Trans-Chalcone Plus Baicalein Synergistically Reduce Intracellular Amyloid Beta (Aβ42) and Protect from Aβ42 Induced Oxidative Damage in Yeast Models of Alzheimer’s Disease
Abstract
1. Introduction
2. Results
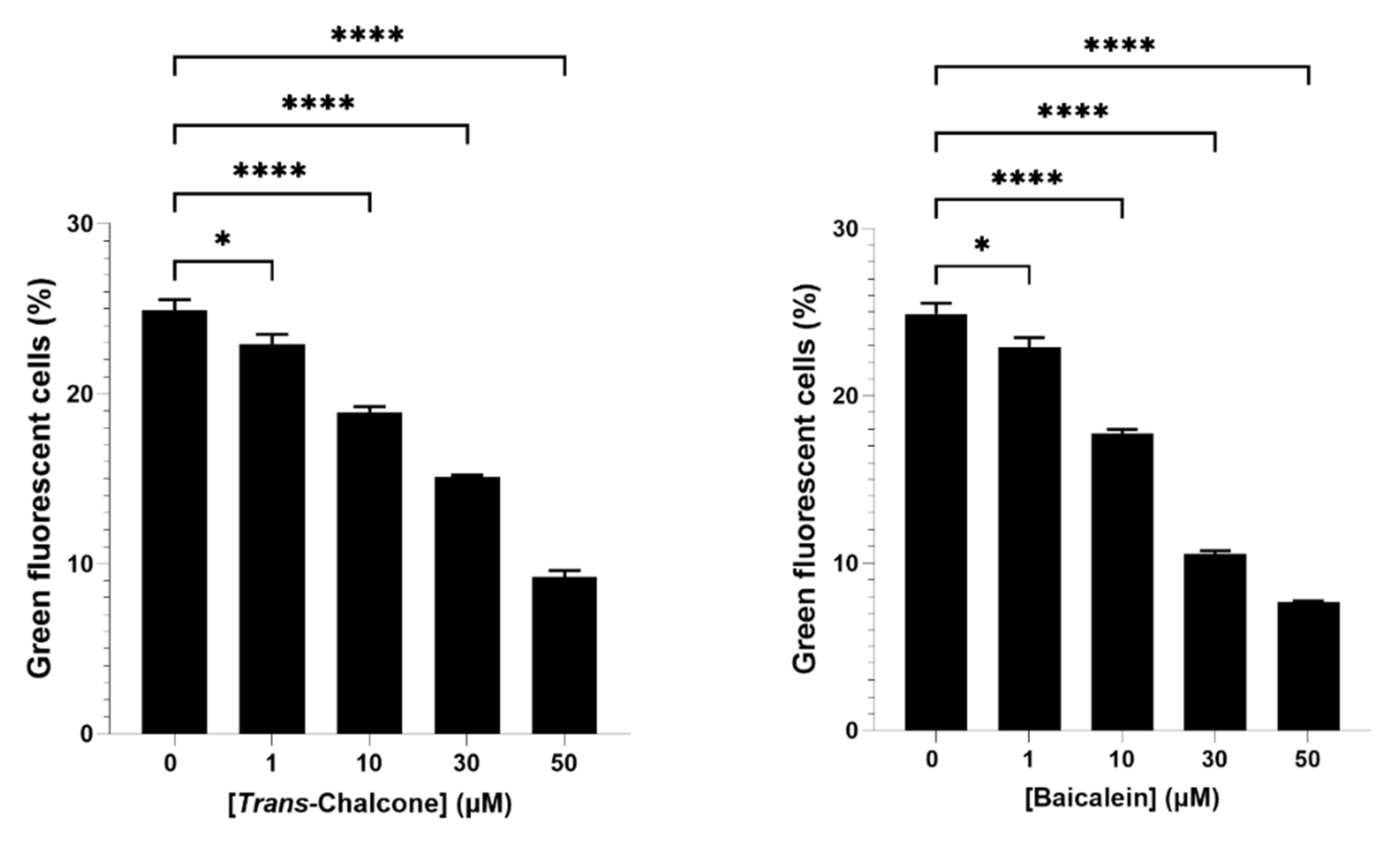
2.1. Baicalein and Trans-Chalcone Were the Most Effective Compounds to Reduce Green Fluorescent Cells
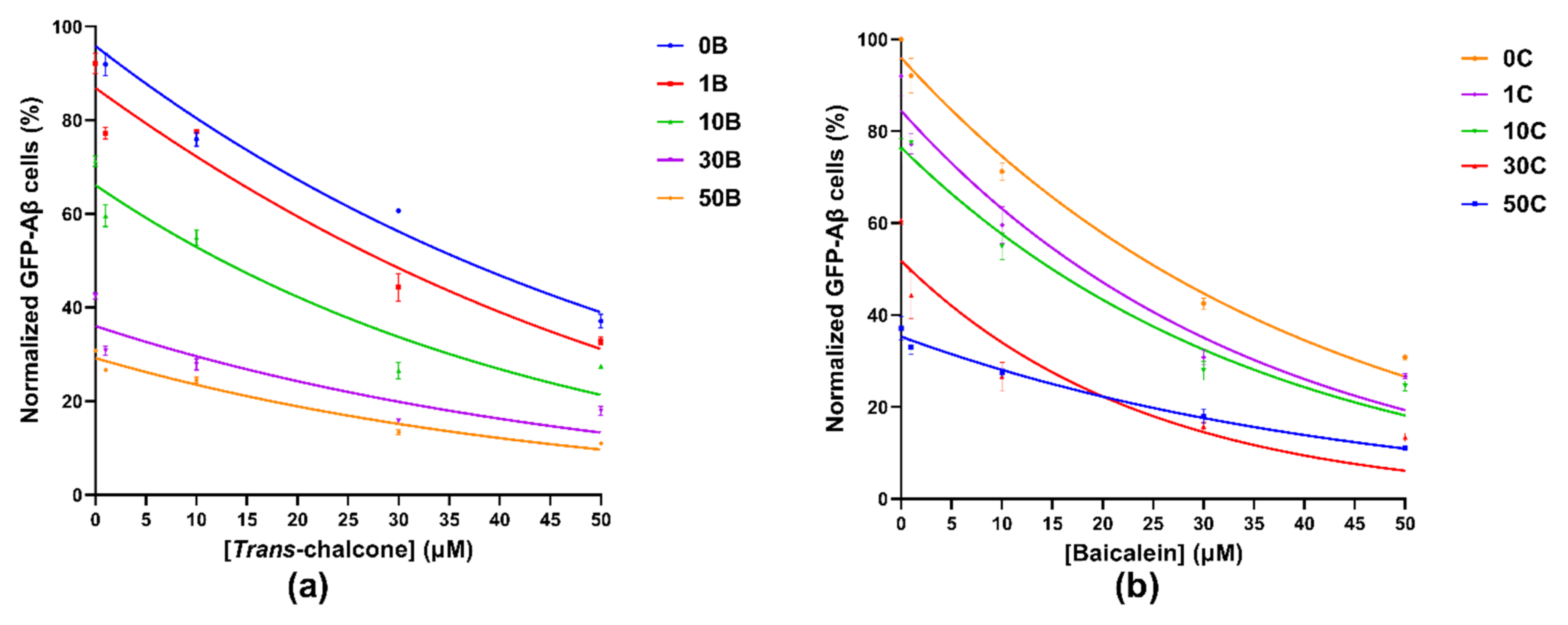
2.2. Combination of Baicalein and Trans-Chalcone Significantly Reduced Levels of GFP-Aβ42 as Compared to Single Compound Treatments
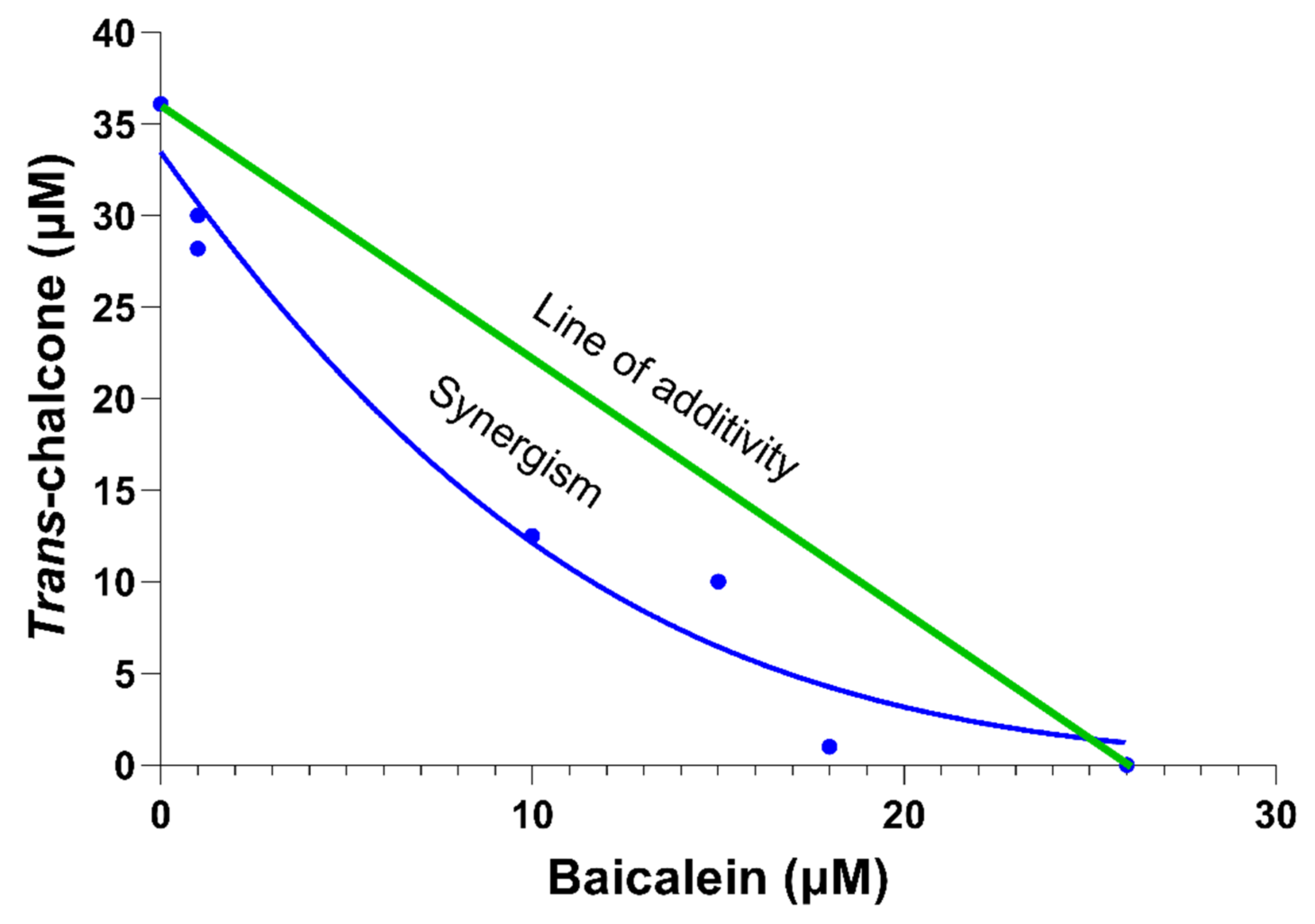
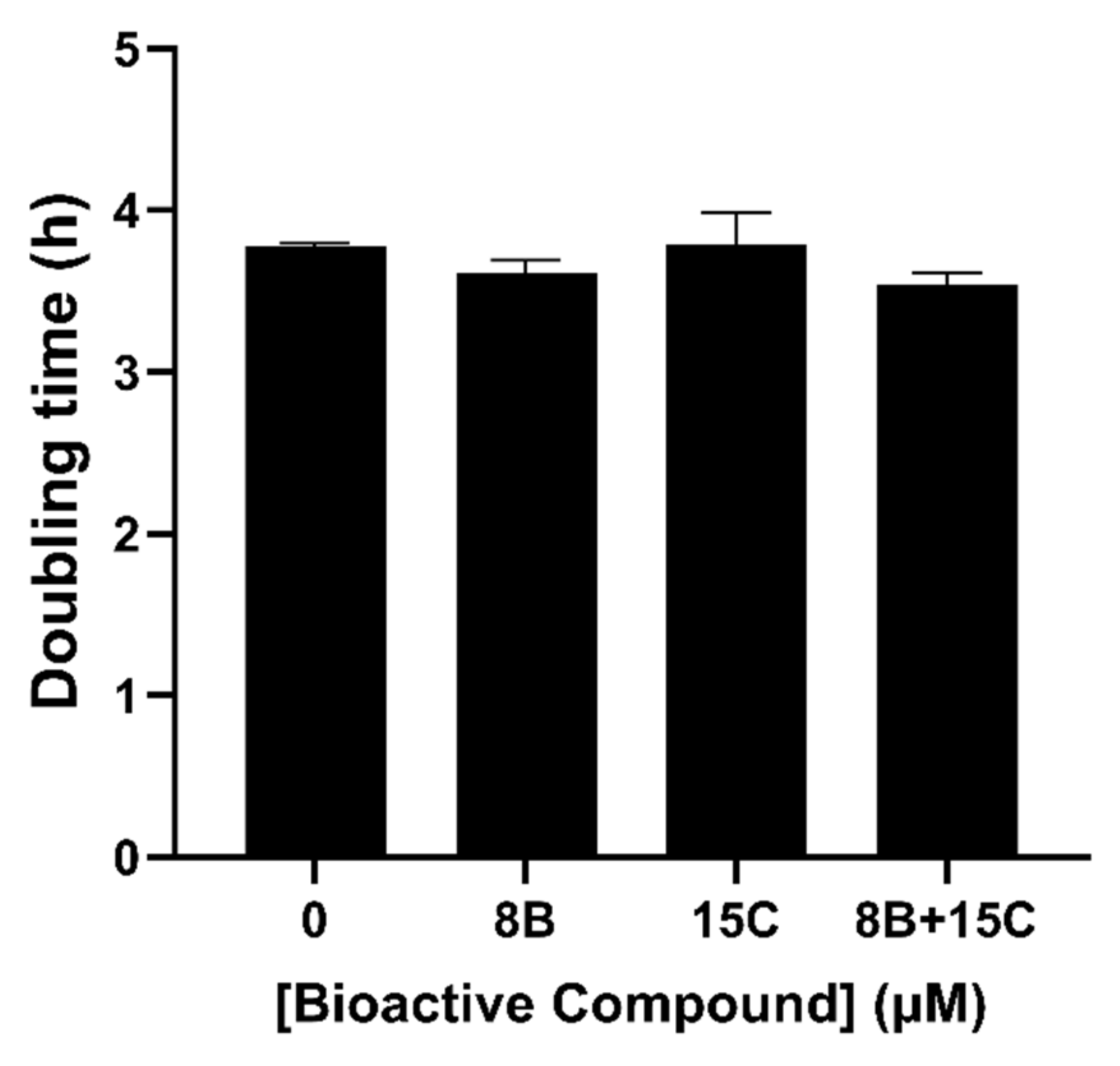
2.3. The Most Effective Combination of Baicalein and Trans-Chalcone to Show Synergy in Reducing GFP-Aβ42 Was Not Growth Inhibitory
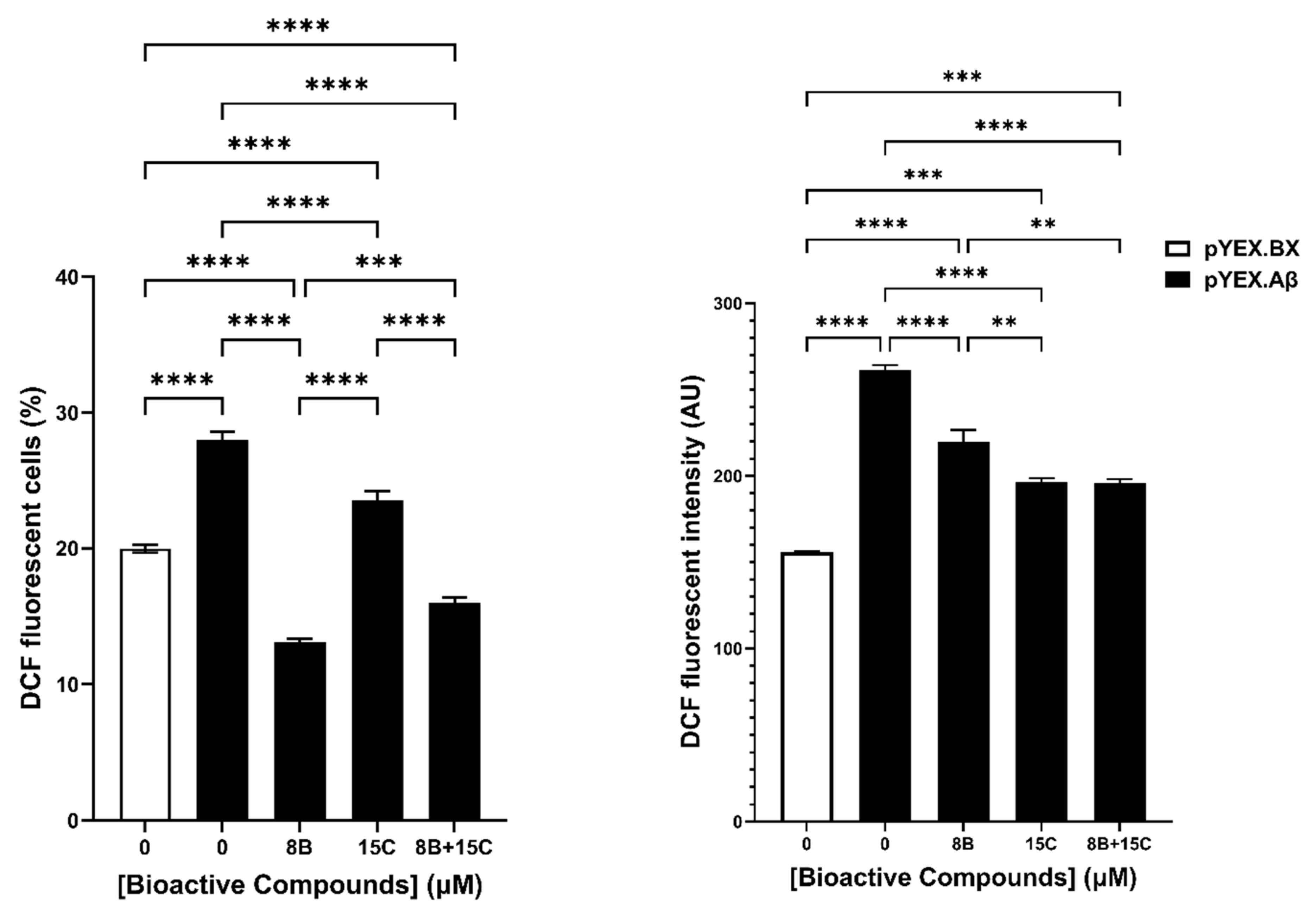
2.4. 8 μM Baicalein Plus 15 μM Trans-Chalcone Does Not Inhibit Growth, Reduces GFP-Aβ42 and Rescues Cells from Aβ42-Induced ROS
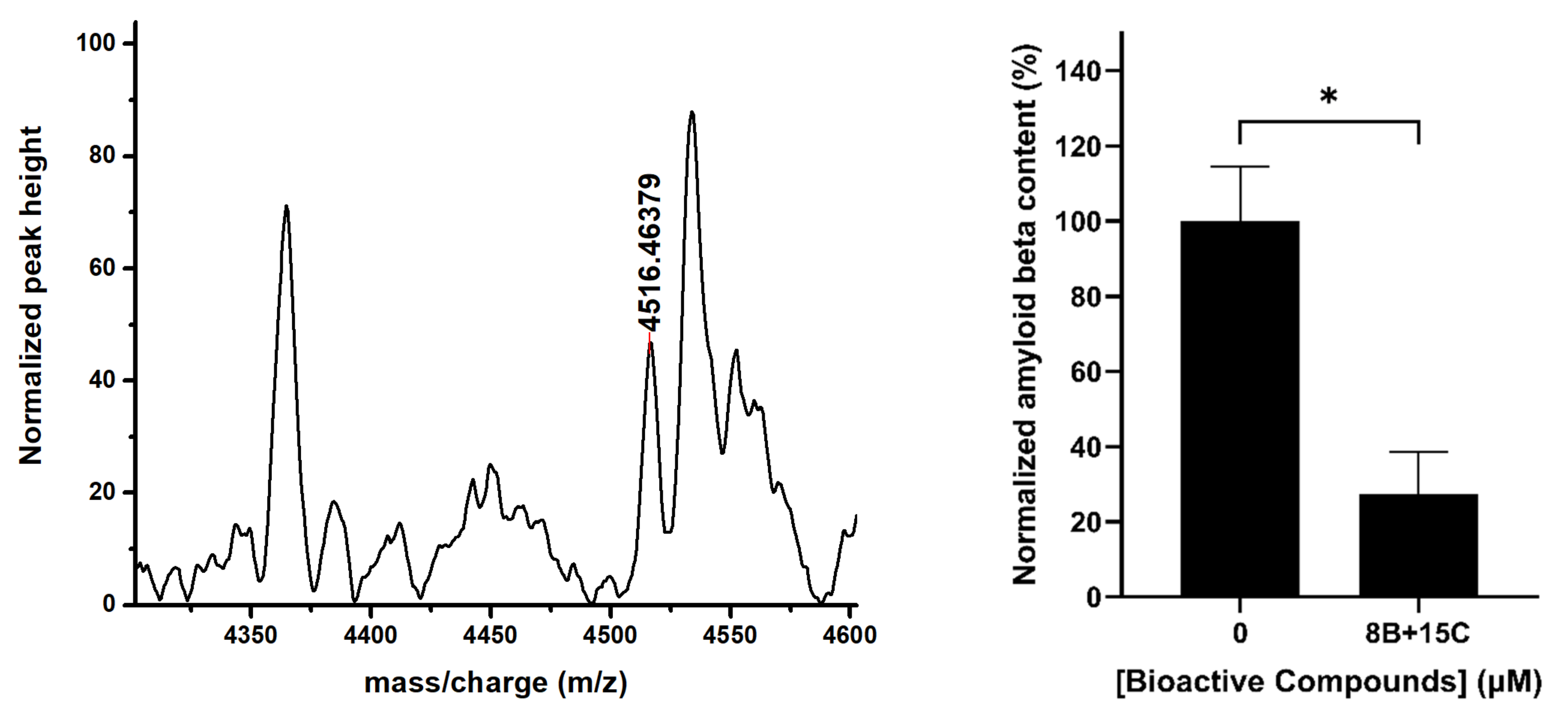
2.5. 15 μM Trans-Chalcone and 8 μM Baicalein Significantly Reduced Aβ42 Levels
3. Discussion
4. Materials and Methods
4.1. Strains of Yeast and Culture Media
4.2. Screening of Bioactive Compounds That Reduce Expression of GFP-Aβ42
4.3. Evaluation of Combination Effect of Two Most Effective Compounds for Their Ability to Reduce GFP-Aβ42 Levels
4.4. Growth Inhibition Assay
4.5. Measurement of ROS Using 2′,7′-Dichlorodihydrofluorescein Diacetate (H2DCFDA) Staining
4.6. Determination of Aβ42 Protein Levels by MALDI-TOF MS
4.6.1. Chemical Treatment of Yeast Cells and Extraction of Cell Lysates for Determination of Amyloid Beta Turnover
4.6.2. MALDI-TOF Analysis of the Aβ42 Levels in Yeast Cell Lysates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhakal, S.; Kushairi, N.; Phan, C.W.; Adhikari, B.; Sabaratnam, V.; Macreadie, I. Dietary polyphenols: A multifactorial strategy to target Alzheimer’s disease. Int. J. Mol. Sci. 2019, 20, 5090. [Google Scholar] [CrossRef] [PubMed]
- Se Thoe, E.; Fauzi, A.; Tang, Y.Q.; Chamyuang, S.; Chia, A.Y.Y. A review on advances of treatment modalities for Alzheimer’s disease. Life Sci. 2021, 276, 119129. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Wightman, E.L. Potential benefits of phytochemicals against Alzheimer’s disease. Proc. Nutr. Soc. 2017, 76, 106–112. [Google Scholar] [CrossRef]
- Choi, D.-Y.; Lee, Y.-J.; Hong, J.T.; Lee, H.-J. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Res. Bull. 2012, 87, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Caine, J.; Sankovich, S.; Antony, H.; Waddington, L.; Macreadie, P.; Varghese, J.; Macreadie, I. Alzheimer’s Aβ fused to green fluorescent protein induces growth stress and a heat shock response. FEMS Yeast Res. 2007, 7, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Macreadie, I.; Luu, Y.N. How Yeast Can Inform Us about Healthy Aging. Open J. Soc. Sci. 2018, 6, 8. [Google Scholar] [CrossRef][Green Version]
- Mcdonald, J.B.; Dhakal, S.; Macreadie, I. Yeast contributions to Alzheimer’s Disease. J. Hum. Clin. Genet. 2020, 2, 1–19. [Google Scholar] [CrossRef]
- Dhakal, S.; Macreadie, I. Protein homeostasis networks and the use of yeast to guide interventions in Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 8014. [Google Scholar] [CrossRef] [PubMed]
- Porzoor, A.; Macreadie, I. Yeast as a model for studies on Aβ aggregation toxicity in Alzheimer’s disease, autophagic responses, and drug screening. Methods Mol. Biol. 2016, 1303, 217–226. [Google Scholar]
- Antony, H. Study of Cellular Responses and Protein Interactions of Abeta in Yeast Models; RMIT University: Melbourne, VIC, Australia, 2008; pp. 1–196. [Google Scholar]
- Macreadie, I.; Dhakal, S. Insights from yeast on oxidative stress in Alzheimer’s Disease, focusing on Ahp1p/Prx5. OBM Geriatr. 2019, 3, 10. [Google Scholar] [CrossRef]
- Porzoor, A.; Alford, B.; Hügel, H.M.; Grando, D.; Caine, J.; Macreadie, I. Anti-amyloidogenic properties of some phenolic compounds. Biomolecules 2015, 5, 505–527. [Google Scholar] [CrossRef] [PubMed]
- Tallarida, R.J. Quantitative methods for assessing drug synergism. Genes Cancer 2011, 2, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Birch-Machin, M.A.; Bowman, A. Oxidative stress and ageing. Br. J. Dermatol. 2016, 175, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Dillin, A.; Cohen, E. Ageing and protein aggregation-mediated disorders: From invertebrates to mammals. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 94–98. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Pagani, L.; Eckert, A. Amyloid-beta interaction with mitochondria. Int. J. Alzheimer’s Dis. 2011, 2011, 925050. [Google Scholar] [CrossRef]
- Sardar Sinha, M.; Ansell-Schultz, A.; Civitelli, L.; Hildesjö, C.; Larsson, M.; Lannfelt, L.; Ingelsson, M.; Hallbeck, M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018, 136, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.R.; Lind, M.; Wagen, A.Z.; Rembach, A.; Frugier, T.; Li, Q.-X.; Ryan, T.M.; McLean, C.A.; Doecke, J.D.; Rowe, C.C.; et al. Biochemically-defined pools of amyloid-β in sporadic Alzheimer’s disease: Correlation with amyloid PET. Brain 2017, 140, 1486–1498. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary anti-aging polyphenols and potential mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef]
- Molino, S.; Dossena, M.; Buonocore, D.; Ferrari, F.; Venturini, L.; Ricevuti, G.; Verri, M. Polyphenols in dementia: From molecular basis to clinical trials. Life Sci. 2016, 161, 69–77. [Google Scholar] [CrossRef]
- Schaffer, S.; Halliwell, B. Do polyphenols enter the brain and does it matter? Some theoretical and practical considerations. Genes Nutr. 2012, 7, 99–109. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Ha, P.T.; Nguyen, A.S.; Nguyen, D.T.; Do, H.D.; Thi, Q.N.; Thi, M.N.H. Curcumin as fluorescent probe for directly monitoring in vitro uptake of curcumin combined paclitaxel loaded PLA-TPGS nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 25001. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Hölscher, C. Therapeutic Potential of Baicalein in Alzheimer’s Disease and Parkinson’s Disease. CNS Drugs 2017, 31, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cui, W.; Li, G.; Yuan, S.; Xu, D.; Hoi, M.P.M.; Lin, Z.; Dou, J.; Han, Y.; Lee, S.M.Y. Baicalein protects against 6-OHDA-induced neurotoxicity through activation of Keap1/Nrf2/HO-1 and involving PKCα and PI3K/AKT signaling pathways. J. Agric. Food Chem. 2012, 60, 8171–8182. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Perez, C.A.; Wei, Y.; Guo, M. Iron-binding and anti-Fenton properties of baicalein and baicalin. J. Inorg. Biochem. 2009, 103, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.H.; Ardah, M.T.; Durairajan, S.S.; Liu, L.F.; Xie, L.X.; Fong, W.F.; Hasan, M.Y.; Huang, J.D.; El-Agnaf, O.M.; Li, M. Baicalein inhibits formation of α-synuclein oligomers within living cells and prevents Aβ peptide fibrillation and oligomerisation. Chembiochem. Eur. J. Chem. Biol. 2011, 12, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Q.; Obregon, D.; Ehrhart, J.; Deng, J.; Tian, J.; Hou, H.; Giunta, B.; Sawmiller, D.; Tan, J. Baicalein reduces β-amyloid and promotes nonamyloidogenic amyloid precursor protein processing in an Alzheimer’s disease transgenic mouse model. J. Neurosci. Res. 2013, 91, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, J.; Wang, K.S.; Mi, C.; Wang, Z.; Piao, L.X.; Xu, G.H.; Li, X.; Lee, J.J.; Jin, X. Baicalein inhibits TNF-α-induced NF-κB activation and expression of NF-κB-regulated target gene products. Oncol. Rep. 2016, 36, 2771–2776. [Google Scholar] [CrossRef]
- Zhu, M.; Rajamani, S.; Kaylor, J.; Han, S.; Zhou, F.; Fink, A.L. The flavonoid baicalein inhibits fibrillation of alpha-synuclein and disaggregates existing fibrils. J. Biol. Chem. 2004, 279, 26846–26857. [Google Scholar] [CrossRef]
- Sonawane, S.K.; Balmik, A.A.; Boral, D.; Ramasamy, S.; Chinnathambi, S. Baicalein suppresses repeat tau fibrillization by sequestering oligomers. Arch. Biochem. Biophys. 2019, 675, 108119. [Google Scholar] [CrossRef]
- Zhang, X.; Du, L.; Zhang, W.; Yang, Y.; Zhou, Q.; Du, G. Therapeutic effects of baicalein on rotenone-induced Parkinson’s disease through protecting mitochondrial function and biogenesis. Sci. Rep. 2017, 7, 9968. [Google Scholar] [CrossRef]
- Zhu, X.; Yao, P.; Liu, J.; Guo, X.; Jiang, C.; Tang, Y. Baicalein attenuates impairment of hepatic lysosomal acidification induced by high fat diet via maintaining V-ATPase assembly. Food Chem. Toxicol. 2020, 136, 110990. [Google Scholar] [CrossRef]
- Han, J.; Ji, Y.; Youn, K.; Lim, G.; Lee, J.; Kim, D.H.; Jun, M. Baicalein as a potential inhibitor against BACE1 and AChE: Mechanistic comprehension through in vitro and computational approaches. Nutrients 2019, 11, 2694. [Google Scholar] [CrossRef]
- Yaghmaei, P.; Kheirbakhsh, R.; Dezfulian, M.; Haeri-Rohani, A.; Larijani, B.; Ebrahim-Habibi, A. Indole and trans-chalcone attenuate amyloid β plaque accumulation in male Wistar rat: In vivo effectiveness of two anti-amyloid scaffolds. Arch. Ital. Biol. 2013, 151, 106–113. [Google Scholar] [PubMed]
- Karimi-Sales, E.; Mohaddes, G.; Alipour, M.R. Chalcones as putative hepatoprotective agents: Preclinical evidence and molecular mechanisms. Pharm. Res. 2018, 129, 177–187. [Google Scholar] [CrossRef]
- Thapa, P.; Upadhyay, S.P.; Suo, W.Z.; Singh, V.; Gurung, P.; Lee, E.S.; Sharma, R.; Sharma, M. Chalcone and its analogs: Therapeutic and diagnostic applications in Alzheimer’s disease. Bioorg. Chem. 2021, 108, 104681. [Google Scholar] [CrossRef] [PubMed]
- Bortolotto, L.F.; Barbosa, F.R.; Silva, G.; Bitencourt, T.A.; Beleboni, R.O.; Baek, S.J.; Marins, M.; Fachin, A.L. Cytotoxicity of trans-chalcone and licochalcone A against breast cancer cells is due to apoptosis induction and cell cycle arrest. Biomed. Pharmacother. 2017, 85, 425–433. [Google Scholar] [CrossRef]
- Koseoglu, M.M.; Norambuena, A.; Sharlow, E.R.; Lazo, J.S.; Bloom, G.S. Aberrant neuronal cell cycle re-entry: The pathological confluence of Alzheimer’s disease and brain insulin resistance, and its relation to cancer. J. Alzheimer’s Dis. 2019, 67, 1–11. [Google Scholar] [CrossRef]
- Dhakal, S.; Subhan, M.; Fraser, J.M.; Gardiner, K.; Macreadie, I. Simvastatin efficiently reduces levels of Alzheimer’s amyloid beta in Yeast. Int. J. Mol. Sci. 2019, 20, 3531. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; Macreadie, I. Tyramine and amyloid beta 42: A toxic synergy. Biomedicines 2020, 8, 145. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhakal, S.; Ramsland, P.A.; Adhikari, B.; Macreadie, I. Trans-Chalcone Plus Baicalein Synergistically Reduce Intracellular Amyloid Beta (Aβ42) and Protect from Aβ42 Induced Oxidative Damage in Yeast Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 9456. https://doi.org/10.3390/ijms22179456
Dhakal S, Ramsland PA, Adhikari B, Macreadie I. Trans-Chalcone Plus Baicalein Synergistically Reduce Intracellular Amyloid Beta (Aβ42) and Protect from Aβ42 Induced Oxidative Damage in Yeast Models of Alzheimer’s Disease. International Journal of Molecular Sciences. 2021; 22(17):9456. https://doi.org/10.3390/ijms22179456
Chicago/Turabian StyleDhakal, Sudip, Paul A. Ramsland, Benu Adhikari, and Ian Macreadie. 2021. "Trans-Chalcone Plus Baicalein Synergistically Reduce Intracellular Amyloid Beta (Aβ42) and Protect from Aβ42 Induced Oxidative Damage in Yeast Models of Alzheimer’s Disease" International Journal of Molecular Sciences 22, no. 17: 9456. https://doi.org/10.3390/ijms22179456
APA StyleDhakal, S., Ramsland, P. A., Adhikari, B., & Macreadie, I. (2021). Trans-Chalcone Plus Baicalein Synergistically Reduce Intracellular Amyloid Beta (Aβ42) and Protect from Aβ42 Induced Oxidative Damage in Yeast Models of Alzheimer’s Disease. International Journal of Molecular Sciences, 22(17), 9456. https://doi.org/10.3390/ijms22179456







