Electrochemical Molecular Conversion of α-Keto Acid to Amino Acid at a Low Overpotential Using a Nanoporous Gold Catalyst
Abstract
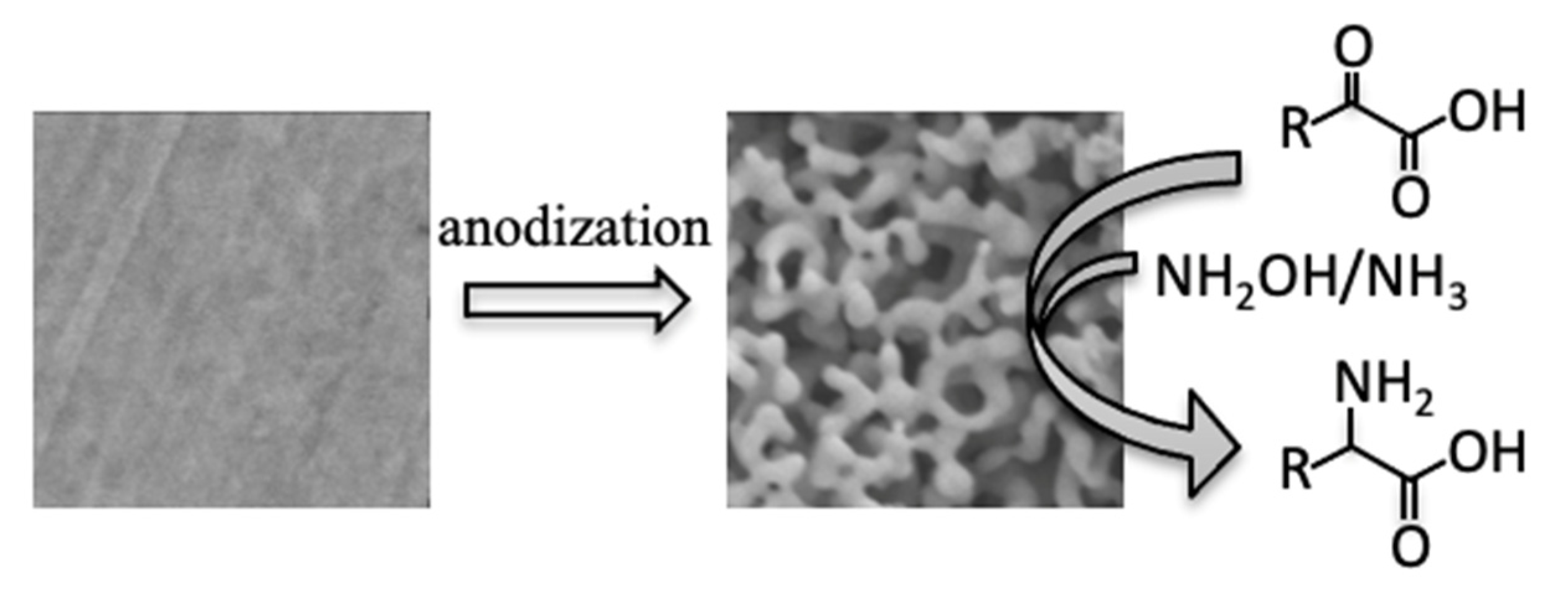
:1. Introduction
2. Results and Discussion
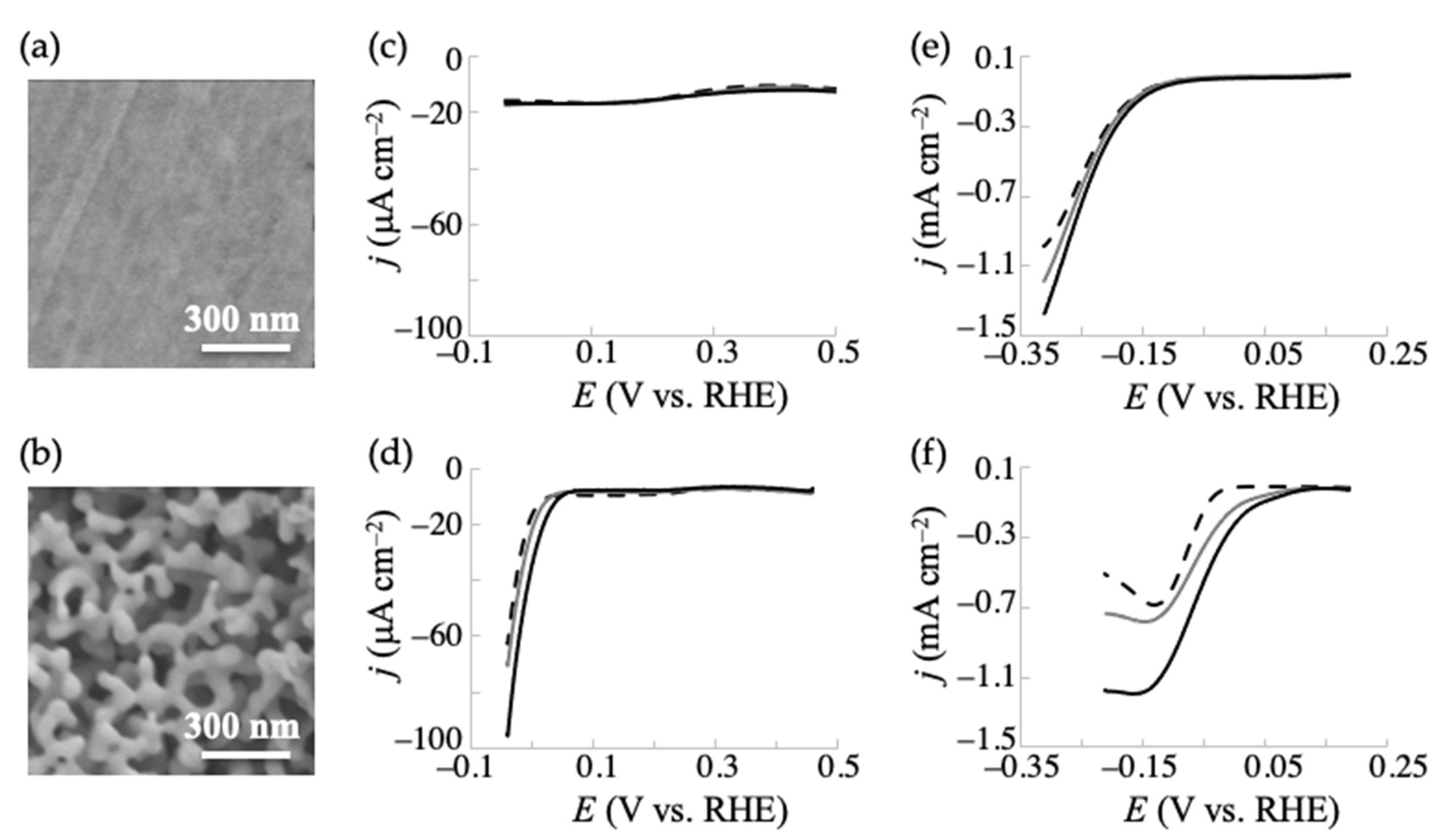
2.1. Electrochemical Reduction of Ketoglutaric Acid at NPG and Conventional (Planar) Gold Electrodes in the Presence of a Nitrogen Source
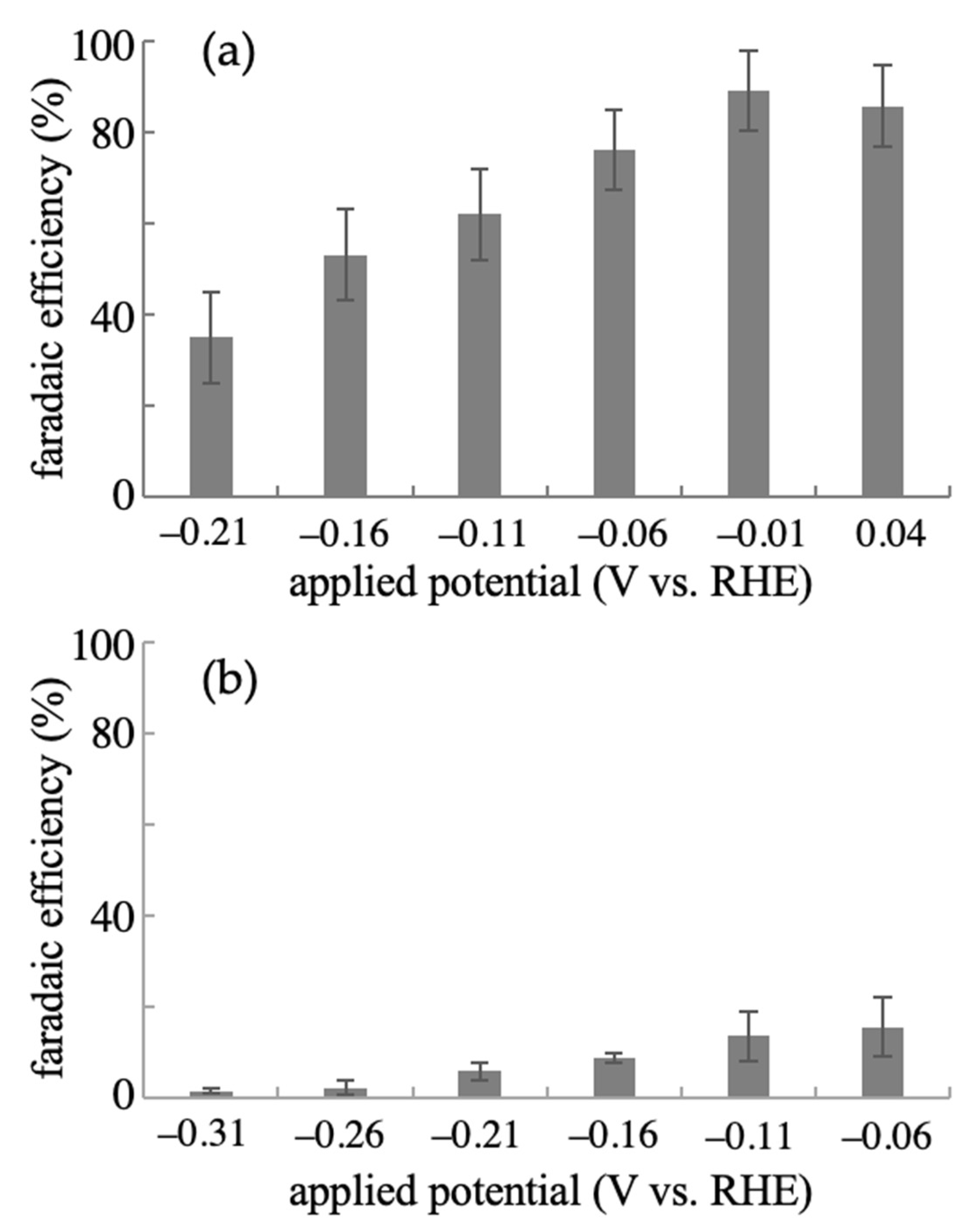
2.2. Effect of Applied Potential on the Electrochemical Production of L-Glutamic Acid
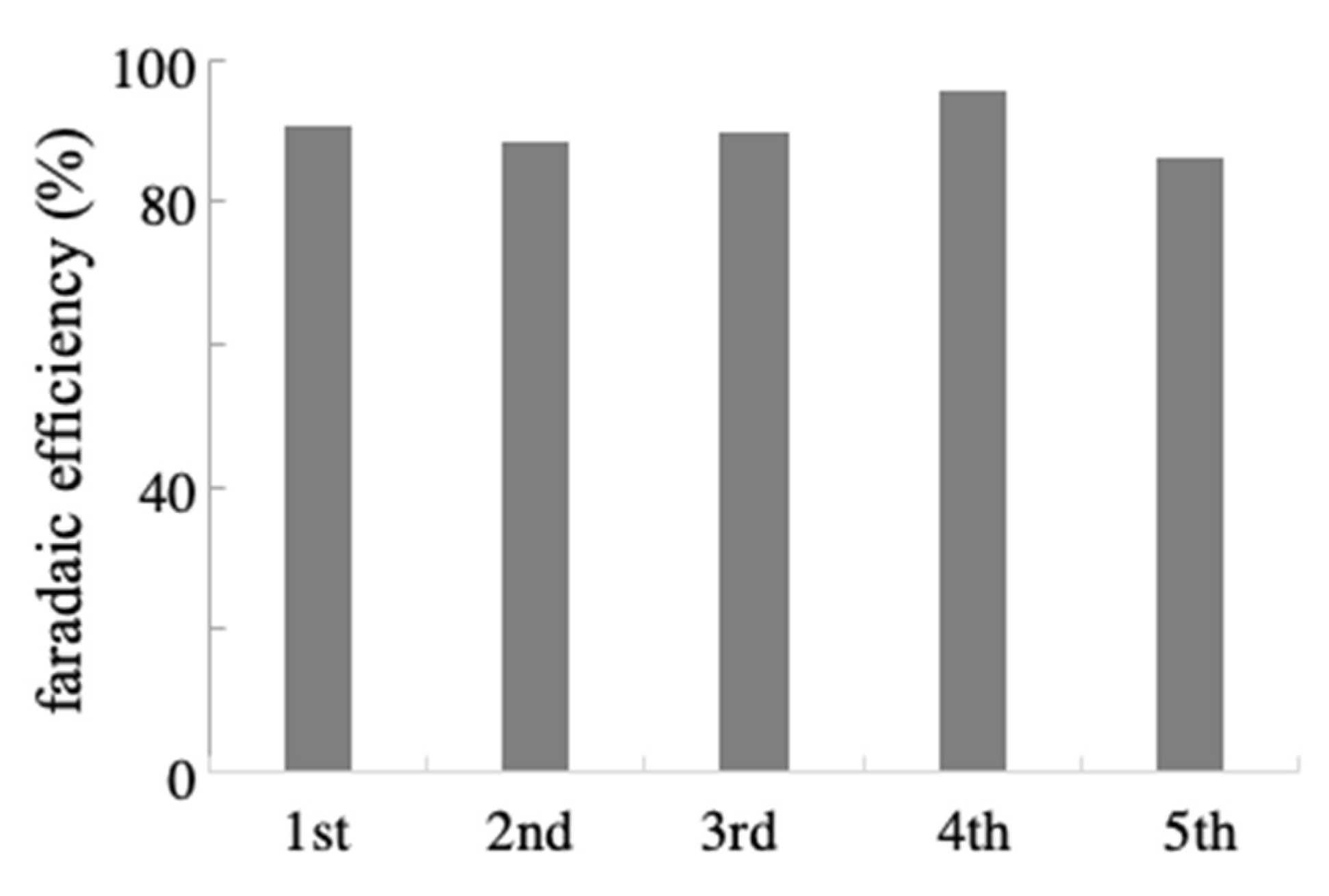
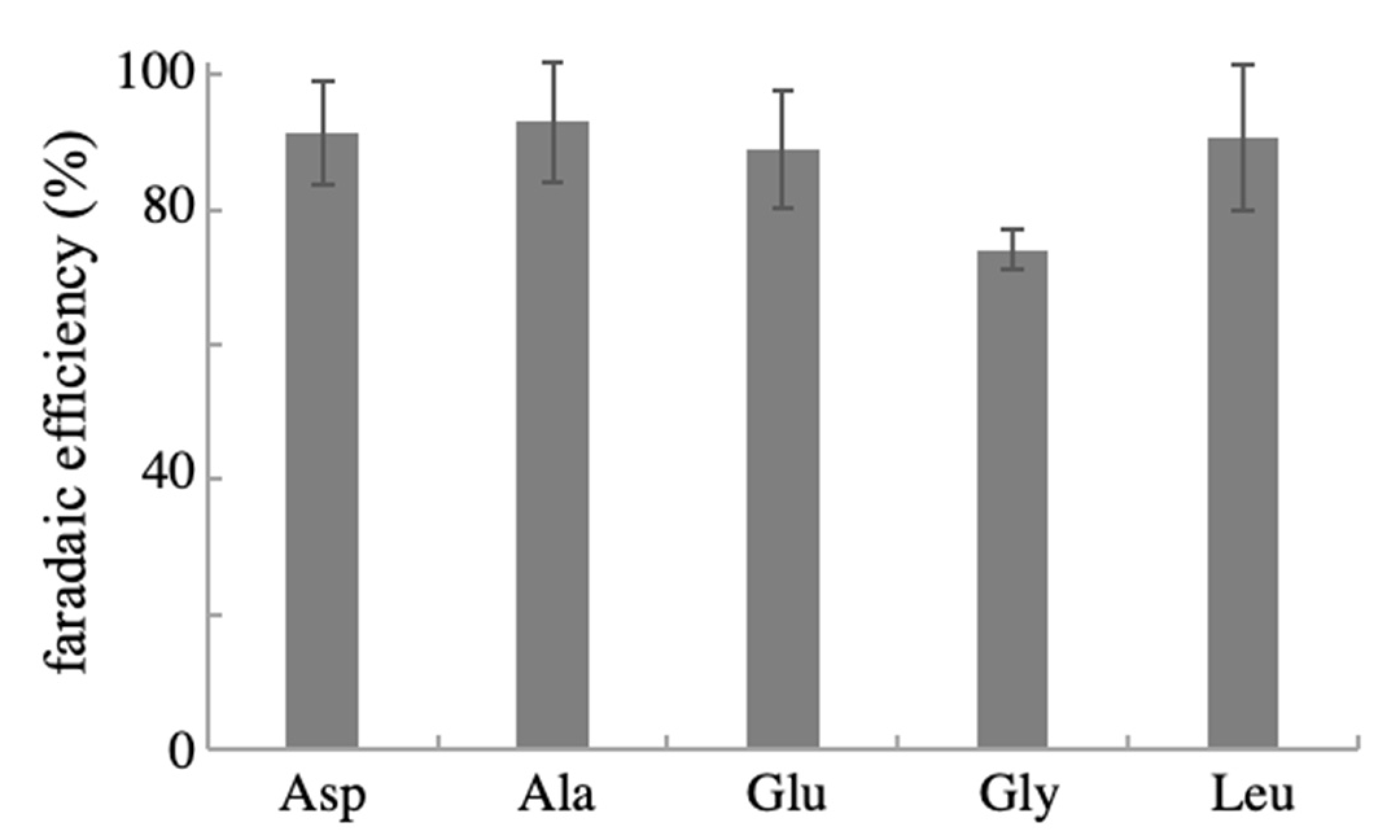
2.3. Reusability and Generality
3. Materials and Methods
3.1. Materials
3.2. Electrode Preparation
3.3. Electrochemical Measurements
3.4. Electrolysis and Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, F.M.; Li, W.J.; Wang, R.; Guo, T.Q.; Sheng, H.Y.; Fu, H.C.; Stahl, S.S.; Jin, S. Modular Electrochemical Synthesis Using a Redox Reservoir Paired with Independent Half-Reactions. Joule 2021, 5, 149–165. [Google Scholar] [CrossRef]
- Tang, C.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Electrocatalytic Refinery for Sustainable Production of Fuels and Chemicals. Angew. Chem. Int. Ed. 2021, 60, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Akhade, S.A.; Singh, N.; Gutierrez, O.Y.; Lopez-Ruiz, J.; Wang, H.M.; Holladay, J.D.; Liu, Y.; Karkamkar, A.; Weber, R.S.; Padmaperuma, A.B.; et al. Electrocatalytic Hydrogenation of Biomass-Derived Organics: A Review. Chem. Rev. 2020, 120, 11370–11419. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.H.; Deng, W.; Lang, L.; Jin, X.; Hu, X.; Wang, Y. Minireview on Bio-Oil Upgrading via Electrocatalytic Hydrogenation: Connecting Biofuel Production with Renewable Power. Energy Fuels 2020, 34, 7915–7928. [Google Scholar] [CrossRef]
- Niwa, O.; Ohta, S.; Takahashi, S.; Zhang, Z.X.; Kamata, T.; Kato, D.; Shiba, S. Hybrid Carbon Film Electrodes for Electroanalysis. Anal. Sci. 2021, 37, 37–47. [Google Scholar] [CrossRef]
- Kang, Y.J.; Yang, P.D.; Markovic, N.M.; Stamenkovic, V.R. Shaping electrocatalysis through tailored nanomaterials. Nano Today 2016, 11, 587–600. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Liu, Q.; Li, T.S.; Luo, Y.L.; Lu, S.Y.; Shi, X.F.; Zhang, F.; Asiri, A.M.; Sun, X.P. Magnetron sputtering enabled sustainable synthesis of nanomaterials for energy electrocatalysis. Green Chem. 2021, 23, 2834–2867. [Google Scholar] [CrossRef]
- Biener, J.; Biener, M.M.; Madix, R.J.; Friend, C.M. Nanoporous Gold: Understanding the Origin of the Reactivity of a 21st Century Catalyst Made by Pre-Columbian Technology. Acs Catal. 2015, 5, 6263–6270. [Google Scholar] [CrossRef]
- Kim, S.H. Nanoporous gold: Preparation and applications to catalysis and sensors. Curr. Appl. Phys. 2018, 18, 810–818. [Google Scholar] [CrossRef]
- Fujita, T.; Guan, P.F.; McKenna, K.; Lang, X.Y.; Hirata, A.; Zhang, L.; Tokunaga, T.; Arai, S.; Yamamoto, Y.; Tanaka, N.; et al. Atomic origins of the high catalytic activity of nanoporous gold. Nat. Mater. 2012, 11, 775–780. [Google Scholar] [CrossRef]
- Qiu, H.J.; Xu, H.T.; Liu, L.; Wang, Y. Correlation of the structure and applications of dealloyed nanoporous metals in catalysis and energy conversion/storage. Nanoscale 2015, 7, 386–400. [Google Scholar] [CrossRef]
- Bhattarai, J.K.; Neupane, D.; Nepal, B.; Mikhaylov, V.; Demchenko, A.V.; Stine, K.J. Preparation, Modification, Characterization, and Biosensing Application of Nanoporous Gold Using Electrochemical Techniques. Nanomaterials 2018, 8, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.N.; Liu, Z.G.; Wen, J.L.; Chen, A.C. Enhanced catalytic activity of nanoporous Au for the efficient electrochemical reduction of carbon dioxide. Appl. Catal. B 2018, 236, 483–489. [Google Scholar] [CrossRef]
- Zeis, R.; Lei, T.; Sieradzki, K.; Snyder, J.; Erlebacher, J. Catalytic reduction of oxygen and hydrogen peroxide by nanoporous gold. J. Catal. 2008, 253, 132–138. [Google Scholar] [CrossRef]
- Xia, H.B.; Ran, Y.; Li, H.S.; Tao, X.T.; Wang, D.Y. Freestanding monolayered nanoporous gold films with high electrocatalytic activity via interfacial self-assembly and overgrowth. J. Mater. Chem. A 2013, 1, 4678–4684. [Google Scholar] [CrossRef]
- Li, Q.Q.; Cui, S.Z.; Yan, X.L. Electrocatalytic oxidation of glucose on nanoporous gold membranes. J. Solid State Electrochem. 2012, 16, 1099–1104. [Google Scholar] [CrossRef]
- Quynh, B.T.P.; Byun, J.Y.; Kim, S.H. Electrochemical Behavior of Aromatic Compounds on Nanoporous Gold Electrode. J. Electrochem. Soc. 2018, 165, B414–B421. [Google Scholar] [CrossRef]
- Takale, B.S.; Feng, X.J.; Lu, Y.; Bao, M.; Jin, T.A.; Minato, T.; Yamamoto, Y. Unsupported Nanoporous Gold Catalyst for Chemoselective Hydrogenation Reactions under Low Pressure: Effect of Residual Silver on the Reaction. J. Am. Chem. Soc. 2016, 138, 10356–10364. [Google Scholar] [CrossRef]
- Yan, M.; Jin, T.; Ishikawa, Y.; Minato, T.; Fujita, T.; Chen, L.Y.; Bao, M.; Asao, N.; Chen, M.W.; Yamamoto, Y. Nanoporous Gold Catalyst for Highly Selective Semihydrogenation of Alkynes: Remarkable Effect of Amine Additives. J. Am. Chem. Soc. 2012, 134, 17536–17542. [Google Scholar] [CrossRef]
- Duan, H.M.; Xu, C.X. Low-temperature CO oxidation over unsupported nanoporous gold catalysts with active or inert oxide residues. J. Catal. 2015, 332, 31–37. [Google Scholar] [CrossRef]
- Dononelli, W.; Tomaschun, G.; Kluner, T.; Moskaleva, L.V. Understanding Oxygen Activation on Nanoporous Gold. ACS Catal. 2019, 9, 5204–5216. [Google Scholar] [CrossRef]
- Xiao, X.X.; Si, P.C.; Magner, E. An overview of dealloyed nanoporous gold in bioelectrochemistry. Bioelectrochemistry 2016, 109, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Ning, S.C.; Liu, P.; Ding, Y.; Hirata, A.; Fujita, T.; Chen, M.W. Tuning Surface Structure of 3D Nanoporous Gold by Surfactant-Free Electrochemical Potential Cycling. Adv. Mater. 2017, 29, 1703601. [Google Scholar] [CrossRef]
- Deng, Y.P.; Huang, W.; Chen, X.; Li, Z.L. Facile fabrication of nanoporous gold film electrodes. Electrochem. Commun. 2008, 10, 810–813. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J. Effect of pH on Anodic Formation of Nanoporous Gold Films in Chloride Solutions: Optimization of Anodization for Ultrahigh Porous Structures. Langmuir 2014, 30, 4844–4851. [Google Scholar] [CrossRef] [PubMed]
- Nishio, K.; Masuda, H. Anodization of Gold in Oxalate Solution To Form a Nanoporous Black Film. Angew. Chem. Int. Ed. 2011, 50, 1603–1607. [Google Scholar] [CrossRef]
- Zhou, C.H.; Xia, Y.; Huang, W.; Li, Z.L. A Rapid Anodic Fabrication of Nanoporous Gold in NH4Cl Solution for Nonenzymatic Glucose Detection. J. Electrochem. Soc. 2014, 161, H802–H808. [Google Scholar] [CrossRef]
- Mie, Y.; Ikegami, M.; Komatsu, Y. Nanoporous Structure of Gold Electrode Fabricated by Anodization and Its Efficacy for Direct Electrochemistry of Human Cytochrome P450. Chem. Lett. 2016, 45, 640–642. [Google Scholar] [CrossRef]
- Mie, Y.; Takayama, H.; Hirano, Y. Facile control of surface crystallographic orientation of anodized nanoporous gold catalyst and its application for highly efficient hydrogen evolution reaction. J. Catal. 2020, 389, 476–482. [Google Scholar] [CrossRef]
- Sukeri, A.; Saravia, L.P.H.; Bertotti, M. A facile electrochemical approach to fabricate a nanoporous gold film electrode and its electrocatalytic activity towards dissolved oxygen reduction. Phys. Chem. Chem. Phys. 2015, 17, 28510–28514. [Google Scholar] [CrossRef]
- Kim, J.; Song, J.T.; Ryoo, H.; Kim, J.G.; Chung, S.Y.; Oh, J. Morphology-controlled Au nanostructures for efficient and selective electrochemical CO2 reduction. J. Mater. Chem. A 2018, 6, 5119–5128. [Google Scholar] [CrossRef]
- Mie, Y.; Katagai, S.; Ikegami, M. Electrochemical Oxidation of Monosaccharides at Nanoporous Gold with Controlled Atomic Surface Orientation and Non-Enzymatic Galactose Sensing. Sensors 2020, 20, 5632. [Google Scholar] [CrossRef]
- Fukushima, T.; Yamauchi, M. Electrosynthesis of amino acids from biomass-derivable acids on titanium dioxide. Chem. Commun. 2019, 55, 14721–14724. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of Biomass into Chemicals over Metal Catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar]
- Zhou, C.H.; Xia, X.; Lin, C.X.; Tong, D.S.; Beltramini, J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011, 40, 5588–5617. [Google Scholar] [CrossRef]
- Ogo, S.; Uehara, K.; Abura, T.; Fukuzumi, S. pH-dependent chemoselective synthesis of alpha-amino acids. Reductive amination of alpha-keto acids with ammonia catalyzed by acid-stable iridium hydride complexes in water. J. Am. Chem. Soc. 2004, 126, 3020–3021. [Google Scholar]
- D’Este, M.; Alvarado-Morales, M.; Angelidaki, I. Amino acids production focusing on fermentation technologies—A review. Biotechnol. Adv. 2018, 36, 14–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Liu, X.H.; Feng, X.M. Asymmetric Strecker Reactions. Chem. Rev. 2011, 111, 6947–6983. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, E.A.; Johansen, O.; Meisters, A. Electrochemical synthesis of amino-acids by reductive amination of keto ACIDS.2. reduction at platinum black and palladium black electrodes. Aust. J. Chem. 1978, 31, 79–84. [Google Scholar] [CrossRef]
- Anne, A.; Daninos, S.; Moiroux, J.; Bourdillon, C. Electrochemical reduction of alpha-ketoglutarate in the presence of ammonia as a means of achieving selectively the reductive amination to glutamate—Thermodynamic and kinetic characteristics of the keto imine equilibrium. New J. Chem. 1994, 18, 1169–1174. [Google Scholar]
- Markle, W.; Lutz, S. Electroenzymatic strategies for deracemization, stereoinversion and asymmetric synthesis of amino acids. Electrochim. Acta 2008, 53, 3175–3180. [Google Scholar] [CrossRef]
- Chen, L.Y.; Lang, X.Y.; Fujita, T.; Chen, M.W. Nanoporous gold for enzyme-free electrochemical glucose sensors. Scripta Mater. 2011, 65, 17–20. [Google Scholar] [CrossRef]
- Xiao, X.X.; Ulstrup, J.; Li, H.; Wang, M.E.; Zhang, J.D.; Si, P.C. Nanoporous gold assembly of glucose oxidase for electrochemical biosensing. Electrochim. Acta 2014, 130, 559–567. [Google Scholar] [CrossRef]
- Ruffino, F.; Grimaldi, M.G. Nanoporous Gold-Based Sensing. Coatings 2020, 10, 899. [Google Scholar] [CrossRef]
- Norskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Norskov, J.K. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, J23–J26. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Sun, Y.M.; Liao, H.B.; Sun, S.N.; Li, S.Z.; Ager, J.W.; Xu, Z.C.J. Activation Effect of Electrochemical Cycling on Gold Nanoparticles towards the Hydrogen Evolution Reaction in Sulfuric Acid. Electrochim. Acta 2016, 209, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Sukeri, A.; Bertotti, M. Nanoporous Gold Surface: An Efficient Platform for Hydrogen Evolution Reaction at Very Low Overpotential. J. Braz. Chem. Soc. 2018, 29, 226–231. [Google Scholar] [CrossRef]
- Sadakiyo, M.; Hata, S.; Fukushima, T.; Juhasz, G.; Yamauchi, M. Electrochemical hydrogenation of non-aromatic carboxylic acid derivatives as a sustainable synthesis process: From catalyst design to device construction. Phys. Chem. Chem. Phys. 2019, 21, 5882–5889. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewski, M.; Soszko, M.; Czerwinski, A. Electrochemical Methods of Real Surface Area Determination of Noble Metal Electrodes—An Overview. Int. J. Electrochem. Sci. 2016, 11, 4442–4469. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mie, Y.; Katagai, S.; Mikami, C. Electrochemical Molecular Conversion of α-Keto Acid to Amino Acid at a Low Overpotential Using a Nanoporous Gold Catalyst. Int. J. Mol. Sci. 2021, 22, 9442. https://doi.org/10.3390/ijms22179442
Mie Y, Katagai S, Mikami C. Electrochemical Molecular Conversion of α-Keto Acid to Amino Acid at a Low Overpotential Using a Nanoporous Gold Catalyst. International Journal of Molecular Sciences. 2021; 22(17):9442. https://doi.org/10.3390/ijms22179442
Chicago/Turabian StyleMie, Yasuhiro, Shizuka Katagai, and Chitose Mikami. 2021. "Electrochemical Molecular Conversion of α-Keto Acid to Amino Acid at a Low Overpotential Using a Nanoporous Gold Catalyst" International Journal of Molecular Sciences 22, no. 17: 9442. https://doi.org/10.3390/ijms22179442
APA StyleMie, Y., Katagai, S., & Mikami, C. (2021). Electrochemical Molecular Conversion of α-Keto Acid to Amino Acid at a Low Overpotential Using a Nanoporous Gold Catalyst. International Journal of Molecular Sciences, 22(17), 9442. https://doi.org/10.3390/ijms22179442





