Functional Characterization of a Cucumber (Cucumis sativus L.) Vacuolar Invertase, CsVI1, Involved in Hexose Accumulation and Response to Low Temperature Stress
Abstract
1. Introduction
2. Results
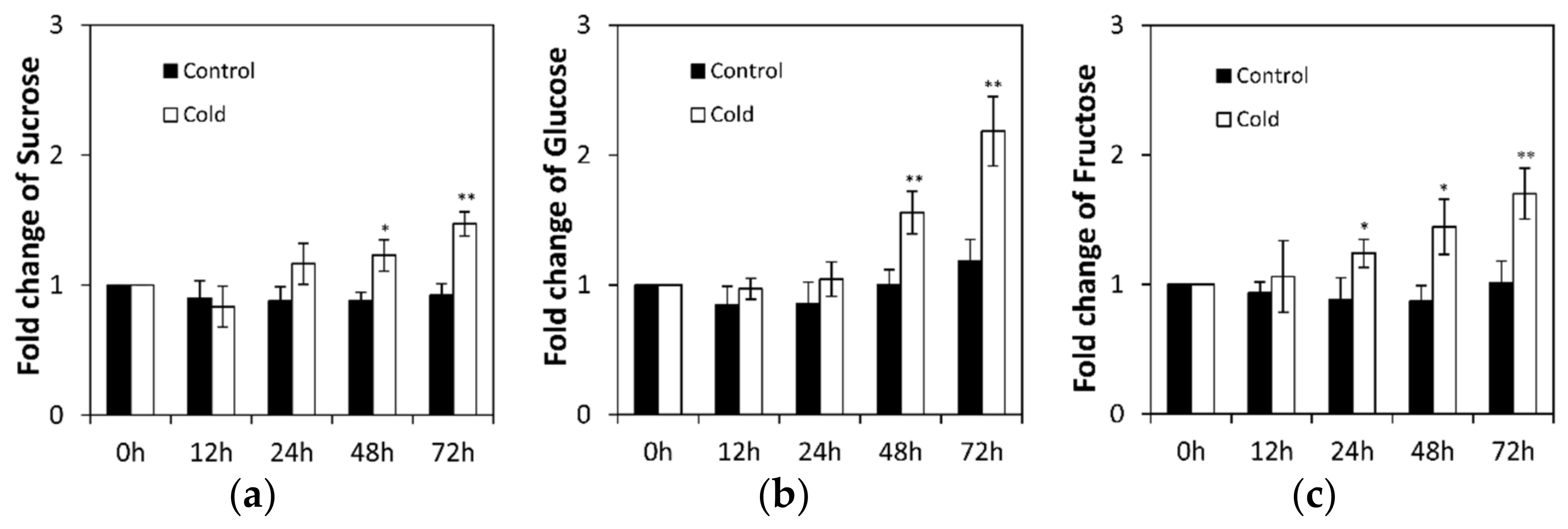
2.1. Low Temperature Affects Invertase in Cucumis sativus Seedling Roots
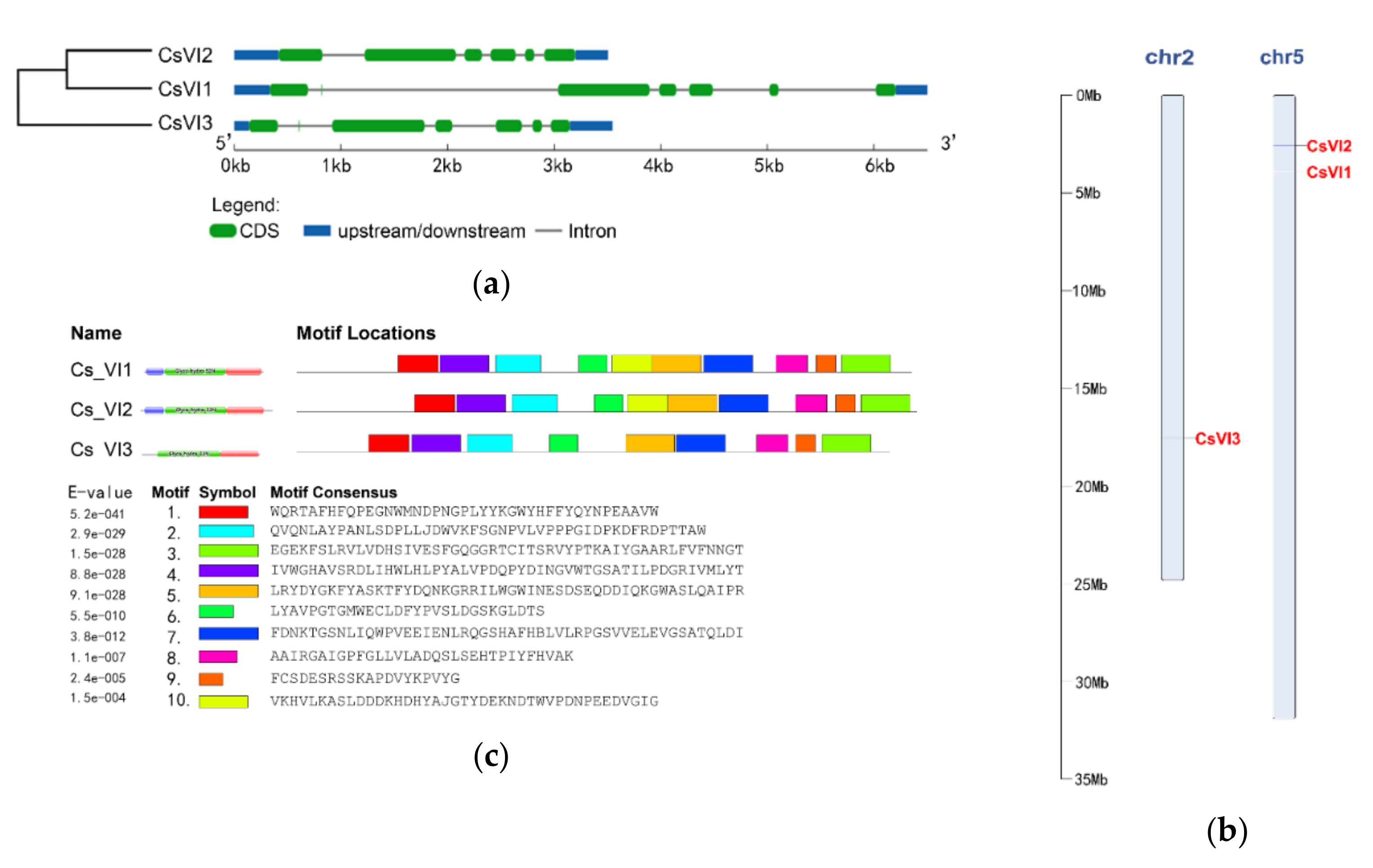
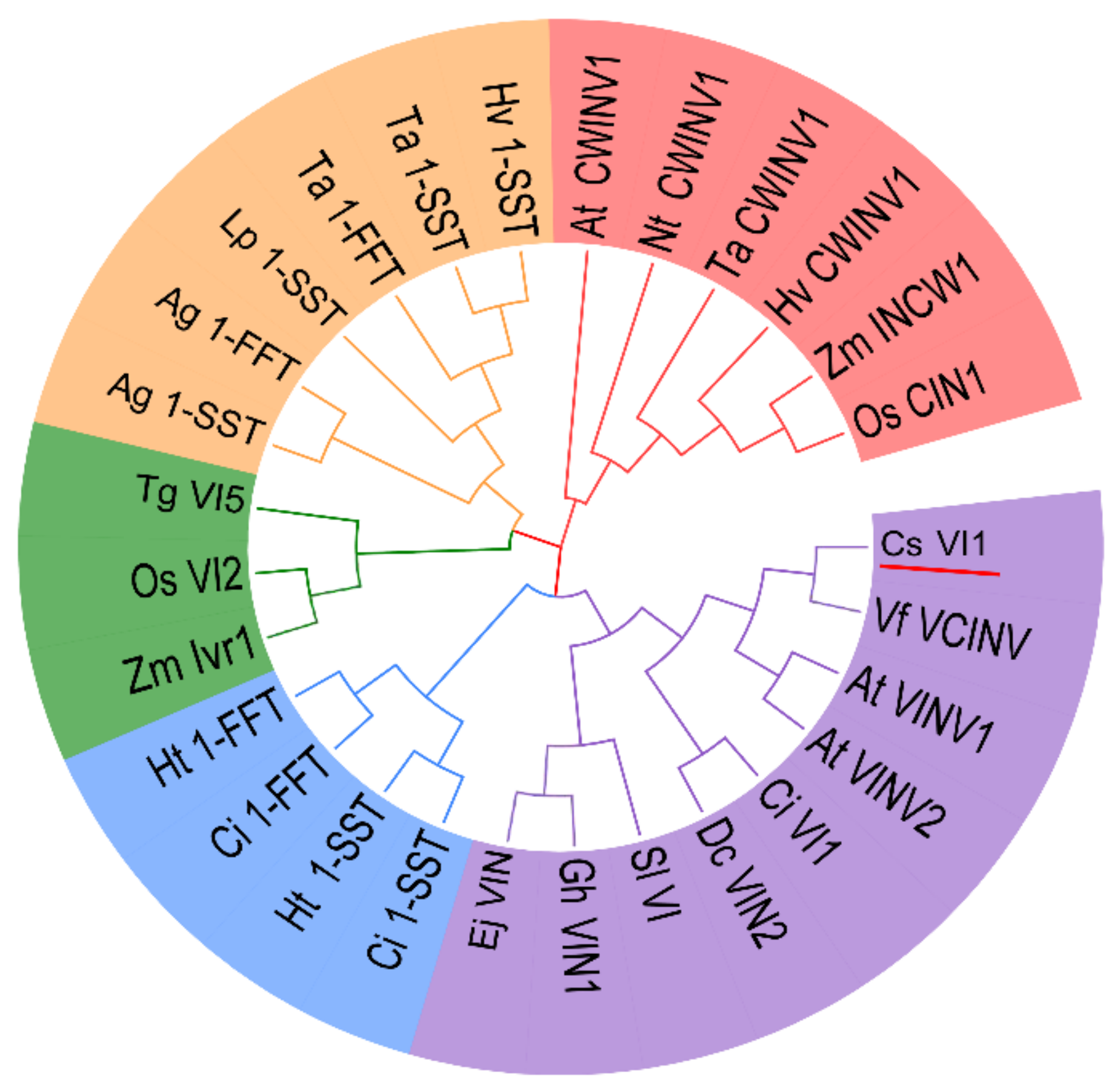
2.2. Genome-Wide Search for Genes Encoding Vacuolar Invertase in Cucumis sativus
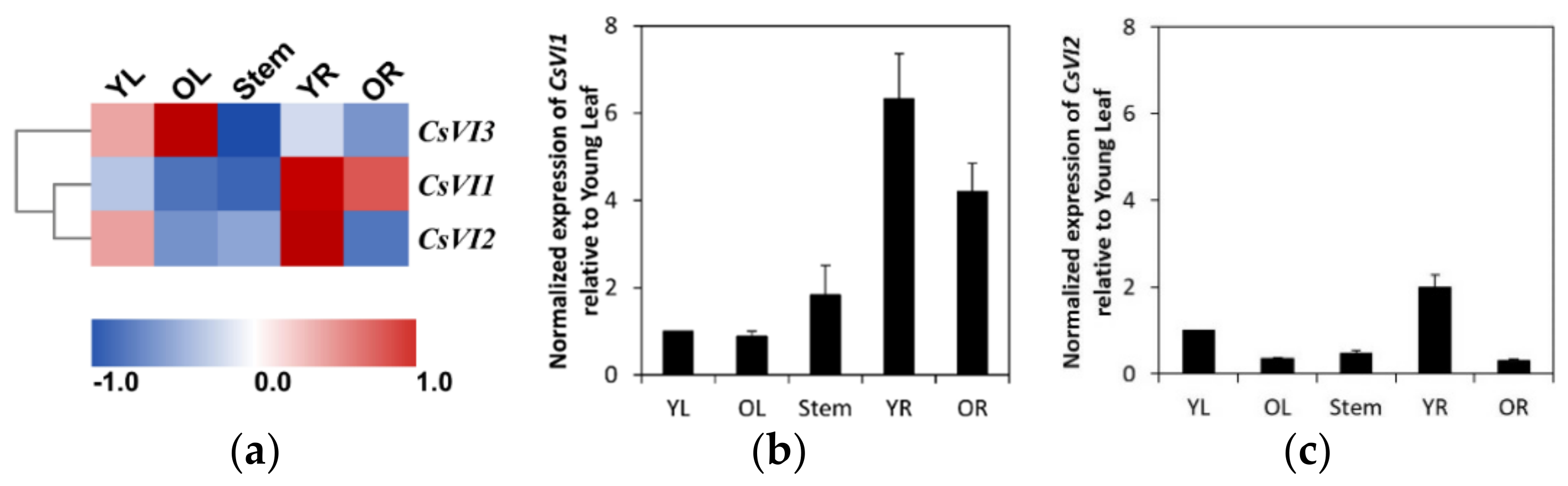
2.3. Expression Profiling of CsVIs in Cucumber Seedlings
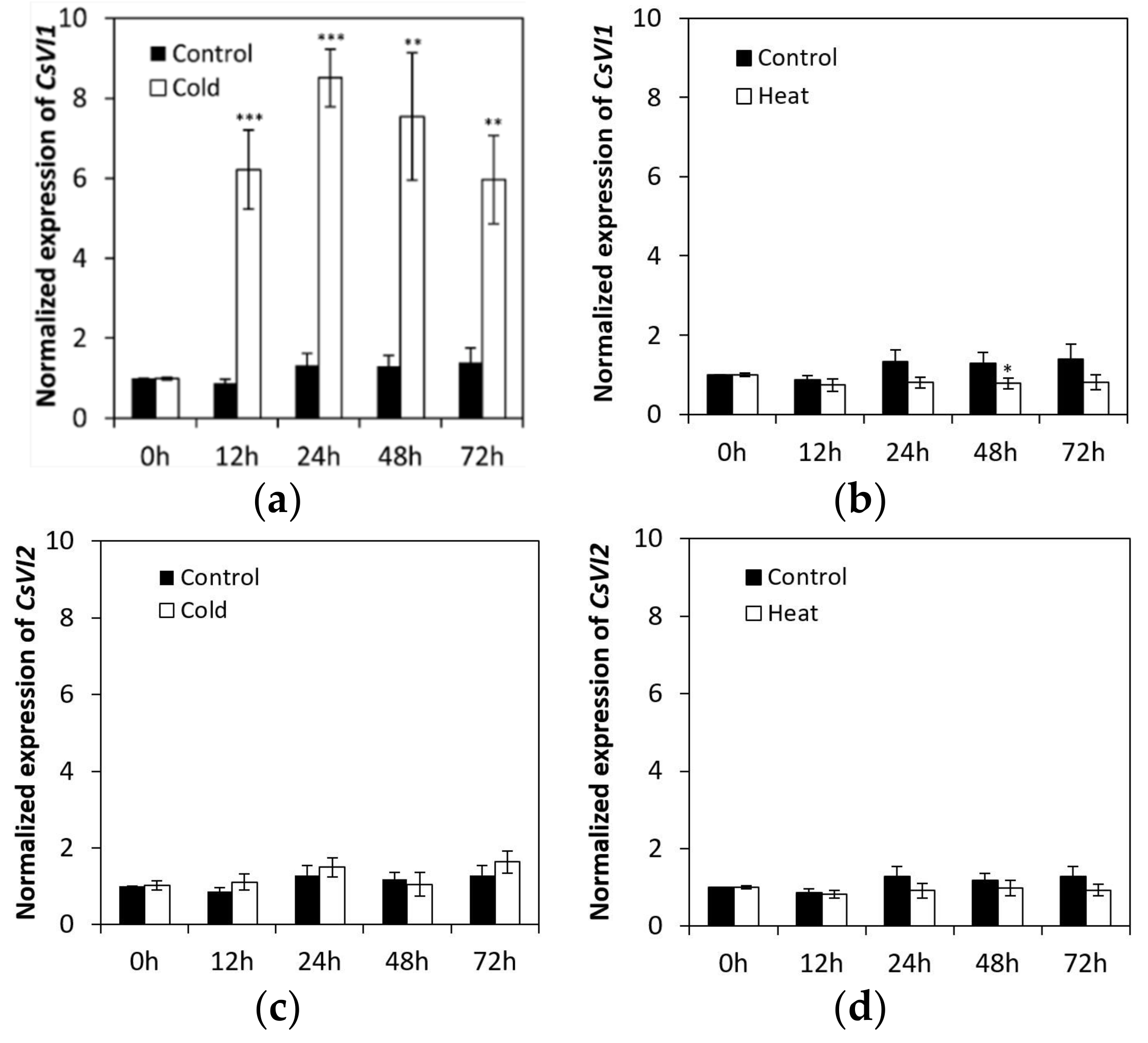
2.4. CsVI1 Transcript Abundance Responds to Low Temperature Stress but Not Heat Stress in Cucumber Seedling Roots
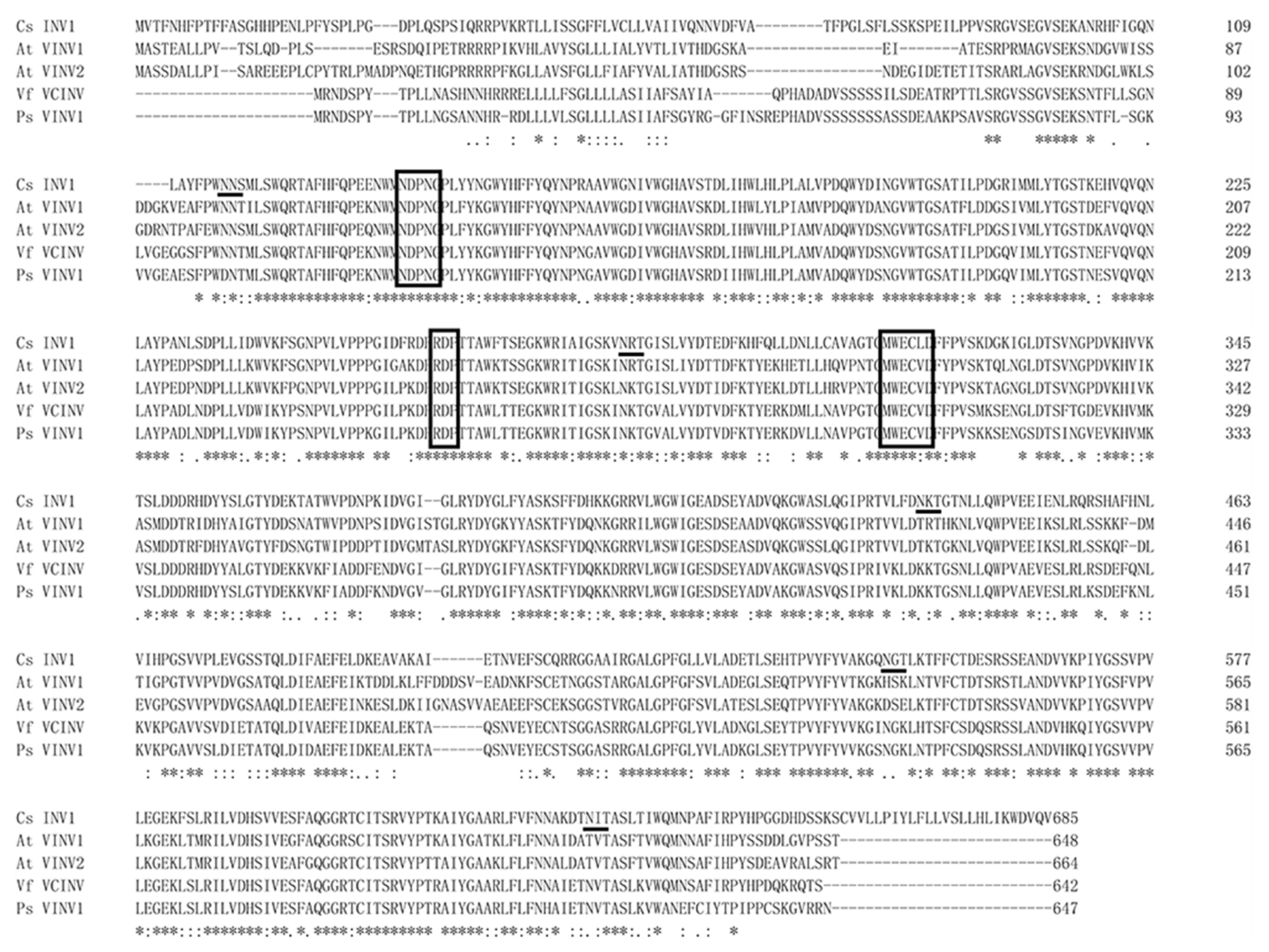
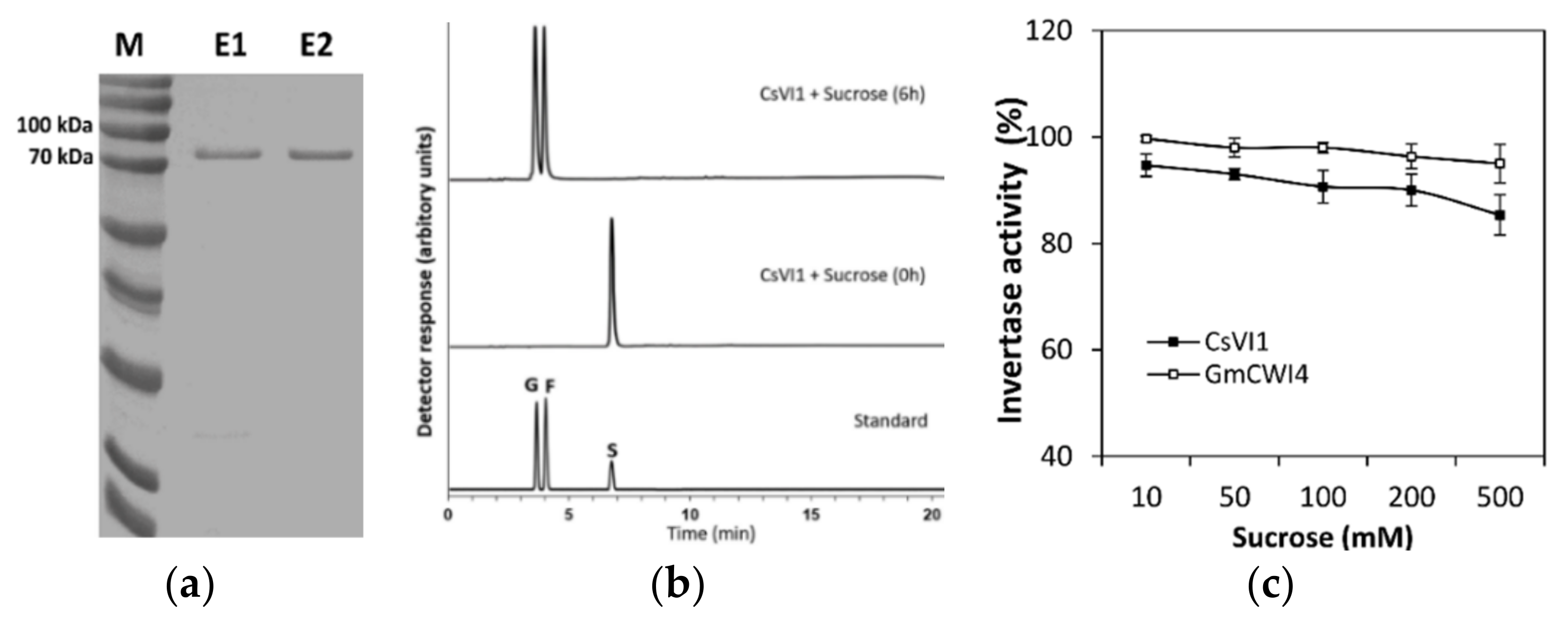
2.5. Characterization of the CsVI1 Protein and Analysis of Invertase Activity
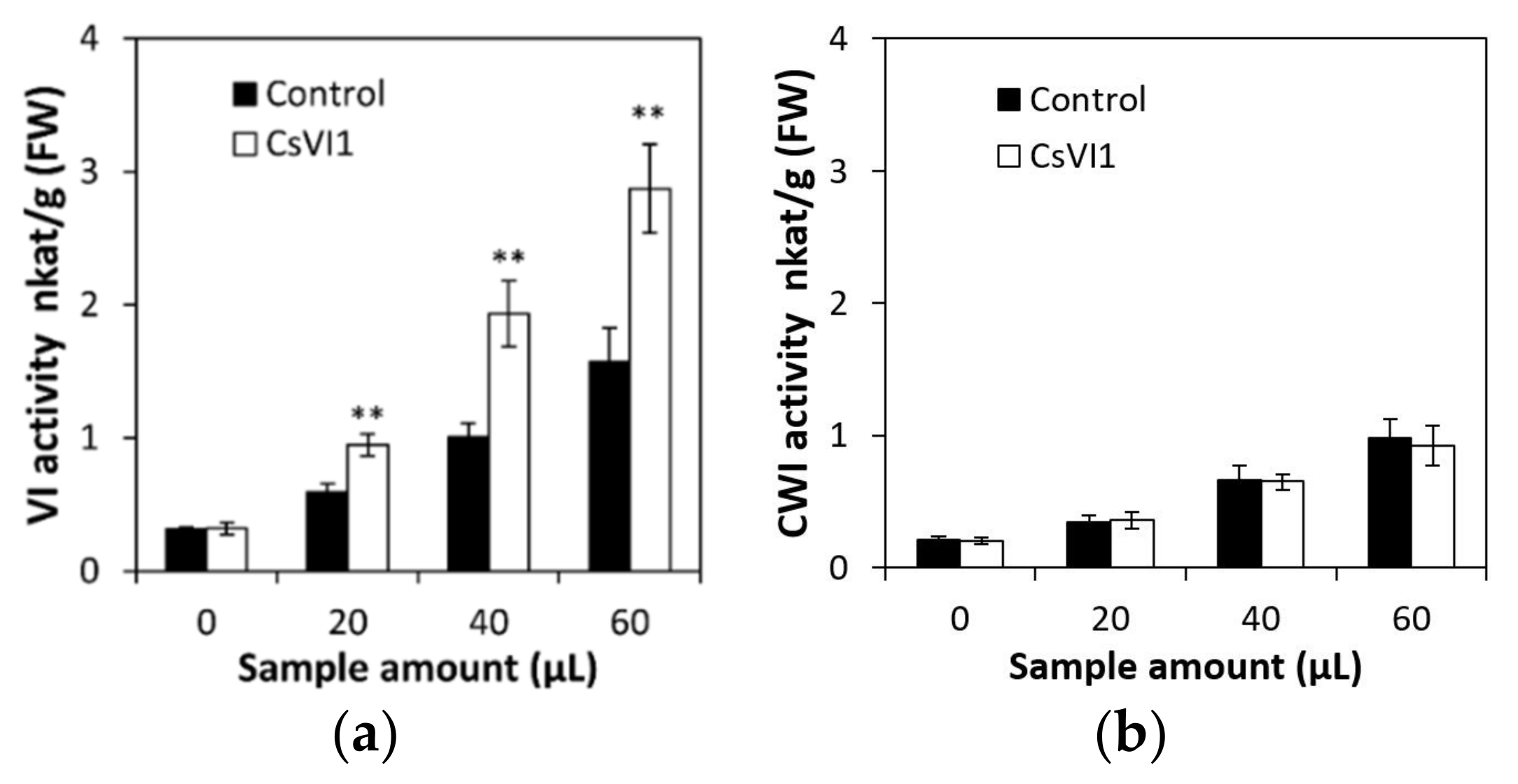
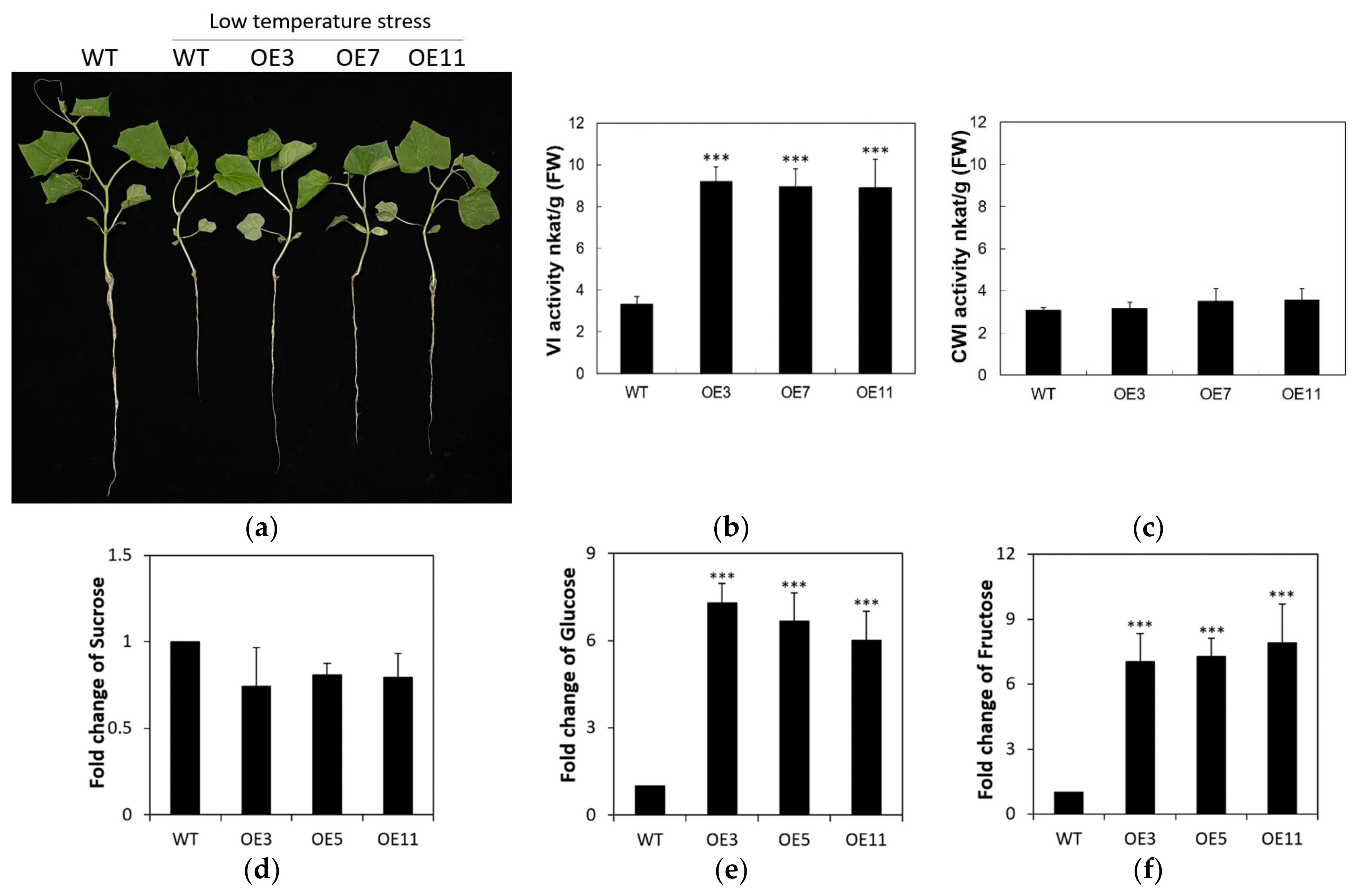
2.6. Overexpression of CsVI1 Increases the Hexose Content and Low Temperature Tolerance of Cucumber Seedling Roots
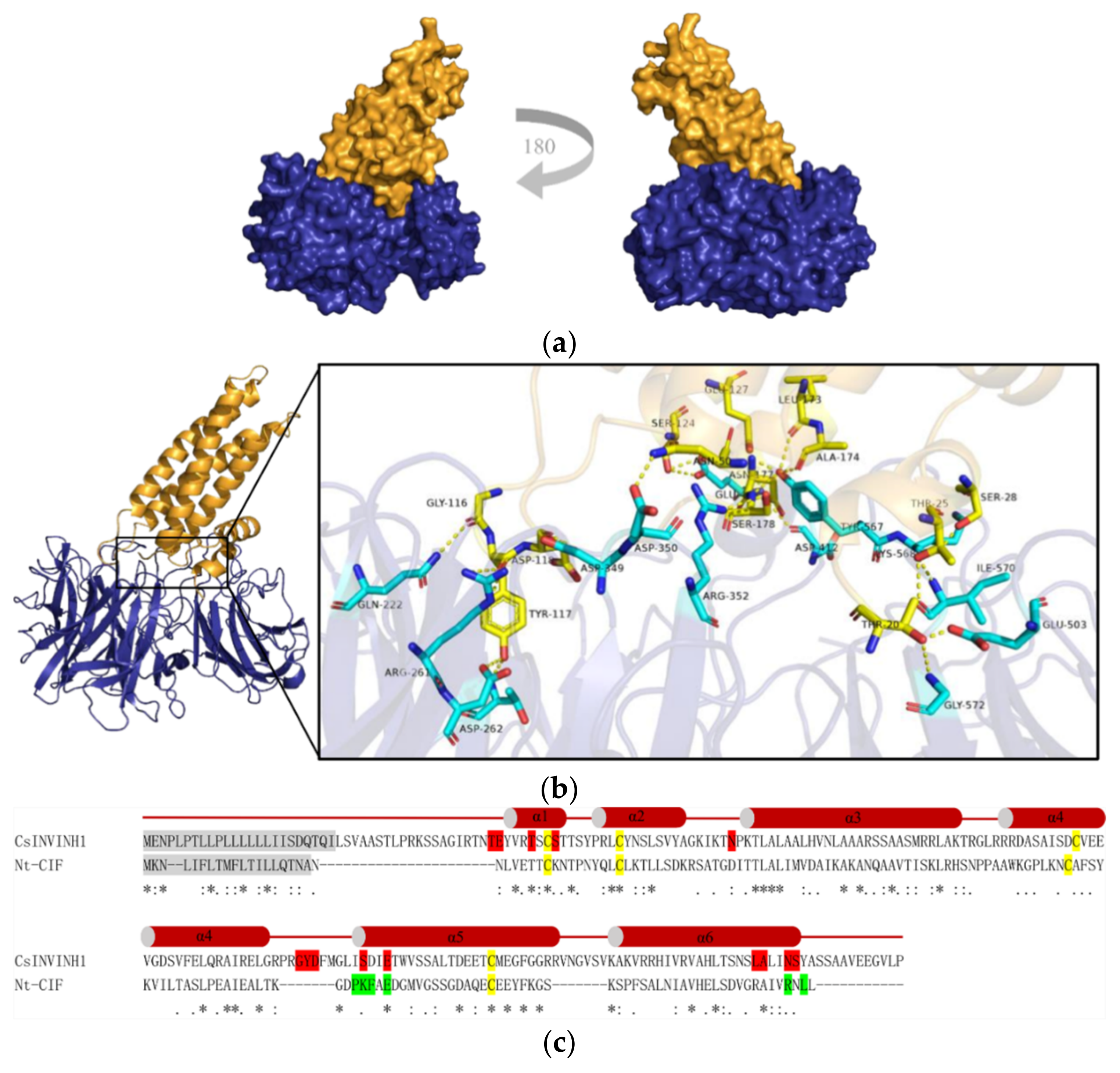
2.7. A Putative Cucumber Invertase Inhibitor CsINVINH1 Can Form a Complex with CsVI1
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Gene Structure, Distribution, Cis-Element, and Conserver Motif Analysis
4.3. Transcriptomic Sequencing and Gene Expression Analysis
4.4. Plant Transformation
4.5. Plant Protein and Soluble Carbohydrate Extraction
4.6. Heterologous Expression and Purification of CsVI1
4.7. Enzyme Activity and Carbohydrate Assay
4.8. Invertase-Invertase Inhibitor Complex Modelling
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, B.; Mitra, S.; Biswas, A.K. Regulation of sugar metabolism in rice (Oryza sativa L.) seedlings under arsenate toxicity and its improvement by phosphate. Physiol. Mol. Biol. Plants 2010, 16, 59–68. [Google Scholar] [CrossRef]
- Koch, K. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef]
- Ruan, Y.-L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Peng, D.; Gu, X.; Xue, L.-J.; Leebens-Mack, J.H.; Tsai, C.-J. Bayesian phylogeny of sucrose transporters: Ancient origins, differential expansion and convergent evolution in monocots and dicots. Front. Plant Sci. 2014, 5, 615. [Google Scholar] [CrossRef]
- Tymowska-Lalanne, Z.; Kreis, M. Expression of the Arabidopsis thaliana invertase gene family. Planta 1998, 207, 259–265. [Google Scholar] [CrossRef]
- Hütsch, B.W.; Jung, S.; Schubert, S. Comparison of salt and drought-stress effects on maize growth and yield formation with regard to acid invertase activity in the kernels. J. Agron. Crop. Sci. 2014, 201, 353–367. [Google Scholar] [CrossRef]
- Barratt, D.H.P.; Derbyshire, P.; Findlay, K.; Pike, M.; Wellner, N.M.; Lunn, J.; Feil, R.; Simpson, C.; Maule, A.J.; Smith, A.M. Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc. Natl. Acad. Sci. USA 2009, 106, 13124–13129. [Google Scholar] [CrossRef]
- Druart, N.; De Roover, J.; Ende, W.V.D.; Goupil, P.; Van Laere, A.; Rambour, S. Sucrose assimilation during early developmental stages of chicory (Cichorium intybus L.) plants. Planta 2001, 212, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Granot, D.; Kelly, G.; Stein, O.; David-Schwartz, R. Substantial roles of hexokinase and fructokinase in the effects of sugars on plant physiology and development. J. Exp. Bot. 2013, 65, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Roitsch, T. Invertases and life beyond sucrose cleavage. Trends Plant Sci. 2000, 5, 47–48. [Google Scholar] [CrossRef]
- Roitsch, T.; González, M.-C. Function and regulation of plant invertases: Sweet sensations. Trends Plant Sci. 2004, 9, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A. Invertases. Primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol. 1999, 121, 1–8. [Google Scholar] [CrossRef]
- Draie, R.; Péron, T.; Pouvreau, J.-B.; Véronési, C.; Jégou, S.; Delavault, P.; Thoiron, S.; Simier, P. Invertases involved in the development of the parasitic plant Phelipanche ramosa: Characterization of the dominant soluble acid isoform, PrSAI1. Mol. Plant Pathol. 2011, 12, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A. Molecular characterization and functional analysis of sucrose-cleaving enzymes in carrot (Daucus carota L.). J. Exp. Bot. 1996, 47, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Fils-Lycaon, B.; Julianus, P.; Chillet, M.; Galas, C.; Hubert, O.; Rinaldo, D.; Mbéguié-A-Mbéguié, D. Acid invertase as a serious candidate to control the balance sucrose versus (glucose + fructose) of banana fruit during ripening. Sci. Hortic. 2011, 129, 197–206. [Google Scholar] [CrossRef]
- Guo, X.; Chen, H.; Liu, Y.; Chen, W.; Ying, Y.; Han, J.; Gui, R.; Zhang, H. The acid invertase gene family is involved in internode elongation in Phyllostachys heterocycla cv. pubescens. Tree Physiol. 2020, 40, 1217–1231. [Google Scholar] [CrossRef]
- Xu, X.; Ren, Y.; Wang, C.; Zhang, H.; Wang, F.; Chen, J.; Liu, X.; Zheng, T.; Cai, M.; Zeng, Z.; et al. OsVIN2 encodes a vacuolar acid invertase that affects grain size by altering sugar metabolism in rice. Plant Cell Rep. 2019, 38, 1273–1290. [Google Scholar] [CrossRef]
- Ni, D.A. Role of vacuolar invertase in regulating Arabidopsis stomatal opening. Acta Physiol. Plant. 2012, 34, 2449–2452. [Google Scholar] [CrossRef]
- Wang, L.; Ruan, Y.-L. Critical roles of vacuolar invertase in floral organ development and male and female fertilities are revealed through characterization of GhVIN1-RNAi cotton plants. Plant Physiol. 2016, 171, 405–423. [Google Scholar] [CrossRef]
- Wang, L.; Cook, A.; Patrick, J.W.; Chen, X.; Ruan, Y.-L. Silencing the vacuolar invertase geneGhVIN1blocks cotton fiber initiation from the ovule epidermis, probably by suppressing a cohort of regulatory genes via sugar signaling. Plant J. 2014, 78, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Yue, C.; Wang, Y.; Cao, H.; Li, N.; Wang, L.; Hao, X.; Wang, X.; Xiao, B.; Yang, Y. Identification of the invertase gene family (INVs) in tea plant and their expression analysis under abiotic stress. Plant Cell Rep. 2016, 35, 2269–2283. [Google Scholar] [CrossRef] [PubMed]
- Tauzin, A.S.; Giardina, T. Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front. Plant Sci. 2014, 5, 293. [Google Scholar] [CrossRef]
- Weiszmann, J.; Fürtauer, L.; Weckwerth, W.; Nägele, T. Vacuolar sucrose cleavage prevents limitation of cytosolic carbohydrate metabolism and stabilizes photosynthesis under abiotic stress. FEBS J. 2018, 285, 4082–4098. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Mahé, A.; Brangeon, J.; Prioul, J.-L. A maize vacuolar invertase, IVR2, is induced by water stress. Organ/tissue specificity and diurnal modulation of expression. Plant Physiol. 2000, 124, 71–84. [Google Scholar] [CrossRef]
- Chen, S.-F.; Liang, K.; Yin, D.-M.; Ni, D.-A.; Zhang, Z.-G.; Ruan, Y.-L. Ectopic expression of a tobacco vacuolar invertase inhibitor in guard cells confers drought tolerance in Arabidopsis. J. Enzym. Inhib. Med. Chem. 2016, 31, 1381–1385. [Google Scholar] [CrossRef]
- Qian, W.; Xiao, B.; Wang, L.; Hao, X.; Yue, C.; Cao, H.; Wang, Y.; Li, N.; Yu, Y.; Zeng, J.; et al. CsINV5, a tea vacuolar invertase gene enhances cold tolerance in transgenic Arabidopsis. BMC Plant Biol. 2018, 18, 228. [Google Scholar] [CrossRef]
- McKenzie, M.J.; Chen, R.K.Y.; Harris, J.C.; Ashworth, M.J.; Brummell, D.A. Post-translational regulation of acid invertase activity by vacuolar invertase inhibitor affects resistance to cold-induced sweetening of potato tubers. Plant Cell Environ. 2012, 36, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Wiberley-Bradford, A.E.; Busse, J.S.; Jiang, J.; Bethke, P.C. Sugar metabolism, chip color, invertase activity, and gene expression during long-term cold storage of potato (Solanum tuberosum) tubers from wild-type and vacuolar invertase silencing lines of Katahdin. BMC Res. 2014, 7, 801. [Google Scholar] [CrossRef]
- Antunes, W.C.; Provart, N.J.; Williams, T.C.R.; Loureiro, M.E. Changes in stomatal function and water use efficiency in potato plants with altered sucrolytic activity. Plant Cell Environ. 2011, 35, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Zhu, J.; Zhu, J.-K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef]
- Smeets, L.; Wehner, T.C. Environmental effects on genetic variation of chilling resistance in cucumber. Euphytica 1997, 97, 217–225. [Google Scholar] [CrossRef]
- Yang, H.; Wu, F.; Cheng, J. Reduced chilling injury in cucumber by nitric oxide and the antioxidant response. Food Chem. 2011, 127, 1237–1242. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Zhu, S.; Wang, Y. Postharvest application of MeJA and NO reduced chilling injury in cucumber (Cucumis sativus) through inhibition of H2O2 accumulation. Postharvest Biol. Technol. 2016, 119, 77–83. [Google Scholar] [CrossRef]
- Kreyszig, E. Advanced Engineering Mathematics, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 1979. [Google Scholar]
- Ende, W.V.D. Multifunctional fructans and raffinose family oligosaccharides. Front. Plant Sci. 2013, 4, 247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Greiner, S.; Scheffzek, K.; Rausch, T.; Wang, G. A 6&1-FEH encodes an enzyme for fructan degradation and interact with invertase inhibitor protein in Maize (Zea mays L.). Int. J. Mol. Sci. 2019, 20, 3807. [Google Scholar] [CrossRef]
- Wan, H.; Wu, L.; Yang, Y.; Zhou, G.; Ruan, Y.-L. Evolution of sucrose metabolism: The dichotomy of invertases and beyond. Trends Plant Sci. 2018, 23, 163–177. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Han, M.; Min, J.; Chen, P.; Mao, Y.; Huang, Q.; Tong, Q.; Liu, Q.; Fang, Y. Genome-wide survey of invertase encoding genes and functional characterization of an extracellular fungal pathogen-responsive invertase in Glycine max. Int. J. Mol. Sci. 2018, 19, 2395. [Google Scholar] [CrossRef]
- Rausch, T.; Greiner, S. Plant protein inhibitors of invertases. BBA Proteins Proteom. 2004, 1696, 253–261. [Google Scholar] [CrossRef]
- Hothorn, M.; Van den Ende, W.; Lammens, W.; Rybin, V.; Sche_zek, K. Structural insights into the pH-controlled targeting of plant cell-wall invertase by a specific inhibitor protein. Proc. Natl. Acad. Sci. USA 2010, 107, 17427–17432. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Osakabe, Y.; Mizoi, J.; Nakashima, K.; Fujita, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of an Arabidopsis thaliana abiotic stress-inducible facilitated diffusion transporter for monosaccharides. J. Biol. Chem. 2010, 285, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Hund, A.; Fracheboud, Y.; Soldati, A.; Stamp, P. Cold tolerance of maize seedlings as determined by root morphology and photosynthetic traits. Eur. J. Agron. 2008, 28, 178–185. [Google Scholar] [CrossRef]
- Sergeeva, L.I.; Keurentjes, J.; Bentsink, L.; Vonk, J.; van der Plas, L.H.W.; Koornneef, M.; Vreugdenhil, D. Vacuolar invertase regulates elongation of Arabidopsis thaliana roots as revealed by QTL and mutant analysis. Proc. Natl. Acad. Sci. USA 2006, 103, 2994–2999. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.-R.; Lian, H.; Ni, D.-A.; He, Y.-K.; Chen, X.; Ruan, Y.-L. Evidence that high activity of vacuolar invertase is required for cotton fiber and Arabidopsis root elongation through osmotic dependent and independent pathways, respectively. Plant Physiol. 2010, 154, 744–756. [Google Scholar] [CrossRef]
- Ruan, Y.-L. Signaling role of sucrose metabolism in development. Mol. Plant 2012, 5, 763–765. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.F. Molecular Analysis of An Acid Invertase Gene Family in Arabidopsis. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2006. [Google Scholar]
- Smeekens, S.; Ma, J.; Hanson, J.; Rolland, F. Sugar signals and molecular networks controlling plant growth. Curr. Opin. Plant Biol. 2010, 13, 273–278. [Google Scholar] [CrossRef]
- Yu, L.; Liu, H.; Shao, X.; Yu, F.; Wei, Y.; Ni, Z.; Xu, F.; Wang, H. Effects of hot air and methyl jasmonate treatment on the metabolism of soluble sugars in peach fruit during cold storage. Postharvest Biol. Technol. 2015, 113, 8–16. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, C.; Brummell, D.A.; Qi, S.; Lin, Q.; Duan, Y. Jasmonic acid treatment alleviates chilling injury in peach fruit by promoting sugar and ethylene metabolism. Food Chem. 2020, 338, 128005. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Xu, X.; Chen, X.; Xue, L.; Cao, B. Cucumber carbohydrate metabolism and translocation under chilling night temperature. J. Plant Physiol. 2007, 164, 621–628. [Google Scholar] [CrossRef]
- Miao, M.; Yang, X.; Han, X.; Wang, K. Sugar signalling is involved in the sex expression response of monoecious cucumber to low temperature. J. Exp. Bot. 2010, 62, 797–804. [Google Scholar] [CrossRef]
- Fryer, M.J.; Andrews, J.R.; Oxborough, K.; Blowers, D.A.; Baker, N.R. Relationship between CO2 assimilation, photosynthetic electron transport, and active O2 metabolism in leaves of maize in the field during periods of low temperature1. Plant Physiol. 1998, 116, 571–580. [Google Scholar] [CrossRef]
- Chen, L.; Liu, X.; Huang, X.; Luo, W.; Long, Y.; Greiner, S.; Rausch, T.; Zhao, H. Functional characterization of a drought-responsive invertase inhibitor from Maize (Zea mays L.). Int. J. Mol. Sci. 2019, 20, 4081. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Rausch, T.; Greiner, S. The N-terminal pro region mediates retention of unprocessed type-I PME in the Golgi apparatus. Plant J. 2009, 58, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, A.; Chowdhury, M.; Abdullah, Z.C.; Napis, S. Influence of silver nitrate (ethylene inhibitor) on cucumber in vitro shoot regeneration. Plant Cell Tissue Organ Cult. 1997, 51, 75–78. [Google Scholar] [CrossRef]
- Link, M.; Rausch, T.; Greiner, S. In Arabidopsis thaliana, the invertase inhibitors AtC/VIF1 and 2 exhibit distinct target enzyme specificities and expression profiles. FEBS Lett. 2004, 573, 105–109. [Google Scholar] [CrossRef]
- Wei, H.; Zhao, H.; Su, T.; Bausewein, A.; Greiner, S.; Harms, K.; Rausch, T. Chicory R2R3-MYB transcription factors CiMYB5 and CiMYB3 regulate fructan 1-exohydrolase expression in response to abiotic stress and hormonal cues. J. Exp. Bot. 2017, 68, 4323–4338. [Google Scholar] [CrossRef]
- Wei, H.; Bausewein, A.; Steininger, H.; Su, T.; Zhao, H.; Harms, K.; Greiner, S.; Rausch, T. Linking expression of fructan active enzymes, cell wall invertases and sucrose transporters with fructan profiles in growing taproot of chicory (Cichorium intybus): Impact of hormonal and environmental cues. Front. Plant Sci. 2016, 7, 1806. [Google Scholar] [CrossRef][Green Version]
- Hothorn, M.; D’Angelo, I.; Márquez, J.A.; Greiner, S.; Scheffzek, K. The invertase inhibitor Nt-CIF from tobacco: A highly thermostable four-helix bundle with an unusual N-terminal extension. J. Mol. Biol. 2003, 335, 987–995. [Google Scholar] [CrossRef]
- Webb, B.; Sali, A. Comparative protein structure modeling using modeller. Curr. Protoc. Protein Sci. 2016, 86, 2.9.1–2.9.37. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Z.; Zheng, F.; Wu, S.; Li, R.; Li, Y.; Zhong, J.; Zhao, H. Functional Characterization of a Cucumber (Cucumis sativus L.) Vacuolar Invertase, CsVI1, Involved in Hexose Accumulation and Response to Low Temperature Stress. Int. J. Mol. Sci. 2021, 22, 9365. https://doi.org/10.3390/ijms22179365
Feng Z, Zheng F, Wu S, Li R, Li Y, Zhong J, Zhao H. Functional Characterization of a Cucumber (Cucumis sativus L.) Vacuolar Invertase, CsVI1, Involved in Hexose Accumulation and Response to Low Temperature Stress. International Journal of Molecular Sciences. 2021; 22(17):9365. https://doi.org/10.3390/ijms22179365
Chicago/Turabian StyleFeng, Zili, Fenghua Zheng, Silin Wu, Rui Li, Yue Li, Jiaxin Zhong, and Hongbo Zhao. 2021. "Functional Characterization of a Cucumber (Cucumis sativus L.) Vacuolar Invertase, CsVI1, Involved in Hexose Accumulation and Response to Low Temperature Stress" International Journal of Molecular Sciences 22, no. 17: 9365. https://doi.org/10.3390/ijms22179365
APA StyleFeng, Z., Zheng, F., Wu, S., Li, R., Li, Y., Zhong, J., & Zhao, H. (2021). Functional Characterization of a Cucumber (Cucumis sativus L.) Vacuolar Invertase, CsVI1, Involved in Hexose Accumulation and Response to Low Temperature Stress. International Journal of Molecular Sciences, 22(17), 9365. https://doi.org/10.3390/ijms22179365




