et al. A global lipid map defines a network essential for Zika virus replication. Nat. Commun. 2020, 11, 3652. [Google Scholar] [CrossRef]
- Plante, J.A.; Liu, Y.; Liu, J.; Xia, H.; Johnson, B.A.; Lokugamage, K.G.; Zhang, X.; Muruato, A.E.; Zou, J.; Fontes-Garfias, C.R.; et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature 2021, 592, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Marfia, G.; Navone, S.; Guarnaccia, L.; Campanella, R.; Mondoni, M.; Locatelli, M.; Barassi, A.; Fontana, L.; Palumbo, F.; Garzia, E.; et al. Decreased serum level of sphingosine-1-phosphate: A novel predictor of clinical severity in COVID-19. EMBO Mol. Med. 2021, 13, e13424. [Google Scholar] [CrossRef] [PubMed]
- Rosen, H.; Oldstone, M.B.A. The riddle of the Sphinx: Why sphingosine-1-phosphate may help define molecular mechanisms underlying risk stratification for serious COVID-19 infections. EMBO Mol. Med. 2021, 13, e13533. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Carpinteiro, A.; Gulbins, E. Acid sphingomyelinase amplifies redox signaling in Pseudomonas aeruginosa-induced macrophage apoptosis. J. Immunol. 2008, 181, 4247–4254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kita, K.; Okino, N.; Ito, M. Reverse hydrolysis reaction of a recombinant alkaline ceramidase of Pseudomonas aeruginosa. Biochim. Biophys. Acta 2000, 1485, 111–120. [Google Scholar] [CrossRef]
- Luberto, C.; Stonehouse, M.J.; Collins, E.A.; Marchesini, N.; El-Bawab, S.; Vasil, A.I.; Vasil, M.L.; Hannun, Y.A. Purification, characterization, and identification of a sphingomyelin synthase from Pseudomonas aeruginosa. PlcH is a multifunctional enzyme. J. Biol. Chem. 2003, 278, 32733–32743. [Google Scholar] [CrossRef] [Green Version]
- Okino, N.; Ito, M. Ceramidase enhances phospholipase C-induced hemolysis by Pseudomonas aeruginosa. J. Biol. Chem. 2007, 282, 6021–6030. [Google Scholar] [CrossRef] [Green Version]
- Koch-Edelmann, S.; Banhart, S.; Saied, E.M.; Rose, L.; Aeberhard, L.; Laue, M.; Doellinger, J.; Arenz, C.; Heuer, D. The cellular ceramide transport protein CERT promotes Chlamydia psittaci infection and controls bacterial sphingolipid uptake. Cell. Microbiol. 2017, 19, e12752. [Google Scholar] [CrossRef] [Green Version]
- Banhart, S.; Schafer, E.K.; Gensch, J.M.; Heuer, D. Sphingolipid Metabolism and Transport in Chlamydia trachomatis and Chlamydia psittaci Infections. Front. Cell Dev. Biol. 2019, 7, 223. [Google Scholar] [CrossRef]
- Elwell, C.A.; Jiang, S.; Kim, J.H.; Lee, A.; Wittmann, T.; Hanada, K.; Melancon, P.; Engel, J.N. Chlamydia trachomatis co-opts GBF1 and CERT to acquire host sphingomyelin for distinct roles during intracellular development. PLoS Pathog. 2011, 7, e1002198. [Google Scholar] [CrossRef] [Green Version]
- Tachida, Y.; Kumagai, K.; Sakai, S.; Ando, S.; Yamaji, T.; Hanada, K. Chlamydia trachomatis-infected human cells convert ceramide to sphingomyelin without sphingomyelin synthases 1 and 2. FEBS Lett. 2020, 594, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Capmany, A.; Gambarte Tudela, J.; Alonso Bivou, M.; Damiani, M.T. Akt/AS160 Signaling Pathway Inhibition Impairs Infection by Decreasing Rab14-Controlled Sphingolipids Delivery to Chlamydial Inclusions. Front. Microbiol. 2019, 10, 666. [Google Scholar] [CrossRef]
- Hotinger, J.A.; May, A.E. Antibodies Inhibiting the Type III Secretion System of Gram-Negative Pathogenic Bacteria. Antibodies 2020, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Coburn, B.; Sekirov, I.; Finlay, B.B. Type III secretion systems and disease. Clin. Microbiol. Rev. 2007, 20, 535–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bah, A.; Sanicas, M.; Nigou, J.; Guilhot, C.; Astarie-Dequeker, C.; Vergne, I. The Lipid Virulence Factors of Mycobacterium tuberculosis Exert Multilayered Control over Autophagy-Related Pathways in Infected Human Macrophages. Cells 2020, 9, 666. [Google Scholar] [CrossRef] [Green Version]
- Bernard, E.M.; Fearns, A.; Bussi, C.; Santucci, P.; Peddie, C.J.; Lai, R.J.; Collinson, L.M.; Gutierrez, M.G.M. tuberculosis infection of human iPSC-derived macrophages reveals complex membrane dynamics during xenophagy evasion. J. Cell Sci. 2020, 134, jcs252973. [Google Scholar]
- Sanjuan, M.A.; Dillon, C.P.; Tait, S.W.; Moshiach, S.; Dorsey, F.; Connell, S.; Komatsu, M.; Tanaka, K.; Cleveland, J.L.; Withoff, S.; et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 2007, 450, 1253–1257. [Google Scholar] [CrossRef]
- Herb, M.; Gluschko, A.; Schramm, M. LC3-associated phagocytosis initiated by integrin ITGAM-ITGB2/Mac-1 enhances immunity to Listeria monocytogenes. Autophagy 2018, 14, 1462–1464. [Google Scholar] [CrossRef] [Green Version]
- Heckmann, B.L.; Boada-Romero, E.; Cunha, L.D.; Magne, J.; Green, D.R. LC3-Associated Phagocytosis and Inflammation. J. Mol. Biol. 2017, 429, 3561–3576. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Malireddi, R.K.; Lu, Q.; Cunha, L.D.; Pelletier, S.; Gingras, S.; Orchard, R.; Guan, J.L.; Tan, H.; Peng, J.; et al. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat. Cell Biol. 2015, 17, 893–906. [Google Scholar] [CrossRef] [Green Version]
- Rolando, M.; Escoll, P.; Buchrieser, C. Legionella pneumophila restrains autophagy by modulating the host’s sphingolipid metabolism. Autophagy 2016, 12, 1053–1054. [Google Scholar] [CrossRef] [Green Version]
- Kunz, T.C.; Kozjak-Pavlovic, V. Diverse Facets of Sphingolipid Involvement in Bacterial Infections. Front. Cell Dev. Biol. 2019, 7, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolando, M.; Escoll, P.; Nora, T.; Botti, J.; Boitez, V.; Bedia, C.; Daniels, C.; Abraham, G.; Stogios, P.J.; Skarina, T.; et al. Legionella pneumophila S1P-lyase targets host sphingolipid metabolism and restrains autophagy. Proc. Natl. Acad. Sci. USA 2016, 113, 1901–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, K.A.; Meyer, C.B.; Bouton, A.H.; Casanova, J.E. Activation of focal adhesion kinase by Salmonella suppresses autophagy via an Akt/mTOR signaling pathway and promotes bacterial survival in macrophages. PLoS Pathog. 2014, 10, e1004159. [Google Scholar] [CrossRef] [Green Version]
- Sakuma, C.; Sekizuka, T.; Kuroda, M.; Hanada, K.; Yamaji, T. Identification of SYS1 as a Host Factor Required for Shiga Toxin-Mediated Cytotoxicity in Vero Cells. Int. J. Mol. Sci. 2021, 22, 4936. [Google Scholar] [CrossRef]
- Yamaji, T.; Hanamatsu, H.; Sekizuka, T.; Kuroda, M.; Iwasaki, N.; Ohnishi, M.; Furukawa, J.I.; Yahiro, K.; Hanada, K. A CRISPR Screen Using Subtilase Cytotoxin Identifies SLC39A9 as a Glycan-Regulating Factor. iScience 2019, 15, 407–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaji, T.; Sekizuka, T.; Tachida, Y.; Sakuma, C.; Morimoto, K.; Kuroda, M.; Hanada, K. A CRISPR Screen Identifies LAPTM4A and TM9SF Proteins as Glycolipid-Regulating Factors. iScience 2019, 11, 409–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capolupo, L.; Khven, I.; Mazzeo, L.; Glousker, G.; Russo, F.; Paz Montoya, J.P.; Ho, S.; Bhandari, D.R.; Bowman, A.P.; Ellis, S.R.; et al. Sphingolipid Control of Fibroblast Heterogeneity Revealed by Single-Cell Lipidomics. bioRxiv 2021. [Google Scholar] [CrossRef]
| GSLs | Co-Receptors | Cell Type | Immune Signaling | Ref. No. |
|---|---|---|---|---|
| GlcCer | TLR4 | Macrophages | Impact on LPS/TLR4 orientation and Mal-associated signaling | [47] |
| GA1 | TLR5 | Lung epithelial cells NCIH292 | Flagellin-mediated autocrine release of ATP | [49] |
| GD1a | TLR2/TLR1 | Monocytes | LT-IIb-B5-mediated NFκB activation | [50] |
| LacCer | CD11b/CD18 | Neutrophils | Lyn and Akt activations, and the resulting phagocytosis of zymosan and mycobacteria | [20,51] |
| Gb3Cer | CD59 | Lung epithelial cells H1299 | PIP3 and flotillin-associated uptake of P. aeruginosa | [52] |
| Neolacto-series GSLs | MHC class I | HAP1 cells | Interference of the accessibility of MHC class I molecules for immune cell receptors and the resulting suppression of CD8+ T-cell activation | [53] |
| GM1, GM3 | CD4, LFA-1 | T-cell line | PI3K and p56lck-associated T-cell responses | [54] |
| a-Series gangliosides | CD4, TCR | T cells | Helper T-cell activation | [55] |
| Asialo-series gangliosides | CD8, TCR | T cells | Killer T-cell activation | [55] |
| GM1 | IgM-BCR | Immature B cells | Removal of autoreactive immature B cells (apoptosis) | [56] |
| GM3 | CD95/Fas | T cells | Formation of death-inducing signaling complex upon CD95/Fas engagement (apoptosis) | [57] |
| GSLs | Immune Functions | Ref. No. |
|---|---|---|
| GM1, GD1a, GD1b | Inhibition of TLRs (TLR2, 3, 4, 6 and 7/8)-mediated IL-6, IL-12 and TNF-α production in monocytes and immature DCs | [110] |
| GM1, GD1a | Inhibition of LPS-induced biological effects in PC12 and epithelial cells | [111] |
| SM4 | Inhibition of LPS-induced TLR4 colocalization with CTxB-positive ganglioside-rich microdomains and HMGB1 secretion in Raw 264.7 cells | [112] |
| Gb4Cer | Inhibition of LPS binding to TLR4 and attenuation of TLR4-MD-2-mediated LPS signaling in vascular endothelial cells | [113] |
| Gb3Cer/Gb4Cer | Enhancement of TLR4-mediated inflammation in mouse BMDMs and human monocytes | [114] |
| GM3 (C22:0, C24:0 or hC24:0 fatty acid) | Enhancement of LPS/HMGB1-associated TLR4 signaling in monocytes | [115] |
| GM3 (C16:0, C18:0 or C24:1 fatty acid) | Inhibition of LPS/HMGB1-associated TLR4 signaling in monocytes | [115] |
| SM4 (C12 or C16 fatty acid) | Activation of TLR4-MD-2 in mouse macrophages | [116] |
| SM4 (C12 or C16 fatty acid) | Antagonizing effect on TLR4-MD-2 activation in human macrophage-like PMA-differentiated THP-1 cells | [116] |
| β-GlcCer | Immunostimulatory factor upon cell damage, endogenous ligand for Mincle | [117] |
| LacCer (C24:0 or C24:1 fatty acid) | Enhancement of activated Lyn-mediated neutrophil functions (chemotaxis, phagocytosis and superoxide generation) in DMSO-treated HL-60 cells | [18,20,51,61] |
| LacCer (C24:0 or C24:1 fatty acid) | Induction of β-glucan binding-dependent SHP-1 phosphorylation through Lyn and the resulting reduction of FcγRIIA affinity in DMF-treated HL-60 cells | [118] |
| Gangliosides | Facilitation of the development of regulatory T-cell activity in murine BMDCs | [119] |
| Gangliosides (tumor derived) | Inhibition of lytic function in CD8+ CTLs | [120] |
| Gangliosides | Cooperative role with IFN-γ to inhibit the immnostimulatory activity of DCs | [121] |
| GQ1b | Facilitation of T-cell-mediated cytokine production, which possibly involves indirect enhancement of B-cell production of Ig | [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokoyama, N.; Hanafusa, K.; Hotta, T.; Oshima, E.; Iwabuchi, K.; Nakayama, H. Multiplicity of Glycosphingolipid-Enriched Microdomain-Driven Immune Signaling. Int. J. Mol. Sci. 2021, 22, 9565. https://doi.org/10.3390/ijms22179565
Yokoyama N, Hanafusa K, Hotta T, Oshima E, Iwabuchi K, Nakayama H. Multiplicity of Glycosphingolipid-Enriched Microdomain-Driven Immune Signaling. International Journal of Molecular Sciences. 2021; 22(17):9565. https://doi.org/10.3390/ijms22179565
Chicago/Turabian StyleYokoyama, Noriko, Kei Hanafusa, Tomomi Hotta, Eriko Oshima, Kazuhisa Iwabuchi, and Hitoshi Nakayama. 2021. "Multiplicity of Glycosphingolipid-Enriched Microdomain-Driven Immune Signaling" International Journal of Molecular Sciences 22, no. 17: 9565. https://doi.org/10.3390/ijms22179565
APA StyleYokoyama, N., Hanafusa, K., Hotta, T., Oshima, E., Iwabuchi, K., & Nakayama, H. (2021). Multiplicity of Glycosphingolipid-Enriched Microdomain-Driven Immune Signaling. International Journal of Molecular Sciences, 22(17), 9565. https://doi.org/10.3390/ijms22179565






