Negative Influence by the Force: Mechanically Induced Hyperpolarization via K2P Background Potassium Channels
Abstract
1. Introduction
2. Mechanical Activation of K2P Channels
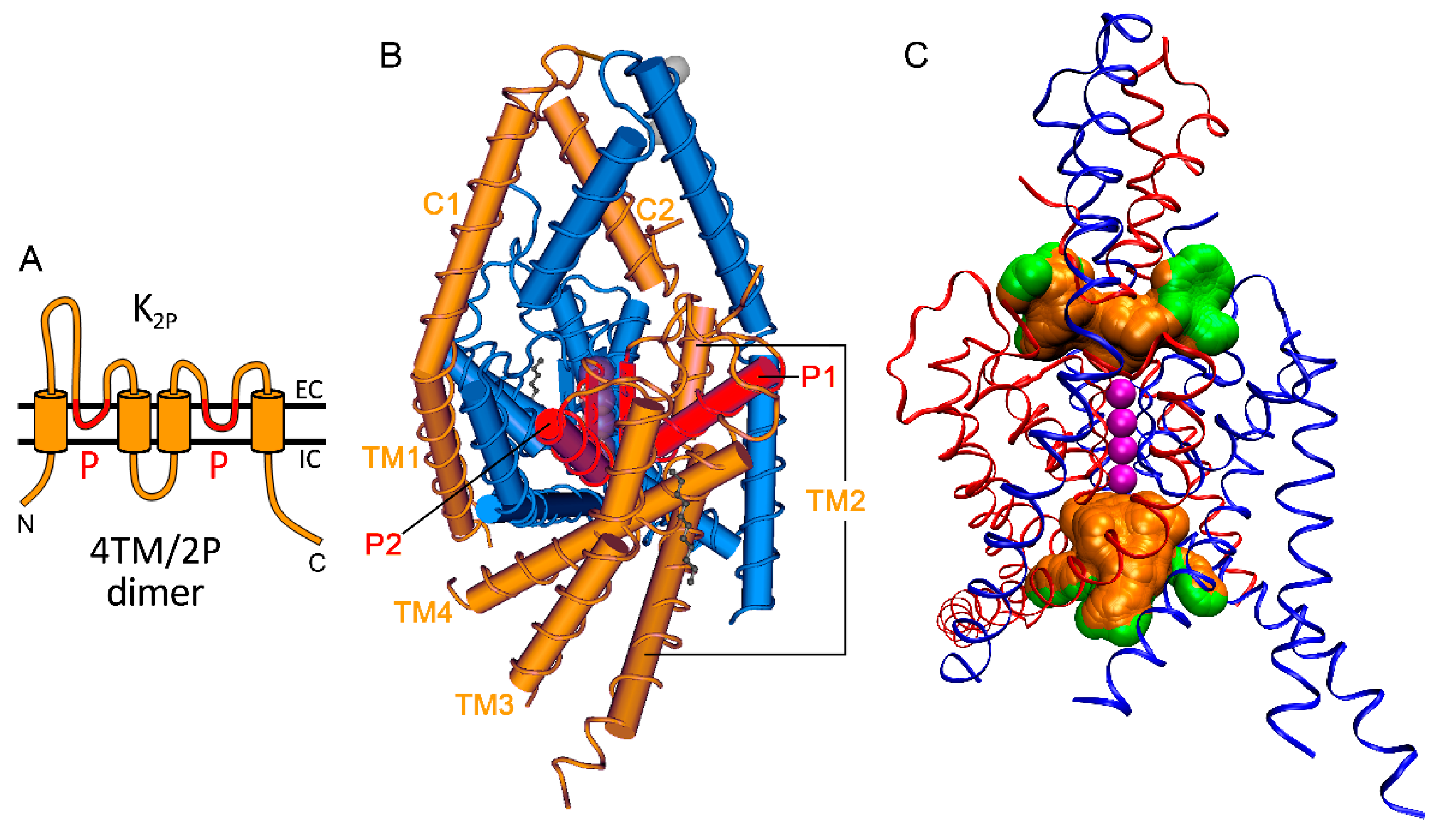
2.1. A General Approach to Mechanosensitivity of K2P Ion Channels
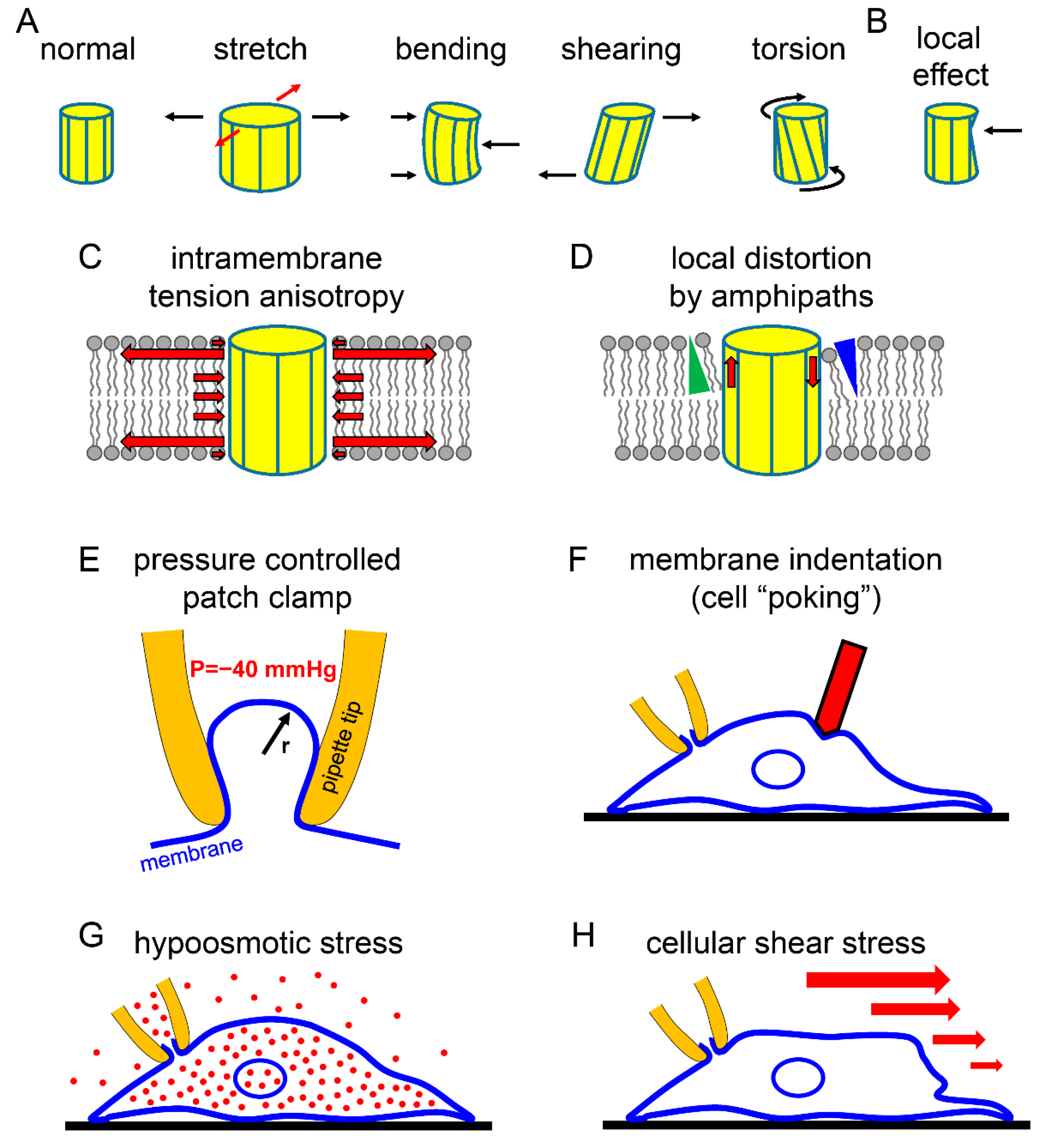
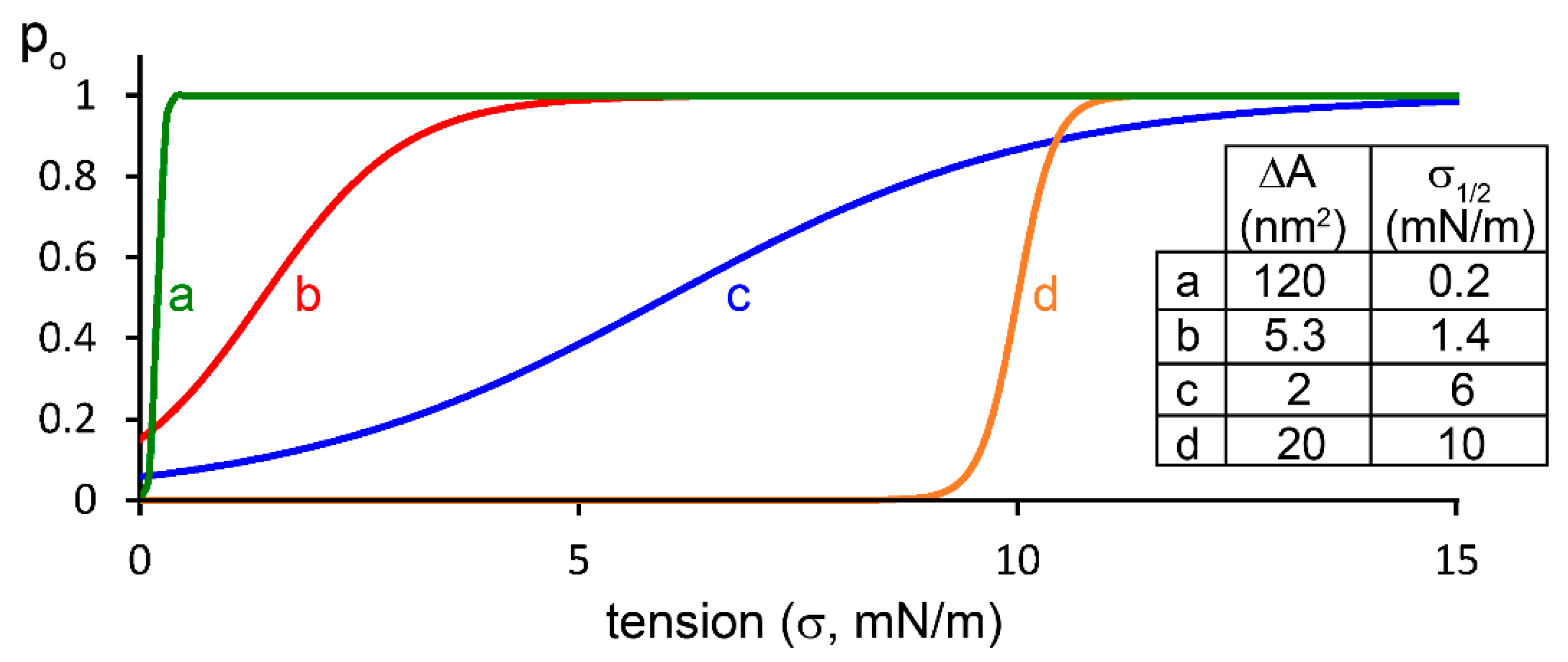
2.2. Mechanosensitive Properties of K2P Channels as Reflected by the Currently Available Methods
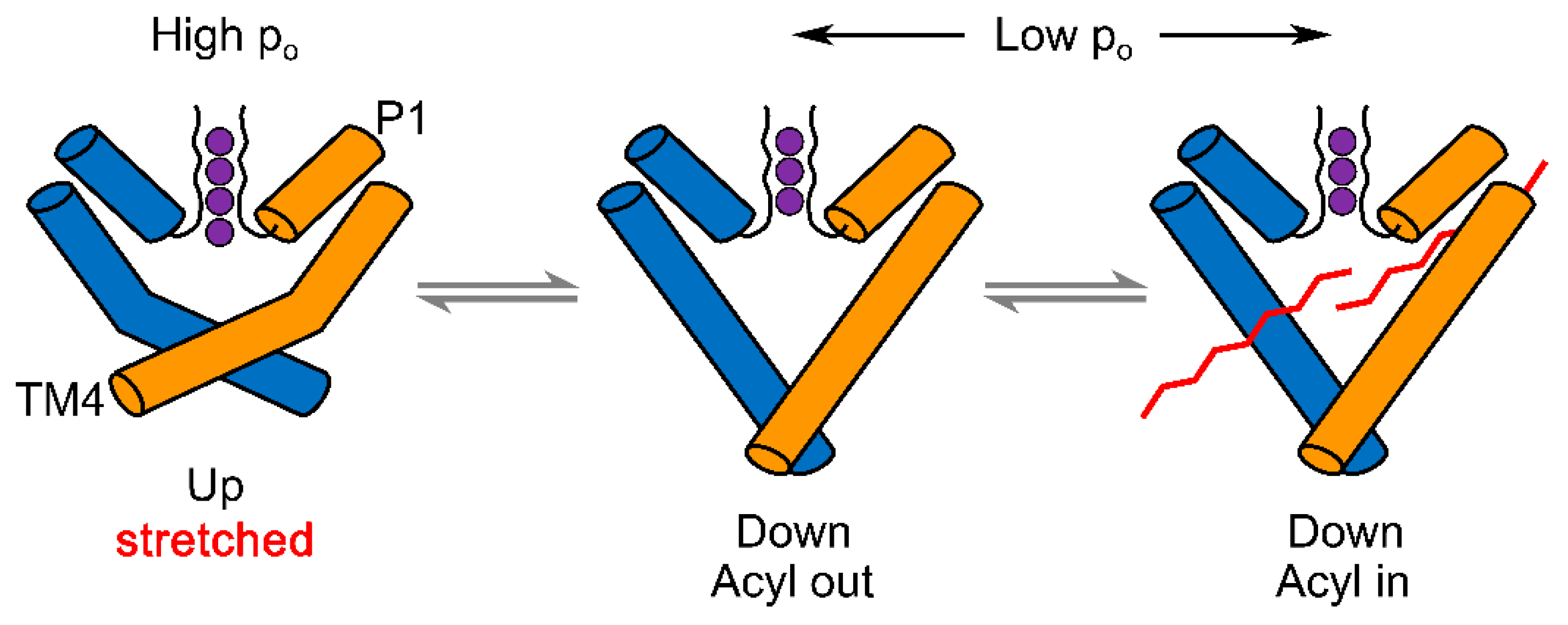
2.3. The Models of TREK/TRAAK Mechanogating
3. The Physiological Roles of K2P Channel Mechanical Activation
3.1. Hyperpolarization and Relaxation of Smooth Muscle Cells in the Wall of Hollow Visceral Organs
3.1.1. Adaptive Relaxation in the Gastrointestinal Tract
3.1.2. Distension of the Urinary Bladder
3.1.3. Relaxation of the Myometrium during Pregnancy
3.2. Putative Role of the Mechanical Activation of K2P Channels in the Cardiovascular System
3.2.1. Mechanical Activation of K2P Channels in the Heart
3.2.2. Mechanical Activation of K2P Channels in the Vasculature
3.3. Mechanical Activation of K2P Channels in the Nervous System
3.3.1. Peripheral Nervous System
3.3.2. Central Nervous System
3.4. Physiological Role of the Mechanical Activation of K2P Channels in Other Locations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Enyedi, P.; Czirják, G. Molecular Background of Leak K+ Currents: Two-Pore Domain Potassium Channels. Physiol. Rev. 2010, 90, 559–605. [Google Scholar] [CrossRef]
- Schewe, M.; Nematian-Ardestani, E.; Sun, H.; Musinszki, M.; Cordeiro, S.; Bucci, G.; de Groot, B.L.; Tucker, S.J.; Rapedius, M.; Baukrowitz, T. A Non-canonical Voltage-Sensing Mechanism Controls Gating in K2P K+ Channels. Cell 2016, 164, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Lolicato, M.; Arrigoni, C.; Mori, T.; Sekioka, Y.; Bryant, C.; Clark, K.A.; Minor, D.L., Jr. K2P2.1 (TREK-1)-activator complexes reveal a cryptic selectivity filter binding site. Nature 2017, 547, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, M.; Czirják, G.; Enyedi, P. TRESK background potassium channel is not gated at the helix bundle crossing near the cytoplasmic end of the pore. PLoS ONE 2018, 13, e0197622. [Google Scholar] [CrossRef] [PubMed]
- Schewe, M.; Sun, H.; Mert, Ü.; Mackenzie, A.; Pike, A.C.W.; Schulz, F.; Constantin, C.; Vowinkel, K.S.; Conrad, L.J.; Kiper, A.K.; et al. A pharmacological master key mechanism that unlocks the selectivity filter gate in K+channels. Science 2019, 363, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Ben Soussia, I.; El Mouridi, S.; Kang, D.; Leclercq-Blondel, A.; Khoubza, L.; Tardy, P.; Zariohi, N.; Gendrel, M.; Lesage, F.; Kim, E.-J.; et al. Mutation of a single residue promotes gating of vertebrate and invertebrate two-pore domain potassium channels. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bustos, D.; Bedoya, M.; Ramírez, D.; Concha, G.; Zúñiga, L.; Decher, N.; Hernández-Rodríguez, E.W.; Sepúlveda, F.V.; Martínez, L.; González, W. Elucidating the Structural Basis of the Intracellular pH Sensing Mechanism of TASK-2 K2P Channels. Int. J. Mol. Sci. 2020, 21, 532. [Google Scholar] [CrossRef]
- Nematian-Ardestani, E.; Abd-Wahab, F.; Chatelain, F.C.; Sun, H.; Schewe, M.; Baukrowitz, T.; Tucker, S.J. Selectivity filter instability dominates the low intrinsic activity of the TWIK-1 K2P K+ channel. J. Biol. Chem. 2020, 295, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Lolicato, M.; Natale, A.M.; Abderemane-Ali, F.; Crottès, D.; Capponi, S.; Duman, R.; Wagner, A.; Rosenberg, J.M.; Grabe, M.; Minor, D.L. K2P channel C-type gating involves asymmetric selectivity filter order-disorder transitions. Sci. Adv. 2020, 6, eabc9174. [Google Scholar] [CrossRef]
- Czirjak, G. PrinCCes: Continuity-based geometric decomposition and systematic visualization of the void repertoire of proteins. J. Mol. Graph. Model. 2015, 62, 118–127. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- González, W.; Zúñiga, L.; Cid, L.P.; Arévalo, B.; Niemeyer, M.I.; Sepúlveda, F.V. An Extracellular Ion Pathway Plays a Central Role in the Cooperative Gating of a K2P K+ Channel by Extracellular pH. J. Biol. Chem. 2013, 288, 5984–5991. [Google Scholar] [CrossRef]
- Braun, G.; Lengyel, M.; Enyedi, P.; Czirják, G. Differential sensitivity of TREK-1, TREK-2 and TRAAK background potassium channels to the polycationic dye ruthenium red. Br. J. Pharmacol. 2015, 172, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Chen, L.; Cheng, X.; Ma, Y.; Li, X.; Zhang, B.; Li, L.; Zhang, S.; Guo, F.; Li, Y.; et al. An allosteric ligand-binding site in the extracellular cap of K2P channels. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Czirják, G.; Enyedi, P. Ruthenium red inhibits TASK-3 potassium channel by interconnecting glutamate 70 of the two subunits. Mol. Pharmacol. 2003, 63, 646–652. [Google Scholar] [CrossRef]
- Pope, L.; Lolicato, M.; Minor, D.L. Polynuclear Ruthenium Amines Inhibit K2P Channels via a Finger in the Dam Mechanism. Cell Chem. Biol. 2020, 27, 511–524.e4. [Google Scholar] [CrossRef]
- Maingret, F.; Honore, E.; Lazdunski, M.; Patel, A.J. Molecular Basis of the Voltage-Dependent Gating of TREK-1, a Mechano-Sensitive K+ Channel. Biochem. Biophys. Res. Commun. 2002, 292, 339–346. [Google Scholar] [CrossRef]
- Fink, M.; Duprat, F.; Lesage, F.; Reyes, R.; Romey, G.; Heurteaux, C.; Lazdunski, M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996, 15, 6854–6862. [Google Scholar] [CrossRef]
- Bang, H.; Kim, Y.; Kim, D. TREK-2, a New Member of the Mechanosensitive Tandem-pore K+ Channel Family. J. Biol. Chem. 2000, 275, 17412–17419. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lewis, A.H.; Grandl, J. Touch, Tension, and Transduction—The Function and Regulation of Piezo Ion Channels. Trends Biochem. Sci. 2017, 42, 57–71. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, X.; Jiang, J.; Xiao, B. Structural Designs and Mechanogating Mechanisms of the Mechanosensitive Piezo Channels. Trends Biochem. Sci. 2021, 46, 472–488. [Google Scholar] [CrossRef]
- Maingret, F.; Patel, A.J.; Lesage, F.; Lazdunski, M.; Honore, E. Mechano- or Acid Stimulation, Two Interactive Modes of Activation of the TREK-1 Potassium Channel. J. Biol. Chem. 1999, 274, 26691–26696. [Google Scholar] [CrossRef]
- Simkin, D.; Cavanaugh, E.J.; Kim, D. Control of the single channel conductance of K2P10.1 (TREK-2) by the amino-terminus: Role of alternative translation initiation. J. Physiol. 2008, 586, 5651–5663. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Plant, L.D.; Wilkens, C.M.; McCrossan, Z.A.; Goldstein, S.A. Alternative Translation Initiation in Rat Brain Yields K2P2.1 Potassium Channels Permeable to Sodium. Neuron 2008, 58, 859–870. [Google Scholar] [CrossRef]
- Honore, E.; Maingret, F.; Lazdunski, M.; Patel, A.J. An intracellular proton sensor commands lipid- and mechano-gating of the K+ channel TREK-1. EMBO J. 2002, 21, 2968–2976. [Google Scholar] [CrossRef]
- Woo, J.; Jun, Y.K.; Zhang, Y.-H.; Nam, J.; Shin, D.H.; Kim, S.J. Identification of critical amino acids in the proximal C-terminal of TREK-2 K+ channel for activation by acidic pHi and ATP-dependent inhibition. Pflügers Arch. Eur. J. Physiol. 2018, 470, 327–337. [Google Scholar] [CrossRef]
- Murbartián, J.; Lei, Q.; Sando, J.J.; Bayliss, D.A. Sequential Phosphorylation Mediates Receptor- and Kinase-induced Inhibition of TREK-1 Background Potassium Channels. J. Biol. Chem. 2005, 280, 30175–30184. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.; Mendez-Resendiz, K.A.; Oviedo, N.; Murbartian, J. PKC- and PKA-dependent phosphorylation modulates TREK-1 function in naive and neuropathic rats. J. Neurochem. 2021, 157, 2039–2054. [Google Scholar] [CrossRef]
- Patel, A.J.; Honore, E.; Maingret, F.; Lesage, F.; Fink, M.; Duprat, F.; Lazdunski, M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998, 17, 4283–4290. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.; Lesage, F.; Duprat, F.; Heurteaux, C.; Reyes, R.; Fosset, M.; Lazdunski, M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 1998, 17, 3297–3308. [Google Scholar] [CrossRef]
- Ben Soussia, I.; Choveau, F.S.; Blin, S.; Kim, E.-J.; Feliciangeli, S.; Chatelain, F.C.; Kang, D.; Bichet, D.; Lesage, F. Antagonistic Effect of a Cytoplasmic Domain on the Basal Activity of Polymodal Potassium Channels. Front. Mol. Neurosci. 2018, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, M.V.; Kotova, P.D.; Bystrova, M.F.; Kabanova, N.V.; Sysoeva, V.Y.; Kolesnikov, S.S. Arachidonic acid hyperpolarizes mesenchymal stromal cells from the human adipose tissue by stimulating TREK1 K+ channels. Channels 2019, 13, 36–47. [Google Scholar] [CrossRef]
- Ma, R.; Lewis, A. Spadin Selectively Antagonizes Arachidonic Acid Activation of TREK-1 Channels. Front. Pharmacol. 2020, 11, 434. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Ling, J.; Tonomura, S.; Noguchi, K.; Matalon, S.; Gu, J.G. TREK-1 and TRAAK Are Principal K+ Channels at the Nodes of Ranvier for Rapid Action Potential Conduction on Mammalian Myelinated Afferent Nerves. Neuron 2019, 104, 960–971.e7. [Google Scholar] [CrossRef] [PubMed]
- Lamas, J.A.; Rueda-Ruzafa, L.; Herrera-Pérez, S. Ion Channels and Thermosensitivity: TRP, TREK, or Both? Int. J. Mol. Sci. 2019, 20, 2371. [Google Scholar] [CrossRef]
- Schneider, E.R.; Anderson, E.O.; Gracheva, E.O.; Bagriantsev, S.N. Temperature Sensitivity of Two-Pore (K2P) Potassium Channels. Curr. Top. Membr. 2014, 74, 113–133. [Google Scholar] [CrossRef]
- Djillani, A.; Pietri, M.; Moreno, S.; Heurteaux, C.; Mazella, J.; Borsotto, M. Shortened Spadin Analogs Display Better TREK-1 Inhibition, In Vivo Stability and Antidepressant Activity. Front. Pharmacol. 2017, 8, 643. [Google Scholar] [CrossRef]
- Djillani, A.; Pietri, M.; Mazella, J.; Heurteaux, C.; Borsotto, M. Fighting against depression with TREK-1 blockers: Past and future. A focus on spadin. Pharmacol. Ther. 2019, 194, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Mazella, J.; Borsotto, M.; Heurteaux, C. The Involvement of Sortilin/NTSR3 in Depression as the Progenitor of Spadin and Its Role in the Membrane Expression of TREK-1. Front. Pharmacol. 2019, 9, 1541. [Google Scholar] [CrossRef]
- Sandoz, G.; Thümmler, S.; Duprat, F.; Feliciangeli, S.; Vinh, J.; Escoubas, P.; Guy, N.; Lazdunski, M.; Lesage, F. AKAP150, a switch to convert mechano-, pH- and arachidonic acid-sensitive TREK K+ channels into open leak channels. EMBO J. 2006, 25, 5864–5872. [Google Scholar] [CrossRef]
- Wague, A.; Joseph, T.; Woll, K.A.; Bu, W.; Vaidya, K.A.; Bhanu, N.V.; Garcia, B.A.; Nimigean, C.M.; Eckenhoff, R.G.; Riegelhaupt, P.M. Mechanistic insights into volatile anesthetic modulation of K2P channels. eLife 2020, 9, 9. [Google Scholar] [CrossRef]
- Zhao, G.; Yang, L.; Wang, S.; Cai, M.; Sun, S.; Dong, H.; Xiong, L. TREK-2 Mediates the Neuroprotective Effect of Isoflurane Preconditioning against Acute Cerebral Ischemia in the Rat. Rejuvenation Res. 2019, 22, 325–334. [Google Scholar] [CrossRef]
- Cai, Y.; Peng, Z.; Guo, H.; Wang, F.; Zeng, Y. TREK-1 pathway mediates isoflurane-induced memory impairment in middle-aged mice. Neurobiol. Learn. Mem. 2017, 145, 199–204. [Google Scholar] [CrossRef]
- Kennard, L.E.; Chumbley, J.R.; Ranatunga, K.M.; Armstrong, S.J.; Veale, E.; Mathie, A. Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Br. J. Pharmacol. 2005, 144, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.Y.; Pike, A.C.W.; Mackenzie, A.; McClenaghan, C.; Aryal, P.; Dong, L.; Quigley, A.; Grieben, M.; Goubin, S.; Mukhopadhyay, S.; et al. K2P channel gating mechanisms revealed by structures of TREK-2 and a complex with Prozac. Science 2015, 347, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, E.-J.; Han, J.; Han, J.; Kang, D. Effects of analgesics and antidepressants on TREK-2 and TRESK currents. Korean J. Physiol. Pharmacol. 2016, 20, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Viatchenko-Karpinski, V.; Ling, J.; Gu, J.G. Characterization of temperature-sensitive leak K+ currents and expression of TRAAK, TREK-1, and TREK2 channels in dorsal root ganglion neurons of rats. Mol. Brain 2018, 11, 40. [Google Scholar] [CrossRef]
- Huang, H.; Li, H.; Shi, K.; Wang, L.; Zhang, X.; Zhu, X. TREK-TRAAK two-pore domain potassium channels protect human retinal pigment epithelium cells from oxidative stress. Int. J. Mol. Med. 2018, 42, 2584–2594. [Google Scholar] [CrossRef]
- Proks, P.; Schewe, M.; Conrad, L.J.; Rao, S.; Rathje, K.; Rödström, K.E.; Carpenter, E.P.; Baukrowitz, T.; Tucker, S.J. Norfluoxetine inhibits TREK-2 K2P channels by multiple mechanisms including state-independent effects on the selectivity filter gate. J. Gen. Physiol. 2021, 153, 8. [Google Scholar] [CrossRef]
- Talley, E.M.; Solórzano, G.; Lei, Q.; Kim, D.; Bayliss, D.A. CNS Distribution of Members of the Two-Pore-Domain (KCNK) Potassium Channel Family. J. Neurosci. 2001, 21, 7491–7505. [Google Scholar] [CrossRef]
- Ren, K.; Liu, H.; Guo, B.; Li, R.; Mao, H.; Xue, Q.; Yao, H.; Wu, S.; Bai, Z.; Wang, W. Quercetin relieves D-amphetamine-induced manic-like behaviour through activating TREK-1 potassium channels in mice. Br. J. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Kim, A.; Jung, H.-G.; Kim, Y.-E.; Kim, S.-C.; Park, J.-Y.; Lee, S.-G.; Hwang, E.M. The Knockdown of TREK-1 in Hippocampal Neurons Attenuate Lipopolysaccharide-Induced Depressive-Like Behavior in Mice. Int. J. Mol. Sci. 2019, 20, 5902. [Google Scholar] [CrossRef]
- Wang, W.; Kiyoshi, C.M.; Du, Y.; Taylor, A.T.; Sheehan, E.R.; Wu, X.; Zhou, M. TREK-1 Null Impairs Neuronal Excitability, Synaptic Plasticity, and Cognitive Function. Mol. Neurobiol. 2019, 57, 1332–1346. [Google Scholar] [CrossRef]
- Brohawn, S.G.; Wang, W.; Handler, A.; Campbell, E.B.; Schwarz, J.R.; MacKinnon, R. The mechanosensitive ion channel TRAAK is localized to the mammalian node of Ranvier. eLife 2019, 8, 8. [Google Scholar] [CrossRef]
- Enyeart, J.J.; Xu, L.; Danthi, S.; Enyeart, J.A. An ACTH- and ATP-regulated background K+ channel in adrenocortical cells is TREK-1. J. Biol. Chem. 2002, 277, 49186–49199. [Google Scholar] [CrossRef]
- Enyeart, J.J.; Enyeart, J.A. Human Adrenal Glomerulosa Cells Express K2P and GIRK Potassium Channels that are inhibited by AngII and ACTH. Am. J. Physiol. Cell Physiol. 2021. [Google Scholar] [CrossRef]
- Aacuternadóttir, J.; Chalfie, M. Eukaryotic Mechanosensitive Channels. Annu. Rev. Biophys. 2010, 39, 111–137. [Google Scholar] [CrossRef]
- Kozlov, M.M.; Chernomordik, L.V. Membrane tension and membrane fusion. Curr. Opin. Struct. Biol. 2015, 33, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, A.-L.; Quiroga, X.; Walani, N.; Arroyo, M.; Roca-Cusachs, P. The plasma membrane as a mechanochemical transducer. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180221. [Google Scholar] [CrossRef]
- Golani, G.; Ariotti, N.; Parton, R.G.; Kozlov, M.M. Membrane Curvature and Tension Control the Formation and Collapse of Caveolar Superstructures. Dev. Cell 2019, 48, 523–538.e4. [Google Scholar] [CrossRef]
- Sharif-Naeini, R.; Folgering, J.H.; Bichet, D.; Duprat, F.; Lauritzen, I.; Arhatte, M.; Jodar, M.; Dedman, A.; Chatelain, F.C.; Schulte, U.; et al. Polycystin-1 and -2 Dosage Regulates Pressure Sensing. Cell 2009, 139, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Brohawn, S.G.; Su, Z.; MacKinnon, R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+channels. Proc. Natl. Acad. Sci. USA 2014, 111, 3614–3619. [Google Scholar] [CrossRef] [PubMed]
- Honore, E.; Patel, A.J.; Chemin, J.; Suchyna, T.; Sachs, F. Desensitization of mechano-gated K2P channels. Proc. Natl. Acad. Sci. USA 2006, 103, 6859–6864. [Google Scholar] [CrossRef]
- Gullingsrud, J.; Schulten, K. Lipid Bilayer Pressure Profiles and Mechanosensitive Channel Gating. Biophys. J. 2004, 86, 3496–3509. [Google Scholar] [CrossRef]
- Martinac, B.; Bavi, N.; Ridone, P.; Nikolaev, Y.A.; Martinac, A.D.; Nakayama, Y.; Rohde, P.R.; Bavi, O. Tuning ion channel mechanosensitivity by asymmetry of the transbilayer pressure profile. Biophys. Rev. 2018, 10, 1377–1384. [Google Scholar] [CrossRef]
- del Marmol, J.; Rietmeijer, R.; Brohawn, S.G. Studying Mechanosensitivity of Two-Pore Domain K+ Channels in Cellular and Reconstituted Proteoliposome Membranes. Breast Cancer 2017, 1684, 129–150. [Google Scholar] [CrossRef]
- Brohawn, S.; Campbell, E.B.; MacKinnon, R. Physical mechanism for gating and mechanosensitivity of the human TRAAK K+ channel. Nat. Cell Biol. 2014, 516, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Kliesch, T.-T.; Dietz, J.; Turco, L.; Halder, P.; Polo, E.; Tarantola, M.; Jahn, R.; Janshoff, A. Membrane tension increases fusion efficiency of model membranes in the presence of SNAREs. Sci. Rep. 2017, 7, 12070. [Google Scholar] [CrossRef]
- Najem, J.S.; Dunlap, M.D.; Rowe, I.D.; Freeman, E.C.; Grant, J.W.; Sukharev, S.; Leo, D.J. Activation of bacterial channel MscL in mechanically stimulated droplet interface bilayers. Sci. Rep. 2015, 5, 13726. [Google Scholar] [CrossRef]
- Nakayama, Y.; Komazawa, K.; Bavi, N.; Hashimoto, K.-I.; Kawasaki, H.; Martinac, B. Evolutionary specialization of MscCG, an MscS-like mechanosensitive channel, in amino acid transport in Corynebacterium glutamicum. Sci. Rep. 2018, 8, 12893. [Google Scholar] [CrossRef]
- Syeda, R.; Florendo, M.N.; Cox, C.D.; Kefauver, J.M.; Santos, J.S.; Martinac, B.; Patapoutian, A. Piezo1 Channels Are Inherently Mechanosensitive. Cell Rep. 2016, 17, 1739–1746. [Google Scholar] [CrossRef]
- Lewis, A.H.; Grandl, J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. eLife 2015, 4, e12088. [Google Scholar] [CrossRef]
- Cox, C.D.; Bae, C.; Ziegler, L.; Hartley, S.; Nikolova-Krstevski, V.; Rohde, P.R.; Ng, C.A.; Sachs, F.; Gottlieb, P.; Martinac, B. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat. Commun. 2016, 7, 10366. [Google Scholar] [CrossRef] [PubMed]
- Aryal, P.; Jarerattanachat, V.; Clausen, M.V.; Schewe, M.; McClenaghan, C.; Argent, L.; Conrad, L.; Dong, Y.; Pike, A.C.; Carpenter, L.; et al. Bilayer-Mediated Structural Transitions Control Mechanosensitivity of the TREK-2 K2P Channel. Structure 2017, 25, 708–718.e2. [Google Scholar] [CrossRef] [PubMed]
- Anishkin, A.; Chiang, C.-S.; Sukharev, S. Gain-of-function Mutations Reveal Expanded Intermediate States and a Sequential Action of Two Gates in MscL. J. Gen. Physiol. 2005, 125, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.R.; MacKinnon, R. Structure-based membrane dome mechanism for Piezo mechanosensitivity. eLife 2017, 6, e33660. [Google Scholar] [CrossRef]
- Clausen, M.V.; Jarerattanachat, V.; Carpenter, L.; Sansom, M.S.P.; Tucker, S.J. Asymmetric mechanosensitivity in a eukaryotic ion channel. Proc. Natl. Acad. Sci. USA 2017, 114, E8343–E8351. [Google Scholar] [CrossRef] [PubMed]
- Fukasaku, M.; Kimura, J.; Yamaguchi, O. Swelling-activated and arachidonic acid-induced currents are TREK-1 in rat bladder smooth muscle cells. Fukushima J. Med. Sci. 2016, 62, 18–26. [Google Scholar] [CrossRef]
- Freed, J.K.; Gutterman, D.D. Communication Is Key: Mechanisms of Intercellular Signaling in Vasodilation. J. Cardiovasc. Pharmacol. 2017, 69, 264–272. [Google Scholar] [CrossRef]
- Carreon, T.A.; Castellanos, A.; Gasull, X.; Bhattacharya, S.K. Interaction of cochlin and mechanosensitive channel TREK-1 in trabecular meshwork cells influences the regulation of intraocular pressure. Sci. Rep. 2017, 7, 452. [Google Scholar] [CrossRef]
- Garry, A.; Fromy, B.; Blondeau, N.; Henrion, D.; Brau, F.; Gounon, P.; Guy, N.; Heurteaux, C.; Lazdunski, M.; Saumet, J.L. Altered acetylcholine, bradykinin and cutaneous pressure-induced vasodilation in mice lacking the TREK1 potassium channel: The endothelial link. EMBO Rep. 2007, 8, 354–359. [Google Scholar] [CrossRef]
- Kubanek, J.; Shi, J.; Marsh, J.; Chen, D.; Deng, C.; Cui, J. Ultrasound modulates ion channel currents. Sci. Rep. 2016, 6, 24170. [Google Scholar] [CrossRef] [PubMed]
- Sorum, B.; Rietmeijer, R.A.; Gopakumar, K.; Adesnik, H.; Brohawn, S.G. Ultrasound activates mechanosensitive TRAAK K+ channels through the lipid membrane. Proc. Natl. Acad. Sci. USA 2021, 118, 6. [Google Scholar] [CrossRef] [PubMed]
- Brohawn, S.G.; del Mármol, J.; MacKinnon, R. Crystal Structure of the Human K2P TRAAK, a Lipid- and Mechano-Sensitive K+ Ion Channel. Science 2012, 335, 436–441. [Google Scholar] [CrossRef]
- Lolicato, M.; Riegelhaupt, P.M.; Arrigoni, C.; Clark, K.A.; Minor, D.L. Transmembrane Helix Straightening and Buckling Underlies Activation of Mechanosensitive and Thermosensitive K2P Channels. Neuron 2014, 84, 1198–1212. [Google Scholar] [CrossRef] [PubMed]
- Brohawn, S.; Campbell, E.B.; MacKinnon, R. Domain-swapped chain connectivity and gated membrane access in a Fab-mediated crystal of the human TRAAK K+ channel. Proc. Natl. Acad. Sci. USA 2013, 110, 2129–2134. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, J.T.; de Groot, B.L. Mechanism of Mechanosensitive Gating of the TREK-2 Potassium Channel. Biophys. J. 2018, 114, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Harrigan, M.P.; McKiernan, K.A.; Shanmugasundaram, V.; Denny, R.A.; Pande, V.S. Markov modeling reveals novel intracellular modulation of the human TREK-2 selectivity filter. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Daday, C.; de Groot, B.L. Lipid-protein forces predict conformational changes in a mechanosensitive channel. Eur. Biophys. J. 2021, 50, 181–186. [Google Scholar] [CrossRef]
- Ma, Y.; Luo, Q.; Fu, J.; Che, Y.; Guo, F.; Mei, L.; Zhang, Q.; Li, Y.; Yang, H. Discovery of an Inhibitor for the TREK-1 Channel Targeting an Intermediate Transition State of Channel Gating. J. Med. Chem. 2020, 63, 10972–10983. [Google Scholar] [CrossRef] [PubMed]
- Ocello, R.; Furini, S.; Lugli, F.; Recanatini, M.; Domene, C.; Masetti, M. Conduction and Gating Properties of the TRAAK Channel from Molecular Dynamics Simulations with Different Force Fields. J. Chem. Inf. Model. 2020, 60, 6532–6543. [Google Scholar] [CrossRef] [PubMed]
- McClenaghan, C.; Schewe, M.; Aryal, P.; Carpenter, L.; Baukrowitz, T.; Tucker, S.J. Polymodal activation of the TREK-2 K2P channel produces structurally distinct open states. J. Gen. Physiol. 2016, 147, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Piechotta, P.L.; Rapedius, M.; Stansfeld, P.J.; Bollepalli, M.K.; Ehrlich, G.; Andres-Enguix, I.; Fritzenschaft, H.; Decher, N.; Sansom, M.S.; Tucker, S.J.; et al. The pore structure and gating mechanism of K2P channels. EMBO J. 2011, 30, 3607–3619. [Google Scholar] [CrossRef] [PubMed]
- Labro, A.J.; Snyders, D.J. Being Flexible: The Voltage-Controllable Activation Gate of Kv Channels. Front. Pharmacol. 2012, 3, 168. [Google Scholar] [CrossRef]
- Meng, X.-Y.; Liu, S.; Cui, M.; Zhou, R.; Logothetis, D.E. The Molecular Mechanism of Opening the Helix Bundle Crossing (HBC) Gate of a Kir Channel. Sci. Rep. 2016, 6, 29399. [Google Scholar] [CrossRef] [PubMed]
- Maeda, R.; Kindt, K.S.; Mo, W.; Morgan, C.P.; Erickson, T.; Zhao, H.; Clemens-Grisham, R.; Barr-Gillespie, P.; Nicolson, T. Tip-link protein protocadherin 15 interacts with transmembrane channel-like proteins TMC1 and TMC2. Proc. Natl. Acad. Sci. USA 2014, 111, 12907–12912. [Google Scholar] [CrossRef]
- Pan, B.; Akyuz, N.; Liu, X.-P.; Asai, Y.; Nist-Lund, C.; Kurima, K.; Derfler, B.H.; György, B.; Limapichat, W.; Walujkar, S.; et al. TMC1 Forms the Pore of Mechanosensory Transduction Channels in Vertebrate Inner Ear Hair Cells. Neuron 2018, 99, 736–753.e6. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhao, Y.; Kusakizako, T.; Wang, Y.; Pan, C.; Zhang, Y.; Nureki, O.; Hattori, M.; Yan, Z. TMC1 and TMC2 Proteins Are Pore-Forming Subunits of Mechanosensitive Ion Channels. Neuron 2020, 105, 310–321.e3. [Google Scholar] [CrossRef]
- Lauritzen, I.; Chemin, J.; Honore, E.; Jodar, M.; Guy, N.; Lazdunski, M.; Jane Patel, A. Cross-talk between the mechano-gated K2P channel TREK-1 and the actin cytoskeleton. EMBO Rep. 2005, 6, 642–648. [Google Scholar] [CrossRef]
- Sitarska, E.; Diz-Muñoz, A. Pay attention to membrane tension: Mechanobiology of the cell surface. Curr. Opin. Cell Biol. 2020, 66, 11–18. [Google Scholar] [CrossRef]
- Fraine, S.L.; Patel, A.; Duprat, F.; Sharif-Naeini, R. Dynamic regulation of TREK1 gating by Polycystin 2 via a Filamin A-mediated cytoskeletal Mechanism. Sci. Rep. 2017, 7, 17403. [Google Scholar] [CrossRef]
- Comoglio, Y.; Levitz, J.; Kienzler, M.A.; Lesage, F.; Isacoff, E.; Sandoz, G. Phospholipase D2 specifically regulates TREK potassium channels via direct interaction and local production of phosphatidic acid. Proc. Natl. Acad. Sci. USA 2014, 111, 13547–13552. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.N.; Pavel, M.A.; Wang, H.; Hansen, S.B. Disruption of palmitate-mediated localization; a shared pathway of force and anesthetic activation of TREK-1 channels. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183091. [Google Scholar] [CrossRef]
- Robinson, C.; Rohacs, T.; Hansen, S.B. Tools for Understanding Nanoscale Lipid Regulation of Ion Channels. Trends Biochem. Sci. 2019, 44, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Levitz, J.; Royal, P.; Comoglio, Y.; Wdziekonski, B.; Schaub, S.; Clemens, D.M.; Isacoff, E.; Sandoz, G. Heterodimerization within the TREK channel subfamily produces a diverse family of highly regulated potassium channels. Proc. Natl. Acad. Sci. USA 2016, 113, 4194–4199. [Google Scholar] [CrossRef]
- Blin, S.; Ben Soussia, I.; Kim, E.-J.; Brau, F.; Kang, D.; Lesage, F.; Bichet, D. Mixing and matching TREK/TRAAK subunits generate heterodimeric K2P channels with unique properties. Proc. Natl. Acad. Sci. USA 2016, 113, 4200–4205. [Google Scholar] [CrossRef] [PubMed]
- Miklós, L.; Czirják, G.; Enyedi, P. Formation of Functional Heterodimers by TREK-1 and TREK-2 Two-pore Domain Potassium Channel Subunits. J. Biol. Chem. 2016, 291, 13649–13661. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.D.; Monaghan, K.; Sergeant, G.P.; Ro, S.; Walker, R.L.; Sanders, K.M.; Horowitz, B. TREK-1 regulation by nitric oxide and cGMP-dependent protein kinase. An essential role in smooth muscle inhibitory neurotransmission. J. Biol. Chem. 2001, 276, 44338–44346. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; O’Kane, N.; Singer, C.; Ward, S.M.; Sanders, K.M.; Koh, S.D. Block of inhibitory junction potentials and TREK-1 channels in murine colon by Ca2+store-active drugs. J. Physiol. 2008, 586, 1169–1184. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.-Q.; Yu, Y.; Mo, L.-H.; Ge, R.-T.; Zhang, H.-P.; Liu, Z.-G.; Zheng, P.; Yang, P.-C. Regulation of TWIK-related potassium channel-1 (Trek1) restitutes intestinal epithelial barrier function. Cell. Mol. Immunol. 2015, 13, 110–118. [Google Scholar] [CrossRef]
- Ma, R.; Seifi, M.; Papanikolaou, M.; Brown, J.F.; Swinny, J.D.; Lewis, A. TREK-1 Channel Expression in Smooth Muscle as a Target for Regulating Murine Intestinal Contractility: Therapeutic Implications for Motility Disorders. Front. Physiol. 2018, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, A.-M.; Nakamura, H.; Parekh, B.; Puri, P. Decreased expression of TRAAK channels in Hirschsprung’s disease: A possible cause of postoperative dysmotility. Pediatr. Surg. Int. 2019, 35, 1431–1435. [Google Scholar] [CrossRef]
- Tomuschat, C.; O’Donnell, A.M.; Coyle, D.; Dreher, N.; Kelly, D.; Puri, P.; Puri, P.L. Altered expression of a two-pore domain (K2P) mechano-gated potassium channel TREK-1 in Hirschsprung’s disease. Pediatr. Res. 2016, 80, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Koh, S.D. Two-pore-domain potassium channels in smooth muscles: New components of myogenic regulation. J. Physiol. 2005, 570, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Pan, X.-Q.; Chang, S.; Malkowicz, S.B.; Guzzo, T.J.; Malykhina, A.P. Response of the human detrusor to stretch is regulated by TREK-1, a two-pore-domain (K2P) mechano-gated potassium channel. J. Physiol. 2014, 592, 3013–3030. [Google Scholar] [CrossRef]
- Pineda, R.H.; Nedumaran, B.; Hypolite, J.; Pan, X.-Q.; Wilson, S.; Meacham, R.B.; Malykhina, A.P. Altered expression and modulation of the two-pore-domain (K2P) mechanogated potassium channel TREK-1 in overactive human detrusor. Am. J. Physiol. Physiol. 2017, 313, F535–F546. [Google Scholar] [CrossRef] [PubMed]
- Tertyshnikova, S.; Knox, R.J.; Plym, M.J.; Thalody, G.; Griffin, C.; Neelands, T.; Harden, D.G.; Signor, L.; Weaver, D.; Myers, R.A.; et al. BL-1249 [(5,6,7,8-Tetrahydro-naphthalen-1-yl)-[2-(1H-tetrazol-5-yl)-phenyl]-amine]: A Putative Potassium Channel Opener with Bladder-Relaxant Properties. J. Pharmacol. Exp. Ther. 2004, 313, 250–259. [Google Scholar] [CrossRef]
- Pineda, R.H.; Hypolite, J.; Lee, S.; Carrasco, A.; Iguchi, N.; Meacham, R.B.; Malykhina, A.P. Altered detrusor contractility and voiding patterns in mice lacking the mechanosensitive TREK-1 channel. BMC Urol. 2019, 19, 40. [Google Scholar] [CrossRef]
- Baker, S.A.; Hatton, W.J.; Han, J.; Hennig, G.W.; Britton, F.C.; Koh, S.D. Role of TREK-1 Potassium Channel in Bladder Overactivity after Partial Bladder Outlet Obstruction in Mouse. J. Urol. 2010, 183, 793–800. [Google Scholar] [CrossRef]
- Nedumaran, B.; Pineda, R.H.; Rudra, P.; Lee, S.; Malykhina, A.P. Association of genetic polymorphisms in the pore domains of mechano-gated TREK-1 channel with overactive lower urinary tract symptoms in humans. Neurourol. Urodyn. 2019, 38, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Buxton, I.L.O.; Singer, C.A.; Tichenor, J.N. Expression of Stretch-Activated Two-Pore Potassium Channels in Human Myometrium in Pregnancy and Labor. PLoS ONE 2010, 5, e12372. [Google Scholar] [CrossRef]
- Heyman, N.S.; Cowles, C.L.; Barnett, S.D.; Wu, Y.Y.; Cullison, C.; Singer, C.A.; Leblanc, N.; Buxton, I.L. TREK-1 currents in smooth muscle cells from pregnant human myometrium. Am. J. Physiol. Cell Physiol. 2013, 305, C632–C642. [Google Scholar] [CrossRef][Green Version]
- Monaghan, K.; Baker, S.A.; Dwyer, L.; Hatton, W.C.; Park, K.S.; Sanders, K.M.; Koh, S.D. The stretch-dependent potassium channel TREK-1 and its function in murine myometrium. J. Physiol. 2011, 589, 1221–1233. [Google Scholar] [CrossRef]
- Yin, Z.; He, W.; Li, Y.; Li, D.; Li, H.; Yang, Y.; Wei, Z.; Shen, B.; Wang, X.; Cao, Y.; et al. Adaptive reduction of human myometrium contractile activity in response to prolonged uterine stretch during term and twin pregnancy. Role of TREK-1 channel. Biochem. Pharmacol. 2018, 152, 252–263. [Google Scholar] [CrossRef]
- Yin, Z.; Li, Y.; He, W.; Li, D.; Li, H.; Yang, Y.; Shen, B.; Wang, X.; Cao, Y.; Khalil, R.A. Progesterone inhibits contraction and increases TREK-1 potassium channel expression in late pregnant rat uterus. Oncotarget 2017, 9, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.-Z.; Marshall, K.L.; Min, S.; Daou, I.; Chapleau, M.W.; Abboud, F.M.; Liberles, S.D.; Patapoutian, A. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 2018, 362, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.M.; Lee, T.E.; Watson, L.J.; Mao, L.; Chandok, G.S.; Wang, H.-G.; Frangakis, S.; Pitt, G.S.; Shah, S.H.; Wolf, M.J.; et al. The two-pore domain potassium channel TREK-1 mediates cardiac fibrosis and diastolic dysfunction. J. Clin. Investig. 2018, 128, 4843–4855. [Google Scholar] [CrossRef] [PubMed]
- Wiedmann, F.; Rinné, S.; Donner, B.; Decher, N.; Katus, H.A.; Schmidt, C. Mechanosensitive TREK-1 two-pore-domain potassium (K2P) channels in the cardiovascular system. Prog. Biophys. Mol. Biol. 2021, 159, 126–135. [Google Scholar] [CrossRef]
- Stewart, L.; Turner, N. Channelling the Force to Reprogram the Matrix: Mechanosensitive Ion Channels in Cardiac Fibroblasts. Cells 2021, 10, 990. [Google Scholar] [CrossRef]
- Herrera-Pérez, S.; Campos-Ríos, A.; Rueda-Ruzafa, L.; Lamas, J. Contribution of K2P Potassium Channels to Cardiac Physiology and Pathophysiology. Int. J. Mol. Sci. 2021, 22, 6635. [Google Scholar] [CrossRef]
- Tan, J.H.C.; Liu, W.; Saint, D.A. Differential expression of the mechanosensitive potassium channelTREK-1in epicardial and endocardial myocytes in rat ventricle. Exp. Physiol. 2004, 89, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.; MacKenzie, L.; Hunter, P.; Smaill, B.; Saint, D. Gene expression of stretch-activated channels and mechanoelectric feedback in the heart. Clin. Exp. Pharmacol. Physiol. 2006, 33, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Stones, R.; Calaghan, S.; Billeter, R.; Harrison, S.M.; White, E. Transmural variations in gene expression of stretch-modulated proteins in the rat left ventricle. Pflügers Arch. Eur. J. Physiol. 2007, 454, 545–549. [Google Scholar] [CrossRef][Green Version]
- Wang, W.; Zhang, M.; Li, P.; Yuan, H.; Feng, N.; Peng, Y.; Wang, L.; Wang, X. An Increased TREK-1–like Potassium Current in Ventricular Myocytes during Rat Cardiac Hypertrophy. J. Cardiovasc. Pharmacol. 2013, 61, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Terrenoire, C.; Lauritzen, I.; Lesage, F.; Romey, G.; Lazdunski, M. A TREK-1-like potassium channel in atrial cells inhibited by beta-adrenergic stimulation and activated by volatile anesthetics. Circ. Res. 2001, 89, 336–342. [Google Scholar] [CrossRef]
- Kim, D. A mechanosensitive K+ channel in heart cells. Activation by arachidonic acid. J. Gen. Physiol. 1992, 100, 1021–1040. [Google Scholar] [CrossRef]
- Tan, J.; Liu, W.; Saint, D. Trek-like Potassium Channels in Rat Cardiac Ventricular Myocytes Are Activated by Intracellular ATP. J. Membr. Biol. 2002, 185, 201–207. [Google Scholar] [CrossRef]
- Quinn, T.A.; Kohl, P. Cardiac Mechano-Electric Coupling: Acute Effects of Mechanical Stimulation on Heart Rate and Rhythm. Physiol. Rev. 2021, 101, 37–92. [Google Scholar] [CrossRef] [PubMed]
- Decher, N.; Kiper, A.K.; Rinné, S. Stretch-activated potassium currents in the heart: Focus on TREK-1 and arrhythmias. Prog. Biophys. Mol. Biol. 2017, 130, 223–232. [Google Scholar] [CrossRef]
- Al-Shammari, H.; Latif, N.; Sarathchandra, P.; McCormack, A.; Rog-Zielinska, E.A.; Raja, S.; Kohl, P.; Yacoub, M.H.; Peyronnet, R.; Chester, A.H. Expression and function of mechanosensitive ion channels in human valve interstitial cells. PLoS ONE 2020, 15, 0240532. [Google Scholar] [CrossRef] [PubMed]
- Unudurthi, S.D.; Wu, X.; Qian, L.; Amari, F.; Onal, B.; Li, N.; Makara, M.A.; Smith, S.A.; Snyder, J.; Fedorov, V.V.; et al. Two-Pore K+ Channel TREK-1 Regulates Sinoatrial Node Membrane Excitability. J. Am. Heart Assoc. 2016, 5, e002865. [Google Scholar] [CrossRef]
- Froese, A.; Breher, S.S.; Waldeyer, C.; Schindler, R.F.; Nikolaev, V.O.; Rinné, S.; Wischmeyer, E.; Schlueter, J.; Becher, J.; Simrick, S.; et al. Popeye domain containing proteins are essential for stress-mediated modulation of cardiac pacemaking in mice. J. Clin. Investig. 2012, 122, 1119–1130. [Google Scholar] [CrossRef]
- Hund, T.J.; Snyder, J.S.; Wu, X.; Glynn, P.; Koval, O.M.; Onal, B.; Leymaster, N.D.; Unudurthi, S.D.; Curran, J.; Camardo, C.; et al. beta(IV)-Spectrin regulates TREK-1 membrane targeting in the heart. Cardiovasc. Res. 2014, 102, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Schindler, R.F.; Scotton, C.; Zhang, J.; Passarelli, C.; Ortiz-Bonnin, B.; Simrick, S.; Schwerte, T.; Poon, K.-L.; Fang, M.; Rinné, S.; et al. POPDC1S201F causes muscular dystrophy and arrhythmia by affecting protein trafficking. J. Clin. Investig. 2015, 126, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Rinné, S.; Ortiz-Bonnin, B.; Stallmeyer, B.; Kiper, A.K.; Fortmüller, L.; Schindler, R.F.; Herbort-Brand, U.; Kabir, N.S.; Dittmann, S.; Friedrich, C.; et al. POPDC2 a novel susceptibility gene for conduction disorders. J. Mol. Cell. Cardiol. 2020, 145, 74–83. [Google Scholar] [CrossRef]
- Macdonald, E.A.; Madl, J.; Greiner, J.; Ramadan, A.F.; Wells, S.M.; Torrente, A.G.; Kohl, P.; Rog-Zielinska, E.A.; Quinn, T.A. Sinoatrial Node Structure, Mechanics, Electrophysiology and the Chronotropic Response to Stretch in Rabbit and Mouse. Front. Physiol. 2020, 11, 809. [Google Scholar] [CrossRef]
- Schmidt, C.; Wiedmann, F.; Kallenberger, S.M.; Ratte, A.; Schulte, J.S.; Scholz, B.; Müller, F.U.; Voigt, N.; Zafeiriou, M.P.; Ehrlich, J.R.; et al. Stretch-activated two-pore-domain (K2P) potassium channels in the heart: Focus on atrial fibrillation and heart failure. Prog. Biophys. Mol. Biol. 2017, 130, 233–243. [Google Scholar] [CrossRef]
- Schmidt, C.; Wiedmann, F.; Tristram, F.; Anand, P.; Wenzel, W.; Lugenbiel, P.; Schweizer, P.A.; Katus, H.A.; Thomas, D. Cardiac expression and atrial fibrillation-associated remodeling of K2P2.1 (TREK-1) K+ channels in a porcine model. Life Sci. 2014, 97, 107–115. [Google Scholar] [CrossRef]
- Lugenbiel, P.; Wenz, F.; Govorov, K.; Syren, P.; Katus, H.A.; Thomas, D. Atrial myofibroblast activation and connective tissue formation in a porcine model of atrial fibrillation and reduced left ventricular function. Life Sci. 2017, 181, 1–8. [Google Scholar] [CrossRef]
- Lugenbiel, P.; Wenz, F.; Syren, P.; Geschwill, P.; Govorov, K.; Seyler, C.; Frank, D.; Schweizer, P.A.; Franke, J.; Weis, T.; et al. TREK-1 (K2P2.1) K+ channels are suppressed in patients with atrial fibrillation and heart failure and provide therapeutic targets for rhythm control. Basic Res. Cardiol. 2017, 112, 8. [Google Scholar] [CrossRef] [PubMed]
- Decher, N.; Ortiz-Bonnin, B.; Friedrich, C.; Schewe, M.; Kiper, A.K.; Rinne, S.; Seemann, G.; Peyronnet, R.; Zumhagen, S.; Bustos, D.; et al. Sodium permeable and "hypersensitive" TREK-1 channels cause ventricular tachycardia. EMBO Mol. Med. 2017, 9, 403–414. [Google Scholar] [CrossRef]
- Wiedmann, F.; Schulte, J.S.; Gomes, B.; Zafeiriou, M.P.; Ratte, A.; Rathjens, F.; Fehrmann, E.; Scholz, B.; Voigt, N.; Müller, F.U.; et al. Atrial fibrillation and heart failure-associated remodeling of two-pore-domain potassium (K2P) channels in murine disease models: Focus on TASK-1. Basic Res. Cardiol. 2018, 113, 27. [Google Scholar] [CrossRef] [PubMed]
- Kamatham, S.; Waters, C.M.; Schwingshackl, A.; Mancarella, S. TREK-1 protects the heart against ischemia-reperfusion-induced injury and from adverse remodeling after myocardial infarction. Pflügers Arch. Eur. J. Physiol. 2019, 471, 1263–1272. [Google Scholar] [CrossRef]
- Gardener, M.J.; Johnson, I.T.; Burnham, M.; Edwards, G.; Heagerty, A.; Weston, A.H. Functional evidence of a role for two-pore domain potassium channels in rat mesenteric and pulmonary arteries. Br. J. Pharmacol. 2004, 142, 192–202. [Google Scholar] [CrossRef]
- Bryan, R.M.; You, J.; Phillips, S.C.; Andresen, J.J.; Lloyd, E.E.; Rogers, P.A.; Dryer, S.E.; Marrelli, S.P. Evidence for two-pore domain potassium channels in rat cerebral arteries. Am. J. Physiol. Circ. Physiol. 2006, 291, H770–H780. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, N.; Pétrault, O.; Manta, S.; Giordanengo, V.; Gounon, P.; Bordet, R.; Lazdunski, M.; Heurteaux, C. Polyunsaturated Fatty Acids Are Cerebral Vasodilators via the TREK-1 Potassium Channel. Circ. Res. 2007, 101, 176–184. [Google Scholar] [CrossRef]
- Namiranian, K.; Lloyd, E.E.; Crossland, R.F.; Marrelli, S.P.; Taffet, G.E.; Reddy, A.K.; Hartley, C.J.; Bryan, R.M. Cerebrovascular responses in mice deficient in the potassium channel, TREK-1. Am. J. Physiol. Integr. Comp. Physiol. 2010, 299, R461–R469. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, G.; Wandall-Frostholm, C.; Sadda, V.; Olivan-Viguera, A.; Lloyd, E.E.; Bryan, R.M., Jr.; Simonsen, U.; Kohler, R. Alterations of N-3 polyunsaturated fatty acid-activated K2P channels in hypoxia-induced pulmonary hypertension. Basic Clin. Pharmacol. Toxicol. 2013, 113, 250–258. [Google Scholar] [CrossRef]
- Kitagawa, M.G.; Reynolds, J.O.; Wehrens, X.H.T.; Bryan, R.M., Jr.; Pandit, L.M. Hemodynamic and Pathologic Characterization of the TASK-1(-/-) Mouse Does Not Demonstrate Pulmonary Hypertension. Front. Med. 2017, 4, 177. [Google Scholar] [CrossRef]
- Lambert, M.; Boet, A.; Rucker-Martin, C.; Ferreira, P.; Capuano, V.; Hatem, S.; Adão, R.; Silva, C.B.; Hautefort, A.; Michel, J.-B.; et al. Loss of KCNK3 is a hallmark of RV hypertrophy/dysfunction associated with pulmonary hypertension. Cardiovasc. Res. 2018, 114, 880–893. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.Y.; Pipkin, F.B.; Khan, R.N. The Effect of pH and Ion Channel Modulators on Human Placental Arteries. PLoS ONE 2014, 9, e114405. [Google Scholar] [CrossRef]
- Manteniotis, S.; Lehmann, R.; Flegel, C.; Vogel, F.; Hofreuter, A.; Schreiner, B.S.P.; Altmüller, J.; Becker, C.; Schöbel, N.; Hatt, H.; et al. Comprehensive RNA-Seq Expression Analysis of Sensory Ganglia with a Focus on Ion Channels and GPCRs in Trigeminal Ganglia. PLoS ONE 2013, 8, e79523. [Google Scholar] [CrossRef]
- Kang, D.; Kim, D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am. J. Physiol. Physiol. 2006, 291, C138–C146. [Google Scholar] [CrossRef] [PubMed]
- Alloui, A.; Zimmermann, K.; Mamet, J.; Duprat, F.; Noel, J.; Chemin, J.; Guy, N.; Blondeau, N.; Voilley, N.; Rubat-Coudert, C.; et al. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 2006, 25, 2368–2376. [Google Scholar] [CrossRef]
- Li, C.L.; Li, K.C.; Wu, D.; Chen, Y.; Luo, H.; Zhao, J.R.; Wang, S.S.; Sun, M.M.; Lu, Y.J.; Zhong, Y.Q.; et al. Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res. 2016, 26, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Usoskin, D.; Furlan, A.; Islam, S.; Abdo, H.; Lönnerberg, P.; Lou, D.; Hjerling-Leffler, J.; Haeggstrom, J.Z.; Kharchenko, O.; Kharchenko, P.V.; et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 2015, 18, 145–153. [Google Scholar] [CrossRef]
- Pereira, V.; Busserolles, J.; Christin, M.; Devilliers, M.; Poupon, L.; Legha, W.; Alloui, A.; Aisouni, Y.; Bourinet, E.; Lesage, F.; et al. Role of the TREK2 potassium channel in cold and warm thermosensation and in pain perception. Pain 2014, 155, 2534–2544. [Google Scholar] [CrossRef]
- Noël, J.; Zimmermann, K.; Busserolles, J.; Deval, E.; Alloui, A.; Diochot, S.; Guy, N.; Borsotto, M.; Reeh, P.; Eschalier, A.; et al. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009, 28, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Royal, P.; Andres-Bilbe, A.; Prado, P.Á.; Verkest, C.; Wdziekonski, B.; Schaub, S.; Baron, A.; Lesage, F.; Gasull, X.; Levitz, J.; et al. Migraine-Associated TRESK Mutations Increase Neuronal Excitability through Alternative Translation Initiation and Inhibition of TREK. Neuron 2019, 101, 232–245.e6. [Google Scholar] [CrossRef] [PubMed]
- Acosta, C.; Djouhri, L.; Watkins, R.; Berry, C.; Bromage, K.; Lawson, S.N. TREK2 Expressed Selectively in IB4-Binding C-Fiber Nociceptors Hyperpolarizes Their Membrane Potentials and Limits Spontaneous Pain. J. Neurosci. 2014, 34, 1494–1509. [Google Scholar] [CrossRef]
- Loucif, A.J.C.; Saintot, P.; Liu, J.; Antonio, B.M.; Zellmer, S.G.; Yoger, K.; Veale, E.L.; Wilbrey, A.; Omoto, K.; Cao, L.; et al. GI-530159, a novel, selective, mechanosensitive two-pore-domain potassium (K2P) channel opener, reduces rat dorsal root ganglion neuron excitability. Br. J. Pharmacol. 2017, 175, 2272–2283. [Google Scholar] [CrossRef]
- Dadi, P.K.; Vierra, N.C.; Days, E.; Dickerson, M.T.; Vinson, P.N.; Weaver, C.D.; Jacobson, D.A. Selective Small Molecule Activators of TREK-2 Channels Stimulate Dorsal Root Ganglion c-Fiber Nociceptor Two-Pore-Domain Potassium Channel Currents and Limit Calcium Influx. ACS Chem. Neurosci. 2017, 8, 558–568. [Google Scholar] [CrossRef]
- Aller, M.; Wisden, W. Changes in expression of some two-pore domain potassium channel genes (KCNK) in selected brain regions of developing mice. Neuroscience 2008, 151, 1154–1172. [Google Scholar] [CrossRef] [PubMed]
- Medhurst, A.D.; Rennie, G.; Chapman, C.G.; Meadows, H.; Duckworth, M.D.; Kelsell, R.E.; Gloger, I.I.; Pangalos, M.N. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Mol. Brain Res. 2001, 86, 101–114. [Google Scholar] [CrossRef]
- Heurteaux, C.; Guy, N.; Laigle, C.; Blondeau, N.; Duprat, F.; Mazzuca, M.; Lang-Lazdunski, L.; Widmann, C.; Zanzouri, M.; Romey, G.; et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004, 23, 2684–2695. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.K.; Calligari, P.; Radio, F.C.; Caputo, V.; Dentici, M.L.; Falah, N.; High, F.; Pantaleoni, F.; Barresi, S.; Ciolfi, A.; et al. Mutations in KCNK4 that Affect Gating Cause a Recognizable Neurodevelopmental Syndrome. Am. J. Hum. Genet. 2018, 103, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.J.; Honore, E.; Lesage, F.; Fink, M.; Romey, G.; Lazdunski, M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat. Neurosci. 1999, 2, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Su, B.; Zhang, H.; Song, W.; Wu, H.; Chen, X.; Zhang, X.; Dong, H.; Xiong, L. TREK1 activation mediates spinal cord ischemic tolerance induced by isoflurane preconditioning in rats. Neurosci. Lett. 2012, 515, 115–120. [Google Scholar] [CrossRef]
- Wang, K.; Kong, X. Isoflurane Preconditioning Induces Neuroprotection by Up-Regulation of TREK1 in a Rat Model of Spinal Cord Ischemic Injury. Biomol. Ther. 2016, 24, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Yang, F.; Lu, C.; Jia, C.; Wang, Q.; Zeng, K. Effects of sevoflurane on rats with ischemic brain injury and the role of the TREK-1 channel. Exp. Ther. Med. 2017, 14, 2937–2942. [Google Scholar] [CrossRef]
- Tong, L.; Cai, M.; Huang, Y.; Zhang, H.; Su, B.; Li, Z.; Dong, H. Activation of K 2 P channel–TREK1 mediates the neuroprotection induced by sevoflurane preconditioning. Br. J. Anaesth. 2014, 113, 157–167. [Google Scholar] [CrossRef]
- Davis, K.A.; Cowley, E.A. Two-pore-domain potassium channels support anion secretion from human airway Calu-3 epithelial cells. Pflügers Arch. Eur. J. Physiol. 2005, 451, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Canella, R.; Martini, M.; Cavicchio, C.; Cervellati, F.; Benedusi, M.; Valacchi, G. Involvement of the TREK-1 channel in human alveolar cell membrane potential and its regulation by inhibitors of the chloride current. J. Cell. Physiol. 2019, 234, 17704–17713. [Google Scholar] [CrossRef]
- Zyrianova, T.; Lopez, B.; Olcese, R.; Belperio, J.; Waters, C.M.; Wong, L.; Nguyen, V.; Talapaneni, S.; Schwingshackl, A. K2P2.1 (TREK-1) potassium channel activation protects against hyperoxia-induced lung injury. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, A.; Lopez, B.; Teng, B.; Luellen, C.; Lesage, F.; Belperio, J.; Olcese, R.; Waters, C.M. Hyperoxia treatment of TREK-1/TREK-2/TRAAK-deficient mice is associated with a reduction in surfactant proteins. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L1030–L1046. [Google Scholar] [CrossRef]
- Schwingshackl, A.; Teng, B.; Ghosh, M.; Lim, K.G.; Tigyi, G.; Narayanan, D.; Jaggar, J.H.; Waters, C.M. Regulation of interleukin-6 secretion by the two-pore-domain potassium channel Trek-1 in alveolar epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2013, 304, L276–L286. [Google Scholar] [CrossRef]
- Schwingshackl, A.; Teng, B.; Ghosh, M.; Waters, C.M. Regulation of Monocyte Chemotactic Protein-1 secretion by the Two-Pore-Domain Potassium (K2P) channel TREK-1 in human alveolar epithelial cells. Am. J. Transl. Res. 2013, 5, 530–542. [Google Scholar] [PubMed]
- Roan, E.; Waters, C.M.; Teng, B.; Ghosh, M.; Schwingshackl, A. The 2-Pore Domain Potassium Channel TREK-1 Regulates Stretch-Induced Detachment of Alveolar Epithelial Cells. PLoS ONE 2014, 9, e89429. [Google Scholar] [CrossRef]
- Goel, M.; Sienkiewicz, A.E.; Picciani, R.; Lee, R.K.; Bhattacharya, S.K. Cochlin Induced TREK-1 Co-Expression and Annexin A2 Secretion: Role in Trabecular Meshwork Cell Elongation and Motility. PLoS ONE 2011, 6, e23070. [Google Scholar] [CrossRef]
- Yarishkin, O.; Phuong, T.T.; Bretz, C.A.; Olsen, K.W.; Baumann, J.M.; Lakk, M.; Crandall, A.; Heurteaux, C.; Hartnett, M.E.; Križaj, D. TREK-1 channels regulate pressure sensitivity and calcium signaling in trabecular meshwork cells. J. Gen. Physiol. 2018, 150, 1660–1675. [Google Scholar] [CrossRef] [PubMed]
- Yarishkin, O.; Phuong, T.T.T.; Križaj, D. Trabecular Meshwork TREK-1 Channels Function as Polymodal Integrators of Pressure and pH. Investig. Opthalmol. Vis. Sci. 2019, 60, 2294–2303. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lengyel, M.; Enyedi, P.; Czirják, G. Negative Influence by the Force: Mechanically Induced Hyperpolarization via K2P Background Potassium Channels. Int. J. Mol. Sci. 2021, 22, 9062. https://doi.org/10.3390/ijms22169062
Lengyel M, Enyedi P, Czirják G. Negative Influence by the Force: Mechanically Induced Hyperpolarization via K2P Background Potassium Channels. International Journal of Molecular Sciences. 2021; 22(16):9062. https://doi.org/10.3390/ijms22169062
Chicago/Turabian StyleLengyel, Miklós, Péter Enyedi, and Gábor Czirják. 2021. "Negative Influence by the Force: Mechanically Induced Hyperpolarization via K2P Background Potassium Channels" International Journal of Molecular Sciences 22, no. 16: 9062. https://doi.org/10.3390/ijms22169062
APA StyleLengyel, M., Enyedi, P., & Czirják, G. (2021). Negative Influence by the Force: Mechanically Induced Hyperpolarization via K2P Background Potassium Channels. International Journal of Molecular Sciences, 22(16), 9062. https://doi.org/10.3390/ijms22169062





