Kisspeptin Neurons and Estrogen–Estrogen Receptor α Signaling: Unraveling the Mystery of Steroid Feedback System Regulating Mammalian Reproduction
Abstract
1. Introduction
2. Feedback Actions of Estrogen on Pulsatile and Surge-Modes of Gonadotropin-Releasing Hormone (GnRH)/Gonadotropin Release
3. Indispensable Role of Estrogen Receptor α for Mammalian Reproduction
4. Possible Targets of the Negative and Positive Feedback Action of Estrogen in the Brain
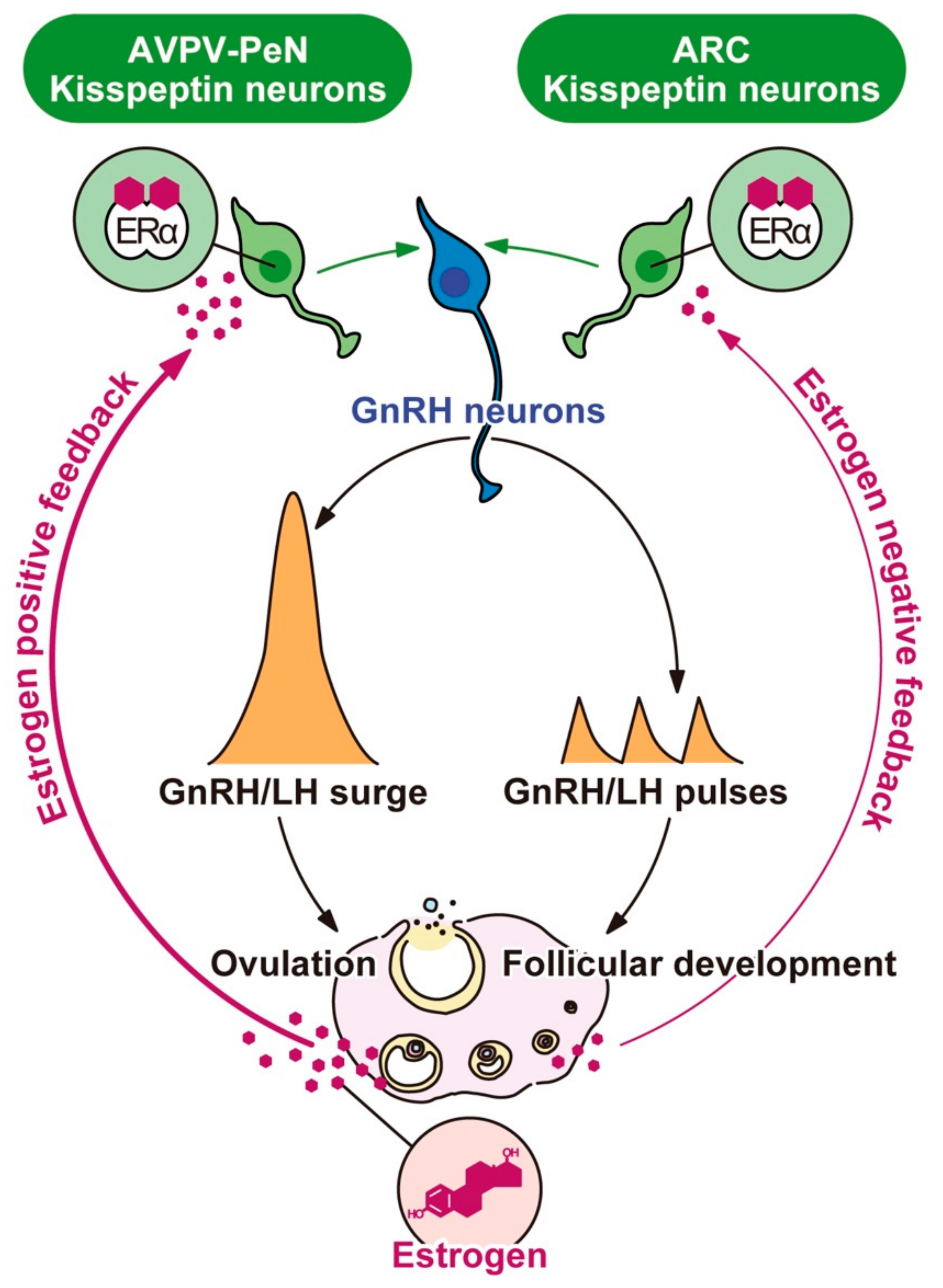
5. Kisspeptin Neurons as Targets of the Negative and Positive Feedback Actions of Estrogen
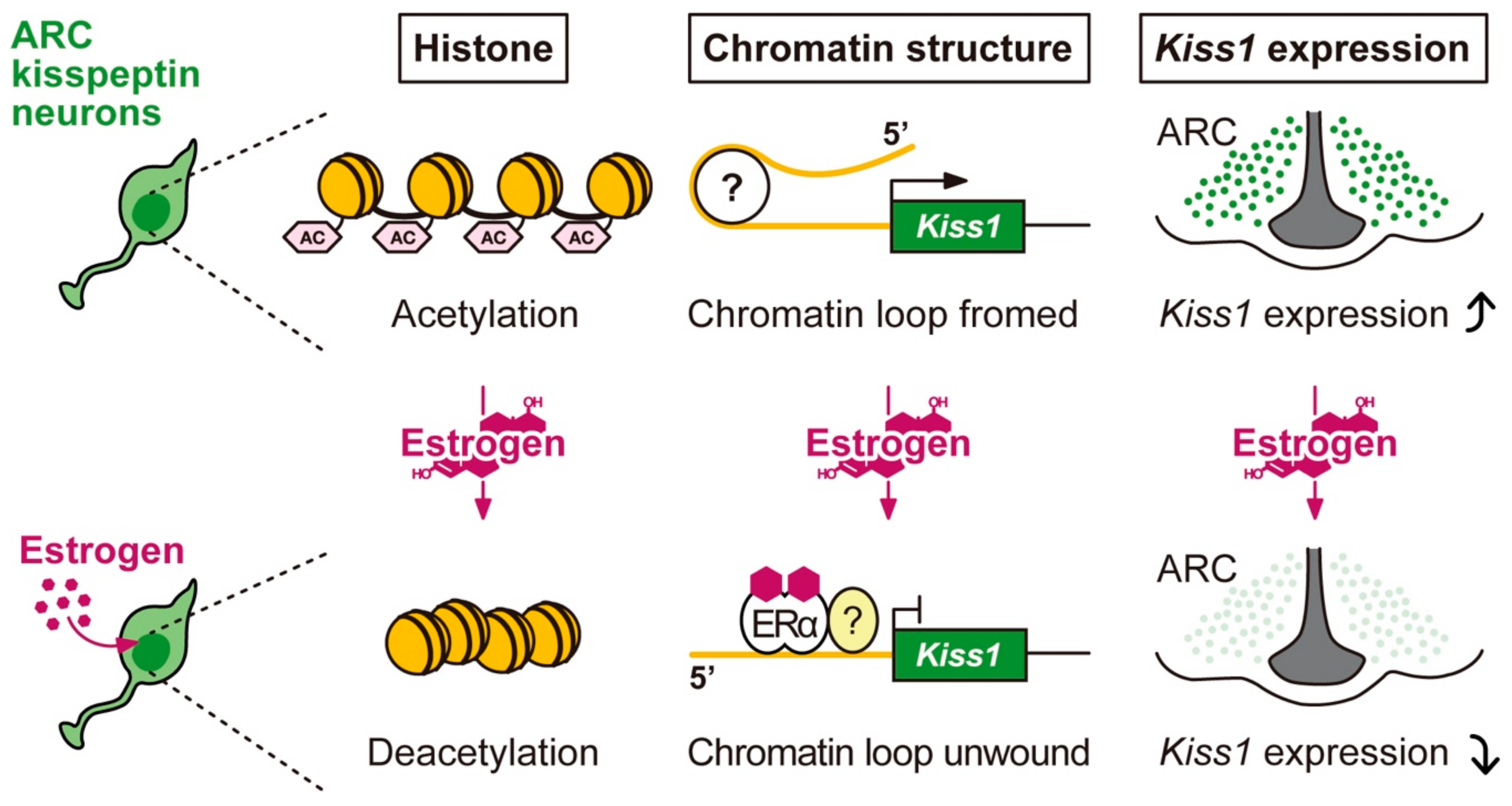
5.1. The Molecular and Epigenetic Mechanism Mediating the Regulation of Arcuate Kiss1 Expression by Estrogen and the Role of arcuate Kisspeptin Neurons as the GnRH Pulse Generator in Mammals
5.2. The Role of Anteroventral Periventricular Nucleus-Periventricular Nucleus (AVPV-PeN)/Preoptic Area (POA) Kisspeptin Neurons as the GnRH/Luteinizing Hormone (LH) Surge Generator and the Molecular and Epigenetic Mechanism Mediating the Regulation of AVPV-PeN/POA Kiss1 Expression by Estrogen Positive Feedback Action
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harris, G.W.; Jacobsohn, D. Functional grafts of the anterior pituitary gland. Proc. R. Soc. Lond. B Biol. Sci. 1952, 139, 263–276. [Google Scholar] [PubMed]
- McCann, S.M. A hypothalamic luteinizing-hormone-releasing factor. Am. J. Physiol. 1962, 202, 395–400. [Google Scholar] [CrossRef]
- Ramirez, V.D.; McCann, S.M. A highly sensitive test for LH-releasing activity: The ovariectomized, estrogen progesterone-blocked rat. Endocrinology 1963, 73, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Neill, J.D.; Johansson, E.D.; Datta, J.K.; Knobil, E. Relationship between the plasma levels of luteinizing hormone and progesterone during the normal menstrual cycle. J. Clin. Endocrinol. Metab. 1967, 27, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Monroe, S.E.; Atkinson, L.E.; Knobil, E. Patterns of circulating luteinizing hormone and their relation to plasma progesterone levels during the menstrual cycle of the Rhesus monkey. Endocrinology 1970, 87, 453–455. [Google Scholar] [CrossRef]
- Atkinson, L.E.; Bhattacharya, A.N.; Monroe, S.E.; Dierschke, D.J.; Knobil, E. Effects of gonadectomy on plasma LH concentration in the rhesus monkey. Endocrinology 1970, 87, 847–849. [Google Scholar] [CrossRef]
- Yamaji, T.; Dierschke, D.J.; Hotchkiss, J.; Bhattacharya, A.N.; Surve, A.H.; Knobil, E. Estrogen induction of LH release in the rhesus monkey. Endocrinology 1971, 89, 1034–1041. [Google Scholar] [CrossRef]
- Karsch, F.J.; Dierschke, D.K.; Weick, R.F.; Yamaji, T.; Hotchkiss, J.; Knobil, E. Positive and negative feedback control by estrogen of luteinizing hormone secretion in the rhesus monkey. Endocrinology 1973, 92, 799–804. [Google Scholar] [CrossRef]
- Matsuo, H.; Baba, Y.; Nair, R.M.; Arimura, A.; Schally, A.V. Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem. Biophys. Res. Commun. 1971, 43, 1334–1339. [Google Scholar] [CrossRef]
- Amoss, M.; Burgus, R.; Blackwell, R.; Vale, W.; Fellows, R.; Guillemin, R. Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing factor (LRF) of ovine origin. Biochem. Biophys. Res. Commun. 1971, 44, 205–210. [Google Scholar] [CrossRef]
- Moenter, S.M.; Caraty, A.; Karsch, F.J. The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology 1990, 127, 1375–1384. [Google Scholar] [CrossRef]
- Moenter, S.M.; Brand, R.M.; Midgley, A.R.; Karsch, F.J. Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology 1992, 130, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Moenter, S.M.; Brand, R.C.; Karsch, F.J. Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: Insights into the mechanism of GnRH surge induction. Endocrinology 1992, 130, 2978–2984. [Google Scholar] [CrossRef]
- Pau, K.Y.; Berria, M.; Hess, D.L.; Spies, H.G. Preovulatory gonadotropin-releasing hormone surge in ovarian-intact rhesus macaques. Endocrinology 1993, 133, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, D.W.; Fraser, H.M.; Lincoln, G.A.; Martin, G.B.; McNeilly, A.S. Hypothalamic pulse generators. Recent Prog. Horm. Res. 1985, 41, 369–419. [Google Scholar] [PubMed]
- Maeda, K.-I.; Tsukamura, H.; Ohkura, S.; Kawakami, S.; Nagabukuro, H.; Yokoyama, A. The LHRH pulse generator: A mediobasal hypothalamic location. Neurosci. Biobehav. Rev. 1995, 19, 427–437. [Google Scholar] [CrossRef]
- Kimura, F.; Funabashi, T. Two subgroups of gonadotropin-releasing hormone neurons control gonadotropin secretion in rats. News Physiol. Sci. 1998, 13, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Couse, J.F.; Korach, K.S. Estrogen receptor null mice: What have we learned and where will they lead us? Endocr. Rev. 1999, 20, 358–417. [Google Scholar] [CrossRef] [PubMed]
- Couse, J.F.; Yates, M.M.; Walker, V.R.; Korach, K.S. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol. Endocrinol. 2003, 17, 1039–1053. [Google Scholar] [CrossRef]
- Rumi, M.A.; Dhakal, P.; Kubota, K.; Chakraborty, D.; Lei, T.; Larson, M.A.; Wolfe, M.W.; Roby, K.F.; Vivian, J.L.; Soares, M.J. Generation of Esr1-knockout rats using zinc finger nuclease-mediated genome editing. Endocrinology 2014, 155, 1991–1999. [Google Scholar] [CrossRef]
- Antonson, P.; Apolinario, L.M.; Shamekh, M.M.; Humire, P.; Poutanen, M.; Ohlsson, C.; Nalvarte, I.; Gustafsson, J.A. Generation of an all-exon Esr2 deleted mouse line: Effects on fertility. Biochem. Biophys. Res. Commun. 2020, 529, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Krege, J.H.; Hodgin, J.B.; Couse, J.F.; Enmark, E.; Warner, M.; Mahler, J.F.; Sar, M.; Korach, K.S.; Gustafsson, J.A.; Smithies, O. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc. Natl. Acad. Sci. USA 1998, 95, 15677–15682. [Google Scholar] [CrossRef]
- Rumi, M.A.; Singh, P.; Roby, K.F.; Zhao, X.; Iqbal, K.; Ratri, A.; Lei, T.; Cui, W.; Borosha, S.; Dhakal, P.; et al. Defining the Role of Estrogen Receptor β in the Regulation of Female Fertility. Endocrinology 2017, 158, 2330–2343. [Google Scholar] [CrossRef]
- Roa, J.; Vigo, E.; Castellano, J.M.; Gaytan, F.; Navarro, V.M.; Aguilar, E.; Dijcks, F.A.; Ederveen, A.G.; Pinilla, L.; van Noort, P.I.; et al. Opposite roles of estrogen receptor (ER)-α and ERβ in the modulation of luteinizing hormone responses to kisspeptin in the female rat: Implications for the generation of the preovulatory surge. Endocrinology 2008, 149, 1627–1637. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogen receptor interaction with co-activators and co-repressors. Steroids 2000, 65, 227–251. [Google Scholar] [CrossRef]
- Stein, B.; Yang, M.X. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-κB and C/EBPβ. Mol. Cell. Biol. 1995, 15, 4971–4979. [Google Scholar] [CrossRef]
- Ray, A.; Prefontaine, K.E.; Ray, P. Down-modulation of interleukin-6 gene expression by 17β-estradiol in the absence of high affinity DNA binding by the estrogen receptor. J. Biol. Chem. 1994, 269, 12940–12946. [Google Scholar] [CrossRef]
- Schmitt, M.; Bausero, P.; Simoni, P.; Queuche, D.; Geoffroy, V.; Marschal, C.; Kempf, J.; Quirin-Stricker, C. Positive and negative effects of nuclear receptors on transcription activation by AP-1 of the human choline acetyltransferase proximal promoter. J. Neurosci. Res. 1995, 40, 152–164. [Google Scholar] [CrossRef]
- Jakacka, M.; Ito, M.; Weiss, J.; Chien, P.Y.; Gehm, B.D.; Jameson, J.L. Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J. Biol. Chem. 2001, 276, 13615–13621. [Google Scholar] [CrossRef]
- Jakacka, M.; Ito, M.; Martinson, F.; Ishikawa, T.; Lee, E.J.; Jameson, J.L. An estrogen receptor (ER)α deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling In Vivo. Mol. Endocrinol. 2002, 16, 2188–2201. [Google Scholar] [CrossRef]
- Glidewell-Kenney, C.; Hurley, L.A.; Pfaff, L.; Weiss, J.; Levine, J.E.; Jameson, J.L. Nonclassical estrogen receptor α signaling mediates negative feedback in the female mouse reproductive axis. Proc. Natl. Acad. Sci. USA 2007, 104, 8173–8177. [Google Scholar] [CrossRef] [PubMed]
- Herbison, A.E.; Pape, J.R. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front. Neuroendocrinol. 2001, 22, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Simerly, R.B.; Chang, C.; Muramatsu, M.; Swanson, L.W. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J. Comp. Neurol. 1990, 294, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Yokosuka, M.; Okamura, H.; Hayashi, S. Postnatal development and sex difference in neurons containing estrogen receptor-α immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J. Comp. Neurol. 1997, 389, 81–93. [Google Scholar] [CrossRef]
- Scott, C.J.; Tilbrook, A.J.; Simmons, D.M.; Rawson, J.A.; Chu, S.; Fuller, P.J.; Ing, N.H.; Clarke, I.J. The distribution of cells containing estrogen receptor-α (ERα) and ERβ messenger ribonucleic acid in the preoptic area and hypothalamus of the sheep: Comparison of males and females. Endocrinology 2000, 141, 2951–2962. [Google Scholar] [CrossRef]
- Lehman, M.N.; Ebling, F.J.; Moenter, S.M.; Karsch, F.J. Distribution of estrogen receptor-immunoreactive cells in the sheep brain. Endocrinology 1993, 133, 876–886. [Google Scholar] [CrossRef]
- Anderson, C.H.; Greenwald, G.S. Autoradiographic analysis of estradiol uptake in the brain and pituitary of the female rat. Endocrinology 1969, 85, 1160–1165. [Google Scholar] [CrossRef]
- Smith, E.R.; Davidson, J.M. Location of feedback receptors: Effects of intracranially implanted steroids on plasma LH and LRF response. Endocrinology 1974, 95, 1566–1573. [Google Scholar] [CrossRef]
- Akema, T.; Tadokoro, Y.; Kawakami, M. Changes in the characteristics of pulsatile LH secretion after estradiol implantation into the preoptic area and the basal hypothalamus in ovariectomized rats. Endocrinol. Jpn. 1983, 30, 281–287. [Google Scholar] [CrossRef]
- Nagatani, S.; Tsukamura, H.; Maeda, K.-I. Estrogen feedback needed at the paraventricular nucleus or A2 to suppress pulsatile luteinizing hormone release in fasting female rats. Endocrinology 1994, 135, 870–875. [Google Scholar] [CrossRef]
- Estacio, M.A.; Yamada, S.; Tsukamura, H.; Hirunagi, K.; Maeda, K. Effect of fasting and immobilization stress on estrogen receptor immunoreactivity in the brain in ovariectomized female rats. Brain Res. 1996, 717, 55–61. [Google Scholar] [CrossRef]
- Kawakami, M.; Konda, N.; Yoshioka, E. Stimulatory feedback action of estradiol and estrone on LH release in the ovariectomized rat: Roles different between limbic system and preoptic area. Endocrinol. Jpn. 1977, 24, 163–172. [Google Scholar] [CrossRef]
- Goodman, R.L. The site of the positive feedback action of estradiol in the rat. Endocrinology 1978, 102, 151–159. [Google Scholar] [CrossRef]
- Wiegand, S.J.; Terasawa, E.; Bridson, W.E.; Goy, R.W. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology 1980, 31, 147–157. [Google Scholar] [CrossRef]
- Wiegand, S.J.; Terasawa, E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology 1982, 34, 395–404. [Google Scholar] [CrossRef]
- Petersen, S.L.; Cheuk, C.; Hartman, R.D.; Barraclough, C.A. Medial preoptic microimplants of the antiestrogen, keoxifene, affect luteinizing hormone-releasing hormone mRNA levels, median eminence luteinizing hormone-releasing hormone concentrations and luteinizing hormone release in ovariectomized, estrogen-treated rats. J. Neuroendocrinol. 1989, 1, 279–283. [Google Scholar]
- Petersen, S.L.; Barraclough, C.A. Suppression of spontaneous LH surges in estrogen-treated ovariectomized rats by microimplants of antiestrogens into the preoptic brain. Brain Res. 1989, 484, 279–289. [Google Scholar] [CrossRef]
- Smith, J.T.; Cunningham, M.J.; Rissman, E.F.; Clifton, D.K.; Steiner, R.A. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 2005, 146, 3686–3692. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Popa, S.M.; Clifton, D.K.; Hoffman, G.E.; Steiner, R.A. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J. Neurosci. 2006, 26, 6687–6694. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Tsukamura, H.; Adachi, S.; Matsui, H.; Uenoyama, Y.; Iwata, K.; Yamada, S.; Inoue, K.; Ohtaki, T.; Matsumoto, H.; et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 2005, 146, 4431–4436. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Yamada, S.; Takatsu, Y.; Matsui, H.; Kinoshita, M.; Takase, K.; Sugiura, H.; Ohtaki, T.; Matsumoto, H.; Uenoyama, Y.; et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J. Reprod. Dev. 2007, 53, 367–378. [Google Scholar] [CrossRef]
- Franceschini, I.; Lomet, D.; Cateau, M.; Delsol, G.; Tillet, Y.; Caraty, A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci. Lett. 2006, 401, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Gottsch, M.L.; Cunningham, M.J.; Smith, J.T.; Popa, S.M.; Acohido, B.V.; Crowley, W.F.; Seminara, S.; Clifton, D.K.; Steiner, R.A. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 2004, 145, 4073–4077. [Google Scholar] [CrossRef] [PubMed]
- Irwig, M.S.; Fraley, G.S.; Smith, J.T.; Acohido, B.V.; Popa, S.M.; Cunningham, M.J.; Gottsch, M.L.; Clifton, D.K.; Steiner, R.A. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 2004, 80, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Takatsu, Y.; Kumano, S.; Matsumoto, H.; Ohtaki, T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem. Biophys. Res. Commun. 2004, 320, 383–388. [Google Scholar] [CrossRef]
- Pheng, V.; Uenoyama, Y.; Homma, T.; Inamoto, Y.; Takase, K.; Yoshizawa-Kumagaye, K.; Isaka, S.; Watanabe, T.X.; Ohkura, S.; Tomikawa, J.; et al. Potencies of centrally- or peripherally-injected full-length kisspeptin or its C-terminal decapeptide on LH release in intact male rats. J. Reprod. Dev. 2009, 55, 378–382. [Google Scholar] [CrossRef][Green Version]
- Navarro, V.M.; Castellano, J.M.; Fernandez-Fernandez, R.; Tovar, S.; Roa, J.; Mayen, A.; Nogueiras, R.; Vazquez, M.J.; Barreiro, M.L.; Magni, P.; et al. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology 2005, 146, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.M.; Castellano, J.M.; Fernandez-Fernandez, R.; Tovar, S.; Roa, J.; Mayen, A.; Barreiro, M.L.; Casanueva, F.F.; Aguilar, E.; Dieguez, C.; et al. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology 2005, 146, 1689–1697. [Google Scholar] [CrossRef]
- Ohkura, S.; Takase, K.; Matsuyama, S.; Mogi, K.; Ichimaru, T.; Wakabayashi, Y.; Uenoyama, Y.; Mori, Y.; Steiner, R.A.; Tsukamura, H.; et al. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J. Neuroendocrinol. 2009, 21, 813–821. [Google Scholar] [CrossRef]
- Naniwa, Y.; Nakatsukasa, K.; Setsuda, S.; Oishi, S.; Fujii, N.; Matsuda, F.; Uenoyama, Y.; Tsukamura, H.; Maeda, K.-I.; Ohkura, S. Effects of full-length kisspeptin administration on follicular development in Japanese Black beef cows. J. Reprod. Dev. 2013, 59, 588–594. [Google Scholar] [CrossRef]
- Dhillo, W.S.; Chaudhri, O.B.; Patterson, M.; Thompson, E.L.; Murphy, K.G.; Badman, M.K.; McGowan, B.M.; Amber, V.; Patel, S.; Ghatei, M.A.; et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J. Clin. Endocrinol. Metab. 2005, 90, 6609–6615. [Google Scholar] [CrossRef]
- Shahab, M.; Mastronardi, C.; Seminara, S.B.; Crowley, W.F.; Ojeda, S.R.; Plant, T.M. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proc. Natl. Acad. Sci. USA 2005, 102, 2129–2134. [Google Scholar] [CrossRef]
- Ohtaki, T.; Shintani, Y.; Honda, S.; Matsumoto, H.; Hori, A.; Kanehashi, K.; Terao, Y.; Kumano, S.; Takatsu, Y.; Masuda, Y.; et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001, 411, 613–617. [Google Scholar] [CrossRef]
- Kotani, M.; Detheux, M.; Vandenbogaerde, A.; Communi, D.; Vanderwinden, J.M.; Le Poul, E.; Brezillon, S.; Tyldesley, R.; Suarez-Huerta, N.; Vandeput, F.; et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J. Biol. Chem. 2001, 276, 34631–34636. [Google Scholar] [CrossRef] [PubMed]
- de Roux, N.; Genin, E.; Carel, J.C.; Matsuda, F.; Chaussain, J.L.; Milgrom, E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. USA 2003, 100, 10972–10976. [Google Scholar] [CrossRef]
- Seminara, S.B.; Messager, S.; Chatzidaki, E.E.; Thresher, R.R.; Acierno, J.S., Jr.; Shagoury, J.K.; Bo-Abbas, Y.; Kuohung, W.; Schwinof, K.M.; Hendrick, A.G.; et al. The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 2003, 349, 1614–1627. [Google Scholar] [CrossRef]
- Topaloglu, A.K.; Tello, J.A.; Kotan, L.D.; Ozbek, M.N.; Yilmaz, M.B.; Erdogan, S.; Gurbuz, F.; Temiz, F.; Millar, R.P.; Yuksel, B. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N. Engl. J. Med. 2012, 366, 629–635. [Google Scholar] [CrossRef]
- Messager, S.; Chatzidaki, E.E.; Ma, D.; Hendrick, A.G.; Zahn, D.; Dixon, J.; Thresher, R.R.; Malinge, I.; Lomet, D.; Carlton, M.B.; et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc. Natl. Acad. Sci. USA 2005, 102, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- d’Anglemont de Tassigny, X.; Fagg, L.A.; Dixon, J.P.; Day, K.; Leitch, H.G.; Hendrick, A.G.; Zahn, D.; Franceschini, I.; Caraty, A.; Carlton, M.B.; et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc. Natl. Acad. Sci. USA 2007, 104, 10714–10719. [Google Scholar] [CrossRef] [PubMed]
- Lapatto, R.; Pallais, J.C.; Zhang, D.; Chan, Y.M.; Mahan, A.; Cerrato, F.; Le, W.W.; Hoffman, G.E.; Seminara, S.B. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology 2007, 148, 4927–4936. [Google Scholar] [CrossRef]
- Chan, Y.M.; Broder-Fingert, S.; Wong, K.M.; Seminara, S.B. Kisspeptin/Gpr54-independent gonadotrophin-releasing hormone activity in Kiss1 and Gpr54 mutant mice. J. Neuroendocrinol. 2009, 21, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Uenoyama, Y.; Nakamura, S.; Hayakawa, Y.; Ikegami, K.; Watanabe, Y.; Deura, C.; Minabe, S.; Tomikawa, J.; Goto, T.; Ieda, N.; et al. Lack of pulse and surge modes and glutamatergic stimulation of LH release in Kiss1 knockout rats. J. Neuroendocrinol. 2015, 27, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Higo, S.; Honda, S.; Iijima, N.; Ozawa, H. Mapping of kisspeptin receptor mRNA in the whole rat brain and its co-localisation with oxytocin in the paraventricular nucleus. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Herbison, A.E.; de Tassigny, X.; Doran, J.; Colledge, W.H. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology 2010, 151, 312–321. [Google Scholar] [CrossRef]
- Han, S.K.; Gottsch, M.L.; Lee, K.J.; Popa, S.M.; Smith, J.T.; Jakawich, S.K.; Clifton, D.K.; Steiner, R.A.; Herbison, A.E. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J. Neurosci. 2005, 25, 11349–11356. [Google Scholar] [CrossRef]
- Kauffman, A.S.; Gottsch, M.L.; Roa, J.; Byquist, A.C.; Crown, A.; Clifton, D.K.; Hoffman, G.E.; Steiner, R.A.; Tena-Sempere, M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 2007, 148, 1774–1783. [Google Scholar] [CrossRef]
- Clarkson, J.; Herbison, A.E. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 2006, 147, 5817–5825. [Google Scholar] [CrossRef]
- Takase, K.; Uenoyama, Y.; Inoue, N.; Matsui, H.; Yamada, S.; Shimizu, M.; Homma, T.; Tomikawa, J.; Kanda, S.; Matsumoto, H.; et al. Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J. Neuroendocrinol. 2009, 21, 527–537. [Google Scholar] [CrossRef]
- Hrabovszky, E.; Takacs, S.; Gocz, B.; Skrapits, K. New Perspectives for Anatomical and Molecular Studies of Kisspeptin Neurons in the Aging Human Brain. Neuroendocrinology 2019, 109, 230–241. [Google Scholar] [CrossRef]
- Rometo, A.M.; Krajewski, S.J.; Voytko, M.L.; Rance, N.E. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J. Clin. Endocrinol. Metab. 2007, 92, 2744–2750. [Google Scholar] [CrossRef]
- Smith, J.T.; Shahab, M.; Pereira, A.; Pau, K.Y.; Clarke, I.J. Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate. Biol. Reprod. 2010, 83, 568–577. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Guerriero, K.A.; Gibbs, R.B.; Plant, T.M. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology 2008, 149, 4387–4395. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Uenoyama, Y.; Suzuki, J.; Takase, K.; Suetomi, Y.; Ohkura, S.; Inoue, N.; Maeda, K.-I.; Tsukamura, H. Oestrogen-induced activation of preoptic kisspeptin neurones may be involved in the luteinizing hormone surge in male and female Japanese monkeys. J. Neuroendocrinol. 2014, 26, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Estrada, K.M.; Clay, C.M.; Pompolo, S.; Smith, J.T.; Clarke, I.J. Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotrophin-releasing hormone/lutenising hormone surge in the ewe suggests a stimulatory role for kisspeptin in oestrogen-positive feedback. J. Neuroendocrinol. 2006, 18, 806–809. [Google Scholar] [CrossRef]
- Smith, J.T.; Li, Q.; Pereira, A.; Clarke, I.J. Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology 2009, 150, 5530–5538. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.L.; Lehman, M.N.; Smith, J.T.; Coolen, L.M.; de Oliveira, C.V.; Jafarzadehshirazi, M.R.; Pereira, A.; Iqbal, J.; Caraty, A.; Ciofi, P.; et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 2007, 148, 5752–5760. [Google Scholar] [CrossRef]
- Wakabayashi, Y.; Nakada, T.; Murata, K.; Ohkura, S.; Mogi, K.; Navarro, V.M.; Clifton, D.K.; Mori, Y.; Tsukamura, H.; Maeda, K.-I.; et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J. Neurosci. 2010, 30, 3124–3132. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, F.; Nakatsukasa, K.; Suetomi, Y.; Naniwa, Y.; Ito, D.; Inoue, N.; Wakabayashi, Y.; Okamura, H.; Maeda, K.-I.; Uenoyama, Y.; et al. The LH surge-generating system is functional in male goats as in females: Involvement of kisspeptin neurones in the medial preoptic area. J. Neuroendocrinol. 2015, 27, 57–65. [Google Scholar] [CrossRef]
- Hassaneen, A.S.A.; Naniwa, Y.; Suetomi, Y.; Matsuyama, S.; Kimura, K.; Ieda, N.; Inoue, N.; Uenoyama, Y.; Tsukamura, H.; Maeda, K.-I.; et al. Immunohistochemical characterization of the arcuate kisspeptin/neurokinin B/dynorphin (KNDy) and preoptic kisspeptin neuronal populations in the hypothalamus during the estrous cycle in heifers. J. Reprod. Dev. 2016, 62, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Decourt, C.; Tillet, Y.; Caraty, A.; Franceschini, I.; Briant, C. Kisspeptin immunoreactive neurons in the equine hypothalamus Interactions with GnRH neuronal system. J. Chem. Neuroanat. 2008, 36, 131–137. [Google Scholar] [CrossRef]
- Tomikawa, J.; Homma, T.; Tajima, S.; Shibata, T.; Inamoto, Y.; Takase, K.; Inoue, N.; Ohkura, S.; Uenoyama, Y.; Maeda, K.-I.; et al. Molecular characterization and estrogen regulation of hypothalamic KISS1 gene in the pig. Biol. Reprod. 2010, 82, 313–319. [Google Scholar] [CrossRef]
- Inoue, N.; Sasagawa, K.; Ikai, K.; Sasaki, Y.; Tomikawa, J.; Oishi, S.; Fujii, N.; Uenoyama, Y.; Ohmori, Y.; Yamamoto, N.; et al. Kisspeptin neurons mediate reflex ovulation in the musk shrew (Suncus murinus). Proc. Natl. Acad. Sci. USA 2011, 108, 17527–17532. [Google Scholar] [CrossRef]
- Smith, J.T.; Clay, C.M.; Caraty, A.; Clarke, I.J. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 2007, 148, 1150–1157. [Google Scholar] [CrossRef]
- Smith, J.T.; Coolen, L.M.; Kriegsfeld, L.J.; Sari, I.P.; Jaafarzadehshirazi, M.R.; Maltby, M.; Bateman, K.; Goodman, R.L.; Tilbrook, A.J.; Ubuka, T.; et al. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: A novel medium for seasonal breeding in the sheep. Endocrinology 2008, 149, 5770–5782. [Google Scholar] [CrossRef] [PubMed]
- Gottsch, M.L.; Navarro, V.M.; Zhao, Z.; Glidewell-Kenney, C.; Weiss, J.; Jameson, J.L.; Clifton, D.K.; Levine, J.E.; Steiner, R.A. Regulation of Kiss1 and Dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J. Neurosci. 2009, 29, 9390–9395. [Google Scholar] [CrossRef]
- Yang, J.A.; Mamounis, K.J.; Yasrebi, A.; Roepke, T.A. Regulation of gene expression by 17β-estradiol in the arcuate nucleus of the mouse through ERE-dependent and ERE-independent mechanisms. Steroids 2016, 107, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.A.; Stires, H.; Belden, W.J.; Roepke, T.A. The arcuate estrogen-regulated transcriptome: Estrogen response element-dependent and -independent signaling of ERα in female mice. Endocrinology 2017, 158, 612–626. [Google Scholar] [PubMed]
- Tomikawa, J.; Uenoyama, Y.; Ozawa, M.; Fukanuma, T.; Takase, K.; Goto, T.; Abe, H.; Ieda, N.; Minabe, S.; Deura, C.; et al. Epigenetic regulation of Kiss1 gene expression mediating estrogen-positive feedback action in the mouse brain. Proc. Natl. Acad. Sci. USA 2012, 109, E1294–E1301. [Google Scholar] [CrossRef]
- Goto, T.; Tomikawa, J.; Ikegami, K.; Minabe, S.; Abe, H.; Fukanuma, T.; Imamura, T.; Takase, K.; Sanbo, M.; Tomita, K.; et al. Identification of hypothalamic arcuate nucleus-specific enhancer region of Kiss1 gene in mice. Mol. Endocrinol. 2015, 29, 121–129. [Google Scholar] [CrossRef]
- Lehman, M.N.; Coolen, L.M.; Goodman, R.L. Minireview: Kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: A central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 2010, 151, 3479–3489. [Google Scholar] [CrossRef]
- Murakawa, H.; Iwata, K.; Takeshita, T.; Ozawa, H. Immunoelectron microscopic observation of the subcellular localization of kisspeptin, neurokinin B and dynorphin A in KNDy neurons in the arcuate nucleus of the female rat. Neurosci. Lett. 2016, 612, 161–166. [Google Scholar] [CrossRef]
- Navarro, V.M.; Gottsch, M.L.; Chavkin, C.; Okamura, H.; Clifton, D.K.; Steiner, R.A. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 2009, 29, 11859–11866. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Ohkura, S.; Uenoyama, Y.; Wakabayashi, Y.; Oka, Y.; Tsukamura, H.; Okamura, H. Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brain Res. 2010, 1364, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Tsukamura, H.; Ohkura, S.; Uenoyama, Y.; Wakabayashi, Y.; Maeda, K.-I. Kisspeptin and GnRH Pulse Generation. In Kisspeptin Signaling in Reproductive Biology; Kauffman, A.S., Smith, J.T., Eds.; Springer: New York, NY, USA, 2013; pp. 297–323. [Google Scholar]
- Goodman, R.L.; Ohkura, S.; Okamura, H.; Coolen, L.M.; Lehman, M.N. KNDy hypothesis for generation of GnRH pulses: Evidence from sheep and goats. In The GnRH Neuron and Its Control; Herbison, A.E., Plant, T.M., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 289–324. [Google Scholar]
- Herbison, A.E. The gonadotropin-releasing hormone pulse generator. Endocrinology 2018, 159, 3723–3736. [Google Scholar] [CrossRef]
- Uenoyama, Y.; Pheng, V.; Tsukamura, H.; Maeda, K.I. The roles of kisspeptin revisited: Inside and outside the hypothalamus. J. Reprod. Dev. 2016, 62, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Nagae, M.; Uenoyama, Y.; Okamoto, S.; Tsuchida, H.; Ikegami, K.; Goto, T.; Majarune, S.; Nakamura, S.; Sanbo, M.; Hirabayashi, M.; et al. Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc. Natl. Acad. Sci. USA 2021, 118, e2009156118. [Google Scholar] [CrossRef]
- Ikegami, K.; Goto, T.; Nakamura, S.; Watanabe, Y.; Sugimoto, A.; Majarune, S.; Horihata, K.; Nagae, M.; Tomikawa, J.; Imamura, T.; et al. Conditional kisspeptin neuron-specific Kiss1 knockout with newly generated Kiss1-floxed and Kiss1-Cre mice replicates a hypogonadal phenotype of global Kiss1 knockout mice. J. Reprod. Dev. 2020, 66, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Minabe, S.; Nakamura, S.; Fukushima, E.; Sato, M.; Ikegami, K.; Goto, T.; Sanbo, M.; Hirabayashi, M.; Tomikawa, J.; Imamura, T.; et al. Inducible Kiss1 knockdown in the hypothalamic arcuate nucleus suppressed pulsatile secretion of luteinizing hormone in male mice. J. Reprod. Dev. 2020, 66, 369–375. [Google Scholar] [CrossRef]
- Clarkson, J.; Han, S.Y.; Piet, R.; McLennan, T.; Kane, G.M.; Ng, J.; Porteous, R.W.; Kim, J.S.; Colledge, W.H.; Iremonger, K.J.; et al. Definition of the hypothalamic GnRH pulse generator in mice. Proc. Natl. Acad. Sci. USA 2017, 114, E10216–E10223. [Google Scholar] [CrossRef]
- Han, S.Y.; Kane, G.; Cheong, I.; Herbison, A.E. Characterization of GnRH Pulse Generator Activity in Male Mice Using GCaMP Fiber Photometry. Endocrinology 2019, 160, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Keen, K.L.; Wegner, F.H.; Bloom, S.R.; Ghatei, M.A.; Terasawa, E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys In Vivo. Endocrinology 2008, 149, 4151–4157. [Google Scholar] [CrossRef] [PubMed]
- Kurian, J.R.; Keen, K.L.; Guerriero, K.A.; Terasawa, E. Tonic control of kisspeptin release in prepubertal monkeys: Implications to the mechanism of puberty onset. Endocrinology 2012, 153, 3331–3336. [Google Scholar] [CrossRef]
- Ikegami, K.; Minabe, S.; Ieda, N.; Goto, T.; Sugimoto, A.; Nakamura, S.; Inoue, N.; Oishi, S.; Maturana, A.D.; Sanbo, M.; et al. Evidence of involvement of neurone-glia/neurone-neurone communications via gap junctions in synchronised activity of KNDy neurones. J. Neuroendocrinol. 2017, 29. [Google Scholar] [CrossRef]
- Navarro, V.M.; Gottsch, M.L.; Wu, M.; Garcia-Galiano, D.; Hobbs, S.J.; Bosch, M.A.; Pinilla, L.; Clifton, D.K.; Dearth, A.; Ronnekleiv, O.K.; et al. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology 2011, 152, 4265–4275. [Google Scholar] [CrossRef]
- Ruka, K.A.; Burger, L.L.; Moenter, S.M. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and kappa-opioid receptors in adult male mice. Endocrinology 2013, 154, 2761–2771. [Google Scholar] [CrossRef]
- Tsuchida, H.; Mostari, P.; Yamada, K.; Miyazaki, S.; Enomoto, Y.; Inoue, N.; Uenoyama, Y.; Tsukamura, H. Paraventricular dynorphin A neurons mediate LH pulse suppression induced by hindbrain glucoprivation in female rats. Endocrinology 2020, 161, bqaa161. [Google Scholar] [CrossRef] [PubMed]
- Assadullah; Ieda, N.; Kawai, N.; Ishii, H.; Ihara, K.; Inoue, N.; Uenoyama, Y.; Tsukamura, H. Co-expression of the calcitonin receptor gene in the hypothalamic kisspeptin neurons in female rats. Reprod. Med. Biol. 2018, 17, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Weems, P.W.; Witty, C.F.; Amstalden, M.; Coolen, L.M.; Goodman, R.L.; Lehman, M.N. Kappa opioid receptor is co-localized in GnRH and KNDy cells in the female ovine and rat brain. Endocrinology 2016, 157, 2367–2379. [Google Scholar] [CrossRef] [PubMed]
- Amstalden, M.; Coolen, L.M.; Hemmerle, A.M.; Billings, H.J.; Connors, J.M.; Goodman, R.L.; Lehman, M.N. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: Colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J. Neuroendocrinol. 2010, 22, 1–12. [Google Scholar] [CrossRef]
- Dellovade, T.L.; Merchenthaler, I. Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor α. Endocrinology 2004, 145, 736–742. [Google Scholar] [CrossRef]
- Pillon, D.; Caraty, A.; Fabre-Nys, C.; Bruneau, G. Short-term effect of oestradiol on neurokinin B mRNA expression in the infundibular nucleus of ewes. J. Neuroendocrinol. 2003, 15, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Abel, T.W.; Voytko, M.L.; Rance, N.E. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J. Clin. Endocrinol. Metab. 1999, 84, 2111–2118. [Google Scholar] [CrossRef]
- Kanaya, M.; Iwata, K.; Ozawa, H. Distinct dynorphin expression patterns with low- and high-dose estrogen treatment in the arcuate nucleus of female rats. Biol. Reprod. 2017, 97, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Dubois, S.L.; Acosta-Martinez, M.; DeJoseph, M.R.; Wolfe, A.; Radovick, S.; Boehm, U.; Urban, J.H.; Levine, J.E. Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor α in kisspeptin neurons. Endocrinology 2015, 156, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Mostari, M.P.; Ieda, N.; Deura, C.; Minabe, S.; Yamada, S.; Uenoyama, Y.; Maeda, K.-I.; Tsukamura, H. Dynorphin-kappa opioid receptor signaling partly mediates estrogen negative feedback effect on LH pulses in female rats. J. Reprod. Dev. 2013, 59, 266–272. [Google Scholar] [CrossRef]
- Stephens, S.B.Z.; Kauffman, A.S. Estrogen regulation of the molecular phenotype and active translatome of AVPV kisspeptin neurons. Endocrinology 2021, 162, bqab080. [Google Scholar] [CrossRef]
- Clarkson, J.; Herbison, A.E. Oestrogen, kisspeptin, GPR54 and the preovulatory luteinising hormone surge. J. Neuroendocrinol. 2009, 21, 305–311. [Google Scholar] [CrossRef]
- Tsukamura, H. Kobayashi Award 2019: The neuroendocrine regulation of the mammalian reproduction. Gen. Comp. Endocrinol. 2021, 113755. [Google Scholar] [CrossRef]
- Tsukamura, H.; Homma, T.; Tomikawa, J.; Uenoyama, Y.; Maeda, K.-I. Sexual differentiation of kisspeptin neurons responsible for sex difference in gonadotropin release in rats. Ann. N. Y. Acad. Sci. 2010, 1200, 95–103. [Google Scholar] [CrossRef]
- Tsukamura, H.; Maeda, K.-I.; Uenoyama, Y. Fetal/perinatal programming causing sexual dimorphism of the kisspeptin–GnRH neuronal network. In The GnRH Neuron and Its Control; Herbison, A.E., Plant, T.M., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 43–60. [Google Scholar]
- Homma, T.; Sakakibara, M.; Yamada, S.; Kinoshita, M.; Iwata, K.; Tomikawa, J.; Kanazawa, T.; Matsui, H.; Takatsu, Y.; Ohtaki, T.; et al. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol. Reprod. 2009, 81, 1216–1225. [Google Scholar] [CrossRef]
- Sakakibara, M.; Deura, C.; Minabe, S.; Iwata, Y.; Uenoyama, Y.; Maeda, K.I.; Tsukamura, H. Different critical perinatal periods and hypothalamic sites of oestradiol action in the defeminization of LH surge and lordosis capacity in the rat. J. Neuroendocrinol. 2013, 25, 251–259. [Google Scholar] [CrossRef]
- Simonian, S.X.; Herbison, A.E. Differential expression of estrogen receptor and neuropeptide Y by brainstem A1 and A2 noradrenaline neurons. Neuroscience 1997, 76, 517–529. [Google Scholar] [CrossRef]
- Jennes, L.; Jennes, M.E.; Purvis, C.; Nees, M. c-fos expression in noradrenergic A2 neurons of the rat during the estrous cycle and after steroid hormone treatments. Brain Res. 1992, 586, 171–175. [Google Scholar] [CrossRef]
- Le, W.W.; Berghorn, K.A.; Smith, M.S.; Hoffman, G.E. Alpha1-adrenergic receptor blockade blocks LH secretion but not LHRH cFos activation. Brain Res. 1997, 747, 236–245. [Google Scholar] [CrossRef]
- Funabashi, T.; Aiba, S.; Sano, A.; Shinohara, K.; Kimura, F. Intracerebroventricular injection of arginine-vasopressin V1 receptor antagonist attenuates the surge of luteinizing hormone and prolactin secretion in proestrous rats. Neurosci. Lett. 1999, 260, 37–40. [Google Scholar] [CrossRef]
- Piet, R.; Fraissenon, A.; Boehm, U.; Herbison, A.E. Estrogen permits vasopressin signaling in preoptic kisspeptin neurons in the female mouse. J. Neurosci. 2015, 35, 6881–6892. [Google Scholar] [CrossRef] [PubMed]
- Yip, S.H.; Boehm, U.; Herbison, A.E.; Campbell, R.E. Conditional viral tract tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin-releasing hormone (GnRH) neurons in the mouse. Endocrinology 2015, 156, 2582–2594. [Google Scholar] [CrossRef]
- Zhang, C.; Roepke, T.A.; Kelly, M.J.; Ronnekleiv, O.K. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J. Neurosci. 2008, 28, 4423–4434. [Google Scholar] [CrossRef]
- Liu, X.; Lee, K.; Herbison, A.E. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology 2008, 149, 4605–4614. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uenoyama, Y.; Inoue, N.; Nakamura, S.; Tsukamura, H. Kisspeptin Neurons and Estrogen–Estrogen Receptor α Signaling: Unraveling the Mystery of Steroid Feedback System Regulating Mammalian Reproduction. Int. J. Mol. Sci. 2021, 22, 9229. https://doi.org/10.3390/ijms22179229
Uenoyama Y, Inoue N, Nakamura S, Tsukamura H. Kisspeptin Neurons and Estrogen–Estrogen Receptor α Signaling: Unraveling the Mystery of Steroid Feedback System Regulating Mammalian Reproduction. International Journal of Molecular Sciences. 2021; 22(17):9229. https://doi.org/10.3390/ijms22179229
Chicago/Turabian StyleUenoyama, Yoshihisa, Naoko Inoue, Sho Nakamura, and Hiroko Tsukamura. 2021. "Kisspeptin Neurons and Estrogen–Estrogen Receptor α Signaling: Unraveling the Mystery of Steroid Feedback System Regulating Mammalian Reproduction" International Journal of Molecular Sciences 22, no. 17: 9229. https://doi.org/10.3390/ijms22179229
APA StyleUenoyama, Y., Inoue, N., Nakamura, S., & Tsukamura, H. (2021). Kisspeptin Neurons and Estrogen–Estrogen Receptor α Signaling: Unraveling the Mystery of Steroid Feedback System Regulating Mammalian Reproduction. International Journal of Molecular Sciences, 22(17), 9229. https://doi.org/10.3390/ijms22179229





