Neuropharmacology of Cevimeline and Muscarinic Drugs—Focus on Cognition and Neurodegeneration
Abstract
:1. Introduction
2. Results and Discussion
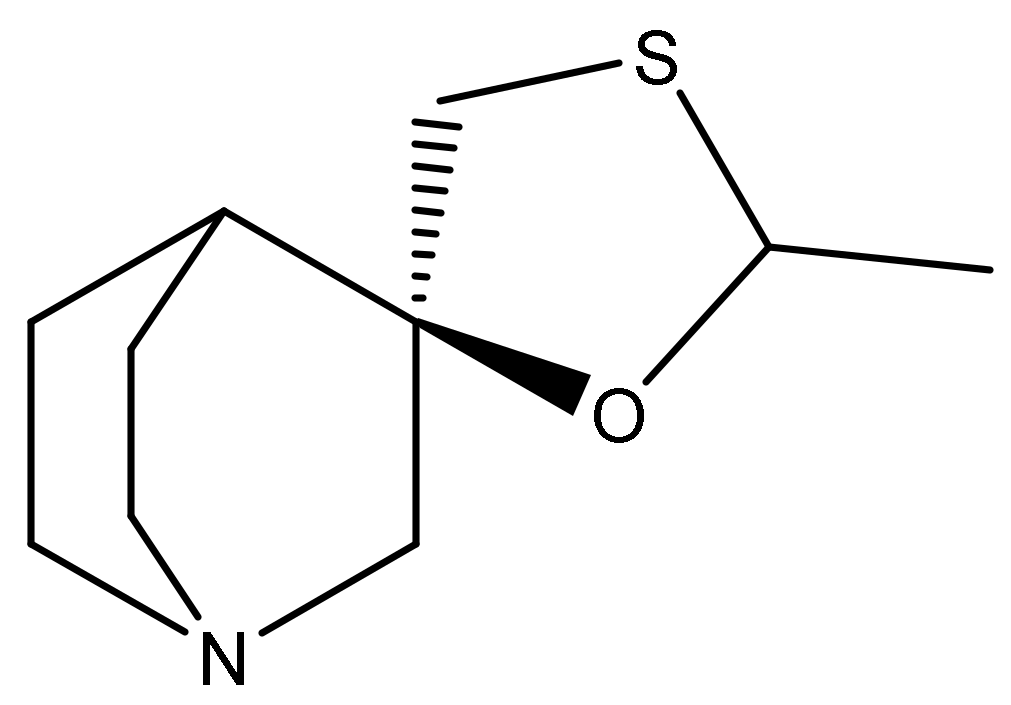
2.1. Pharmacology of Cevimeline
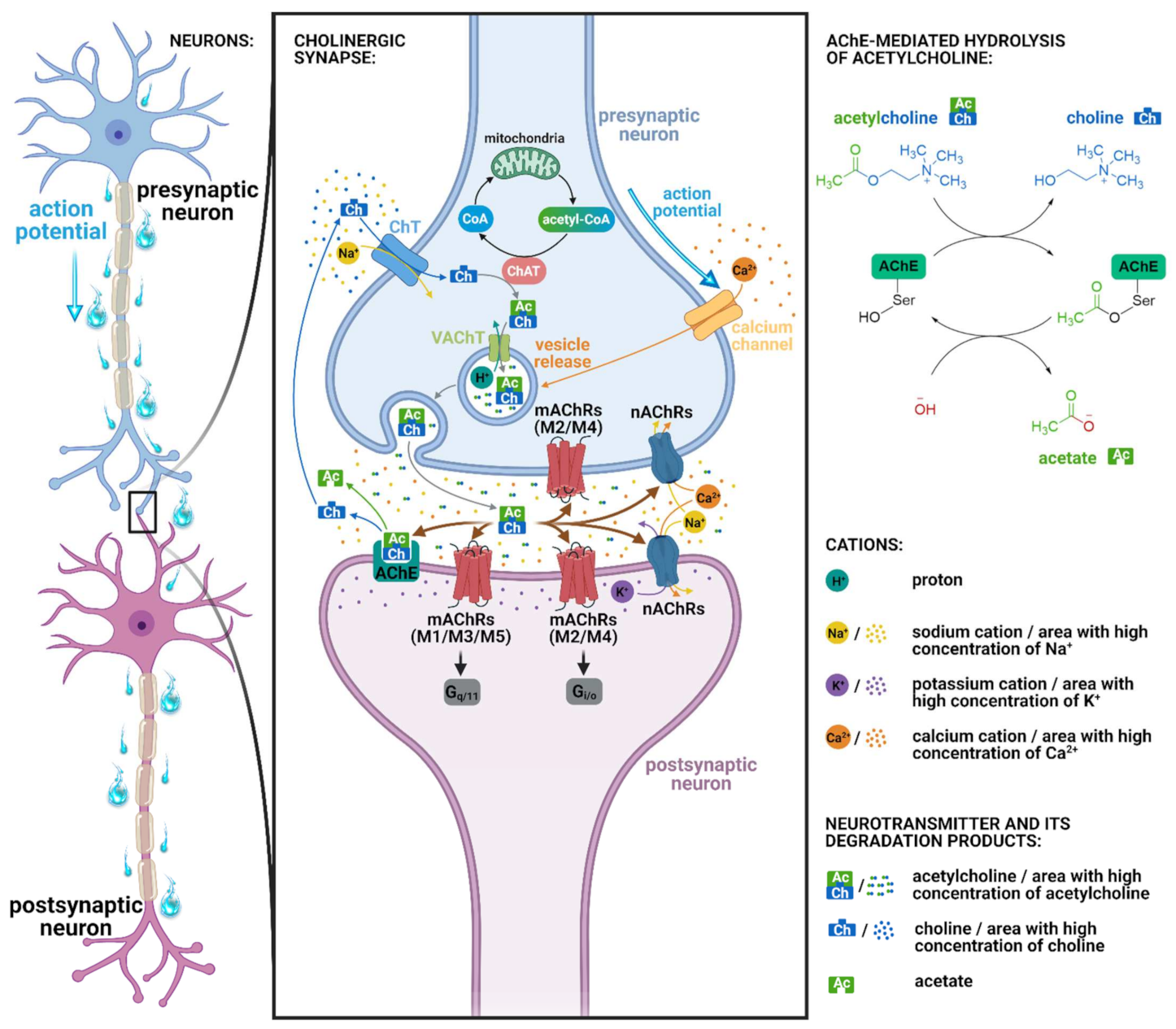
2.2. Muscarinic Acetylcholine Receptors and Cognition
2.3. Cevimeline and Cognition
2.4. Cevimeline and Alzheimer’s Disease
3. Evoxac® (Cevimeline Hydrochloride) in the Treatment of Sjögren’s Syndrome
4. Methods
- Only peer-reviewed English-written full-text journal articles were involved.
- The time of publishing the article was limited by 31 March 2020.
- Reviews.
- The articles focusing on different research topics (such as xerostomia, dry mouth, etc.).
- Outcomes were not reported or were inconsistent.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- NIH. Alzheimer’s Disease Fact Sheet. 2019. Available online: https://www.Nia.Nih.Gov/Health/Alzheimers-Disease-Fact-Sheet (accessed on 15 March 2021).
- Klimova, B.; Maresova, P.; Valis, M.; Hort, J.; Kuca, K. Alzheimer’s Disease and Language Impairments: Social Intervention and Medical Treatment. Clin. Interv. Aging 2015, 10, 1401–1407. [Google Scholar] [CrossRef] [Green Version]
- EFPIA. 2021. Available online: https://Efpia.Eu/about-Medicines/Use-of-Medicines/Disease-Specific-Groups/Wewontrest-until-Alzheimer-s-Patients-Have-a-Brighter-Future (accessed on 10 March 2021).
- Klimova, B.; Kuca, K. Alzheimer’s Disease: Potential Preventive, Non-Invasive, Intervention Strategies in Lowering the Risk of Cognitive Decline—A Review Study. J. Appl. Biomed. 2015, 13, 257–261. [Google Scholar] [CrossRef]
- Heerema, E. Short-Term Memory and How It’s Affected by Alzheimer’s. 2020. Available online: https://www.Verywellhealth.Com/Short-Term-Memory-Affected-by-Alzheimers-98569 (accessed on 10 March 2021).
- Alzheimer’s Disease Epidemic in Europe. 2020. Available online: https://www.Politico.Eu/Sponsored-Content/Alzheimers-Disease-Epidemic-in-Europe/ (accessed on 15 March 2021).
- Klimova, B.; Maresova, P.; Kuca, K. Non-Pharmacological Approaches to the Prevention and Treatment of Alzheimer’s Disease with Respect to the Rising Treatment Costs. Curr. Alzheimer Res. 2016, 13, 1249–1258. [Google Scholar] [CrossRef]
- Pakala, R.S.; Brown, K.N.; Preuss, C.V. Cholinergic Medications. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- EVOXAC® Capsules. Available online: www.Accessdata.Fda.Gov/Drugsatfda_docs/Label/2006/020989s008lbl.Pdf (accessed on 15 March 2021).
- Al-Hashimi, I.; Taylor, S.E. A New Medication for Treatment of Dry Mouth in Sjögren’s Syndrome. Tex. Dent. J. 2001, 118, 262–266. [Google Scholar] [PubMed]
- Yasuda, H.; Niki, H. Review of the Pharmacological Properties and Clinical Usefulness of Muscarinic Agonists for Xerostomia in Patients with Sjögren’s Syndrome. Clin. Drug Investig. 2002, 22, 67–73. [Google Scholar] [CrossRef]
- Iwabuchi, Y.; Katagiri, M.; Masuhara, T. Salivary Secretion and Histopathological Effects after Single Administration of the Muscarinic Agonist SNI-2011 in MRL/Lpr Mice. Arch. Int. Pharmacodyn. Ther. 1994, 328, 315–325. [Google Scholar]
- Washio, T.; Kohsaka, K.; Arisawa, H.; Masunaga, H.; Nagatsuka, S.; Satoh, Y. Pharmacokinetics and Metabolism of Radiolabelled SNI-2011, a Novel Muscarinic Receptor Agonist, in Healthy Volunteers. Comprehensive Understanding of Absorption, Metabolism and Excretion Using Radiolabelled SNI-2011. Arzneimittelforschung 2003, 53, 80–86. [Google Scholar] [CrossRef]
- Washio, T.; Arisawa, H.; Kohsaka, K.; Yasuda, H. Identification of Human Drug-Metabolizing Enzymes Involved in the Metabolism of SNI-2011. Biol. Pharm. Bull. 2001, 24, 1263–1266. [Google Scholar] [CrossRef] [Green Version]
- Washio, T.; Kohsaka, K.; Arisawa, H.; Masunaga, H. Pharmacokinetics and Metabolism of the Novel Muscarinic Receptor Agonist SNI-2011 in Rats and Dogs. Arzneimittelforschung 2003, 53, 26–33. [Google Scholar] [CrossRef]
- Heinrich, J.N.; Butera, J.A.; Carrick, T.; Kramer, A.; Kowal, D.; Lock, T.; Marquis, K.L.; Pausch, M.H.; Popiolek, M.; Sun, S.-C.; et al. Pharmacological Comparison of Muscarinic Ligands: Historical versus More Recent Muscarinic M1-Preferring Receptor Agonists. Eur. J. Pharmacol. 2009, 605, 53–56. [Google Scholar] [CrossRef]
- Vuckovic, Z.; Gentry, P.R.; Berizzi, A.E.; Hirata, K.; Varghese, S.; Thompson, G.; van der Westhuizen, E.T.; Burger, W.A.C.; Rahmani, R.; Valant, C.; et al. Crystal Structure of the M5 Muscarinic Acetylcholine Receptor. Proc. Natl. Acad. Sci. USA 2019, 116, 26001–26007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitoh, Y.; Ueda, H.; Ichikawa, H.; Fujita, M.; Kobashi, M.; Matsuo, R. Effects of Cevimeline on Excitability of Parasympathetic Preganglionic Neurons in the Superior Salivatory Nucleus of Rats. Auton. Neurosci. 2017, 206, 1–7. [Google Scholar] [CrossRef]
- Voskoboynik, B.; Babu, K.; Hack, J.B. Cevimeline (Evoxac®) Overdose. J. Med. Toxicol. 2011, 7, 57–59. [Google Scholar] [CrossRef] [Green Version]
- Haga, T. Molecular Properties of Muscarinic Acetylcholine Receptors. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2013, 89, 226–256. [Google Scholar] [CrossRef] [Green Version]
- Papke, R.L.; Lindstrom, J.M. Nicotinic Acetylcholine Receptors: Conventional and Unconventional Ligands and Signaling. Neuropharmacology 2020, 168, 108021. [Google Scholar] [CrossRef]
- Zhang, B.; Madden, P.; Gu, J.; Xing, X.; Sankar, S.; Flynn, J.; Kroll, K.; Wang, T. Uncovering the Transcriptomic and Epigenomic Landscape of Nicotinic Receptor Genes in Non-Neuronal Tissues. BMC Genom. 2017, 18, 439. [Google Scholar] [CrossRef] [Green Version]
- Unwin, N. Refined Structure of the Nicotinic Acetylcholine Receptor at 4 Å Resolution. J. Mol. Biol. 2005, 346, 967–989. [Google Scholar] [CrossRef]
- Pedersen, J.E.; Bergqvist, C.A.; Larhammar, D. Evolution of Vertebrate Nicotinic Acetylcholine Receptors. BMC Evol. Biol. 2019, 19, 38. [Google Scholar] [CrossRef] [Green Version]
- Young, G.T.; Zwart, R.; Walker, A.S.; Sher, E.; Millar, N.S. Potentiation of A7 Nicotinic Acetylcholine Receptors via an Allosteric Transmembrane Site. Proc. Natl. Acad. Sci. USA 2008, 105, 14686–14691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millar, N.S.; Gotti, C. Diversity of Vertebrate Nicotinic Acetylcholine Receptors. Neuropharmacology 2009, 56, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.I.; Kitt, C.A.; Simonds, W.F.; Price, D.L.; Brann, M.R. Identification and Localization of Muscarinic Acetylcholine Receptor Proteins in Brain with Subtype-Specific Antibodies. J. Neurosci. 1991, 11, 3218–3226. [Google Scholar] [CrossRef] [Green Version]
- Hersch, S.M.; Gutekunst, C.A.; Rees, H.D.; Heilman, C.J.; Levey, A.I. Distribution of M1–M4 Muscarinic Receptor Proteins in the Rat Striatum: Light and Electron Microscopic Immunocytochemistry Using Subtype-Specific Antibodies. J. Neurosci. 1994, 14, 3351–3363. [Google Scholar] [CrossRef] [Green Version]
- Fisher, A. M1 Muscarinic Agonists Target Major Hallmarks of Alzheimer’s Disease—The Pivotal Role of Brain M1 Receptors. Neurodegener. Dis. 2008, 5, 237–240. [Google Scholar] [CrossRef]
- Jones, C.K.; Brady, A.E.; Davis, A.A.; Xiang, Z.; Bubser, M.; Tantawy, M.N.; Kane, A.S.; Bridges, T.M.; Kennedy, J.P.; Bradley, S.R.; et al. Novel Selective Allosteric Activator of the M1 Muscarinic Acetylcholine Receptor Regulates Amyloid Processing and Produces Antipsychotic-like Activity in Rats. J. Neurosci. 2008, 28, 10422–10433. [Google Scholar] [CrossRef] [PubMed]
- McArthur, R.A.; Gray, J.; Schreiber, R. Cognitive Effects of Muscarinic M1 Functional Agonists in Non-Human Primates and Clinical Trials. Curr. Opin. Investig. Drugs 2010, 11, 740–760. [Google Scholar] [PubMed]
- Hasselmo, M.E.; Sarter, M. Modes and Models of Forebrain Cholinergic Neuromodulation of Cognition. Neuropsychopharmacology 2011, 36, 52–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Puertas, R.; Pascual, J.; Vilaró, T.; Pazos, A. Autoradiographic Distribution of M1, M2, M3, and M4 Muscarinic Receptor Subtypes in Alzheimer’s Disease. Synapse 1997, 26, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Pakrasi, S.; Colloby, S.J.; Firbank, M.J.; Perry, E.K.; Wyper, D.J.; Owens, J.; McKeith, I.G.; Williams, E.D.; O’Brien, J.T. Muscarinic Acetylcholine Receptor Status in Alzheimer’s Disease Assessed Using (R, R) 123I-QNB SPECT. J. Neurol. 2007, 254, 907–913. [Google Scholar] [CrossRef]
- Fisher, A.; Brandeis, R.; Bar-Ner, R.H.N.; Kliger-Spatz, M.; Natan, N.; Sonego, H.; Marcovitch, I.; Pittel, Z. AF150(S) and AF267B: M1 Muscarinic Agonists as Innovative Therapies for Alzheimer’s Disease. J. Mol. Neurosci. 2002, 19, 145–153. [Google Scholar] [CrossRef]
- Watt, M.L.; Schober, D.A.; Hitchcock, S.; Liu, B.; Chesterfield, A.K.; McKinzie, D.; Felder, C.C. Pharmacological Characterization of LY593093, an M1 Muscarinic Acetylcholine Receptor-Selective Partial Orthosteric Agonist. J. Pharmacol. Exp. Ther. 2011, 338, 622–632. [Google Scholar] [CrossRef] [Green Version]
- Shirey, J.K.; Brady, A.E.; Jones, P.J.; Davis, A.A.; Bridges, T.M.; Kennedy, J.P.; Jadhav, S.B.; Menon, U.N.; Xiang, Z.; Watson, M.L.; et al. A Selective Allosteric Potentiator of the M1 Muscarinic Acetylcholine Receptor Increases Activity of Medial Prefrontal Cortical Neurons and Restores Impairments in Reversal Learning. J. Neurosci. 2009, 29, 14271–14286. [Google Scholar] [CrossRef]
- Chambon, C.; Jatzke, C.; Wegener, N.; Gravius, A.; Danysz, W. Using Cholinergic M1 Receptor Positive Allosteric Modulators to Improve Memory via Enhancement of Brain Cholinergic Communication. Eur. J. Pharmacol. 2012, 697, 73–80. [Google Scholar] [CrossRef]
- Tsang, S.W.Y.; Francis, P.T.; Esiri, M.M.; Wong, P.T.H.; Chen, C.P.L.H.; Lai, M.K.P. Loss of [3H]4-DAMP Binding to Muscarinic Receptors in the Orbitofrontal Cortex of Alzheimer’s Disease Patients with Psychosis. Psychopharmacology 2008, 198, 251–259. [Google Scholar] [CrossRef]
- Züchner, T.; Perez-Polo, J.R.; Schliebs, R. Beta-Secretase BACE1 Is Differentially Controlled through Muscarinic Acetylcholine Receptor Signaling. J. Neurosci. Res. 2004, 77, 250–257. [Google Scholar] [CrossRef]
- Zuchner, T.; Schliebs, R.; Perez-Polo, J.R. Down-Regulation of Muscarinic Acetylcholine Receptor M2 Adversely Affects the Expression of Alzheimer’s Disease-Relevant Genes and Proteins. J. Neurochem. 2005, 95, 20–32. [Google Scholar] [CrossRef]
- Liu, J.; Rasul, I.; Sun, Y.; Wu, G.; Li, L.; Premont, R.T.; Suo, W.Z. GRK5 Deficiency Leads to Reduced Hippocampal Acetylcholine Level via Impaired Presynaptic M2/M4 Autoreceptor Desensitization. J. Biol. Chem. 2009, 284, 19564–19571. [Google Scholar] [CrossRef] [Green Version]
- Nitsch, R.M.; Slack, B.E.; Wurtman, R.J.; Growdon, J.H. Release of Alzheimer Amyloid Precursor Derivatives Stimulated by Activation of Muscarinic Acetylcholine Receptors. Science 1992, 258, 304–307. [Google Scholar] [CrossRef]
- Scarpa, M.; Hesse, S.; Bradley, S.J. M1 Muscarinic Acetylcholine Receptors: A Therapeutic Strategy for Symptomatic and Disease-Modifying Effects in Alzheimer’s Disease? Adv. Pharmacol. 2020, 88, 277–310. [Google Scholar] [CrossRef]
- Harries, M.H.; Samson, N.A.; Cilia, J.; Hunter, A.J. The Profile of Sabcomeline (SB-202026), a Functionally Selective M1 Receptor Partial Agonist, in the Marmoset. Br. J. Pharmacol. 1998, 124, 409–415. [Google Scholar] [CrossRef]
- Clader, J.W.; Wang, Y. Muscarinic Receptor Agonists and Antagonists in the Treatment of Alzheimer’s Disease. Curr. Pharm. Des. 2005, 11, 3353–3361. [Google Scholar] [CrossRef] [PubMed]
- Beach, T.G.; Walker, D.G.; Sue, L.I.; Scott, S.; Layne, K.J.; Newell, A.J.; Potter, P.E.; Durham, R.A.; Emmerling, M.R.; Webster, S.D. Immunotoxin Lesion of the Cholinergic Nucleus Basalis Causes Aβ Deposition: Towards a Physiologic Animal Model of Alzheimers Disease. Curr. Med. Chem. Immunol. Endocr. Metab. Agents 2003, 3, 57–75. [Google Scholar] [CrossRef]
- Bartolomeo, A.C.; Morris, H.; Buccafusco, J.J.; Kille, N.; Rosenzweig-Lipson, S.; Husbands, M.G.; Sabb, A.L.; Abou-Gharbia, M.; Moyer, J.A.; Boast, C.A. The Preclinical Pharmacological Profile of WAY-132983, a Potent M1 Preferring Agonist. J. Pharmacol. Exp. Ther. 2000, 292, 584–596. [Google Scholar]
- Bodick, N.C.; Offen, W.W.; Levey, A.I.; Cutler, N.R.; Gauthier, S.G.; Satlin, A.; Shannon, H.E.; Tollefson, G.D.; Rasmussen, K.; Bymaster, F.P.; et al. Effects of Xanomeline, a Selective Muscarinic Receptor Agonist, on Cognitive Function and Behavioral Symptoms in Alzheimer Disease. Arch. Neurol. 1997, 54, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Knapp, M.J.; Knopman, D.S.; Solomon, P.R.; Pendlebury, W.W.; Davis, C.S.; Gracon, S.I. A 30-Week Randomized Controlled Trial of High-Dose Tacrine in Patients with Alzheimer’s Disease. The Tacrine Study Group. JAMA 1994, 271, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.J.; Choi, D.L.; Conn, P.J.; Rook, J.M. Activation of M1 and M4 Muscarinic Receptors as Potential Treatments for Alzheimer’s Disease and Schizophrenia. Neuropsychiatr. Dis. Treat. 2014, 10, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Shekhar, A.; Potter, W.Z.; Lightfoot, J.; Lienemann, J.; Dubé, S.; Mallinckrodt, C.; Bymaster, F.P.; McKinzie, D.L.; Felder, C.C. Selective Muscarinic Receptor Agonist Xanomeline as a Novel Treatment Approach for Schizophrenia. Am. J. Psychiatry 2008, 165, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Brannan, S.K.; Sawchak, S.; Miller, A.C.; Lieberman, J.A.; Paul, S.M.; Breier, A. Muscarinic Cholinergic Receptor Agonist and Peripheral Antagonist for Schizophrenia. N. Engl. J. Med. 2021, 384, 717–726. [Google Scholar] [CrossRef]
- Gould, R.W.; Dencker, D.; Grannan, M.; Bubser, M.; Zhan, X.; Wess, J.; Xiang, Z.; Locuson, C.; Lindsley, C.W.; Conn, P.J.; et al. Role for the M1 Muscarinic Acetylcholine Receptor in Top-Down Cognitive Processing Using a Touchscreen Visual Discrimination Task in Mice. ACS Chem. Neurosci. 2015, 6, 1683–1695. [Google Scholar] [CrossRef] [Green Version]
- Uslaner, J.M.; Eddins, D.; Puri, V.; Cannon, C.E.; Sutcliffe, J.; Chew, C.S.; Pearson, M.; Vivian, J.A.; Chang, R.K.; Ray, W.J.; et al. The Muscarinic M1 Receptor Positive Allosteric Modulator PQCA Improves Cognitive Measures in Rat, Cynomolgus Macaque, and Rhesus Macaque. Psychopharmacology 2013, 225, 21–30. [Google Scholar] [CrossRef]
- Beshore, D.C.; Di Marco, C.N.; Chang, R.K.; Greshock, T.J.; Ma, L.; Wittmann, M.; Seager, M.A.; Koeplinger, K.A.; Thompson, C.D.; Fuerst, J.; et al. MK-7622: A First-in-Class M1 Positive Allosteric Modulator Development Candidate. ACS Med. Chem. Lett. 2018, 9, 652–656. [Google Scholar] [CrossRef]
- Voss, T.; Li, J.; Cummings, J.; Farlow, M.; Assaid, C.; Froman, S.; Leibensperger, H.; Snow-Adami, L.; McMahon, K.B.; Egan, M.; et al. Randomized, Controlled, Proof-of-Concept Trial of MK-7622 in Alzheimer’s Disease. Alzheimers Dement (N. Y.) 2018, 4, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, N.; Iga, Y.; Mizobe, F.; Kawanishi, G. Amelioration of Experimental Amnesia (Passive Avoidance Failure) in Rodents by the Selective M1 Agonist AF102B. Jpn. J. Pharmacol. 1988, 48, 502–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahara, N.; Iga, Y.; Saito, Y.; Mizobe, F.; Kawanishi, G. Beneficial Effects of FKS-508 (AF102B), a Selective M1 Agonist, on the Impaired Working Memory in AF64A-Treated Rats. Jpn. J. Pharmacol. 1989, 51, 539–547. [Google Scholar] [CrossRef]
- Fisher, A.; Brandeis, R.; Karton, I.; Pittel, Z.; Gurwitz, D.; Haring, R.; Sapir, M.; Levy, A.; Heldman, E. (+−)-Cis-2-Methyl-Spiro(1,3-Oxathiolane-5,3′)Quinuclidine, an M1 Selective Cholinergic Agonist, Attenuates Cognitive Dysfunctions in an Animal Model of Alzheimer’s Disease. J. Pharmacol. Exp. Ther. 1991, 257, 392–403. [Google Scholar]
- Brandeis, R.; Dachir, S.; Sapir, M.; Levy, A.; Fisher, A. Reversal of Age-Related Cognitive Impairments by an M1 Cholinergic Agonist, AF102B. Pharmacol. Biochem. Behav. 1990, 36, 89–95. [Google Scholar] [CrossRef]
- Vincent, G.P.; Sepinwall, J. AF102B, a Novel M1 Agonist, Enhanced Spatial Learning in C57BL/10 Mice with a Long Duration of Action. Brain Res. 1992, 597, 264–268. [Google Scholar] [CrossRef]
- Dawson, G.R.; Bayley, P.; Channell, S.; Iversen, S.D. A Comparison of the Effects of the Novel Muscarinic Receptor Agonists L-689,660 and AF102B in Tests of Reference and Working Memory. Psychopharmacology 1994, 113, 361–368. [Google Scholar] [CrossRef]
- Ohno, M.; Yamamoto, T.; Watanabe, S. Blockade of Hippocampal M1 Muscarinic Receptors Impairs Working Memory Performance of Rats. Brain Res. 1994, 650, 260–266. [Google Scholar] [CrossRef]
- Suzuki, M.; Yamaguchi, T.; Ozawa, Y.; Iwai, A.; Yamamoto, M. Effect of YM796, a Novel Muscarinic Agonist, on the Impairment of Passive Avoidance Response in Senescence-Accelerated Mice. Pharmacol. Biochem. Behav. 1995, 51, 623–626. [Google Scholar] [CrossRef]
- Iga, Y.; Arisawa, H.; Ise, M.; Yasuda, H.; Takeshita, Y. Modulation of Rhythmical Slow Activity, Long-Term Potentiation and Memory by Muscarinic Receptor Agonists. Eur. J. Pharmacol. 1996, 308, 13–19. [Google Scholar] [CrossRef]
- Young, M.B.; Thomas, S.A. M1-Muscarinic Receptors Promote Fear Memory Consolidation via Phospholipase C and the M-Current. J. Neurosci. 2014, 34, 1570–1578. [Google Scholar] [CrossRef]
- Dawson, G.R.; Iversen, S.D. The Effects of Novel Cholinesterase Inhibitors and Selective Muscarinic Receptor Agonists in Tests of Reference and Working Memory. Behav. Brain Res. 1993, 57, 143–153. [Google Scholar] [CrossRef]
- Suzuki, M.; Yamaguchi, T.; Ozawa, Y.; Ohyama, M.; Yamamoto, M. Effects of (-)-S-2,8-Dimethyl-3-Methylene-1-Oxa-8-Azaspiro[4,5]Decane L-Tartrate Monohydrate (YM796), a Novel Muscarinic Agonist, on Disturbance of Passive Avoidance Learning Behavior in Drug-Treated and Senescence-Accelerated Mice. J. Pharmacol. Exp. Ther. 1995, 275, 728–736. [Google Scholar]
- O’Neill, J.; Fitten, L.J.; Siembieda, D.; Halgren, E.; Kim, E.; Fisher, A.; Perryman, K. Effects of AF102B and Tacrine on Delayed Match-to-Sample in Monkeys. Prog. Neuropsychopharmacol. Biol. Psychiatry 1998, 22, 665–678. [Google Scholar] [CrossRef]
- Fisher, A.; Bezprozvanny, I.; Wu, L.; Ryskamp, D.A.; Bar-Ner, N.; Natan, N.; Brandeis, R.; Elkon, H.; Nahum, V.; Gershonov, E.; et al. AF710B, a Novel M1/Σ1 Agonist with Therapeutic Efficacy in Animal Models of Alzheimer’s Disease. NDD 2016, 16, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Nitsch, R.M.; Deng, M.; Tennis, M.; Schoenfeld, D.; Growdon, J.H. The Selective Muscarinic M1 Agonist AF102B Decreases Levels of Total Abeta in Cerebrospinal Fluid of Patients with Alzheimer’s Disease. Ann. Neurol. 2000, 48, 913–918. [Google Scholar] [CrossRef]
- Fisher, A.; Heldman, E.; Gurwitz, D.; Haring, R.; Karton, Y.; Meshulam, H.; Pittel, Z.; Marciano, D.; Brandeis, R.; Sadot, E.; et al. M1 Agonists for the Treatment of Alzheimer’s Disease. Novel Properties and Clinical Update. Ann. N. Y. Acad. Sci. 1996, 777, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Haring, R.; Gurwitz, D.; Barg, J.; Pinkas-Kramarski, R.; Heldman, E.; Pittel, Z.; Wengier, A.; Meshulam, H.; Marciano, D.; Karton, Y. Amyloid Precursor Protein Secretion via Muscarinic Receptors: Reduced Desensitization Using the M1-Selective Agonist AF102B. Biochem. Biophys. Res. Commun. 1994, 203, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Haring, R.; Gurwitz, D.; Barg, J.; Pinkas-Kramarski, R.; Heldman, E.; Pittel, Z.; Danenberg, H.D.; Wengier, A.; Meshulam, H.; Marciano, D. NGF Promotes Amyloid Precursor Protein Secretion via Muscarinic Receptor Activation. Biochem. Biophys. Res. Commun. 1995, 213, 15–23. [Google Scholar] [CrossRef]
- Gurwitz, D.; Haring, R.; Pinkas-Kramarski, R.; Stein, R.; Heldman, E.; Karton, Y.; Fisher, A. NGF-Dependent Neurotrophic-like Effects of AF102B, an M1 Muscarinic Agonist, in PC12M1 Cells. Neuroreport 1995, 6, 485–488. [Google Scholar] [CrossRef]
- Sadot, E.; Gurwitz, D.; Barg, J.; Behar, L.; Ginzburg, I.; Fisher, A. Activation of M1 Muscarinic Acetylcholine Receptor Regulates Tau Phosphorylation in Transfected PC12 Cells. J. Neurochem. 1996, 66, 877–880. [Google Scholar] [CrossRef]
- Beach, T.G.; Walker, D.G.; Potter, P.E.; Sue, L.I.; Fisher, A. Reduction of Cerebrospinal Fluid Amyloid Beta after Systemic Administration of M1 Muscarinic Agonists. Brain Res. 2001, 905, 220–223. [Google Scholar] [CrossRef]
- Welt, T.; Kulic, L.; Hoey, S.E.; McAfoose, J.; Späni, C.; Chadha, A.S.; Fisher, A.; Nitsch, R.M. Acute Effects of Muscarinic M1 Receptor Modulation on AβPP Metabolism and Amyloid-β Levels in Vivo: A Microdialysis Study. J. Alzheimers Dis. 2015, 46, 971–982. [Google Scholar] [CrossRef]
- van der Westhuizen, E.T.; Choy, K.H.C.; Valant, C.; McKenzie-Nickson, S.; Bradley, S.J.; Tobin, A.B.; Sexton, P.M.; Christopoulos, A. Fine Tuning Muscarinic Acetylcholine Receptor Signaling through Allostery and Bias. Front. Pharmacol. 2021, 11, 2217. [Google Scholar] [CrossRef] [PubMed]
- Petrone, D.; Condemi, J.J.; Fife, R.; Gluck, O.; Cohen, S.; Dalgin, P. A Double-Blind, Randomized, Placebo-Controlled Study of Cevimeline in Sjögren’s Syndrome Patients with Xerostomia and Keratoconjunctivitis Sicca. Arthritis Rheum. 2002, 46, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Fife, R.S.; Chase, W.F.; Dore, R.K.; Wiesenhutter, C.W.; Lockhart, P.B.; Tindall, E.; Suen, J.Y. Cevimeline for the Treatment of Xerostomia in Patients With Sjögren Syndrome: A Randomized Trial. Arch. Intern. Med. 2002, 162, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Evoxac—FDA Prescribing Information, Side Effects and Uses. Available online: https://www.drugs.com/pro/evoxac.html (accessed on 2 August 2021).
- Fox, R.I.; Fox, C.M.; Gottenberg, J.E.; Dörner, T. Treatment of Sjögren’s Syndrome: Current Therapy and Future Directions. Rheumatology 2021, 60, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zeron, P.; Siso-Almirall, A.; Bosch, X.; Tzioufas, A.G. Topical and Systemic Medications for the Treatment of Primary Sjögren’s Syndrome. Nat. Rev. Rheumatol. 2012, 8, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.M.; Holliday, C.; Cimmino, J.; Roomian, T.; Papas, A. Comparing the Effectiveness and Adverse Effects of Pilocarpine and Cevimeline in Patients with Hyposalivation. Oral Dis. 2019, 25, 1937–1944. [Google Scholar] [CrossRef]


| Total Number of AD Patients in the Trials (Female/Male) | Age [Years] (Mean ± SD) | Illness Duration [Years] (Mean ± SD) | IMC Score * (Mean ± SD) | Baseline CSF Aβtotal (Mean ± SD) | Treatment CSF Aβtotal (Mean ± SD) | |
|---|---|---|---|---|---|---|
| Cevimeline | 19 (10/9) | 72.2 ± 10.1 | 4.9 ± 2.9 | 19.4 ± 9.8 | 22.8 ± 7.5 | 19.9 ± 7.9 |
| Hydroxychloroquine | 10 (3/7) | 68.3 ± 6.9 | 3.8 ± 1.9 | 19.5 ± 13.1 | 23.5 ± 10.1 | 24.0 ± 8.1 |
| Physostigmine | 9 (6/3) | 67.7 ± 9.3 | 3.8 ± 1.5 | 13.2 ± 8.9 | 21.2 ± 7.9 | 21.0 ± 6.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oleksak, P.; Novotny, M.; Patocka, J.; Nepovimova, E.; Hort, J.; Pavlik, J.; Klimova, B.; Valis, M.; Kuca, K. Neuropharmacology of Cevimeline and Muscarinic Drugs—Focus on Cognition and Neurodegeneration. Int. J. Mol. Sci. 2021, 22, 8908. https://doi.org/10.3390/ijms22168908
Oleksak P, Novotny M, Patocka J, Nepovimova E, Hort J, Pavlik J, Klimova B, Valis M, Kuca K. Neuropharmacology of Cevimeline and Muscarinic Drugs—Focus on Cognition and Neurodegeneration. International Journal of Molecular Sciences. 2021; 22(16):8908. https://doi.org/10.3390/ijms22168908
Chicago/Turabian StyleOleksak, Patrik, Michal Novotny, Jiri Patocka, Eugenie Nepovimova, Jakub Hort, Jan Pavlik, Blanka Klimova, Martin Valis, and Kamil Kuca. 2021. "Neuropharmacology of Cevimeline and Muscarinic Drugs—Focus on Cognition and Neurodegeneration" International Journal of Molecular Sciences 22, no. 16: 8908. https://doi.org/10.3390/ijms22168908
APA StyleOleksak, P., Novotny, M., Patocka, J., Nepovimova, E., Hort, J., Pavlik, J., Klimova, B., Valis, M., & Kuca, K. (2021). Neuropharmacology of Cevimeline and Muscarinic Drugs—Focus on Cognition and Neurodegeneration. International Journal of Molecular Sciences, 22(16), 8908. https://doi.org/10.3390/ijms22168908









