Photodynamic Inactivation of Legionella pneumophila Biofilm Formation by Cationic Tetra- and Tripyridylporphyrins in Waters of Different Hardness
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of Water Samples
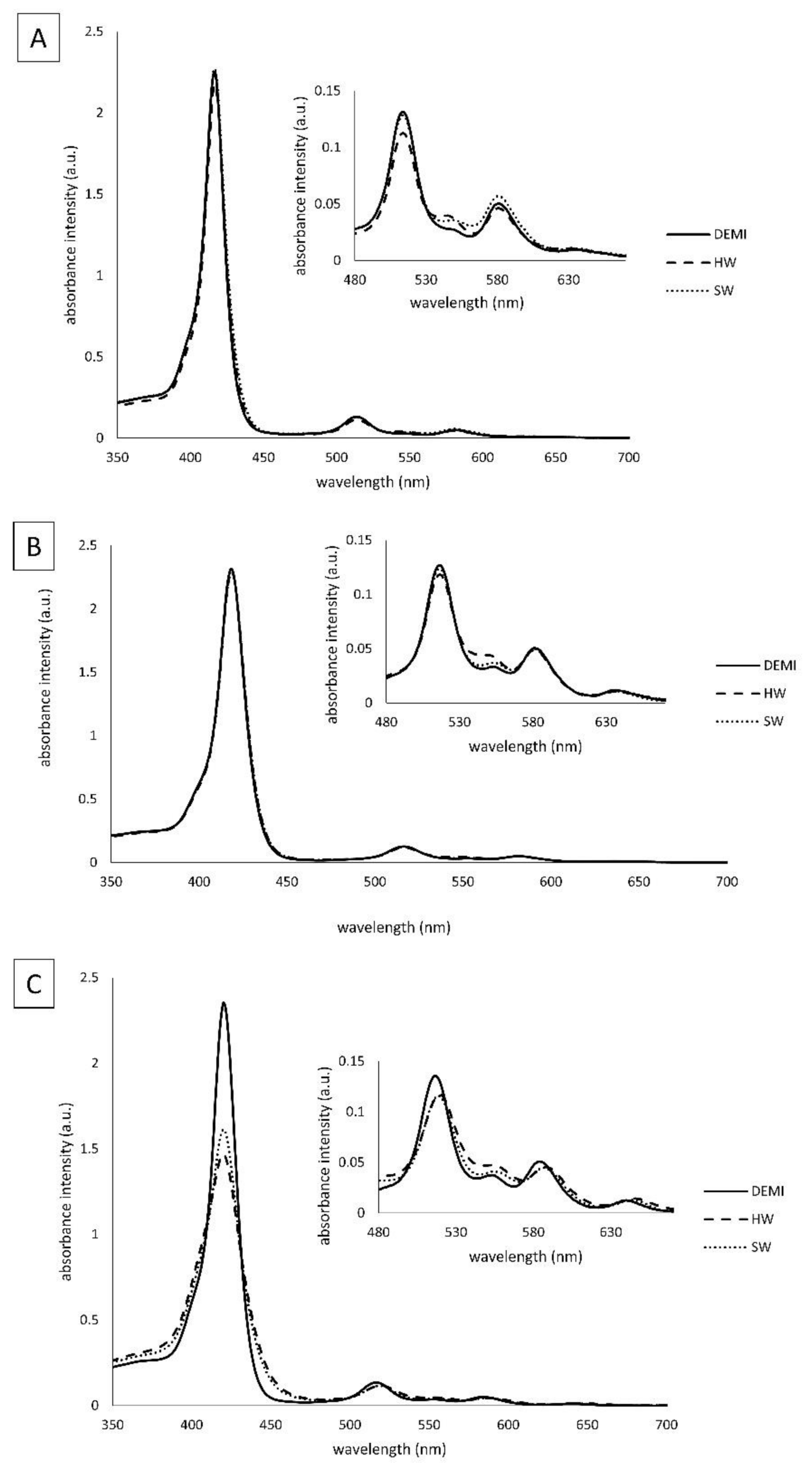
2.2. Spectroscopic Properties of the Tested PSs in Waters of Different Hardness
2.3. Photostability of the PSs in Different Water Samples
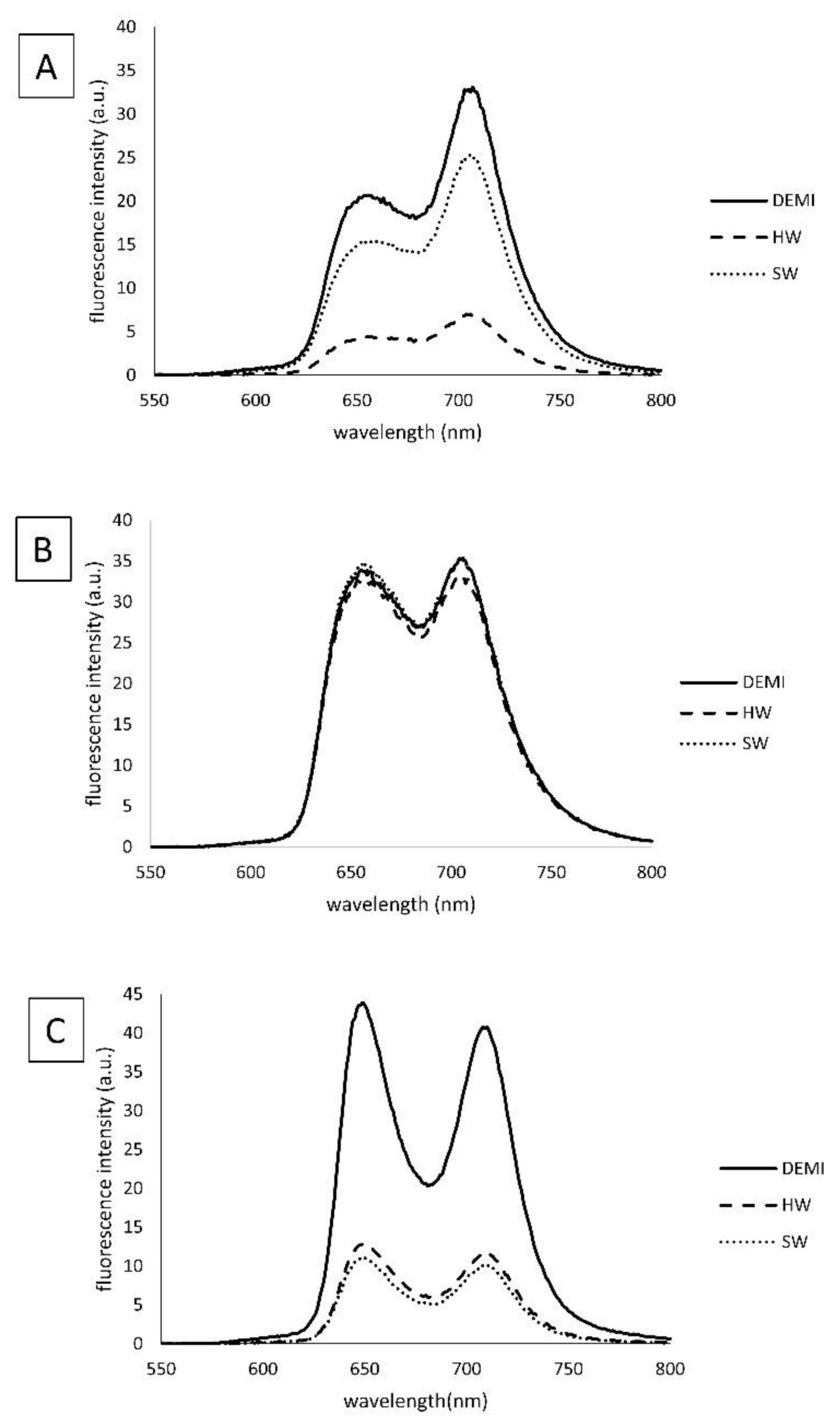
2.4. Singlet Oxygen Production
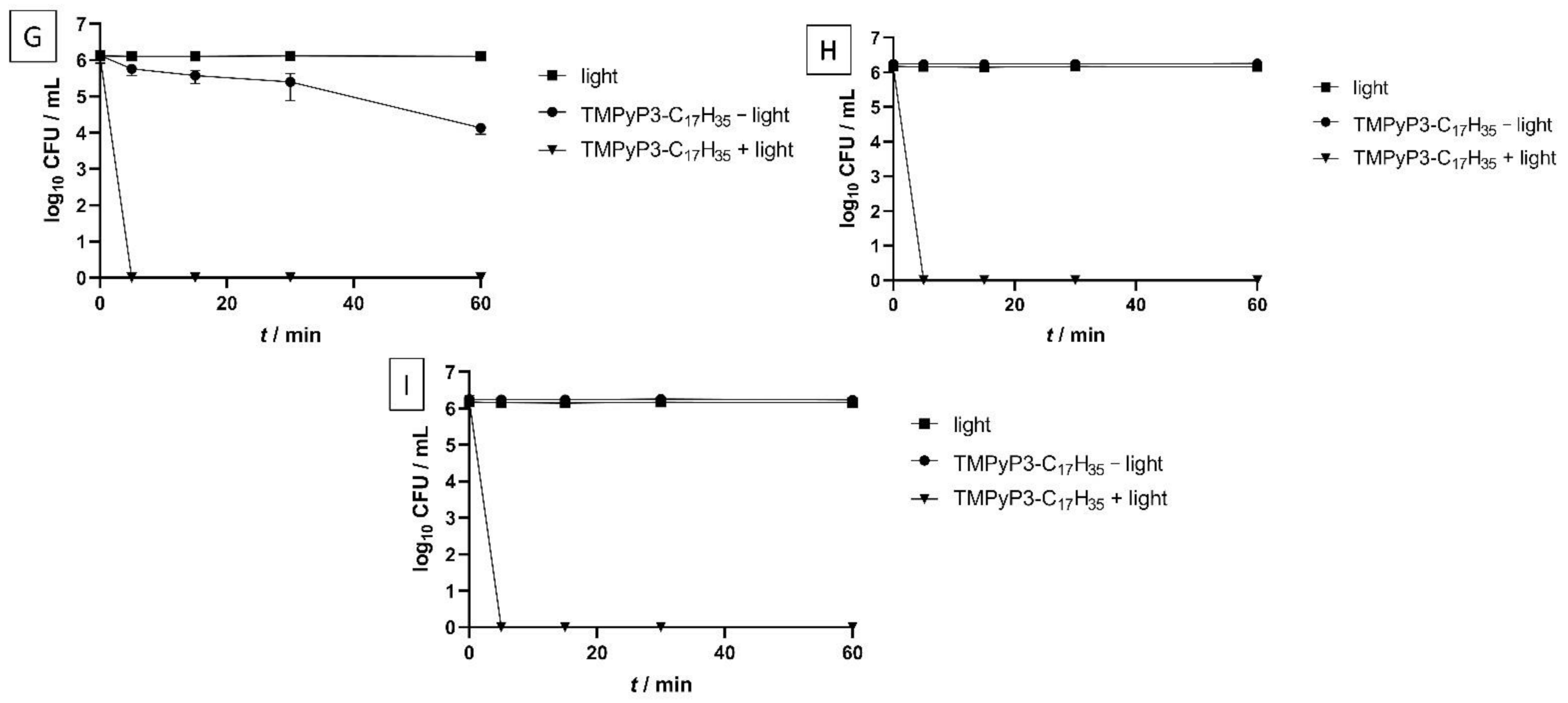
2.5. Minimal Effective Concentrations (MECs) and Photoinactivation (PDI) Studies of the PSs on Legionella pneumophila
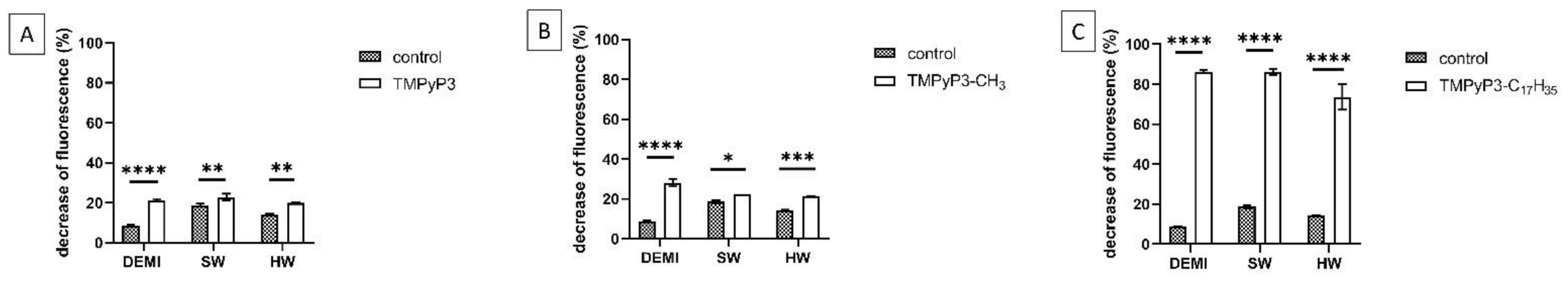
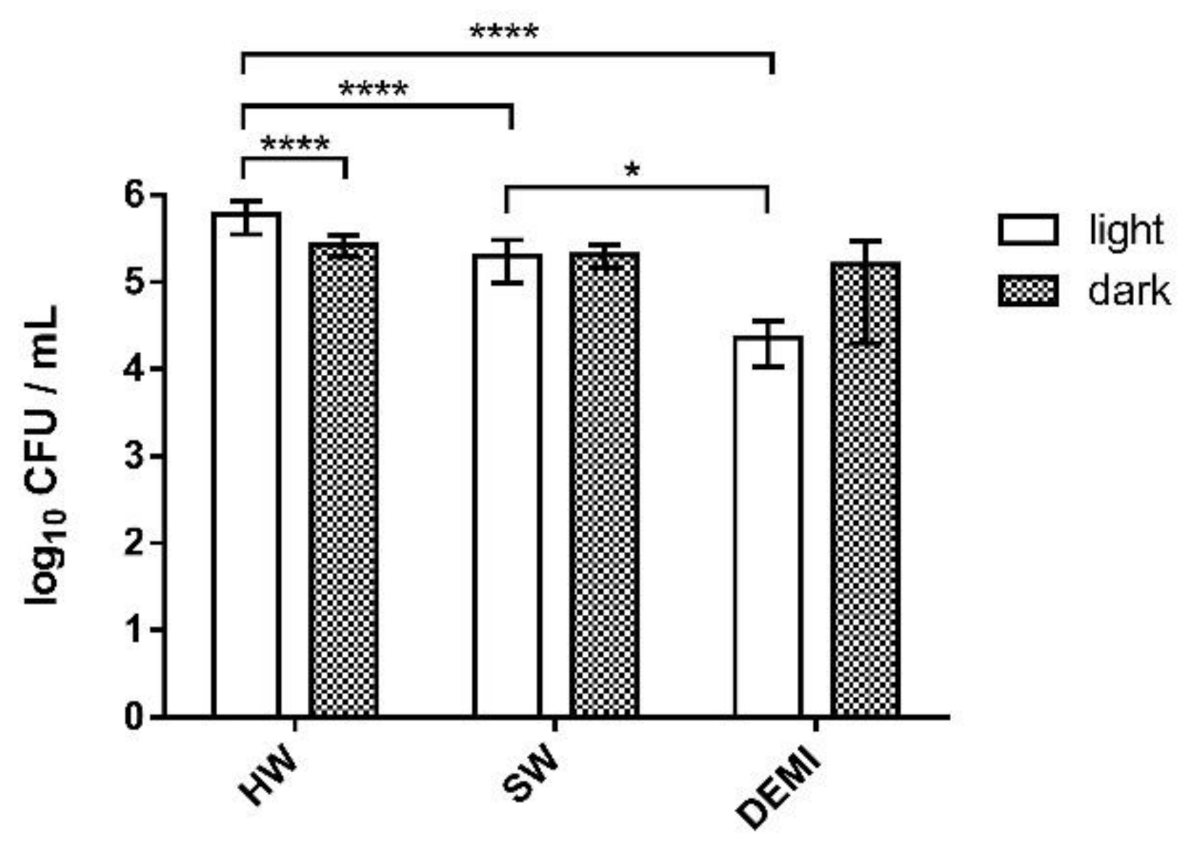
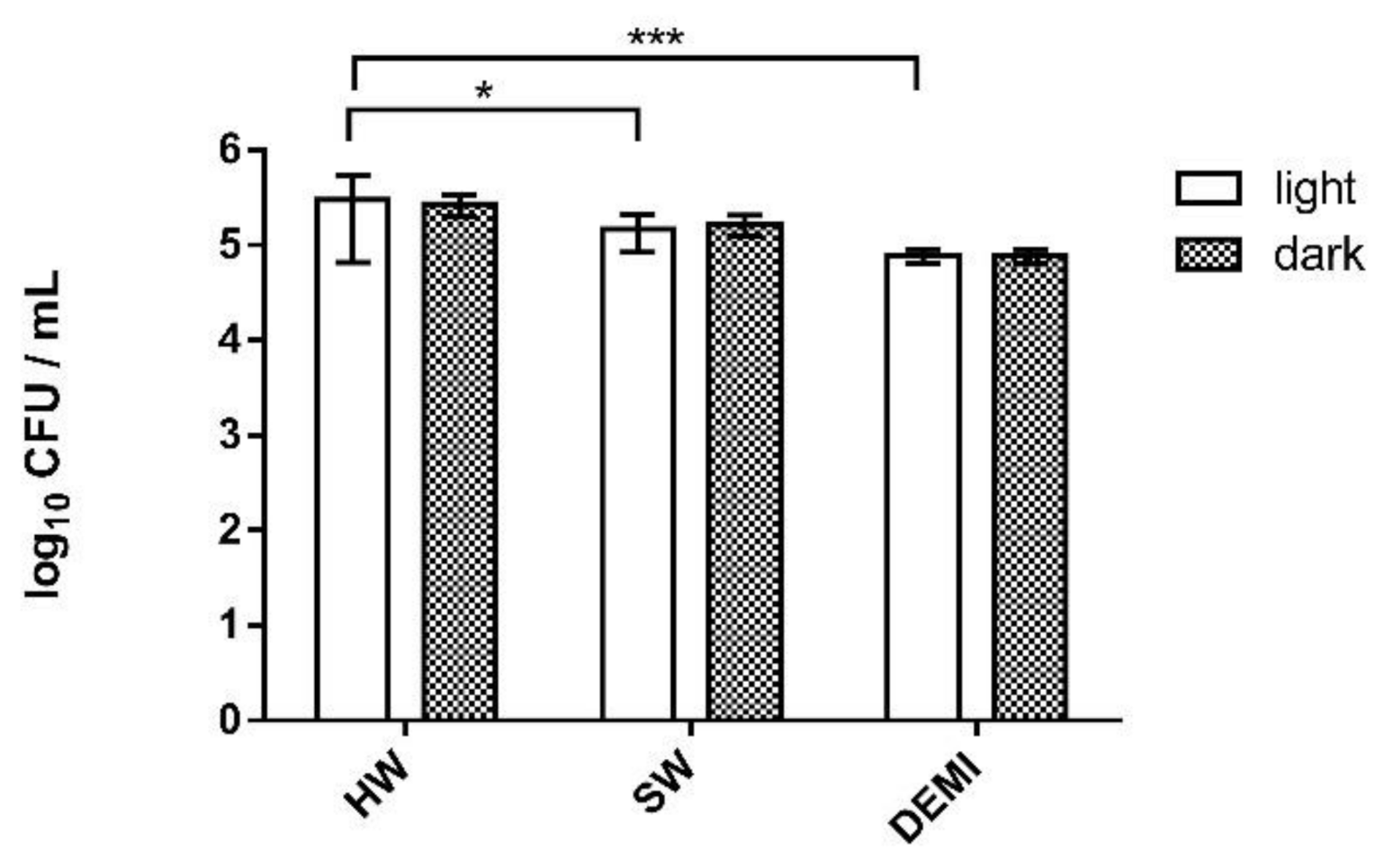
2.6. Impact of PDI Activity on L. pneumophila Adhesion to Polystyrene
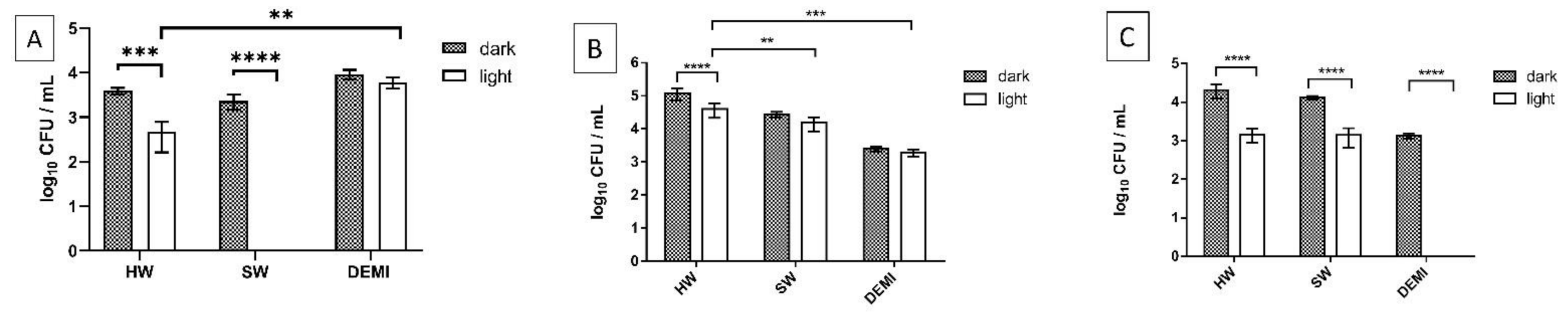
2.7. Impact of PDI Activity on L. pneumophila Biofilm Formation
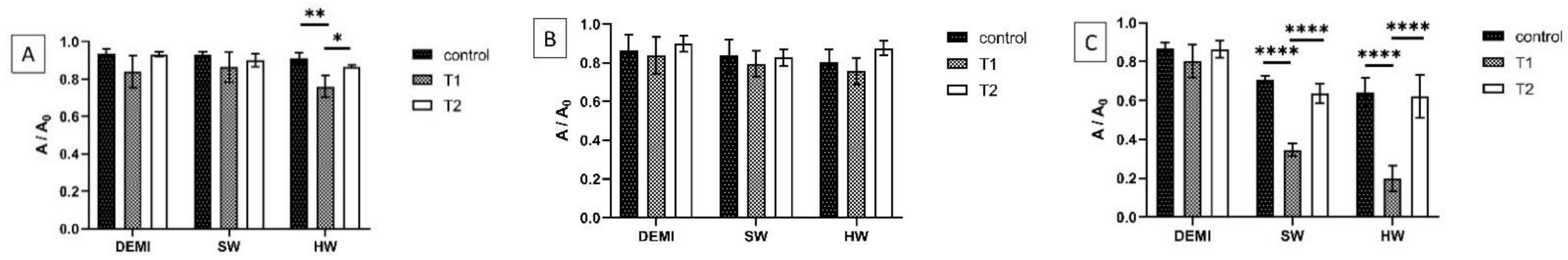
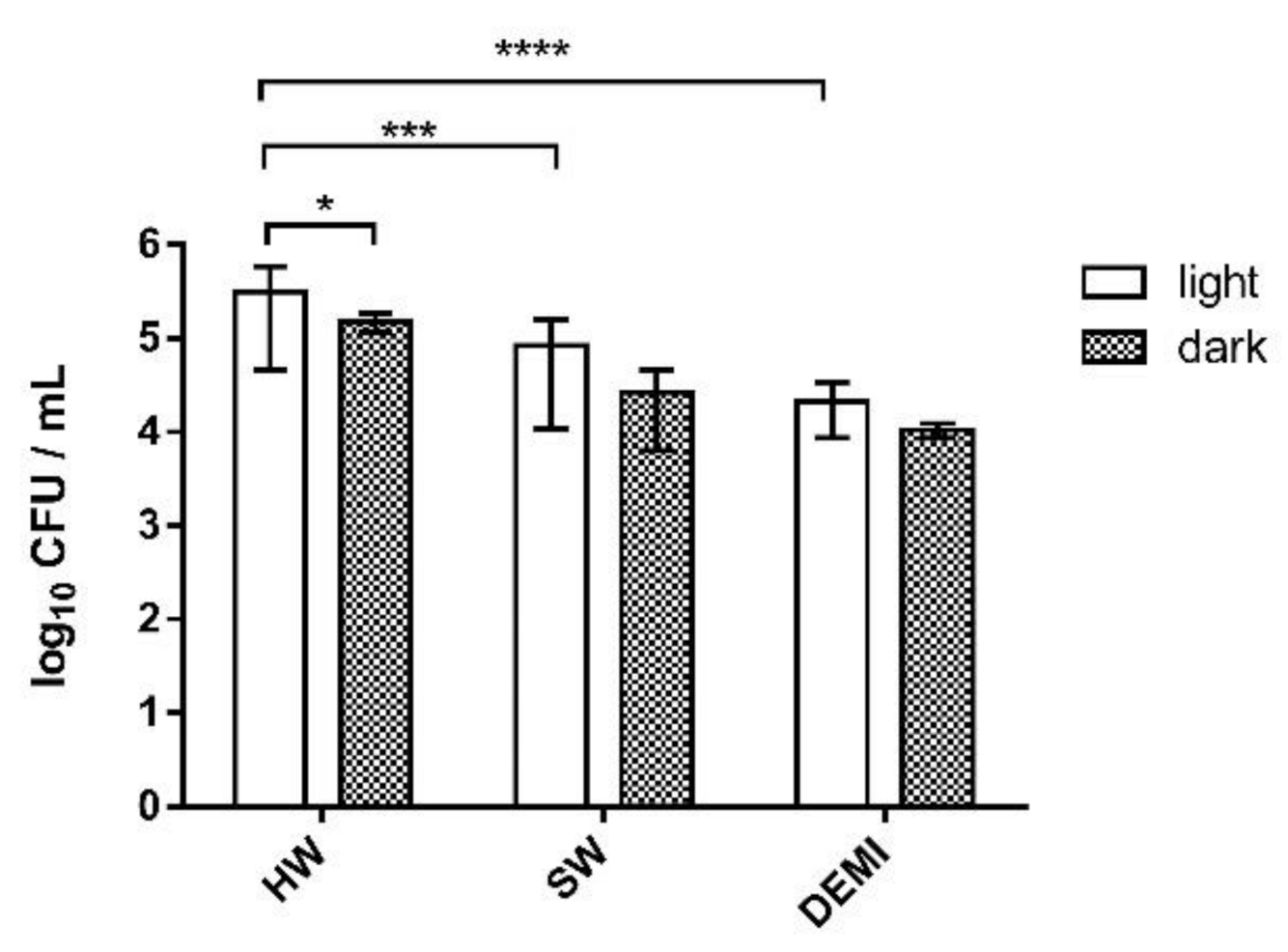
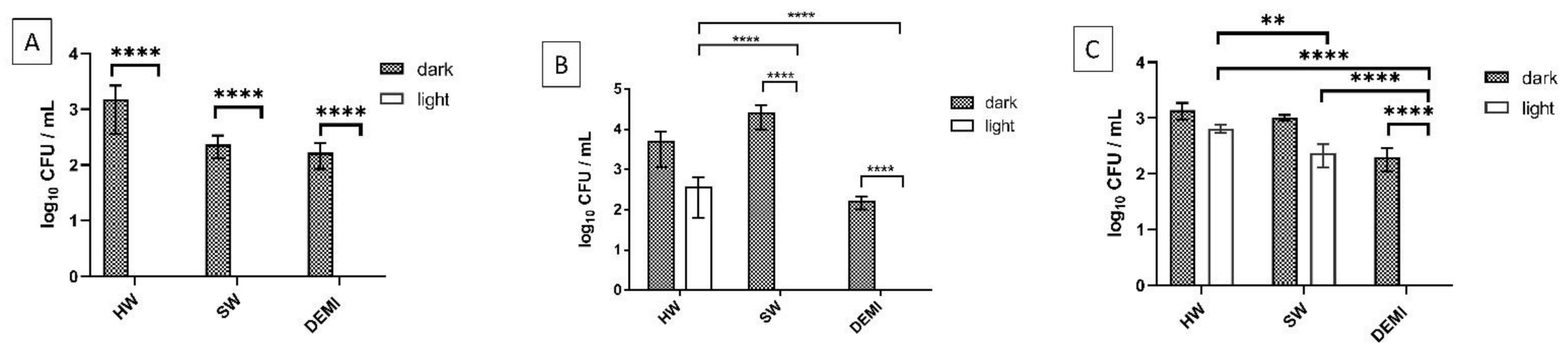
2.8. L. pneumophila Biofilm Destruction in Different Water Hardness
3. Materials and Methods
3.1. Porphyrins Used as PSs
3.2. Water Samples
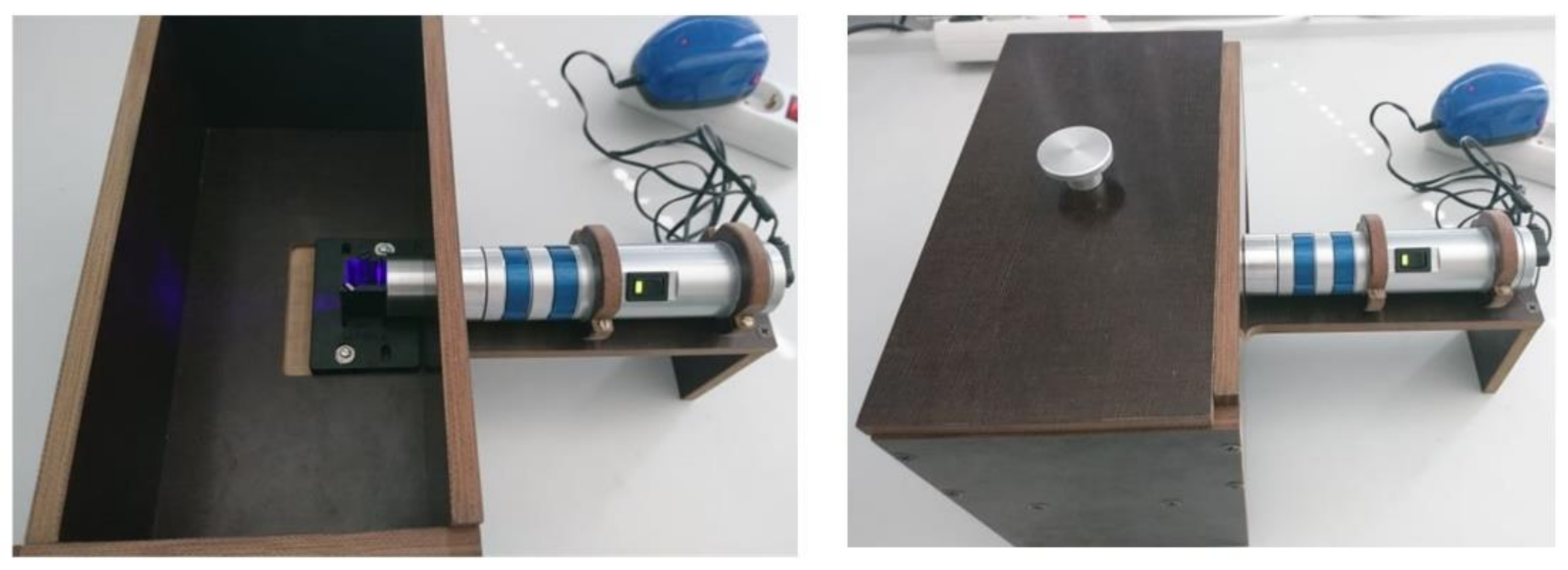
3.3. Light Sources
3.4. Spectroscopic Properties
3.5. Photostability Measurements
3.6. Singlet Oxygen (1O2) Detection
3.7. Bacteria Strain and Growth Conditions
3.8. Determining Minimum Effective Concentration (MEC) of the PSs in Water Samples of Different Hardness
3.9. PDI Studies in Water Samples of Different Hardness
3.10. Antiadhesion and Antibiofilm Properties of the PSs in Water Samples of Different Hardness on Polystyrene
3.11. Effectiveness of the PSs and Their PDI Activity on Biofilm Destruction in Waters of Different Hardness
3.12. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niculescu, A.-G.; Grumezescu, A.M. Photodynamic Therapy—An Up-to-Date Review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Ladeira, B.M.F.; Dias, C.J.; Gomes, A.T.P.C.; Tomé, A.C.; Neves, M.G.P.M.S.; Moura, N.M.M.; Almeida, A.; Faustino, M.A.F. Cationic Pyrrolidine/Pyrroline-Substituted Porphyrins as Efficient Photosensitizers against E. coli. Molecules 2021, 26, 464. [Google Scholar] [CrossRef]
- Skwor, T.A.; Klemm, S.; Zhang, H.; Schardt, B.; Blaszczyk, S.; Bork, M.A. Photodynamic inactivation of methicillin-resistant Staphylococcus aureus and Escherichia coli: A metalloporphyrin comparison. J. Photochem. Photobiol. B Biol. 2016, 165, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Amos-Tautua, M.B.; Songca, P.S.; Oluwafemi, S.O. Application of Porphyrins in Antibacterial Photodynamic Therapy. Molecules 2019, 24, 2456. [Google Scholar] [CrossRef] [Green Version]
- Sobotta, L.; Skupin-Mrugalska, P.; Piskorz, J.; Mielcarek, J. Porphyrinoid photosensitizers mediated photodynamic inactivation against bacteria. Eur. J. Med. Chem. 2019, 175, 72–106. [Google Scholar] [CrossRef] [PubMed]
- Street, C.N.; Gibbs, A.; Pedigo, L.; Andersen, D.; Loebel, N.G. In vitro photodynamic eradication of Pseudomonas aeruginosa in planktonic and biofilm culture. Photochem. Photobiol. 2009, 85, 137–143. [Google Scholar] [CrossRef]
- Soukos, N.S.; Goodson, J.M. Photodynamic therapy in the control of oral biofilms. Periodontol. 2000 2011, 55, 143–166. [Google Scholar] [CrossRef]
- Santin, G.C.; Oliveira, D.S.B.; Galo, R.; Borsatto, M.C.; Corona, S.A.M. Antimicrobial photodynamic therapy and dental plaque: A systematic review of the literature. Sci. World J. 2014, 824538. [Google Scholar] [CrossRef] [Green Version]
- Vassena, C.; Fenu, S.; Giuliani, F.; Fantetti, L.; Roncucci, G.; Simonutti, G.; Romanò, C.L.; De Francesco, R.; Drago, L. Photodynamic antibacterial and antibiofilm activity of RLP068/Cl against Staphylococcus aureus and Pseudomonas aeruginosa forming biofilms on prosthetic material. Int. J. Antimicrob. Agents 2014, 44, 47–55. [Google Scholar] [CrossRef]
- Dai, T.; Huang, Y.-Y.; Hamblin, M.R. Photodynamic therapy for localized infections—State of the art. Photodiagnosis Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef] [Green Version]
- Alves, E.; Costa, L.; Carvalho, C.M.B.; Tomé, J.P.C.; Faustino, M.A.; Neves, M.G.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A. Charge effect on the photoinactivation of Gram-negative and Gram-positive bacteria by cationic meso-substituted porphyrins. BMC Microbiol. 2009, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- George, S.; Hamblin, M.R.; Kishen, A. Uptake pathways of anionic and cationic photosensitizers into bacteria. Photochem. Photobiol. Sci. 2009, 8, 788–795. [Google Scholar] [CrossRef] [Green Version]
- Falkinham, O.J.; Pruden, A.; Edwards, M. Opportunistic Premise Plumbing Pathogens: Increasingly Important Pathogens in Drinking Water. Pathogens 2015, 4, 373–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fields, B.S.; Benson, R.F.; Besser, R.E. Legionella and Legionnaires’ Disease: 25 Years of Investigation. Clin. Microbiol. Rev. 2002, 15, 506–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, J.; Dowsett, A.B.; Dennis, P.J.; Lee, J.V.; Keevil, C.W. Influence of temperature and plumbing material selection on biofilm formation and growth of Legionella pneumophila in a model potable water system containing complex microbial flora. Appl. Environ. Microbiol. 1994, 60, 1585–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Kooij, D.; Veenendaal, H.R.; Scheffer, W.J.H. Biofilm formation and multiplication of Legionella in a model warm water system with pipes of copper, stainless steel and cross-linked polyethylene. Water Res. 2005, 39, 2789–2798. [Google Scholar] [CrossRef]
- Abdel-Nour, M.; Duncan, C.; Low, D.E.; Guyard, C. Biofilms: The stronghold of Legionella pneumophila. Int. J. Mol. Sci. 2013, 14, 21660–21675. [Google Scholar] [CrossRef] [Green Version]
- Abu Khweek, A.; Amer, A.O. Factors Mediating Environmental Biofilm Formation by Legionella pneumophila. Front. Cell. Infect. Microbiol. 2018, 8, 38. [Google Scholar] [CrossRef]
- Lesar, A.; Mušković, M.; Begić, G.; Lončarić, M.; Linšak, D.T.; Malatesti, N.; Gobin, I. Cationic porphyrins as effective agents in photodynamic inactivation of opportunistic plumbing pathogen Legionella pneumophila. Int. J. Mol. Sci. 2020, 21, 5367. [Google Scholar] [CrossRef]
- Szysz, M.P. Biological Role of Legionella Pneumophila lipopolysaccharide. Biomed. J. Sci. Tech. Res. 2018, 4, 3739–3741. [Google Scholar] [CrossRef]
- Herschmann, J.R.; Ali, A.; Harris, M.; McClinton, M.; Zamadar, M. Effect of Toxic Metal Ions on Photosensitized Singlet Oxygen Generation for Photodegradation of Polyaromatic Hydrocarbon Derivatives and Inactivation of Escherichia coli. Photochem. Photobiol. 2019, 95, 823–832. [Google Scholar] [CrossRef]
- Sengupta, P. Potential health impacts of hard water. Int. J. Prev. Med. 2013, 4, 866–875. [Google Scholar] [PubMed]
- Malatesti, N.; Harej, A.; Kraljević Pavelić, S.; Lončarić, M.; Zorc, H.; Wittine, K.; Andjelkovic, U.; Josic, D. Synthesis, characterisation and in vitro investigation of photodynamic activity of 5-(4-octadecanamidophenyl)-10,15,20-tris(N-methylpyridinium-3-yl)porphyrin trichloride on HeLa cells using low light fluence rate. Photodiagnosis Photodyn. Ther. 2016, 15, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Gradova, M.A.; Gradov, O.V.; Zhdanova, K.A.; Bragina, N.A.; Lobanov, A.V. Self-assembly of amphiphilic meso-aryl-substituted porphyrin derivatives in the presence of surfactants. J. Porphyr. Phthalocyanines 2019, 24, 505–514. [Google Scholar] [CrossRef]
- Entradas, T.; Waldron, S.; Volk, M. The detection sensitivity of commonly used singlet oxygen probes in aqueous environments. J. Photochem. Photobiol. B Biol. 2020, 204, 111787. [Google Scholar] [CrossRef]
- Carloni, P.; Damiani, E.; Greci, L.; Stipa, P.; Tanfani, F.; Tartaglini, E.; Wozniak, M. On the use of 1,3-diphenylisobenzofuran (DPBF). Reactions with carbon and oxygen centered radicals in model and natural systems. Res. Chem. Intermed. 1993, 19, 395–405. [Google Scholar] [CrossRef]
- Żamojć, K.; Zdrowowicz, M.; Rudnicki-Velasquez, P.B.; Krzymiński, K.; Zaborowski, B.; Niedziałkowski, P.; Jacewicz, D.; Chmurzyński, L. The development of 1,3-diphenylisobenzofuran as a highly selective probe for the detection and quantitative determination of hydrogen peroxide. Free Radic. Res. 2017, 51, 38–46. [Google Scholar] [CrossRef]
- Jelovica, M.; Grbčić, P.; Mušković, M.; Sedić, M.; Pavelić, S.K.; Lončarić, M.; Malatesti, N. In Vitro Photodynamic Activity of N-Methylated and N-Oxidised Tripyridyl Porphyrins with Long Alkyl Chains and Their Inhibitory Activity in Sphingolipid Metabolism. ChemMedChem 2018, 13, 360–372. [Google Scholar] [CrossRef]
- Schenning, A.P.H.J.; Feiters, M.C.; Nolte, R.J.M. An amphiphilic porphyrin with unexpected aggregation behaviour. Tetrahedron Lett. 1993, 34, 7077–7080. [Google Scholar] [CrossRef]
- Rapozzi, V.; Zorzet, S.; Zacchigna, M.; Drioli, S.; Xodo, L.E. The PDT activity of free and pegylated pheophorbide a against an amelanotic melanoma transplanted in C57/BL6 mice. Invest. New Drugs 2013, 31, 192–199. [Google Scholar] [CrossRef]
- Sułek, A.; Pucelik, B.; Kobielusz, M.; Barzowska, A.; Dąbrowski, J.M. Photodynamic inactivation of bacteria with porphyrin derivatives: Effect of charge, lipophilicity, ros generation, and cellular uptake on their biological activity in vitro. Int. J. Mol. Sci. 2020, 21, 8716. [Google Scholar] [CrossRef] [PubMed]
- Bargellini, A.; Marchesi, I.; Righi, E.; Ferrari, A.; Cencetti, S.; Borella, P.; Rovesi, S. Parameters predictive of Legionella contamination in hot water systems: Association with trace elements and heterotropic plate counts. Water Res. 2011, 45, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Prochnow, E.P.; Martins, M.R.; Campagnolo, C.B.; Santos, R.C.; Villetti, M.A.; Kantorski, K.Z. Antimicrobial photodynamic effect of phenothiazinic photosensitizers in formulations with ethanol on Pseudomonas aeruginosa biofilms. Photodiagnosis Photodyn. Ther. 2016, 13, 291–296. [Google Scholar] [CrossRef]
- Fekrirad, Z.; Darabpour, E.; Kashef, N. Eradication of Acinetobacter baumannii Planktonic and Biofilm Cells Through Erythrosine-Mediated Photodynamic Inactivation Augmented by Acetic Acid and Chitosan. Curr. Microbiol. 2021, 78, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, A.; Kruszewska, B.; Pierański, M.K.; Rychłowski, M.; Grinholc, M. Antimicrobial photodynamic inactivation affects the antibiotic susceptibility of Enterococcus spp. clinical isolates in biofilm and planktonic cultures. Biomolecules 2021, 11, 693. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Martegani, E.; Bolognese, F.; Trivellin, N.; Garzotto, F.; Caruso, E. Photoinactivation of Pseudomonas aeruginosa Biofilm by Dicationic Diaryl-Porphyrin. Int. J. Mol. Sci. 2021, 22, 6808. [Google Scholar] [CrossRef]
- Patel, N.; Swavey, S.; Robinson, J. A Cationic Porphyrin, ZnPor, Disassembles Pseudomonas aeruginosa Biofilm Matrix, Kills Cells Directly, and Enhances Antibiotic Activity of Tobramycin. Antibiotics 2020, 9, 875. [Google Scholar] [CrossRef] [PubMed]



| Title 1 | Water Samples | ||
|---|---|---|---|
| DEMI | SW | HW | |
| CaCO3 (mg/L) a | / | 231 | 403 |
| pH a,b | 7.00 | 7.66 | 7.43 |
| Conductivity (S/cm) (t = 20 °C) b | 4 | 403 | 678 |
| Cl− (mg/L) a | / | 8.53 | 20.08 |
| K+ (mg/L) a | / | 1.7 | 1.8 |
| Na+ (mg/L) a | / | 5.1 | 5.2 |
| Mg2+ (mg/L) a | / | 7.7 | 23.3 |
| Ca2+ (mg/L) a | / | / | 112.0 |
| Hardness (mg/L) a | 0.2 | 240 | 396 |
| Oxygen levels (mg/L) b | 2.55 | 9.54 | 9.80 |
| MEC (μM) | |||
|---|---|---|---|
| Dark | λ = 395 nm | ||
| TMPyP3 | DEMI | 6.250 | 3.125 |
| SW | >5 | 6.250 | |
| HW | >25 | 12.50 | |
| TMPyP3-CH3 | DEMI | 3.125 | 0.780 |
| SW | >5 | 3.125 | |
| HW | >25 | 6.250 | |
| TMPyP3-C17H35 | DEMI | 3.125 | 0.780 |
| SW | 6.250 | 3.125 | |
| HW | >25 | 6.250 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mušković, M.; Ćavar, I.; Lesar, A.; Lončarić, M.; Malatesti, N.; Gobin, I. Photodynamic Inactivation of Legionella pneumophila Biofilm Formation by Cationic Tetra- and Tripyridylporphyrins in Waters of Different Hardness. Int. J. Mol. Sci. 2021, 22, 9095. https://doi.org/10.3390/ijms22169095
Mušković M, Ćavar I, Lesar A, Lončarić M, Malatesti N, Gobin I. Photodynamic Inactivation of Legionella pneumophila Biofilm Formation by Cationic Tetra- and Tripyridylporphyrins in Waters of Different Hardness. International Journal of Molecular Sciences. 2021; 22(16):9095. https://doi.org/10.3390/ijms22169095
Chicago/Turabian StyleMušković, Martina, Iva Ćavar, Andrija Lesar, Martin Lončarić, Nela Malatesti, and Ivana Gobin. 2021. "Photodynamic Inactivation of Legionella pneumophila Biofilm Formation by Cationic Tetra- and Tripyridylporphyrins in Waters of Different Hardness" International Journal of Molecular Sciences 22, no. 16: 9095. https://doi.org/10.3390/ijms22169095
APA StyleMušković, M., Ćavar, I., Lesar, A., Lončarić, M., Malatesti, N., & Gobin, I. (2021). Photodynamic Inactivation of Legionella pneumophila Biofilm Formation by Cationic Tetra- and Tripyridylporphyrins in Waters of Different Hardness. International Journal of Molecular Sciences, 22(16), 9095. https://doi.org/10.3390/ijms22169095







