Dihydroisotanshinone I as a Treatment Option for Head and Neck Squamous Cell Carcinomas
Abstract
:1. Introduction
2. Results
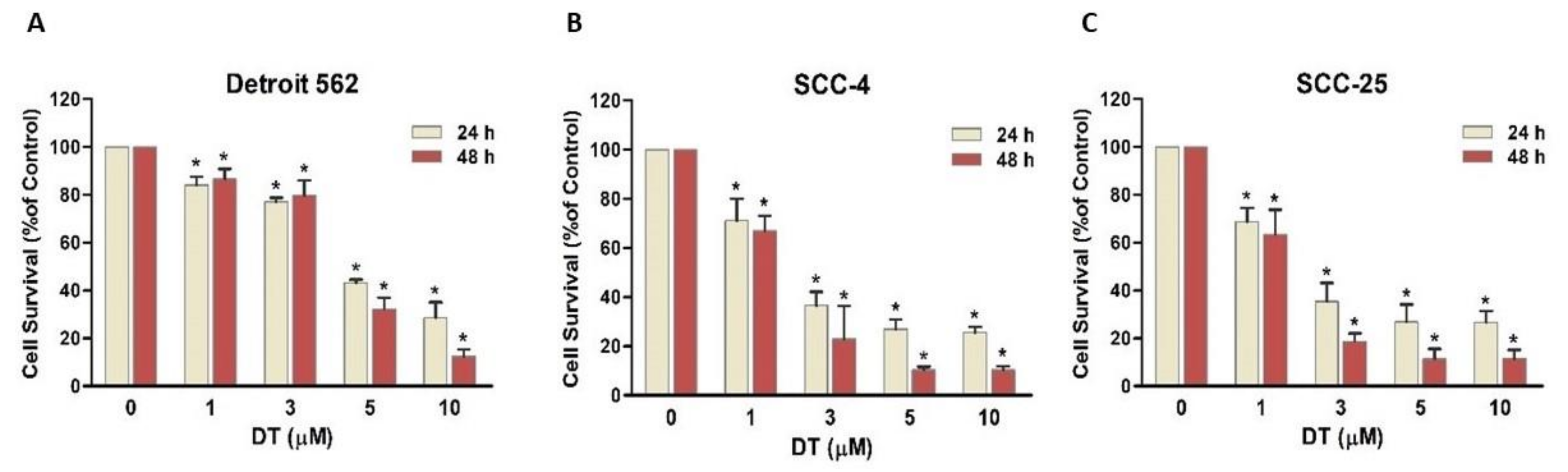
2.1. DT Inhibited Cell Survival of Human HNSCCs Cells
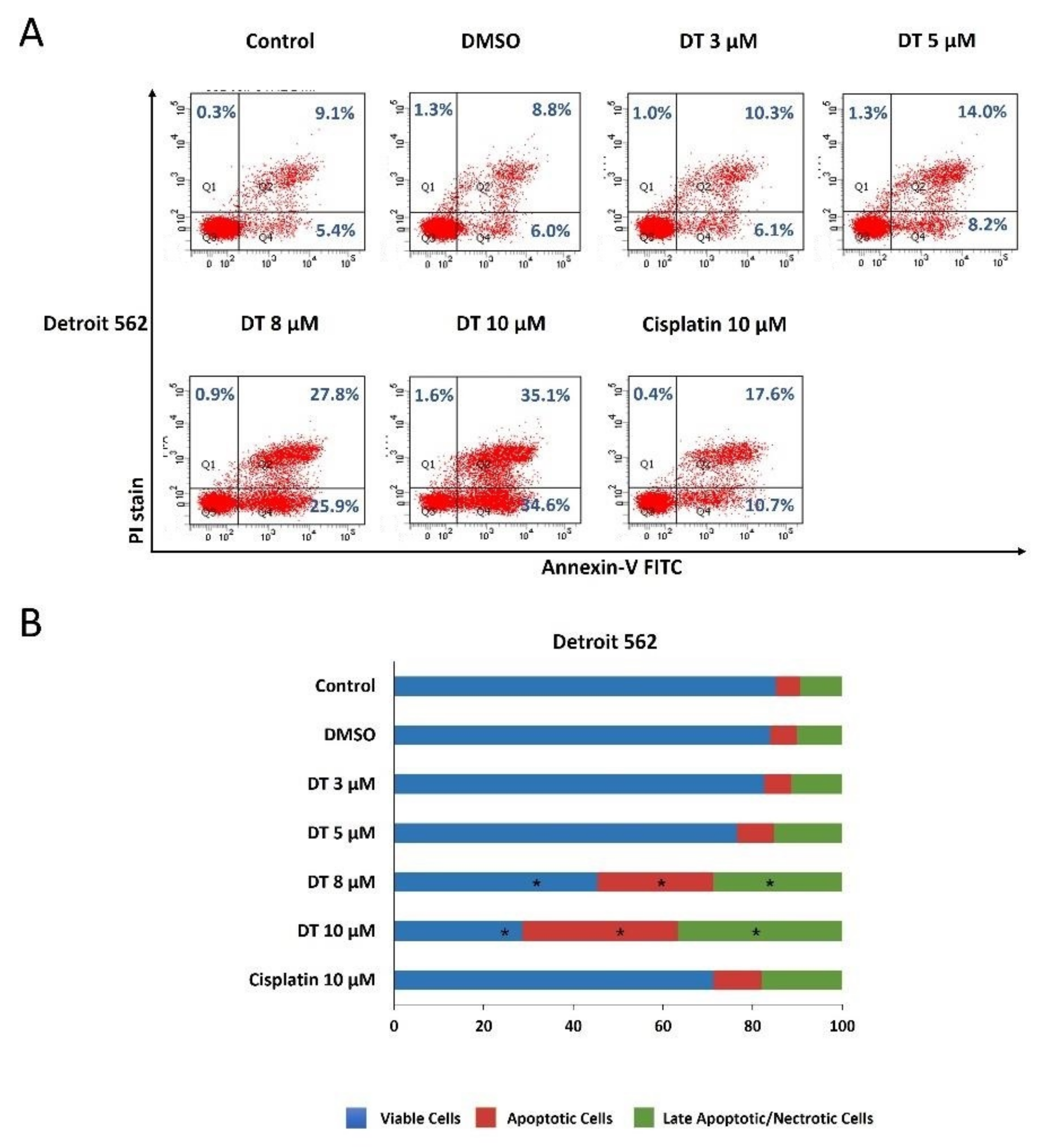
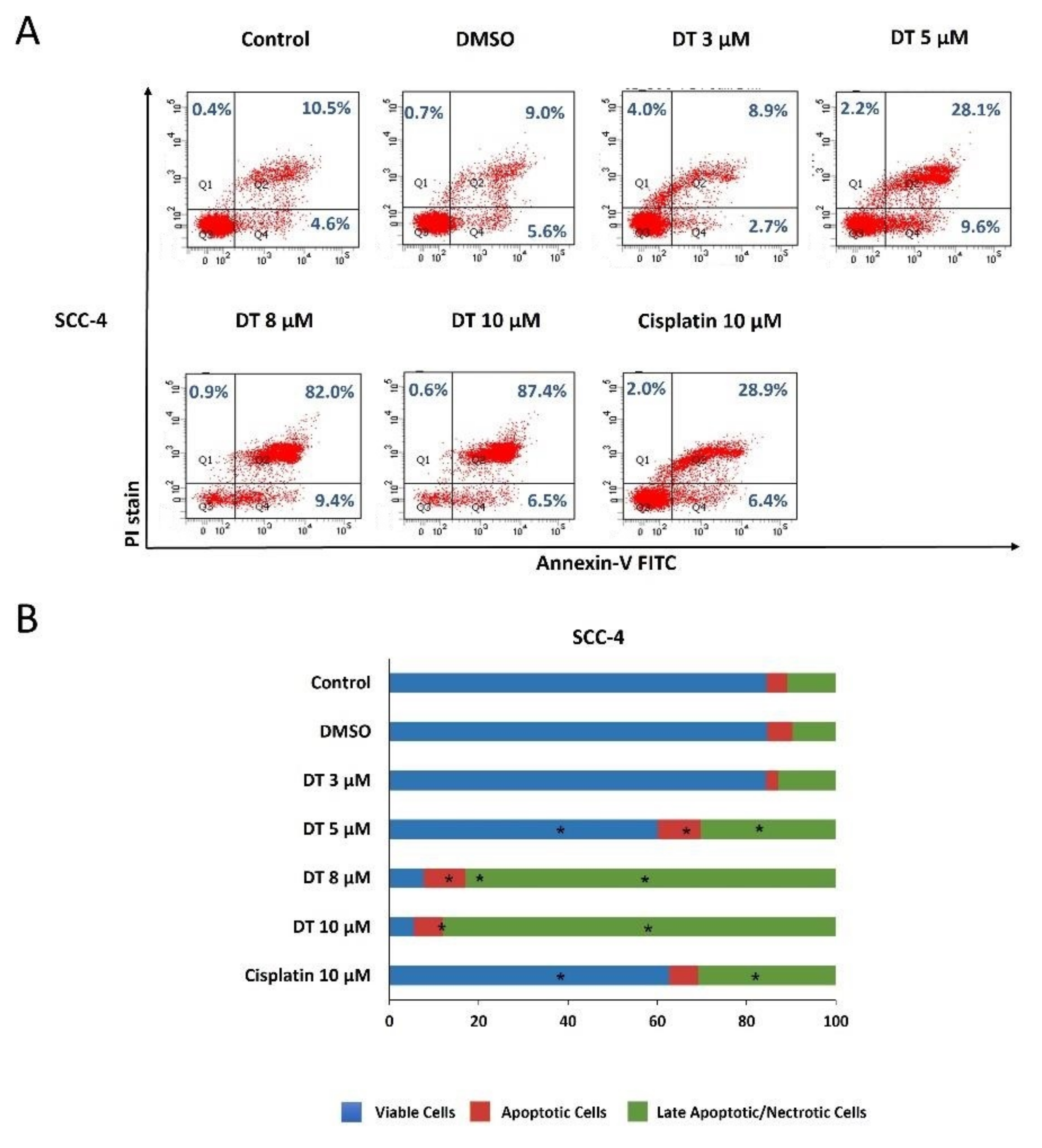
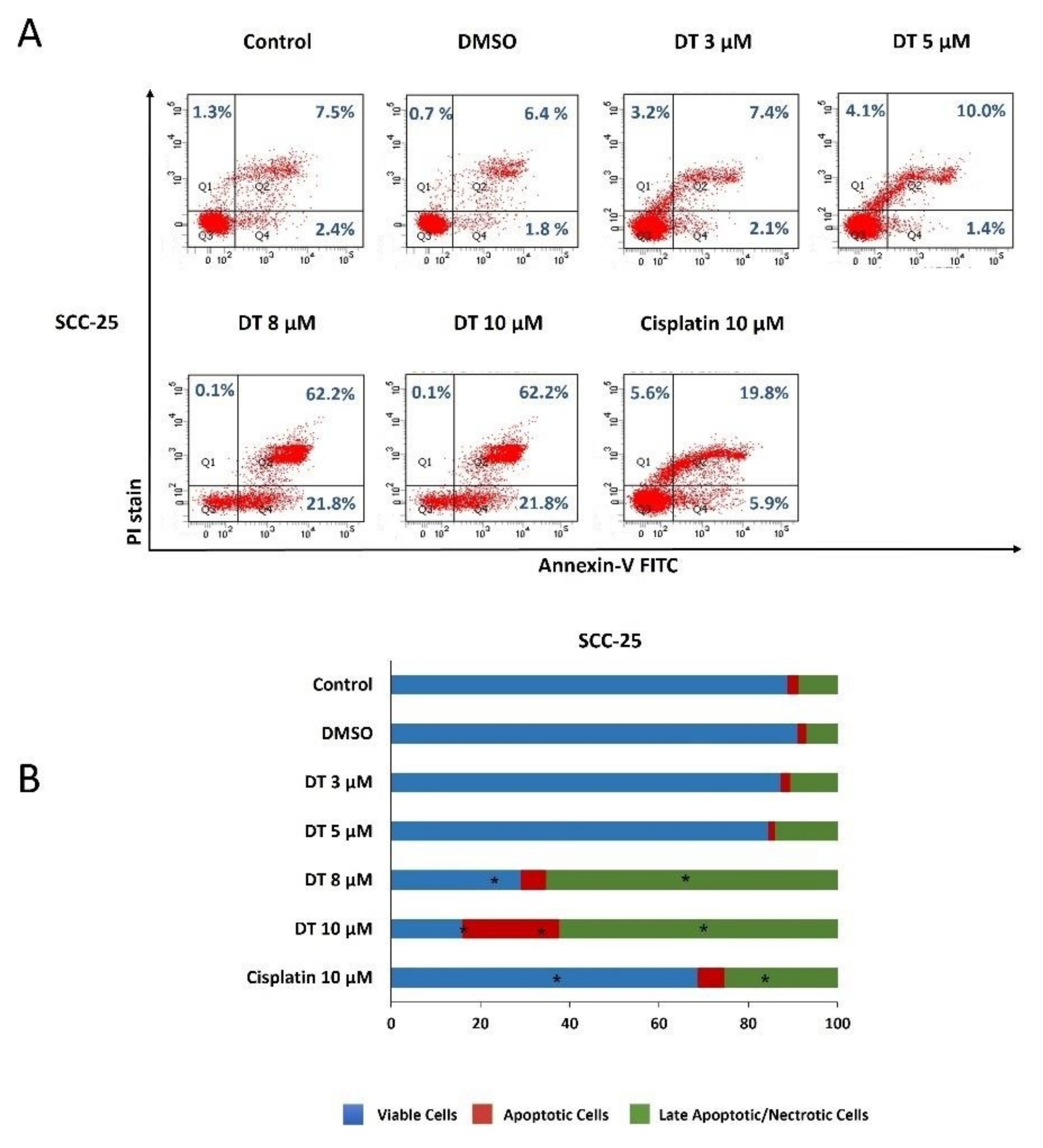
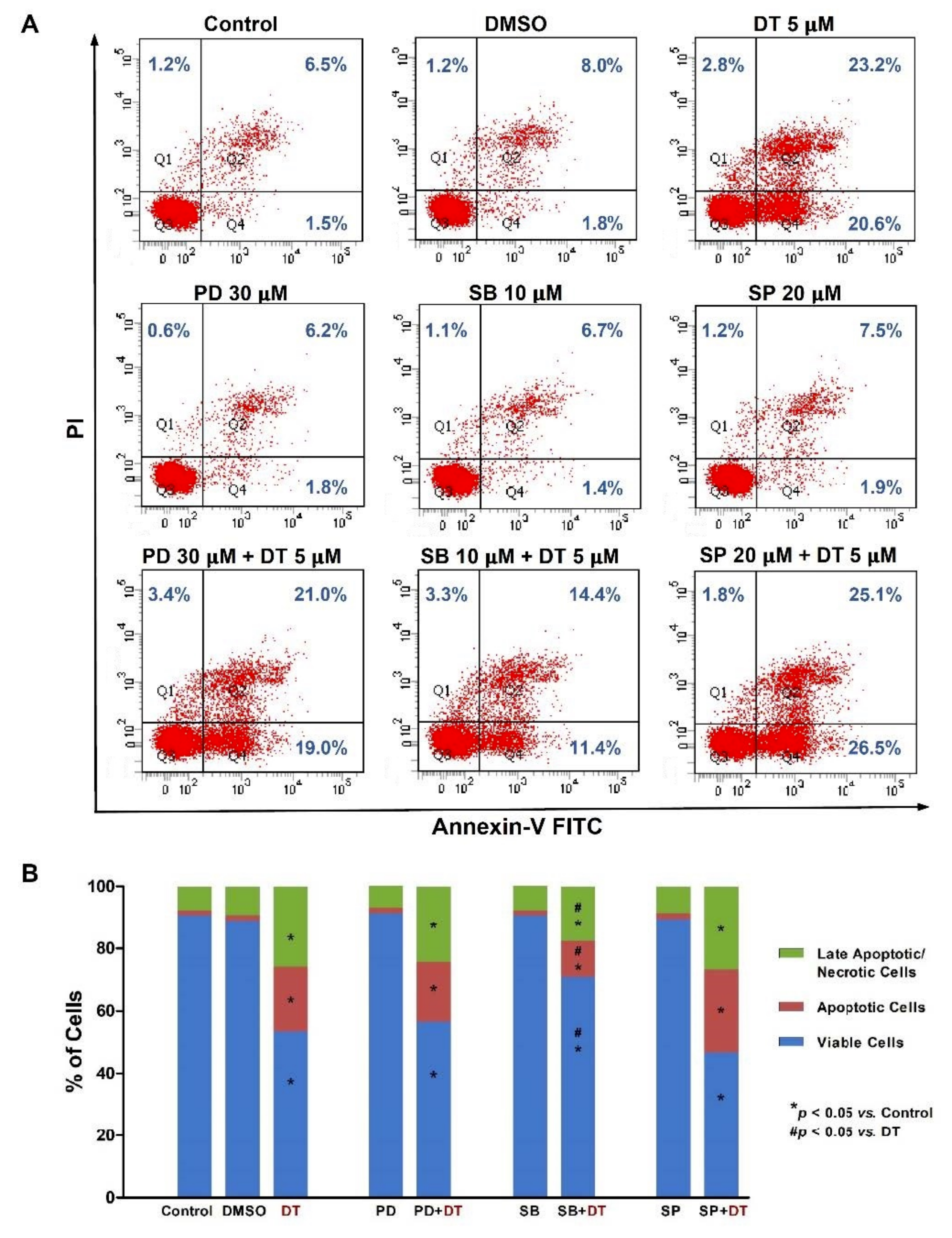
2.2. DT-Induced Apoptosis in HNSCCs Cells
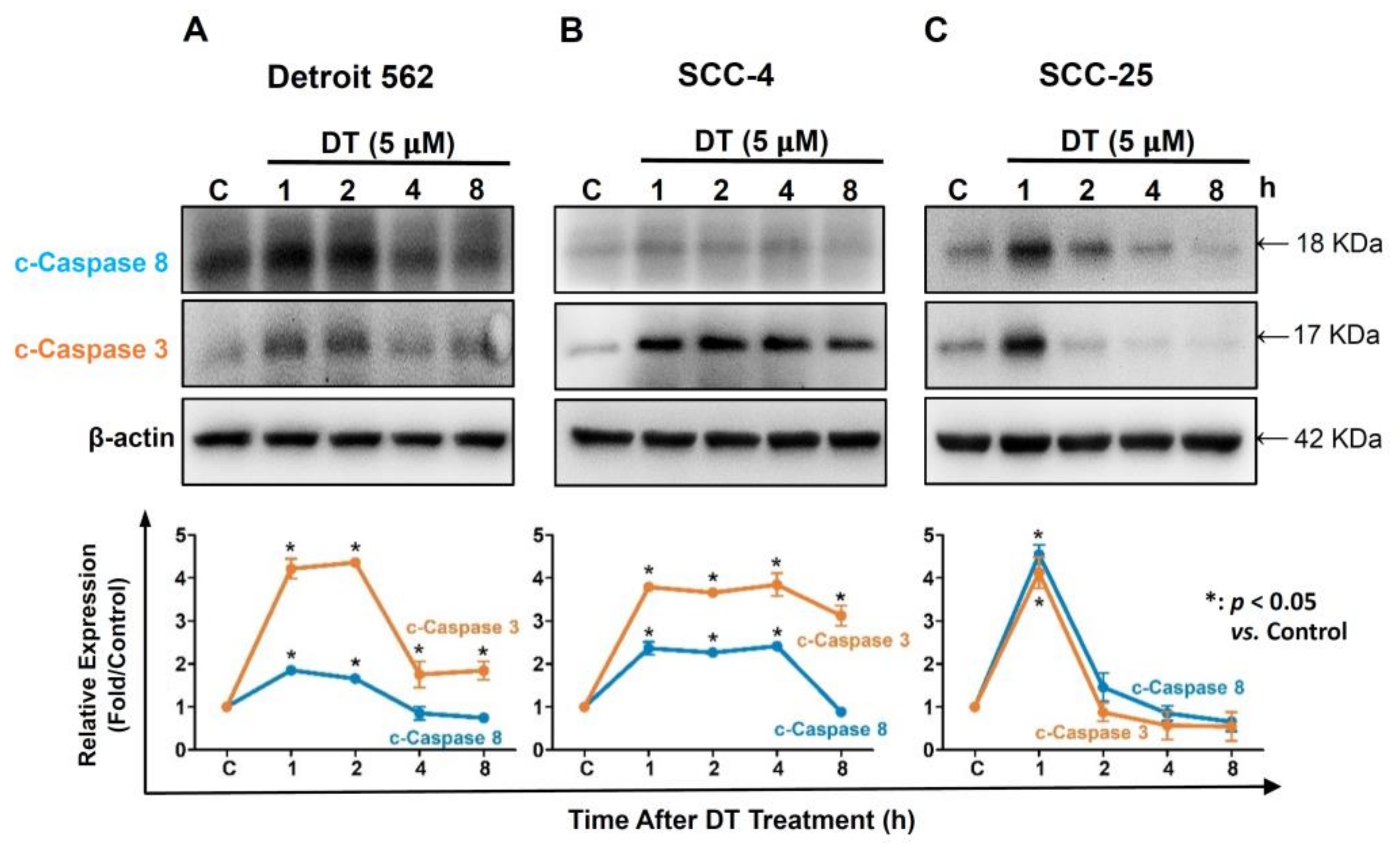
2.3. DT Increased Expression of Caspase-3 and Caspase-8 in HNSCCs Cells
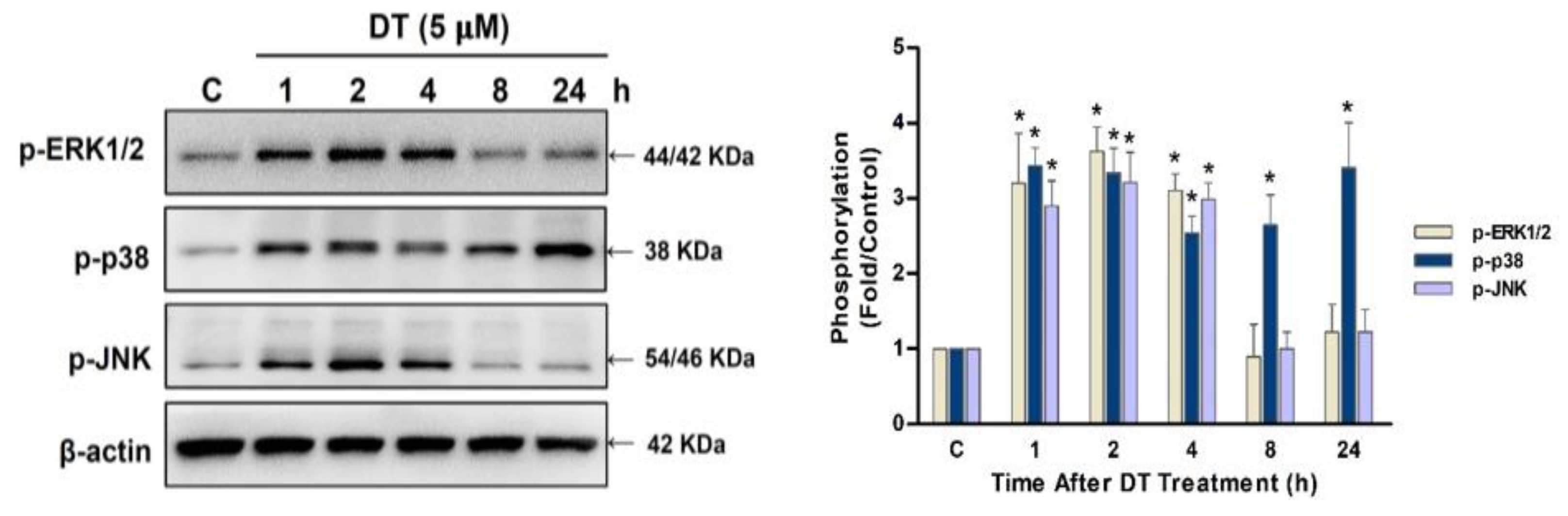
2.4. p38 Signaling Regulated DT-Induced Apoptosis in Detroit 562 Cells
2.5. DT Suppressed Detroit 562 Tumor Growth in BALB/cAnN.Cg Nude Mice
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. MTT Assay
4.3. Western Blotting
4.4. Annexin V–FITC/PI Staining for Flow Cytometry
4.5. Mouse Xenograft Model
4.6. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mourad, M.; Jetmore, T.; Jategaonkar, A.A.; Moubayed, S.; Moshier, E.; Urken, M.L. Epidemiological Trends of Head and Neck Cancer in the United States: A SEER Population Study. J. Oral Maxillofac. Surg. 2017, 75, 2562–2572. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Mehanna, H.; Paleri, V.; West, C.M.; Nutting, C. Head and neck cancer-part 1: Epidemiology, presentation, and preservation. Clin. Otolaryngol. 2011, 36, 65–68. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Health Promotion Administration Ministry of Health and Welfare, Taiwan. Cancer Registry Annual Report. 2020. Available online: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=269&pid=13498 (accessed on 22 July 2021).
- Haller, J.R.; Mountain, R.E.; Schuller, D.E.; Nag, S. Mortality and morbidity with intraoperative radiotherapy for head and neck cancer. Am. J. Otolaryngol. 1996, 17, 308–310. [Google Scholar] [CrossRef]
- Hussain, M.; Kish, J.A.; Crane, L.; Uwayda, A.; Cummings, G.; Ensley, J.F.; Tapazoglou, E.; Al-Sarraf, M. The role of infection in the morbidity and mortality of patients with head and neck cancer undergoing multimodality therapy. Cancer 1991, 67, 716–721. [Google Scholar] [CrossRef]
- Joo, Y.H.; Cho, K.J.; Park, J.O.; Kim, S.Y.; Kim, M.S. Surgical morbidity and mortality in patients after microvascular reconstruction for head and neck cancer. Clin. Otolaryngol. 2018, 43, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.H.; Roh, J.L.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Incidence and risk factors for morbidity and mortality in elderly head and neck cancer patients undergoing major oncological surgery. J. Cancer Res. Clin. Oncol. 2016, 142, 1343–1351. [Google Scholar] [CrossRef]
- Shi, M.; Huang, F.; Deng, C.; Wang, Y.; Kai, G. Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit. Rev. Food Sci. Nutr. 2019, 59, 953–964. [Google Scholar] [CrossRef]
- Fu, L.; Han, B.; Zhou, Y.; Ren, J.; Cao, W.; Patel, G.; Kai, G.; Zhang, J. The Anticancer Properties of Tanshinones and the Pharmacological Effects of Their Active Ingredients. Front. Pharmacol. 2020, 11, 193. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Guo, J.; Bao, J.; Lu, J.; Wang, Y. The anticancer properties of Salvia miltiorrhiza Bunge (Danshen): A systematic review. Med. Res. Rev. 2014, 34, 768–794. [Google Scholar] [CrossRef]
- Stumpf, C.; Fan, Q.; Hintermann, C.; Raaz, D.; Kurfurst, I.; Losert, S.; Pflederer, W.; Achenbach, S.; Daniel, W.G.; Garlichs, C.D. Anti-inflammatory effects of danshen on human vascular endothelial cells in culture. Am. J. Chin. Med. 2013, 41, 1065–1077. [Google Scholar] [CrossRef]
- Maione, F.; Mascolo, N. Danshen and the Cardiovascular System: New Advances for an Old Remedy. Semin. Thromb. Hemost. 2016, 42, 321–322. [Google Scholar]
- Ma, L.; Jiang, H.; Xu, X.; Zhang, C.; Niu, Y.; Wang, Z.; Tao, Y.; Li, Y.; Cai, F.; Zhang, X.; et al. Tanshinone IIA mediates SMAD7-YAP interaction to inhibit liver cancer growth by inactivating the transforming growth factor beta signaling pathway. Aging 2019, 11, 9719–9737. [Google Scholar] [CrossRef]
- He, L.; Gu, K. Tanshinone IIA regulates colorectal cancer apoptosis via attenuation of Parkinmediated mitophagy by suppressing AMPK/Skp2 pathways. Mol. Med. Rep. 2018, 18, 1692–1703. [Google Scholar] [PubMed] [Green Version]
- Lin, Y.S.; Shen, Y.C.; Wu, C.Y.; Tsai, Y.Y.; Yang, Y.H.; Lin, Y.Y.; Kuan, F.C.; Lu, C.N.; Chang, G.H.; Tsai, M.S.; et al. Danshen Improves Survival of Patients With Breast Cancer and Dihydroisotanshinone I Induces Ferroptosis and Apoptosis of Breast Cancer Cells. Front. Pharmacol. 2019, 10, 1226. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.Y.; Cherng, J.Y.; Yang, Y.H.; Lin, C.L.; Kuan, F.C.; Lin, Y.Y.; Lin, Y.S.; Shu, L.H.; Cheng, Y.C.; Liu, H.T.; et al. Danshen improves survival of patients with advanced lung cancer and targeting the relationship between macrophages and lung cancer cells. Oncotarget 2017, 8, 90925–90947. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, Y.; Chen, L.; Liu, F.; Qi, Z.; Cheng, X.; Wang, Z. Cryptotanshinone suppresses cell proliferation and glucose metabolism via STAT3/SIRT3 signaling pathway in ovarian cancer cells. Cancer Med. 2018, 7, 4610–4618. [Google Scholar] [CrossRef]
- Chiu, T.L.; Su, C.C. Tanshinone IIA increases protein expression levels of PERK, ATF6, IRE1alpha, CHOP, caspase3 and caspase12 in pancreatic cancer BxPC3 cellderived xenograft tumors. Mol. Med. Rep. 2017, 15, 3259–3263. [Google Scholar] [CrossRef]
- Guan, Z.; Chen, J.; Li, X.; Dong, N. Tanshinone IIA induces ferroptosis in gastric cancer cells through p53-mediated SLC7A11 down-regulation. Biosci. Rep. 2020, 40, BSR20201807. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, L.; Su, J.; Li, S.; Duncan, S.E.; Liu, Z.; Fan, G. Pharmacological Activity and Mechanism of Tanshinone IIA in Related Diseases. Drug Des. Devel. Ther. 2020, 14, 4735–4748. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wawrose, J.S.; Gooding, W.E.; Garrway, L.A.; Liu, V.W.Y.; Peyser, N.D.; Grandis, J.R. Genomic analysis of head and neck squamous cell carcinoma cell lines and human tumors: A rational approach to preclinical model selection. Mol. Cancer Res. 2014 12, 571–582. [CrossRef] [Green Version]
- Yang, M.; Zhu, S.J.; Shen, C.; Zhai, R.; Li, D.D.; Fang, M.; Xu, J.N.; Gan, Y.N.; Yang, L.; Ren, Z.Y.; et al. Clinical Application of Chinese Herbal Injection for Cancer Care: Evidence-Mapping of the Systematic Reviews, Meta-analyses, and Randomized Controlled Trials. Front. Pharmacol. 2021, 12, 666368. [Google Scholar] [CrossRef] [PubMed]
- Matos, L.C.; Machado, J.P.; Monteiro, F.J.; Greten, H.J. Understanding Traditional Chinese Medicine Therapeutics: An Overview of the Basics and Clinical Applications. Healthcare 2021, 9, 257. [Google Scholar] [CrossRef]
- Ren, J.; Fu, L.; Nile, S.H.; Zhang, J.; Kai, G. Salvia miltiorrhiza in Treating Cardiovascular Diseases: A Review on Its Pharmacological and Clinical Applications. Front. Pharmacol. 2019, 10, 753. [Google Scholar] [CrossRef]
- Deng, Y.; Ng, E.S.; Kwan, Y.W.; Lau, C.B.; Cheung, D.W.; Koon, J.C.; Zhang, Z.; Zuo, Z.; Leung, P.C.; Fung, K.P.; et al. Cerebral vasodilator properties of Danshen and Gegen: A study of their combined efficacy and mechanisms of actions. Phytomedicine 2014, 21, 391–399. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, S.; Qian, L.; Tian, Y.; Chen, Y.N.; Bi, L.; Chen, W.P. OCF can repress tumor metastasis by inhibiting epithelial-mesenchymal transition involved in PTEN/PI3K/AKT pathway in lung cancer cells. PLoS ONE 2017, 16, e0174021. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Yang, L.; Zhang, B.; Chen, S. Tanshinone IIA effects on ovarian cancer cell line. J. Pharm. Pharmacol. 2018, 70, 1369–1377. [Google Scholar] [CrossRef]
- Yan, Y.; Su, W.; Zeng, S.; Qian, L.; Chen, X.; Wei, J.; Chen, N.; Gong, Z.; Xu, Z. Effect and Mechanism of Tanshinone I on the Radiosensitivity of Lung Cancer Cells. Mol. Pharm. 2018, 15, 4843–4853. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, Y.Y.; Chen, H.; Wu, Y.C.; Zhang, L. Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif. 2020, 53, e12739. [Google Scholar] [CrossRef]
- Luo, Y.; Song, L.; Wang, X.; Huang, Y.; Liu, Y.; Wang, Q.; Hong, M.; Yuan, Z. Uncovering the Mechanisms of Cryptotanshinone as a Therapeutic Agent Against Hepatocellular Carcinoma. Front. Pharmacol. 2020, 11, 1264. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jiang, Y.Y.; Wang, X.X.; Wang, H.P.; Chen, H.; Wu, Y.C.; Wang, L.; Pu, X.; Yue, G.Z.; Zhang, L. Tanshinone IIA suppresses ovarian cancer growth through inhibiting malignant properties and angiogenesis. Ann. Transl. Med. 2020, 8, 1295. [Google Scholar] [CrossRef] [PubMed]
- Jieensinue, S.; Zhu, H.; Li, G.; Dong, K.; Liang, M.; Li, Y. Tanshinone IIA reduces SW837 colorectal cancer cell viability via the promotion of mitochondrial fission by activating JNK-Mff signaling pathways. BMC Cell Biol. 2018, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Yang, Y.H.; Lin, Y.Y.; Kuan, F.C.; Lin, Y.S.; Lin, W.Y.; Tsai, M.Y.; Yang, J.J.; Cheng, Y.C.; Shu, L.H.; et al. Anti-cancer effect of danshen and dihydroisotanshinone I on prostate cancer: Targeting the crosstalk between macrophages and cancer cells via inhibition of the STAT3/CCL2 signaling pathway. Oncotarget 2017, 8, 40246–40263. [Google Scholar] [CrossRef] [PubMed]
- Picon, H.; Guddati, A.K. Mechanisms of resistance in head and neck cancer. Am. J. Cancer Res. 2020, 10, 2742–2751. [Google Scholar]
- Kanno, Y.; Chen, C.Y.; Lee, H.L.; Chiou, J.F.; Chen, Y.J. Molecular Mechanisms of Chemotherapy Resistance in Head and Neck Cancers. Front. Oncol. 2021, 11, 640392. [Google Scholar] [CrossRef]
- Prasanna, P.L.; Renu, K.; Valsala Gopalakrishnan, A. New molecular and biochemical insights of doxorubicin-induced hepatotoxicity. Life Sci. 2020, 250, 117599. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Lee, I.Y.; Huang, W.S.; Lin, Y.S.; Kuan, F.C.; Shu, L.H.; Cheng, Y.C.; Yang, Y.H.; Wu, C.Y. Danshen improves survival of patients with colon cancer and dihydroisotanshinone I inhibit the proliferation of colon cancer cells via apoptosis and skp2 signaling pathway. J. Ethnopharmacol. 2017, 209, 305–316. [Google Scholar] [CrossRef]
- Huang, S.T.; Huang, C.C.; Huang, W.L.; Lin, T.K.; Liao, P.L.; Wang, P.W.; Liou, C.W.; Chuang, J.H. Tanshinone IIA induces intrinsic apoptosis in osteosarcoma cells both in vivo and in vitro associated with mitochondrial dysfunction. Sci. Rep. 2017, 7, 40382. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.Y.; Lin, Y.Y.; Yang, Y.H.; Lin, Y.S.; Lin, C.L.; Lin, W.Y.; Cheng, Y.C.; Shu, L.H.; Wu, C.Y. Dihydroisotanshinone I combined with radiation inhibits the migration ability of prostate cancer cells through DNA damage and CCL2 pathway. BMC Pharmacol. Toxicol. 2018, 19, 5. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-M.; Yang, M.-Y.; Tsai, M.-S.; Chang, G.-H.; Yang, Y.-H.; Tsai, Y.-T.; Wu, C.-Y.; Chang, S.-F. Dihydroisotanshinone I as a Treatment Option for Head and Neck Squamous Cell Carcinomas. Int. J. Mol. Sci. 2021, 22, 8881. https://doi.org/10.3390/ijms22168881
Hsu C-M, Yang M-Y, Tsai M-S, Chang G-H, Yang Y-H, Tsai Y-T, Wu C-Y, Chang S-F. Dihydroisotanshinone I as a Treatment Option for Head and Neck Squamous Cell Carcinomas. International Journal of Molecular Sciences. 2021; 22(16):8881. https://doi.org/10.3390/ijms22168881
Chicago/Turabian StyleHsu, Cheng-Ming, Ming-Yu Yang, Ming-Shao Tsai, Geng-He Chang, Yao-Hsu Yang, Yao-Te Tsai, Ching-Yuan Wu, and Shun-Fu Chang. 2021. "Dihydroisotanshinone I as a Treatment Option for Head and Neck Squamous Cell Carcinomas" International Journal of Molecular Sciences 22, no. 16: 8881. https://doi.org/10.3390/ijms22168881
APA StyleHsu, C.-M., Yang, M.-Y., Tsai, M.-S., Chang, G.-H., Yang, Y.-H., Tsai, Y.-T., Wu, C.-Y., & Chang, S.-F. (2021). Dihydroisotanshinone I as a Treatment Option for Head and Neck Squamous Cell Carcinomas. International Journal of Molecular Sciences, 22(16), 8881. https://doi.org/10.3390/ijms22168881







