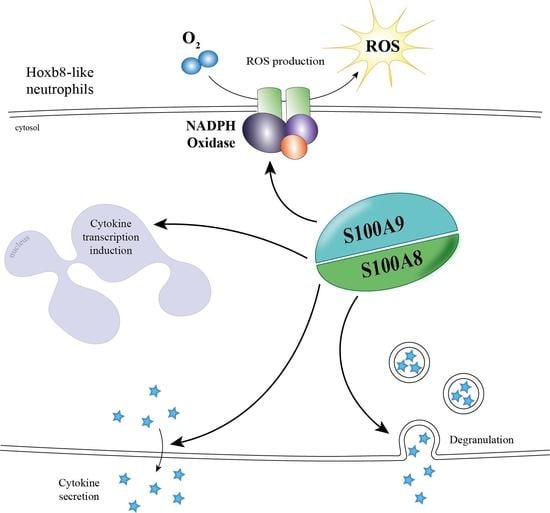
Role of S100A8/A9 for Cytokine Secretion, Revealed in Neutrophils Derived from ER-Hoxb8 Progenitors
Abstract
1. Introduction
2. Results
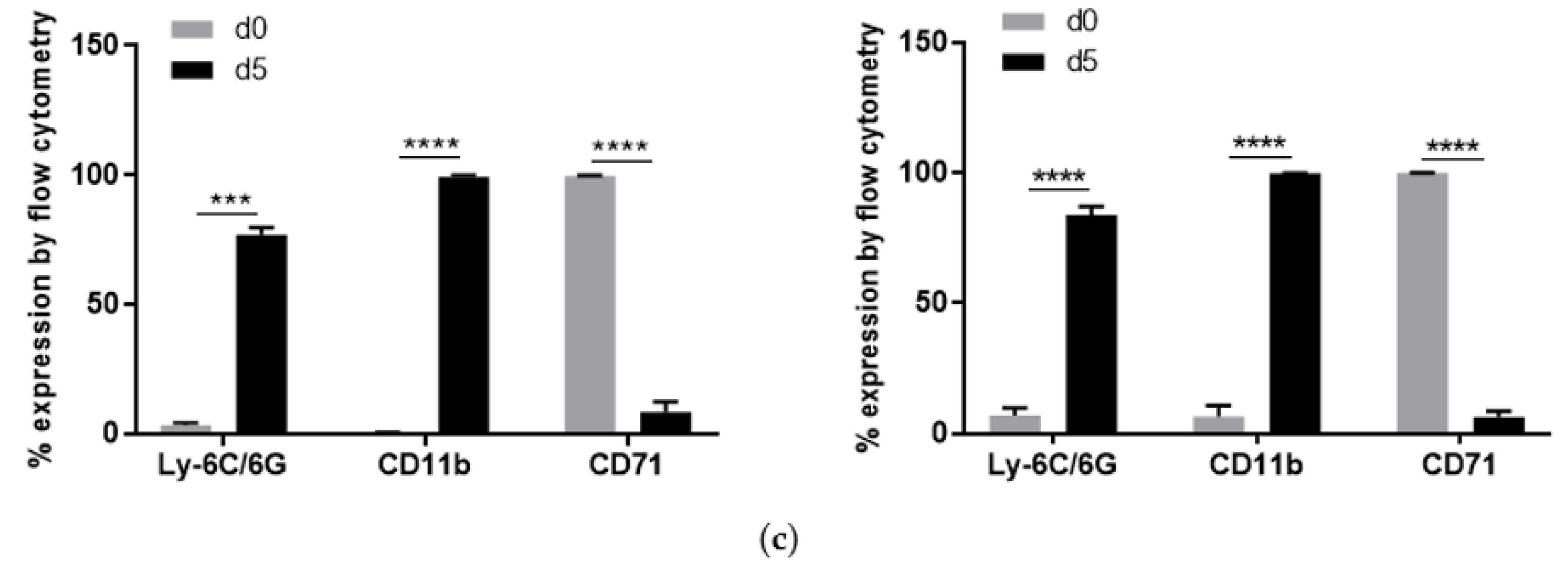
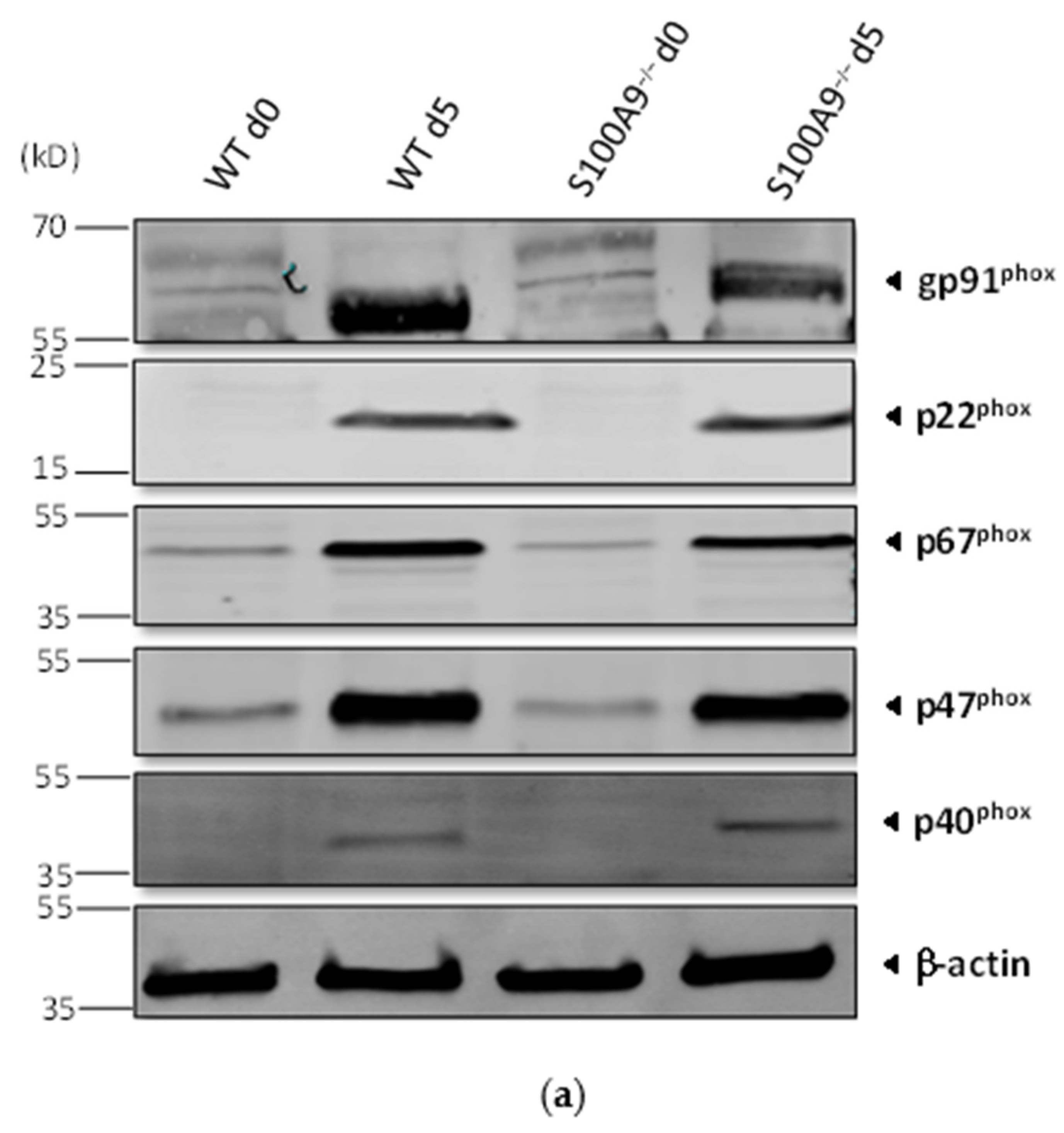
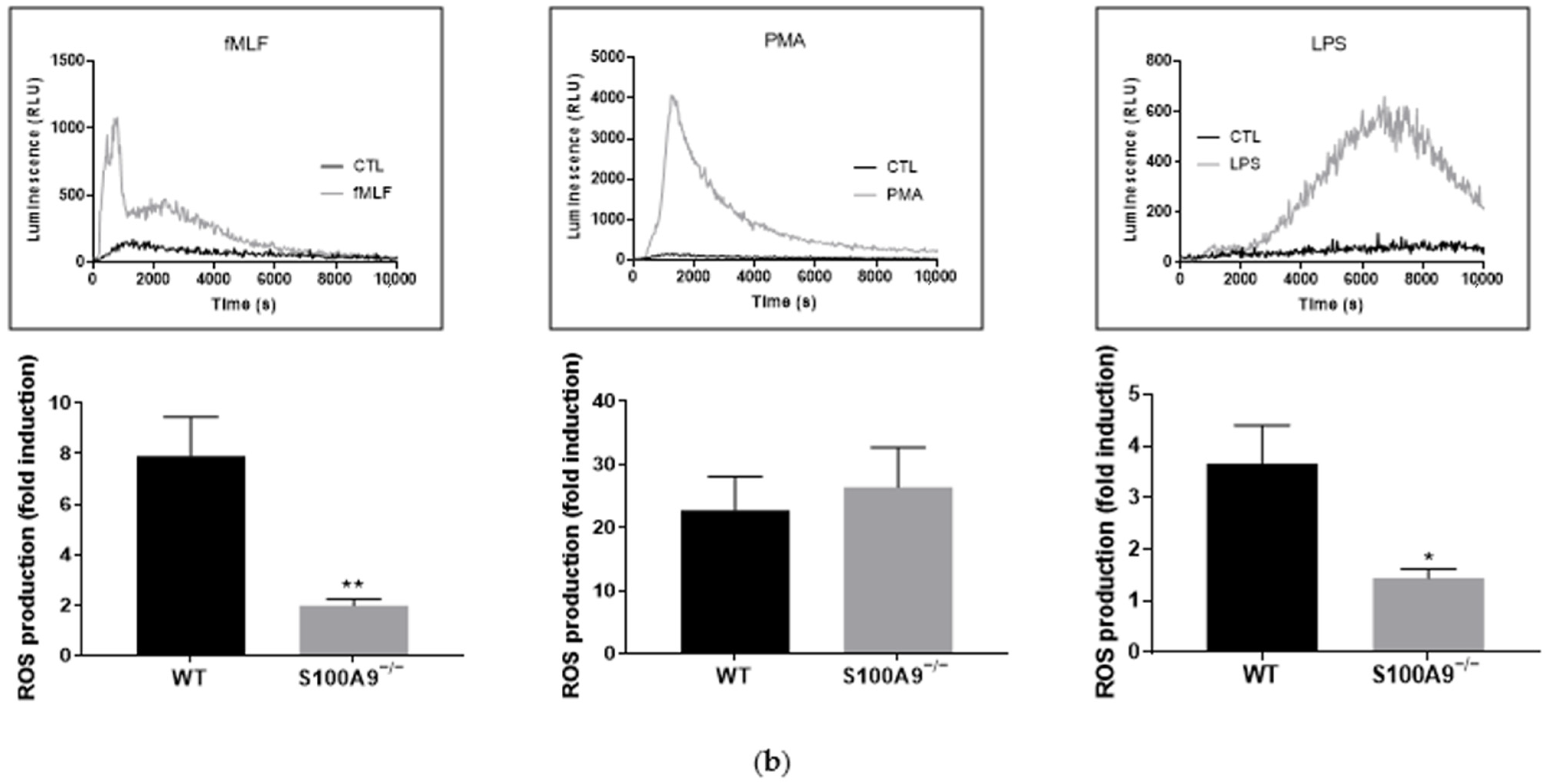
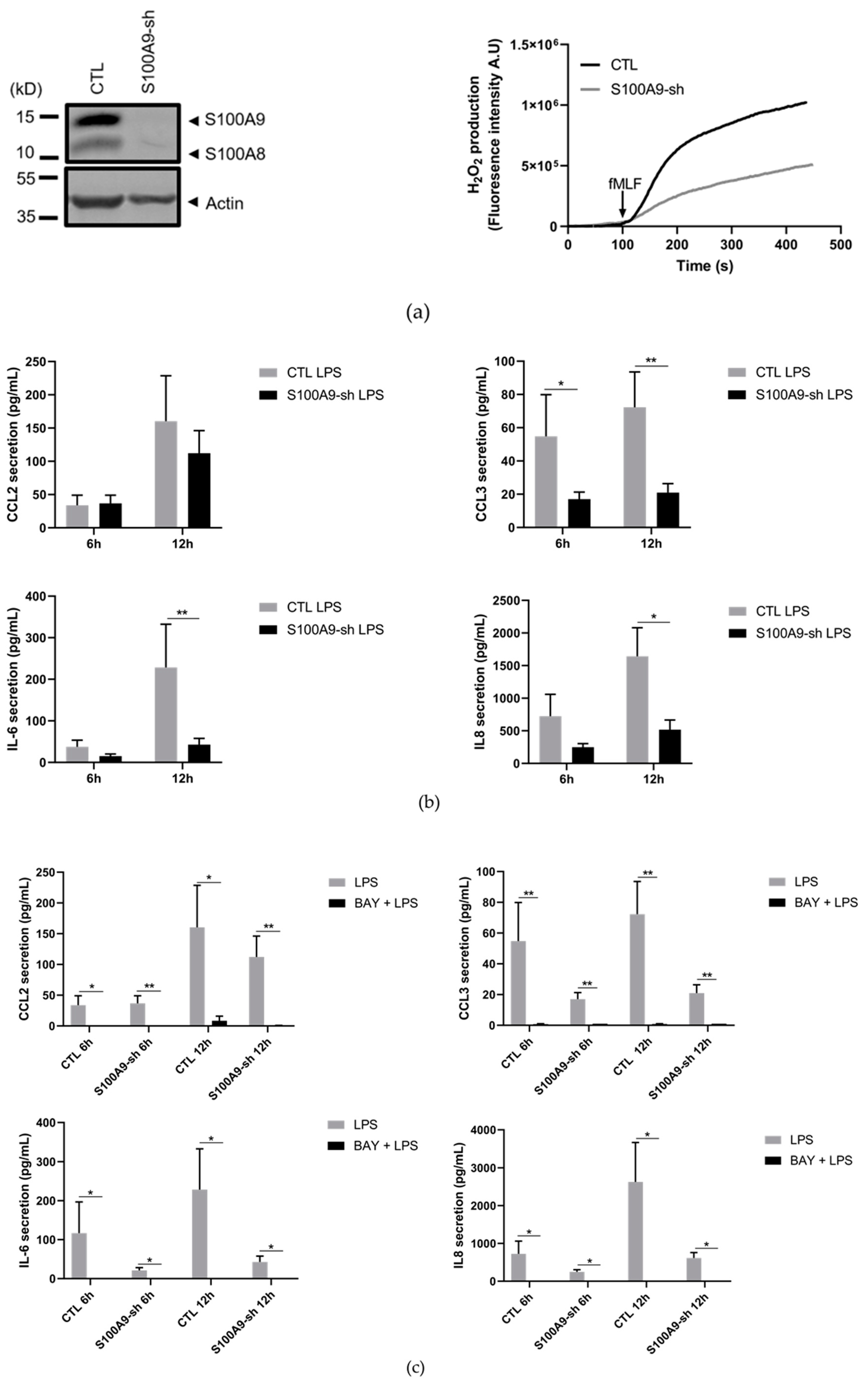
2.1. Characterization of S100A9−/− Hoxb8 Neutrophil-like Cells
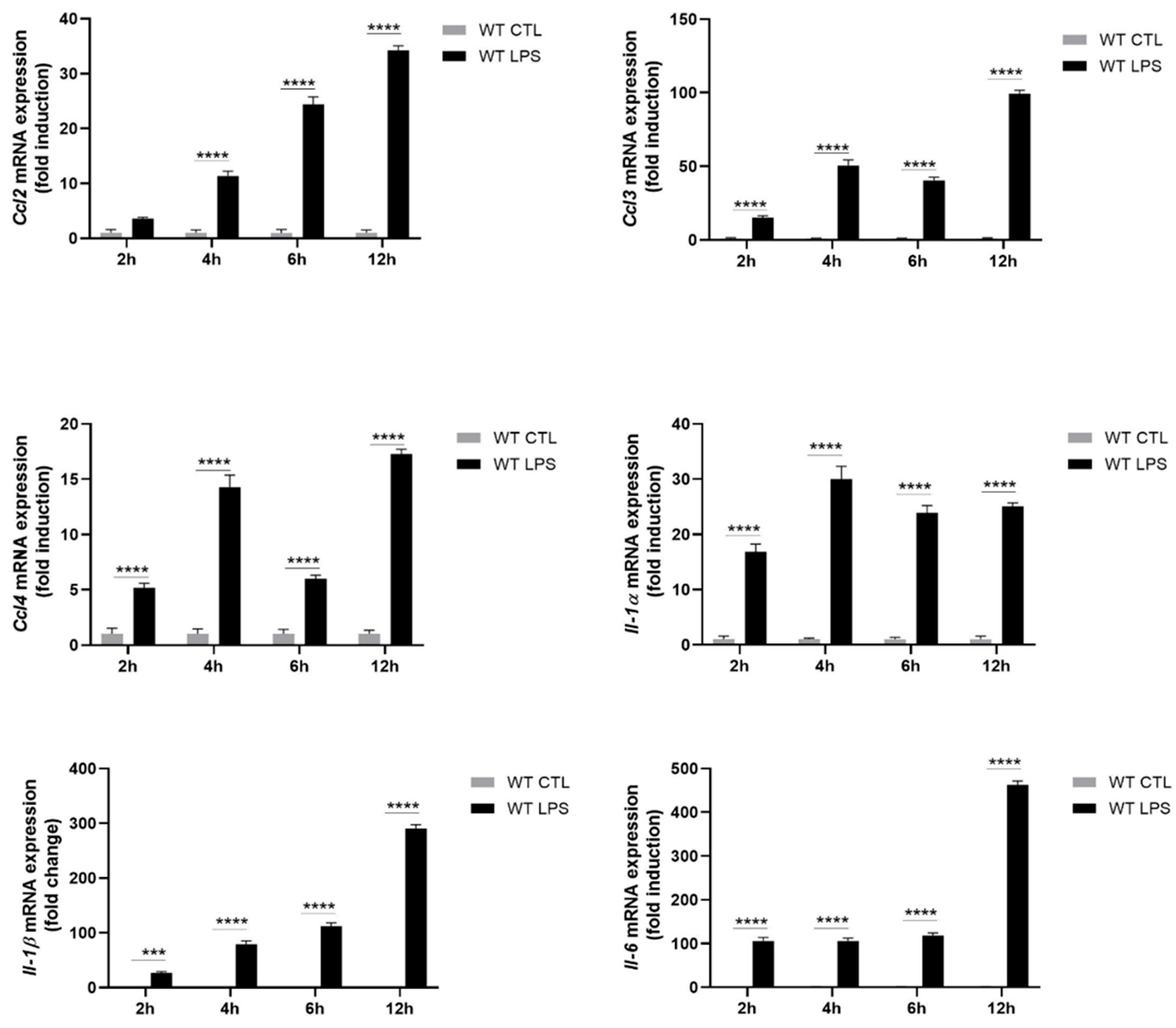
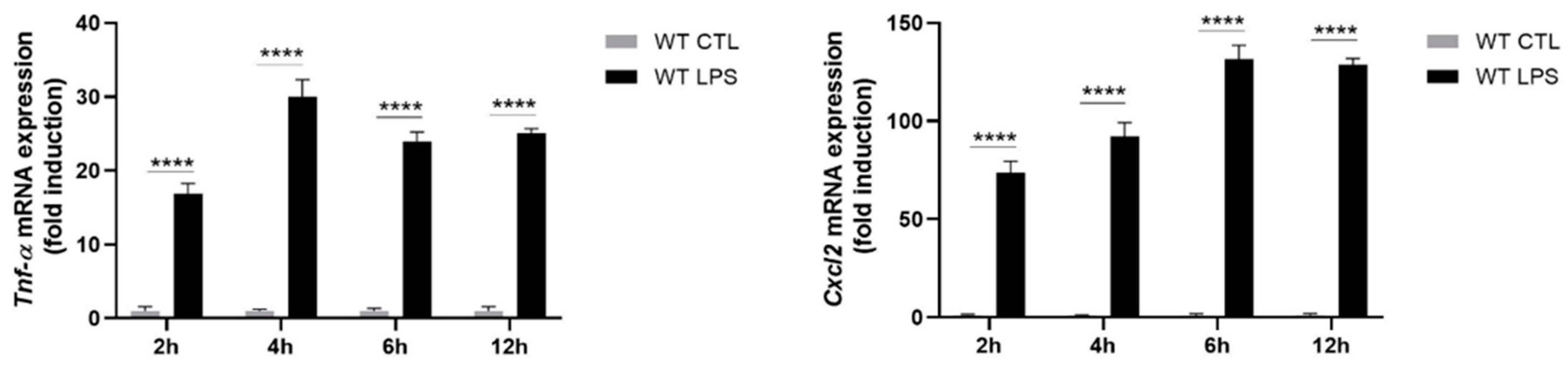
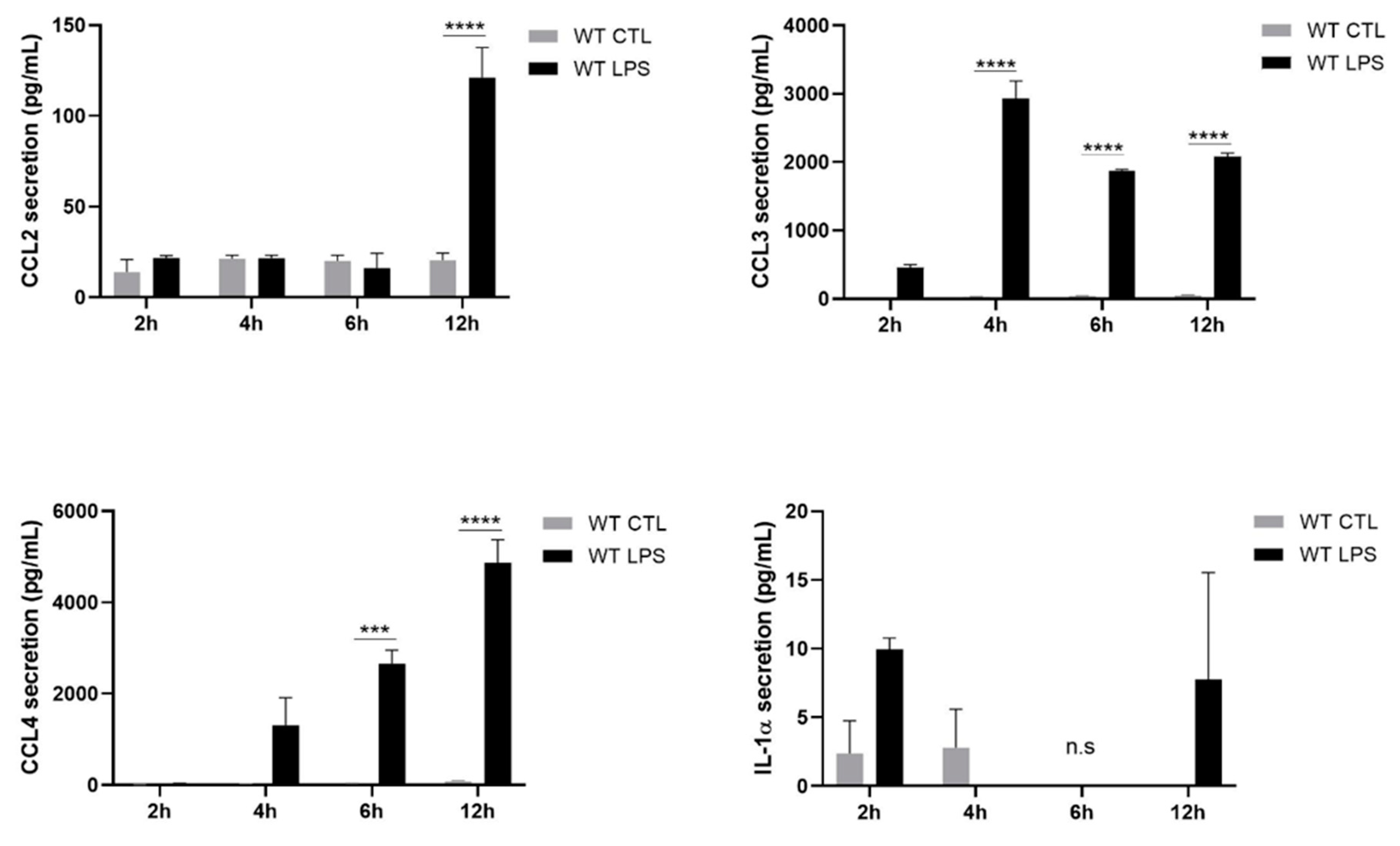
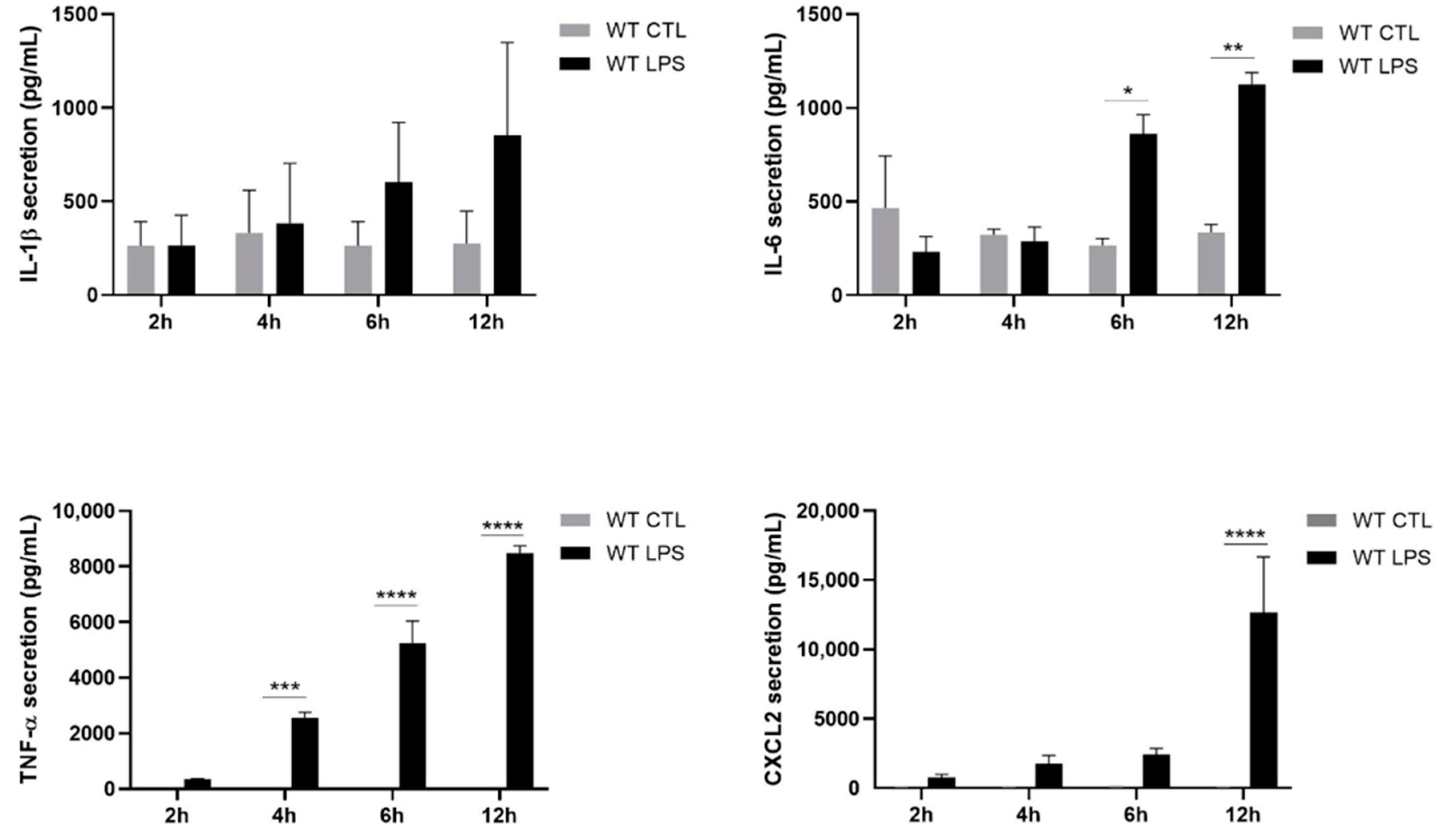
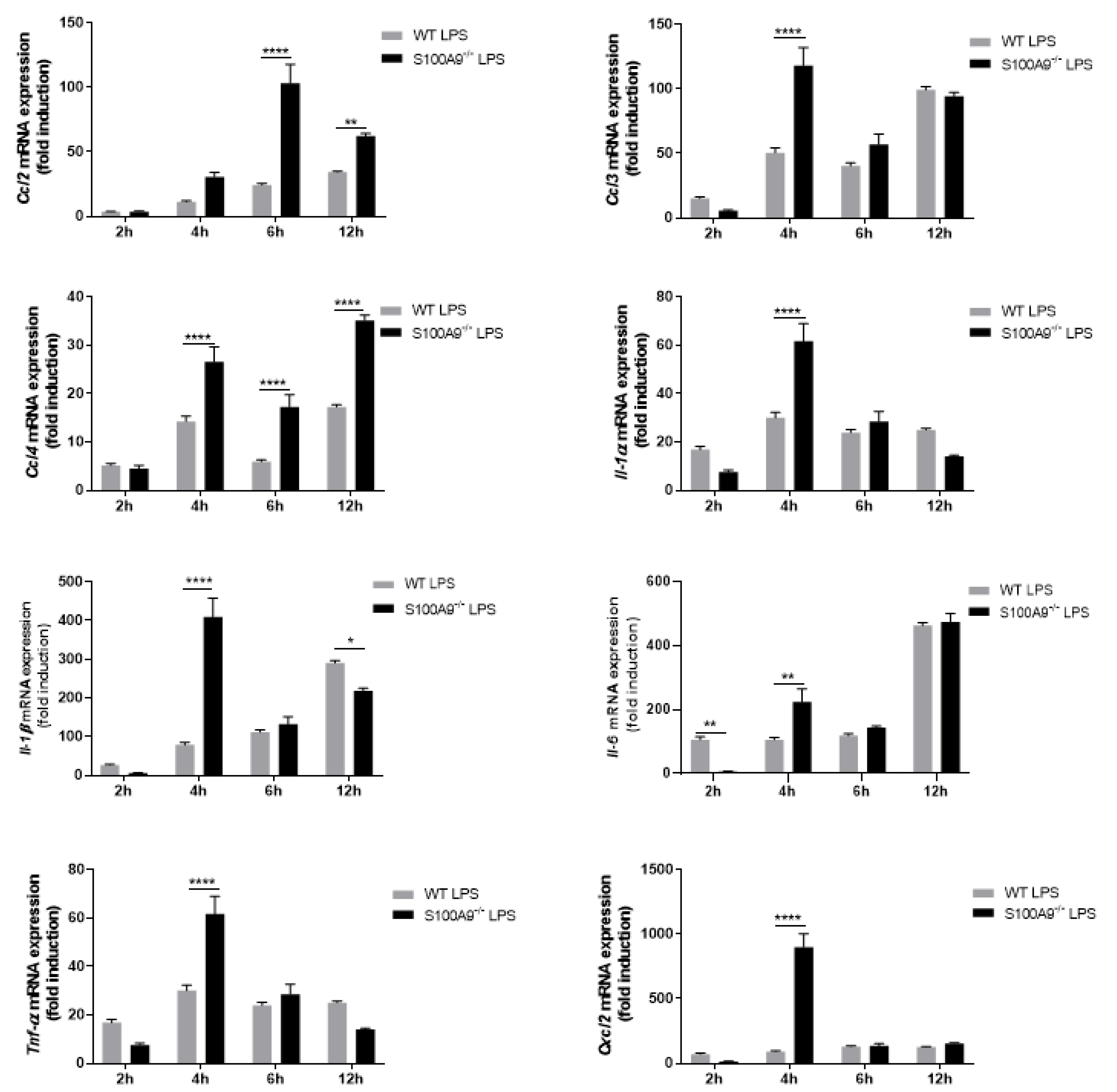
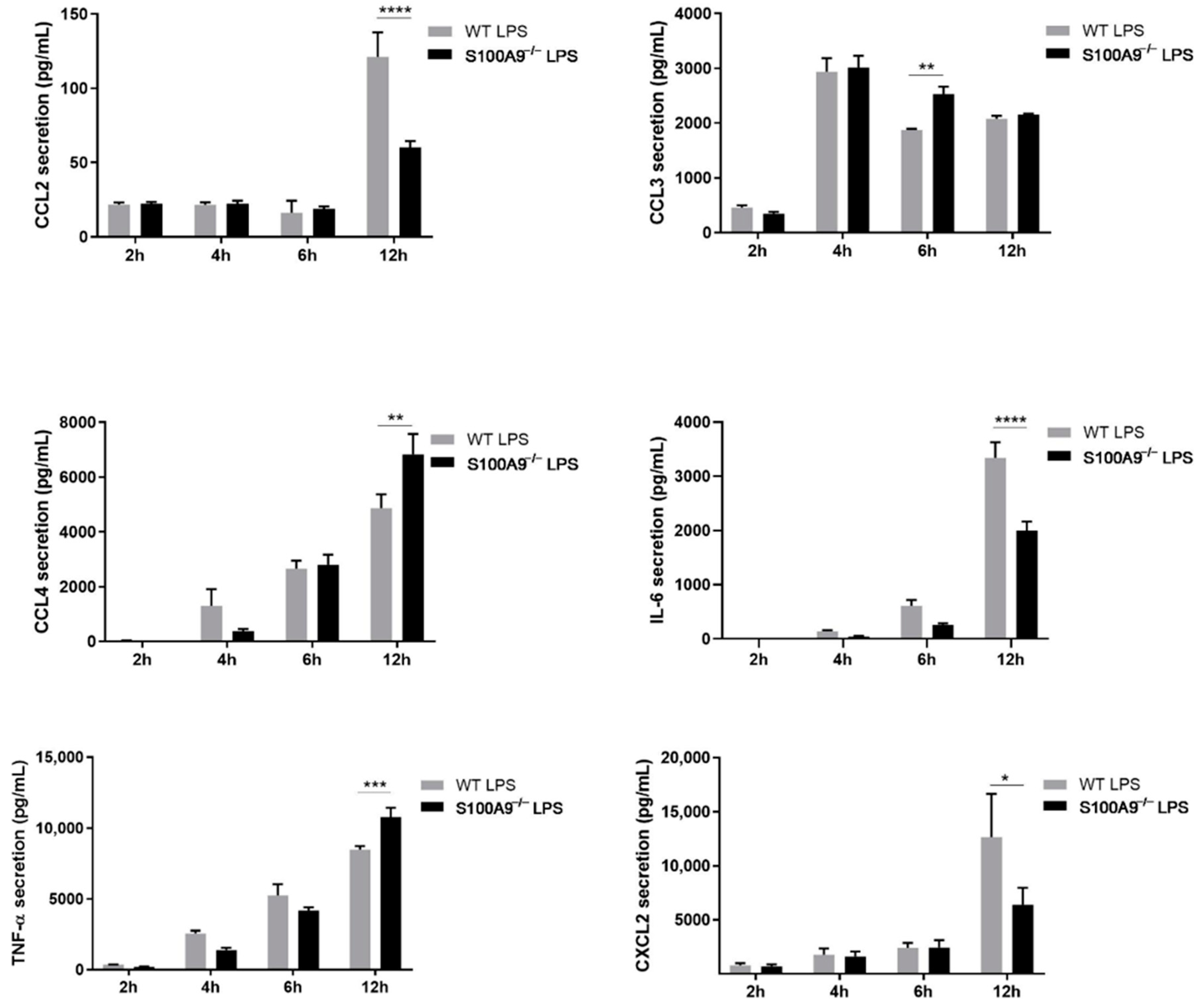
2.2. S100A8/A9 Modulates Cytokine Secretion
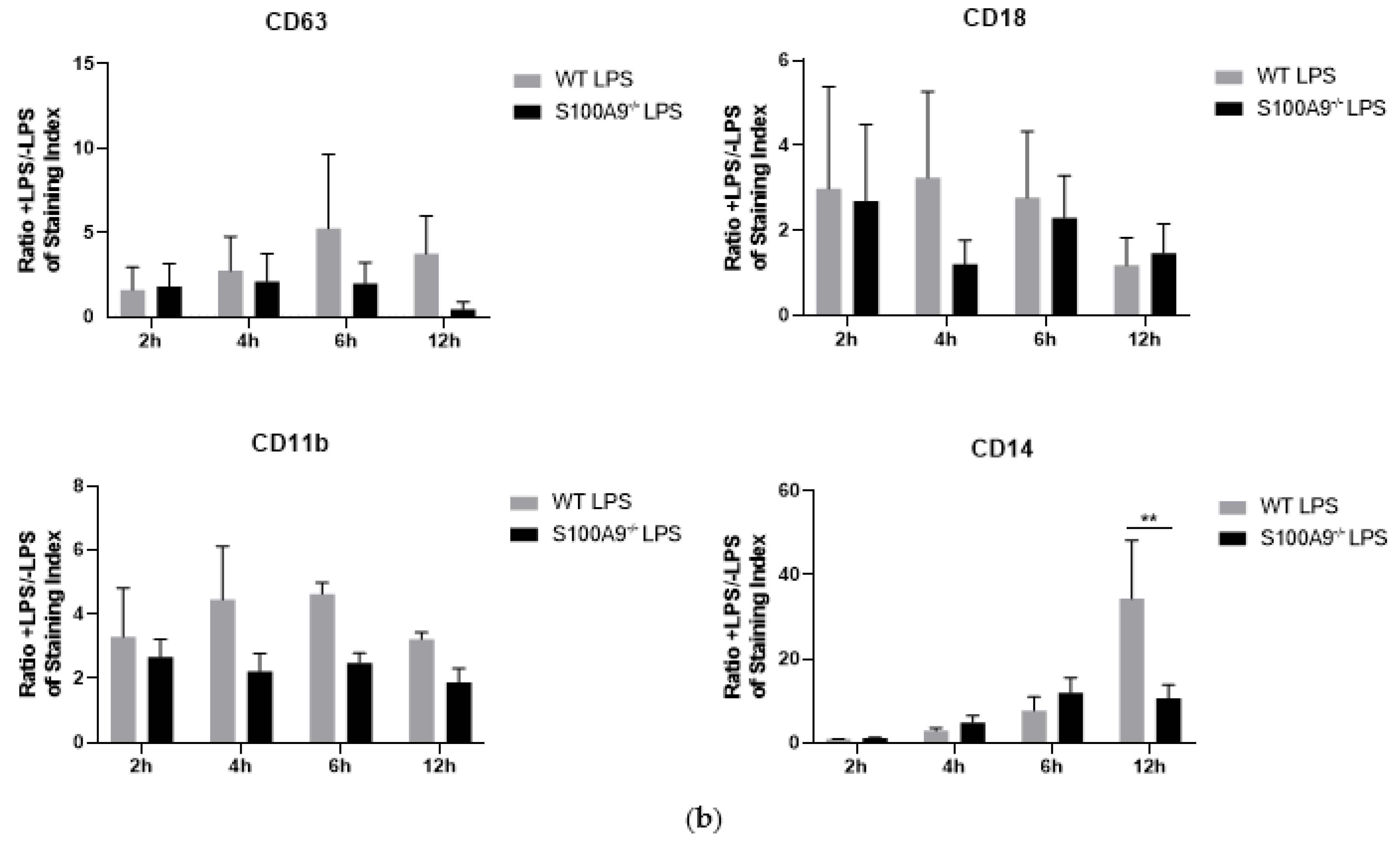
2.3. Is Cytokine Secretion Mediated by the Process of Degranulation?
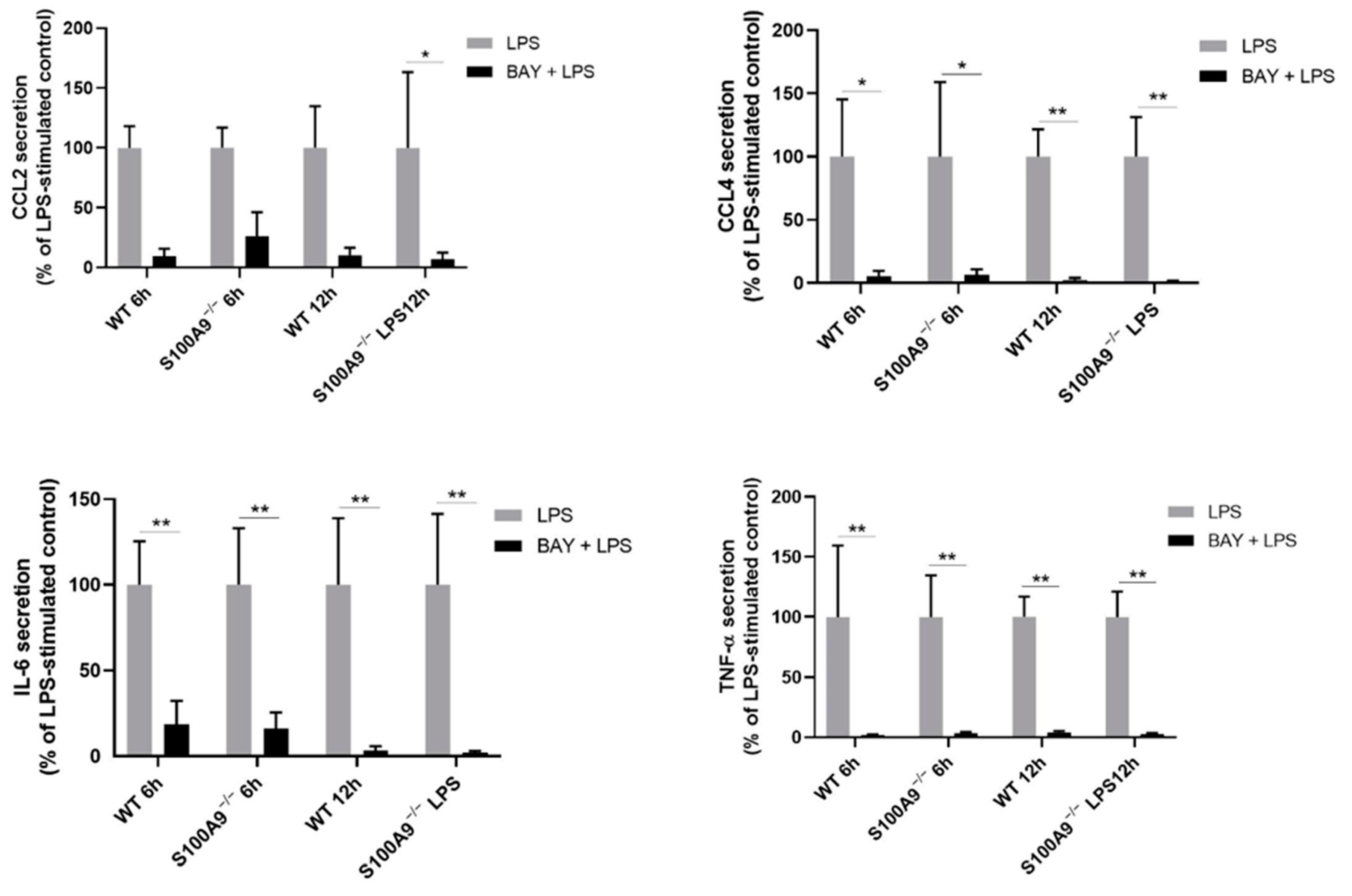
2.4. The Effects of S100A9 on Cytokine Secretion Are Mediated by NF-κB
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Differentiation of ER-Hoxb8 Progenitors to Neutrophil-like Cells
4.2. May-Grünwald Giemsa Staining
4.3. Cell Viability
4.4. Analysis of Surface Marker Expression by Flow Cytometry
4.5. Total RNA Extraction and Quantitative RT-PCR
4.6. Knock-Down of S100A9 in HL-60 Cell by shRNA Interference
4.7. Western Blot
4.8. ROS Production
4.9. Measurement of Cytokine Secretion by Cytometric Bead Array (CBA) and ELISA
4.10. Degranulation Analysis by Flow Cytometry
4.11. Statistics
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 7-AAD | 7 amino actinomycin D |
| DPI | DiPhenyleneIodonium |
| ER-Hoxb8 | Estrogen Regulated-Homeobox b8 |
| ROS | Reactive Oxygen Species |
| SCF | Stem Cell Factor |
| WT | Wild Type |
References
- Edgeworth, J.; Gorman, M.; Bennett, R.; Freemont, P.; Hogg, N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J. Biol. Chem. 1991, 266, 7706–7713. [Google Scholar] [CrossRef]
- Vogl, T.; Leukert, N.; Barczyk, K.; Strupat, K.; Roth, J. Biophysical characterization of S100A8 and S100A9 in the absence and presence of bivalent cations. Biochim. Biophys. Acta 2006, 1763, 1298–1306. [Google Scholar] [CrossRef]
- Korndörfer, I.P.; Brueckner, F.; Skerra, A. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting α-helices can determine specific association of two EF-hand proteins. J. Mol. Biol. 2007, 370, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Leukert, N.; Vogl, T.; Strupat, K.; Reichelt, R.; Sorg, C.; Roth, J. Calcium-dependent tetramer formation of S100A8 and S100A9 is essential for biological activity. J. Mol. Biol. 2006, 359, 961–972. [Google Scholar] [CrossRef]
- Vogl, T.; Gharibyan, A.; Morozova-Roche, A. Pro-Inflammatory S100A8 and S100A9 proteins: Self-assembly into multifunctional native and amyloid complexes. Int. J. Mol. Sci. 2012, 13, 2893–2917. [Google Scholar] [CrossRef]
- Vogl, T.; Tenbrock, K.; Ludwig, S.; Leukert, N.; Ehrhardt, C.; van Zoelen, M.A.D.; Nacken, W.; Foell, D.; van der Poll, T.; Sorg, C.; et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 2007, 13, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, H.; Chen, X.; Jiang, Y.; Huang, Q. Functional characterization of S100A8 and S100A9 in altering monolayer permeability of human umbilical endothelial cells. PLoS ONE 2014, 9, e90472. [Google Scholar] [CrossRef] [PubMed]
- Narumi, K.; Miyakawa, R.; Ueda, R.; Hashimoto, H.; Yamamoto, Y.; Yoshida, T.; Aoki, K. Proinflammatory proteins S100A8/S100A9 activate NK cells via interaction with RAGE. J. Immunol. 2015, 194, 5539–5548. [Google Scholar] [CrossRef]
- Lackmann, M.; Rajasekariah, P.; Iismaa, S.E.; Jones, G.; Cornish, C.J.; Hu, S.; Simpson, R.J.; Moritz, R.L.; Geczy, C.L. Identification of a chemotactic domain of the pro-inflammatory S100 protein CP10. J. Immunol. 1993, 150, 2981–2991. [Google Scholar]
- Ehlermann, P.; Eggers, K.; Bierhaus, A.; Most, P.; Weichenhan, D.; Greten, J.; Nawroth, P.P.; Katus, H.A.; Remppis, A. Increased proinflammatory endothelial response to S100A8/A9 after preactivation through advanced glycation end products. Cardiovasc. Diabetol. 2006, 5, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, P.; Corzo, C.A.; Luetteke, N.; Yu, B.; Nagaraj, S.; Bui, M.M.; Ortiz, M.; Nacken, W.; Sorg, C.; Vogl, T.; et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med. 2008, 205, 2235–2249. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.-C.; Noël, C.; Tessier, P.A.; Girard, D. Human S100A9 potentiates IL-8 production in response to GM-CSF or fMLP via activation of a different set of transcription factors in neutrophils. FEBS Lett. 2014, 588, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Sunahori, K.; Yamamura, M.; Yamana, J.; Takasugi, K.; Kawashima, M.; Yamamoto, H.; Chazin, W.J.; Nakatani, Y.; Yui, S.; Makino, H. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen activated protein kinase in rheumatoid arthritis. Arthritis Res. Ther. 2006, 8, R69. [Google Scholar] [CrossRef]
- Berthier, S.; Paclet, M.-H.; Lerouge, S.; Roux, F.; Vergnaud, S.; Coleman, A.W.; Morel, F. Changing the conformation state of cytochrome b558 initiates NADPH oxidase activation. J. Biol. Chem. 2003, 278, 25499–25508. [Google Scholar] [CrossRef]
- Kerkhoff, C.; Eue, I.; Sorg, C. The Regulatory role of MRP8 (S100A8) and MRP14 (S100A9) in the transendothelial migration of human leukocytes. Pathobiology 1999, 67, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Tak, T.; Tesselaar, K.; Pillay, J.; Borghans, J.A.; Koenderman, L. What’s your age again? Determination of human neutrophil half-lives revisited. J. Leukoc. Biol. 2013, 94, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.G.; Calvo, K.R.; Pasillas, M.P.; Sykes, D.B.; Häcker, H.; Kamps, M.P. Quantitative production of macrophages or neutrophils ex vivo using conditional Hoxb8. Nat. Methods 2006, 3, 287–293. [Google Scholar] [CrossRef]
- McDonald, J.U.; Cortini, A.; Rosas, M.; Fossati-Jimack, L.; Ling, G.S.; Lewis, K.J.; Dewitt, S.; Liddiard, K.; Brown, G.D.; Jones, S.A.; et al. In vivo functional analysis and genetic modification of in vitro-derived mouse neutrophils. FASEB J. 2011, 25, 1972–1982. [Google Scholar] [CrossRef]
- Redecke, V.; Wu, R.; Zhou, J.; Finkelstein, D.; Chaturvedi, V.; High, A.A.; Häcker, H. Hematopoietic progenitor cell lines with myeloid and lymphoid potential. Nat. Methods 2013, 10, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.; Burwinkel, F.; van den Bos, C.; Goebeler, M.; Vollmer, E.; Sorg, C. MRP8 and MRP14, S-100-like proteins associated with myeloid differentiation, are translocated to plasma membrane and intermediate filaments in a calcium-dependent manner. Blood 1993, 82, 1875–1883. [Google Scholar] [CrossRef]
- Manitz, M.P.; Horst, B.; Seeliger, S.; Strey, A.; Skryabin, B.V.; Gunzer, M.; Frings, W.; Schönlau, F.; Roth, J.; Sorg, C.; et al. Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol. Cell Biol. 2003, 23, 1034–1043. [Google Scholar] [CrossRef]
- Hobbs, J.A.; May, R.; Tanousis, K.; McNeill, E.; Mathies, M.; Gebhardt, C.; Henderson, R.; Robinson, M.J.; Hogg, N. Myeloid cell function in MRP-14 (S100A9) null mice. Mol. Cell Biol. 2003, 23, 2564–2576. [Google Scholar] [CrossRef]
- Vogl, T.; Stratis, A.; Wixler, V.; Völler, T.; Thurainayagam, S.; Jorch, S.K.; Zenker, S.; Dreiling, A.; Chakraborty, D.; Fröhling, M.; et al. Autoinhibitory regulation of S100A8/S100A9 alarmin activity locally restricts sterile inflammation. J. Clin. Investig. 2018, 128, 1852–1866. [Google Scholar] [CrossRef]
- Swamydas, M.; Luo, Y.; Dorf, M.E.; Lionakis, M.S. Isolation of mouse neutrophils. Curr. Protoc. Immunol. 2015, 110, 3–20. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef]
- Doussiere, J.; Bouzidi, F.; Vignais, P.V. The S100A8/A9 protein as a partner for the cytosolic factors of NADPH oxidase activation in neutrophils. Eur. J. Biochem. 2002, 269, 3246–3255. [Google Scholar] [CrossRef]
- Paclet, M.H.; Berthier, S.; Kuhn, L.; Garin, J.; Morel, F. Regulation of phagocyte NADPH oxidase activity: Identification of two cytochrome b558 activation states. FASEB J. 2007, 4, 1244–1255. [Google Scholar] [CrossRef]
- Schenten, V.; Melchior, C.; Steinckwich, N.; Tschirhart, E.J.; Bréchard, S. Sphingosine kinases regulate NOX2 activity via p38 MAPK-dependent translocation of S100A8/A9. J. Leukoc. Biol. 2011, 89, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Naegelen, I.; Plançon, S.; Nicot, N.; Kaoma, T.; Muller, A.; Vallar, L.; Tschirhart, E.J.; Bréchard, S. An essential role of syntaxin 3 protein for granule exocytosis and secretion of IL-1α, IL-1β, IL-12b, and CCL4 from differentiated HL-60 cells. J. Leukoc. Biol. 2015, 97, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.Y.; McCormick, B.; Mazelyte, G.; Michael, M.; Vermeren, S. HoxB8 neutrophils replicate Fcγ receptor and integrin-induced neutrophil signaling and functions. J. Leukoc. Biol. 2019, 105, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Saul, S.; Castelbou, C.; Fickentscher, C.; Demaurex, N. Signaling and functional competency of neutrophils derived from bone-marrow cells expressing the ER-HOXB8 oncoprotein. J. Leukoc. Biol. 2019, 106, 1101–1115. [Google Scholar] [CrossRef]
- Simard, J.-C.; Cesaro, A.; Chapeton-Montes, J.; Tardif, M.; Antoine, F.; Girard, D.; Tessier, P.A. S100A8 and S100A9 induce cytokine expression and regulate the NLRP3 inflammasome via ROS-dependent activation of NF-κB1. PLoS ONE 2013, 8, e72138. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Hughes, C.C.W. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Tamassia, N.; Bianchetto-Aguilera, F.; Arruda-Silva, F.; Gardiman, E.; Gasperini, S.; Calzetti, F.; Cassatella, M.A. Cytokine production by human neutrophils: Revisiting the dark side of the moon. Eur. J. Clin. Investig. 2018, 48, e12952. [Google Scholar] [CrossRef] [PubMed]
- Foell, D.; Frosch, M.; Sorg, C.; Roth, J. Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin. Chim. Acta 2004, 344, 37–51. [Google Scholar] [CrossRef]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- Beil, W.J.; Weller, P.F.; Peppercorn, M.A.; Galli, S.J.; Dvorak, A.M. Ultrastructural immunogold localization of subcellular sites of TNF-alpha in colonic Crohn’s disease. J. Leukoc. Biol. 1995, 58, 284–298. [Google Scholar] [CrossRef]
- Bliss, S.K.; Marshall, A.J.; Zhang, Y.; Denkers, E.Y. Human polymorphonuclear leukocytes produce IL-12, TNF-alpha, and the chemokines macrophage-inflammatory protein-1 alpha and -1 beta in response to Toxoplasma gondii antigens. J. Immunol. 1999, 162, 7369–7375. [Google Scholar]
- Brandt, E.; Colombel, J.F.; Ectors, N.; Gambiez, L.; Emilie, D.; Geboes, K.; Capron, M.; Desreumaux, P. Enhanced production of IL-8 in chronic but not in early ileal lesions of Crohn’s disease (CD). Clin. Exp. Immunol. 2000, 122, 180–185. [Google Scholar] [CrossRef]
- Matzer, S.P.; Baumann, T.; Lukacs, N.W.; Röllinghoff, M.; Beuscher, H.U. Constitutive expression of macrophage-inflammatory protein 2 (MIP-2) mRNA in bone marrow gives rise to peripheral neutrophils with preformed MIP-2 Protein. J. Immunol. 2001, 167, 4635–4643. [Google Scholar] [CrossRef]
- Denkers, E.Y.; Del Rio, L.; Bennouna, S. Neutrophil production of IL-12 and other cytokines during microbial infection. Chem. Immunol. Allergy 2003, 83, 95–114. [Google Scholar] [PubMed]
- Brough, D.; Rothwell, N.J. Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J. Cell Sci. 2007, 120, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Ehrchen, J.M.; Sunderkötter, C.; Foell, D.; Vogl, T.; Roth, J. The endogenous Toll like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 2009, 86, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Sjolin, C.; Stendahl, O.; Dahlgren, C.; Stendahlt, O.; Dahlgren, C. Calcium-induced translocation of annexins to subcellular organelles of human neutrophils. Biochem. J. 1994, 300, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Lindmark, I.M.; Karlsson, A.; Serrander, L.; Francois, P.; Lew, D.; Rasmusson, B.; Stendahl, O.; Nüsse, O. Synaptotagmin II could confer Ca(2+) sensitivity to phagocytosis in human neutrophils. Biochim. Biophys. Acta 2002, 1590, 159–166. [Google Scholar] [CrossRef][Green Version]
- Chasserot-Golaz, S.; Vitale, N.; Umbrecht-Jenck, E.; Knight, D.; Gerke, V.; Bader, M.F. Annexin 2 promotes the formation of lipid microdomains required for calcium-regulated exocytosis of dense-core vesicles. Mol. Biol. 2005, 16, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.-C.; Girard, D.; Tessier, P.A. Induction of neutrophil degranulation by S100A9 via a MAPK-dependent mechanism. J. Leukoc. Biol. 2010, 87, 905–914. [Google Scholar] [CrossRef]
- Clemens, R.A.; Chong, J.; Grimes, D.; Hu, Y.; Lowell, C.A. STIM1 and STIM2 cooperatively regulate mouse neutrophil store-operated calcium entry and cytokine production. Blood 2017, 130, 1565–1577. [Google Scholar] [CrossRef]
- Kabe, Y.; Ando, K.; Hirao, S.; Yoshida, M.; Handa, H. Redox Regulation of NF-ΚB Activation: Distinct Redox Regulation between the Cytoplasm and the Nucleus. Antioxid. Redox Signal. 2005, 7, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.B.; Leonard, W.J. Modulation of transcription factor NF-κB binding activity by oxidation-reduction in vitro. Proc. Natl. Acad. Sci. USA 1991, 88, 4328–4332. [Google Scholar] [CrossRef] [PubMed]
- Schenten, V.; Bréchard, S.; Plançon, S.; Melchior, C.; Frippiat, J.P.; Tschirhart, E.J. IPLA2, a novel determinant in Ca2+- and phosphorylation-dependent S100A8/A9 regulated NOX2 activity. Biochim. Biophys. Acta 2010, 1803, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.S.; Chang, C.M.; Liu, X.; Huang, W.C.; Chang, W.C. Characterization of cytokinome landscape for clinical responses in human cancers. Oncoimmunology 2016, 5, e1214789. [Google Scholar] [CrossRef] [PubMed]
- Tecchio, C.; Scapini, P.; Pizzolo, G.; Cassatella, M.A. On the cytokines produced by human neutrophils in tumors. Sem. Cancer Biol. 2013, 23, 159–170. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Brennan, F.M.; McInnes, I.B. Evidence that cytokines play a role in rheumatoid arthritis. J. Clin. Investig. 2008, 118, 3537–3545. [Google Scholar] [CrossRef] [PubMed]
- Tamassia, N.; Zimmermann, M.; Castellucci, M.; Ostuni, R.; Bruderek, K.; Schilling, B.; Brandau, S.; Bazzoni, F.; Natoli, G.; Cassatella, M.A. Cutting edge: An inactive chromatin configuration at the IL-10 locus in human neutrophils. J. Immunol. 2013, 190, 1921–1925. [Google Scholar] [CrossRef]
- Chang, H.H.; Oh, P.Y.; Ingber, D.E.; Huang, S. Multistable and multistep dynamics in neutrophil differentiation. BMC Cell Biol. 2006, 7, 11. [Google Scholar] [CrossRef]
- Lee, P.Y.; Wang, J.-X.; Parisini, E.; Dascher, C.C.; Nigrovic, P.A. Ly6 family proteins in neutrophil biology. J. Leukoc. Biol. 2013, 94, 585–594. [Google Scholar] [CrossRef]
- Collins, S.J.; Gallo, R.C.; Gallagher, R.E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature 1977, 270, 347–349. [Google Scholar] [CrossRef]
- Jiang, W.; Hua, R.; Wei, M.; Li, C.; Qiu, Z.; Yang, X.; Zhang, C. An optimized method for high-titer lentivirus preparations without ultracentrifugation. Sci. Rep. 2015, 5, 13875. [Google Scholar] [CrossRef]
- Dodo, K.; Chono, H.; Saito, N.; Tanaka, Y.; Tahara, K.; Nukaya, I.; Mineno, J. An efficient large-scale retroviral transduction method involving preloading the vector into a RetroNectin-coated bag with low-temperature shaking. PLoS ONE 2014, 9, e86275. [Google Scholar] [CrossRef] [PubMed]
- Schenten, V.; Plançon, S.; Jung, N.; Hann, J.; Bueb, J.-L.; Bréchard, S.; Tschirhart, E.J.; Tolle, F. Secretion of the phosphorylated form of S100A9 from neutrophils is essential for the proinflammatory functions of extracellular S100A8/A9. Front. Immunol. 2018, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Maecker, H.T.; Frey, T.; Nomura, L.E.; Trotter, J. Selecting fluorochrome conjugates for maximum sensitivity. Cytom. A 2004, 62, 169–173. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| S100a8 | GTCCTCAGTTTGTGCAGAATA | CACCATCGCAAGGAACTC |
| S100a9 | TTTAGCTTGAAGAGCAAGAAG | TGTCCTTCCTTCCTAGAGTATTG |
| Ccl2 | ATCATCCCTGCGAGCCTATCCT | GACCTTTTTTGGCTAAACGCTTTC |
| Ccl3 | ACTGCCTGCTGCTTCTCCTACA | ATGACACCTGGCTGGGAGCAAA |
| Ccl4 | ACCCTCCCACTTCCTGCTGTTT | CTGTCTGCCTCTTTTGGTCAGG |
| Il-1α | ACGGCTGAGTTTCAGTGAGACC | CACTCTGGTAGGTGTAAGGTGC |
| Il-1β | TGGACCTTCCAGGATGAGGACA | GTTCATCTCGGAGCCTGTAGTG |
| Il-6 | TACCACTTCACAAGTCGGAGGC | CTGCAAGTGCATCATCGTTGTTC |
| Tnf-α | GGTGCCTATGTCTCAGCCTCTT | GCCATAGAACTGATGAGAGGGAG |
| Cxcl2 | CCAAGGGTTGACTTCAAGAAC | TGAGAGTGGCTATGACTTCTG |
| Actβ | AGCCTTCCTTCTTGGGTATG | AGCACTGTGTTGGCATAGA |
| 18s | AGCGAGTGATCACCATCAT | CCAGAACCTGGCTGTACTT |
| B2m | TCACACTGAATTCACCCCCAC | TGATCACATGTCTCGATCCCAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Hann, J.; Schenten, V.; Plançon, S.; Bueb, J.-L.; Tolle, F.; Bréchard, S. Role of S100A8/A9 for Cytokine Secretion, Revealed in Neutrophils Derived from ER-Hoxb8 Progenitors. Int. J. Mol. Sci. 2021, 22, 8845. https://doi.org/10.3390/ijms22168845
Zhou Y, Hann J, Schenten V, Plançon S, Bueb J-L, Tolle F, Bréchard S. Role of S100A8/A9 for Cytokine Secretion, Revealed in Neutrophils Derived from ER-Hoxb8 Progenitors. International Journal of Molecular Sciences. 2021; 22(16):8845. https://doi.org/10.3390/ijms22168845
Chicago/Turabian StyleZhou, Yang, Justine Hann, Véronique Schenten, Sébastien Plançon, Jean-Luc Bueb, Fabrice Tolle, and Sabrina Bréchard. 2021. "Role of S100A8/A9 for Cytokine Secretion, Revealed in Neutrophils Derived from ER-Hoxb8 Progenitors" International Journal of Molecular Sciences 22, no. 16: 8845. https://doi.org/10.3390/ijms22168845
APA StyleZhou, Y., Hann, J., Schenten, V., Plançon, S., Bueb, J.-L., Tolle, F., & Bréchard, S. (2021). Role of S100A8/A9 for Cytokine Secretion, Revealed in Neutrophils Derived from ER-Hoxb8 Progenitors. International Journal of Molecular Sciences, 22(16), 8845. https://doi.org/10.3390/ijms22168845