Protective, Antioxidant and Antiproliferative Activity of Grapefruit IntegroPectin on SH-SY5Y Cells
Abstract
:1. Introduction
2. Results
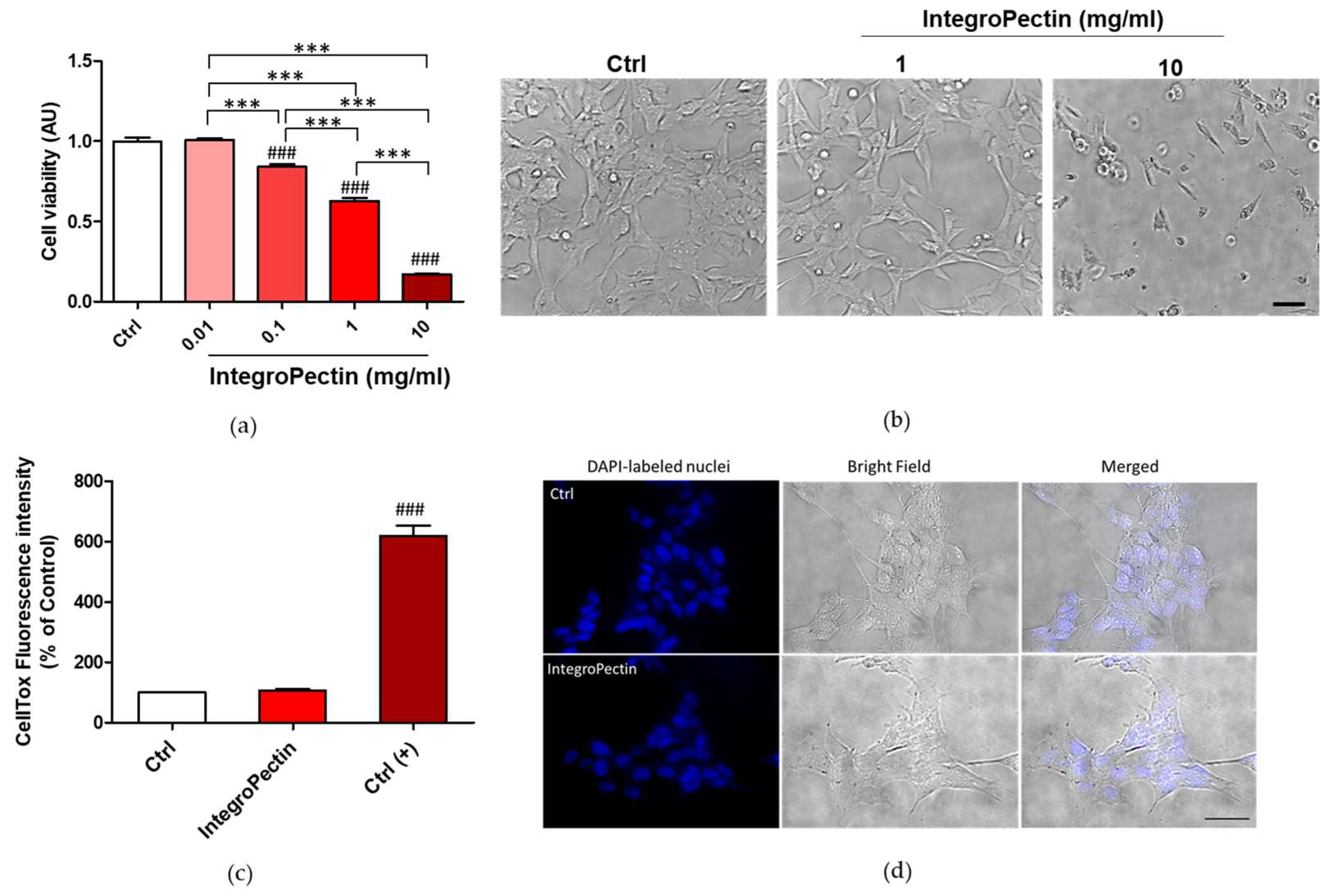
2.1. Effect on SH-SY5Y Cell Viability
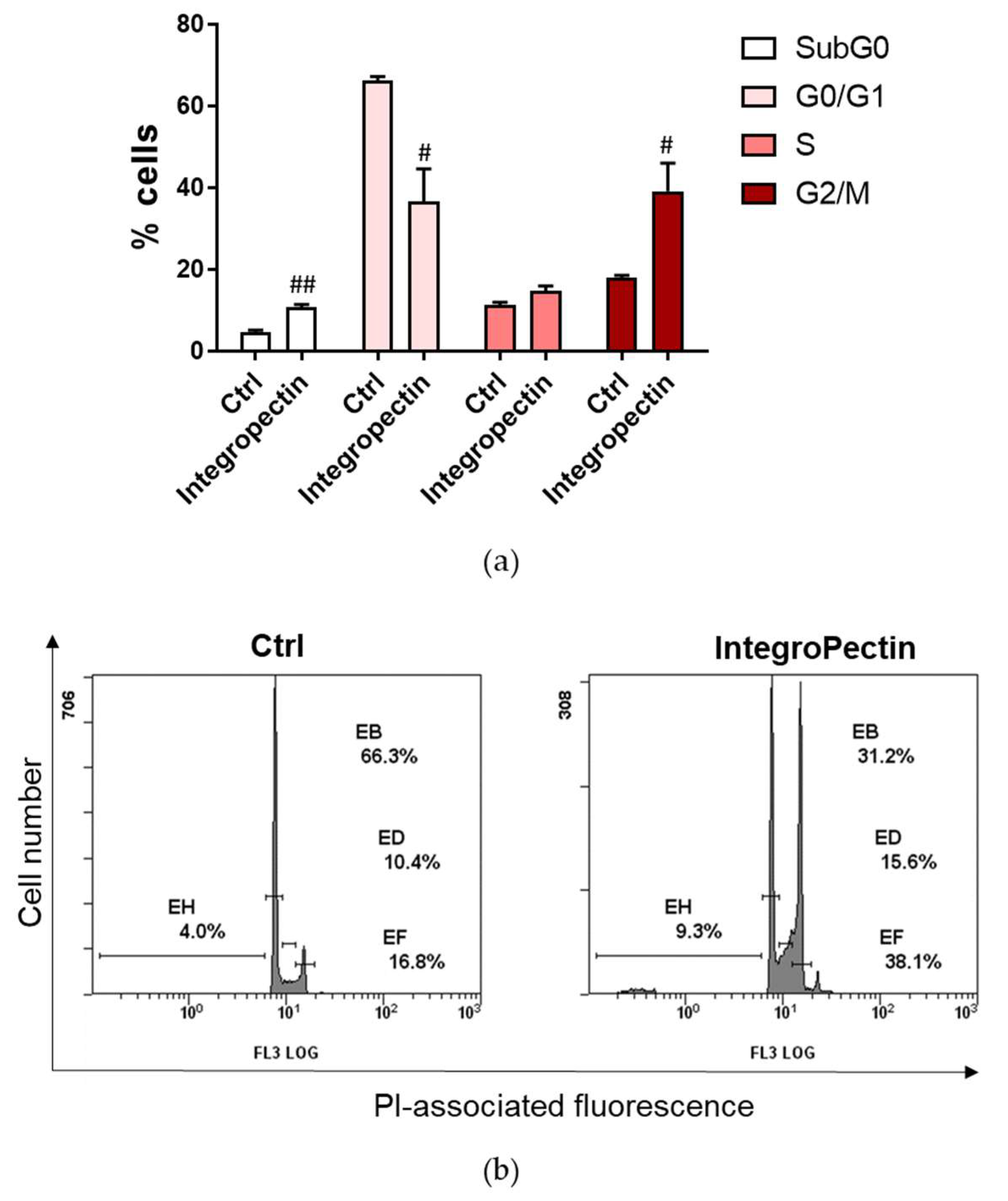
2.2. Cytostatic Effect on SH-SY5Y Cells
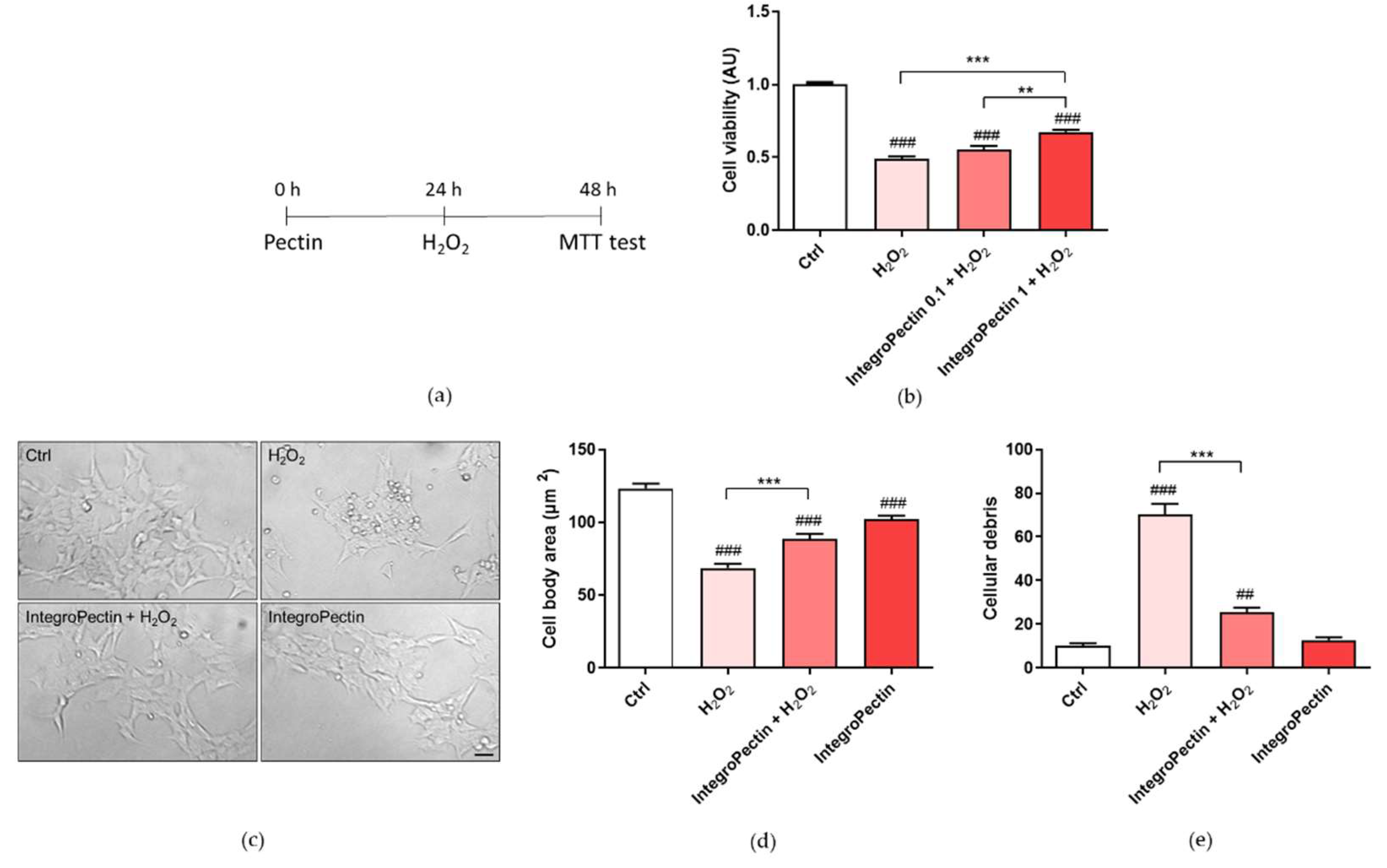
2.3. Protective Effect on SH-SY5Y Cells
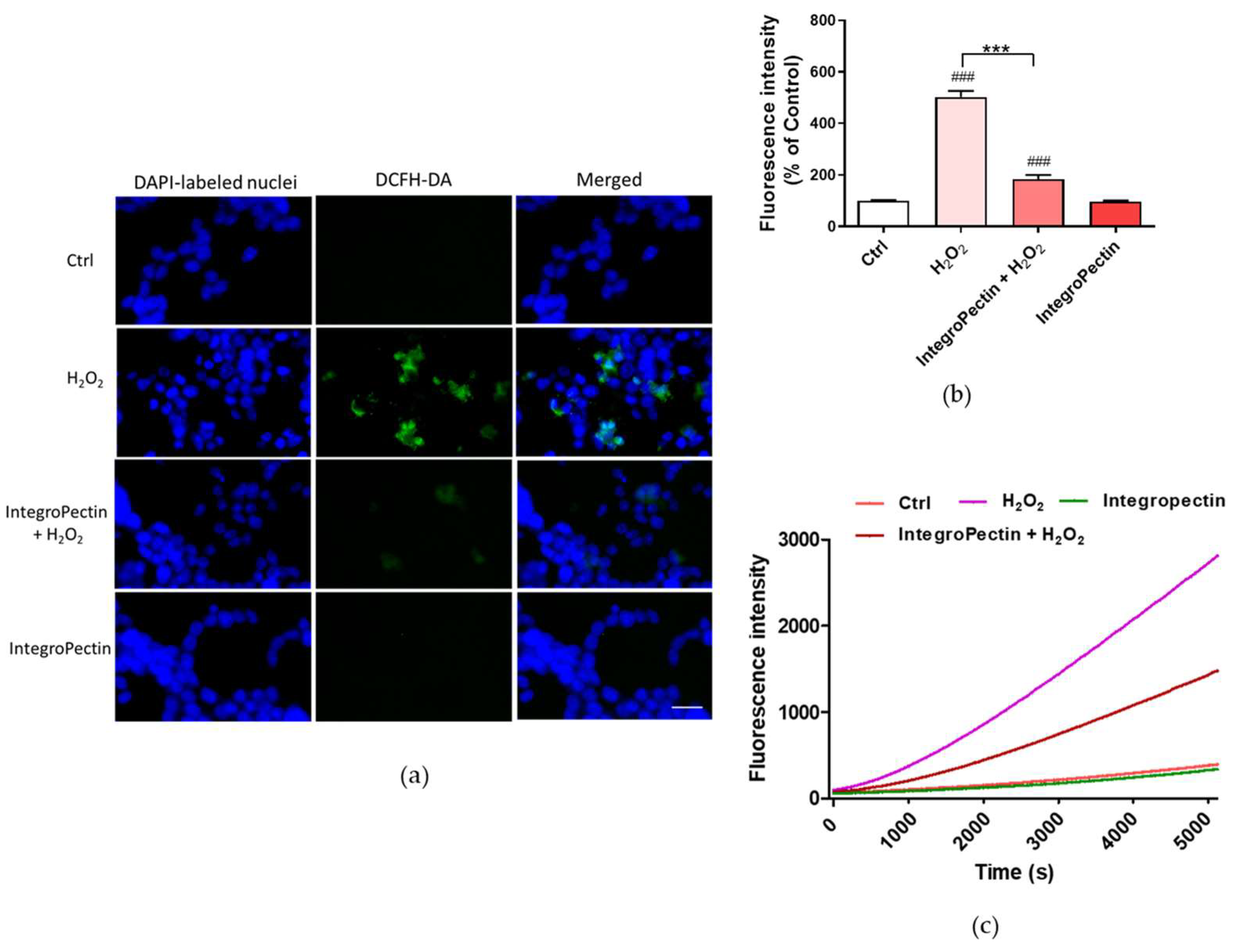
2.4. Antioxidative Effect on SH-SY5Y Cells
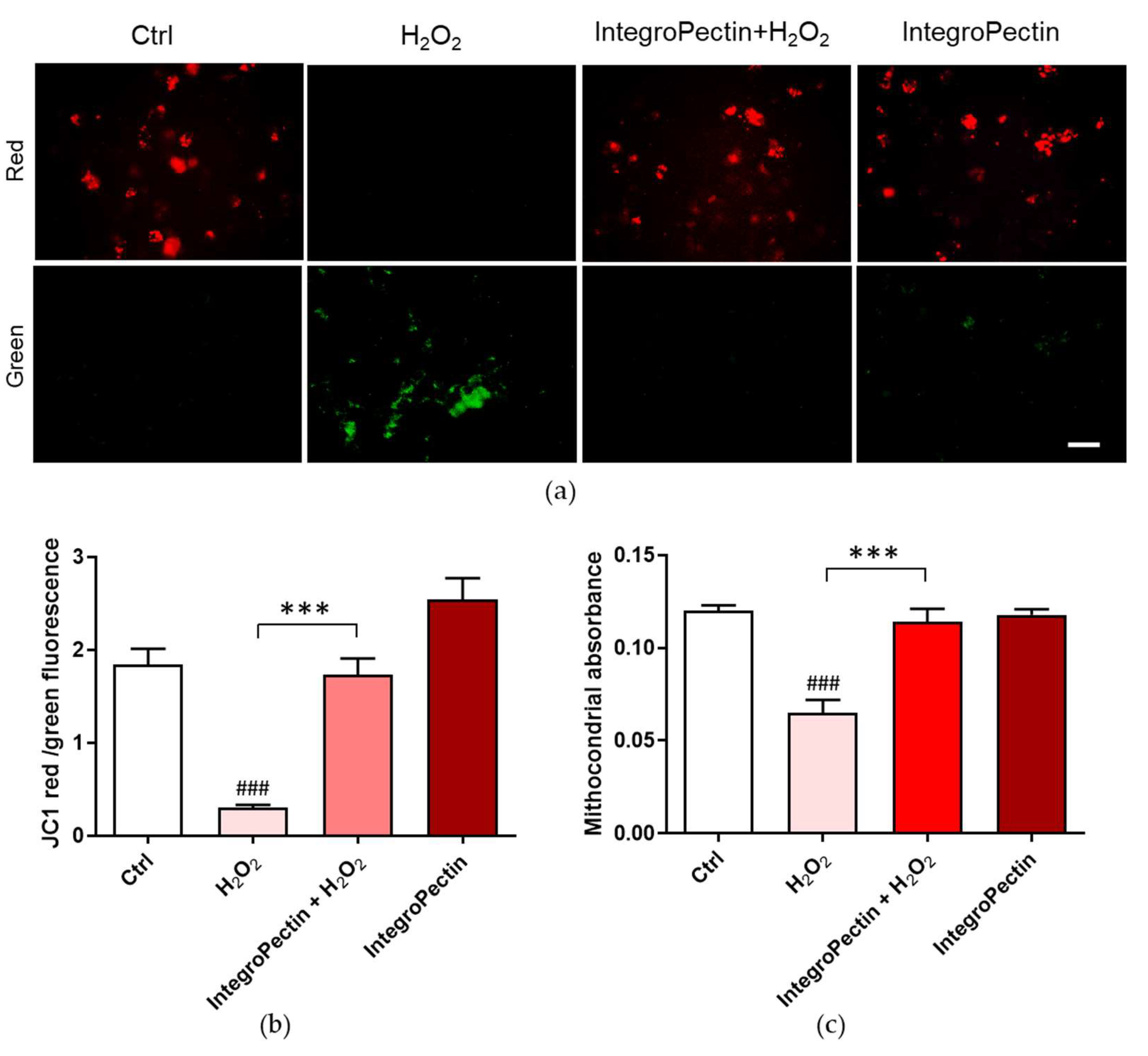
2.5. Mitoprotective Effect on SH-SY5Y Cells
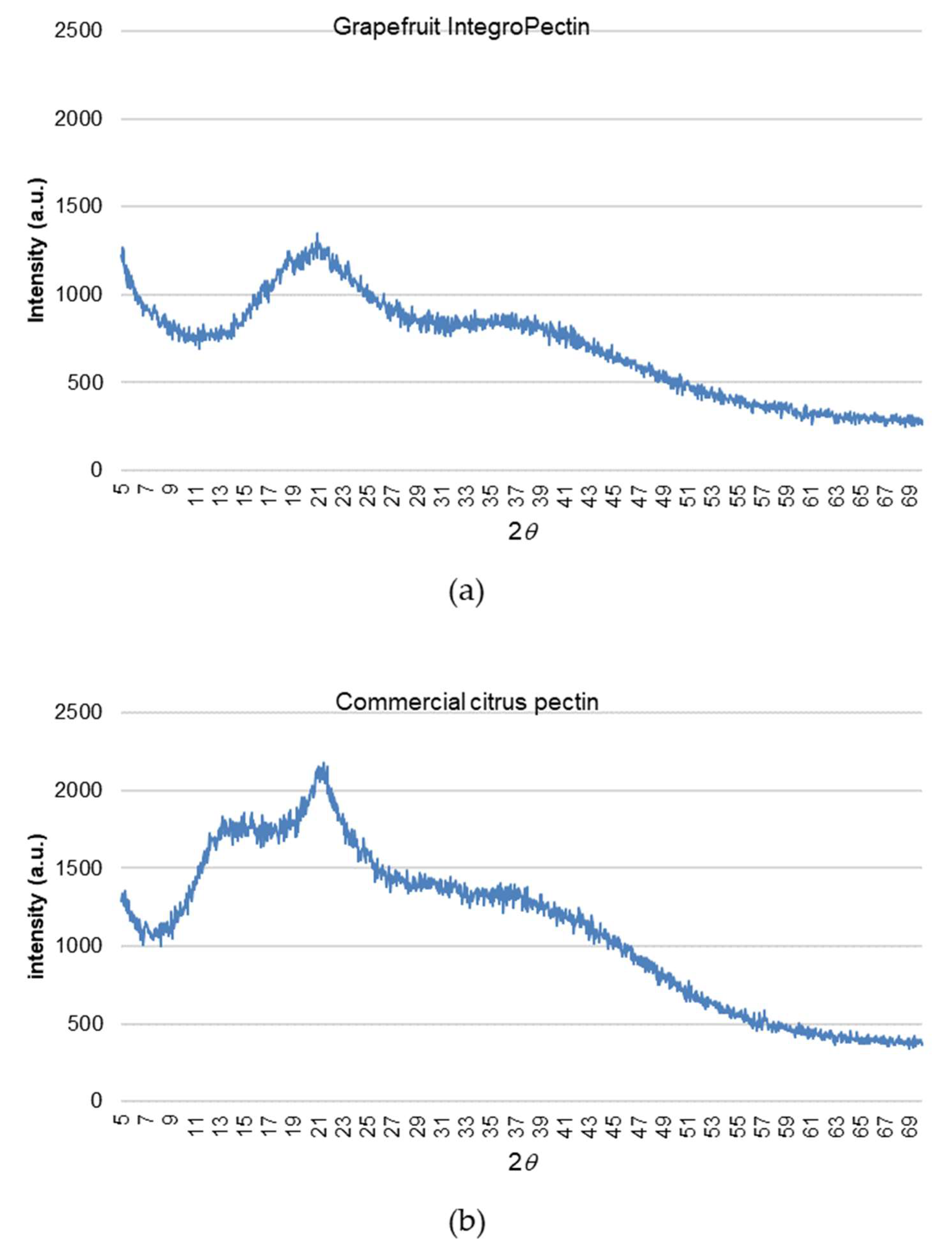
2.6. Structure of Grapefruit IntegroPectin
3. Discussion
4. Materials and Methods
4.1. Solubilization of IntegroPectin
4.2. Cell Cultures and Treatment
4.3. Cell Viability and Cell Morphology
4.4. CellTox Green Cytotoxicity Assay
4.5. Flow Cytometry Analysis of Cell Cycle
4.6. Analysis of ROS and Oxidation Kinetics
4.7. Mitochondrial Membrane Potential Analysis
4.8. Swelling of Isolated Mitochondria
4.9. XRD Measurements
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [CrossRef] [Green Version]
- Picone, P.; Di Carlo, M.; Nuzzo, D. Obesity and Alzheimer’s disease: Molecular bases. Eur. J. Neurosci. 2020, 52, 3944–3950. [Google Scholar] [CrossRef]
- Nuzzo, D.; Picone, P. Potential neurological effects of severe COVID-19 infection. Neurosci. Res. 2020, 158, 1–5. [Google Scholar] [CrossRef]
- Picone, P.; Nuzzo, D.; Caruana, L.; Scafidi, V.; Di Carlo, M. Mitochondrial dysfunction: Different routes to Alzheimer’s disease therapy. Oxid. Med. Cell Longev. 2014, 2014, 780179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picone, P.; Nuzzo, D.; Giacomazza, D.; Di Carlo, M. β-Amyloid peptide: The cell compartment multi-faceted interaction in Alzheimer’s disease. Neurotox. Res. 2020, 37, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, D.; Presti, G.; Picone, P.; Galizzi, G.; Gulotta, E.; Giuliano, S.; Mannino, C.; Gambino, V.; Scoglio, S.; Di Carlo, M. Effects of Aphanizomenon flos-aquae (Klamin) extract on a cell model of neurodegeneration. Oxid. Med. Cell Longev. 2018, 2018, 9089016. [Google Scholar] [CrossRef]
- Nuzzo, D.; Contardi, M.; Kossyvaki, D.; Picone, P.; Cristaldi, L.; Galizzi, G.; Bosco, G.; Scoglio, S.; Athanassiou, A.; Di Carlo, M. Heat-resistant Aphanizomenon flos-aquae (AFA) extract (Klamin) as a functional ingredient in food strategy for prevention of oxidative stress. Oxid. Med. Cell Longev. 2019, 2019, 9481390. [Google Scholar] [CrossRef] [Green Version]
- Kahl, R.; Kappus, H. Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Z. Lebensm. Unters. Forsch. 1993, 196, 329–338. [Google Scholar] [CrossRef]
- Nuzzo, D. Role of Natural Antioxidants on Neuroprotection and Neuroinflammation. Antioxidants 2021, 10, 608. [Google Scholar] [CrossRef]
- Zaitseva, O.; Khudyakov, A.; Sergushkina, M.; Solomina, O.; Polezhaeva, T. Pectins as a universal medicine. Fitoterapia 2020, 146, 104676. [Google Scholar] [CrossRef]
- Beukema, M.; Faas, M.M.; de Vos, P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef]
- Daher Firas, B.; Braybrook, S.A. How to let go: Pectin and plant cell adhesion. Front. Plant Sci. 2015, 6, 523. [Google Scholar] [CrossRef] [Green Version]
- Seisun, D.; Zalesny, N. Strides in food texture and hydrocolloids. Food Hydrocoll. 2021, 117, 106575. [Google Scholar] [CrossRef]
- Ciriminna, R.; Chavarría-Hernández, N.; Hernández, A.R.; Pagliaro, M. Pectin: A new perspective from the biorefinery standpoint. Biofuel. Bioprod. Bioref. 2015, 9, 368–377. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Delisi, R.; Ilharco, L.M.; Pagliaro, M. Pectin production and global market. Agro Food Ind. Hi-Tech 2016, 27, 17–20. [Google Scholar]
- Mao, G.; Wu, D.; Wei, C.; Tao, W.; Ye, X.; Linhardt, R.J.; Orfila, C.; Chen, S. Reconsidering conventional and innovative methods for pectin extraction from fruit and vegetable waste: Targeting rhamnogalacturonan I. Trends Food Sci. Technol. 2019, 94, 65–78. [Google Scholar] [CrossRef]
- Wu, D.; Zheng, J.; Mao, G.; Hu, W.; Ye, X.; Linhardt, R.J.; Chen, S. Rethinking the impact of RG-I mainly from fruits and vegetables on dietary health. Crit. Rev. Food Sci. Nutr. 2020, 60, 2938–2960. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Brunetti, C.; Fidalgo, A.; Ciriminna, R.; Delisi, R.; Albanese, L.; Zabini, F.; Gori, A.; dos Santos Nascimento, L.B.; De Carlo, A.; et al. Real-scale integral valorization of waste orange peel via hydrodynamic cavitation. Processes 2019, 7, 581. [Google Scholar] [CrossRef] [Green Version]
- Nuzzo, D.; Cristaldi, L.; Sciortino, M.; Albanese, L.; Scurria, A.; Zabini, F.; Lino, C.; Pagliaro, M.; Meneguzzo, F.; Di Carlo, M.; et al. Exceptional antioxidant, non-cytotoxic activity of integral lemon pectin from hydrodynamic cavitation. ChemistrySelect 2020, 5, 5066–5071. [Google Scholar] [CrossRef]
- Scurria, A.; Sciortino, M.; Albanese, L.; Nuzzo, D.; Zabini, F.; Meneguzzo, F.; Alduina, R.V.; Presentato, A.; Pagliaro, M.; Avellone, G.; et al. Flavonoids in lemon and grapefruit IntegroPectin. Preprints 2021, 2021020620. [Google Scholar] [CrossRef]
- Scurria, A.; Sciortino, M.; Presentato, A.; Lino, C.; Piacenza, E.; Albanese, L.; Zabini, F.; Meneguzzo, F.; Nuzzo, D.; Pagliaro, M.; et al. Volatile compounds of lemon and grapefruit IntegroPectin. Molecules 2021, 26, 51. [Google Scholar] [CrossRef] [PubMed]
- Presentato, A.; Scurria, A.; Albanese, L.; Lino, C.; Sciortino, M.; Pagliaro, M.; Zabini, F.; Meneguzzo, F.; Alduina, R.; Nuzzo, D.; et al. Superior antibacterial activity of integral lemon pectin from hydrodynamic cavitation. ChemistryOpen 2020, 9, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Presentato, A.; Piacenza, E.; Scurria, A.; Albanese, L.; Zabini, F.; Meneguzzo, F.; Nuzzo, D.; Pagliaro, M.; Chillura Martino, D.; Alduina, R.; et al. A new water-soluble bactericidal agent for the treatment of infections caused by Gram-positive and Gram-negative bacterial strains. Antibiotics 2020, 9, 586. [Google Scholar] [CrossRef]
- Nuzzo, D.; Picone, P.; Giardina, C.; Scordino, M.; Mudò, G.; Pagliaro, M.; Scurria, A.; Meneguzzo, F.; Ilharco, L.M.; Fidalgo, A.; et al. New neuroprotective effect of lemon IntegroPectin on neuronal cellular model. Antioxidants 2021, 10, 669. [Google Scholar] [CrossRef]
- Victor, M.M.; David, J.M.; Sakukuma, M.C.K.; França, E.L.; Nunes, A.V.J. A simple and efficient process for the extraction of naringin from grapefruit peel waste. Green Process. Synth. 2018, 7, 524–529. [Google Scholar] [CrossRef]
- Collin, F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [Green Version]
- Lezaja, A.; Altmeyer, M. Inherited DNA lesions determine G1 duration in the next cell cycle. Cell Cycle 2018, 17, 24–32. [Google Scholar] [CrossRef]
- Schwartz, G.K.; Shah, M.A. Targeting the cell cycle: A new approach to cancer therapy. J. Clin. Oncol. 2005, 23, 9408–9421. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.A.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J., II; Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the mitochondrial membrane potential using the cationic JC-1 dye as a sensitive fluorescent probe. Biol. Protoc. 2019, 9, e3128. [Google Scholar] [CrossRef]
- Sharma, R.; Kamboj, S.; Khurana, R.; Singh, G.; Rana, V. Physicochemical and functional performance of pectin extracted by QbD approach from Tamarindus indica L. pulp. Carbohydr. Polym. 2015, 134, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Palmer, K.J.; Hartzog, M.B. An X-ray diffraction investigation of sodium pectate. J. Am. Chem. Soc. 1945, 67, 2122–2127. [Google Scholar] [CrossRef]
- Gohil, R.M. Synergistic blends of natural polymers, pectin and sodium alginate. J. Appl. Polym. Sci. 2010, 120, 2324–2336. [Google Scholar] [CrossRef]
- Forster, J.I.; Köglsberger, S.; Trefois, C.; Boyd, O.; Baumuratov, A.S.; Buck, L.; Balling, R.; Antony, P.M.A. Characterization of differentiated SH-SY5Y as neuronal screening model reveals increased oxidative vulnerability. J. Biomol. Screen. 2016, 21, 496–509. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari-Movahed, M.; Jackson, G.; Farzaei, M.H.; Bishayee, A. A systematic review of the preventive and therapeutic effects of naringin against human malignancies. Front. Pharmacol. 2021, 12, 639840. [Google Scholar] [CrossRef] [PubMed]
- Pulley, G.N. Solubility of naringin in water. Ind. Eng. Chem. Anal. Ed. 1936, 8, 360. [Google Scholar] [CrossRef]
- Migheli, R.; Lostia, G.; Galleri, G.; Rocchitta, G.; Serra, P.A.; Bassareo, V.; Acquas, E.; Peana, A.T. Neuroprotective effect of (R)-(-)-linalool on oxidative stress in PC12 cells. Phytomedicine Plus 2021, 1, 100073. [Google Scholar] [CrossRef]
- Negromonte Souto-Maior, F.; Vilar da Fonsêca, D.; Rodrigues Salgado, P.R.; de Oliveira Monte, L.; Pergentino de Sousa, D.; Nóbrega de Almeida, R. Antinociceptive and anticonvulsant effects of the monoterpene linalool oxide. Pharm. Biol. 2017, 55, 63–67. [Google Scholar] [CrossRef]
- Ro, J.; Kim, Y.; Kim, H.; Jang, S.B.; Lee, H.J.; Chakma, S.; Jeong, J.H.; Lee, J. Anti-oxidative activity of pectin and its stabilizing effect on retinyl palmitate. Korean J. Physiol. Pharmacol. 2013, 17, 197–201. [Google Scholar] [CrossRef]
- Wikiera, A.; Grabacka, M.; Byczyński, L.; Stodolak, B.; Mika, M. Enzymatically extracted apple pectin possesses antioxidant and antitumor activity. Molecules 2021, 26, 1434. [Google Scholar] [CrossRef] [PubMed]
- Restivo, I.; Tesoriere, L.; Frazzitta, A.; Livrea, M.A.; Attanzio, A.; Allegra, M. Anti-poliferative activity of a hydrophilic extract of manna from Fraxinus angustifolia Vahl through mitochondrial pathway-mediated apoptosis and cell cycle arrest in human colon cancer cells. Molecules 2020, 25, 5055. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food), Scientific Opinion on the re-evaluation of pectin (E 440i) and amidated pectin (E 440ii) as food additives. EFSA J. 2017, 15, 4866. [CrossRef]
- Ahmed, O.M.; AbouZid, S.F.; Ahmed, N.A.; Zaky, M.Y.; Liu, H. An up-to-date review on citrus flavonoids: Chemistry and benefits in health and diseases. Curr. Pharm. Des. 2021, 27, 513–530. [Google Scholar] [CrossRef]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus essential oils (CEOs) and their applications in food: An overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Karakhanova, S.; Hartwig, W.; D’Haese, J.G.; Philippov, P.P.; Werner, J.; Bazhin, A.V. Mitochondria and mitochondrial ROS in cancer: Novel targets for anticancer therapy. J. Cell Physiol. 2016, 231, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Esquivel-Chirino, C.; Esquivel-Soto, J.; Morales-González, J.A.; Montes Sánchez, D.; Ventura-Gallegos, J.L.; Hernández-Mora, L.E.; Zentella-Dehesa, A. Inflammatory Environmental, Oxidative Stress in Tumoral Progression. In Oxidative Stress and Chronic Degenerative Diseases-A Role for Antioxidants; Intech Open: Zagreb, Croatia, 2013; pp. 187–208. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuzzo, D.; Scordino, M.; Scurria, A.; Giardina, C.; Giordano, F.; Meneguzzo, F.; Mudò, G.; Pagliaro, M.; Picone, P.; Attanzio, A.; et al. Protective, Antioxidant and Antiproliferative Activity of Grapefruit IntegroPectin on SH-SY5Y Cells. Int. J. Mol. Sci. 2021, 22, 9368. https://doi.org/10.3390/ijms22179368
Nuzzo D, Scordino M, Scurria A, Giardina C, Giordano F, Meneguzzo F, Mudò G, Pagliaro M, Picone P, Attanzio A, et al. Protective, Antioxidant and Antiproliferative Activity of Grapefruit IntegroPectin on SH-SY5Y Cells. International Journal of Molecular Sciences. 2021; 22(17):9368. https://doi.org/10.3390/ijms22179368
Chicago/Turabian StyleNuzzo, Domenico, Miriana Scordino, Antonino Scurria, Costanza Giardina, Francesco Giordano, Francesco Meneguzzo, Giuseppa Mudò, Mario Pagliaro, Pasquale Picone, Alessandro Attanzio, and et al. 2021. "Protective, Antioxidant and Antiproliferative Activity of Grapefruit IntegroPectin on SH-SY5Y Cells" International Journal of Molecular Sciences 22, no. 17: 9368. https://doi.org/10.3390/ijms22179368
APA StyleNuzzo, D., Scordino, M., Scurria, A., Giardina, C., Giordano, F., Meneguzzo, F., Mudò, G., Pagliaro, M., Picone, P., Attanzio, A., Raimondo, S., Ciriminna, R., & Di Liberto, V. (2021). Protective, Antioxidant and Antiproliferative Activity of Grapefruit IntegroPectin on SH-SY5Y Cells. International Journal of Molecular Sciences, 22(17), 9368. https://doi.org/10.3390/ijms22179368













