Angioedema and Fatty Acids
Abstract
:1. Introduction
2. Angioedema Pathogenesis
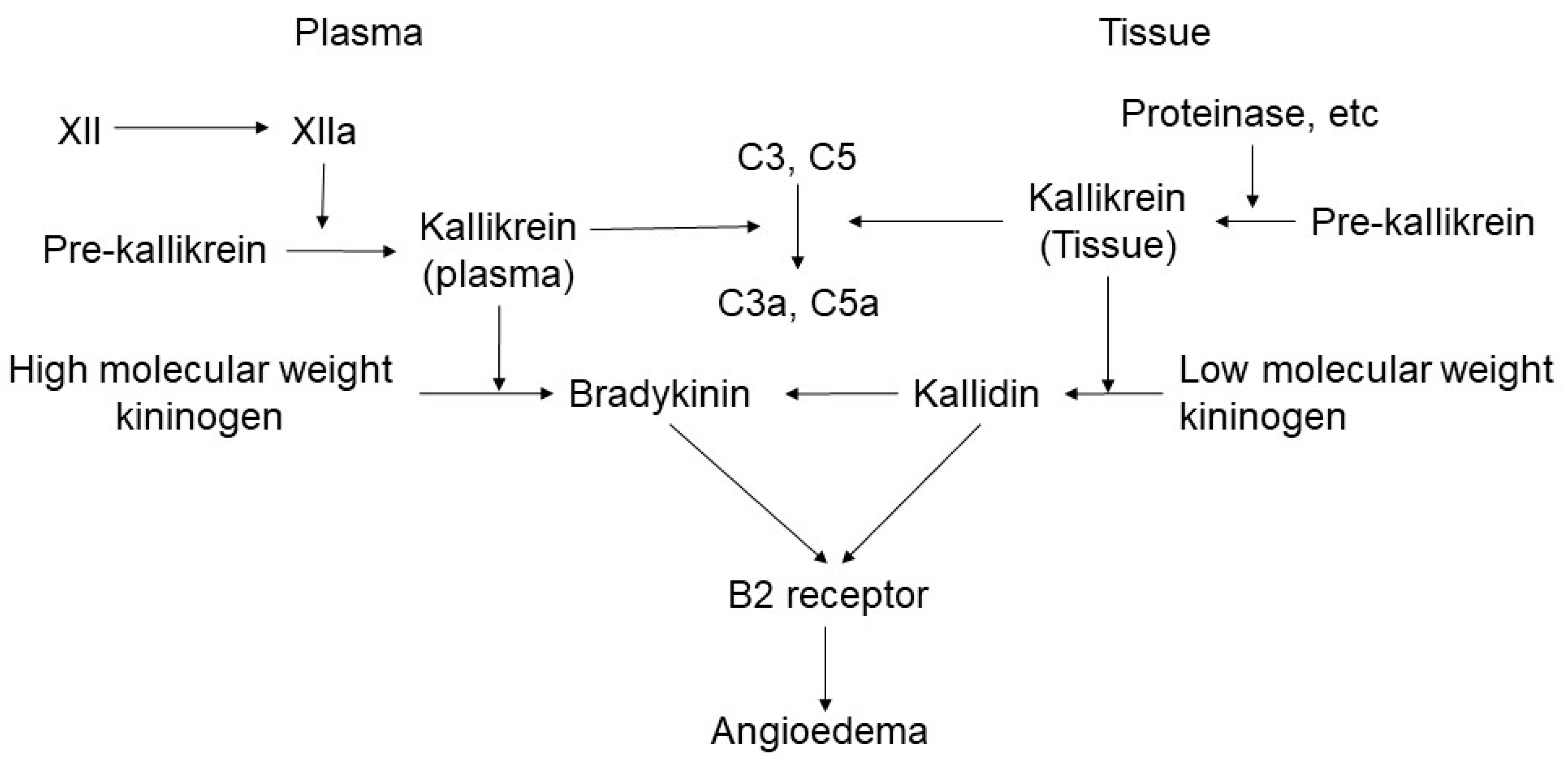
2.1. Bradykinin-Mediated Mechanism
2.2. Histamine-Mediated Mechanism as IgE-Mediated Angioedema
2.3. Medical-Agent-Mediated Angioedema as Non-IgE Mediated Angioedema
2.4. Recent Advancement of Angioedema Treatment
3. Prostanoids and Their Metabolisms
4. Evidence of Prostanoid-Related Angioedema
4.1. COX-Related Angioedema
4.1.1. PGE2 and Angioedema
4.1.2. PGD2 and Angioedema
4.1.3. PGF2 and Angioedema
4.1.4. PGI2 and Angioedema
4.1.5. Thromboxane A2 (TxA2) and Angioedema
4.1.6. Phospholipase A2 (PLA2) and Angioedema
4.2. Leukotrienes
4.2.1. LTB4 and Angioedema
4.2.2. LTC4 and Angioedema
4.2.3. LTD4 and Angioedema
4.3. PUFA and Angioedema
4.4. Short-Chain Fatty Acid and Angioedema
5. Therapeutic Potential for Fatty Acids Involving Angioedema
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Egawa, G.; Kabashima, K. Skin as a peripheral lymphoid organ: Revisiting the concept of skin-associated lymphoid tissues. J. Investig. Dermatol. 2011, 131, 2178–2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef]
- Sawada, Y.; Saito-Sasaki, N.; Mashima, E.; Nakamura, M. Daily Lifestyle and Inflammatory Skin Diseases. Int. J. Mol. Sci. 2021, 22, 5204. [Google Scholar] [CrossRef]
- Steinberg, P.; Ishizaka, K.; Norman, P.S. Possible role of IgE-mediated reaction in immunity. J. Allergy Clin. Immunol. 1974, 54, 359–366. [Google Scholar] [CrossRef]
- Bork, K.; Frank, J.; Grundt, B.; Schlattmann, P.; Nussberger, J.; Kreuz, W. Treatment of acute edema attacks in hereditary angioedema with a bradykinin receptor-2 antagonist (Icatibant). J. Allergy Clin. Immunol. 2007, 119, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Valerieva, A.; Staevska, M.T.; Grivcheva-Panovska, V.; Jesenak, M.; Kőhalmi, K.V.; Hrubiskova, K.; Zanichelli, A.; Bellizzi, L.; Relan, A.; Hakl, R.; et al. Recombinant human C1 esterase inhibitor for hereditary angioedema attacks: A European registry. World Allergy Organ. J. 2021, 14, 100535. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Saito-Sasaki, N.; Nakamura, M. Omega 3 Fatty Acid and Skin Diseases. Front. Immunol. 2020, 11, 623052. [Google Scholar] [CrossRef]
- Higashi, N.; Taniguchi, M.; Mita, H.; Higashi, A.; Akiyama, K. Aspirin-induced urticaria and angioedema, but not bronchoconstriction, associated with cysteinyl leukotriene overproduction in 2 patients with asthma. J. Allergy Clin. Immunol. 2002, 110, 666–667. [Google Scholar] [CrossRef]
- Weldon, D. Differential diagnosis of angioedema. Immunol. Allergy Clin. N. Am. 2006, 26, 603–613. [Google Scholar] [CrossRef]
- Depetri, F.; Tedeschi, A.; Cugno, M. Angioedema and emergency medicine: From pathophysiology to diagnosis and treatment. Eur. J. Intern. Med. 2019, 59, 8–13. [Google Scholar] [CrossRef]
- Bindke, G.; Gehring, M.; Wieczorek, D.; Kapp, A.; Buhl, T.; Wedi, B. Identification of novel biomarkers to distinguish bradykinin-mediated angioedema from mast cell-/histamine-mediated angioedema. Allergy 2021, in press. [Google Scholar] [CrossRef]
- Bircher, A.J. Drug-induced urticaria and angioedema caused by non-IgE mediated pathomechanisms. Eur. J. Dermatol. EJD 1999, 9, 657–663, quiz 663. [Google Scholar] [PubMed]
- Tachdjian, R.; Johnston, D.T. Angioedema: Differential diagnosis and acute management. Postgrad. Med. 2021, 26, 1–6. [Google Scholar] [CrossRef]
- Ocak, M.; Nain, E.; Şahiner, Ü.M.; Akin, M.; Karabiber, E.; Şekerel, B.E.; Soyer, Ö. Recurrent angioedema in childhood: Hereditary angioedema or histaminergic angioedema? Pediatr. Dermatol. 2021, 38, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.A.; Kocatürk, E.; Bauer, A.; Aygören-Pürsün, E. What’s New in the Treatment of Urticaria and Angioedema. J. Allergy Clin. Immunol. Pract. 2021, 9, 2170–2184. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, S.; Wilkerson, R.G.; Winters, M.E.; Vilke, G.M.; Wu, M.Y.C. Clinical Practice Statement: What is the Emergency Department Management of Patients with Angioedema Secondary to an ACE-Inhibitor? J. Emerg. Med. 2021, 61, 105–112. [Google Scholar] [CrossRef]
- Giard, C.; Nicolie, B.; Drouet, M.; Lefebvre-Lacoeuille, C.; Le Sellin, J.; Bonneau, J.C.; Maillard, H.; Rénier, G.; Cichon, S.; Ponard, D.; et al. Angio-oedema induced by oestrogen contraceptives is mediated by bradykinin and is frequently associated with urticaria. Dermatology 2012, 225, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Zeerleder, S.; Levi, M. Hereditary and acquired C1-inhibitor-dependent angioedema: From pathophysiology to treatment. Ann. Med. 2016, 48, 256–267. [Google Scholar] [CrossRef] [Green Version]
- Ravhe, I.S.; Krishnan, A.; Manoj, N. Evolutionary history of histamine receptors: Early vertebrate origin and expansion of the H(3)–H(4) subtypes. Mol. Phylogenetics Evol. 2021, 154, 106989. [Google Scholar] [CrossRef]
- Maciel-Guerra, H.; Penha, M.; Jorge, M.F.S.; Libório, R.D.S.; Carrijo, A.; Parise-Fortes, M.R.; Miot, H.A. Suppression of wheal and flare in histamine test by the main H1 antihistamines commercialized in Brazil. An. Bras. Dermatol. 2018, 93, 233–237. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Y.; Che, D.; Liu, R.; Yang, L.; Zhang, T.; He, L. H(1)R mediates local anesthetic-induced vascular permeability in angioedema. Toxicol. Appl. Pharmacol. 2020, 392, 114921. [Google Scholar] [CrossRef]
- Busse, P.J.; Smith, T. Histaminergic Angioedema. Immunol. Allergy Clin. N. Am. 2017, 37, 467–481. [Google Scholar] [CrossRef]
- Flynn, S.B.; Owen, D.A. Histamine H1- and H2-receptor antagonists reduce histamine-induced increases in vascular permeability and oedema formation in cat skeletal muscle. Agents Actions 1979, 9, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Argenbright, L.W.; Forbes, P.D.; Stewart, G.J. Quantitation of phototoxic hyperemia and permeability to protein: II. Inhibition by histamine (H1 and H2) receptor antagonists in mouse skin. J. Investig. Dermatol. 1980, 75, 417–420. [Google Scholar] [CrossRef]
- Mortillaro, N.A.; Granger, D.N.; Kvietys, P.R.; Rutili, G.; Taylor, A.E. Effects of histamine and histamine antagonists on intestinal capillary permeability. Am. J. Physiol. 1981, 240, G381–G386. [Google Scholar] [CrossRef]
- McLeod, R.L.; Mingo, G.G.; Kreutner, W.; Hey, J.A. Effect of combined histamine H1 and H3 receptor blockade on cutaneous microvascular permeability elicited by compound 48/80. Life Sci. 2005, 76, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Le, T.A.; Smith, W.; Hissaria, P. Real-world off-label use of icatibant for acute management of non-hereditary angioedema. Intern. Med. J. 2021, 51, 419–423. [Google Scholar] [CrossRef]
- Napolitano, F.; Montuori, N. The Role of the Plasminogen Activation System in Angioedema: Novel Insights on the Pathogenesis. J. Clin. Med. 2021, 10, 518. [Google Scholar] [CrossRef]
- Iaboni, A.; Kanani, A.; Lacuesta, G.; Song, C.; Kan, M.; Betschel, S.D. Impact of lanadelumab in hereditary angioedema: A case series of 12 patients in Canada. Allergy Asthma Clin. Immunol. 2021, 17, 78. [Google Scholar] [CrossRef]
- Banerji, A.; Bernstein, J.A.; Johnston, D.T.; Lumry, W.R.; Magerl, M.; Maurer, M.; Martinez-Saguer, I.; Zanichelli, A.; Hao, J.; Inhaber, N.; et al. Long-term prevention of hereditary angioedema attacks with lanadelumab: The Help Ole Study. Allergy 2021, in press. [Google Scholar] [CrossRef]
- Johnston, D.T.; Busse, P.J.; Riedl, M.A.; Maurer, M.; Anderson, J.; Nurse, C.; Inhaber, N.; Yu, M.; Banerji, A. Effectiveness of lanadelumab for preventing hereditary angioedema attacks: Subgroup analyses from the HELP study. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Buttgereit, T.; Vera, C.; Weller, K.; Gutsche, A.; Grekowitz, E.M.; Aykanat, S.; Wahn, V.; Krüger, R.; Maurer, M.; Magerl, M. Lanadelumab Efficacy, Safety, and Injection Interval Extension in HAE: A Real-Life Study. J. Allergy Clin. Immunol. Pract. 2021, in press. [Google Scholar] [CrossRef]
- Wedner, H.J.; Aygören-Pürsün, E.; Bernstein, J.; Craig, T.; Gower, R.; Jacobs, J.S.; Johnston, D.T.; Lumry, W.R.; Zuraw, B.L.; Best, J.M.; et al. Randomized Trial of the Efficacy and Safety of Berotralstat (BCX7353) as an Oral Prophylactic Therapy for Hereditary Angioedema: Results of APeX-2 Through 48 Weeks (Part 2). J. Allergy Clin. Immunol. Pract. 2021, 9, 2305–2314.e4. [Google Scholar] [CrossRef]
- Manning, M.E.; Kashkin, J.M. Berotralstat (BCX7353) is a novel oral prophylactic treatment for hereditary angioedema: Review of phase II and III studies. Allergy Asthma Proc. 2021, 42, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Farkas, H.; Stobiecki, M.; Peter, J.; Kinaciyan, T.; Maurer, M.; Aygören-Pürsün, E.; Kiani-Alikhan, S.; Wu, A.; Reshef, A.; Bygum, A.; et al. Long-term safety and effectiveness of berotralstat for hereditary angioedema: The open-label APeX-S study. Clin. Transl. Allergy 2021, 11, e12035. [Google Scholar] [CrossRef]
- Powell, J.; Piszczatoski, C.; Rubido, E. Orladeyo (Berotralstat): A Novel Oral Therapy for the Prevention of Hereditary Angioedema. Ann. Pharmacother. 2021, in press. [Google Scholar] [CrossRef]
- Sawada, Y.; Honda, T.; Nakamizo, S.; Otsuka, A.; Ogawa, N.; Kobayashi, Y.; Nakamura, M.; Kabashima, K. Resolvin E1 attenuates murine psoriatic dermatitis. Sci. Rep. 2018, 8, 11873. [Google Scholar] [CrossRef] [Green Version]
- Sawada, Y.; Honda, T.; Hanakawa, S.; Nakamizo, S.; Murata, T.; Ueharaguchi-Tanada, Y.; Ono, S.; Amano, W.; Nakajima, S.; Egawa, G.; et al. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J. Exp. Med. 2015, 212, 1921–1930. [Google Scholar] [CrossRef] [Green Version]
- Saito-Sasaki, N.; Sawada, Y.; Mashima, E.; Yamaguchi, T.; Ohmori, S.; Yoshioka, H.; Haruyama, S.; Okada, E.; Nakamura, M. Maresin-1 suppresses imiquimod-induced skin inflammation by regulating IL-23 receptor expression. Sci. Rep. 2018, 8, 5522. [Google Scholar] [CrossRef]
- Germann, B.; Neuhaus, W.; Hofer-Warbinek, R.; Noe, C.R. Applying blood-brain barrier in vitro models to study the influence of drugs on endothelial cells--effects of selected COX-inhibitors. Pharmazie 2008, 63, 303–307. [Google Scholar]
- Gholamreza-Fahimi, E.; Bisha, M.; Hahn, J.; Straßen, U.; Krybus, M.; Khosravani, F.; Hoffmann, T.K.; Hohlfeld, T.; Greve, J.; Bas, M.; et al. Cyclooxygenase activity in bradykinin-induced dermal extravasation. A study in mice and humans. Biomed. Pharmacother. 2020, 123, 109797. [Google Scholar] [CrossRef]
- Sawada, Y.; Honda, T.; Nakamizo, S.; Nakajima, S.; Nonomura, Y.; Otsuka, A.; Egawa, G.; Yoshimoto, T.; Nakamura, M.; Narumiya, S.; et al. Prostaglandin E(2) (PGE(2))-EP2 signaling negatively regulates murine atopic dermatitis-like skin inflammation by suppressing thymic stromal lymphopoietin expression. J. Allergy Clin. Immunol. 2019, 144, 1265–1273.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueharaguchi, Y.; Honda, T.; Kusuba, N.; Hanakawa, S.; Adachi, A.; Sawada, Y.; Otsuka, A.; Kitoh, A.; Dainichi, T.; Egawa, G.; et al. Thromboxane A(2) facilitates IL-17A production from Vγ4(+) γδ T cells and promotes psoriatic dermatitis in mice. J. Allergy Clin. Immunol. 2018, 142, 680–683.e2. [Google Scholar] [CrossRef] [Green Version]
- McLeish, K.R.; Wellhausen, S.R.; Stelzer, G.T. Mechanism of prostaglandin E2 inhibition of acute changes in vascular permeability. Inflammation 1987, 11, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Kida, T.; Hori, M.; Ozaki, H.; Murata, T. Multiple roles of the PGE2 -EP receptor signal in vascular permeability. Br. J. Pharmacol. 2014, 171, 4879–4889. [Google Scholar] [CrossRef] [Green Version]
- Konya, V.; Üllen, A.; Kampitsch, N.; Theiler, A.; Philipose, S.; Parzmair, G.P.; Marsche, G.; Peskar, B.A.; Schuligoi, R.; Sattler, W.; et al. Endothelial E-type prostanoid 4 receptors promote barrier function and inhibit neutrophil trafficking. J. Allergy Clin. Immunol. 2013, 131, 532–540.e1–2. [Google Scholar] [CrossRef]
- Frankowski, J.C.; DeMars, K.M.; Ahmad, A.S.; Hawkins, K.E.; Yang, C.; Leclerc, J.L.; Doré, S.; Candelario-Jalil, E. Detrimental role of the EP1 prostanoid receptor in blood-brain barrier damage following experimental ischemic stroke. Sci. Rep. 2015, 5, 17956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, S.M.; Cruse, G.; Cockerill, S.L.; Brightling, C.E.; Bradding, P. Engagement of the EP2 prostanoid receptor closes the K+ channel KCa3.1 in human lung mast cells and attenuates their migration. Eur. J. Immunol. 2008, 38, 2548–2556. [Google Scholar] [CrossRef]
- Kay, L.J.; Yeo, W.W.; Peachell, P.T. Prostaglandin E2 activates EP2 receptors to inhibit human lung mast cell degranulation. Br. J. Pharmacol. 2006, 147, 707–713. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, K.; Shirata, N.; Taketomi, Y.; Tsuchiya, S.; Segi-Nishida, E.; Inazumi, T.; Kabashima, K.; Tanaka, S.; Murakami, M.; Narumiya, S.; et al. Prostaglandin E2-EP3 signaling induces inflammatory swelling by mast cell activation. J. Immunol. 2014, 192, 1130–1137. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.S.; Lau, H.Y. Prostaglandin E potentiates the immunologically stimulated histamine release from human peripheral blood-derived mast cells through EP1/EP3 receptors. Allergy 2006, 61, 503–506. [Google Scholar] [CrossRef]
- Whelan, C.J.; Head, S.A.; Poll, C.T.; Coleman, R.A. Prostaglandin (PG) modulation of bradykinin-induced hyperalgesia and oedema in the guinea-pig paw--effects of PGD2, PGE2 and PGI2. Agents Actions Suppl. 1991, 32, 107–111. [Google Scholar]
- Roberts, L.J., 2nd; Sweetman, B.J.; Lewis, R.A.; Austen, K.F.; Oates, J.A. Increased production of prostaglandin D2 in patients with systemic mastocytosis. N. Engl. J. Med. 1980, 303, 1400–1404. [Google Scholar] [CrossRef] [PubMed]
- Heavey, D.J.; Kobza-Black, A.; Barrow, S.E.; Chappell, C.G.; Greaves, M.W.; Dollery, C.T. Prostaglandin D2 and histamine release in cold urticaria. J. Allergy Clin. Immunol. 1986, 78 Pt 1, 458–461. [Google Scholar] [CrossRef]
- Koro, O.; Dover, J.S.; Francis, D.M.; Kobza Black, A.; Kelly, R.W.; Barr, R.M.; Greaves, M.W. Release of prostaglandin D2 and histamine in a case of localized heat urticaria, and effect of treatments. Br. J. Dermato. 1986, 115, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Okayama, Y.; Benyon, R.C.; Lowman, M.A.; Church, M.K. In vitro effects of H1-antihistamines on histamine and PGD2 release from mast cells of human lung, tonsil, and skin. Allergy 1994, 49, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Arai, I.; Tanaka, M.; Nakaike, S. Prostaglandin D2 inhibits IgE-mediated scratching by suppressing histamine release from mast cells. J. Pharmacol. Sci. 2005, 98, 90–93. [Google Scholar] [CrossRef] [Green Version]
- Palikhe, N.S.; Kim, S.H.; Ye, Y.M.; Hur, G.Y.; Cho, B.Y.; Park, H.S. Association of CRTH2 gene polymorphisms with the required dose of antihistamines in patients with chronic urticaria. Pharmacogenomics 2009, 10, 375–383. [Google Scholar] [CrossRef]
- Horikami, D.; Toya, N.; Kobayashi, K.; Omori, K.; Nagata, N.; Murata, T. L-PGDS-derived PGD(2) attenuates acute lung injury by enhancing endothelial barrier formation. J. Pathol. 2019, 248, 280–290. [Google Scholar] [CrossRef]
- Ke, Y.; Oskolkova, O.V.; Sarich, N.; Tian, Y.; Sitikov, A.; Tulapurkar, M.E.; Son, S.; Birukova, A.A.; Birukov, K.G. Effects of prostaglandin lipid mediators on agonist-induced lung endothelial permeability and inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L710–L721. [Google Scholar] [CrossRef]
- Sarashina, H.; Tsubosaka, Y.; Omori, K.; Aritake, K.; Nakagawa, T.; Hori, M.; Hirai, H.; Nakamura, M.; Narumiya, S.; Urade, Y.; et al. Opposing immunomodulatory roles of prostaglandin D2 during the progression of skin inflammation. J. Immunol. 2014, 192, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Tsubosaka, Y.; Hori, M.; Narumiya, S.; Ozaki, H.; Murata, T. Prostaglandin D2-DP signaling promotes endothelial barrier function via the cAMP/PKA/Tiam1/Rac1 pathway. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 565–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, T.; Fujiwara, Y.; Yamada, R.; Fujii, W.; Hamabata, T.; Lee, M.Y.; Maeda, S.; Aritake, K.; Roers, A.; Sessa, W.C.; et al. Mast cell-derived prostaglandin D(2) attenuates anaphylactic reactions in mice. J. Allergy Clin. Immunol. 2017, 140, 630–632.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marks, J.G., Jr.; Trautlein, J.J.; Zwillich, C.W.; Demers, L.M. Contact urticaria and airway obstruction from carbonless copy paper. JAMA 1984, 252, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Svanborg, N.; Hedqvist, P.; Gréen, K. Aspects of prostaglandin action in asthma. Adv. Prostaglandin Thromboxane Res. 1976, 1, 439–447. [Google Scholar] [PubMed]
- Divekar, R.; Butterfield, J. Urinary 11β-PGF2α and N-methyl histamine correlate with bone marrow biopsy findings in mast cell disorders. Allergy 2015, 70, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Efimova, O.; Volokhov, A.; Hales, C.A. Injection of prostaglandin F2alpha into the bronchial artery in sheep increases the pulmonary vascular permeability to protein. Pulm. Pharmacol. Ther. 2007, 20, 167–171. [Google Scholar] [CrossRef]
- Platshon, L.F.; Kaliner, M. The effects of the immunologic release of histamine upon human lung cyclic nucleotide levels and prostaglandin generation. J. Clin. Investig. 1978, 62, 1113–1121. [Google Scholar] [CrossRef] [Green Version]
- Burka, J.F.; Garland, L.G. A possible modulatory role for prostacyclin (PGI2) INIgGa-induced release of slow-reacting substance of anaphylaxis in rats. Br. J. Pharmacol. 1977, 61, 697–699. [Google Scholar] [CrossRef]
- Forrest, M.J.; Jose, P.J.; Williams, T.J. Kinetics of the generation and action of chemical mediators in zymosan-induced inflammation of the rabbit peritoneal cavity. Br. J. Pharmacol. 1986, 89, 719–730. [Google Scholar] [CrossRef] [Green Version]
- Komoriya, K.; Ohmori, H.; Azuma, A.; Kurozumi, S.; Hashimoto, Y.; Nicolaou, K.C.; Barnette, W.E.; Magolda, R.L. Prostaglandin I2 as a potentiator of acute inflammation in rats. Prostaglandins 1978, 15, 557–564. [Google Scholar] [CrossRef]
- Neppl, H.; Neuhof, H.; Afflerbach, F.; Llach Puig Neppl, J.; Berghöfer, A. Bradykinin-induced oedema formation proceeds from B2 receptor stimulation and is potentiated by concomitantly released prostaglandins. Acta Physiol. Scand. 1991, 142, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.J.; Eichelberg, D.; Schmutzler, W. The effects of prostaglandins of the E and I type on histamine release from human adenoidal mast cells. Agents Actions 1986, 18, 113–114. [Google Scholar] [CrossRef]
- Mita, H.; Ishii, T.; Akiyama, K. Generation of thromboxane A2 from highly purified human sinus mast cells after immunological stimulation. Prostaglandins Leukot. Essent. Fatty Acids 1999, 60, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Berti, F.; Folco, G.C.; Nicosia, S.; Omini, C.; Pasargiklian, R. The role of histamine H1- and H2-receptors in the generation of thromboxane A2 in perfused guinea-pig lungs. Br. J. Pharmacol. 1979, 65, 629–633. [Google Scholar] [CrossRef]
- Garcia-Szabo, R.R.; Peterson, M.B.; Watkins, W.D.; Bizios, R.; Kong, D.L.; Malik, A.B. Thromboxane generation after thrombin. Protective effect of thromboxane synthetase inhibition on lung fluid balance. Circ. Res. 1983, 53, 214–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeger, W.; Ernst, C.; Walmrath, D.; Neuhof, H.; Roka, L. Influence of the thromboxane antagonist BM 13.177 on the arachidonic acid-induced increase in pulmonary vascular resistance and permeability in rabbit lungs. Thromb. Res. 1985, 40, 793–805. [Google Scholar] [CrossRef]
- Yamasaki, M.; Matsumoto, T.; Fukuda, S.; Nakayama, T.; Nagaya, H.; Ashida, Y. Involvement of thromboxane A2 and histamine in experimental allergic rhinitis of guinea pigs. J. Pharmacol. Exp. Ther. 1997, 280, 1471–1479. [Google Scholar] [PubMed]
- Kim, S.R.; Bae, S.K.; Park, H.J.; Kim, M.K.; Kim, K.; Park, S.Y.; Jang, H.O.; Yun, I.; Kim, Y.J.; Yoo, M.A.; et al. Thromboxane A(2) increases endothelial permeability through upregulation of interleukin-8. Biochem. Biophys. Res. Commun. 2010, 397, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, S.; Ferrara, A.L.; Bova, M.; Borriello, F.; Suffritti, C.; Veszeli, N.; Petraroli, A.; Galdiero, M.R.; Varricchi, G.; Granata, F.; et al. Secreted Phospholipases A(2) in Hereditary Angioedema With C1-Inhibitor Deficiency. Front. Immunol. 2018, 9, 1721. [Google Scholar] [CrossRef]
- Jurado-Escobar, R.; Doña, I.; Triano-Cornejo, J.; Perkins, J.R.; Pérez-Sánchez, N.; Testera-Montes, A.; Labella, M.; Bartra, J.; Laguna, J.J.; Estravís, M.; et al. Genetic Variants in Cytosolic Phospholipase A2 Associated with Nonsteroidal Anti-Inflammatory Drug-Induced Acute Urticaria/Angioedema. Front. Pharmacol. 2021, 12, 667824. [Google Scholar] [CrossRef]
- Rådmark, O.; Werz, O.; Steinhilber, D.; Samuelsson, B. 5-Lipoxygenase: Regulation of expression and enzyme activity. Trends Biochem. Sci. 2007, 32, 332–341. [Google Scholar] [CrossRef]
- Erlansson, M.; Svensjö, E.; Bergqvist, D. Leukotriene B4-induced permeability increase in postcapillary venules and its inhibition by three different antiinflammatory drugs. Inflammation 1989, 13, 693–705. [Google Scholar] [CrossRef]
- Griswold, D.E.; Webb, E.F.; Hillegass, L.M. Induction of plasma exudation and inflammatory cell infiltration by leukotriene C4 and leukotriene B4 in mouse peritonitis. Inflammation 1991, 15, 251–258. [Google Scholar] [CrossRef]
- Soter, N.A.; Lewis, R.A.; Corey, E.J.; Austen, K.F. Local effects of synthetic leukotrienes (LTC4, LTD4, LTE4, and LTB4) in human skin. J. Investig. Dermatol. 1983, 80, 115–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, D.G.; Coutinho, S.F.; Silveira, M.R.; Cara, D.C.; Teixeira, M.M. Effects of a BLT receptor antagonist on local and remote reperfusion injuries after transient ischemia of the superior mesenteric artery in rats. Eur. J. Pharmacol. 2000, 403, 121–128. [Google Scholar] [CrossRef]
- Souza, D.G.; Pinho, V.; Cassali, G.D.; Poole, S.; Teixeira, M.M. Effect of a BLT receptor antagonist in a model of severe ischemia and reperfusion injury in the rat. Eur. J. Pharmacol. 2002, 440, 61–69. [Google Scholar] [CrossRef]
- Shigematsu, M.; Koga, T.; Ishimori, A.; Saeki, K.; Ishii, Y.; Taketomi, Y.; Ohba, M.; Jo-Watanabe, A.; Okuno, T.; Harada, N.; et al. Leukotriene B(4) receptor type 2 protects against pneumolysin-dependent acute lung injury. Sci. Rep. 2016, 6, 34560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkin, S.W.; Fraser, L.; Showell, H.J.; Williams, T.J.; Warren, J.B. Prolonged microvascular vasodilation induced by leukotriene B4 in human skin is cyclooxygenase independent. J. Pharmacol. Exp. Ther. 1995, 272, 392–398. [Google Scholar] [PubMed]
- Aoki, Y.; Qiu, D.; Zhao, G.H.; Kao, P.N. Leukotriene B4 mediates histamine induction of NF-kappaB and IL-8 in human bronchial epithelial cells. Am. J. Physiol. 1998, 274, L1030–L1039. [Google Scholar] [PubMed]
- Koyama, S.; Rennard, S.I.; Robbins, R.A. Bradykinin stimulates bronchial epithelial cells to release neutrophil and monocyte chemotactic activity. Am. J. Physiol. 1995, 269 Pt 1, L38–L44. [Google Scholar] [CrossRef]
- Bigby, T.D. The leukotriene C(4) synthase gene and asthma. Am. J. Respir. Cell Mol. Biol. 2000, 23, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Ueno, A.; Tanaka, K.; Katori, M.; Hayashi, M.; Arai, Y. Species difference in increased vascular permeability by synthetic leukotriene C4 and D4. Prostaglandins 1981, 21, 637–648. [Google Scholar] [CrossRef]
- Rinkema, L.E.; Bemis, K.G.; Fleisch, J.H. Production and antagonism of cutaneous vascular permeability in the guinea pig in response to histamine, leukotrienes and A23187. J. Pharmacol. Exp. Ther. 1984, 230, 550–557. [Google Scholar] [PubMed]
- Inagaki, N.; Goto, S.; Yamasaki, M.; Nagai, H.; Koda, A. Studies on vascular permeability increasing factors involved in 48-hour homologous PCA in the mouse ear. Int. Arch. Allergy Appl. Immunol. 1986, 80, 285–290. [Google Scholar] [CrossRef]
- Chan, C.C.; Ford-Hutchinson, A. Effects of synthetic leukotrienes on local blood flow and vascular permeability in porcine skin. J. Investig. Dermatol. 1985, 84, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, H.; Lerche, A.; Kristensen, J.K. Leukotriene- and histamine-induced increases in vascular permeability and interstitial transport in the skin. J. Investig. Dermatol. 1985, 84, 427–429. [Google Scholar] [CrossRef] [Green Version]
- Bryan, D.L.; Forsyth, K.D.; Hart, P.H.; Gibson, R.A. Polyunsaturated fatty acids regulate cytokine and prostaglandin E2 production by respiratory cells in response to mast cell mediators. Lipids 2006, 41, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Gueck, T.; Seidel, A.; Fuhrmann, H. Consequences of eicosapentaenoic acid (n-3) and arachidonic acid (n-6) supplementation on mast cell mediators. J. Anim. Physiol. Anim. Nutr. 2004, 88, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Saito, P.; Melo, C.P.B.; Martinez, R.M.; Fattori, V.; Cezar, T.L.C.; Pinto, I.C.; Bussmann, A.J.C.; Vignoli, J.A.; Georgetti, S.R.; Baracat, M.M.; et al. The Lipid Mediator Resolvin D1 Reduces the Skin Inflammation and Oxidative Stress Induced by UV Irradiation in Hairless Mice. Front. Pharmacol. 2018, 9, 1242. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, G.D.; Jin, Y.H.; Park, Y.S.; Park, C.S. Omega-3 fatty acid-derived mediator, Resolvin E1, ameliorates 2,4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice. Int. Immunopharmacol. 2012, 14, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Cezar, T.L.C.; Martinez, R.M.; Rocha, C.D.; Melo, C.P.B.; Vale, D.L.; Borghi, S.M.; Fattori, V.; Vignoli, J.A.; Camilios-Neto, D.; Baracat, M.M.; et al. Treatment with maresin 1, a docosahexaenoic acid-derived pro-resolution lipid, protects skin from inflammation and oxidative stress caused by UVB irradiation. Sci. Rep. 2019, 9, 3062. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H.; Wang, L.; Yao, C.; Yuan, R.; Wu, Q. Resolvin D1 reduces deterioration of tight junction proteins by upregulating HO-1 in LPS-induced mice. Lab. Investig. 2013, 93, 991–1000. [Google Scholar] [CrossRef] [Green Version]
- Sawada, Y.; Nakatsuji, T.; Dokoshi, T.; Kulkarni, N.N.; Liggins, M.C.; Sen, G.; Gallo, R.L. Cutaneous innate immune tolerance is mediated by epigenetic control of MAP2K3 by HDAC8/9. Sci. Immunol. 2021, 6, eabe1935. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Gallo, R.L. Role of Epigenetics in the Regulation of Immune Functions of the Skin. J. Investig. Dermatol. 2021, 141, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, M.; Usami, M.; Ohata, A. Short-chain fatty acids and trichostatin A alter tight junction permeability in human umbilical vein endothelial cells. Nutrition 2008, 24, 1189–1198. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Yu, Z.; Zhang, F.; Huang, L.; Xing, C.; Liu, N.; Xu, Y.; Wang, X. HDAC3 inhibition prevents oxygen glucose deprivation/reoxygenation-induced transendothelial permeability by elevating PPARγ activity in vitro. J. Neurochem. 2019, 149, 298–310. [Google Scholar] [CrossRef]
- Shi, W.; Wei, X.; Wang, Z.; Han, H.; Fu, Y.; Liu, J.; Zhang, Y.; Guo, J.; Dong, C.; Zhou, D.; et al. HDAC9 exacerbates endothelial injury in cerebral ischaemia/reperfusion injury. J. Cell Mol. Med. 2016, 20, 1139–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, O.; Altintas, D.; Rondon, C.; Cingi, C.; Oghan, F. Effectiveness of montelukast in pediatric patients with allergic rhinitis. Int. J. Pediatric Otorhinolaryngol. 2013, 77, 1922–1924. [Google Scholar] [CrossRef]
- Braido, F.; Riccio, A.M.; Rogkakou, A.; Massacane, P.; Guerra, L.; Fumagalli, F.; Stagi, E.; Balestracci, S.; Porcu, A.; Canonica, G.W. Montelukast effects on inflammation in allergic rhinitis: A double blind placebo controlled pilot study. Eur. Ann. Allergy Clin. Immunol. 2012, 44, 48–53. [Google Scholar] [PubMed]
- Wightman, F.; Lu, H.K.; Solomon, A.E.; Saleh, S.; Harman, A.N.; Cunningham, A.L.; Gray, L.; Churchill, M.; Cameron, P.U.; Dear, A.E.; et al. Entinostat is a histone deacetylase inhibitor selective for class 1 histone deacetylases and activates HIV production from latently infected primary T cells. AIDS 2013, 27, 2853–2862. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Xie, X.; Gong, P.; Wei, Y.; Du, H.; Xu, Y.; Xu, Q.; Jing, Y.; Zhao, L. A 18β-glycyrrhetinic acid conjugate with Vorinostat degrades HDAC3 and HDAC6 with improved antitumor effects. Eur. J. Med. Chem. 2020, 188, 111991. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, A.; Sawada, Y.; Sugino, H.; Nakamura, M. Angioedema and Fatty Acids. Int. J. Mol. Sci. 2021, 22, 9000. https://doi.org/10.3390/ijms22169000
Wada A, Sawada Y, Sugino H, Nakamura M. Angioedema and Fatty Acids. International Journal of Molecular Sciences. 2021; 22(16):9000. https://doi.org/10.3390/ijms22169000
Chicago/Turabian StyleWada, Akane, Yu Sawada, Hitomi Sugino, and Motonobu Nakamura. 2021. "Angioedema and Fatty Acids" International Journal of Molecular Sciences 22, no. 16: 9000. https://doi.org/10.3390/ijms22169000
APA StyleWada, A., Sawada, Y., Sugino, H., & Nakamura, M. (2021). Angioedema and Fatty Acids. International Journal of Molecular Sciences, 22(16), 9000. https://doi.org/10.3390/ijms22169000




