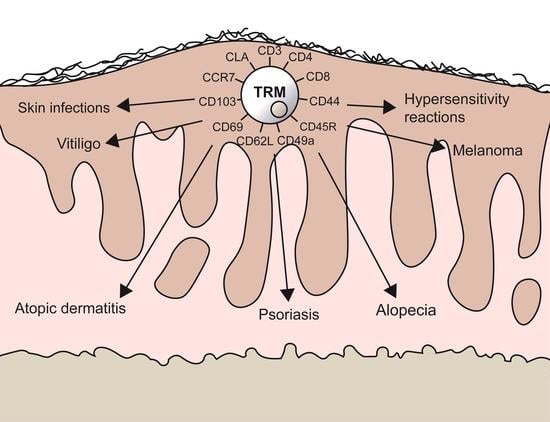
Tissue-Resident Memory T Cells in Skin Diseases: A Systematic Review
Abstract
:1. Introduction
1.1. Generation and Definition of TRMs
1.2. TRMs in Skin Diseases
2. Materials and Methods
2.1. Protocol and Registration:
2.2. Search Strategy
2.3. Study Inclusion Criteria
2.4. Study Exclusion Criteria
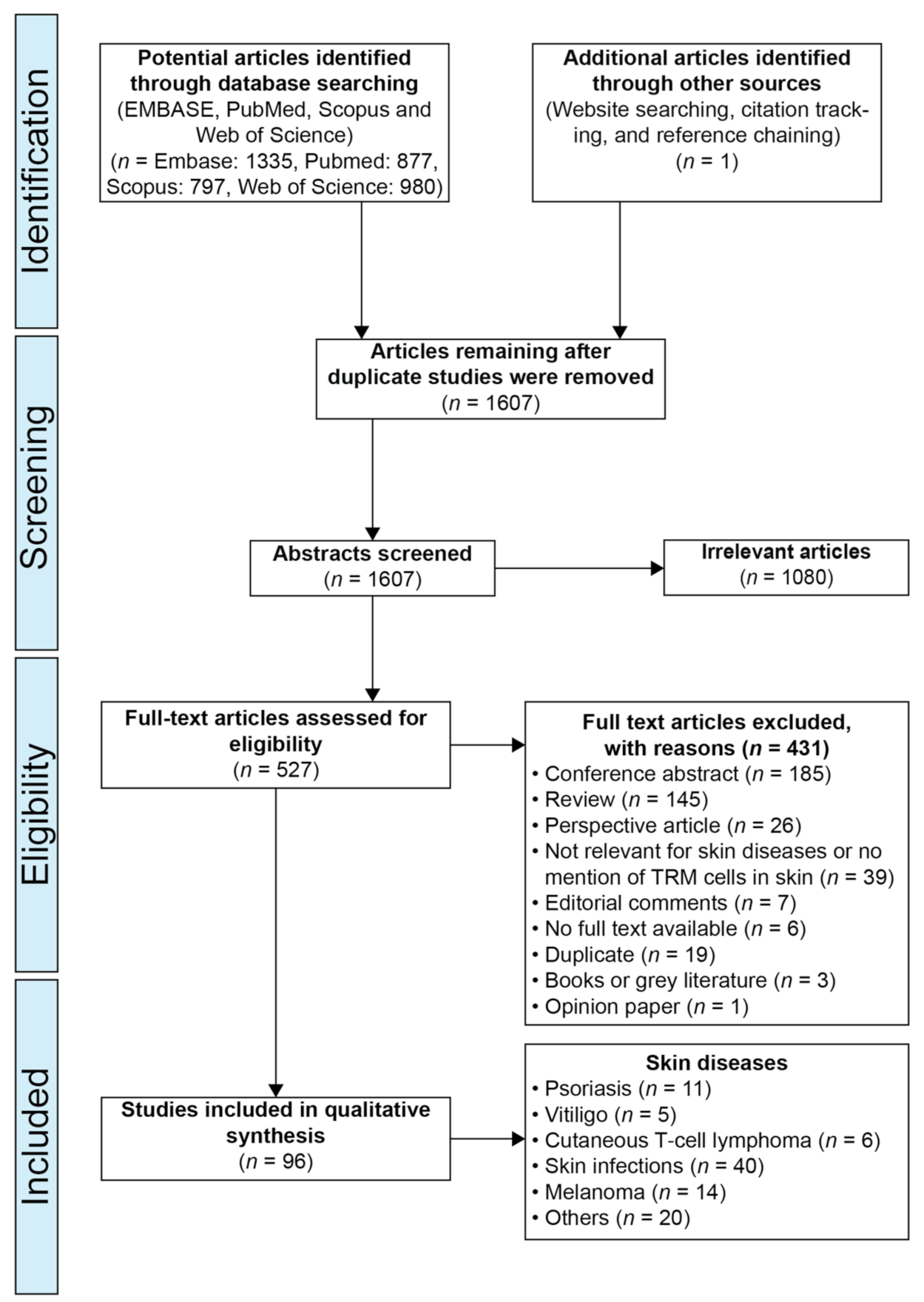
2.5. Study Selection
3. Results
3.1. TRMs and Skin Infections
3.2. Psoriasis
3.3. Vitiligo
3.4. Melanoma
3.5. Cutaneous T-Cell Lymphoma
3.6. Others
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Laidlaw, B.J.; Craft, J.E.; Kaech, S.M. The multifaceted role of CD4+ T cells in CD8+ T cell memory. Nat. Rev. Immunol. 2016, 16, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Purwar, R.; Campbell, J.; Murphy, G.; Richards, W.G.; Clark, R.A.; Kupper, T.S. Resident Memory T Cells (TRM) Are Abundant in Human Lung: Diversity, Function, and Antigen Specificity. PLoS ONE 2011, 6, e16245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackay, L.; Rahimpour, A.; Ma, J.; Collins, N.C.; Stock, A.T.; Hafon, M.-L.; Vega-Ramos, J.; Lauzurica, P.; Mueller, S.; Stefanovic, T.; et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 2013, 14, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Guggino, G.; Rizzo, A.; Mauro, D.; Macaluso, F.; Ciccia, F. Gut-derived CD8+ tissue-resident memory T cells are expanded in the peripheral blood and synovia of SpA patients. Ann. Rheum. Dis. 2019, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Yao, Y.; Li, L.; Yang, S.; Chu, H.; Tsuneyama, K.; Li, X.; Gershwin, M.E.; Lian, Z. Tissue-Resident Memory CD 8+ T Cells Acting as Mediators of Salivary Gland Damage in a Murine Model of Sjögren’s Syndrome. Arthritis Rheumatol. 2019, 71, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Smolders, J.; Heutinck, K.M.; Fransen, N.L.; Remmerswaal, E.B.M.; Hombrink, P.; Berge, I.J.M.T.; Van Lier, R.A.W.; Huitinga, I.; Hamann, J. Tissue-resident memory T cells populate the human brain. Nat. Commun. 2018, 9, 4593. [Google Scholar] [CrossRef] [Green Version]
- Hedin, C.R.H.; Vavricka, S.R.; Stagg, A.J.; Schoepfer, A.; Raine, T.; Puig, L.; Pleyer, U.; Navarini, A.; van der Meulen-de Jong, A.E.; Maul, J.; et al. Gene and Mirna Regulatory Networks During Different Stages of Crohn’s Disease. J. Crohn’s Colitis. 2019, 13, 541–554. [Google Scholar] [CrossRef]
- Okhrimenko, A.; Grün, J.R.; Westendorf, K.; Fang, Z.; Reinke, S.; von Roth, P.; Wassilew, G.; Kühl, A.A.; Kudernatsch, R.; Demski, S.; et al. Human memory T cells from the bone marrow are resting and maintain long-lasting systemic memory. Proc. Natl. Acad. Sci. USA 2014, 111, 9229–9234. [Google Scholar] [CrossRef] [Green Version]
- Pallett, L.J.; Davies, J.; Colbeck, E.J.; Robertson, F.; Hansi, N.; Easom, N.J.W.; Burton, A.R.; Stegmann, K.A.; Schurich, A.; Swadling, L.; et al. IL-2high tissue-resident T cells in the human liver: Sentinels for hepatotropic infec-tion. J. Exp. Med. 2017, 214, 1567–1580. [Google Scholar] [CrossRef]
- Enamorado, M.; Khouili, S.C.; Iborra, S.; Sancho, D. Genealogy, Dendritic Cell Priming, and Differentiation of Tissue-Resident Memory CD8+ T Cells. Front. Immunol. 2018, 9, 1751. [Google Scholar] [CrossRef] [Green Version]
- Masopust, D.; Vezys, V.; Marzo, A.L.; Lefrançois, L. Preferential Localization of Effector Memory Cells in Nonlymphoid Tissue. Science 2001, 291, 2413–2417. [Google Scholar] [CrossRef] [Green Version]
- Schenkel, J.; Masopust, D. Tissue-Resident Memory T Cells. Immunity 2014, 41, 886–897. [Google Scholar] [CrossRef] [Green Version]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central Memory and Effector Memory T Cell Subsets: Function, Generation, and Maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef]
- Herndler-Brandstetter, D.; Ishigame, H.; Shinnakasu, R.; Plajer, V.; Stecher, C.; Zhao, J.; Lietzenmayer, M.; Kroehling, L.; Takumi, A.; Kometani, K.; et al. KLRG1+ Effector CD8+ T Cells Lose KLRG1, Differentiate into All Memory T Cell Lineages, and Convey Enhanced Protective Immunity. Immunity 2018, 48, 716–729.e8. [Google Scholar] [CrossRef] [Green Version]
- Kane, C.J.; Knapp, A.M.; Mansbridge, J.N.; Hanawalt, P.C. Transforming growth factor-beta 1 localization in normal and psoriatic epidermal keratinocytes in situ. J. Cell. Physiol. 1990, 144, 144–150. [Google Scholar] [CrossRef]
- Hofmann, M.; Pircher, H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc. Natl. Acad. Sci. USA 2011, 108, 16741–16746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masopust, D.; Choo, D.; Vezys, V.; Wherry, E.J.; Duraiswamy, J.; Akondy, R.; Wang, J.; Casey, K.A.; Barber, D.L.; Kawamura, K.S.; et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 2010, 207, 553–564. [Google Scholar] [CrossRef] [Green Version]
- Kok, L.; Dijkgraaf, F.E.; Urbanus, J.; Bresser, K.; Vredevoogd, D.W.; Cardoso, R.F.; Perié, L.; Beltman, J.B.; Schumacher, T.N. A committed tissue-resident memory T cell precursor within the circulating CD8+ effector T cell pool. J. Exp. Med. 2020, 217, 711. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Lenig, D.; Forster, R.; Lipp, M.; Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401, 708–712. [Google Scholar] [CrossRef]
- Klonowski, K.D.; Marzo, A.L.; Williams, K.J.; Lee, S.-J.; Pham, Q.-M.; Lefrançois, L. CD8 T Cell Recall Responses Are Regulated by the Tissue Tropism of the Memory Cell and Pathogen. J. Immunol. 2006, 177, 6738–6746. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.V.; Ma, W.; Miron, M.; Granot, T.; Guyer, R.S.; Carpenter, D.J.; Senda, T.; Sun, X.; Ho, S.-H.; Lerner, H.; et al. Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep. 2017, 20, 2921–2934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabo, P.A.; Miron, M.; Farber, D.L. Location, location, location: Tissue resident memory T cells in mice and humans. Sci. Immunol. 2019, 4, 9673. [Google Scholar] [CrossRef] [PubMed]
- Park, C.O.; Kupper, T.S. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat. Med. 2015, 21, 688–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanasetty, N.K.; Pai, V.V.; Athanikar, S.B. Annular lesions in dermatology. Indian J. Dermatol. 2013, 58, 157. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, T.; Lybaek, D.; Johansen, C.; Iversen, L. Non-random Plaque-site Recurrence of Psoriasis in Patients Treated with Dead Sea Climatotherapy. Acta Derm. Venereol. 2019, 99, 909–910. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.J.; Clark, R.A.; Watanabe, R.; Kupper, T.S. Sézary syndrome and mycosis fungoides arise from distinct T-cell subsets: A biologic rationale for their distinct clinical behaviors. Blood 2010, 116, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, S.H.; Schlums, H.; Sérézal, I.G.; Martini, E.; Chiang, S.; Marquardt, N.; Gibbs, A.; Detlofsson, E.; Introini, A.; Forkel, M.; et al. CD49a Expression Defines Tissue-Resident CD8 + T Cells Poised for Cytotoxic Function in Human Skin. Immunty 2017, 46, 287–300. [Google Scholar] [CrossRef] [Green Version]
- Richmond, J.M.; Strassner, J.P.; Rashighi, M.; Agarwal, P.; Garg, M.; Essien, K.I.; Pell, L.S.; Harris, J.E. Resident Memory and Recirculating Memory T Cells Cooperate to Maintain Disease in a Mouse Model of Vitiligo. J. Investig. Dermatol. 2019, 139, 769–778. [Google Scholar] [CrossRef] [Green Version]
- Azzolino, V.; Zapata, L.; Garg, M.; Gjoni, M.; Riding, R.L.; Strassner, J.P.; Richmond, J.M.; Harris, J.E. Jak Inhibitors Reverse Vitiligo in Mice but Do Not Deplete Skin Resident Memory T Cells. J. Investig. Dermatol. 2021, 141, 182–184. [Google Scholar] [CrossRef]
- Gálvez-Cancino, F.; López, E.; Menares, E.; Díaz, X.; Flores, C.; Cáceres, P.; Hidalgo, S.; Chovar, O.; Alcántara-Hernández, M.; Borgna, V.; et al. Vaccination-induced skin-resident memory CD8+T cells mediate strong protection against cutaneous melanoma. OncoImmunology 2018, 7, e1442163. [Google Scholar] [CrossRef] [Green Version]
- Hochheiser, K.; Aw Yeang, H.X.; Wagner, T.; Tutuka, C.; Behren, A.; Waithman, J.; Angel, C.; Neeson, P.J.; Gebhardt, T.; Gyorki, D.E. Accumulation of CD103 + CD8 + T Cells in a Cutaneous Melanoma Micrometastasis. Clin. Transl. Immunol. 2019, 8, e1100. [Google Scholar] [CrossRef] [Green Version]
- Duhen, T.; Duhen, R.; Montler, R.; Moses, J.; Moudgil, T.; de Miranda, N.; Goodall, C.P.; Blair, T.C.; Fox, B.A.; McDermott, J.E.; et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 2018, 9, 2724. [Google Scholar] [CrossRef] [Green Version]
- Kurihara, K.; Shimauchi, T.; Tokura, Y. Indolent Multipapular Adult T-Cell Leukemia/Lymphoma with Phenotype of Resi-dent Memory T Cells. Blackwell Publ. Ltd. 2020, 47, e280–e281. [Google Scholar]
- Miyagawa, F.; Iioka, H.; Fukumoto, T.; Kobayashi, N.; Asada, H. A case ofCD8+primary cutaneous peripheral T-cell lymphoma arising from tissue-resident memory T cells in the skin. Br. J. Dermatol. 2015, 173, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Kobayashi, T.; Sugihara, E.; Yamada, T.; Ikuta, K.; Pittaluga, S.; Saya, H.; Amagai, M.; Nagao, K. Hair Follicle–Derived IL-7 and IL-15 Mediate Skin-Resident Memory T Cell Homeostasis and Lymphoma. Nat. Med. 2015, 21, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.; Bueno, E.M.; Granter, S.R.; Laga, A.C.; Saavedra, A.P.; Lin, W.M.; Susa, J.S.; Zhan, Q.; Chandraker, A.K.; Tullius, S.G.; et al. Biomarker evaluation of face transplant rejection: Association of donor T cells with target cell injury. Mod. Pathol. 2014, 27, 788–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsutsumi, R.; Yoshida, Y.; Adachi, K.; Nanba, E.; Yamamoto, O. Acute localized exanthematous pustulosis caused by a herbal medicine, dai-kenchu-to. Contact Dermat. 2018, 79, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Boopathy, A.V.; Lam, L.K.W.; Moynihan, K.D.; Welch, M.E.; Bennett, N.R.; Turvey, M.E.; Thai, N.; Van, J.H.; Love, J.C.; et al. Cell and fluid sampling microneedle patches for monitoring skin-resident immunity. Sci. Transl. Med. 2018, 10, 2227. [Google Scholar] [CrossRef]
- Koguchi-Yoshioka, H.; Watanabe, R.; Fujisawa, Y.; Ishitsuka, Y.; Nakamura, Y.; Okiyama, N.; Fujimoto, M. Skin Resident Memory T-cell Population Is Not Constructed Effectively in Systemic Sclerosis. Br. J. Dermatol. 2019, 180, 219–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trubiano, J.A.; Gordon, C.L.; Castellucci, C.; Christo, S.N.; Park, S.L.; Mouhtouris, E.; Konvinse, K.; Rose, M.; Goh, M.; Boyd, A.S.; et al. Analysis of Skin-Resident Memory T Cells Following Drug Hypersensitivity Reactions. J. Investig. Dermatol. 2020, 140, 1442–1445.e4. [Google Scholar] [CrossRef]
- Gadsbøll, A.-S.Ø.; Jee, M.H.; Funch, A.B.; Alhede, M.; Mraz, V.; Weber, J.F.; Callender, L.A.; Carroll, E.C.; Bjarnsholt, T.; Woetmann, A.; et al. Pathogenic CD8+ Epidermis-Resident Memory T Cells Displace Dendritic Epidermal T Cells in Allergic Dermatitis. J. Investig. Dermatol. 2020, 140, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Murata, A.; Hayashi, S.-I. CD4+ Resident Memory T Cells Mediate Long-Term Local Skin Immune Memory of Contact Hy-persensitivity in BALB/c Mice. Front. Immunol. 2020, 11, 775. [Google Scholar] [CrossRef]
- Gimenez-Rivera, V.-A.; Siebenhaar, F.; Zimmermann, C.; Siiskonen, H.; Metz, M.; Maurer, M. Mast Cells Limit the Exacerbation of Chronic Allergic Contact Dermatitis in Response to Repeated Allergen Exposure. J. Immunol. 2016, 197, 4240–4246. [Google Scholar] [CrossRef] [Green Version]
- McCully, M.L.; Ladell, K.; Andrews, R.; Jones, R.E.; Miners, K.L.; Roger, L.; Baird, D.M.; Cameron, M.J.; Jessop, Z.M.; Whitaker, I.S.; et al. CCR8 Expression Defines Tissue-Resident Memory T Cells in Human Skin. J. Immunol. 2018, 200, 1639–1650. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, A.R.; Tabacchi, M.; Ngo, K.H.; Wallendorf, M.; Rosman, I.S.; Cornelius, L.A.; Demehri, S. Skin Cancer Precursor Immunotherapy for Squamous Cell Carcinoma Prevention. JCI Insight 2019, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhong, Q.; Tian, T.; Dubin, K.; Athale, S.K.; Kupper, T.S. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell–mediated immunity. Nat. Med. 2010, 16, 224–227. [Google Scholar] [CrossRef] [Green Version]
- Hirai, T.; Zenke, Y.; Yang, Y.; Bartholin, L.; Beura, L.K.; Masopust, D.; Kaplan, D.H. Keratinocyte-Mediated Activation of the Cytokine TGF-β Maintains Skin Recirculating Memory CD8+ T Cells. Immunity 2019, 50, 1249–1261.e5. [Google Scholar] [CrossRef]
- Mani, V.; Bromley, S.K.; Äijö, T.; Mora-Buch, R.; Carrizosa, E.; Warner, R.D.; Hamze, M.; Sen, D.R.; Chasse, A.Y.; Lorant, A.; et al. Migratory DCs activate TGF-β to precondition naïve CD8+ T cells for tissue-resident memory fate. Science 2019, 366, 5728. [Google Scholar] [CrossRef]
- Seneschal, J.; Clark, R.A.; Gehad, A.; Baecher-Allan, C.; Kupper, T.S. Human Epidermal Langerhans Cells Maintain Immune Homeostasis in Skin by Activating Skin Resident Regulatory T Cells. Immunity 2012, 36, 873–884. [Google Scholar] [CrossRef] [Green Version]
- Ohta, T.; Yoshikawa, S.; Tabakawa, Y.; Yamaji, K.; Ishiwata, K.; Shitara, H.; Taya, C.; Oh-Hora, M.; Kawano, Y.; Miyake, K.; et al. Skin CD4+ Memory T Cells Play an Essential Role in Acquired Anti-Tick Immunity through Interleukin-3-Mediated Basophil Recruitment to Tick-Feeding Sites. Front. Immunol. 2017, 8, 1348. [Google Scholar] [CrossRef] [Green Version]
- Cheuk, S.H.; Wikén, M.; Blomqvist, L.; Nylén, S.; Talme, T.; Ståhle, M.; Eidsmo, L. Epidermal Th22 and Tc17 Cells Form a Localized Disease Memory in Clinically Healed Psoriasis. J. Immunol. 2014, 192, 3111–3120. [Google Scholar] [CrossRef] [Green Version]
- Kurihara, K.; Fujiyama, T.; Phadungsaksawasdi, P.; Ito, T.; Tokura, Y. Significance of IL-17A-producing CD8+CD103+ skin resident memory T cells in psoriasis lesion and their possible relationship to clinical course. J. Dermatol. Sci. 2019, 95, 21–27. [Google Scholar] [CrossRef]
- Lauron, E.J.; Yang, L.; Harvey, I.B.; Sojka, D.K.; Williams, G.; Paley, M.A.; Bern, M.D.; Park, E.; Victorino, F.; Boon, A.C.; et al. Viral MHCI inhibition evades tissue-resident memory T cell formation and responses. J. Exp. Med. 2018, 216, 117–132. [Google Scholar] [CrossRef]
- Klicznik, M.; Benedetti, A.; Gail, L.M.; Varkhande, S.R.; Holly, R.; Laimer, M.; Stoecklinger, A.; Sir, A.; Reitsamer, R.; Neuper, T.; et al. A novel humanized mouse model to study the function of human cutaneous memory T cells in vivo in human skin. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Glennie, N.D.; Yeramilli, V.A.; Beiting, D.P.; Volk, S.W.; Weaver, C.T.; Scott, P. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J. Exp. Med. 2015, 212, 1405–1414. [Google Scholar] [CrossRef]
- Glennie, N.D.; Volk, S.W.; Scott, P. Skin-resident CD4+ T cells protect against Leishmania major by recruiting and activating inflammatory monocytes. PLoS Pathog. 2017, 13, e1006349. [Google Scholar] [CrossRef]
- Boniface, K.; Jacquemin, C.; Darrigade, A.-S.; Dessarthe, B.; Martins, C.; Boukhedouni, N.; Vernisse, C.; Grasseau, A.; Thiolat, D.; Rambert, J.; et al. Vitiligo Skin Is Imprinted with Resident Memory CD8 T Cells Expressing CXCR3. J. Investig. Dermatol. 2018, 138, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Vo, S.; Watanabe, R.; Koguchi-Yoshioka, H.; Matsumura, Y.; Ishitsuka, Y.; Nakamura, Y.; Okiyama, N.; Fujisawa, Y.; Fujimoto, M. CD 8 resident memory T cells with interleukin 17A-producing potential are accumulated in disease-naïve nonlesional sites of psoriasis possibly in correlation with disease duration. Br. J. Dermatol. 2019, 181, 410–412. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, Y.; Liu, H.; Qiu, F.; Liang, C.-L.; Zhang, Q.; Huang, R.-Y.; Han, L.; Lu, C.; Dai, Z. Dihydroartemisinin ameliorates psoriatic skin inflammation and its relapse by diminishing CD8+ T-cell memory in wild-type and humanized mice. Theranostics 2020, 10, 10466–10482. [Google Scholar] [CrossRef]
- Lai, J.C.Y.; Cheng, W.K.; Hopkins, P.D.; Komba, M.; Carlow, D.A.; Dutz, J.P. Topical Adjuvant Application during Subcutaneous Vaccination Promotes Resident Memory T Cell Generation. J. Immunol. 2019, 203, 2443–2450. [Google Scholar] [CrossRef]
- Antonio-Herrera, L.; Badillo, O.; Medina-Contreras, O.; Tepale-Segura, A.; García-Lozano, A.; Gutierrez-Xicotencatl, L.; Soldevila, G.; Esquivel-Guadarrama, F.R.; Idoyaga, J.; Bonifaz, L.C. The Nontoxic Cholera B Subunit Is a Potent Adjuvant for Intradermal DC-Targeted Vaccination. Front. Immunol. 2018, 9, 2212. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; Watanabe, R.; Teague, J.E.; Schlapbach, C.; Tawa, M.C.; Adams, N.; Dorosario, A.A.; Chaney, K.S.; Cutler, C.S.; Leboeuf, N.R.; et al. Skin Effector Memory T Cells Do Not Recirculate and Provide Immune Protection in Alemtuzumab-Treated CTCL Patients. Sci. Transl. Med. 2012, 4, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, R.; Gehad, A.; Yang, C.; Scott, L.L.; Teague, J.E.; Schlapbach, C.; Elco, C.P.; Huang, V.; Matos, T.R.; Kupper, T.S.; et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci. Transl. Med. 2015, 7, 279. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Zeng, D.; Wu, X.; Wang, B.; Yang, S.; Song, Q.; Zhu, Y.; Salas, M.; Qin, H.; Nasri, U.; et al. Tissue-resident PSGL1loCD4+ T cells promote B cell differentiation and chronic graft-versus-host disease–associated autoimmunity. J. Clin. Investig. 2021, 131, 468. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, T.; Whitney, P.; Zaid, A.; Mackay, L.; Brooks, A.; Heath, W.; Carbone, F.R.; Mueller, S. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 2011, 477, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Iriki, H.; Adachi, T.; Mori, M.; Tanese, K.; Funakoshi, T.; Karigane, D.; Shimizu, T.; Okamoto, S.; Nagao, K. Toxic epidermal necrolysis in the absence of circulating T cells: A possible role for resident memory T cells. J. Am. Acad. Dermatol. 2014, 71, e214–e216. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Suryawanshi, H.; Morozov, P.; Gay-Mimbrera, J.; Del Duca, E.; Kim, H.J.; Kameyama, N.; Estrada, Y.; Der, E.; Krueger, J.G.; et al. Single-cell transcriptome analysis of human skin identifies novel fibroblast subpopulation and enrichment of immune subsets in atopic dermatitis. J. Allergy Clin. Immunol. 2020, 145, 1615–1628. [Google Scholar] [CrossRef] [Green Version]
- Petrelli, A.; Mijnheer, G.; van Konijnenburg, D.H.; Van Der Wal, M.M.; Giovannone, B.; Mocholi, E.; Vazirpanah, N.; Broen, J.C.; Hijnen, D.; Oldenburg, B.; et al. PD-1+CD8+ T cells are clonally expanding effectors in human chronic inflammation. J. Clin. Investig. 2018, 128, 4669–4681. [Google Scholar] [CrossRef]
- Schmidt, J.D.; Ahlström, M.G.; Johansen, J.D.; Dyring-Andersen, B.; Agerbeck, C.; Nielsen, M.M.; Poulsen, S.S.; Woetmann, A.; Ødum, N.; Thomsen, A.R.; et al. Rapid allergen-induced interleukin-17 and interferon-γ secretion by skin-resident memory CD8+T cells. Contact Dermat. 2017, 76, 218–227. [Google Scholar] [CrossRef]
- Gamradt, P.; Laoubi, L.; Nosbaum, A.; Mutez, V.; Lenief, V.; Grande, S.; Redoulès, D.; Schmitt, A.-M.; Nicolas, J.F.; Vocanson, M. Inhibitory checkpoint receptors control CD8+ resident memory T cells to prevent skin allergy. J. Allergy Clin. Immunol. 2019, 143, 2147–2157.e9. [Google Scholar] [CrossRef]
- Gebhardt, T.; Wakim, L.M.; Eidsmo, L.; Reading, P.; Heath, W.; Carbone, F.R. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 2009, 10, 524–530. [Google Scholar] [CrossRef]
- Gaide, O.; Emerson, R.O.; Jiang, X.; Gulati, N.; Nizza, S.T.; Desmarais, C.; Robins, H.; Krueger, J.G.; Clark, R.A.; Kupper, T.S. Common clonal origin of central and resident memory T cells following skin immunization. Nat. Med. 2015, 21, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Boddupalli, C.S.; Bar, N.; Kadaveru, K.; Krauthammer, M.; Pornputtapong, N.; Mai, Z.; Ariyan, S.; Narayan, D.; Kluger, H.; Deng, Y.; et al. Interlesional diversity of T cell receptors in melanoma with immune checkpoints enriched in tissue-resident memory T cells. JCI Insight 2016, 1, e88955. [Google Scholar] [CrossRef]
- Ise, M.; Tanese, K.; Adachi, T.; Du, W.; Amagai, M.; Ohyama, M. Postherpetic Wolf’s isotopic response: Possible contribution of resident memory T cells to the pathogenesis of lichenoid reaction. Br. J. Dermatol. 2015, 173, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.P.; Vukmanovic-Stejic, M.; Suarez-Farinas, M.; Chambers, E.S.; Sandhu, D.; Fuentes-Duculan, J.; Mabbott, N.A.; Rustin, M.H.A.; Krueger, J.; Akbar, A.N. Impact of Zostavax Vaccination on T-Cell Accumulation and Cutaneous Gene Expression in the Skin of Older Humans After Varicella Zoster Virus Antigen–Specific Challenge. J. Infect. Dis. 2018, 218, S88–S98. [Google Scholar] [CrossRef]
- Jiang, X.; Clark, R.A.; Liu, L.; Wagers, A.J.; Fuhlbrigge, R.C.; Kupper, T.S. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 2012, 483, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, S.J.; Nolz, J.C. Targeted Expansion of Tissue-Resident CD8+ T Cells to Boost Cellular Immunity in the Skin. Cell Rep. 2019, 29, 2990–2997.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijkgraaf, F.E.; Matos, T.R.; Hoogenboezem, M.; Toebes, M.; Vredevoogd, D.; Mertz, M.; Broek, B.V.D.; Song, J.-Y.; Teunissen, M.B.M.; Luiten, R.M.; et al. Tissue patrol by resident memory CD8+ T cells in human skin. Nat. Immunol. 2019, 20, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Mackay, L.; Stock, A.T.; Ma, J.; Jones, C.; Kent, S.; Mueller, S.; Heath, W.; Carbone, F.R.; Gebhardt, T. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl. Acad. Sci. USA 2012, 109, 7037–7042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bromley, S.K.; Akbaba, H.; Mani, V.; Mora-Buch, R.; Chasse, A.Y.; Sama, A.; Luster, A.D. CD49a Regulates Cutaneous Resident Memory CD8+ T Cell Persistence and Response. Cell Rep. 2020, 32, 108085. [Google Scholar] [CrossRef]
- Walsh, D.A.; Da Silva, H.B.; Beura, L.K.; Peng, C.; Hamilton, S.E.; Masopust, D.; Jameson, S.C. The Functional Requirement for CD69 in Establishment of Resident Memory CD8+T Cells Varies with Tissue Location. J. Immunol. 2019, 203, 946–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muschaweckh, A.; Buchholz, V.R.; Fellenzer, A.; Hessel, C.; König, P.-A.; Tao, S.; Tao, R.; Heikenwälder, M.; Busch, D.H.; Korn, T.; et al. Antigen-dependent competition shapes the local repertoire of tissue-resident memory CD8+ T cells. J. Exp. Med. 2016, 213, 3075–3086. [Google Scholar] [CrossRef] [Green Version]
- Osborn, J.F.; Hobbs, S.J.; Mooster, J.L.; Khan, T.N.; Kilgore, A.M.; Harbour, J.; Nolz, J.C. Central memory CD8+ T cells become CD69+ tissue-residents during viral skin infection independent of CD62L-mediated lymph node surveillance. PLoS Pathog. 2019, 15, e1007633. [Google Scholar] [CrossRef]
- Fonseca, R.; Beura, L.K.; Quarnstrom, C.F.; Ghoneim, H.E.; Fan, Y.; Zebley, C.C.; Scott, M.C.; Fares-Frederickson, N.J.; Wijeyesinghe, S.; Thompson, E.A.; et al. Developmental plasticity allows outside-in immune responses by resident memory T cells. Nat. Immunol. 2020, 21, 412–421. [Google Scholar] [CrossRef]
- Enamorado, M.; Iborra, S.; Priego, E.; Cueto, F.J.; Quintana, J.A.; Martínez-Cano, S.; Mejías-Pérez, E.; Esteban, M.; Melero, I.; Hidalgo, A.; et al. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8+ T cells. Nat. Commun. 2017, 8, 16073. [Google Scholar] [CrossRef]
- Danahy, D.B.; Anthony, S.M.; Jensen, I.J.; Hartwig, S.M.; Shan, Q.; Xue, H.-H.; Harty, J.T.; Griffith, T.S.; Badovinac, V.P. Polymicrobial sepsis impairs bystander recruitment of effector cells to infected skin despite optimal sensing and alarming function of skin resident memory CD8 T cells. PLoS Pathog. 2017, 13, e1006569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, T.N.; Mooster, J.L.; Kilgore, A.M.; Osborn, J.F.; Nolz, J.C. Local antigen in nonlymphoid tissue promotes resident memory CD8+ T cell formation during viral infection. J. Exp. Med. 2016, 213, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Iborra, S.; Martínez-López, M.; Khouili, S.C.; Enamorado, M.; Cueto, F.J.; Conde-Garrosa, R.; del Fresno, C.; Sancho, D. Optimal Generation of Tissue-Resident but Not Circulating Memory T Cells during Viral Infection Requires Crosspriming by DNGR-1 + Dendritic Cells. Immunity 2016, 45, 847–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaid, A.; Hor, J.L.; Christo, S.N.; Groom, J.R.; Heath, W.R.; Mackay, L.K.; Mueller, S.N. Chemokine Receptor–Dependent Control of Skin Tissue–Resident Memory T Cell Formation. J. Immunol. 2017, 199, 2451–2459. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Dove, C.G.; Hor, J.L.; Murdock, H.; Strauss-Albee, D.M.; Garcia, J.; Mandl, J.N.; Grodick, R.A.; Jing, H.; Chandler-Brown, D.B.; et al. DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J. Exp. Med. 2014, 211, 2549–2566. [Google Scholar] [CrossRef] [Green Version]
- Nolz, J.C.; Harty, J.T. IL-15 regulates memory CD8+ T cell O-glycan synthesis and affects trafficking. J. Clin. Investig. 2014, 124, 1013–1026. [Google Scholar] [CrossRef] [Green Version]
- Frizzell, H.; Fonseca, R.; Christo, S.N.; Evrard, M.; Cruz-Gomez, S.; Zanluqui, N.G.; von Scheidt, B.; Freestone, D.; Park, S.L.; McWilliam, H.E.G.; et al. Organ-specific isoform selection of fatty acid–binding proteins in tissue-resident lymphocytes. Sci. Immunol. 2020, 5, 9283. [Google Scholar] [CrossRef] [PubMed]
- Mackay, L.K.; Braun, A.; Macleod, B.L.; Collins, N.; Tebartz, C.; Bedoui, S.; Carbone, F.R.; Gebhardt, T. Cutting Edge: CD69 Interference with Sphingosine-1-Phosphate Receptor Function Regulates Peripheral T Cell Retention. J. Immunol. 2015, 194, 2059–2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, T.; Kurihara, K.; Tokura, Y. Tissue resident memory T cells in lesional and non-lesional psoriatic skin on a scar. J. Dermatol. 2020, 47, e210–e211. [Google Scholar] [CrossRef]
- Richmond, J.M.; Strassner, J.P.; Zapata, L.; Garg, M.; Riding, R.L.; Refat, M.A.; Fan, X.; Azzolino, V.; Tovar-Garza, A.; Tsurushita, N.; et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci. Transl. Med. 2018, 10, 7710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, C.E.; Thompson, E.A.; Quarnstrom, C.F.; Fraser, K.A.; Seelig, D.M.; Bhela, S.; Burbach, B.; Masopust, D.; Vezys, V. Robust Iterative Stimulation with Self-Antigens Overcomes CD8+ T Cell Tolerance to Self- and Tumor Antigens. Cell Rep. 2019, 28, 3092–3104.e5. [Google Scholar] [CrossRef] [Green Version]
- Mintern, J.; Guillonneau, C.; Carbone, F.R.; Doherty, P.C.; Turner, S.J. Cutting Edge: Tissue-Resident Memory CTL Down-Regulate Cytolytic Molecule Expression following Virus Clearance. J. Immunol. 2007, 179, 7220–7224. [Google Scholar] [CrossRef]
- Menares, E.; Gálvez-Cancino, F.; Cáceres-Morgado, P.; Ghorani, E.; López, E.; Díaz, X.; Saavedra-Almarza, J.; Figueroa, D.A.; Roa, E.; Quezada, S.A.; et al. Tissue-resident memory CD8+ T cells amplify anti-tumor immunity by triggering antigen spreading through dendritic cells. Nat. Commun. 2019, 10, 4401. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Buzzai, A.; Rautela, J.; Hor, J.L.; Hochheiser, K.; Effern, M.; McBain, N.; Wagner, T.; Edwards, J.; McConville, R.; et al. Tissue-resident memory CD8+ T cells promote melanoma–immune equilibrium in skin. Nature 2019, 565, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.; Marraco, S.A.F.; Baumgaertner, P.; Bordry, N.; Cagnon, L.; Donda, A.; Romero, P.; Verdeil, G.; Speiser, D.E. Very Late Antigen-1 Marks Functional Tumor-Resident CD8 T Cells and Correlates with Survival of Melanoma Patients. Front. Immunol. 2016, 7, 573. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, C.; Ishida, Y.; Nakagawa, K.; Irie, H.; Hirata, M.; Kataoka, T.; Otsuka, A.; Kabashima, K. Identification of CD 49a+ CD 8+ resident memory T cells in vitiligo-like lesions associated with nivolumab treatment for melanoma. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e79–e82. [Google Scholar] [CrossRef]
- Milner, J.J.; Toma, C.; Yu, B.; Zhang, K.; Omilusik, K.; Phan, A.T.; Wang, D.; Getzler, A.; Nguyen, T.; Crotty, S.; et al. Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature 2017, 552, 253–257. [Google Scholar] [CrossRef]
- Davies, B.; Prier, J.; Jones, C.M.; Gebhardt, T.; Carbone, F.R.; Mackay, L.K. Cutting Edge: Tissue-Resident Memory T Cells Generated by Multiple Immunizations or Localized Deposition Provide Enhanced Immunity. J. Immunol. 2017, 198, 2233–2237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, S.L.; Tse, J.Y.; Fisher, D.C.; Lebeouf, N.R.; Murphy, G.F.; Kupper, T.S.; Clark, R.A.; Lian, C.G. Histopathologic spectrum of hypersensitivity reactions associated with anti-CD52 therapy (alemtuzumab). J. Cutan. Pathol. 2016, 43, 989–993. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Zaid, A.; Hor, J.L.; Christo, S.N.; Prier, J.; Davies, B.; Alexandre, Y.O.; Gregory, J.L.; Russell, T.; Gebhardt, T.; et al. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat. Immunol. 2018, 19, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Hain, T.; Melchior, F.; Kamenjarin, N.; Muth, S.; Weslati, H.; Clausen, B.E.; Mahnke, K.; Silva-Vilches, C.; Schütze, K.; Sohl, J.; et al. Dermal CD207-Negative Migratory Dendritic Cells Are Fully Competent to Prime Protective, Skin Homing Cytotoxic T-Lymphocyte Responses. J. Investig. Dermatol. 2019, 139, 422–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaid, A.; Mackay, L.; Rahimpour, A.; Braun, A.; Veldhoen, M.; Carbone, F.R.; Manton, J.H.; Heath, W.; Mueller, S.N. Persistence of skin-resident memory T cells within an epidermal niche. Proc. Natl. Acad. Sci. USA 2014, 111, 5307–5312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackay, L.K.; Wynne-Jones, E.; Freestone, D.; Pellicci, D.; Mielke, L.; Newman, D.M.; Braun, A.; Masson, F.; Kallies, A.; Belz, G.; et al. T-box Transcription Factors Combine with the Cytokines TGF-β and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity 2015, 43, 1101–1111. [Google Scholar] [CrossRef] [Green Version]
- Ariotti, S.; Beltman, J.B.; Chodaczek, G.; Hoekstra, M.E.; van Beek, A.; Gomez-Eerland, R.; Ritsma, L.; van Rheenen, J.; Marée, A.F.M.; Zal, T.; et al. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc. Natl. Acad. Sci. USA 2012, 109, 19739–19744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariotti, S.; Hogenbirk, M.A.; Dijkgraaf, F.E.; Visser, L.L.; Hoekstra, M.E.; Song, J.-Y.; Jacobs, H.; Haanen, J.B.; Schumacher, T.N. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science 2014, 346, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Beckhove, P.; Feuerer, M.; Dolenc, M.; Schuetz, F.; Choi, C.; Sommerfeldt, N.; Schwendemann, J.; Ehlert, K.; Altevogt, P.; Bastert, G.; et al. Specifically activated memory T cell subsets from cancer patients recognize and reject xenotransplanted autologous tumors. J. Clin. Investig. 2004, 114, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Park, C.O.; Fu, X.; Jiang, X.; Pan, Y.; Teague, J.E.; Collins, N.; Tian, T.; O’Malley, J.T.; Emerson, R.O.; Kim, J.H.; et al. Staged development of long-lived T-cell receptor αβ T H 17 resident memory T-cell population to Candida albicans after skin infection. J. Allergy Clin. Immunol. 2018, 142, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Egawa, G.; Roediger, B.; Tay, S.S.; LCavanagh, L.; VGuy, T.; Fazekas, B.; Brzoska, A.J.; Firth, N.; Weninger, W. Bacterial antigen is directly delivered to the draining lymph nodes and activates CD8+ T cells during Staphylococcus aureus skin infection. Immunol. Cell Biol. 2021, 99, 299–308. [Google Scholar] [CrossRef]
- Pan, Y.; Tian, T.; Park, C.O.; Lofftus, S.Y.; Mei, S.; Liu, X.; Luo, C.; O’Malley, J.T.; Gehad, A.; Teague, J.E.; et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 2017, 543, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Del Duca, E.; Ruano Ruiz, J.; Pavel, A.B.; Sanyal, R.D.; Song, T.; Gay-Mimbrera, J.; Zhang, N.; Estrada, Y.D.; Peng, X.; Renert-Yuval, Y.; et al. Frontal fibrosing alopecia shows robust T helper 1 and Janus kinase 3 skewing. Br. J. Dermatol. 2020, 183, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Mashiko, S.; Edelmayer, R.M.; Bi, Y.; Olson, L.M.; Wetter, J.B.; Wang, J.; Maari, C.; Proulx, E.S.-C.; Kaimal, V.; Li, X.; et al. Persistence of Inflammatory Phenotype in Residual Psoriatic Plaques in Patients on Effective Biologic Therapy. J. Investig. Dermatol. 2020, 140, 1015–1025.e4. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, T.; Pantelyushin, S.; Croxford, A.L.; Kulig, P.; Becher, B. Dermal IL-17-Producing Γδ T Cells Establish Long-Lived Memory in the Skin. Eur. J. Immunol. 2015, 45, 3022–3033. [Google Scholar] [CrossRef] [PubMed]
- Iversen, L.; Eidsmo, L.; Austad, J.; Rie, M.; Osmancevic, A.; Skov, L.; Talme, T.; Bachmann, I.; Kerkhof, P.; Stahle, M.; et al. Secukinumab Treatment in New-onset Psoriasis: Aiming to Understand the Potential for Disease Modification–Rationale and Design of the Randomized, Multicenter STEPI n Study. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1930–1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, J.; Wilmott, J.; Madore, J.; Gide, T.; Quek, C.; Tasker, A.; Ferguson, A.; Chen, J.; Hewavisenti, R.; Hersey, P.; et al. CD103+ Tumor-Resident CD8+ T Cells Are Associated with Improved Survival in Immunotherapy-Naïve Melanoma Patients and Expand Significantly During Anti–PD-1 Treatment. Clin. Cancer Res. 2018, 24, 3036–3045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, B.T.; Byrne, K.T.; Vella, J.L.; Zhang, P.; Shabaneh, T.B.; Steinberg, S.M.; Molodtsov, A.K.; Bowers, J.S.; Angeles, C.V.; Paulos, C.M.; et al. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci. Immunol. 2017, 2, 6346. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Nicolaidou, E.; Antoniou, C.; Stratigos, A.J.; Stefanaki, C.; Katsambas, A.D. Efficacy, predictors of response, and long-term follow-up in patients with vitiligo treated with narrowband UVB phototherapy. J. Am. Acad. Dermatol. 2007, 56, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Cavalié, M.; Ezzedine, K.; Fontas, E.; Montaudié, H.; Castela, E.; Bahadoran, P.; Taïeb, A.; Lacour, J.-P.; Passeron, T. Maintenance Therapy of Adult Vitiligo with 0.1% Tacrolimus Ointment: A Randomized, Double Blind, Placebo–Controlled Study. J. Investig. Dermatol. 2015, 135, 970–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iijima, N.; Iwasaki, A. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 2014, 346, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogan, R.J.; Zhong, W.; Usherwood, E.J.; Cookenham, T.; Roberts, A.D.; Woodland, D.L. Protection from Respiratory Virus Infections Can Be Mediated by Antigen-Specific Cd4+ T Cells That Persist in the Lungs. J. Exp. Med. 2001, 193, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, B.J.; Zhang, N.; Marshall, H.D.; Staron, M.M.; Guan, T.; Hu, Y.; Cauley, L.S.; Craft, J.; Kaech, S.M. CD4+ T Cell Help Guides Formation of CD103+ Lung-Resident Memory CD8+ T Cells during Influenza Viral Infection. Immunity 2014, 41, 633–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, Y.; Hirahara, K.; Kiuchi, M.; Wada, T.; Ichikawa, T.; Kanno, T.; Okano, M.; Kokubo, K.; Onodera, A.; Sakurai, D.; et al. Amphiregulin-Producing Pathogenic Memory T Helper 2 Cells Instruct Eosinophils to Secrete Osteopontin and Facilitate Airway Fibrosis. Immunity 2018, 49, 134–150.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishu, S.; El Zaatari, M.; Hayashi, A.; Hou, G.; Bowers, N.; Kinnucan, J.; Manoogian, B.; Muza-Moons, M.; Zhang, M.; Grasberger, H.; et al. CD4+ Tissue-resident Memory T Cells Expand and Are a Major Source of Mucosal Tumour Necrosis Factor α in Active Crohn’s Disease. J. Crohn’s Coliti. 2019, 13, 905–915. [Google Scholar] [CrossRef]
- Brehm, M.A.; Shultz, L.D.; Luban, J.; Greiner, D.L. Overcoming Current Limitations in Humanized Mouse Research. J. Infect. Dis. 2013, 208, S125–S130. [Google Scholar] [CrossRef] [Green Version]
| Disease | Study |
|---|---|
| Skin infections | |
| Viral skin infection | [14,38,46,47,48,53,54,75,76,77,82,83,84,85,86,87,88,90,91,92,105] |
| Herpes simplex virus-1 | [18,65,71,78,79,80,89,93,97,103,107,108,109,110] |
| Candida albicans | [49,54,112] |
| Tick infection | [50] |
| Staphylococcus aureus | [113] |
| Herpes zoster | [74,81] |
| Leishmaniasis | [55,56] |
| Psoriasis | [27,51,52,57,58,59,94,114,116,117,118] |
| Melanoma | [30,31,32,60,61,73,85,96,98,99,100,101,102,119] |
| Cutaneous T-cell lymphoma or mycosis fungoides | [26,33,34,35,62,63] |
| Vitiligo | [27,28,29,57,95,120] |
| Others | |
| Acute localized exanthematous pustulosis | [37] |
| Drug reaction with eosinophilia and systemic symptoms | [40] |
| Skin allograft rejection | [36] |
| Chronic antigen exposure | [44] |
| Contact hypersensitivity | [42,43,70] |
| Skin contact hypersensitivity | [72] |
| Dinitrofluorobenzene-induced skin inflammation | [105,106] |
| Frontal fifibrosing alopecia | [115] |
| Drug hypersensitivity reaction | [104] |
| Contact dermatitis | [41] |
| Atopic dermatitis | [67,68] |
| Contact allergy | [69] |
| Actinic keratosis | [45] |
| Systemic sclerosis | [39] |
| Graft-versus-host disease | [64] |
| Toxic epidermal necrolysis | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emmanuel, T.; Mistegård, J.; Bregnhøj, A.; Johansen, C.; Iversen, L. Tissue-Resident Memory T Cells in Skin Diseases: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 9004. https://doi.org/10.3390/ijms22169004
Emmanuel T, Mistegård J, Bregnhøj A, Johansen C, Iversen L. Tissue-Resident Memory T Cells in Skin Diseases: A Systematic Review. International Journal of Molecular Sciences. 2021; 22(16):9004. https://doi.org/10.3390/ijms22169004
Chicago/Turabian StyleEmmanuel, Thomas, Josephine Mistegård, Anne Bregnhøj, Claus Johansen, and Lars Iversen. 2021. "Tissue-Resident Memory T Cells in Skin Diseases: A Systematic Review" International Journal of Molecular Sciences 22, no. 16: 9004. https://doi.org/10.3390/ijms22169004
APA StyleEmmanuel, T., Mistegård, J., Bregnhøj, A., Johansen, C., & Iversen, L. (2021). Tissue-Resident Memory T Cells in Skin Diseases: A Systematic Review. International Journal of Molecular Sciences, 22(16), 9004. https://doi.org/10.3390/ijms22169004