Influence of Hyperglycemia and Diabetes on Cardioprotection by Humoral Factors Released after Remote Ischemic Preconditioning (RIPC)
Abstract
:1. Introduction
2. Results
2.1. Animal Characteristics and Glucose Values
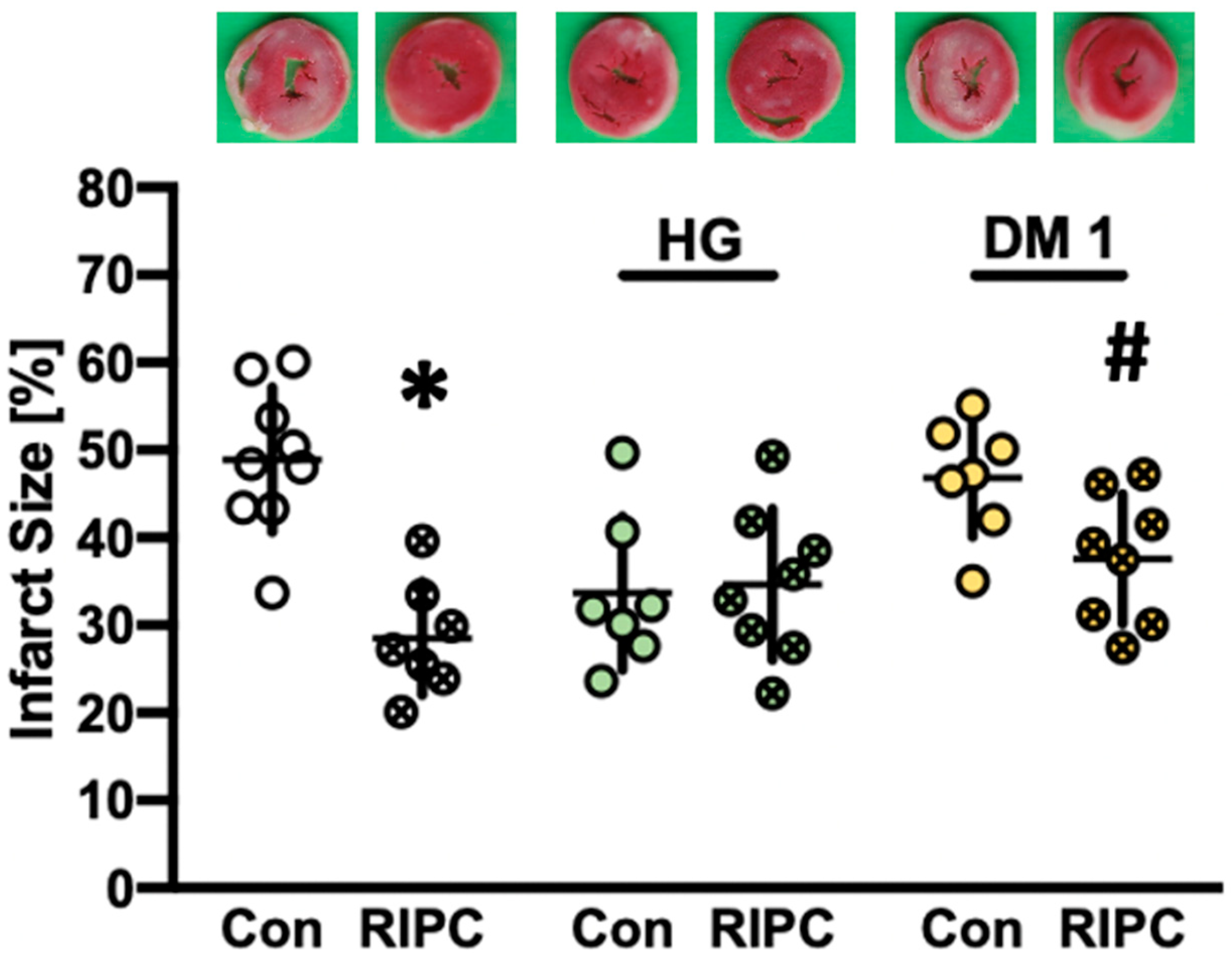
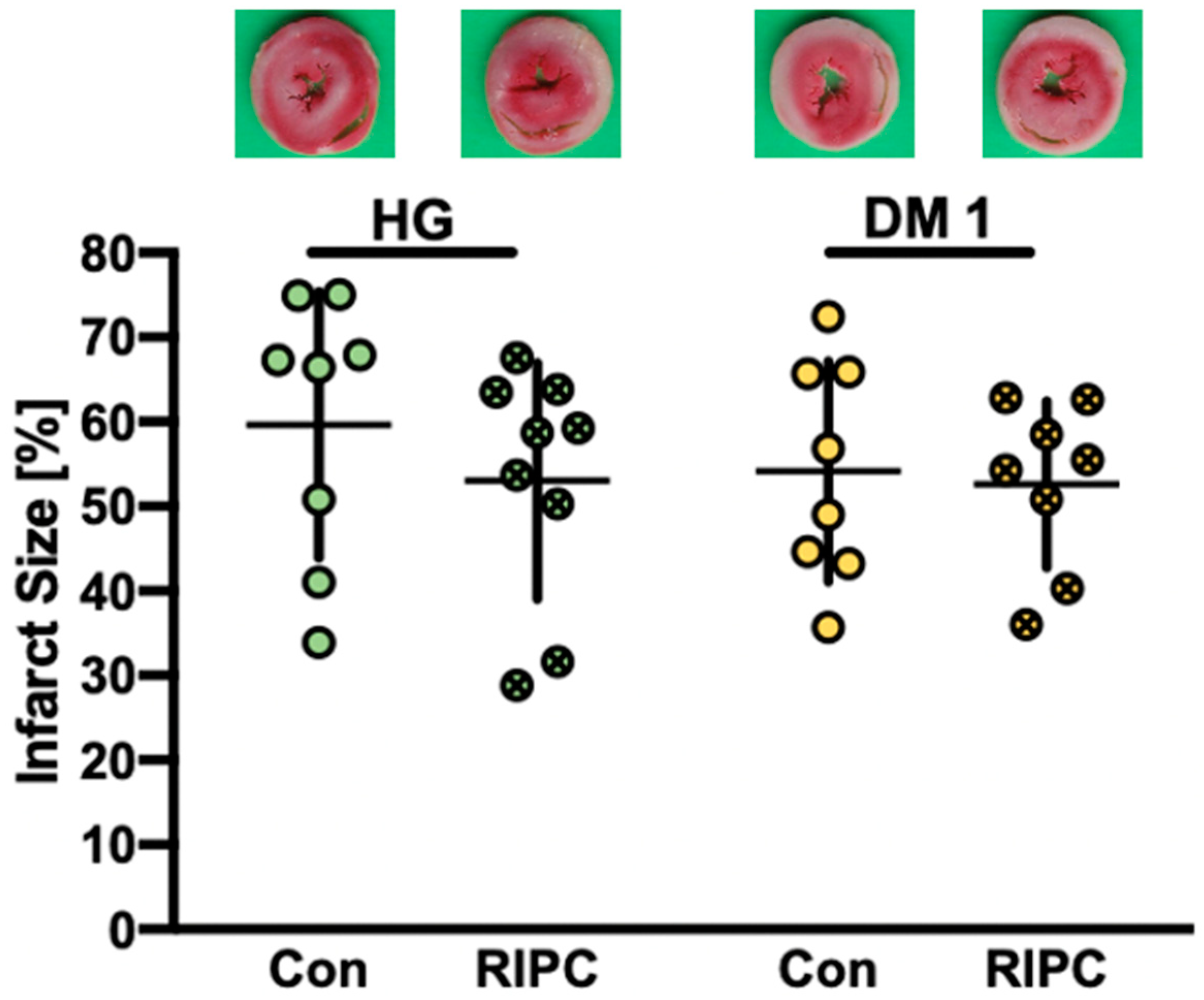
2.2. Infarct Size Measurements
2.3. Cardiac Function
3. Discussion
4. Materials and Methods
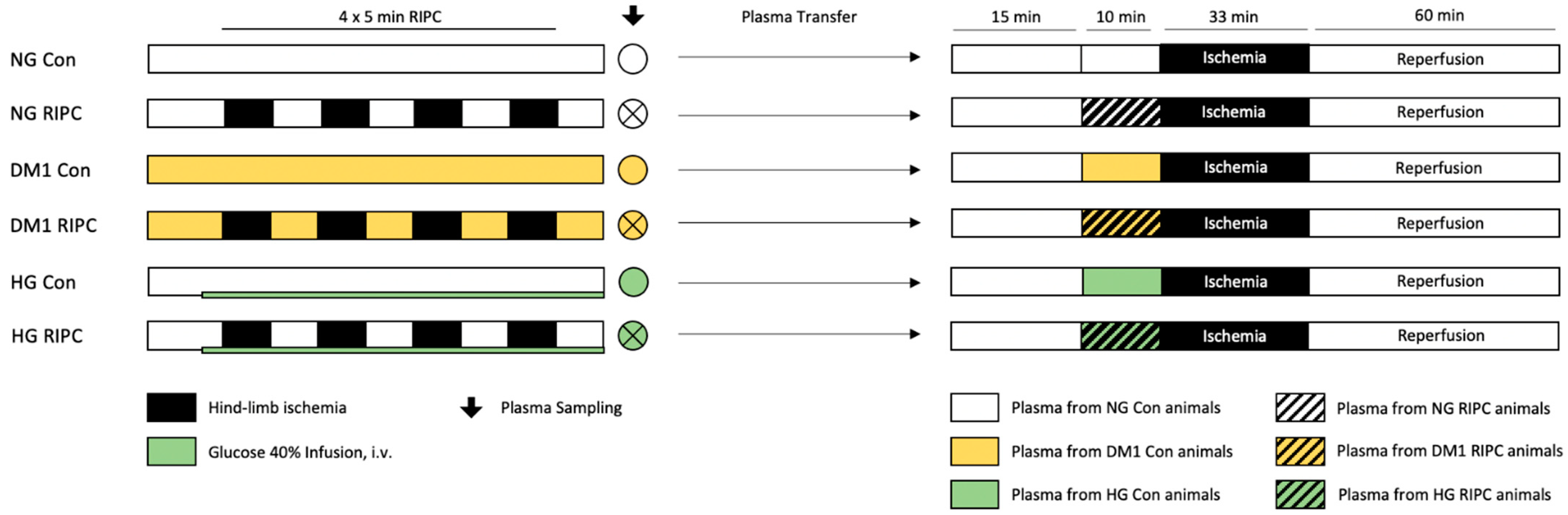
4.1. In Vivo Experiments and Plasma Sampling
4.1.1. Surgical Preparation and RIPC Protocol
4.1.2. Induction of DM1 and HG
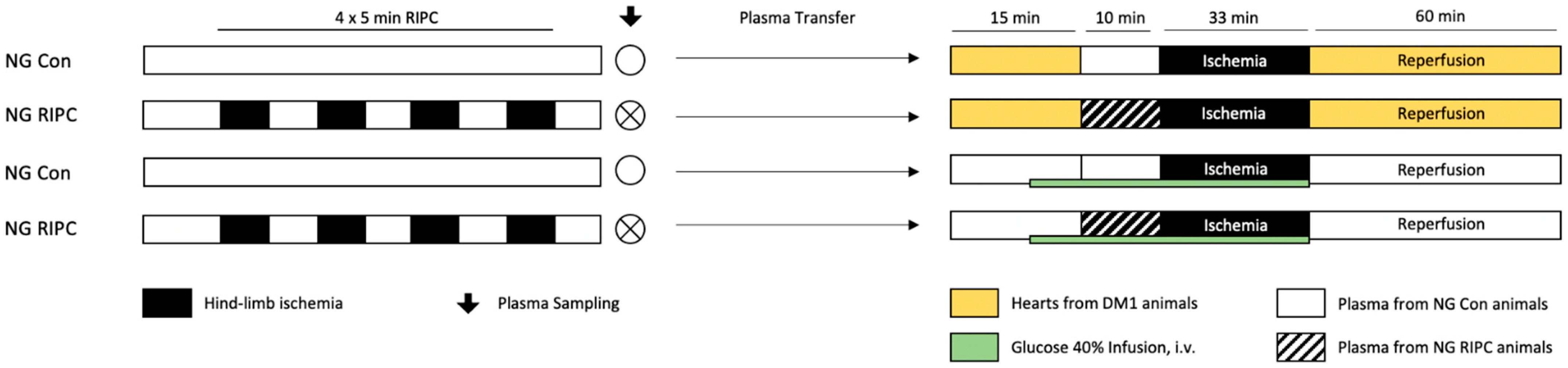
4.2. In Vitro Experiments and Plasma Transfer
4.2.1. Surgical Preparation
4.2.2. Langendorff Protocol
4.2.3. Induction of HG
4.2.4. Part 1: “Release of Humoral Factors”—Plasma Transfer: Diseased → Healthy
4.2.5. Part 2: “Influence of Diseased Myocardium”—Plasma Transfer: Healthy → Diseased
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buddeke, J.; Bots, M.L.; van Dis, I.; Visseren, F.L.; Hollander, M.; Schellevis, F.G.; Vaartjes, I. Comorbidity in patients with cardiovascular disease in primary care: A cohort study with routine healthcare data. Br. J. Gen. Pract. 2019, 69, e398–e406. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelesoff, N.E.; Feinglos, M.; Granger, C.B.; Califf, R.M. Outcomes of diabetic patients following acute myocardial infarction: A review of the major thrombolytic trials. Coron. Artery Dis. 1996, 7, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Bellodi, G.; Manicardi, V.; Malavasi, V.; Veneri, L.; Bernini, G.; Bossini, P.; Distefano, S.; Magnanini, G.; Muratori, L.; Rossi, G.; et al. Hyperglycemia and prognosis of acute myocardial infarction in patients without diabetes mellitus. Am. J. Cardiol. 1989, 64, 885–888. [Google Scholar] [CrossRef]
- Wei, C.H.; Litwin, S.E. Hyperglycemia and Adverse Outcomes in Acute Coronary Syndromes: Is Serum Glucose the Provocateur or Innocent Bystander? Diabetes 2014, 63, 2209–2212. [Google Scholar] [CrossRef] [Green Version]
- Timmer, J.R.; Hoekstra, M.; Nijsten, M.W.; van der Horst, I.C.; Ottervanger, J.P.; Slingerland, R.J.; Dambrink, J.H.; Bilo, H.J.; Zijlstra, F.; van’t Hof, A.W. Prognostic value of admission glycosylated hemoglobin and glucose in nondiabetic patients with ST-segment-elevation myocardial infarction treated with percutaneous coronary intervention. Circulation 2011, 124, 704–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceriello, A. Acute hyperglycaemia: A ‘new’ risk factor during myocardial infarction. Eur. Heart J. 2004, 26, 328–331. [Google Scholar] [CrossRef]
- Torregroza, C.; Raupach, A.; Feige, K.; Weber, N.C.; Hollmann, M.W.; Huhn, R. Perioperative Cardioprotection: General Mechanisms and Pharmacological Approaches. Anesth. Analg. 2020, 131, 1765–1780. [Google Scholar] [CrossRef]
- Yellon, D.M.; Hausenloy, D.J. Myocardial Reperfusion Injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef]
- Hausenloy, D.; Lim, S. Remote Ischemic Conditioning: From Bench to Bedside. Front. Physiol. 2012, 3, 27. [Google Scholar] [CrossRef] [Green Version]
- Billah, M.; Ridiandries, A.; Allahwala, U.; Mudaliar, H.; Dona, A.; Hunyor, S.; Khachigian, L.M.; Bhindi, R. Circulating mediators of remote ischemic preconditioning: Search for the missing link between non-lethal ischemia and cardioprotection. Oncotarget 2019, 10, 216–244. [Google Scholar] [CrossRef]
- Heusch, G. Critical Issues for the Translation of Cardioprotection. Circ. Res. 2017, 120, 1477–1486. [Google Scholar] [CrossRef] [Green Version]
- Roth, S.; Torregroza, C.; Huhn, R.; Hollmann, M.W.; Preckel, B. Perioperative Cardioprotection: Clinical Implications. Anesth. Analg. 2020, 131, 1751–1764. [Google Scholar] [CrossRef] [PubMed]
- Meybohm, P.; Kohlhaas, M.; Stoppe, C.; Gruenewald, M.; Renner, J.; Bein, B.; Albrecht, M.; Cremer, J.; Coburn, M.; Schaelte, G.; et al. RIPHeart (Remote Ischemic Preconditioning for Heart Surgery) Study: Myocardial Dysfunction, Postoperative Neurocognitive Dysfunction, and 1 Year Follow-Up. J. Am. Heart Assoc. 2018, 7, e008077. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Candilio, L.; Evans, R.; Ariti, C.; Jenkins, D.P.; Kolvekar, S.; Knight, R.; Kunst, G.; Laing, C.; Nicholas, J.; et al. Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. N. Engl. J. Med. 2015, 373, 1408–1417. [Google Scholar] [CrossRef]
- Kristiansen, S.B.; Paelestik, K.B.; Johnsen, J.; Jespersen, N.R.; Pryds, K.; Hjortbak, M.V.; Jensen, R.V.; Botker, H.E. Impact of hyperglycemia on myocardial ischemia-reperfusion susceptibility and ischemic preconditioning in hearts from rats with type 2 diabetes. Cardiovasc. Diabetol. 2019, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Inoue, I.; Kawagoe, T.; Shimatani, Y.; Kurisu, S.; Nishioka, K.; Kouno, Y.; Umemura, T.; Nakamura, S.; Sato, H. Diabetes mellitus prevents ischemic preconditioning in patients with a first acute anterior wall myocardial infarction. J. Am. Coll. Cardiol. 2001, 38, 1007–1011. [Google Scholar] [CrossRef] [Green Version]
- Penna, C.; Andreadou, I.; Aragno, M.; Beauloye, C.; Bertrand, L.; Lazou, A.; Falcão-Pires, I.; Bell, R.; Zuurbier, C.J.; Pagliaro, P.; et al. Effect of hyperglycaemia and diabetes on acute myocardial ischaemia-reperfusion injury and cardioprotection by ischaemic conditioning protocols. Br. J. Pharmacol. 2020, 177, 5312–5335. [Google Scholar] [CrossRef]
- Tsang, A.; Hausenloy, D.J.; Mocanu, M.M.; Carr, R.D.; Yellon, D.M. Preconditioning the Diabetic Heart. The Importance of Akt Phosphorylation. Diabetes 2005, 54, 2360–2364. [Google Scholar] [CrossRef] [Green Version]
- Rehni, A.K.; Dave, K.R. Ameliorative potential of conditioning on ischemia-reperfusion injury in diabetes. Cond. Med. 2018, 1, 105–115. [Google Scholar]
- Yadav, H.N.; Singh, M.; Sharma, P.L. Involvement of GSK-3β in attenuation of the cardioprotective effect of ischemic preconditioning in diabetic rat heart. Mol. Cell. Biochem. 2010, 343, 75–81. [Google Scholar] [CrossRef]
- Juhaszova, M.; Zorov, D.B.; Yaniv, Y.; Nuss, H.B.; Wang, S.; Sollott, S.J. Role of glycogen synthase kinase-3beta in cardioprotection. Circ. Res. 2009, 104, 1240–1252. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Tian, Y.; Liu, Y.; Hennessy, S.; Kron, I.L.; French, B.A. Acute hyperglycemia abolishes ischemic preconditioning by inhibiting Akt phosphorylation: Normalizing blood glucose before ischemia restores ischemic preconditioning. Oxid. Med. Cell. Longev. 2013, 2013, 329183. [Google Scholar] [CrossRef]
- Kersten, J.R.; Montgomery, M.W.; Ghassemi, T.; Gross, E.R.; Toller, W.G.; Pagel, P.S.; Warltier, D.C. Diabetes and hyperglycemia impair activation of mitochondrial K(ATP) channels. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1744–H1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranyai, T.; Nagy, C.T.; Koncsos, G.; Onodi, Z.; Karolyi-Szabo, M.; Makkos, A.; Varga, Z.V.; Ferdinandy, P.; Giricz, Z. Acute hyperglycemia abolishes cardioprotection by remote ischemic perconditioning. Cardiovasc. Diabetol. 2015, 14, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunte, S.; Behmenburg, F.; Eckelskemper, F.; Mohr, F.; Stroethoff, M.; Raupach, A.; Heinen, A.; Hollmann, M.W.; Huhn, R. Cardioprotection by Humoral Factors Released After Remote Ischemic Preconditioning Depends on Anesthetic Regimen. Crit. Care Med. 2019, 47, e250–e255. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.M.; Davidson, S.M.; Hausenloy, D.J.; Yellon, D.M. Co-dependence of the neural and humoral pathways in the mechanism of remote ischemic conditioning. Basic Res. Cardiol. 2016, 111, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, R.V.; Støttrup, N.B.; Kristiansen, S.B.; Bøtker, H.E. Release of a humoral circulating cardioprotective factor by remote ischemic preconditioning is dependent on preserved neural pathways in diabetic patients. Basic Res. Cardiol. 2012, 107, 285. [Google Scholar] [CrossRef]
- Gu, W.; Pagel, P.S.; Warltier, D.C.; Kersten, J.R. Modifying cardiovascular risk in diabetes mellitus. Anesthesiology 2003, 98, 774–779. [Google Scholar] [CrossRef]
- Lin, K.Y.; Ito, A.; Asagami, T.; Tsao, P.S.; Adimoolam, S.; Kimoto, M.; Tsuji, H.; Reaven, G.M.; Cooke, J.P. Impaired nitric oxide synthase pathway in diabetes mellitus: Role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation 2002, 106, 987–992. [Google Scholar] [CrossRef] [Green Version]
- Hassouna, A.; Loubani, M.; Matata, B.M.; Fowler, A.; Standen, N.B.; Galiñanes, M. Mitochondrial dysfunction as the cause of the failure to precondition the diabetic human myocardium. Cardiovasc. Res. 2006, 69, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Saeid, F.; Aniseh, J.; Reza, B.; Manouchehr, V.S. Signaling mediators modulated by cardioprotective interventions in healthy and diabetic myocardium with ischaemia-reperfusion injury. Eur. J. Prev. Cardiol. 2018, 25, 1463–1481. [Google Scholar] [CrossRef]
- Heusch, G. Molecular basis of cardioprotection: Signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 2015, 116, 674–699. [Google Scholar] [CrossRef]
- Torregroza, C.; Feige, K.; Schneider, L.; Bunte, S.; Stroethoff, M.; Heinen, A.; Hollmann, M.W.; Huhn, R.; Raupach, A. Influence of Hyperglycemia on Dexmedetomidine-Induced Cardioprotection in the Isolated Perfused Rat Heart. J. Clin. Med. 2020, 9, 1445. [Google Scholar] [CrossRef]
- Grill, V.; Adamson, U.; Cerasi, E. Immediate and time-dependent effects of glucose on insulin release from rat pancreatic tissue. Evidence for different mechanisms of action. J. Clin. Investig. 1978, 61, 1034–1043. [Google Scholar] [CrossRef] [Green Version]
- Nesher, R.; Cerasi, E. Modeling phasic insulin release: Immediate and time-dependent effects of glucose. Diabetes 2002, 51 (Suppl. 1), S53–S59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, K.W.; Allen, M.L.; Desai, A.; Macrae, D.; Pathan, N. Cardioprotective Effects of Insulin. Circulation 2012, 125, 721–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Zhang, A.-M.; Xiao, Y.-B.; Weng, Y.-G.; Hetzer, R. Glucose–insulin–potassium therapy in adult patients undergoing cardiac surgery: A meta-analysis. Eur. J. Cardio Thorac. Surg. 2011, 40, 192–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marfella, R.; Sasso, F.C.; Cacciapuoti, F.; Portoghese, M.; Rizzo, M.R.; Siniscalchi, M.; Carbonara, O.; Ferraraccio, F.; Torella, M.; Petrella, A.; et al. Tight Glycemic Control May Increase Regenerative Potential of Myocardium during Acute Infarction. J. Clin. Endocrinol. Metab. 2012, 97, 933–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasso, F.C.; Rinaldi, L.; Lascar, N.; Marrone, A.; Pafundi, P.C.; Adinolfi, L.E.; Marfella, R. Role of Tight Glycemic Control during Acute Coronary Syndrome on CV Outcome in Type 2 Diabetes. J. Diabetes Res. 2018, 2018, 3106056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbarzadeh, A.; Norouzian, D.; Mehrabi, M.R.; Jamshidi, S.; Farhangi, A.; Verdi, A.A.; Mofidian, S.M.; Rad, B.L. Induction of diabetes by Streptozotocin in rats. Indian J. Clin. Biochem. 2007, 22, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Stanley, W.C.; Dabkowski, E.R.; Ribeiro, R.F., Jr.; O’Connell, K.A. Dietary fat and heart failure: Moving from lipotoxicity to lipoprotection. Circ. Res. 2012, 110, 764–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Sasso, F.C.; Pafundi, P.C.; Simeon, V.; De Nicola, L.; Chiodini, P.; Galiero, R.; Rinaldi, L.; Nevola, R.; Salvatore, T.; Sardu, C.; et al. Efficacy and durability of multifactorial intervention on mortality and MACEs: A randomized clinical trial in type-2 diabetic kidney disease. Cardiovasc. Diabetol. 2021, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Torregroza, C.; Feige, K.; Preckel, B.; Hollmann, M.W.; Weber, N.C.; Huhn, R. Pharmacological Conditioning of the Heart: An Update on Experimental Developments and Clinical Implications. Int. J. Mol. Sci. 2021, 22, 2519. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Ferdinandy, P.; Andreadou, I.; Bøtker, H.E.; Heusch, G.; Ibáñez, B.; Ovize, M.; Schulz, R.; Yellon, D.M.; Hausenloy, D.J.; et al. Multitarget Strategies to Reduce Myocardial Ischemia/Reperfusion Injury: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 89–99. [Google Scholar] [CrossRef]
- Heusch, G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef]
- National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press (US): Washington, DC, USA, 2010. [Google Scholar]
- Behmenburg, F.; van Caster, P.; Bunte, S.; Brandenburger, T.; Heinen, A.; Hollmann, M.W.; Huhn, R. Impact of Anesthetic Regimen on Remote Ischemic Preconditioning in the Rat Heart In Vivo. Anesth. Analg. 2018, 126, 1377–1380. [Google Scholar] [CrossRef]
- Ahmet, I.; Wan, R.; Mattson, M.P.; Lakatta, E.G.; Talan, M. Cardioprotection by intermittent fasting in rats. Circulation 2005, 112, 3115–3121. [Google Scholar] [CrossRef] [Green Version]
- Snorek, M.; Hodyc, D.; Sedivý, V.; Durišová, J.; Skoumalová, A.; Wilhelm, J.; Neckář, J.; Kolář, F.; Herget, J. Short-term fasting reduces the extent of myocardial infarction and incidence of reperfusion arrhythmias in rats. Physiol. Res. 2012, 61, 567–574. [Google Scholar] [CrossRef]
- Al-Awar, A.; Kupai, K.; Veszelka, M.; Szűcs, G.; Attieh, Z.; Murlasits, Z.; Török, S.; Pósa, A.; Varga, C. Experimental Diabetes Mellitus in Different Animal Models. J. Diabetes Res. 2016, 2016, 9051426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Yan, L.J. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab. Syndr. Obes. 2015, 8, 181–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5–47. [Google Scholar] [CrossRef]
- Raupach, A.; Reinle, J.; Stroethoff, M.; Mathes, A.; Heinen, A.; Hollmann, M.W.; Huhn, R.; Bunte, S. Milrinone-Induced Pharmacological Preconditioning in Cardioprotection: Hints for a Role of Mitochondrial Mechanisms. J. Clin. Med. 2019, 8, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behmenburg, F.; Dorsch, M.; Huhn, R.; Mally, D.; Heinen, A.; Hollmann, M.W.; Berger, M.M. Impact of Mitochondrial Ca2+-Sensitive Potassium (mBKCa) Channels in Sildenafil-Induced Cardioprotection in Rats. PLoS ONE 2015, 10, e0144737. [Google Scholar] [CrossRef]
| n | Body Weight (g) | Heart Weight Wet (g) | Heart Weight Dry (g) | Time of Max. Ischemic Contracture (min) | Level of Max. Ischemic Contracture (mmHg) | ||
|---|---|---|---|---|---|---|---|
| Part 1—plasma transfer diseased → healthy | |||||||
| NG | Con | 9 | 288 ± 16 | 1.25 ± 0.11 | 0.10 ± 0.02 | 15 ± 2 | 77 ± 11 |
| RIPC | 7 | 285 ± 21 | 1.22 ± 0.09 | 0.09 ± 0.02 | 15 ± 1 | 73 ± 13 | |
| HG | Con | 7 | 300 ± 20 | 1.26 ± 0.07 | 0.10 ± 0.01 | 16 ± 2 | 62 ± 13 |
| RIPC | 8 | 299 ± 21 | 1.26 ± 0.10 | 0.09 ± 0.01 | 14 ± 1 | 74 ± 11 | |
| DM1 | Con | 7 | 288 ± 20 | 1.22 ± 0.11 | 0.10 ± 0.01 | 15 ± 2 | 73 ± 11 |
| RIPC | 8 | 281 ± 13 | 1.21 ± 0.04 | 0.10 ± 0.01 | 16 ± 3 | 66 ± 14 | |
| Part 2—plasma transfer healthy → diseased | |||||||
| HG | Con | 8 | 283 ± 15 | 1.25 ± 0.07 | 0.11 ± 0.01 | 17 ± 1 | 99 ± 12 |
| RIPC | 9 | 290 ± 15 | 1.25 ± 0.08 | 0.11 ± 0.01 | 19 ± 2 | 96 ± 15 | |
| DM1 | Con | 8 | 257 ± 39 | 1.15 ± 0.13 | 0.10 ± 0.01 | 20 ± 3 | 77 ± 9 |
| RIPC | 8 | 246 ± 28 | 1.14 ± 0.14 | 0.10 ± 0.01 | 20 ± 2 | 74 ± 11 | |
| Baseline | PC | Reperfusion | |||
|---|---|---|---|---|---|
| 33 min | 60 min | ||||
| Heart Rate (bpm) | |||||
| NG | Con | 312 ± 37 | 286 ± 46 | 205 ± 69 | 227 ± 75 |
| RIPC | 301 ± 56 | 269 ± 52 | 265 ± 55 | 244 ± 51 | |
| HG | Con | 288 ± 25 | 257 ± 36 | 224 ± 51 | 272 ± 34 |
| RIPC | 303 ± 30 | 278 ± 39 | 229 ± 74 | 220 ± 72 | |
| DM1 | Con | 315 ± 28 | 311 ± 36 | 302 ± 55 | 236 ± 64 |
| RIPC | 310 ± 23 | 274 ± 53 | 256 ± 52 | 229 ± 59 | |
| Left Ventricular Developed Pressure (mmHg) | |||||
| NG | Con | 143 ± 15 | 132 ± 24 | 22 ± 12 * | 28 ± 7 * |
| RIPC | 152 ± 20 | 132 ± 27 * | 45 ± 9 * | 46 ± 20 * | |
| HG | Con | 141 ± 22 | 123 ± 35 | 27 ± 13 * | 34 ± 13 * |
| RIPC | 142 ± 18 | 135 ± 19 | 25 ± 11 * | 34 ± 9 * | |
| DM1 | Con | 146 ± 29 | 131 ± 25 | 17 ± 5 * | 27 ± 11 * |
| RIPC | 132 ± 32 | 119 ± 32 | 31 ± 12 * | 40 ± 12 * | |
| Coronary Flow (ml/min) | |||||
| NG | Con | 16 ± 3 | 14 ± 5 | 7 ± 3 * | 6 ± 2 * |
| RIPC | 14 ± 3 | 11 ± 3 * | 9 ± 3 * | 7 ± 3 * | |
| HG | Con | 18 ± 3 | 13 ± 4 * | 9 ± 1 * | 7 ± 1 * |
| RIPC | 17 ± 5 | 16 ± 5 | 8 ± 3 * | 7 ± 2 * | |
| DM1 | Con | 15 ± 4 | 13 ± 3 | 7 ± 2 * | 6 ± 2 * |
| RIPC | 13 ± 3 | 10 ± 2 * | 7 ± 1 * | 7 ± 2 * | |
| Baseline | PC | Reperfusion | |||
|---|---|---|---|---|---|
| 33 min | 60 min | ||||
| Heart Rate (bpm) | |||||
| HG | Con | 324 ± 24 | 283 ± 34 | 267 ± 96 | 255 ± 92 |
| RIPC | 280 ± 16 | 267 ± 22 | 249 ± 71 | 223 ± 47 | |
| DM1 | Con | 258 ± 40 | 230 ± 28 | 238 ± 62 | 222 ± 39 |
| RIPC | 253 ± 30 | 236 ± 22 | 224 ± 44 | 221 ± 52 | |
| Left Ventricular Developed Pressure (mmHg) | |||||
| HG | Con | 122 ± 34 | 94 ± 20 | 31 ± 12 * | 35 ± 12 * |
| RIPC | 127 ± 30 | 110 ± 21 | 40 ± 11 * | 48 ± 11 * | |
| DM1 | Con | 131 ± 20 | 114 ± 18 | 34 ± 7 * | 36 ± 20 * |
| RIPC | 116 ± 28 | 106 ± 29 | 39 ± 9 * | 40 ± 8 * | |
| Coronary Flow (ml/min) | |||||
| HG | Con | 13 ± 3 | 10 ± 2 | 5 ± 1 * | 5 ± 1 * |
| RIPC | 11 ± 3 | 10 ± 1 | 6 ± 2 * | 6 ± 2 * | |
| DM1 | Con | 11 ± 2 | 8 ± 2 | 7 ± 2 * | 7 ± 2 * |
| RIPC | 10 ± 2 | 8 ± 2 | 6 ± 1 * | 6 ± 1 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torregroza, C.; Gnaegy, L.; Raupach, A.; Stroethoff, M.; Feige, K.; Heinen, A.; Hollmann, M.W.; Huhn, R. Influence of Hyperglycemia and Diabetes on Cardioprotection by Humoral Factors Released after Remote Ischemic Preconditioning (RIPC). Int. J. Mol. Sci. 2021, 22, 8880. https://doi.org/10.3390/ijms22168880
Torregroza C, Gnaegy L, Raupach A, Stroethoff M, Feige K, Heinen A, Hollmann MW, Huhn R. Influence of Hyperglycemia and Diabetes on Cardioprotection by Humoral Factors Released after Remote Ischemic Preconditioning (RIPC). International Journal of Molecular Sciences. 2021; 22(16):8880. https://doi.org/10.3390/ijms22168880
Chicago/Turabian StyleTorregroza, Carolin, Lara Gnaegy, Annika Raupach, Martin Stroethoff, Katharina Feige, André Heinen, Markus W. Hollmann, and Ragnar Huhn. 2021. "Influence of Hyperglycemia and Diabetes on Cardioprotection by Humoral Factors Released after Remote Ischemic Preconditioning (RIPC)" International Journal of Molecular Sciences 22, no. 16: 8880. https://doi.org/10.3390/ijms22168880
APA StyleTorregroza, C., Gnaegy, L., Raupach, A., Stroethoff, M., Feige, K., Heinen, A., Hollmann, M. W., & Huhn, R. (2021). Influence of Hyperglycemia and Diabetes on Cardioprotection by Humoral Factors Released after Remote Ischemic Preconditioning (RIPC). International Journal of Molecular Sciences, 22(16), 8880. https://doi.org/10.3390/ijms22168880






