Epigenetic Modulation of Vasopressin Expression in Health and Disease
Abstract
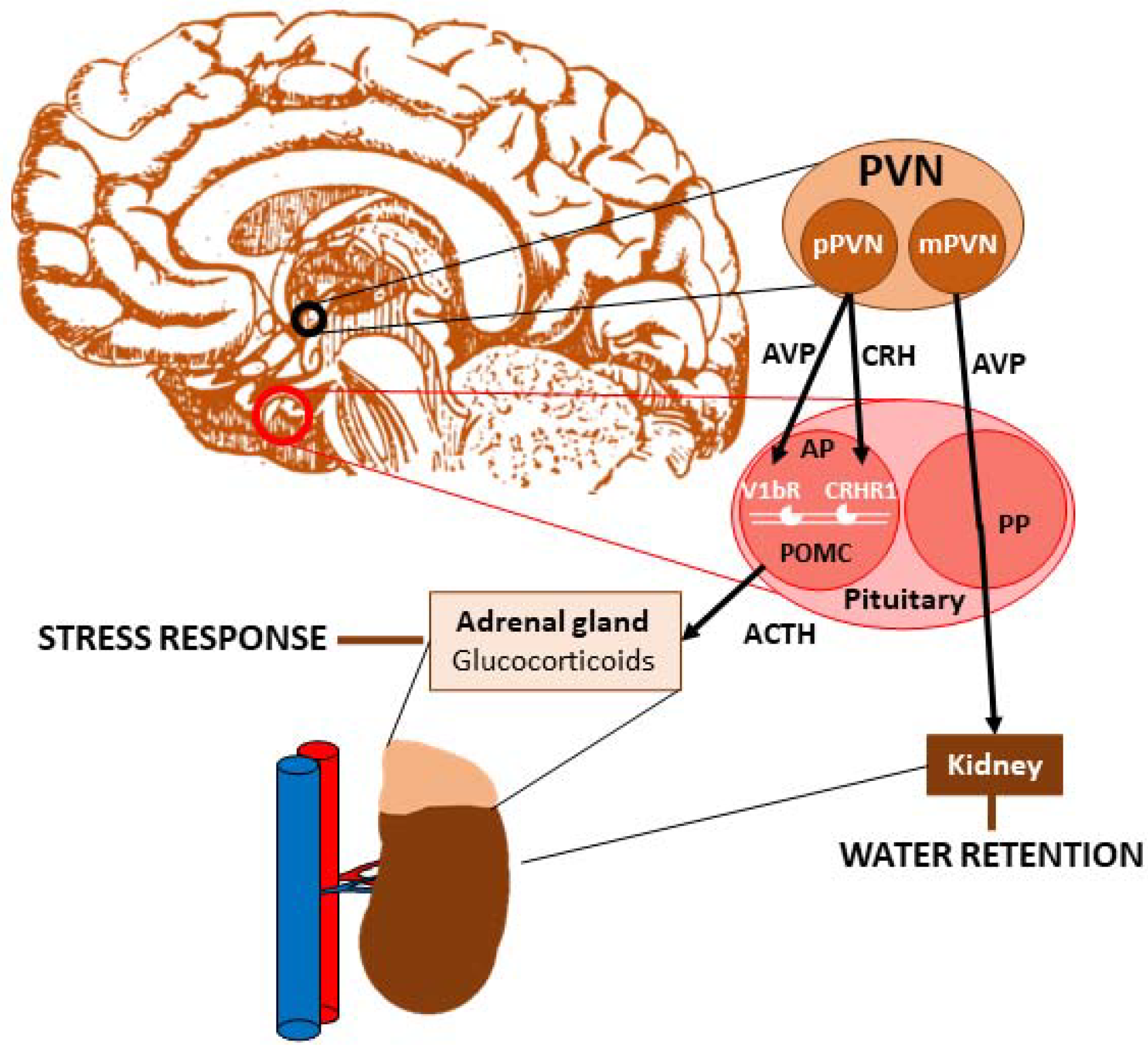
1. Vasopressin
Vasopressin and Stress
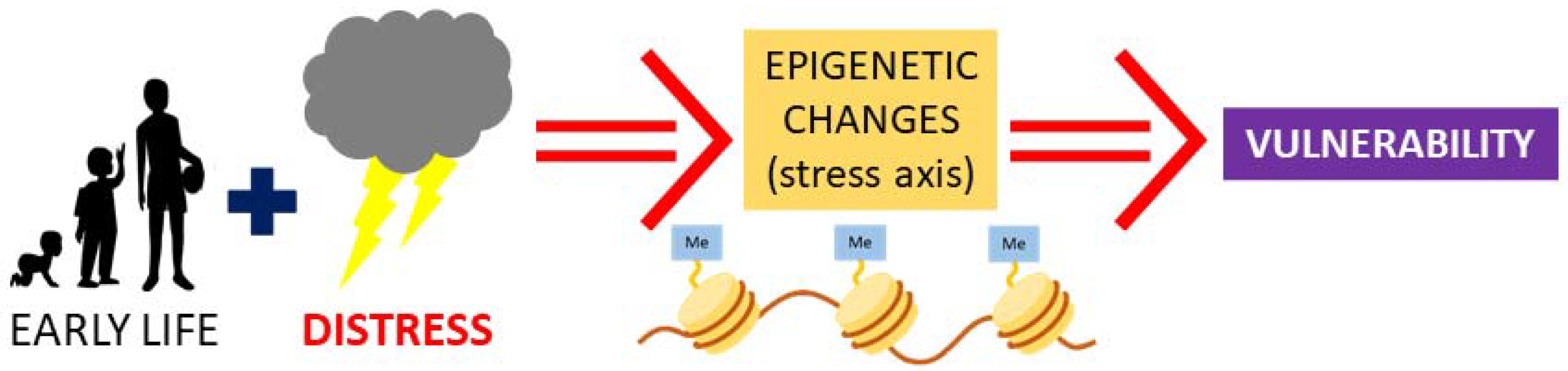
2. Early Life Stress and Vasopressin: Possibility for Epigenetic Programming
3. Epigenetic Changes in the Vasopressinergic System
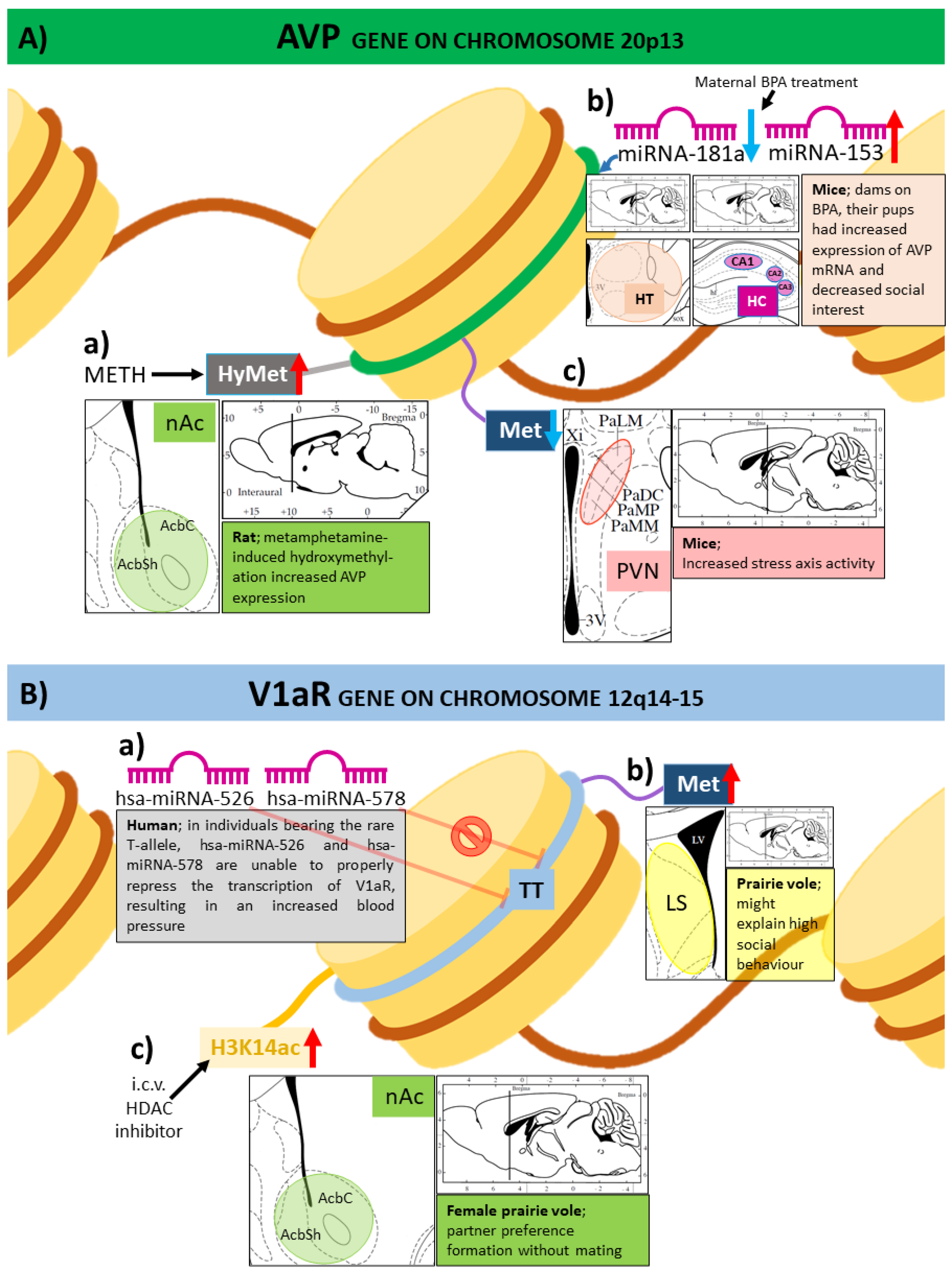
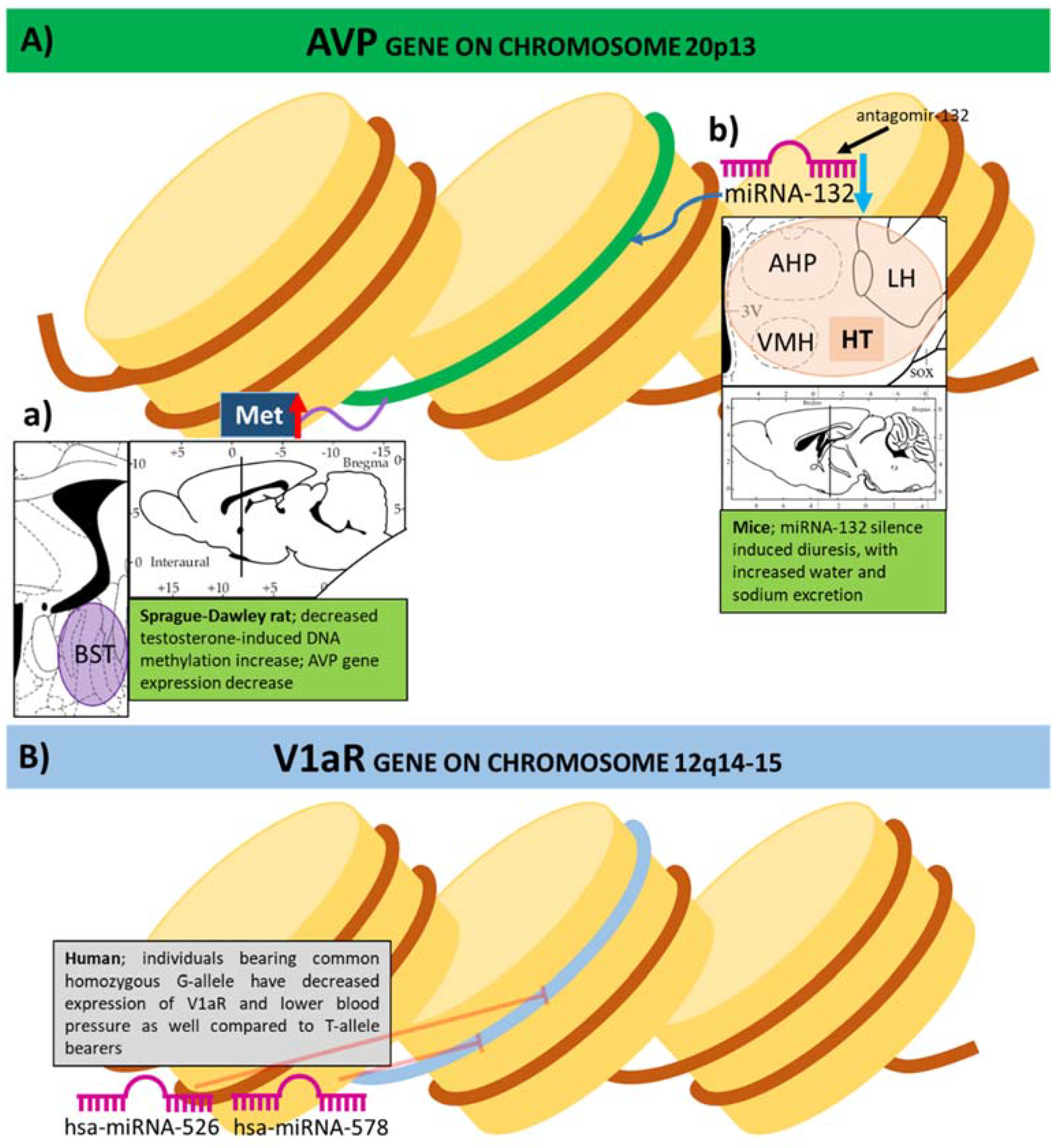
3.1. Expression of Genes of the Vasopressinergic System Influenced by DNA Methylation
3.2. Histone Modifications and Vasopressin
3.3. MicroRNAs in the Epigenetic Regulation of Vasopressin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tong, W.H.; Abdulai-Saiku, S.; Vyas, A. Medial Amygdala Arginine Vasopressin Neurons Regulate Innate Aversion to Cat Odors in Male Mice. Neuroendocrinology 2021, 111, 505–520. [Google Scholar] [CrossRef]
- Marie-Luce, C.; Raskin, K.; Bolborea, M.; Monin, M.; Picot, M.; Mhaouty-Kodja, S. Effects of neural androgen receptor disruption on aggressive behavior, arginine vasopressin and galanin systems in the bed nucleus of stria terminalis and lateral septum. Gen. Comp. Endocrinol. 2013, 188, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Dubey, R.; Phillips, J.K.; Matsumoto, S.; Lipski, J. Effects of vasopressin on isolated rat adrenal chromaffin cells. Regul. Pept. 2002, 106, 55–65. [Google Scholar] [CrossRef]
- Zelena, D. Vasopressin in health and disease with a focus on affective disorders. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 286–303. [Google Scholar] [CrossRef] [PubMed]
- Bundzikova, J.; Pirnik, Z.; Zelena, D.; Mikkelsen, J.D.; Kiss, A. Response of substances co-expressed in hypothalamic magnocellular neurons to osmotic challenges in normal and Brattleboro rats. Cell. Mol. Neurobiol. 2008, 28, 1033–1047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hernandez, V.S.; Zetter, M.A.; Eiden, L.E. VGLUT-VGAT expression delineates functionally specialised populations of vasopressin-containing neurones including a glutamatergic perforant path-projecting cell group to the hippocampus in rat and mouse brain. J. Neuroendocrinol. 2020, 32, e12831. [Google Scholar] [CrossRef]
- Ponzio, T.A.; Ni, Y.; Montana, V.; Parpura, V.; Hatton, G.I. Vesicular glutamate transporter expression in supraoptic neurones suggests a glutamatergic phenotype. J. Neuroendocrinol. 2006, 18, 253–265. [Google Scholar] [CrossRef]
- Raadsheer, F.C.; Sluiter, A.A.; Ravid, R.; Tilders, F.J.; Swaab, D.F. Localization of corticotropin-releasing hormone (CRH) neurons in the paraventricular nucleus of the human hypothalamus; age-dependent colocalization with vasopressin. Brain Res. 1993, 615, 50–62. [Google Scholar] [CrossRef]
- Engelmann, M. Vasopressin in the septum: Not important versus causally involved in learning and memory—two faces of the same coin? Prog. Brain Res. 2008, 170, 389–395. [Google Scholar] [CrossRef]
- Liu, J.J.; Lou, F.; Lavebratt, C.; Forsell, Y. Impact of Childhood Adversity and Vasopressin receptor 1a Variation on Social Interaction in Adulthood: A Cross-Sectional Study. PLoS ONE 2015, 10, e0136436. [Google Scholar] [CrossRef]
- Fodor, A.; Barsvari, B.; Aliczki, M.; Balogh, Z.; Zelena, D.; Goldberg, S.R.; Haller, J. The effects of vasopressin deficiency on aggression and impulsiveness in male and female rats. Psychoneuroendocrinology 2014, 47, 141–150. [Google Scholar] [CrossRef]
- Csikota, P.; Fodor, A.; Balazsfi, D.; Pinter, O.; Mizukami, H.; Weger, S.; Heilbronn, R.; Engelmann, M.; Zelena, D. Vasopressinergic control of stress-related behavior: Studies in Brattleboro rats. Stress 2016, 19, 349–361. [Google Scholar] [CrossRef]
- Varga, J.; Klausz, B.; Domokos, A.; Kalman, S.; Pakaski, M.; Szucs, S.; Garab, D.; Zvara, A.; Puskas, L.; Kalman, J.; et al. Increase in Alzheimer’s related markers preceeds memory disturbances: Studies in vasopressin-deficient Brattleboro rat. Brain Res. Bull. 2014, 100, 6–13. [Google Scholar] [CrossRef]
- Sipos, E.; Torok, B.; Barna, I.; Engelmann, M.; Zelena, D. Vasopressin and post-traumatic stress disorder. Stress 2020, 23, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Fodor, A.; Klausz, B.; Toth, B.; Zelena, D. The prepulse inhibition deficit appearance is largely independent on the circadian cycle, body weight, and the gender of vasopressin deficient Brattleboro rat. Endocr. Regul. 2016, 50, 16–23. [Google Scholar] [CrossRef]
- Demeter, K.; Torok, B.; Fodor, A.; Varga, J.; Ferenczi, S.; Kovacs, K.J.; Eszik, I.; Szegedi, V.; Zelena, D. Possible contribution of epigenetic changes in the development of schizophrenia-like behavior in vasopressin-deficient Brattleboro rats. Behav. Brain Res. 2016, 300, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Török, B.; Zelena, D. Vasopressin and Schizophrenia. In Advances in Health and Disease; Duncan, L.T., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2018; Chapter 7. [Google Scholar]
- Natochin, Y.V.; Golosova, D.V. Vasopressin receptor subtypes and renal sodium transport. Vitam. Horm. 2020, 113, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Young, L.J.; Toloczko, D.; Insel, T.R. Localization of vasopressin (V1a) receptor binding and mRNA in the rhesus monkey brain. J. Neuroendocrinol. 1999, 11, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Fodor, A.; Pinter, O.; Domokos, A.; Langnaese, K.; Barna, I.; Engelmann, M.; Zelena, D. Blunted HPA axis response in lactating, vasopressin-deficient Brattleboro rats. J. Endocrinol. 2013, 219, 89–100. [Google Scholar] [CrossRef][Green Version]
- Melander, O. Vasopressin, from Regulator to Disease Predictor for Diabetes and Cardiometabolic Risk. Ann. Nutr. Metab. 2016, 68 (Suppl. S2), 24–28. [Google Scholar] [CrossRef] [PubMed]
- Torok, B.; Fodor, A.; Zsebok, S.; Sipos, E.; Zelena, D. The Effect of Vasopressin Antagonists on Maternal-Separation-Induced Ultrasonic Vocalization and Stress-Hormone Level Increase during the Early Postnatal Period. Brain Sci. 2021, 11, 444. [Google Scholar] [CrossRef]
- Chowdrey, H.S.; Larsen, P.J.; Harbuz, M.S.; Jessop, D.S.; Aguilera, G.; Eckland, D.J.; Lightman, S.L. Evidence for arginine vasopressin as the primary activator of the HPA axis during adjuvant-induced arthritis. Br. J. Pharmacol. 1995, 116, 2417–2424. [Google Scholar] [CrossRef]
- Wigger, A.; Sanchez, M.M.; Mathys, K.C.; Ebner, K.; Frank, E.; Liu, D.; Kresse, A.; Neumann, I.D.; Holsboer, F.; Plotsky, P.M.; et al. Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: Critical role of vasopressin. Neuropsychopharmacology 2004, 29, 1–14. [Google Scholar] [CrossRef]
- Landgraf, R.; Kessler, M.S.; Bunck, M.; Murgatroyd, C.; Spengler, D.; Zimbelmann, M.; Nussbaumer, M.; Czibere, L.; Turck, C.W.; Singewald, N.; et al. Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: Focus on vasopressin and glyoxalase-I. Neurosci. Biobehav. Rev. 2007, 31, 89–102. [Google Scholar] [CrossRef]
- Murgatroyd, C.; Wigger, A.; Frank, E.; Singewald, N.; Bunck, M.; Holsboer, F.; Landgraf, R.; Spengler, D. Impaired repression at a vasopressin promoter polymorphism underlies overexpression of vasopressin in a rat model of trait anxiety. J. Neurosci. 2004, 24, 7762–7770. [Google Scholar] [CrossRef]
- Zelena, D.; Pinter, O.; Balazsfi, D.G.; Langnaese, K.; Richter, K.; Landgraf, R.; Makara, G.B.; Engelmann, M. Vasopressin signaling at brain level controls stress hormone release: The vasopressin-deficient Brattleboro rat as a model. Amino Acids 2015, 47, 2245–2253. [Google Scholar] [CrossRef]
- Lesse, A.; Rether, K.; Groger, N.; Braun, K.; Bock, J. Chronic Postnatal Stress Induces Depressive-like Behavior in Male Mice and Programs second-Hit Stress-Induced Gene Expression Patterns of OxtR and AvpR1a in Adulthood. Mol. Neurobiol. 2017, 54, 4813–4819. [Google Scholar] [CrossRef] [PubMed]
- Serradeil-Le Gal, C.; Wagnon, J., 3rd; Tonnerre, B.; Roux, R.; Garcia, G.; Griebel, G.; Aulombard, A. An overview of SSR149415, a selective nonpeptide vasopressin V(1b) receptor antagonist for the treatment of stress-related disorders. CNS Drug Rev. 2005, 11, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Griebel, G.; Beeske, S.; Stahl, S.M. The vasopressin V(1b) receptor antagonist SSR149415 in the treatment of major depressive and generalized anxiety disorders: Results from 4 randomized, double-blind, placebo-controlled studies. J. Clin. Psychiatry 2012, 73, 1403–1411. [Google Scholar] [CrossRef]
- Chaki, S. Vasopressin V1B Receptor Antagonists as Potential Antidepressants. Int. J. Neuropsychopharmacol. 2021, 24, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Inatani, S.; Mizuno-Yasuhira, A.; Kamiya, M.; Nishino, I.; Sabia, H.D.; Endo, H. Prediction of a clinically effective dose of THY1773, a novel V1B receptor antagonist, based on preclinical data. Biopharm. Drug Dispos. 2021, 42, 204–217. [Google Scholar] [CrossRef]
- Engel, G.L. The biopsychosocial model and the education of health professionals. Ann. N. Y. Acad. Sci. 1978, 310, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, N.P.; Bagot, R.C.; Parker, K.J.; Vinkers, C.H.; de Kloet, E.R. The three-hit concept of vulnerability and resilience: Toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology 2013, 38, 1858–1873. [Google Scholar] [CrossRef] [PubMed]
- Tsankova, N.; Renthal, W.; Kumar, A.; Nestler, E.J. Epigenetic regulation in psychiatric disorders. Nat. Rev. Neurosci. 2007, 8, 355–367. [Google Scholar] [CrossRef]
- Flavell, S.W.; Greenberg, M.E. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu. Rev. Neurosci. 2008, 31, 563–590. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.J.; Austin, E.; Knapp, R.; Vaiano, T.; Galbally, M. Perinatal Maternal Mental Health, Fetal Programming and Child Development. Healthcare 2015, 3, 1212–1227. [Google Scholar] [CrossRef]
- Mastorakos, G.; Ilias, I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann. N. Y. Acad. Sci. 2003, 997, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Brunton, P.J.; Russell, J.A. Endocrine induced changes in brain function during pregnancy. Brain Res. 2010, 1364, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Brunton, P.J.; Russell, J.A. The expectant brain: Adapting for motherhood. Nat. Rev. Neurosci. 2008, 9, 11–25. [Google Scholar] [CrossRef]
- Wellmann, S.; Koslowski, A.; Spanaus, K.; Zimmermann, R.; Burkhardt, T. Fetal Release of Copeptin in Response to Maternal Oxytocin Administration: A Randomized Controlled Trial. Obstet. Gynecol. 2016, 128, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Szczepanska-Sadowska, E.; Cudnoch-Jedrzejewska, A.; Wsol, A. The role of oxytocin and vasopressin in the pathophysiology of heart failure in pregnancy and in fetal and neonatal life. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H639–H651. [Google Scholar] [CrossRef]
- Hammock, E.A. Developmental perspectives on oxytocin and vasopressin. Neuropsychopharmacology 2015, 40, 24–42. [Google Scholar] [CrossRef] [PubMed]
- Lukas, M.; Bredewold, R.; Neumann, I.D.; Veenema, A.H. Maternal separation interferes with developmental changes in brain vasopressin and oxytocin receptor binding in male rats. Neuropharmacology 2010, 58, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Korosi, A.; Baram, T.Z. Plasticity of the stress response early in life: Mechanisms and significance. Dev. Psychobiol. 2010, 52, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Branchi, I. The mouse communal nest: Investigating the epigenetic influences of the early social environment on brain and behavior development. Neurosci. Biobehav. Rev. 2009, 33, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Levine, S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology 2005, 30, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Meaney, M.J. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001, 24, 1161–1192. [Google Scholar] [CrossRef]
- Murgatroyd, C.; Patchev, A.V.; Wu, Y.; Micale, V.; Bockmuhl, Y.; Fischer, D.; Holsboer, F.; Wotjak, C.T.; Almeida, O.F.; Spengler, D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat. Neurosci. 2009, 12, 1559–1566. [Google Scholar] [CrossRef]
- Kompier, N.F.; Keysers, C.; Gazzola, V.; Lucassen, P.J.; Krugers, H.J. Early Life Adversity and Adult Social Behavior: Focus on Arginine Vasopressin and Oxytocin as Potential Mediators. Front. Behav. Neurosci. 2019, 13, 143. [Google Scholar] [CrossRef]
- Kelly, A.M.; Ong, J.Y.; Witmer, R.A.; Ophir, A.G. Paternal deprivation impairs social behavior putatively via epigenetic modification to lateral septum vasopressin receptor. Sci. Adv. 2020, 6. [Google Scholar] [CrossRef]
- Engelmann, M.; Landgraf, R.; Wotjak, C.T. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: An old concept revisited. Front. Neuroendocrinol. 2004, 25, 132–149. [Google Scholar] [CrossRef]
- Wotjak, C.T.; Landgraf, R.; Engelmann, M. Listening to neuropeptides by microdialysis: Echoes and new sounds? Pharmacol. Biochem. Behav. 2008, 90, 125–134. [Google Scholar] [CrossRef]
- Auger, C.J.; Coss, D.; Auger, A.P.; Forbes-Lorman, R.M. Epigenetic control of vasopressin expression is maintained by steroid hormones in the adult male rat brain. Proc. Natl. Acad. Sci. USA 2011, 108, 4242–4247. [Google Scholar] [CrossRef]
- Garcia, A.N.; Bezner, K.; Depena, C.; Yin, W.; Gore, A.C. The effects of long-term estradiol treatment on social behavior and gene expression in adult female rats. Horm. Behav. 2017, 87, 145–154. [Google Scholar] [CrossRef]
- Forbes-Lorman, R.M.; Rautio, J.J.; Kurian, J.R.; Auger, A.P.; Auger, C.J. Neonatal MeCP2 is important for the organization of sex differences in vasopressin expression. Epigenetics 2012, 7, 230–238. [Google Scholar] [CrossRef][Green Version]
- Fodor, A.; Kovacs, K.B.; Balazsfi, D.; Klausz, B.; Pinter, O.; Demeter, K.; Daviu, N.; Rabasa, C.; Rotllant, D.; Nadal, R.; et al. Depressive- and anxiety-like behaviors and stress-related neuronal activation in vasopressin-deficient female Brattleboro rats. Physiol. Behav. 2016, 158, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, S.; Gonzalez, B.; McCoy, M.T.; Ladenheim, B.; Bisagno, V.; Cadet, J.L. Methamphetamine Induces TET1- and TET3-Dependent DNA Hydroxymethylation of Crh and Avp Genes in the Rat Nucleus Accumbens. Mol. Neurobiol. 2018, 55, 5154–5166. [Google Scholar] [CrossRef] [PubMed]
- Unternaehrer, E.; Luers, P.; Mill, J.; Dempster, E.; Meyer, A.H.; Staehli, S.; Lieb, R.; Hellhammer, D.H.; Meinlschmidt, G. Dynamic changes in DNA methylation of stress-associated genes (OXTR, BDNF) after acute psychosocial stress. Transl. Psychiatry 2012, 2, e150. [Google Scholar] [CrossRef] [PubMed]
- Hillemacher, T.; Frieling, H.; Luber, K.; Yazici, A.; Muschler, M.A.; Lenz, B.; Wilhelm, J.; Kornhuber, J.; Bleich, S. Epigenetic regulation and gene expression of vasopressin and atrial natriuretic peptide in alcohol withdrawal. Psychoneuroendocrinology 2009, 34, 555–560. [Google Scholar] [CrossRef]
- Schroeder, F.A.; Penta, K.L.; Matevossian, A.; Jones, S.R.; Konradi, C.; Tapper, A.R.; Akbarian, S. Drug-induced activation of dopamine D(1) receptor signaling and inhibition of class I/II histone deacetylase induce chromatin remodeling in reward circuitry and modulate cocaine-related behaviors. Neuropsychopharmacology 2008, 33, 2981–2992. [Google Scholar] [CrossRef]
- Torrisi, S.A.; Laudani, S.; Contarini, G.; De Luca, A.; Geraci, F.; Manago, F.; Papaleo, F.; Salomone, S.; Drago, F.; Leggio, G.M. Dopamine, Cognitive Impairments and Second-Generation Antipsychotics: From Mechanistic Advances to More Personalized Treatments. Pharmaceuticals 2020, 13, 365. [Google Scholar] [CrossRef]
- Shilling, P.D.; Kinkead, B.; Murray, T.; Melendez, G.; Nemeroff, C.B.; Feifel, D. Upregulation of striatal dopamine-2 receptors in Brattleboro rats with prepulse inhibition deficits. Biol. Psychiatry 2006, 60, 1278–1281. [Google Scholar] [CrossRef]
- Cilia, J.; Gartlon, J.E.; Shilliam, C.; Dawson, L.A.; Moore, S.H.; Jones, D.N. Further neurochemical and behavioural investigation of Brattleboro rats as a putative model of schizophrenia. J. Psychopharmacol. 2010, 24, 407–419. [Google Scholar] [CrossRef]
- Tickerhoof, M.C.; Smith, A.S. Vasopressinergic Neurocircuitry Regulating Social Attachment in a Monogamous Species. Front. Endocrinol. 2017, 8, 265. [Google Scholar] [CrossRef]
- Wang, H.; Duclot, F.; Liu, Y.; Wang, Z.; Kabbaj, M. Histone deacetylase inhibitors facilitate partner preference formation in female prairie voles. Nat. Neurosci. 2013, 16, 919–924. [Google Scholar] [CrossRef]
- Zhao, S.; Allis, C.D.; Wang, G.G. The language of chromatin modification in human cancers. Nat. Rev. Cancer 2021, 21, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A brief review on the mechanisms of miRNA regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Liu, J. Control of protein synthesis and mRNA degradation by microRNAs. Curr. Opin. Cell. Biol. 2008, 20, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Jung, H.J.; Lee, Y.J.; Kwon, T.H. Vasopressin-regulated miRNAs and AQP2-targeting miRNAs in kidney collecting duct cells. Am. J. Physiol. Renal Physiol. 2015, 308, F749–764. [Google Scholar] [CrossRef] [PubMed]
- Bijkerk, R.; Trimpert, C.; van Solingen, C.; de Bruin, R.G.; Florijn, B.W.; Kooijman, S.; van den Berg, R.; van der Veer, E.P.; Bredewold, E.O.W.; Rensen, P.C.N.; et al. MicroRNA-132 controls water homeostasis through regulating MECP2-mediated vasopressin synthesis. Am. J. Physiol. Renal Physiol. 2018, 315, F1129–F1138. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, M.; Di Mise, A.; Centrone, M.; D’Agostino, M.; Tingskov, S.J.; Venneri, M.; Pellegrino, T.; Difonzo, G.; Caponio, F.; Norregaard, R.; et al. Olive Leaf Extract (OLE) impaired vasopressin-induced aquaporin-2 trafficking through the activation of the calcium-sensing receptor. Sci. Rep. 2021, 11, 4537. [Google Scholar] [CrossRef]
- Ranieri, M.; Di Mise, A.; Tamma, G.; Valenti, G. Calcium sensing receptor exerts a negative regulatory action toward vasopressin-induced aquaporin-2 expression and trafficking in renal collecting duct. Vitam. Horm. 2020, 112, 289–310. [Google Scholar] [CrossRef] [PubMed]
- Oosthuyzen, W.; Scullion, K.M.; Ivy, J.R.; Morrison, E.E.; Hunter, R.W.; Starkey Lewis, P.J.; O’Duibhir, E.; Street, J.M.; Caporali, A.; Gregory, C.D.; et al. Vasopressin Regulates Extracellular Vesicle Uptake by Kidney Collecting Duct Cells. J. Am. Soc. Nephrol. 2016, 27, 3345–3355. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Groot, M.; Pinilla-Vera, M.; Fredenburgh, L.E.; Jin, Y. Identification of miRNA-rich vesicles in bronchoalveolar lavage fluid: Insights into the function and heterogeneity of extracellular vesicles. J. Control. Release 2019, 294, 43–52. [Google Scholar] [CrossRef]
- Butler, M.C.; Long, C.N.; Kinkade, J.A.; Green, M.T.; Martin, R.E.; Marshall, B.L.; Willemse, T.E.; Schenk, A.K.; Mao, J.; Rosenfeld, C.S. Endocrine disruption of gene expression and microRNA profiles in hippocampus and hypothalamus of California mice: Association of gene expression changes with behavioural outcomes. J. Neuroendocrinol. 2020, 32, e12847. [Google Scholar] [CrossRef]
- Nossent, A.Y.; Hansen, J.L.; Doggen, C.; Quax, P.H.; Sheikh, S.P.; Rosendaal, F.R. SNPs in microRNA binding sites in 3’-UTRs of RAAS genes influence arterial blood pressure and risk of myocardial infarction. Am. J. Hypertens. 2011, 24, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- McAdams, R.M.; McPherson, R.J.; Beyer, R.P.; Bammler, T.K.; Farin, F.M.; Juul, S.E. Dose-dependent effects of morphine exposure on mRNA and microRNA (miR) expression in hippocampus of stressed neonatal mice. PLoS ONE 2015, 10, e0123047. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Török, B.; Fazekas, C.L.; Szabó, A.; Zelena, D. Epigenetic Modulation of Vasopressin Expression in Health and Disease. Int. J. Mol. Sci. 2021, 22, 9415. https://doi.org/10.3390/ijms22179415
Török B, Fazekas CL, Szabó A, Zelena D. Epigenetic Modulation of Vasopressin Expression in Health and Disease. International Journal of Molecular Sciences. 2021; 22(17):9415. https://doi.org/10.3390/ijms22179415
Chicago/Turabian StyleTörök, Bibiána, Csilla Lea Fazekas, Adrienn Szabó, and Dóra Zelena. 2021. "Epigenetic Modulation of Vasopressin Expression in Health and Disease" International Journal of Molecular Sciences 22, no. 17: 9415. https://doi.org/10.3390/ijms22179415
APA StyleTörök, B., Fazekas, C. L., Szabó, A., & Zelena, D. (2021). Epigenetic Modulation of Vasopressin Expression in Health and Disease. International Journal of Molecular Sciences, 22(17), 9415. https://doi.org/10.3390/ijms22179415




