Evaluation of the In Vitro and In Vivo Efficacy of Ruthenium Polypyridyl Compounds against Breast Cancer
Abstract
:1. Introduction
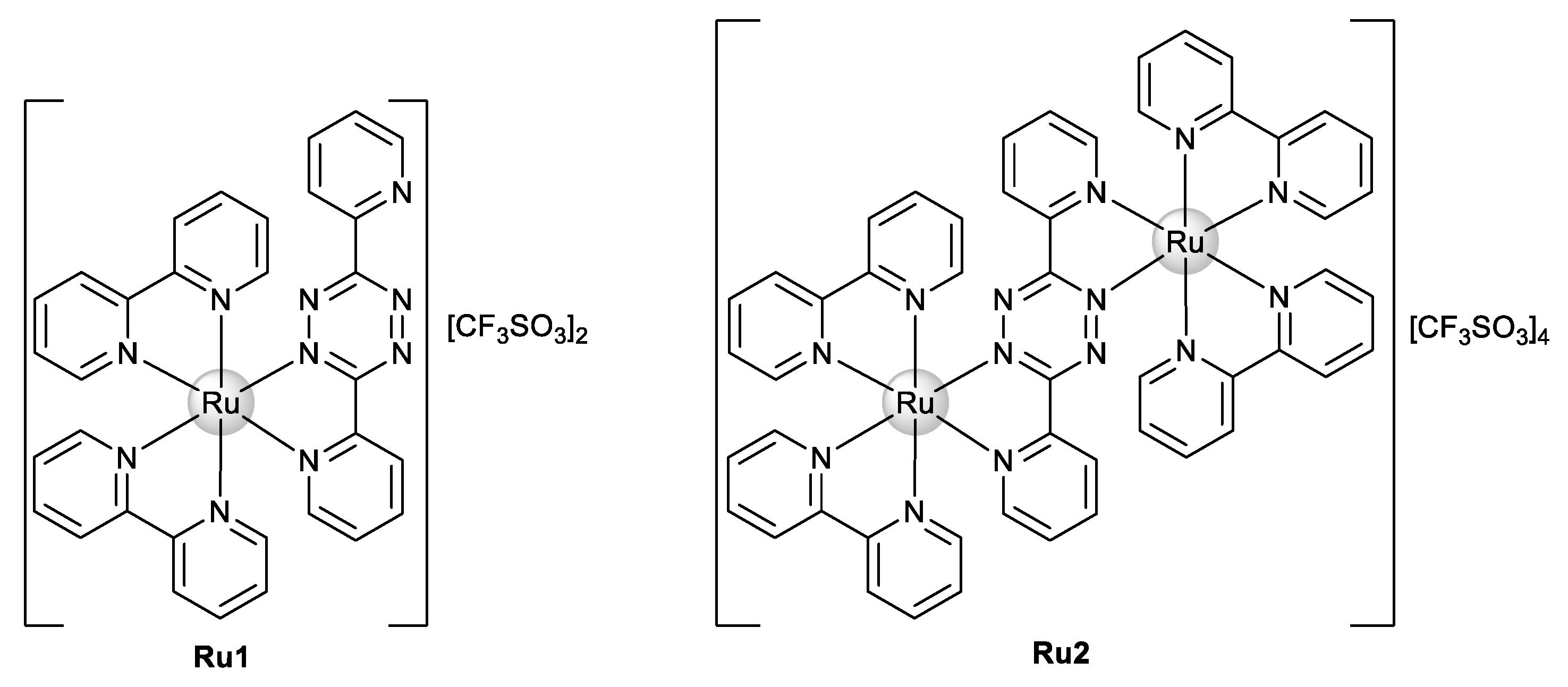
2. Results and Discussion
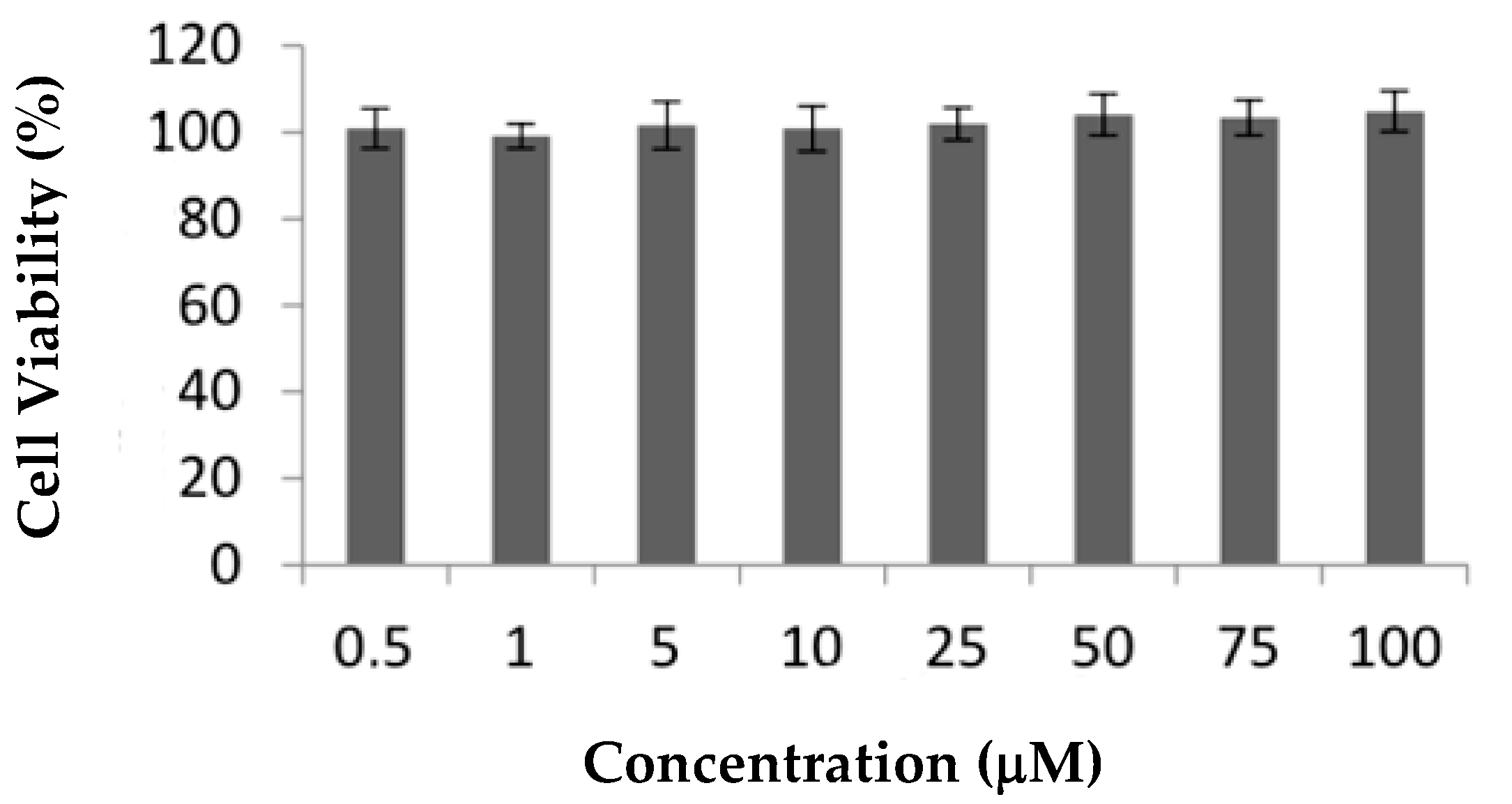
2.1. In Vitro Assays
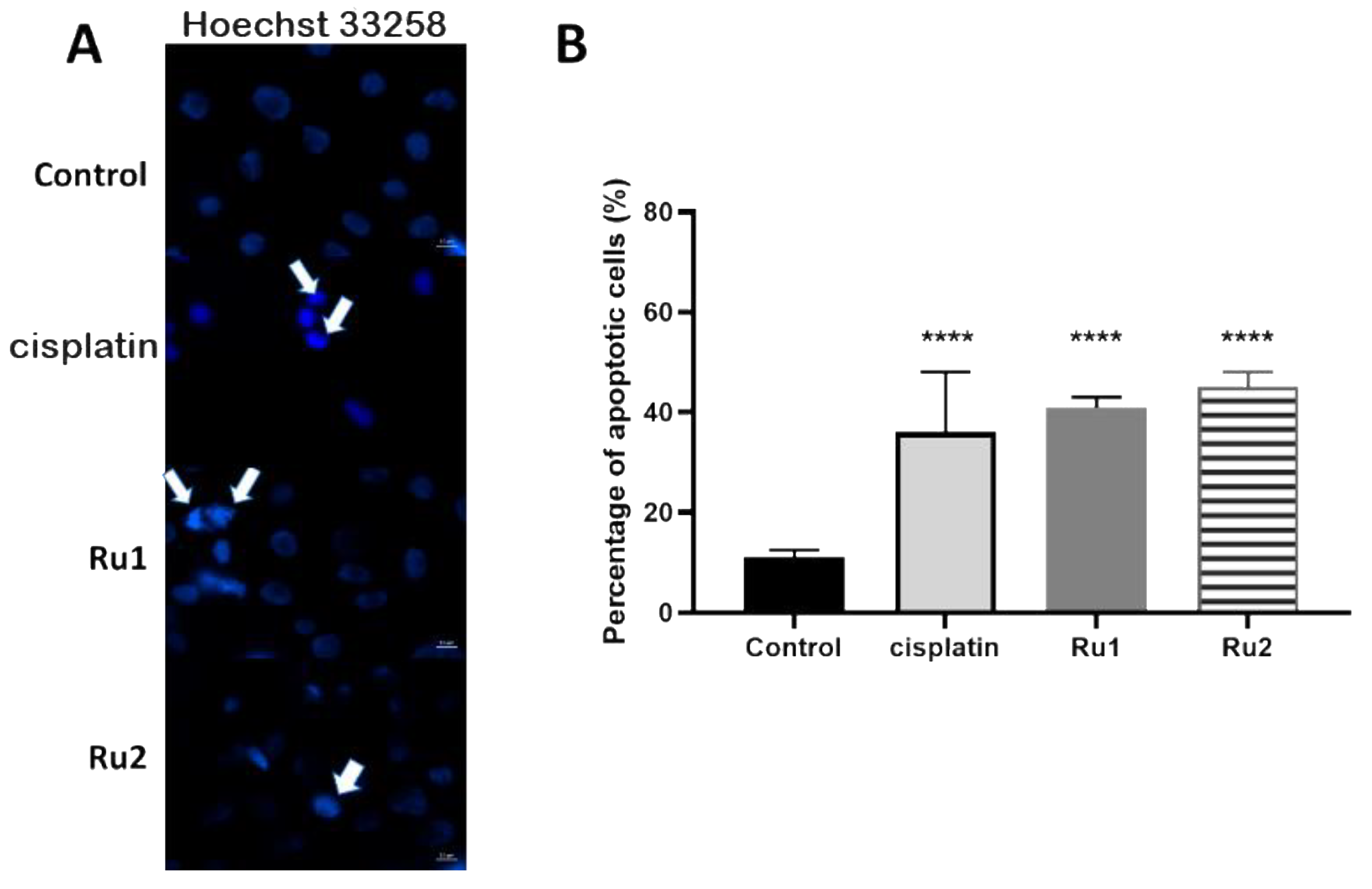
2.2. Cell Death Mechanism
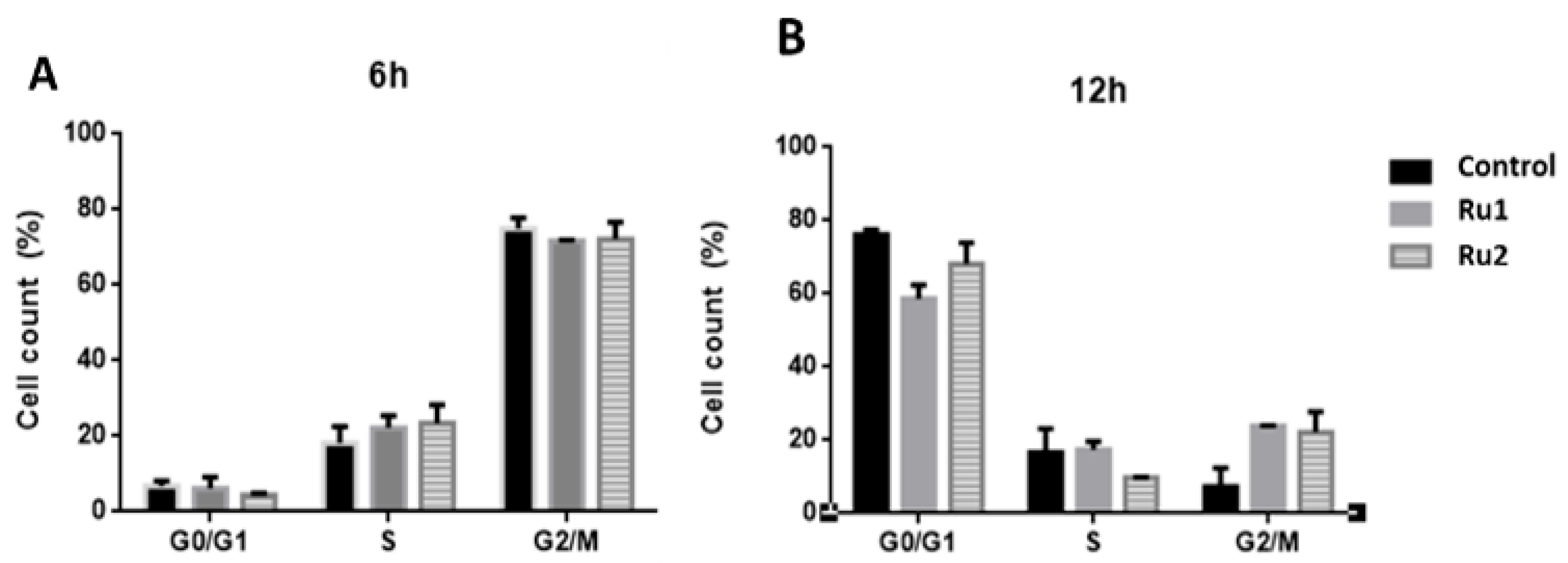
2.3. Cell-Cycle Progression
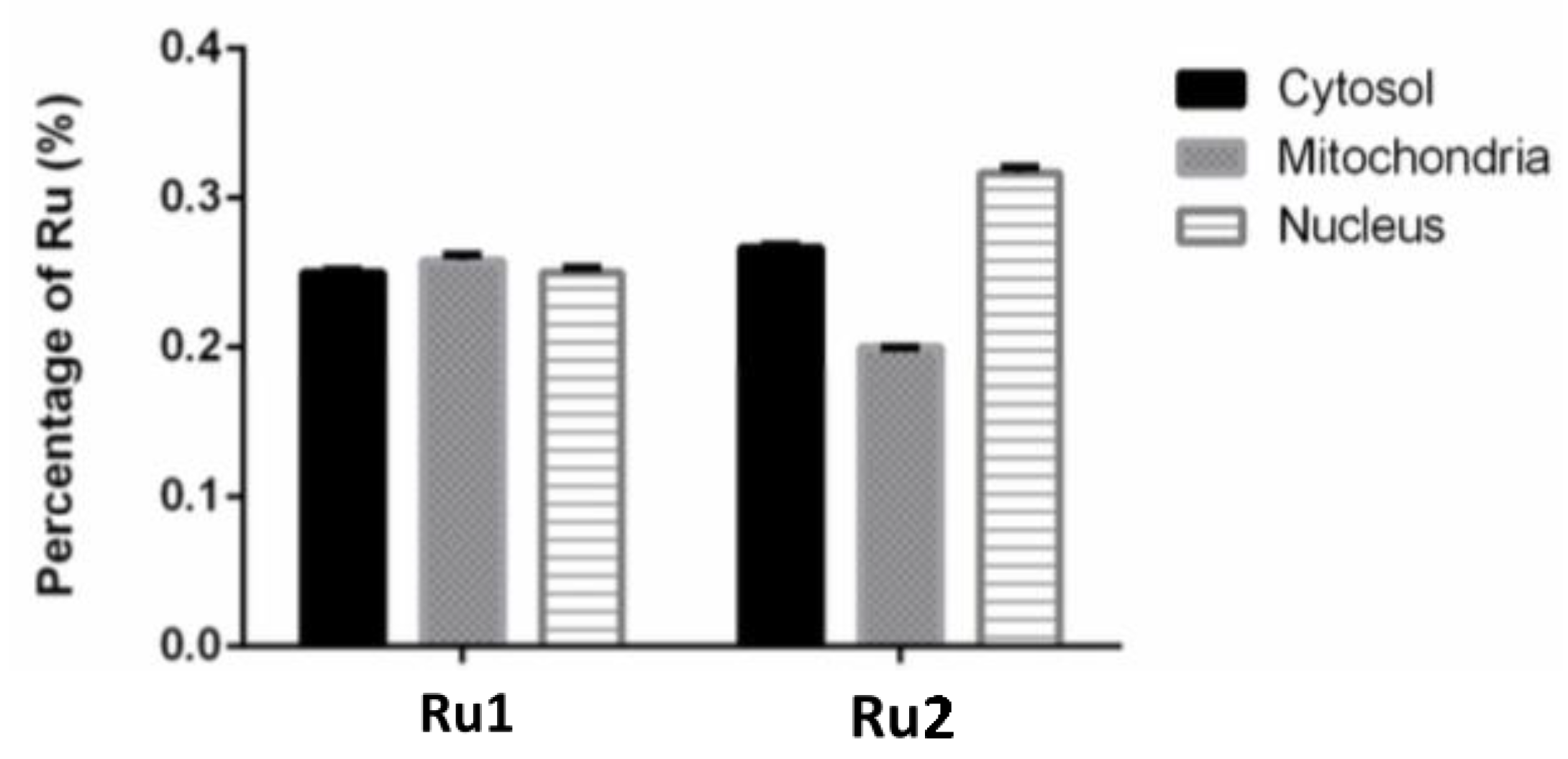
2.4. Subcellular Distribution
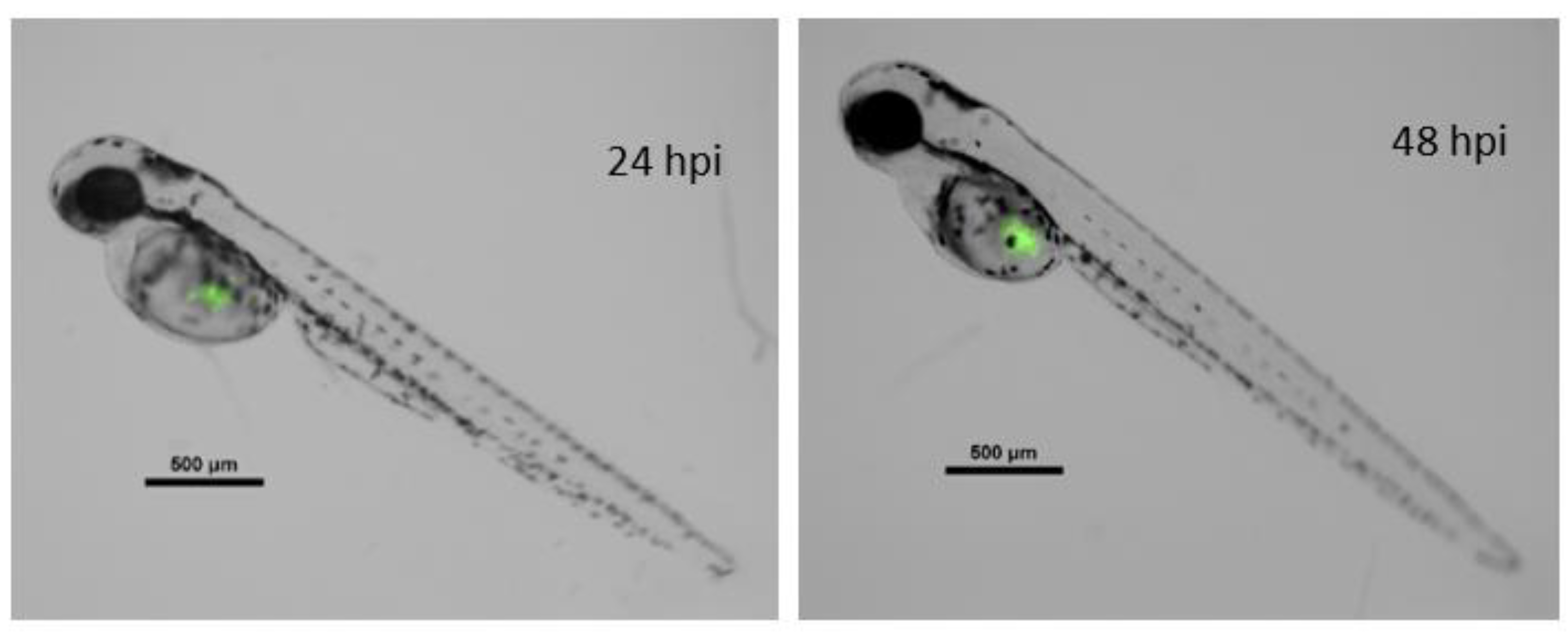
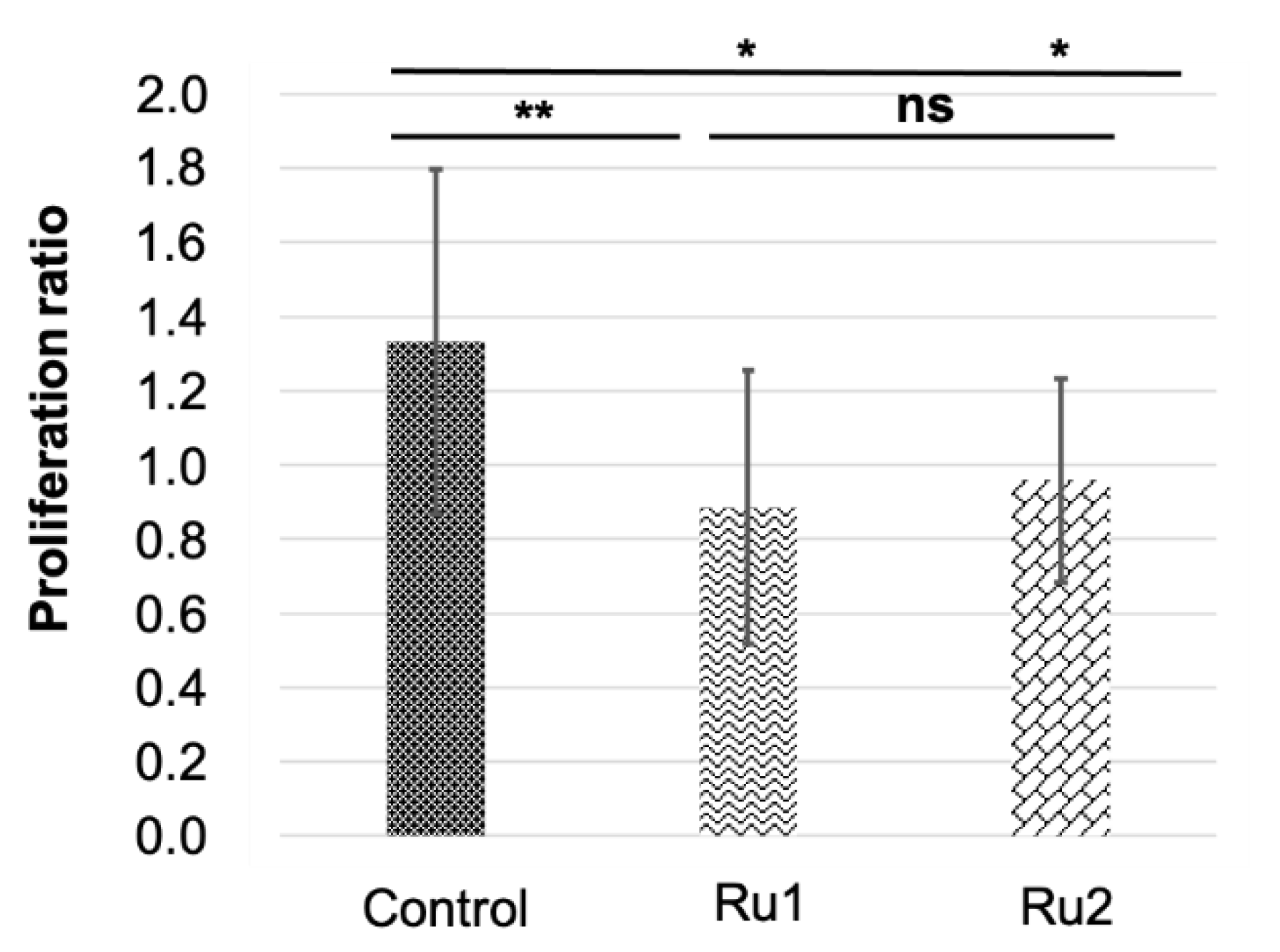
2.5. In Vivo Assays
3. Materials and Methods
3.1. In Vitro Assays
3.1.1. Cell Culture and Maintenance
3.1.2. Cytotoxic Activity Assay in Normal Human Fibroblasts and MCF7
3.1.3. Hoechst 33258 Staining
3.1.4. Cell-Cycle Analysis
3.1.5. Subcellular Distribution
3.1.6. Statistical Analysis
3.2. In Vivo Assays
3.2.1. Zebrafish Maintenance
3.2.2. Toxicological Analysis
3.2.3. Zebrafish Xenografts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Horn, L.; Garassino, M. COVID-19 in patients with cancer: Managing a pandemic within a pandemic. Nat. Rev. Clin. Oncol. 2021, 18, 1–2. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Lal, S.; Lee, J.E.; Choi, Y.-L.; Wen, J.; Ram, S.; Ding, Y.; Lee, S.-H.; Powell, E.; Lee, S.K.; et al. Chemotherapy induces dynamic immune responses in breast cancers that impact treatment outcome. Nat. Commun. 2020, 11, 6175. [Google Scholar] [CrossRef] [PubMed]
- Avan, A.; Postma, T.J.; Ceresa, C.; Avan, A.; Cavaletti, G.; Giovannetti, E.; Peters, G.J. Platinum-induced neurotoxicity and preventive strategies: Past, present, and future. Oncologist 2015, 20, 411–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.-S. The development of anticancer ruthenium(II) complexes: From single molecule compounds to nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef]
- Monro, S.; Colón, K.L.; Yin, H.; Roque, J.; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition metal complexes and photodynamic therapy from a tumor-centered approach: Challenges, opportunities, and highlights from the development of TLD1433. Chem. Rev. 2019, 119, 797–828. [Google Scholar] [CrossRef]
- Notaro, A.; Gasser, G. Monomeric and dimeric coordinatively saturated and substitutionally inert Ru(ii) polypyridyl complexes as anticancer drug candidates. Chem. Soc. Rev. 2017, 46, 7317–7337. [Google Scholar] [CrossRef]
- Poynton, F.E.; Bright, S.A.; Blasco, S.; Williams, D.C.; Kelly, J.M.; Gunnlaugsson, T. The development of ruthenium(II) polypyridyl complexes and conjugates for in vitro cellular and in vivo applications. Chem Soc. Rev. 2017, 46, 7706–7756. [Google Scholar] [CrossRef]
- Golbaghi, G.; Castonguay, A. Rationally designed ruthenium complexes for breast cancer therapy. Molecules 2020, 25, 265. [Google Scholar] [CrossRef] [Green Version]
- Schobert, R.; Seibt, S.; Effenberger-Neidnicht, K.; Underhill, C.; Biersack, B.; Hammond, G.L. (Arene)Cl₂Ru(II) complexes with N-coordinated estrogen and androgen isonicotinates: Interaction with sex hormone binding globulin and anticancer activity. Steroids 2011, 76, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Rodríguez, V.; Cutillas, N.; Espinosa, A.; Hannon, M.J. A potent ruthenium(II) antitumor complex bearing a lipophilic levonorgestrel group. Inorg. Chem. 2011, 50, 9164–9171. [Google Scholar] [CrossRef]
- Lv, G.; Qiu, L.; Li, K.; Liu, Q.; Li, X.; Peng, Y.; Wang, S.; Lin, J. Enhancement of therapeutic effect in breast cancer with a steroid-conjugated ruthenium complex. New J. Chem. 2019, 43, 3419–3427. [Google Scholar] [CrossRef]
- Mandal, P.; Kundu, B.K.; Vyas, K.; Sabu, V.; Helen, A.; Dhankhar, S.S.; Nagaraja, C.M.; Bhattacherjee, D.; Bhabak, K.P.; Mukhopadhyay, S. Ruthenium(ii) arene NSAID complexes: Inhibition of cyclooxygenase and antiproliferative activity against cancer cell lines. Dalton Trans. 2018, 47, 517–527. [Google Scholar] [CrossRef]
- Smith, G.S.; Therrien, B. Targeted and multifunctional arene ruthenium chemotherapeutics. Dalton Trans. 2011, 40, 10793–10800. [Google Scholar] [CrossRef]
- Du, J.; Zhang, E.; Zhao, Y.; Zheng, W.; Zhang, Y.; Lin, Y.; Wang, Z.; Luo, Q.; Wu, K.; Wang, F. Discovery of a dual-targeting organometallic ruthenium complex with high activity inducing early stage apoptosis of cancer cells. Metallomics 2015, 7, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qian, H.; Yiu, S.-M.; Sun, J.; Zhu, G. Multi-targeted organometallic ruthenium(II)-arene anticancer complexes bearing inhibitors of poly(ADP-ribose) polymerase-1: A strategy to improve cytotoxicity. J. Inorg. Biochem. 2014, 131, 47–55. [Google Scholar] [CrossRef]
- Pracharova, J.; Novohradsky, V.; Kostrhunova, H.; Starha, P.; Travnicek, Z.; Kasparkova, J.; Brabec, V. Half-sandwich Os(II) and Ru(II) bathophenanthroline complexes: Anticancer drug candidates with unusual potency and cellular activity profile in highly invasive triple-negative breast cancer cells. Dalton Trans. 2018. [Google Scholar] [CrossRef]
- Montani, M.; Pazmay, G.V.B.; Hysi, A.; Lupidi, G.; Pettinari, R.; Gambini, V.; Tilio, M.; Marchetti, F.; Pettinari, C.; Ferraro, S.; et al. The water soluble ruthenium(II) organometallic compound [Ru(p-cymene)(bis(3,5 dimethylpyrazol-1-yl)methane)Cl]Cl suppresses triple negative breast cancer growth by inhibiting tumor infiltration of regulatory T cells. Pharmacol. Res. 2016, 107, 282–290. [Google Scholar] [CrossRef]
- Frik, M.; Martínez, A.; Elie, B.T.; Gonzalo, O.; Ramírez de Mingo, D.; Sanaú, M.; Sánchez-Delgado, R.; Sadhukha, T.; Prabha, S.; Ramos, J.W.; et al. In vitro and in vivo evaluation of water-soluble iminophosphorane ruthenium(II) compounds. A potential chemotherapeutic agent for triple negative breast cancer. J. Med. Chem. 2014, 57, 9995–10012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colina-Vegas, L.; Oliveira, K.; Cunha, B.; Cominetti, M.; Navarro, M.; Azevedo Batista, A. Anti-proliferative and anti-migration activity of Arene–ruthenium(II) complexes with azole therapeutic agents. Inorganics 2018, 6, 132. [Google Scholar] [CrossRef] [Green Version]
- Thota, S.; Rodrigues, D.A.; Crans, D.C.; Barreiro, E.J. Ru(II) compounds: Next-generation anticancer metallotherapeutics? J. Med. Chem. 2018, 61, 5805–5821. [Google Scholar] [CrossRef]
- Golbaghi, G.; Pitard, I.; Lucas, M.; Haghdoost, M.M.; de Los Santos, Y.L.; Doucet, N.; Patten, S.A.; Sanderson, J.T.; Castonguay, A. Synthesis and biological assessment of a ruthenium(II) cyclopentadienyl complex in breast cancer cells and on the development of zebrafish embryos. Eur. J. Med. Chem. 2020, 188, 112030. [Google Scholar] [CrossRef] [PubMed]
- Côrte-Real, L.; Karas, B.; Brás, A.R.; Pilon, A.; Avecilla, F.; Marques, F.; Preto, A.; Buckley, B.T.; Cooper, K.R.; Doherty, C.; et al. Ruthenium-cyclopentadienyl bipyridine-biotin based compounds: Synthesis and biological effect. Inorg. Chem. 2019, 58, 9135–9149. [Google Scholar] [CrossRef] [PubMed]
- Côrte-Real, L.; Karas, B.; Gírio, P.; Moreno, A.; Avecilla, F.; Marques, F.; Buckley, B.T.; Cooper, K.R.; Doherty, C.; Falson, P.; et al. Unprecedented inhibition of P-gp activity by a novel ruthenium-cyclopentadienyl compound bearing a bipyridine-biotin ligand. Eur. J. Med. Chem. 2019, 163, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.S.; Silva, T.J.L.; Marques, F.; Robalo, M.P.; Avecilla, F.; Amorim Madeira, P.J.; Mendes, P.J.G.; Santos, I.; Garcia, M.H. Synthesis of organometallic ruthenium(II) complexes with strong activity against several human cancer cell lines. J. Inorg. Biochem. 2012, 114, 65–74. [Google Scholar] [CrossRef]
- Valle, S.; Alcalá, S.; Martin-Hijano, L.; Cabezas-Sáinz, P.; Navarro, D.; Muñoz, E.R.; Yuste, L.; Tiwary, K.; Walter, K.; Ruiz-Cañas, L.; et al. Exploiting oxidative phosphorylation to promote the stem and immunoevasive properties of pancreatic cancer stem cells. Nat. Commun. 2020, 11, 5265. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Glasgow, E.; Agarwal, S. Zebrafish xenografts for drug discovery and personalized medicine. Trends Cancer 2020, 6, 569–579. [Google Scholar] [CrossRef]
- Cabezas-Sáinz, P.; Pensado-López, A.; Sáinz, B.; Sánchez, L. Modeling cancer using zebrafish xenografts: Drawbacks for mimicking the human microenvironment. Cells 2020, 9, 1978. [Google Scholar] [CrossRef]
- Roel, M.; Rubiolo, J.A.; Guerra-Varela, J.; Silva, S.B.L.; Thomas, O.P.; Cabezas-Sainz, P.; Sánchez, L.; López, R.; Botana, L.M. Marine guanidine alkaloids crambescidins inhibit tumor growth and activate intrinsic apoptotic signaling inducing tumor regression in a colorectal carcinoma zebrafish xenograft model. Oncotarget 2016, 7, 83071–83087. [Google Scholar] [CrossRef] [Green Version]
- Lenis-Rojas, O.A.; Fernandes, A.R.; Roma-Rodrigues, C.; Baptista, P.V.; Marques, F.; Pérez-Fernández, D.; Guerra-Varela, J.; Sánchez, L.; Vázquez-García, D.; Torres, M.L.; et al. Heteroleptic mononuclear compounds of ruthenium(II): Synthesis, structural analyses, in vitro antitumor activity and in vivo toxicity on zebrafish embryos. Dalton Trans. 2016, 45, 19127–19140. [Google Scholar] [CrossRef]
- Lenis-Rojas, O.A.; Roma-Rodrigues, C.; Fernandes, A.R.; Marques, F.; Pérez-Fernández, D.; Guerra-Varela, J.; Sánchez, L.; Vázquez-García, D.; López-Torres, M.; Fernández, A.; et al. Dinuclear RuII(bipy)2 Derivatives: Structural, biological, and in vivo zebrafish toxicity evaluation. Inorg. Chem. 2017, 56, 7127–7144. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Fernandes, A.R.; Baptista, P.V. Exosome in tumour microenvironment: Overview of the crosstalk between normal and cancer cells. Biomed. Res. Int. 2014, 2014, 179486. [Google Scholar] [CrossRef]
- Silva, T.F.S.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Kuznetsov, M.L.; Fernandes, A.R.; Silva, A.; Pan, C.-J.; Lee, J.-F.; Hwang, B.-J.; Pombeiro, A.J.L. Cobalt complexes with pyrazole ligands as catalyst precursors for the peroxidative oxidation of cyclohexane: X-ray absorption spectroscopy studies and biological applications. Chem. Asian J. 2014, 9, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.R.; Jesus, J.; Martins, P.; Figueiredo, S.; Rosa, D.; Martins, L.M.R.D.R.S.; Corvo, M.L.; Carvalheiro, M.C.; Costa, P.M.; Baptista, P.V. Multifunctional gold-nanoparticles: A nanovectorization tool for the targeted delivery of novel chemotherapeutic agents. J. Control. Release 2017, 245, 52–61. [Google Scholar] [CrossRef]
- Martins, M.; Baptista, P.V.; Mendo, A.S.; Correia, C.; Videira, P.; Rodrigues, A.S.; Muthukumaran, J.; Santos-Silva, T.; Silva, A.; da Silva, M.F.C.G.; et al. In vitro and in vivo biological characterization of the anti-proliferative potential of a cyclic trinuclear organotin(iv) complex. Mol. Biosyst. 2016, 12, 1015–1023. [Google Scholar] [CrossRef]
- Luís, D.V.; Silva, J.; Tomaz, A.I.; de Almeida, R.F.M.; Larguinho, M.; Baptista, P.V.; Martins, L.M.D.R.S.; Silva, T.F.S.; Borralho, P.M.; Rodrigues, C.M.P.; et al. Insights into the mechanisms underlying the antiproliferative potential of a Co(II) coordination compound bearing 1,10-phenanthroline-5,6-dione: DNA and protein interaction studies. J. Biol. Inorg. Chem. 2014, 19, 787–803. [Google Scholar] [CrossRef]
- Groessl, M.; Zava, O.; Dyson, P.J. Cellular uptake and subcellular distribution of ruthenium-based metallodrugs under clinical investigation versus cisplatin. Metallomics 2011, 3, 591–599. [Google Scholar] [CrossRef]
- Perdisatt, L.; Moqadasi, S.; O’Neill, L.; Hessman, G.; Ghion, A.; Warraich, M.Q.M.; Casey, A.; O’Connor, C. Synthesis, characterisation and DNA intercalation studies of regioisomers of ruthenium (II) polypyridyl complexes. J. Inorg. Biochem. 2018, 182, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, M.; Sun, W.; Fan, J.; Du, J.; Peng, X. An estrogen receptor targeted ruthenium complex as a two-photon photodynamic therapy agent for breast cancer cells. Chem. Commun. 2018, 54, 7038–7041. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, G.; Zhu, Y.; Zhao, J.; Gou, S. Bifunctional ruthenium(II) polypyridyl complexes of curcumin as potential anticancer agents. Dalton Trans. 2020, 49, 9454–9463. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redfern, W.S.; Waldron, G.; Winter, M.J.; Butler, P.; Holbrook, M.; Wallis, R.; Valentin, J.-P. Zebrafish assays as early safety pharmacology screens: Paradigm shift or red herring? J. Pharmacol. Toxicol. Methods 2008, 58, 110–117. [Google Scholar] [CrossRef]
- Silva, A.; Luís, D.; Santos, S.; Silva, J.; Mendo, A.S.; Coito, L.; Silva, T.F.S.; da Silva, M.F.C.G.; Martins, L.M.D.R.S.; Pombeiro, A.J.L.; et al. Biological characterization of the antiproliferative potential of Co(II) and Sn(IV) coordination compounds in human cancer cell lines: A comparative proteomic approach. Drug Metab. Drug Interact. 2013, 28, 167–176. [Google Scholar] [CrossRef]
- Silva, T.F.S.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Fernandes, A.R.; Silva, A.; Borralho, P.M.; Santos, S.; Rodrigues, C.M.P.; Pombeiro, A.J.L. Cobalt complexes bearing scorpionate ligands: Synthesis, characterization, cytotoxicity and DNA cleavage. Dalton Trans. 2012, 41, 12888–12897. [Google Scholar] [CrossRef]
- Stirling, D.; Tomlinson, G. Quantifish—A Zebrafish Fluorescence Analyser; Zenodo: Geneve, Switzerland, 2017. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenis-Rojas, O.A.; Roma-Rodrigues, C.; Fernandes, A.R.; Carvalho, A.; Cordeiro, S.; Guerra-Varela, J.; Sánchez, L.; Vázquez-García, D.; López-Torres, M.; Fernández, A.; et al. Evaluation of the In Vitro and In Vivo Efficacy of Ruthenium Polypyridyl Compounds against Breast Cancer. Int. J. Mol. Sci. 2021, 22, 8916. https://doi.org/10.3390/ijms22168916
Lenis-Rojas OA, Roma-Rodrigues C, Fernandes AR, Carvalho A, Cordeiro S, Guerra-Varela J, Sánchez L, Vázquez-García D, López-Torres M, Fernández A, et al. Evaluation of the In Vitro and In Vivo Efficacy of Ruthenium Polypyridyl Compounds against Breast Cancer. International Journal of Molecular Sciences. 2021; 22(16):8916. https://doi.org/10.3390/ijms22168916
Chicago/Turabian StyleLenis-Rojas, Oscar A., Catarina Roma-Rodrigues, Alexandra R. Fernandes, Andreia Carvalho, Sandra Cordeiro, Jorge Guerra-Varela, Laura Sánchez, Digna Vázquez-García, Margarita López-Torres, Alberto Fernández, and et al. 2021. "Evaluation of the In Vitro and In Vivo Efficacy of Ruthenium Polypyridyl Compounds against Breast Cancer" International Journal of Molecular Sciences 22, no. 16: 8916. https://doi.org/10.3390/ijms22168916
APA StyleLenis-Rojas, O. A., Roma-Rodrigues, C., Fernandes, A. R., Carvalho, A., Cordeiro, S., Guerra-Varela, J., Sánchez, L., Vázquez-García, D., López-Torres, M., Fernández, A., & Fernández, J. J. (2021). Evaluation of the In Vitro and In Vivo Efficacy of Ruthenium Polypyridyl Compounds against Breast Cancer. International Journal of Molecular Sciences, 22(16), 8916. https://doi.org/10.3390/ijms22168916








