Characterization of Osteogenesis and Chondrogenesis of Human Decellularized Allogeneic Bone with Mesenchymal Stem Cells Derived from Bone Marrow, Adipose Tissue, and Wharton’s Jelly
Abstract
:1. Introduction
2. Results
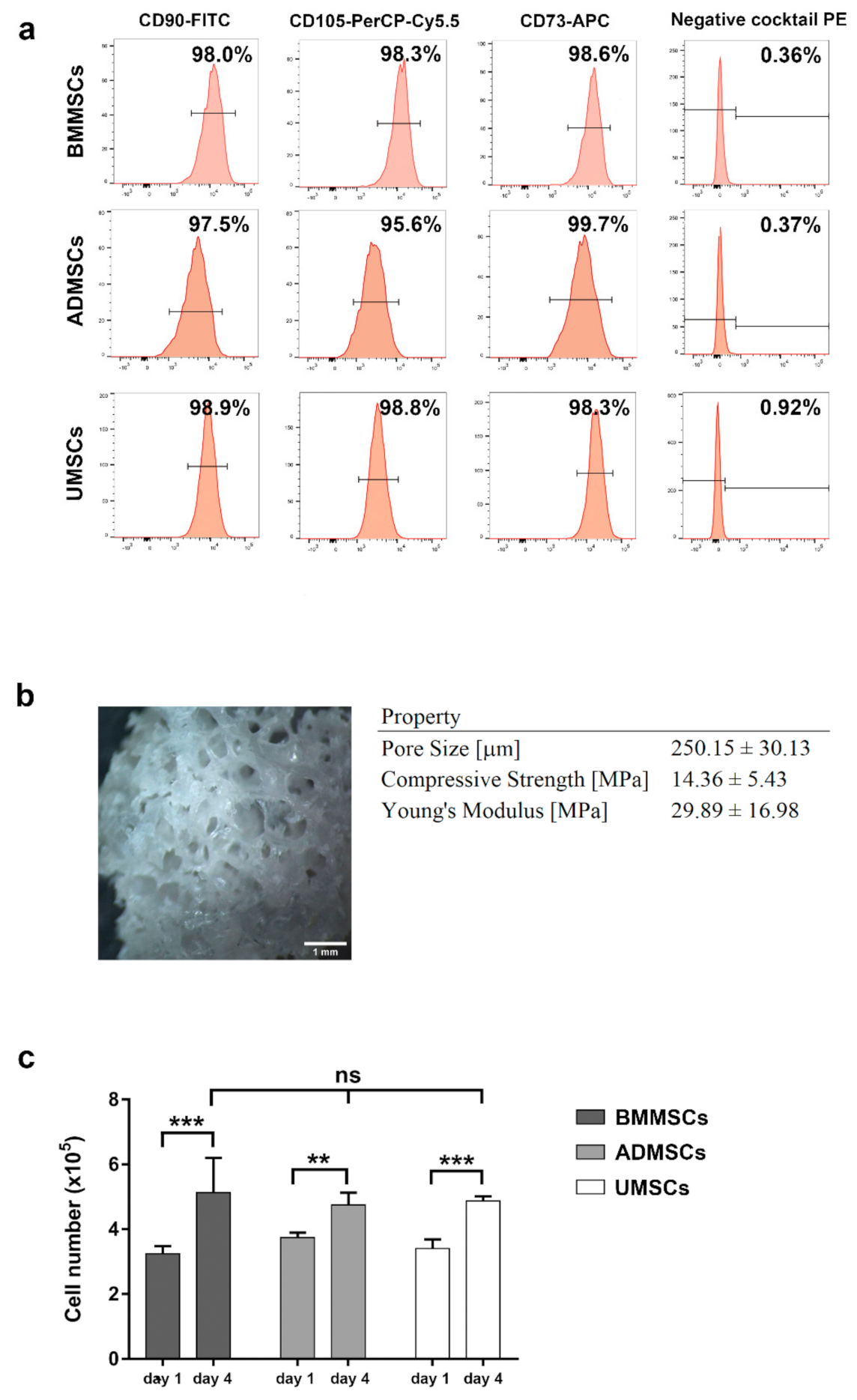
2.1. Characterization of hMSCs and hDCB
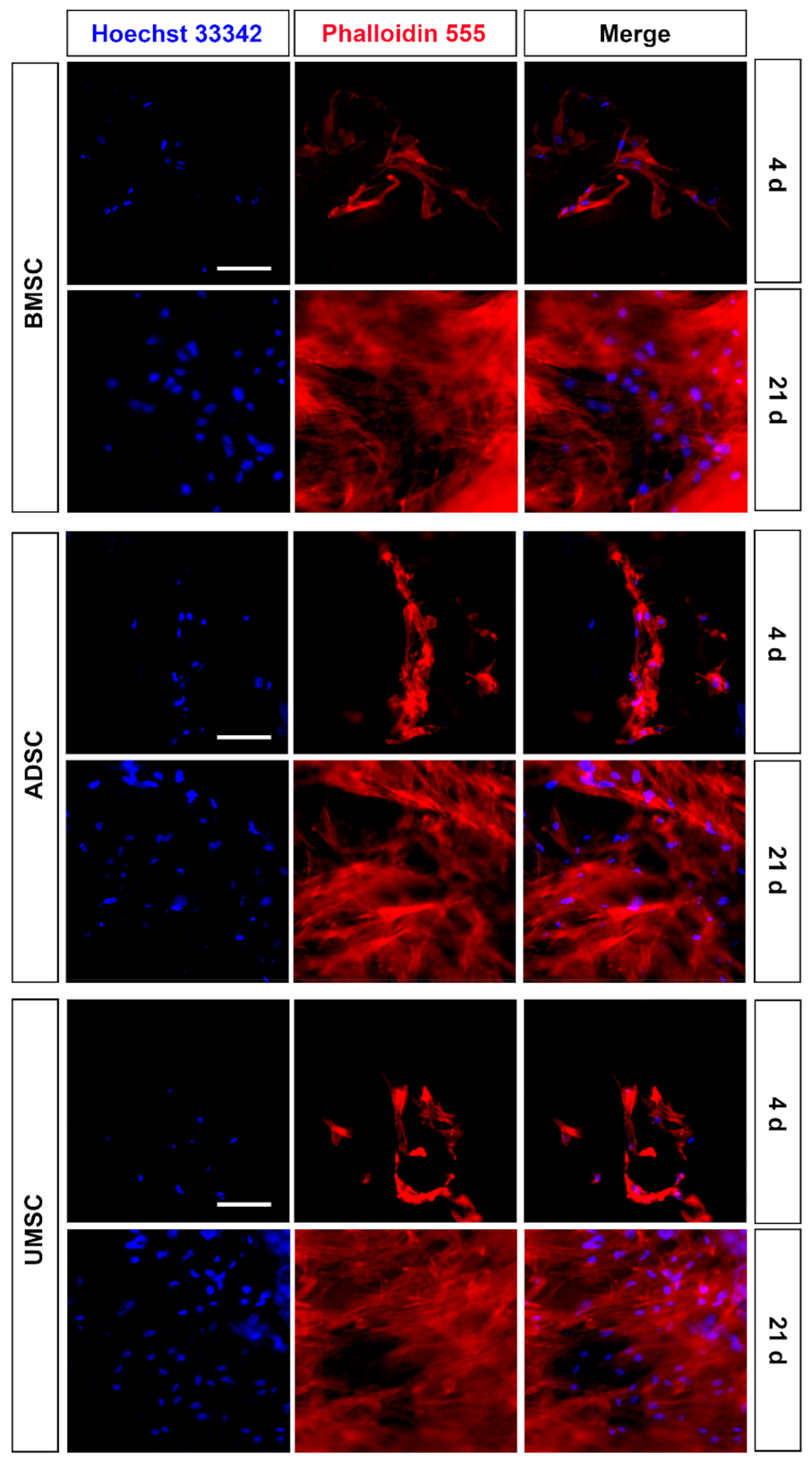
2.2. Proliferation and Adhesion of hMSCs on hDCB Blocks
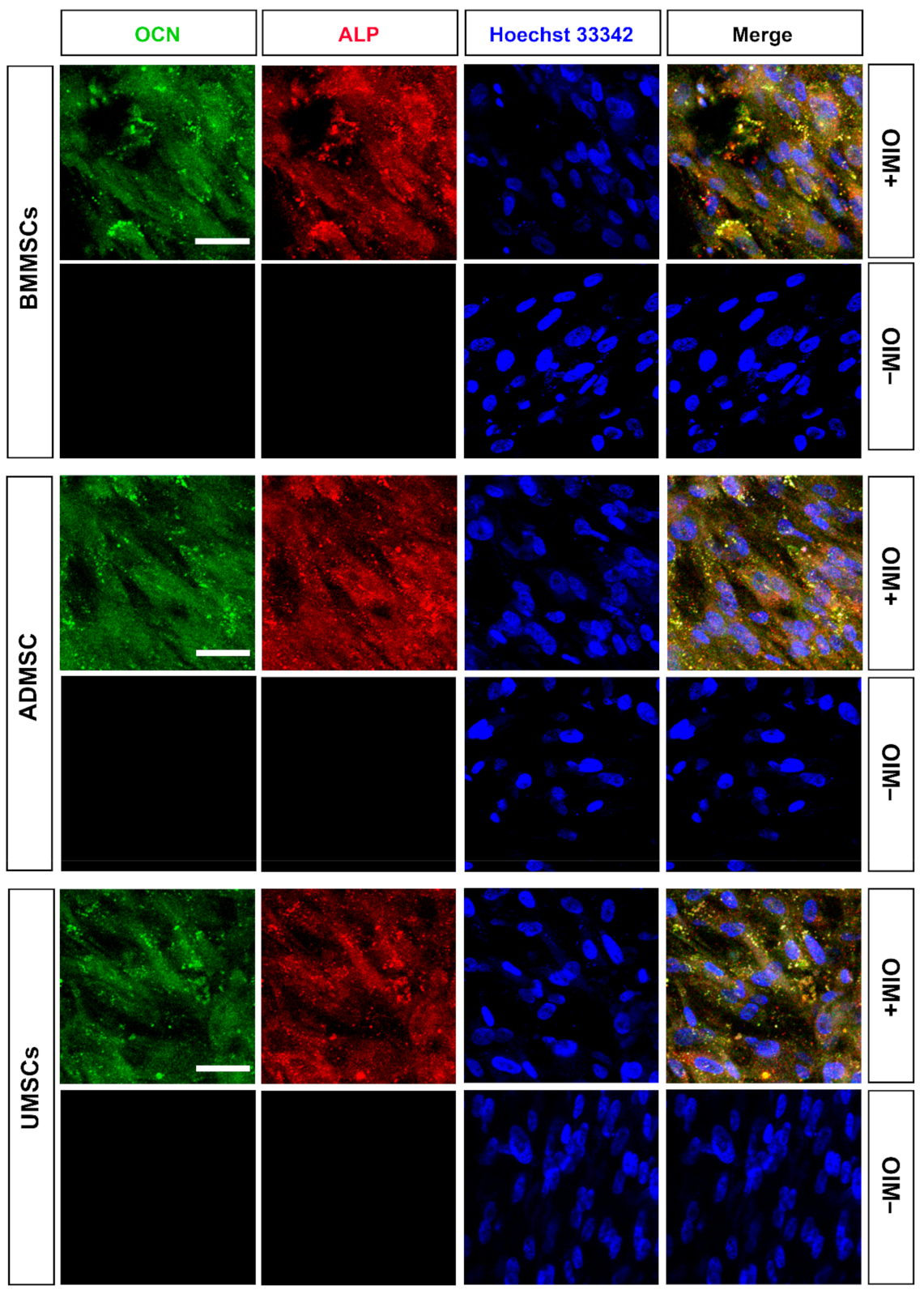
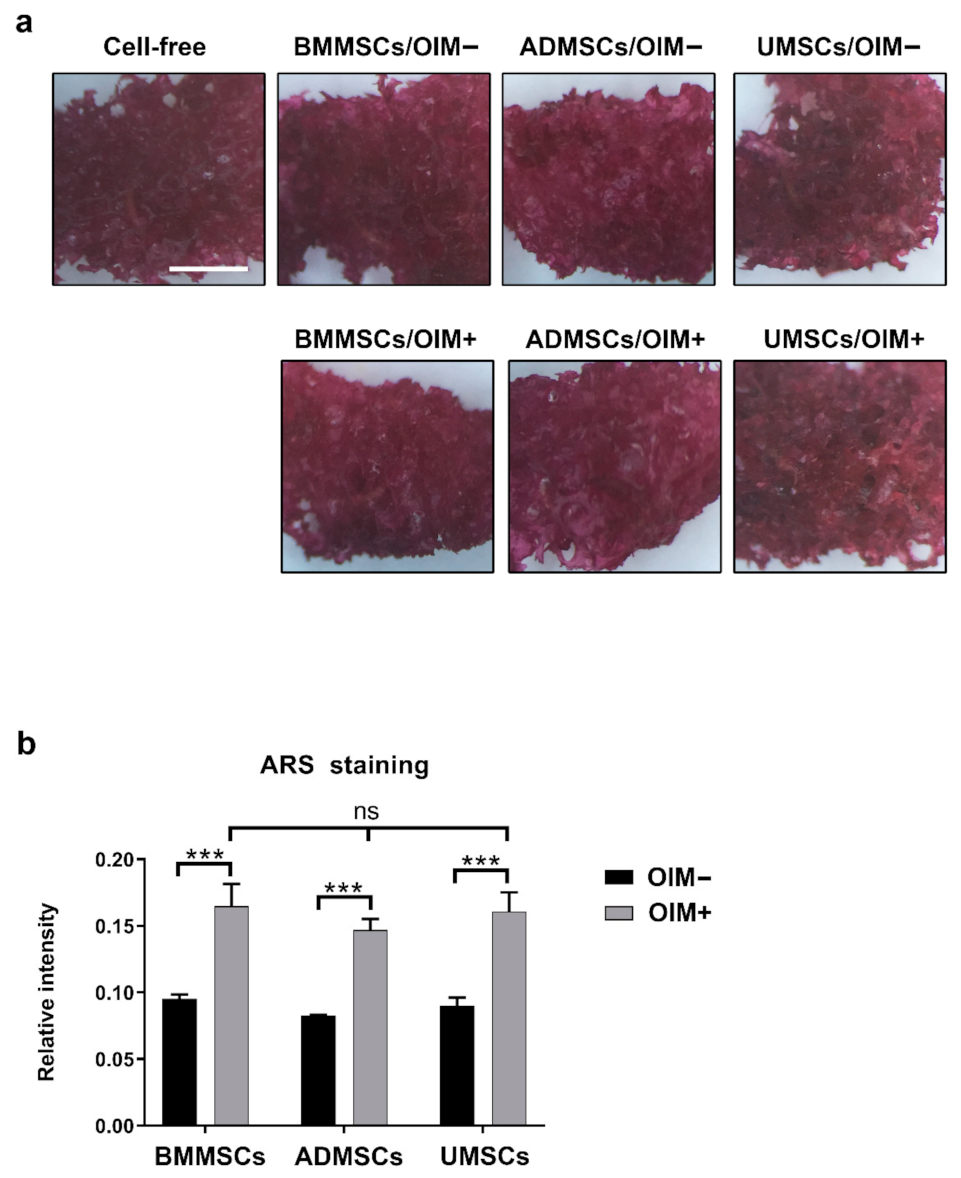
2.3. Production of Osteogenic Proteins and Calcium Deposition of hMSCs on hDCB Blocks
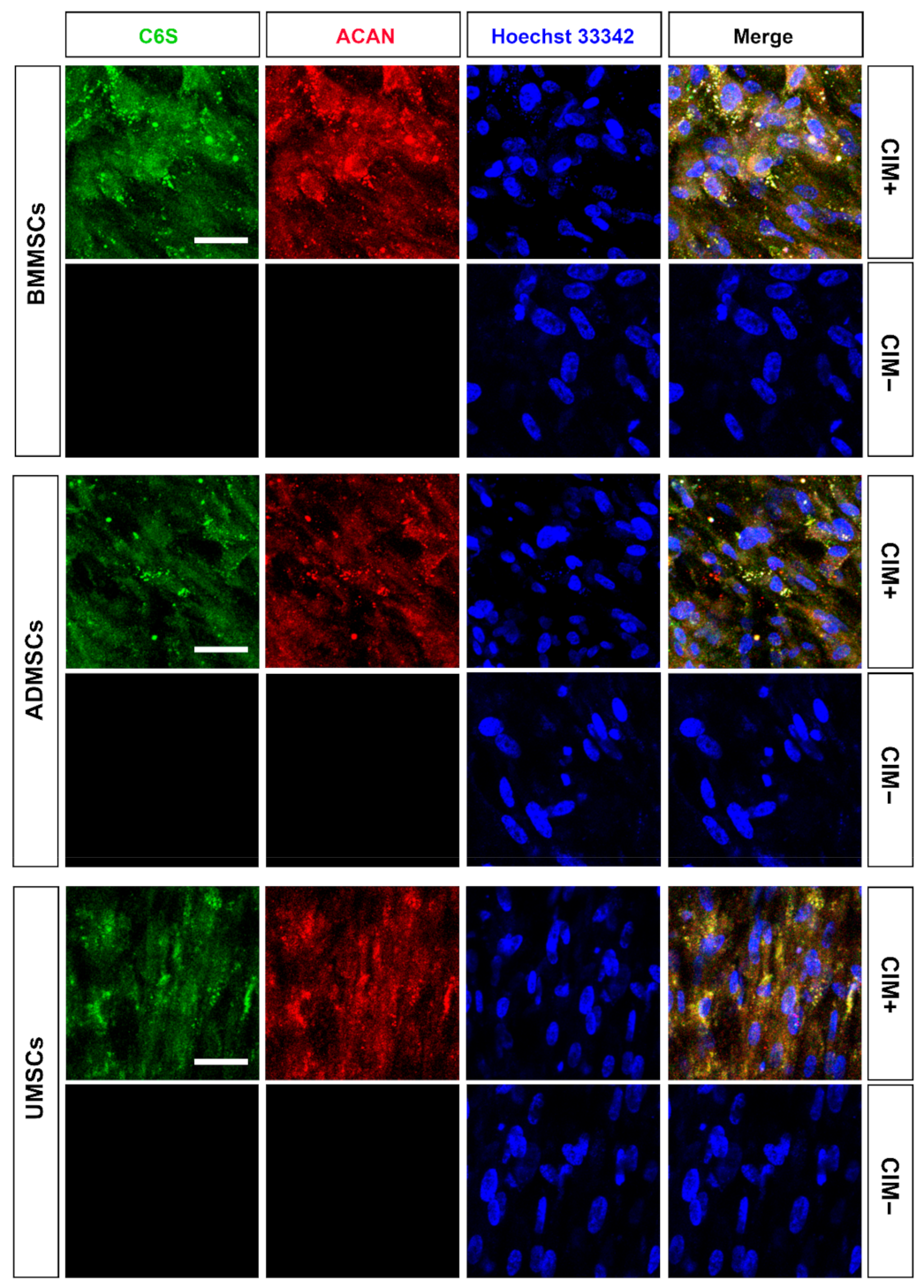
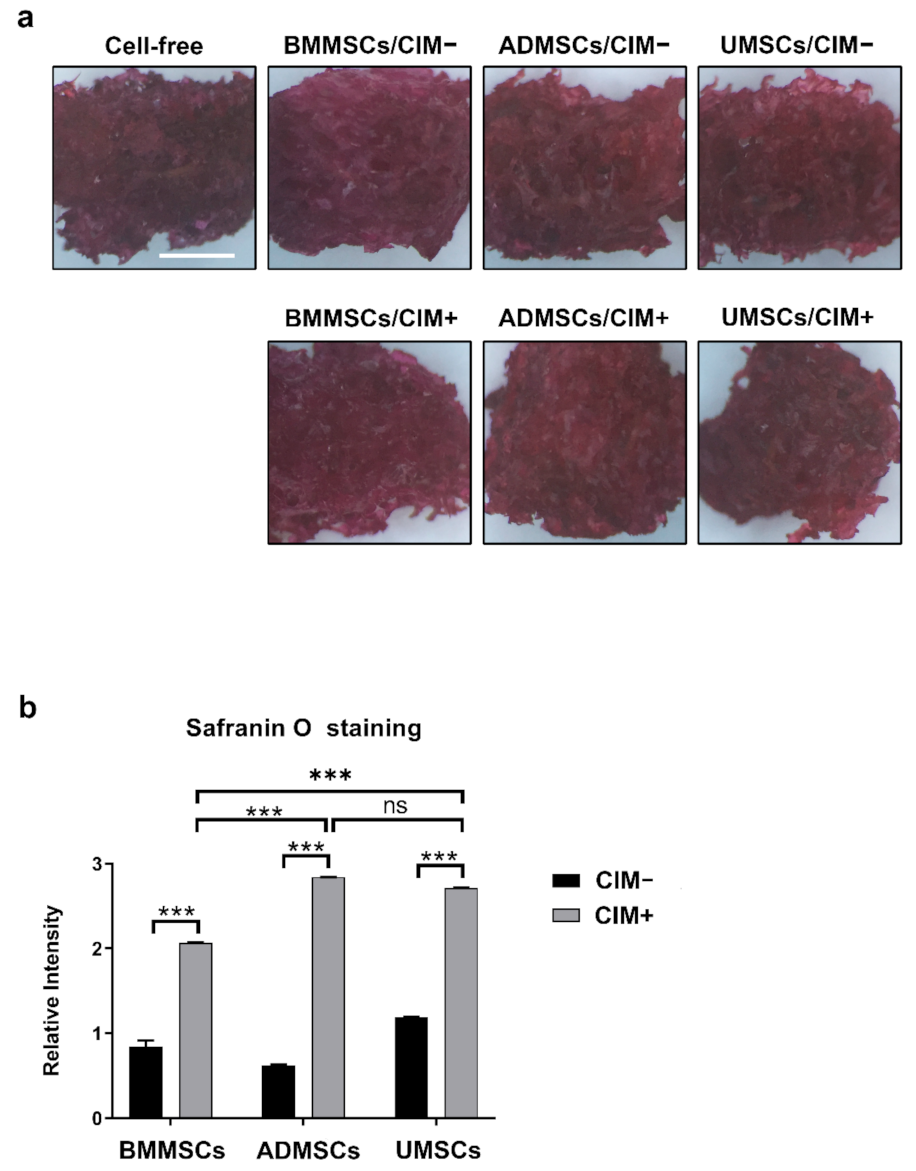
2.4. Production of Chondrogenic Proteins and Proteoglycans of hMSCs on hDCB Blocks
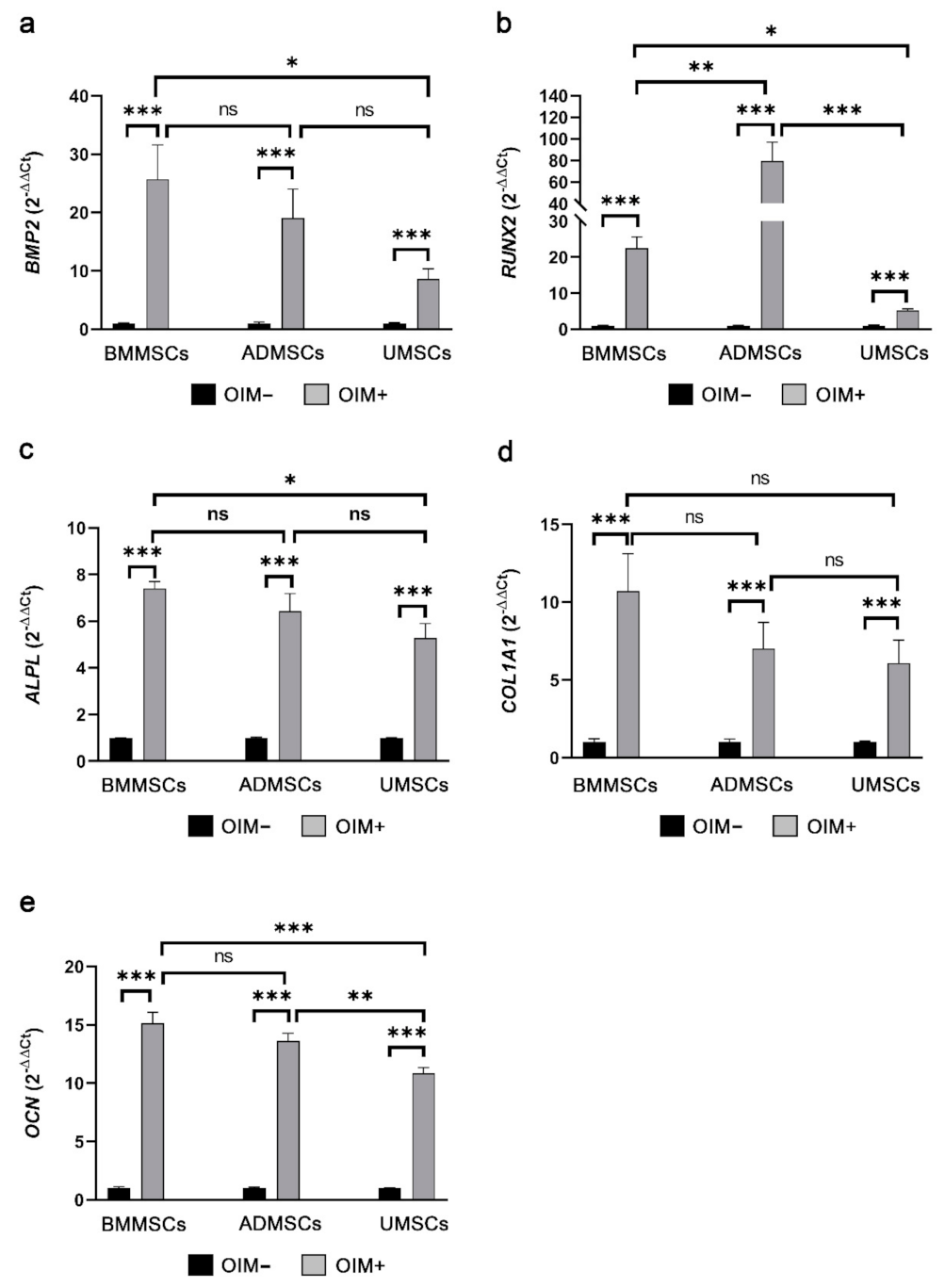
2.5. Comparison of Osteogenic and Chondrogenic Gene Expression Levels of hMSCs on hDCB Blocks Using Real-Time RT-qPCR
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Isolation and Expansion of Human MSCs
5.2. Processing of Allogeneic Bone
5.3. Characterization of hDCB Block
5.4. Osteogenic and Chondrogenic Differentiation of hMSCs
5.5. MTT Assay
5.6. Immunofluorescence
5.7. Alizarin Red S Staining
5.8. Safranin O Staining
5.9. Real-Time Quantitative Polymerase Chain Reaction Analysis of Osteogenic and Chondrogenic Genes
5.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, S.; Tapasvi, S.R. Osteochondral autografts. Curr. Rev. Musculoskelet. Med. 2015, 8, 423–428. [Google Scholar] [CrossRef] [Green Version]
- Rothrauff, B.B.; Tuan, R.S. Decellularized bone extracellular matrix in skeletal tissue engineering. Biochem. Soc. Trans. 2020, 48, 755–764. [Google Scholar] [CrossRef]
- Chen, G.; Lv, Y. Decellularized Bone Matrix Scaffold for Bone Regeneration. Methods Mol. Biol. 2018, 1577, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Beane, O.S.; Darling, E.M. Isolation, characterization, and differentiation of stem cells for cartilage regeneration. Ann. Biomed. Eng. 2012, 40, 2079–2097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, H.; Xu, W.; Zhuang, X.; Chang, F.; Wang, Y.; Ding, J. Mesenchymal stem cells for cartilage regeneration. J. Tissue Eng. 2020, 11, 2041731420943839. [Google Scholar] [CrossRef]
- Iijima, K.; Otsuka, H. Cell Scaffolds for Bone Tissue Engineering. Bioengineering 2020, 7, 119. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [Green Version]
- Bianco, P.; Riminucci, M.; Gronthos, S.; Robey, P.G. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells 2001, 19, 180–192. [Google Scholar] [CrossRef] [Green Version]
- Kemp, K.C.; Hows, J.; Donaldson, C. Bone marrow-derived mesenchymal stem cells. Leuk. Lymphoma 2005, 46, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- Minteer, D.; Marra, K.G.; Rubin, J.P. Adipose-derived mesenchymal stem cells: Biology and potential applications. Adv. Biochem. Eng. Biotechnol. 2013, 129, 59–71. [Google Scholar] [CrossRef]
- Bongso, A.; Fong, C.Y. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton’s jelly of the human umbilical cord. Stem Cell Rev. Rep. 2013, 9, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.; Niemeyer, P.; Salzmann, G.; Sudkamp, N.P.; Hube, R.; Klehm, J.; Menzel, M.; von Eisenhart-Rothe, R.; Bohner, M.; Gorz, L.; et al. Microporous calcium phosphate ceramics as tissue engineering scaffolds for the repair of osteochondral defects: Histological results. Acta Biomater. 2013, 9, 7490–7505. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.G.; Shah, K.; Freitag, J.; Cromer, B.; Sumer, H. Differentiation Potential of Early- and Late-Passage Adipose-Derived Mesenchymal Stem Cells Cultured under Hypoxia and Normoxia. Stem Cells Int. 2020, 2020, 8898221. [Google Scholar] [CrossRef]
- Wall, M.E.; Bernacki, S.H.; Loboa, E.G. Effects of serial passaging on the adipogenic and osteogenic differentiation potential of adipose-derived human mesenchymal stem cells. Tissue Eng. 2007, 13, 1291–1298. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Chi, Y.; Zhang, Q.; Xu, F.; Yang, Z.; Meng, L.; Yang, S.; Yan, S.; Mao, A.; et al. Long-term cultured mesenchymal stem cells frequently develop genomic mutations but do not undergo malignant transformation. Cell Death Dis. 2013, 4, e950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lembong, J.; Kirian, R.; Takacs, J.D.; Olsen, T.R.; Lock, L.T.; Rowley, J.A.; Ahsan, T. Bioreactor Parameters for Microcarrier-Based Human MSC Expansion under Xeno-Free Conditions in a Vertical-Wheel System. Bioengineering 2020, 7, 73. [Google Scholar] [CrossRef]
- Chen, X.; Fan, H.; Deng, X.; Wu, L.; Yi, T.; Gu, L.; Zhou, C.; Fan, Y.; Zhang, X. Scaffold Structural Microenvironmental Cues to Guide Tissue Regeneration in Bone Tissue Applications. Nanomaterials 2018, 8, 960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, S.; Mancardi, D.; Merlino, A.; Bussolati, B.; Munaron, L. Hypoxia and hydrogen sulfide differentially affect normal and tumor-derived vascular endothelium. Redox Biol. 2017, 12, 499–504. [Google Scholar] [CrossRef]
- Lim, T.C.; Chian, K.S.; Leong, K.F. Cryogenic prototyping of chitosan scaffolds with controlled micro and macro architecture and their effect on in vivo neo-vascularization and cellular infiltration. J. Biomed. Mater. Res. A 2010, 94, 1303–1311. [Google Scholar] [CrossRef]
- Fu, Q.; Rahaman, M.N.; Bal, B.S.; Brown, R.F. Preparation and in vitro evaluation of bioactive glass (13–93) scaffolds with oriented microstructures for repair and regeneration of load-bearing bones. J. Biomed. Mater. Res. A 2010, 93, 1380–1390. [Google Scholar] [CrossRef]
- Im, G.I.; Ko, J.Y.; Lee, J.H. Chondrogenesis of adipose stem cells in a porous polymer scaffold: Influence of the pore size. Cell Transpl. 2012, 21, 2397–2405. [Google Scholar] [CrossRef]
- Gerhardt, L.C.; Boccaccini, A.R. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [Green Version]
- Guthold, M.; Liu, W.; Sparks, E.A.; Jawerth, L.M.; Peng, L.; Falvo, M.; Superfine, R.; Hantgan, R.R.; Lord, S.T. A comparison of the mechanical and structural properties of fibrin fibers with other protein fibers. Cell Biochem. Biophys. 2007, 49, 165–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izadifar, Z.; Chen, X.; Kulyk, W. Strategic design and fabrication of engineered scaffolds for articular cartilage repair. J. Funct. Biomater 2012, 3, 799–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, Y.S.; Chen, W.C.; Huang, C.H.; Cheng, C.K.; Chan, K.K.; Chang, T.K. The effect of graft strength on knee laxity and graft in-situ forces after posterior cruciate ligament reconstruction. PLoS ONE 2015, 10, e0127293. [Google Scholar] [CrossRef] [Green Version]
- Labutin, D.; Vorobyov, K.; Bozhkova, S.; Polyakova, E.; Vodopyanova, T. Human bone graft cytocompatibility with mesenchymal stromal cells is comparable after thermal sterilization and washing followed by gamma-irradiation: An in vitro study. Regen. Biomater. 2018, 5, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.S.; Choi, Y.; Kim, H.S.; Kim, H.O. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int. J. Mol. Med. 2016, 37, 115–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasch, A.; Naujokat, H.; Wang, F.; Seekamp, A.; Fuchs, S.; Kluter, T. Evaluation of bone allograft processing methods: Impact on decellularization efficacy, biocompatibility and mesenchymal stem cell functionality. PLoS ONE 2019, 14, e0218404. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.J.; Jiang, D.; Zhang, Z.Z.; Huang, A.B.; Qi, Y.S.; Wang, H.J.; Zhang, J.Y.; Yu, J.K. Chondrogenic Potential of Peripheral Blood Derived Mesenchymal Stem Cells Seeded on Demineralized Cancellous Bone Scaffolds. Sci. Rep. 2016, 6, 36400. [Google Scholar] [CrossRef] [Green Version]
- Motamedian, S.R.; Tabatabaei, F.S.; Akhlaghi, F.; Torshabi, M.; Gholamin, P.; Khojasteh, A. Response of Dental Pulp Stem Cells to Synthetic, Allograft, and Xenograft Bone Scaffolds. Int. J. Periodontics Restor. Dent. 2017, 37, 49–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rath, S.N.; Nooeaid, P.; Arkudas, A.; Beier, J.P.; Strobel, L.A.; Brandl, A.; Roether, J.A.; Horch, R.E.; Boccaccini, A.R.; Kneser, U. Adipose- and bone marrow-derived mesenchymal stem cells display different osteogenic differentiation patterns in 3D bioactive glass-based scaffolds. J. Tissue Eng. Regen. Med. 2016, 10, E497–E509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Teoh, S.H.; Chong, M.S.; Schantz, J.T.; Fisk, N.M.; Choolani, M.A.; Chan, J. Superior osteogenic capacity for bone tissue engineering of fetal compared with perinatal and adult mesenchymal stem cells. Stem Cells 2009, 27, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Mozafari, M.; Hashemian, S.J.; Brouki Milan, P.; Hamzehlou, S.; Soleimani, M.; Joghataei, M.T.; Gholipourmalekabadi, M.; Korourian, A.; Mousavizadeh, K.; et al. Osteogenic potential of stem cells-seeded bioactive nanocomposite scaffolds: A comparative study between human mesenchymal stem cells derived from bone, umbilical cord Wharton’s jelly, and adipose tissue. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 61–72. [Google Scholar] [CrossRef]
- Jakobsen, R.B.; Shahdadfar, A.; Reinholt, F.P.; Brinchmann, J.E. Chondrogenesis in a hyaluronic acid scaffold: Comparison between chondrocytes and MSC from bone marrow and adipose tissue. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 1407–1416. [Google Scholar] [CrossRef] [Green Version]
- Fabre, H.; Ducret, M.; Degoul, O.; Rodriguez, J.; Perrier-Groult, E.; Aubert-Foucher, E.; Pasdeloup, M.; Auxenfans, C.; McGuckin, C.; Forraz, N.; et al. Characterization of Different Sources of Human MSCs Expanded in Serum-Free Conditions with Quantification of Chondrogenic Induction in 3D. Stem Cells Int. 2019, 2019, 2186728. [Google Scholar] [CrossRef]
- Xie, X.; Wang, Y.; Zhao, C.; Guo, S.; Liu, S.; Jia, W.; Tuan, R.S.; Zhang, C. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials 2012, 33, 7008–7018. [Google Scholar] [CrossRef]
- Reppel, L.; Schiavi, J.; Charif, N.; Leger, L.; Yu, H.; Pinzano, A.; Henrionnet, C.; Stoltz, J.F.; Bensoussan, D.; Huselstein, C. Chondrogenic induction of mesenchymal stromal/stem cells from Wharton’s jelly embedded in alginate hydrogel and without added growth factor: An alternative stem cell source for cartilage tissue engineering. Stem Cell Res. Ther. 2015, 6, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Feng, Q.; Bian, L. Differential effect of hypoxia on human mesenchymal stem cell chondrogenesis and hypertrophy in hyaluronic acid hydrogels. Acta Biomater. 2014, 10, 1333–1340. [Google Scholar] [CrossRef]
- Meretoja, V.V.; Dahlin, R.L.; Wright, S.; Kasper, F.K.; Mikos, A.G. The effect of hypoxia on the chondrogenic differentiation of co-cultured articular chondrocytes and mesenchymal stem cells in scaffolds. Biomaterials 2013, 34, 4266–4273. [Google Scholar] [CrossRef] [Green Version]
- Puetzer, J.L.; Petitte, J.N.; Loboa, E.G. Comparative review of growth factors for induction of three-dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue. Tissue Eng. Part. B Rev. 2010, 16, 435–444. [Google Scholar] [CrossRef]
- Wei, Y.; Sun, X.; Wang, W.; Hu, Y. Adipose-derived stem cells and chondrogenesis. Cytotherapy 2007, 9, 712–716. [Google Scholar] [CrossRef]
- Pelttari, K.; Winter, A.; Steck, E.; Goetzke, K.; Hennig, T.; Ochs, B.G.; Aigner, T.; Richter, W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006, 54, 3254–3266. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.H.J.; Kunze, M.; Mulet-Sierra, A.; Williams, L.; Ansari, K.; Osswald, M.; Adesida, A.B. Strategies to Mitigate Variability in Engineering Human Nasal Cartilage. Sci. Rep. 2017, 7, 6490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.P.; Liao, Y.T.; Wu, S.H.; Chiang, E.R.; Hsu, S.H.; Tseng, T.C.; Hung, S.C. Mesenchymal stem cells from a hypoxic culture improve nerve regeneration. J. Tissue Eng. Regen. Med. 2020, 14, 1804–1814. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Liao, Y.T.; Wu, S.H.; Huang, H.K.; Chou, P.H.; Chiang, E.R. Adipose Derived Mesenchymal Stem Cells from a Hypoxic Culture Reduce Cartilage Damage. Stem Cell Rev. Rep. 2021. [Google Scholar] [CrossRef]
- Chu, K.A.; Wang, S.Y.; Yeh, C.C.; Fu, T.W.; Fu, Y.Y.; Ko, T.L.; Chiu, M.M.; Chen, T.H.; Tsai, P.J.; Fu, Y.S. Reversal of bleomycin-induced rat pulmonary fibrosis by a xenograft of human umbilical mesenchymal stem cells from Wharton’s jelly. Theranostics 2019, 9, 6646–6664. [Google Scholar] [CrossRef]
- Calabrese, G.; Giuffrida, R.; Forte, S.; Fabbi, C.; Figallo, E.; Salvatorelli, L.; Memeo, L.; Parenti, R.; Gulisano, M.; Gulino, R. Human adipose-derived mesenchymal stem cells seeded into a collagen-hydroxyapatite scaffold promote bone augmentation after implantation in the mouse. Sci. Rep. 2017, 7, 7110. [Google Scholar] [CrossRef]
- Wang, H.S.; Hung, S.C.; Peng, S.T.; Huang, C.C.; Wei, H.M.; Guo, Y.J.; Fu, Y.S.; Lai, M.C.; Chen, C.C. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 2004, 22, 1330–1337. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Lu, H.; Kawazoe, N.; Chen, G. Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomater. 2014, 10, 2005–2013. [Google Scholar] [CrossRef]
- Coulson-Thomas, Y.M.; Coulson-Thomas, V.J.; Norton, A.L.; Gesteira, T.F.; Cavalheiro, R.P.; Meneghetti, M.C.; Martins, J.R.; Dixon, R.A.; Nader, H.B. The identification of proteoglycans and glycosaminoglycans in archaeological human bones and teeth. PLoS ONE 2015, 10, e0131105. [Google Scholar] [CrossRef]
- Ruhl, T.; Beier, J.P. Quantification of chondrogenic differentiation in monolayer cultures of mesenchymal stromal cells. Anal. Biochem. 2019, 582, 113356. [Google Scholar] [CrossRef] [PubMed]

| Target Gene | Directions | Sequences | Accession No. | Product Size |
|---|---|---|---|---|
| ALPL | Forward | ACAGATGCCAACTTCCCACACG | NM_001200 | 112 bp |
| Reverse | GCGGCAGACTTTGGTTTCTTGG | |||
| COL1A1 | Forward | TCCCCTCCACTCCTTCCCAAA | NM_000088 | 146 bp |
| Reverse | GGCCACTTGGGTGTTTGAGCA | |||
| COL2A1 | Forward | TGGCTGACCTGACCTGATGTCC | NM_001844 | 95 bp |
| Reverse | TGCAGTCTGCCCAGTTCAGGTC | |||
| COL10A1 | Forward | AGGCCCACTACCCAACACCAAGA | NM_000493 | 161 bp |
| Reverse | CGTAGCCTGGTTTTCCTGGTGGTC | |||
| GAPDH | Forward | TGAGCACCAGGTGGTCTCCTCTGAC | NM_001256799 | 147 bp |
| Reverse | TCCACCACCCTGTTGCTGTAGCCA | |||
| Reverse | TGGGAGCAGCTGGGATGATG | |||
| RUNX2 | Forward | ATGACGTCCCCGTCCATCCA | NM_001024630 | 135 bp |
| Reverse | GGAAGGCCAGAGGCAGAAGTCA | |||
| SOX9 | Forward | CCAAGCGCATTACCCACTTGTG | NM_000346 | 130 bp |
| Reverse | CGATTCTCCATCATCCTCCACG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-F.; Chen, Y.-C.; Fu, Y.-S.; Tsai, S.-W.; Wu, P.-K.; Chen, C.-M.; Chang, M.-C.; Chen, W.-M. Characterization of Osteogenesis and Chondrogenesis of Human Decellularized Allogeneic Bone with Mesenchymal Stem Cells Derived from Bone Marrow, Adipose Tissue, and Wharton’s Jelly. Int. J. Mol. Sci. 2021, 22, 8987. https://doi.org/10.3390/ijms22168987
Chen C-F, Chen Y-C, Fu Y-S, Tsai S-W, Wu P-K, Chen C-M, Chang M-C, Chen W-M. Characterization of Osteogenesis and Chondrogenesis of Human Decellularized Allogeneic Bone with Mesenchymal Stem Cells Derived from Bone Marrow, Adipose Tissue, and Wharton’s Jelly. International Journal of Molecular Sciences. 2021; 22(16):8987. https://doi.org/10.3390/ijms22168987
Chicago/Turabian StyleChen, Cheng-Fong, Yi-Chun Chen, Yu-Show Fu, Shang-Wen Tsai, Po-Kuei Wu, Chao-Ming Chen, Ming-Chau Chang, and Wei-Ming Chen. 2021. "Characterization of Osteogenesis and Chondrogenesis of Human Decellularized Allogeneic Bone with Mesenchymal Stem Cells Derived from Bone Marrow, Adipose Tissue, and Wharton’s Jelly" International Journal of Molecular Sciences 22, no. 16: 8987. https://doi.org/10.3390/ijms22168987
APA StyleChen, C.-F., Chen, Y.-C., Fu, Y.-S., Tsai, S.-W., Wu, P.-K., Chen, C.-M., Chang, M.-C., & Chen, W.-M. (2021). Characterization of Osteogenesis and Chondrogenesis of Human Decellularized Allogeneic Bone with Mesenchymal Stem Cells Derived from Bone Marrow, Adipose Tissue, and Wharton’s Jelly. International Journal of Molecular Sciences, 22(16), 8987. https://doi.org/10.3390/ijms22168987






