Ganglioside Composition Distinguishes Anaplastic Ganglioglioma Tumor Tissue from Peritumoral Brain Tissue: Complementary Mass Spectrometry and Thin-Layer Chromatography Evidence
Abstract
1. Introduction
2. Results
2.1. Quantitative Ganglioside Analysis
2.2. High-Performance Thin-Layer Chromatography (HPTLC) Analysis of GG Mixtures
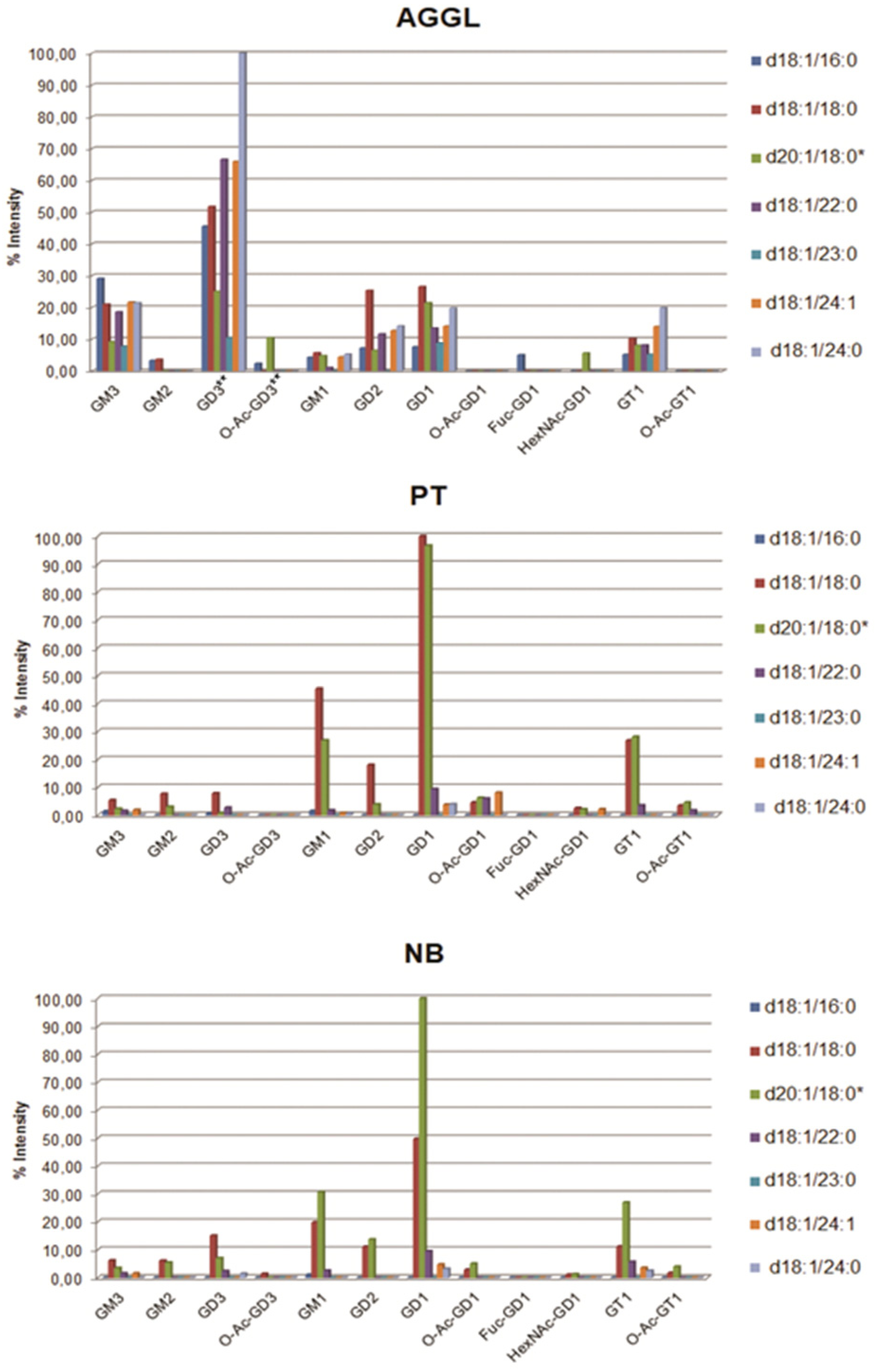
2.3. Compositional Analysis of AGGL, PT, and NB Tissue GG Mixtures via MS
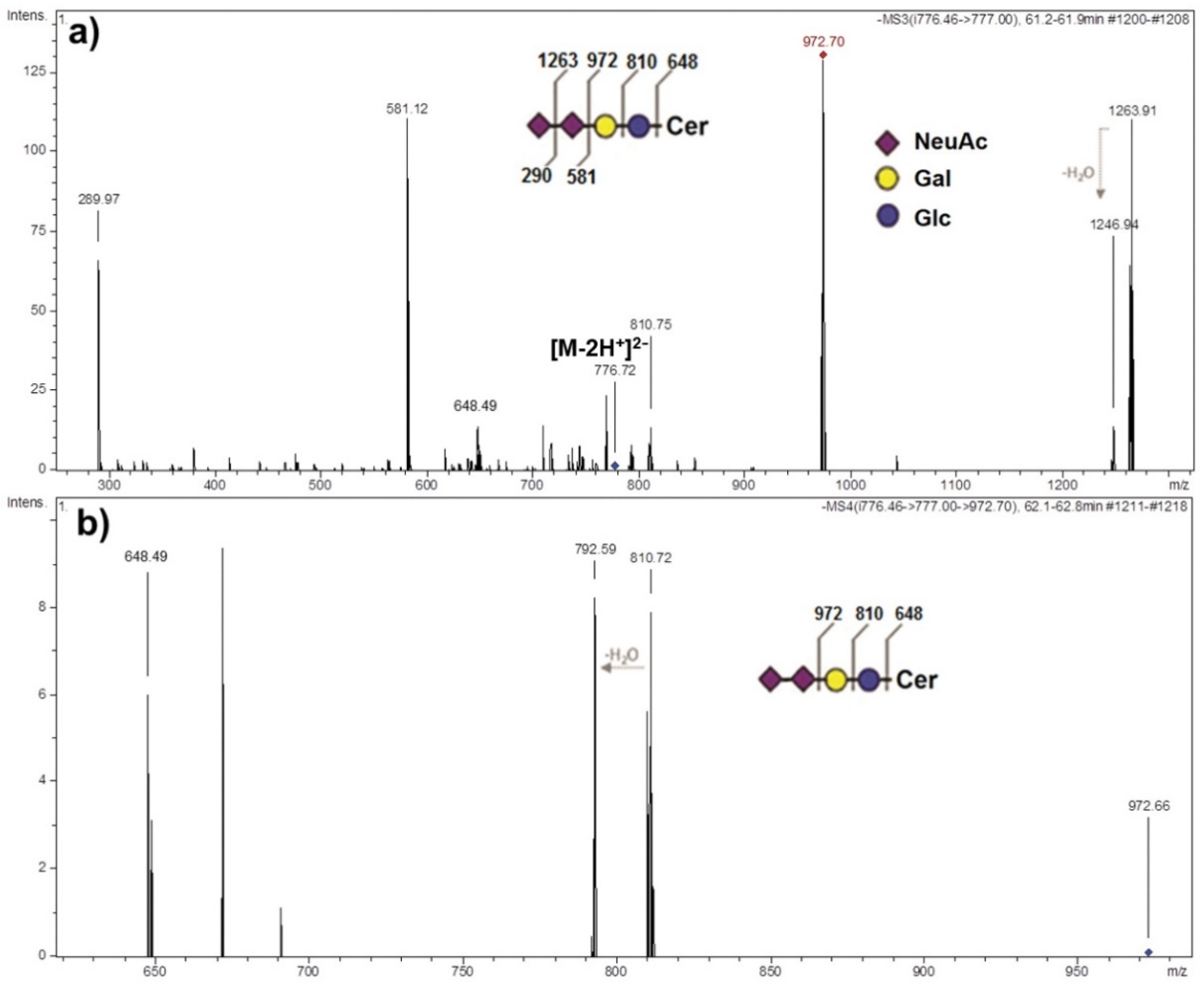
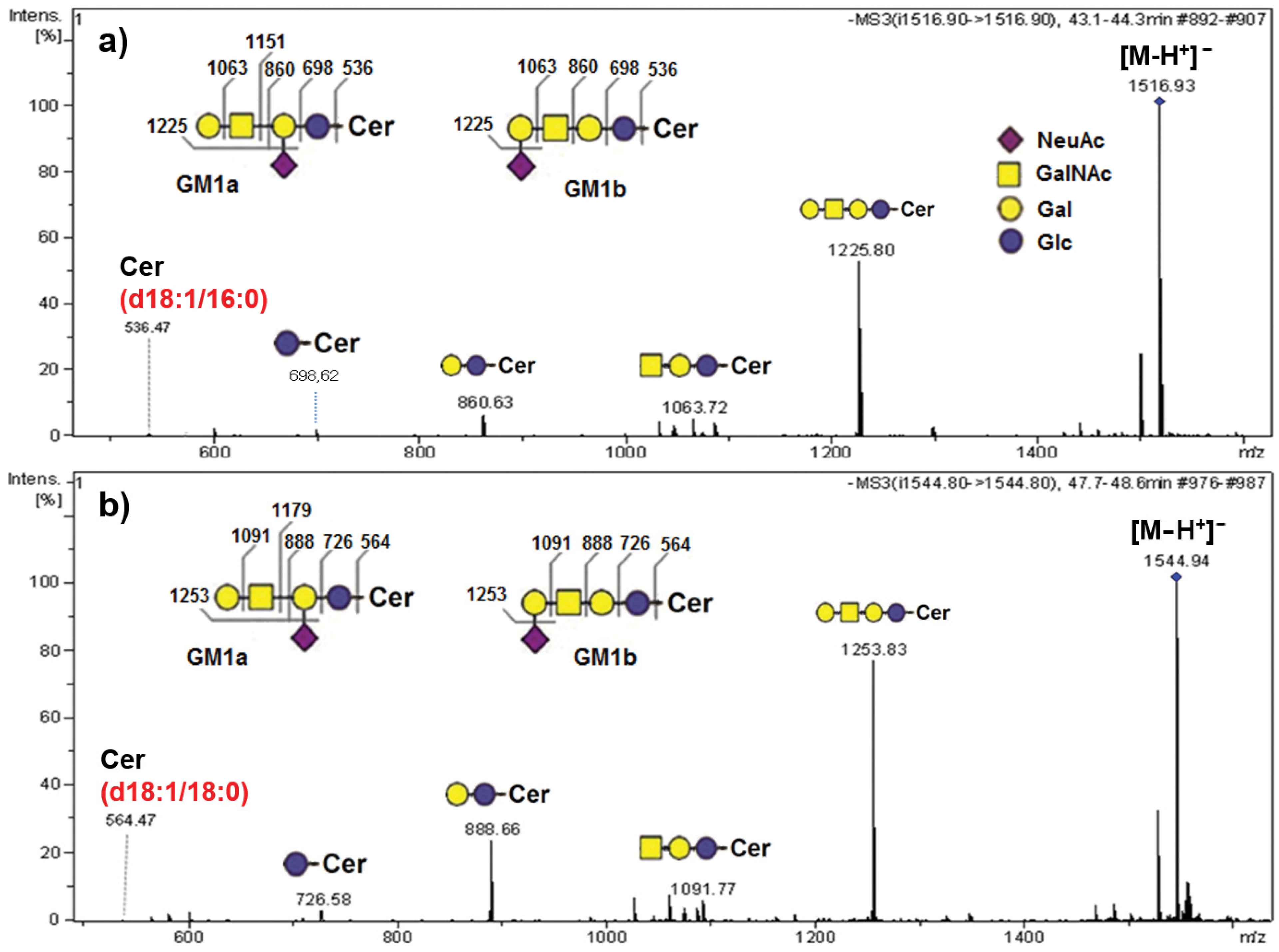
2.4. Structural Identification of AGGL Ganglioside Species via MS Sequencing
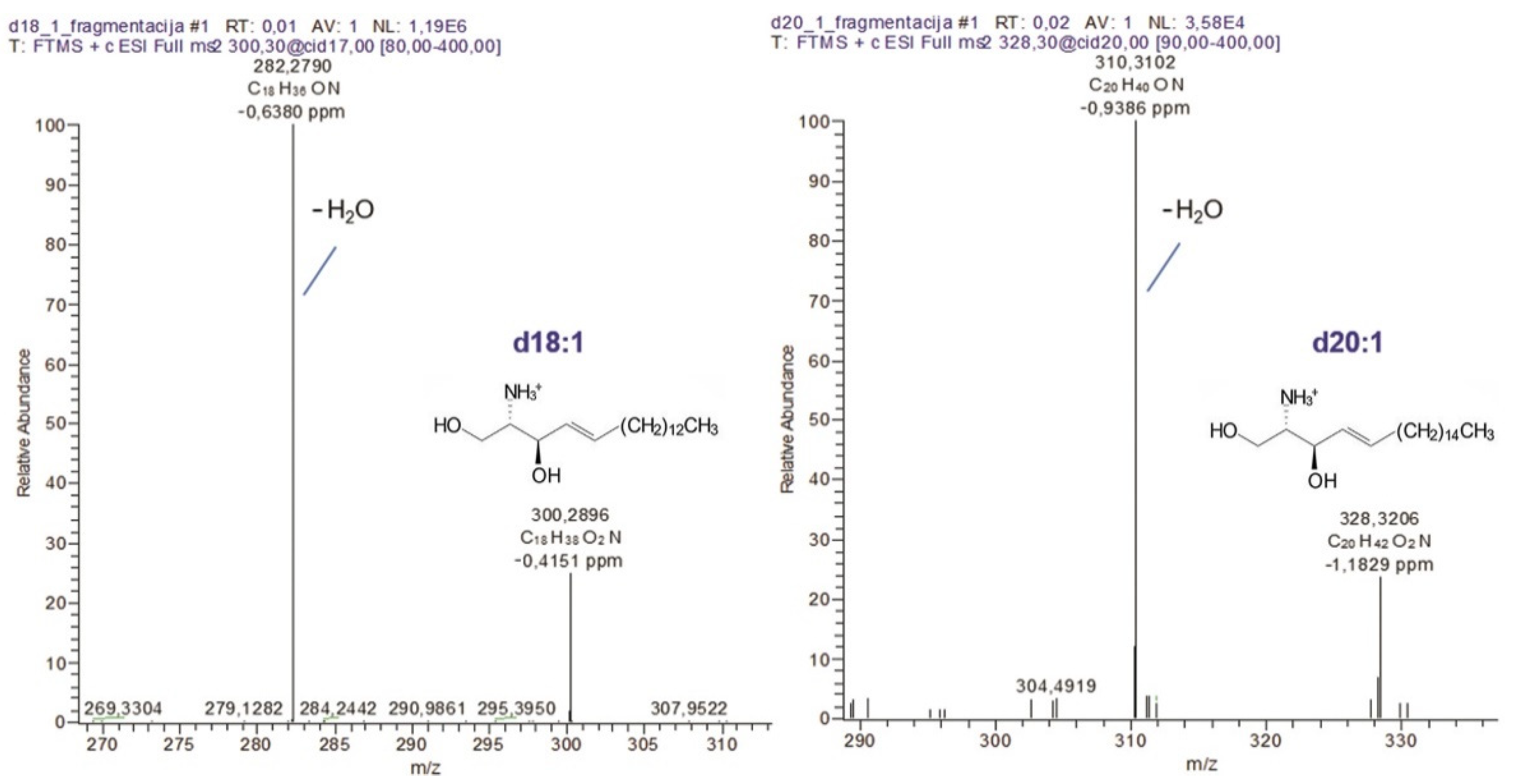
2.5. Structural Characterization of Sphingoid Bases Obtained from AGGL Gangliosides Ceramides
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Sampling and Characterization of Tumor, Peritumoral Tissue, and Normal Brain Tissue
4.2. Gangliosides Extraction and Purification
4.3. Quantitative GG Mixture Analysis
4.4. High-Performance Thin-Layer Chromatography Analysis of GG Mixtures
4.5. MS Characterization of Ganglioside Mixtures
4.6. Sphingoid Base Extraction and Purification
4.7. HR-MS Characterization of Sphingoid Bases
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LacCer | Galβ4Glcβ1Cer; |
| GA2 | Gg3Cer, GalNAcβ4Galβ4Glcβ1Cer; |
| GA1 | Gg4Cer, Galβ3GalNAcβ4Galβ4Glcβ1Cer; |
| Lc3 | GlcNAcβ3Galβ4Glcβ1Cer; |
| Lc4 | Galβ3GlcNAcβ3Galβ4Glcβ1Cer; |
| nLc4 | Galβ4GlcNAcβ3Galβ4Glcβ1Cer; |
| nLc6 | Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer; |
| Gb3 | Galα4Galβ4Glcβ1Cer; |
| Gb4 | GalNAcβ3Galα4Galβ4Glcβ1Cer; |
| iGb3 | Galα3Galβ4Glcβ1Cer; |
| iGb4 | GalNAcβ3Galα3Galβ4Glcβ1Cer; |
| GM3 | II3-α-Neu5Ac-LacCer; |
| GD3 | II3-α-(Neu5Ac)2-LacCer; |
| GT3 | II3-α-(Neu5Ac)3-LacCer; |
| GM2 | II3-α-Neu5Ac-Gg3Cer; |
| GD2 | II3-α-(Neu5Ac)2-Gg3Cer; |
| GM1a or GM1 | II3-α-Neu5Ac-Gg4Cer; |
| GM1b | IV3-α-Neu5Ac-Gg4Cer; |
| LM1 | IV3-α-Neu5Ac- Lc4Cer; |
| nLM1 | IV3-α-Neu5Ac- nLc4Cer; |
| GD1a | IV3-α-Neu5Ac,II3-α-Neu5Ac-Gg4Cer; |
| GD1b | II3-α-(Neu5Ac)2-Gg4Cer; |
| LD1 | IV3-α-Neu5Ac,II6-α-Neu5Ac-Lc4Cer; |
| nLD1 | IV3-α-Neu5Ac,II6-α-Neu5Ac-nLc4Cer; |
| GT1b | V3-α-Neu5Ac,II3-α-(Neu5Ac)2-Gg4Cer; |
| GQ1b | IV3-α-(Neu5Ac)2,II3-α-(Neu5Ac)2-Gg4Cer. |
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Pandita, A.; Balasubramaniam, A.; Perrin, R.; Shannon, P.; Guha, A. Malignant and benign ganglioglioma: A pathological and molecular study. Neuro Oncol. 2007, 9, 124–134. [Google Scholar] [CrossRef]
- Gatto, L.; Franceschi, E.; Nunno, V.D.; Tomasello, C.; Bartolini, S.; Brandes, A.A. Glioneuronal tumors: Clinicopathological findings and treatment options. Future Neurol. 2020, 15, 47. [Google Scholar] [CrossRef]
- Lin, X.; Huang, R.; Zhang, P.; Sun, J.; Dong, G.; Huang, Y.; Tian, X. Low-grade gangliogliomas in adults: A population-based study. Cancer Med. 2020, 10, 3577. [Google Scholar] [CrossRef]
- Ajithkumar, T.; Imbulgoda, N.; Rees, E.; Harris, F.; Horan, G.; Burke, A.; Jefferies, S.; Price, S.; Cross, J.; Allinson, K. Uncommon low-grade brain tumors. Neuro Oncol. 2019, 21, 151–166. [Google Scholar] [CrossRef]
- Lundar, T.; Due-Tønnessen, B.J.; Fric, R.; Egge, A.; Krossnes, B.; Due-Tønnessen, P.; Stensvold, E.; Brandal, P. Neurosurgical treatment of gangliogliomas in children and adolescents: Long-term follow-up of a single-institution series of 32 patients. Acta Neurochir. 2018, 160, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Mittelbronn, M.; Schittenhelm, J.; Lemke, D.; Ritz, R.; Nägele, T.; Weller, M.; Meyermann, R.; Beschorner, R. Low grade ganglioglioma rapidly progressing to a WHO grade IV tumor showing malignant transformation in both astroglial and neuronal cell components. Neuropathology 2007, 27, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Selvanathan, S.K.; Hammouche, S.; Salminen, H.J.; Jenkinson, M.D. Outcome and prognostic features in anaplastic ganglioglioma: Analysis of cases from the SEER database. J. Neurooncol. 2011, 105, 539–545. [Google Scholar] [CrossRef]
- Demarchi, R.; Abu-Abed, S.; Munoz, D.; Loch MacDonald, R. Malignant ganglioglioma: Case report and review of literature. J. Neurooncol. 2011, 101, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Karabekir, H.S.; Balci, C.; Tokyol, C. Primary Spinal Anaplastic Ganglioglioma. Pediatr. Neurosurg. 2006, 42, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Zanello, M.; Pagès, M.; Roux, A.; Peeters, S.; Dezamis, E.; Puget, S.; Devaux, B.; Sainte-Rose, C.; Zerah, M.; Louvel, G.; et al. Epileptic seizures in anaplastic gangliogliomas. Br. J. Neurosurg. 2017, 31, 227–233. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef]
- Furukawa, K.; Ohmi, Y.; Ohkawa, Y.; Bhuiyan, R.H.; Zhang, P.; Tajima, O.; Hashimoto, N.; Hamamura, K.; Furukawa, K. New era of research on cancer-associated glycosphingolipids. Cancer Sci. 2019, 110, 1544–1551. [Google Scholar] [CrossRef]
- Sonnino, S.; Chiricozzi, E.; Grassi, S.; Mauri, L.; Prioni, S.; Prinetti, A. Gangliosides in Membrane Organization. Prog. Mol. Biol. Transl. Sci. 2018, 156, 83–120. [Google Scholar] [CrossRef]
- Merrill, A.H. Sphingolipid and Glycosphingolipid Metabolic Pathways in the Era of Sphingolipidomics. Chem. Rev. 2011, 111, 6387–6422. [Google Scholar] [CrossRef]
- Grösch, S.; Schiffmann, S.; Geisslinger, G. Chain length-specific properties of ceramides. Prog. Lipid Res. 2012, 51, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Brachtendorf, S.; El-Hindi, K.; Grösch, S. Ceramide synthases in cancer therapy and chemoresistance. Prog. Lipid Res. 2019, 74, 160–185. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, K.; Nakayama, H.; Iwahara, C.; Takamori, K. Significance of glycosphingolipid fatty acid chain length on membrane microdomain-mediated signal transduction. FEBS Lett. 2010, 584, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Hakomori, S. Glycosynaptic microdomains controlling tumor cell phenotype through alteration of cell growth, adhesion, and motility. FEBS Lett. 2010, 584, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Cavdarli, S.; Groux-Degroote, S.; Delannoy, P. Gangliosides: The Double-Edge Sword of Neuro-Ectodermal Derived Tumors. Biomolecules 2019, 9, 311. [Google Scholar] [CrossRef]
- Groux-Degroote, S.; Guérardel, Y.; Delannoy, P. Gangliosides: Structures, Biosynthesis, Analysis, and Roles in Cancer. Chembiochem 2017, 18, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Fleurence, J.; Bahri, M.; Fougeray, S.; Faraj, S.; Vermeulen, S.; Pinault, E.; Geraldo, F.; Oliver, L.; Véziers, J.; Marquet, P.; et al. Impairing temozolomide resistance driven by glioma stem-like cells with adjuvant immunotherapy targeting O-acetyl GD2 ganglioside. Int. J. Cancer 2020, 146, 424–438. [Google Scholar] [CrossRef]
- Nagashima, G.; Suzuki, R.; Hokaku, H.; Takahashi, M.; Miyo, T.; Asai, J.; Nakagawa, N.; Fujimoto, T. Graphic analysis of microscopic tumor cell infiltration, proliferative potential, and vascular endothelial growth factor expression in an autopsy brain with glioblastoma. Surg. Neurol. 1999, 51, 292–299. [Google Scholar] [CrossRef]
- Lemée, J.-M.; Clavreul, A.; Aubry, M.; Com, E.; de Tayrac, M.; Eliat, P.-A.; Henry, C.; Rousseau, A.; Mosser, J.; Menei, P. Characterizing the peritumoral brain zone in glioblastoma: A multidisciplinary analysis. J. Neurooncol. 2015, 122, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Sampedro, M.; Valle-Argos, B.; Gómez-Nicola, D.; Fernández-Mayoralas, A.; Nieto-Díaz, M. Inhibitors of glioma growth that reveal the tumour to the immune system. Clin. Med. Insights Oncol. 2011, 5, 265–314. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, K.M.; Mahesparan, R.; Read, T.A.; Tysnes, B.B.; Thorsen, F.; Visted, T.; Bjerkvig, R.; Fredman, P. The glioma-associated gangliosides 3′-isoLM1, GD3 and GM2 show selective area expression in human glioblastoma xenografts in nude rat brains. Neuropathol. Appl. Neurobiol. 2001, 27, 451–464. [Google Scholar] [CrossRef]
- Iwasawa, T.; Zhang, P.; Ohkawa, Y.; Momota, H.; Wakabayashi, T.; Ohmi, Y.; Bhuiyan, R.; Furukawa, K.; Furukawa, K. Enhancement of malignant properties of human glioma cells by ganglioside GD3/GD2. Int. J. Oncol. 2018, 52, 1255–1266. [Google Scholar] [CrossRef]
- Yeh, S.-C.; Wang, P.-Y.; Lou, Y.-W.; Khoo, K.-H.; Hsiao, M.; Hsu, T.-L.; Wong, C.-H. Glycolipid GD3 and GD3 synthase are key drivers for glioblastoma stem cells and tumorigenicity. Proc. Natl. Acad. Sci. USA 2016, 113, 5592–5597. [Google Scholar] [CrossRef]
- Mount, C.W.; Majzner, R.G.; Sundaresh, S.; Arnold, E.P.; Kadapakkam, M.; Haile, S.; Labanieh, L.; Hulleman, E.; Woo, P.J.; Rietberg, S.P.; et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M+ diffuse midline gliomas letter. Nat. Med. 2018, 24, 572–579. [Google Scholar] [CrossRef]
- Nazha, B.; Inal, C.; Owonikoko, T.K. Disialoganglioside GD2 Expression in Solid Tumors and Role as a Target for Cancer Therapy. Front. Oncol. 2020, 10, 1000. [Google Scholar] [CrossRef]
- Ploessl, C.; Pan, A.; Maples, K.T.; Lowe, D.K. Dinutuximab: An Anti-GD2 Monoclonal Antibody for High-Risk Neuroblastoma. Ann. Pharmacother. 2016, 50, 416–422. [Google Scholar] [CrossRef]
- Mora, J.; Chan, G.C.-F.; Morgenstern, D.A.; Nysom, K.; Bear, M.K.; Dalby, L.W.; Lisby, S.; Kushner, B.H. Naxitamab, a new generation anti-GD2 monoclonal antibody (mAb) for treatment of relapsed/refractory high-risk neuroblastoma (HR-NB). J. Clin. Oncol. 2020, 38, 10543. [Google Scholar] [CrossRef]
- Moghimi, B.; Muthugounder, S.; Jambon, S.; Tibbetts, R.; Hung, L.; Bassiri, H.; Hogarty, M.D.; Barrett, D.M.; Shimada, H.; Asgharzadeh, S. Preclinical assessment of the efficacy and specificity of GD2-B7H3 SynNotch CAR-T in metastatic neuroblastoma. Nat. Commun. 2021, 12, 511. [Google Scholar] [CrossRef]
- Becker, R.; Rohlfs, J.; Jennemann, R.; Wiegandt, H.; Mennel, H.D.; Bauer, B.L. Glycosphingolipid component profiles of human gliomas—Correlation to survival time and histopathological malignancy grading. Clin. Neuropathol. 2000, 19, 119–125. [Google Scholar]
- Fabris, D.; Rožman, M.; Sajko, T.; Vukelić, Ž. Aberrant ganglioside composition in glioblastoma multiforme and peritumoral tissue: A mass spectrometry characterization. Biochimie 2017, 137, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Vukelić, Z.; Kalanj-Bognar, S.; Froesch, M.; Bîndila, L.; Radić, B.; Allen, M.; Peter-Katalinić, J.; Zamfir, A.D. Human gliosarcoma-associated ganglioside composition is complex and distinctive as evidenced by high-performance mass spectrometric determination and structural characterization. Glycobiology 2007, 17, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, Y.; Momota, H.; Kato, A.; Hashimoto, N.; Tsuda, Y.; Kotani, N.; Honke, K.; Suzumura, A.; Furukawa, K.; Ohmi, Y.; et al. Ganglioside GD3 Enhances Invasiveness of Gliomas by Forming a Complex with Platelet-derived Growth Factor Receptor α and Yes Kinase. J. Biol. Chem. 2015, 290, 16043–16058. [Google Scholar] [CrossRef] [PubMed]
- Noll, E.N.; Lin, J.; Nakatsuji, Y.; Miller, R.H.; Black, P.M. GM3 as a novel growth regulator for human gliomas. Exp. Neurol. 2001, 168, 300–309. [Google Scholar] [CrossRef]
- Todeschini, R.A.; Hakomori, S.I.; Todeschini, R.A.; Hakomori, S. itiroh Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 421–433. [Google Scholar] [CrossRef]
- Hettmer, S.; McCarter, R.; Ladisch, S.; Kaucic, K. Alterations in neuroblastoma ganglioside synthesis by induction of GD1b synthase by retinoic acid. Br. J. Cancer 2004, 91, 389–397. [Google Scholar] [CrossRef]
- Sonnino, S.; Chigorno, V. Ganglioside molecular species containing C18- and C20-sphingosine in mammalian nervous tissues and neuronal cell cultures. Biochim. Biophys. Acta Rev. Biomembr. 2000, 1469, 63–77. [Google Scholar] [CrossRef]
- Jensen, S.A.; Calvert, A.E.; Volpert, G.; Kouri, F.M.; Hurley, L.A.; Luciano, J.P.; Wu, Y.; Chalastanis, A.; Futerman, A.H.; Stegh, A.H. Bcl2L13 is a ceramide synthase inhibitor in glioblastoma. Proc. Natl. Acad. Sci. USA 2014, 111, 5682–5687. [Google Scholar] [CrossRef]
- Stiban, J.; Tidhar, R.; Futerman, A.H. Ceramide synthases: Roles in cell physiology and signaling. Adv. Exp. Med. Biol. 2010, 688, 60–71. [Google Scholar]
- Park, J.W.; Park, W.J.; Futerman, A.H. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 671–681. [Google Scholar] [CrossRef]
- Corte, E.L.; Cas, M.D.; Raggi, A.; Patanè, M.; Broggi, M.; Schiavolin, S.; Calatozzolo, C.; Pollo, B.; Pipolo, C.; Bruzzone, M.G.; et al. Long and Very-Long-Chain Ceramides Correlate with A More Aggressive Behavior in Skull Base Chordoma Patients. Int. J. Mol. Sci. 2019, 20, 4480. [Google Scholar] [CrossRef]
- Das, A.; Zdzislaw, S.; Cachia, D.; Patel, S.J.; Ogretmen, B. Exth-51. C18-Ceramide analogue drug overcomes resistance to temozolomide in glioblastoma. Neuro Oncol. 2018, 20, vi96. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Wen, L.; Zhu, F.; Wang, Y.; Xie, Q.; Chen, Z.; Li, Y. Overexpression of ceramide synthase 1 increases C18-ceramide and leads to lethal autophagy in human glioma. Oncotarget 2017, 8, 104022–104036. [Google Scholar] [CrossRef]
- O’Brien, J.S.; Sampson, E.L. Lipid composition of the normal human brain: Gray matter, white matter, and myelin. J. Lipid Res. 1965, 6, 537–544. [Google Scholar] [CrossRef]
- Svennerholm, L.; Fredman, P. A procedure for the quantitative isolation of brain gangliosides. Biochim. Biophys. Acta 1980, 617, 97–109. [Google Scholar] [CrossRef]
- Vukelić, Z.; Metelmann, W.; Müthing, J.; Kos, M.; Peter-Katalinić, J. Anencephaly: Structural characterization of gangliosides in defined brain regions. Biol. Chem. 2001, 382, 259–274. [Google Scholar] [CrossRef]
- Schiopu, C.; Vukelić, Ž.; Capitan, F.; Kalanj-Bognar, S.; Sisu, E.; Zamfir, A.D. Chip-nanoelectrospray quadrupole time-of-flight tandem mass spectrometry of meningioma gangliosides: A preliminary study. Electrophoresis 2012, 33, 1778–1786. [Google Scholar] [CrossRef]
- Zamfir, A.D.; Serb, A.; Vukelić, Ž.; Flangea, C.; Schiopu, C.; Fabris, D.; Kalanj-Bognar, S.; Capitan, F.; Sisu, E. Assessment of the molecular expression and structure of gangliosides in brain metastasis of lung adenocarcinoma by an advanced approach based on fully automated chip-nanoelectrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 2011, 22, 2145–2159. [Google Scholar] [CrossRef]
- Svennerholm, L. Quantitive estimation of sialic acids: II. A colorimetric resorcinol-hydrochloric acid method. Biochim. Biophys. Acta 1957, 24, 604–611. [Google Scholar] [CrossRef]
- Miettinen, T.; Takki-Luukkainen, T. Use of Butyl Acetate in Determination of Sialic Acid. Acta Chem. Scand. 1959, 13, 656–658. [Google Scholar] [CrossRef]
- Rožman, M.; Fabris, D.; Mrla, T.; Vukelić, Ž. Database and data analysis application for structural characterization of gangliosides and sulfated glycosphingolipids by negative ion mass spectrometry. Carbohydr. Res. 2014, 400, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoo, H.-S.; Norred, W.P.; Riley, R.T. A rapid method for quantifying free sphingoid bases and complex sphingolipids in microgram amounts of cells following exposure to fumonisin B1. Toxicol. Vitr. 1996, 10, 77–84. [Google Scholar] [CrossRef]
- Angelucci, C.; D’Alessio, A.; Sorrentino, S.; Biamonte, F.; Moscato, U.; Mangiola, A.; Sica, G.; Iacopino, F. Immunohistochemical Analysis of DNA Repair- and Drug-Efflux-Associated Molecules in Tumor and Peritumor Areas of Glioblastoma. Int. J. Mol. Sci. 2021, 22, 1620. [Google Scholar] [CrossRef]
- Svennerholm, L. Chromatographic separation of human brain gangliosides. J. Neurochem. 1963, 10, 613–623. [Google Scholar] [CrossRef]
- Svennerholm, L. Ganglioside designation. Adv. Exp. Med. Biol. 1980, 125, 11. [Google Scholar]
- Svennerholm, L. Designation and schematic structure of gangliosides and allied glycosphingolipids. Prog. Brain Res. 1994, 101, 51–55. [Google Scholar] [CrossRef]
- Chester, M.A.; IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature of glycolipids—Recommendations 1997. Eur. J. Biochem. 1998, 257, 293–298. [Google Scholar] [CrossRef]
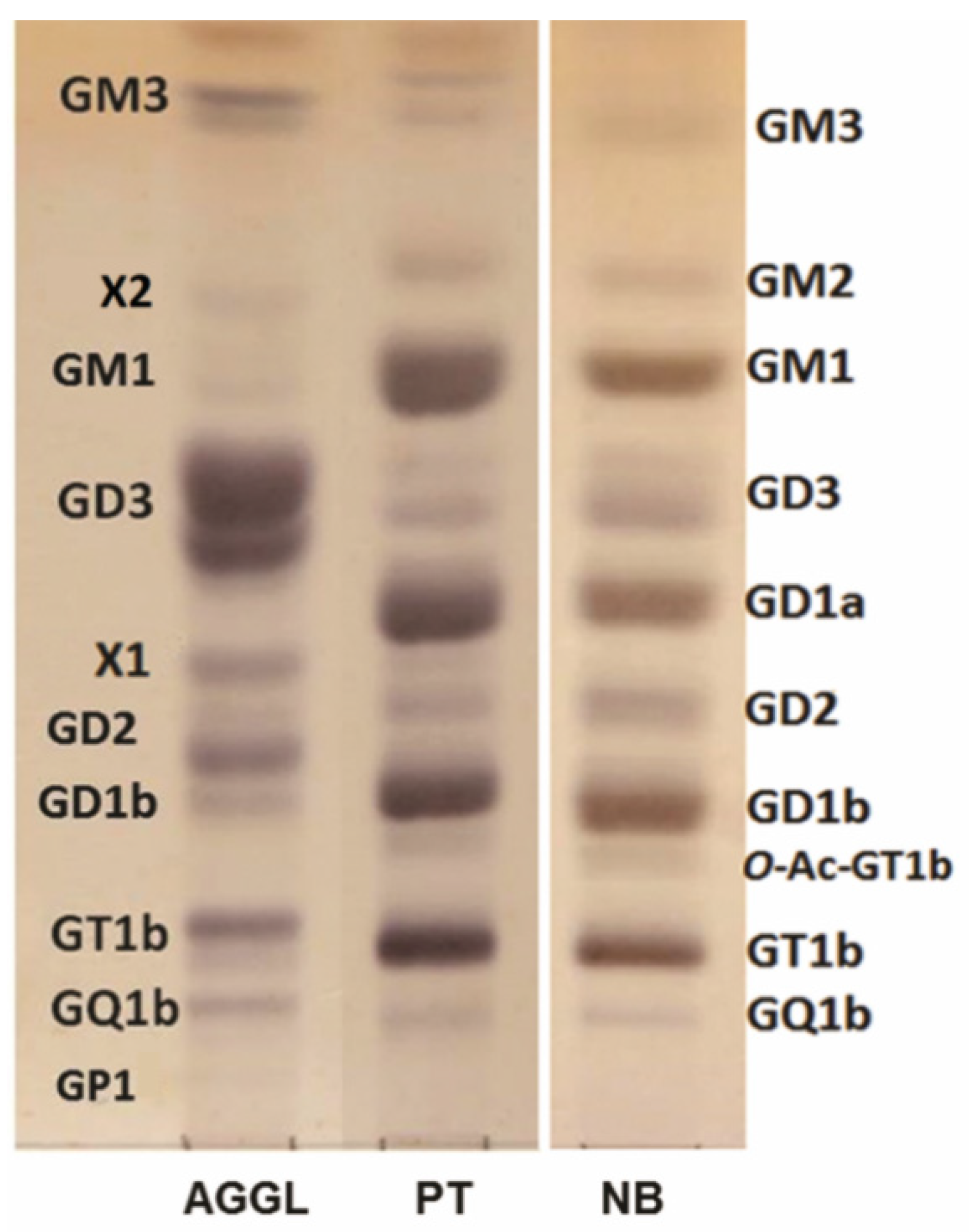

| Ganglioside Fractions (%) Migrating as: | AGGL | PT | NB |
|---|---|---|---|
| GM3 a | 6.2 | 1.3 | 3.1 |
| GM2 | n.d. | 4.7 | 2.2 |
| X2 | 1.5 | n.d. | n.d. |
| GM1 | 1.4 | 23.4 | 15.7 |
| GD3 a | 52.4 | 4.0 | 9.7 |
| GD1a | n.d. | 22.3 | 17.3 |
| X1 | 6.1 | n.d. | n.d. |
| GD2 a | 12.1 | 2.1 | 6.4 |
| GD1b | 4.0 | 19.4 | 20.0 |
| O-Ac-GT1b | n.d. | 1.5 | 2.8 |
| GT1b | 11.3 | 19.2 | 20.7 |
| GQ1b | 4.7 | 2.1 | 1.8 |
| GP1 | 0.4 | n.d. | n.d. |
| Sphingoid Base Type | m/z | Molecular Formula | m/z | Molecular Formula | ||||
|---|---|---|---|---|---|---|---|---|
| Theor. | Experim. | ppm | Theor. | Experim. | ppm | |||
| [M+H+]+ | [M+H+-H2O]+ | |||||||
| d18:1 | 300.2897 | 300.2896 | −0.4151 | C18H38O2N | 282.2786 | 282.2790 | −0.6380 | C18H36ON |
| d20:1 | 328.3210 | 328.3206 | −1.1829 | C20H42O2N | 310.3104 | 310.3102 | −0.9386 | C20H40ON |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabris, D.; Karmelić, I.; Muharemović, H.; Sajko, T.; Jurilj, M.; Potočki, S.; Novak, R.; Vukelić, Ž. Ganglioside Composition Distinguishes Anaplastic Ganglioglioma Tumor Tissue from Peritumoral Brain Tissue: Complementary Mass Spectrometry and Thin-Layer Chromatography Evidence. Int. J. Mol. Sci. 2021, 22, 8844. https://doi.org/10.3390/ijms22168844
Fabris D, Karmelić I, Muharemović H, Sajko T, Jurilj M, Potočki S, Novak R, Vukelić Ž. Ganglioside Composition Distinguishes Anaplastic Ganglioglioma Tumor Tissue from Peritumoral Brain Tissue: Complementary Mass Spectrometry and Thin-Layer Chromatography Evidence. International Journal of Molecular Sciences. 2021; 22(16):8844. https://doi.org/10.3390/ijms22168844
Chicago/Turabian StyleFabris, Dragana, Ivana Karmelić, Hasan Muharemović, Tomislav Sajko, Mia Jurilj, Slavica Potočki, Ruđer Novak, and Željka Vukelić. 2021. "Ganglioside Composition Distinguishes Anaplastic Ganglioglioma Tumor Tissue from Peritumoral Brain Tissue: Complementary Mass Spectrometry and Thin-Layer Chromatography Evidence" International Journal of Molecular Sciences 22, no. 16: 8844. https://doi.org/10.3390/ijms22168844
APA StyleFabris, D., Karmelić, I., Muharemović, H., Sajko, T., Jurilj, M., Potočki, S., Novak, R., & Vukelić, Ž. (2021). Ganglioside Composition Distinguishes Anaplastic Ganglioglioma Tumor Tissue from Peritumoral Brain Tissue: Complementary Mass Spectrometry and Thin-Layer Chromatography Evidence. International Journal of Molecular Sciences, 22(16), 8844. https://doi.org/10.3390/ijms22168844