Abstract
Temperature stress is one of the major abiotic stresses that adversely affect agricultural productivity worldwide. Temperatures beyond a plant’s physiological optimum can trigger significant physiological and biochemical perturbations, reducing plant growth and tolerance to stress. Improving a plant’s tolerance to these temperature fluctuations requires a deep understanding of its responses to environmental change. To adapt to temperature fluctuations, plants tailor their acclimatory signal transduction events, and specifically, cellular redox state, that are governed by plant hormones, reactive oxygen species (ROS) regulatory systems, and other molecular components. The role of ROS in plants as important signaling molecules during stress acclimation has recently been established. Here, hormone-triggered ROS produced by NADPH oxidases, feedback regulation, and integrated signaling events during temperature stress activate stress-response pathways and induce acclimation or defense mechanisms. At the other extreme, excess ROS accumulation, following temperature-induced oxidative stress, can have negative consequences on plant growth and stress acclimation. The excessive ROS is regulated by the ROS scavenging system, which subsequently promotes plant tolerance. All these signaling events, including crosstalk between hormones and ROS, modify the plant’s transcriptomic, metabolomic, and biochemical states and promote plant acclimation, tolerance, and survival. Here, we provide a comprehensive review of the ROS, hormones, and their joint role in shaping a plant’s responses to high and low temperatures, and we conclude by outlining hormone/ROS-regulated plant responsive strategies for developing stress-tolerant crops to combat temperature changes.
1. Introduction
In natural habitats, plants are often exposed to multiple stresses individually or in combination. These stressors, either biotic or abiotic, are potentially unfavorable, interfering with and attenuating the plant’s normal biological functions [1]. Temperature is one of the primary physical parameters that govern a plant’s health. Atmospheric temperatures have increased over the last two decades and are expected to continue to rise 1.0 to 1.7 °C by 2050 and 4.0 to 5.0 °C by the end of the 21st century, at which point, high temperature stress will become one of the most frequent abiotic stresses confronted by plants [2,3,4]. In contrast, low temperatures limit the geographical distribution of plant species and negatively affect their biological functions. In total, temperature extremes, together with other abiotic stresses, reduce average crop yields by more than 50% and continue to pose a risk to agricultural and forest production [5,6,7].
By definition, any fluctuation in temperature that induces irreversible damage to the plant’s metabolism or growth is considered stress and is categorized into high temperature or low-temperature stress [8]. High temperature stress results in many cellular and physiological changes, e.g., protein denaturation, loss of membrane integrity, reduced cellular function, and reduced plant growth. High temperatures also result in drought stress, another global issue. Drought and high temperature stress conditions individually or in their combination cause oxidative and osmotic stress, contributing to reduced plant growth and development [9,10].
The temperature is initially sensed by plant thermosensors, including calcium channels in the plasma membrane, histone sensors in the nucleus, reactive oxygen species (ROS) in the cell, and denatured proteins in the endoplasmic reticulum and cytosol. These sensors trigger signaling pathways that ultimately result in the expression of heat stress-responsive genes, contributing to plant thermotolerance [11,12]. In addition, plants have developed a wide range of survival strategies to overcome temperature changes. The ability of the plants to survive abrupt temperature increases is known as basal thermotolerance [11]. Plants also possess acclimatory responses known as acquired thermotolerance, where the pre-exposure to sublethal heat stimuli can aid in cellular reprogramming and increase the thermotolerance [13,14].
Low temperature stresses negatively impact plant growth and development by inhibiting the activity of the metabolic enzymes, altering the gene expression, and influencing the plant metabolism and transcriptome, thus delaying many developmental processes and growth [15]. Cold stress includes chilling stress (0–20 °C) and freezing stress (<0 °C), and stress tolerance varies depending on the plant species and temperature ranges [16]. Interestingly, high and low temperatures share some common responses, including changes in the membrane fluidity, alterations in the levels of plant hormones, the production and accumulation of ROS, calcium, nitric oxide (NO) signaling, Mitogen-activated protein kinase (MAPK) signaling, protein sumoylation and proteasomal degradation, the reprogramming of transcriptomic or metabolomic signatures, and other stress-responsive signaling cascades [17,18]. In contrast, some of the distinct signaling components in high- and low-temperature response pathways include the up- and downregulation of transcription factors (TFs) such as heat shock factor (HSF) family TFs and Inducer of CBF expression (ICE1) TFs that regulate the heat and cold stress responses, respectively, and the formation and accumulation of secondary metabolites, sugars, and amino acids.
Plant responses to abnormal or extreme temperature changes are primarily mediated by plant hormones that control and mediate complex stress-adaptive signaling cascades and induce heat or cold stress responses [19,20]. One of the mechanisms by which plant hormones induce thermotolerance is by inducing ROS production, activating NADPH oxidases, and/or by altering the redox signaling that regulates various cellular and physiological responses in response to temperature changes [21,22]. The extensive crosstalk between the ROS and hormone signaling pathways aids in plant development and acclimation responses (Figure 1) [22,23,24,25,26]. A few studies also suggest that ROS can mediate signaling crosstalk events between different hormones, resulting in improved thermotolerance [27,28,29,30]. In addition, plant hormones and ROS interact with other key signaling molecules or components, such as TFs, Ca2+, NO, kinases such as calcium-dependent protein kinase (CDPKs) and mitogen-activated protein kinases (MAPKs), and phosphatases, to orchestrate the required molecular and physiological responses to survive extreme temperatures and regulate plant developments [11,31,32,33]. Although several studies have demonstrated the role of ROS as secondary messengers in hormone signal transduction pathways, the in-depth and precise mechanisms of ROS and hormone interactions during temperature stress remain to be fully elucidated. In this review article, we highlight the recent and early studies on the plant tolerance to temperature changes, especially mediated through the underlying signal transduction pathways of the ROS, hormones, and their integration. From this perspective, we provide insights on strategies to improve crop tolerance to temperature stress that will aid in coping with the global temperature fluctuations.
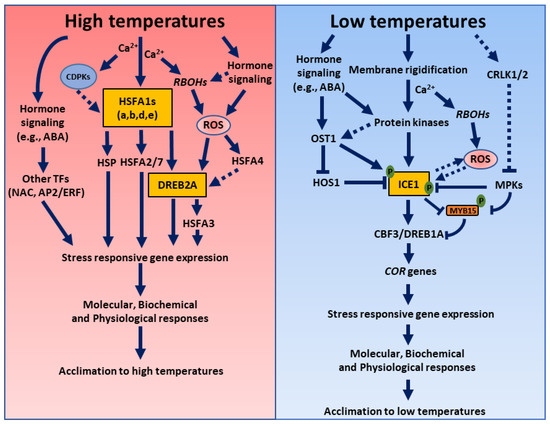
Figure 1.
A schematic model showing coordination between various signaling components to induce acclimation responses to temperatures stress. The interplay between the ROS; hormones; and other signaling components such as Ca2+, CDPKs, MAPKs, and TFs determines the plant’s molecular, biochemical, metabolic and physiological responses to high or low temperatures. Left panel: Heat stress affects the membrane fluidity and simultaneously triggers hormone signaling pathways, resulting in increased cytosolic calcium, reactive oxygen species, and the activation of other cytoplasmic proteins. The intercellular calcium activates CDPKs or RBOHs, which result in ROS production. Hormones and ROS, along with other signaling components, interdependently activate multiple signaling pathways to alter the activity of the heat shock factors (e.g., HSFA1), which, in turn, activate other transcription factors like Dehydration-Responsive Element-Binding Protein2a (DREB2A), inducing the appropriate stress-responsive gene expression. Right panel: Low temperatures trigger plasma membrane rigidification, and activate the Ca2+ channels, leading to increased Ca2+ concentrations in the cytosol. The cytosolic calcium activates protein kinases such as CDPKs that, in turn, phosphorylate the transcription factor ICEAlternatively, ICE1 can be phosphorylated by ABA-induced OSTICE1 activates the expression of the CBF genes by directly binding to their promoters at low temperatures, inducing the appropriate COR gene expression. Some of the common features between high- and low-temperature response pathways include changes in the membrane fluidity, alterations in the levels of plant hormones, the production and accumulation of ROS, calcium, NO and MAPK signaling, protein sumoylation and proteasomal degradation, the reprogramming of transcriptomic or metabolomic signatures, and other stress-responsive signaling cascades. Distinct signaling components in high- and low-temperature response pathways include TFs, proteins, stress-responsive genes, the formation and accumulation of secondary metabolites, sugars, amino acids, etc. ABA, abscisic acid; AP2/ERF, Apetala2/Ethylene Responsive Factor; Ca2+, calcium; CBF, C-repeat-binding factor; CDPK, calcium-dependent protein kinase; COR, cold-response genes; CRLK, calcium/calmodulin-regulated receptor-like kinases; DREBs, dehydration response element-binding factors; HOS1, HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENE1; HSP, heat shock proteins; HSF, HEAT SHOCK FACTOR; ICE1, inducer of CBF expression 1; MPKs, mitogen-activated protein kinase; MYB, Myb-like transcription factors; NAC, NAM, ATAF, and CUC family TF; OST1, OPEN STOMATA 1; RBOHs, respiratory burst oxidase homologs; ROS, reactive oxygen species; and SnRK2, SNF1-related protein kinase 2.
2. ROS Signaling
In contrast to its damaging effects and role in thermotolerance, ROS in their optimal levels serve as signaling molecules generated in response to stress perception by stress sensors [11,22,34]. As signaling molecules, ROS are distributed in all the metabolically active plant tissues and are controlled by the ROS gene network [11,35,36]. During temperature stress—specifically heat stress, although the chloroplast is the major site of ROS production in plants—the signaling ROS are known to be mediated by calcium or by the activation of NADPH oxidases at the plasma membrane and act as a heat stress signal transducer [36,37,38,39,40].
ROS, along with other signals such as Ca2+, are also involved in the long-distance systemic signaling required for the activation of systemic acquired acclimation in response to heat or other abiotic stresses [21,41,42,43,44,45]. For example, upon the perception of heat stress by sensors, plant hormones such as abscisic acid (ABA) and jasmonic acid (JA) trigger ROS production, which, in turn, activates the RBOH proteins in the neighboring cells to further generate ROS, initiating a systemic signal (the ROS wave) [38,46]. The hormone-triggered ROS move via cell-to-cell propagation, forming an amplification loop, and activate the systemic acquired acclimation responses [33,43,45]. Studies have shown that the local application of heat or cold stimuli induced similar stress transcriptional responses in both local and systemic tissues that were shown to be ROS wave-dependent [42,45,47]. Similarly, ROS activate calcium channels, which, in turn, activate the Two Pore Channel1 (TPC1), a vacuolar calcium channel that transports vacuolar-stored Ca2+, resulting in the activation of the respiratory burst oxidase homolog D (RBOHD) proteins [48]. This mechanism occurs in a feedback loop activating ROS and calcium, thus inducing a whole-plant acclimation response to high or low temperatures [36,41,42,45].
3. High Temperatures Stress
Heat stress affects the membrane fluidity, resulting in increased cytosolic calcium, which initiates downstream calcium signaling mediated by the plasma membrane-localized Cyclic Nucleotide Gated Channel (CNGC) family proteins or several Ca2+ sensors like calmodulins (CaMs), including CMLs (CaM-like proteins), calcineurin B-like proteins (CBLs), CDPKs, and Ca2+ binding proteins [49]. The Ca2+/CaM-binding protein kinase AtCBK3 participates in heat stress responses by targeting AtHSFA1a [50]. On the other hand, the HS-initiated Ca2+ signaling is transduced via RBOH proteins, initiating the ROS burst at the apoplast. The generated ROS by RBOHs is transported into the cell via the aquaporins to trigger the further release of Ca2+ by TPC1 channels to regulate different signaling and stress responses [21,51]. Thus, calcium-ROS signaling, along with other hormone signaling components, activates multiple downstream signaling pathways that regulate the heat shock transcription factors (HSFs) via post-translational modifications, thereby inducing heat stress responses (Figure 1).
3.1. Role of ROS during High Temperatures Stress
The perception of temperature changes by the plant receptors or sensors initiates several complex signaling networks that result in decreased membrane thermostability; higher malondialdehyde (MDA) accumulation; and the generation of ROS such as hydrogen peroxide (H2O2), singlet oxygen (1O2), superoxide (O2−), or hydroxyl radical [11,52]. In plants, ROS are mainly produced by the Respiratory Burst Oxidase Homolog (RBOH) proteins, the plasma membrane-localized NADPH oxidases, and several peroxidases that are controlled by the ROS gene network [11,35,36]. The ROS generated in plants are decoded by various ROS sensors, including Glutathione peroxidases (GPXs), plasma membrane-localized receptor-like kinases (RLKs), Cys-rich receptor-like kinases (CRKs), serine/threonine protein kinase (OXI1), cyclic nucleotide-gated channels activated by heat stress, and redox response transcription factors like HsfA4a and, thereafter, initiate stress-specific signals that induce the required gene expression and protein synthesis [11,53,54,55,56,57]. However, if uncontrolled, ROS overproduction results in oxidative stress that can damage proteins, biomolecules, and membranes, thereby affecting the photosynthetic machinery and overall leaf physiology [58,59,60]. For example, exposing chickpea or rice plants to heat stress of (32/20 °C Day/Night, 7 d) resulted in a 6.5-fold increase of H2O2 accumulation and resulted in oxidative damage compared to the controls [61,62]. Nevertheless, many studies have revealed the importance of ROS and its regulatory systems at various stages of plant development in response to heat stress [63,64]. ROS were shown to regulate the plant’s basal and acquired thermotolerance. In Arabidopsis, Davletova et al. and Miller et al. showed that mutants deficient in the antioxidant pathways, such as ascorbate peroxidase 1 (apx1), were defective in basal thermotolerance and showed a greater sensitivity to heat stress [65,66]. Davletova et al. (2005) also showed, using knockout (KO) mutants of cytosolic ascorbate peroxidase, an ROS scavenging enzyme and that heat shock transcription factor 21 (HSF21/AtHSFA4) is involved in H2O2 stress sensing, suggesting a close link between the HSF and ROS [65]. A few other major ROS responsive TFs include MYB domain protein 44 (MYB44), Heat Stress transcription factor A-4A (HSFA4A), and Ethylene Responsive Element-Binding Factor 6 (ERF6) [53,67,68,69,70,71]. Similarly, heat stress induction has been shown to trigger the expression of heat shock proteins HSP17.6 and HSP18.2 in Arabidopsis cell cultures. On the other hand, the application of ROS inhibitors such as diphenyleneiodonium chloride significantly reduced the HSP gene expression, suggesting that heat stress induced H2O2 is required for the effective expression of heat shock genes in Arabidopsis [70,72].
In response to the excess production of ROS under high temperatures, plants not only initiate heat stress (HS) responsive pathways but also induce expression of stress-related and antioxidant proteins that result in higher antioxidant activities of ascorbate peroxidase (APX), catalase (CAT), or superoxide dismutase (SOD), thereby reducing the negative effects of oxidative damage caused by ROS [35]. Studies using the KO mutants of ROS-scavenging enzymes were shown to be HS-sensitive, suggesting that they are necessary for the detoxification of excess ROS under high temperatures. Similarly, in tomatoes, the HS-induced negative effects of ROS, such as reduced growth, were reversed when treated with a ROS-scavenging antioxidant ascorbic acid. Studies using phyB mutants have suggested that the light priming of ROS detoxification via APX2 is a key component of thermotolerant adaptation [73,74,75]. Another study reported that an RNA-binding protein, flowering control locus A, is required to induce thermotolerance by triggering antioxidant accumulation under heat stress conditions [76]. Recent studies have also shown that miRNAs, such as miR398, regulate the heat response TF by negatively regulating several ROS-scavenging enzymes, resulting in an ROS accumulation that triggers HSFA1 to induce heat stress responses [77,78,79]. For instance, in rice, the NAC transcription factor gene SNAC3 (ONAC003, LOC_Os01g09550) modulates the H2O2 homeostasis by controlling the expression of ROS-associated genes, thus serving as a positive regulator under high temperatures [80]. In total, the evidence suggests that ROS-scavenging systems prevent an excess accumulation of toxic ROS and regulate the thermotolerance in plants [81,82]. Likewise, the studies using ROS biosynthesis mutants, such as NADPH oxidase (atrbohB and atrbohD), showed a reduced thermotolerance due to the lower levels of ROS, indicating that plants are required to maintain ROS at the optimal levels for attaining thermotolerance [63,83]. Similarly, studies have shown that priming plants with heat improved the antioxidant capacity, accumulated lower ROS, and delayed the ROS-induced cell death [84,85]. Heat priming also improved photosynthesis by enhancing stomatal conductance and by synthesizing secondary compounds with antioxidative characteristics that helped maintain the leaf membrane integrity under high temperature stress [86,87]. Taken together, these studies suggest that ROS regulate the trade-off between the growth, acclimation, or defense responses at altered temperatures [88,89].
3.2. Role of Hormones during High Temperatures Stress
The plant responses to abnormal or extreme temperature changes are primarily mediated by plant hormones, including abscisic acid (ABA), brassinosteroids (BRs), cytokinins (CKs), salicylic acid (SA), jasmonic acid (JA), and ethylene (ET). These hormones control and mediate complex stress-adaptive signaling cascades and induce stress-response TFs [19,20]. Various studies on the transcriptional regulation of heat and cold via TFs have uncovered both ABA-dependent and ABA-independent components and pathways, such as ABA-responsive element-binding protein/ABA-binding factor (AREB/ABF) or cold-binding factor/dehydration responsive element-binding transcription factors (CBF/DREB) [17,90,91,92].
3.2.1. Role of ABA and JA during High Temperatures
Phytohormone ABA is one of the extensively studied hormones involved in regulating heat stress responses and is required during most of the developmental stages to induce thermotolerance. Upon the perception of heat stress, ABA is released and perceived by the PYR/PYL receptors to form the ABA-PYR/PYL complex that interacts and suppresses the activity of protein phosphatase 2Cs (PP2Cs). This interaction releases (SnRK2)/Open Stomata 1 (OST1), a positive regulator of the ABA response. The released SnRK2s autophosphorylate and trigger the activity of different TFs, such as heat shock transcription factors (HSF), MYB1, or ABA-Responsive Promoter Elements (ABREs)-Binding Factors (ABFs) (AREB/ABFs), to induce heat stress responses (Figure 2) [21,93]. ABA is required during most of the developmental stages to induce thermotolerance. During the reproductive stage in Arabidopsis, ABA was shown to induce the SPL transcription factors conferring thermotolerance [94]. Heat stress induces a rapid increase in the endogenous ABA concentration and enhances the antioxidant ability to confer heat tolerance in plants [95,96,97]. For example, Arabidopsis and tomato mutant plants, deficient in ABA biosynthesis and signaling, exhibited heat-sensitive responses and had lower photochemical efficiency than the wild type [98]. Likewise, the plants treated with ABA showed a higher accumulation of H2O2 that mediated the induction of heat tolerance [99]. Microarray studies, using Arabidopsis, suggest that ABA can induce thermotolerance by inducing the expression of HSFA6b, a class A HSF [100]. In line with these findings, promoter mutagenesis analyses in wheat showed that transcriptional activator TaHsfC2a-B activates heat protection genes via the ABA-mediated pathway, and its overexpression improved the thermotolerance in wheat grains [97]. ABA also induces other TFs, like HSF6b and SQUAMOSA Promoter-Binding Proteinlike (SPL), required to acquire thermotolerance [94,100]. The activated HSF6b then directly binds to the promoter of Dehydration-Responsive Element-Binding Protein2a, enhancing its expression and, thus, inducing thermotolerance. Similarly, the TFs SPL1 and SPL12 confer thermotolerance via PYL-mediated ABA signaling [94,100].
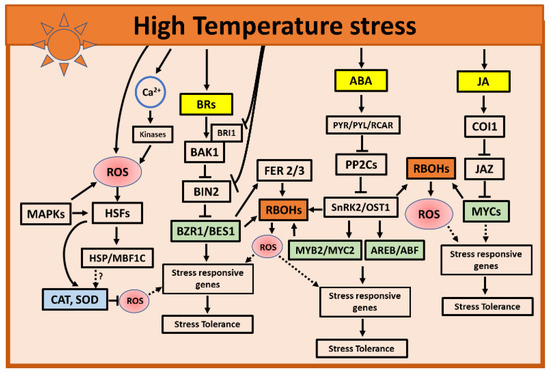
Figure 2.
Selected hormone and ROS interactions in response to high-temperature stress. During high temperatures, ABA-regulated kinases such as SnRK2 or TFs activate RBOHs to generate ROS. Similarly, in the BR-signaling pathway TF BZR1 activates RBOHs to generate ROS. Along with TFs, the generated ROS may activate and induce the expression of stress-responsive genes to orchestrate the appropriate molecular and physiological acclimation responses to high temperatures. Other plant hormones such as ET, SA, and CKs may also play roles in inducing stress responses to high temperatures. Only ABA, BRs, and JA were selected to be presented in the mode due to the availability of a relatively large amount of data supporting their roles in ROS interactions in response to high-temperature stress. Solid arrows and blocked arrows indicate confirmed positive and negative regulations. Dashed lines indicate potential interactions. The hormones are indicated in yellow, transcription factors in green, ROS in red, and ROS-producing and -scavenging enzymes are indicated in brown and blue, respectively. ABA, abscisic acid; AREB/ABF, ABA-responsive element-binding protein/ABA-binding factor; BR, brassinosteroid; BAK1, BRI1-ASSOCIATED KINASE1; BES1, BRI1-EMS SUPPRESSOR1; BIN2, brassinosteroid insensitive 2; BRI1, BRASSINOSTEROID INSENSITIVE1; BZR1, BRASSINAZOLE-RESISTANT1; Ca2+, calcium; CAT, catalase; COI1, CORONATINE-INSENSITIVE 1; FER, FERONIA; HSF, HEAT SHOCK FACTOR; HSP, heat shock proteins; JA, Jasmonic acid; JAZ, JASMONATE-ZIM-DOMAIN; MAPK, mitogen-activated protein kinase; MBF1C, MULTIPROTEIN BRIDGING FACTOR 1C; MYB/C, MYB/C transcription factors; OST1, OPEN STOMATA 1; PP2C, protein phosphatase 2Cs; PYR/PYL/RCAR, pyrabactin resistance protein or PYR-like proteins; RBOHs, respiratory burst oxidase homologs; ROS, reactive oxygen species; SnRK2, SNF1-related protein kinase 2; and SOD, superoxide dismutase.
Like ABA, plants have been shown to accumulate JA and its precursors OPDA or MeJA in response to heat stress [101,102]. Studies in JA biosynthesis or signaling mutants, such as jar1 or coi1, displayed lower heat tolerances, suggesting that JA is involved in regulating plants’ basal thermotolerance [103]. In contrast, pretreating Arabidopsis plants with MeJA upregulated the antioxidant enzyme activity and conferred heat tolerance, suggesting that jasmonic acid is required for acquiring thermotolerance [103,104]. At the molecular level, JA transcriptionally induces WRKY or MYC TFs to induce thermotolerance [102,105,106,107].
3.2.2. Role of BRs during High Temperatures
The perception of BR by its receptor BRASSINOSTEROID INSENSITIVE1 (BRI1) facilitates the interaction of the active BR1 with a coreceptor BRI1-ASSOCIATED KINASE1 (BAK1). This interaction positively regulates the BR-signaling components BRASSINAZOLE-RESISTANT1 (BZR1) and BRI1-EMS SUPPRESSOR1 (BES1) to enhance the expression of heat-responsive genes (Figure 2) [108]. For example, at high temperatures, the accumulation of BES1 and BZR1 increases, and BZR1 binds to the E-box and G-box elements of the PHYTOCHROME INTERACTING FACTOR 4 (PIF4) promotor to regulate its expression. This interaction induces the expression of growth-promoting genes, thus regulating the thermomorphogenesis [109]. Additionally, thermomorphogenic genes are activated by the abundant levels of the BES1-PIF4 complexes, highlighting the roles of BR-induced TFs in attaining thermotolerance [110,111]. A recent study in Arabidopsis also indicated that a loss-of-function of BRI1-EMS-SUPPRESSOR 1 (bes1)-mediated BR signaling, exhibited the most sensitive characteristics to heat stress, such as a lower PSII photochemistry efficiency (Fv/Fm), higher lipid hydroperoxide contents, higher photoinhibition, and photo-oxidative stress compared to the wild type [112]. In barley (Hordeum vulgare), the mutants deficient in BR biosynthesis and signaling negatively affected the accumulation of the HSP transcripts and heat-shock proteins, demonstrating the role of BRs in attaining plant acclimation to high temperatures [113,114]. In addition, BRs were shown to be involved in signaling crosstalks with other hormones to coordinate stress responses [23]. For example, BRs were shown to interact and enhance the endogenous level of ABA in Chlorella vulgaris by regulating ABA biosynthesis, thus enhancing the tolerance to heat [115,116]. Taken together, these results highlight the role of BR signaling in attaining heat stress acclimation in plants.
3.2.3. Role of ET during High Temperatures
Ethylene is another hormone that is involved in the heat stress response and modulates the plant growth and development through transcription factor ETHYLENE RESPONSE FACTOR 74 (ERF74). Studies using overexpression lines of ERF74 showed increased ROS-dependent dichlorofluorescein fluorescence and displayed enhanced basal thermotolerance, whereas the KO mutants accumulated lower ROS levels and showed reduced basal thermotolerance, suggesting that ERF74 possibly regulates the RBOHD expression at high temperatures [117]. In addition, it has been shown that erf74 and erf74;erf75 lines lack the ROS burst in the early stages of heat and other stresses as a result of the lower expression level of the RbohD proteins [117,118]. Together, these studies suggest that ERF74 acts as a switch to control the RBOHD-dependent mechanism to maintain H2O2 homeostasis and that the induction of a ROS-scavenging enzyme is dependent on the ERF74-RBOHD-ROS-signaling pathway [117,118]. Similarly, studies using the erf6 mutants suggest that the AP2/ERF domain-containing TF ERF6 regulates ROS signaling in Arabidopsis during heat and other abiotic stresses, indicating that ERFs regulate the stress tolerance by controlling ROS homeostasis in an RBOHD-dependent manner [118,119,120]. More recently, using ethylene signaling-defective mutants, the role of additional ethylene response factors, ERF95 and ERF97, involved in the basal thermotolerance of Arabidopsis were uncovered, suggesting that ERF95 and ERF97 genetically function downstream of EIN3 and that the ectopic, constitutive expression of ERF95 or ERF97 increases the basal thermotolerance [121]. It has been reported that ERF95 and ERF97 regulate the heat-responsive genes and directly bind to the promoter of HSFA2, thus establishing a connection between ethylene and its downstream regulation in the basal thermotolerance of plants [120,121].
3.2.4. Role of Salicylic Acid during High Temperatures
Salicylic acid has been reported to be a key signaling molecule in plants under high temperatures. Earlier studies suggest that SA is primarily involved in promoting the basal thermotolerance by inducing several HSPs [122]. The SA levels increase upon heat stress in plants, which were shown to improve the photosynthetic capacity by protecting the photosystem II (PSII) complex from higher levels of ROS during heat stress [123,124,125]. A few studies suggest that the exogenous application of SA increased the activity of antioxidant enzymes such as CAT and alleviated the heat-induced reduction in pollen viability and floret fertility by regulating the ROS level in developing anthers [126]. Another study showed that H2O2-mediated SA prevented the pollen abortion caused by heat stress [127]. In Medicago sativa L., SA treatments triggered the heat shock proteins in the organelles and improved the activity of PSII and altered the rate of electron transport, thereby reducing the damage caused by heat stress [128]. These findings indicate the role of SA in regulating the antioxidant defense system and improving the photosynthetic efficiency of plants, resulting in enhanced thermotolerance [126,127,128,129].
3.2.5. Role of CKs during High Temperatures
CKs are primarily involved in plant growth and developmental functions, including regulating the organ size, a role in the meristem activity of shoots and roots, and branching [130]. Although the exact nature of CKs during high temperatures is yet to be clarified, a few studies suggest their role in adaptive mechanisms during heat stress. Earlier studies found that the CK contents are generally reduced during high temperatures [131]. Recent studies indicated that heat stress induces a rapid but transient increase in active CK contents, followed by its depletion, signifying that CKs could serve as primary receptors in temperature sensing and serve as the first signal for thermomorphogenesis [132,133]. Transcriptomic and proteomic studies in Arabidopsis have indicated a large overlap of CK- and heat-responsive transcriptomic changes [132,133,134]. The exogenous application of CKs induced heat shock responses by upregulating the heat shock proteins, regulating several phosphoproteins and increasing the activity of the antioxidant system [132,133,134]. In Arabidopsis, the application of INCYDE (2-fluoro-6-(3-methoxyphenyl) aminopurine), an inhibitor of CK oxidase/dehydrogenase (CKX) enzymes, inhibited cytokinin degradation and mildly promoted the heat stress tolerance [135]. Taken together, these studies highlight the positive role of CKs during the high temperature response in plants [134,135].
3.3. Hormone and ROS Crosstalk during High Temperatures Stress
The ROS and hormonal signaling are tightly synchronized to regulate plant growth and development (Figure 1) [22,23,24,25,26]. At their optimal levels, the ROS and hormones are involved in the various developmental process, including seed germination, apoptosis, stomatal responses, root hair growth and elongation, and lignin synthesis, and their coordination is required to induce stress responses during high or low temperatures [21,136].
Once induced by heat stress, hormones alter the ROS levels, primarily by activating RBOHs and/or by altering redox signaling [21,22,23,32]. Nevertheless, ROS have been shown to act both upstream and downstream of the hormones, and the interplay between them can act as an amplification loop to control the gene expression and induce heat and other abiotic stress responses [21,23,54,137,138]. In addition, ROS can mediate the signaling crosstalk between different hormones. For example, in response to heat stress in tomatoes, the ROS produced via RBOHs were shown to mediate an interaction between ABA and BRs, resulting in an improved heat stress tolerance [28].
The transcriptional regulation mediated by hormones and/or ROS play a key role in mediating the heat stress responses in plants. ABA is reported as one of the early response hormones during high temperatures. ABA regulates its key TFs, AREB/ABFs, to mediate the ABA-dependent gene expression, which, in turn, influences the ROS production or enhances the antioxidant capacity, depending on the nature and severity of stress (Figure 2) [93,139]. ROS can act as a hormonal response signal, with ABA-induced ROS bursts emerging as an example [34,140,141]. Thus, the ROS regulatory systems and hormone signaling events need to be strictly coordinated to fine-tune the heat stress responses [34,141]. During heat stress, ABA also mediates HSP accumulation that, in turn, acts as a molecular chaperone to mediate heat tolerance by enhancing the scavenging of ROS and inducing the expression of HS-responsive genes [70,134]. Grafting studies in cucumbers showed that ABA-induced HSP accumulation occurred in an apoplastic ROS-dependent manner, indicating that inhibiting ROS production impaired the HSP accumulation and heat tolerance in the presence of ABA [98].
Plant development and heat stress tolerance are highly dependent on the interaction between BRs and ROS signaling. For example, similar to ABA, the BRs regulator BZR1 directly binds to the promoter of RBOH1, and RBOH1-mediated ROS triggers programmed cell death (PCD) and tapetal cell degradation [142,143]. In line with these observations, tomato plants overexpressing BZR1 showed an enhanced production of apoplastic H2O2 that interacts and binds to the promoters of FERONIA (FER 2/3) receptor-like kinase, resulting in heat stress tolerance [144]. During high temperatures stress, ROS mediate the signaling crosstalk between hormones, regulating the gene expression and inducing stress tolerance [69,71]. For example, the study by Zhou et al. showed that BRs-induced ROS mediated an interaction between ABA and BRs by activating RBOHs and resulted in an improved stress tolerance to heat in tomatoes [28].
The exact mechanisms by which SA induces ROS or ROS generated in the cells triggers SA biosynthesis remains unidentified. However, earlier studies indicate that SA inhibits the catalase activity and disturbs the cellular redox homeostasis, thus resulting in a higher ROS accumulation [145]. On the other hand, higher levels of SA were observed in plants with sustained ROS production in peroxisomes or the chloroplast [146]. Taken together, these results suggest that peroxisomes or chloroplast ROS trigger SA biosynthesis, which is required for the defense response [146]. The mutant analysis of SA biosynthesis or catalase-deficient mutants suggest that ROS can also function upstream of SA biosynthesis, suggesting that ROS and SA function in a self-amplifying feedback loop [23,54]. For example, by analyzing the mutants deficient in SA signaling (sid2), it was shown that the mutants accumulated higher ROS levels and showed tolerance to a combination of heat and drought stress [129,147]. Another study suggested that the exogenous application of SA increased the heat tolerance in tomatoes and barley by improving the antioxidant defense system through the scavenging of ROS [148,149]. Like SA, CKs are also proven to enhance the heat tolerance in plants. Although previous studies have shown that heat inhibits the cytokinin levels by downregulating histidine kinases genes, treating plants with CK upregulated antioxidant system activity triggered HSPs, which lead to heat tolerance [133,134].
4. Low Temperatures Stress
Sudden or extremely low temperatures cause adverse effects on a plant’s metabolism, growth, and stress responses [150]. Some of the adverse effects of cold stress include membrane rigidification, the modification of stability and degradation of proteins, a higher accumulation of toxic metabolites, a reduced efficiency of ROS scavenging enzymes, and a lower photosynthesis rate [151]. Low temperatures negatively affect the gene expression, protein synthesis, and stress-responsive transcripts. At extreme low temperatures (<0 °C), ice crystals can form, which influences a plant’s water potential, leading to an osmotic imbalance and oxidative stress, thereby impacting the normal cellular and metabolic processes of the cell [152,153,154].
Plants have evolved sophisticated perception, signaling, and acclimation strategies that allow their survival at low temperatures, even at the cost of reduced growth and yield [155]. Changes in the lipid and protein compositions of the cellular membranes, the accumulation of cryoprotective polypeptides, soluble sugars, higher antioxidant enzymes, higher hormone accumulation, anti-freezing proteins (AFPs), and cold shock proteins (CSPs) are common at low temperatures [156,157,158]. Low temperatures are also sensed by changes in the membrane fluidity, such as the reduction of cell membrane fluidity and activate cold-responsive Ca2+ channels like mid1-complementing activity 1 and 2 (MCA1 and MCA2), or other Ca2+ signatures that result in a rise of the cytosolic Ca2+ levels [159]. The higher Ca2+ levels lead to the activation of RBOHs, thereby generating ROS. For example, a recent study in tomato plants suggested that CDPKs like CDPK27 induce crosstalk between ROS and other signaling molecules, leading to the activation of ABA. This suggests that CDPKs may function as a positive regulator of hormones in plant adaptation to cold stress [160]. It should also be noted that, apart from CDPKs, CaM-regulated receptor-like kinases 1 (CRLK1) via interactions with calmodulin (CaM) were shown to be important for plant responses to cold stress [160,161]. Thus, calcium, along with other secondary metabolites and signals, including ROS, NO, or hormones, mediate the electrophysiological responses and initiate cold stress responses [151,162,163]. In most plant species, the cold response is primarily mediated by the hormone-induced C REPEAT-BINDING FACTOR- COLD RESPONSIVE (CBF-COR) signaling pathway, where CBF directly binds to the promoters of the COR genes and induces their expression, thus enhancing the freezing tolerance (Figure 1) [118]. These CBFs, in turn, regulate the expression of the genes involved in ROS detoxification, hormone metabolism, and other presumed cellular protective functions [17]. Early molecular studies revealed that an MYC-type transcription factor INDUCER OF CBF EXPRESSION (ICE1) regulates the activity of CBF/DREB1 genes [17,164], and these TFs and other downstream signaling components are regulated by a coordination between different hormones and ROS (Figure 1 and Figure 3).
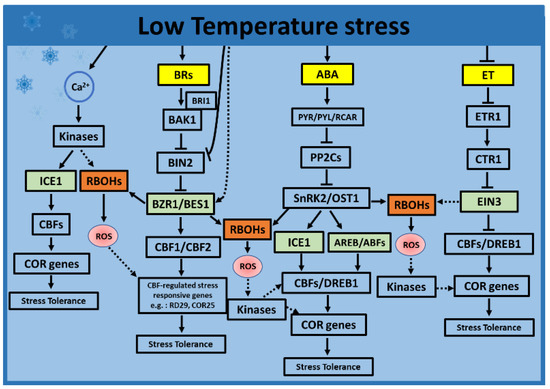
Figure 3.
Selected hormone and ROS interactions in response to low-temperature stress. During low temperatures, ABA-regulated kinase SnRK2 regulates the stability and transcriptional activity of ICE1, causing the activation of CBF/COR. Parallelly, SnRK2 could phosphorylate RBOHs, generating ROS. Similarly, TFs BZR1 and EIN3 possibly induce RBOHs to generate ROS via the BR and ET-signaling pathways, respectively. The ROS generated in a feedback loop could induce the expression of CBFs via kinases. Alternatively, low temperatures cause membrane rigidification, which might induce a calcium signature that activates kinases. The activated kinases could phosphorylate ICE1 or RBOHs, thus inducing the expression of COR genes to orchestrate the appropriate molecular and physiological acclimation responses to low temperatures. Other plant hormones such as JA and SA may also play roles in inducing the stress responses to low temperatures. Only ABA, BRs, and ET were selected to be presented in the mode due to the availability of a relatively large amount of data supporting their roles in ROS interactions in response to low-temperature stress. Solid arrows and blocked arrows indicate confirmed positive and negative regulations. Dashed lines indicate potential interactions. The hormones are indicated in yellow, transcription factors in green, ROS in red, and the ROS-producing and -scavenging enzymes are indicated in brown and blue, respectively. ABA, abscisic acid; AREB/ABF, ABA-responsive element-binding protein/ABA-binding factor; BR, brassinosteroid; BAK1, BRI1-ASSOCIATED KINASE1; BES1, BRI1-EMS SUPPRESSOR1; BIN2, brassinosteroid insensitive 2; BRI1, BRASSINOSTEROID INSENSITIVE1; BZR1, BRASSINAZOLE-RESISTANT1; Ca2+, calcium; CBF, C-repeat-binding factor; COR, cold-response genes; CTR1, CONSTITUTIVE TRIPLE RESPONSE1; DREB1, dehydration response element binding factor1; ET, ethylene; EIN3, ETHYLENE INSENSITIVE3; ETR1, ethylene receptors; ICE1, inducer of CBF expression 1; OST1, OPEN STOMATA 1; PP2C, protein phosphatase 2Cs; PYR/PYL/RCAR, pyrabactin resistance protein or PYR-like proteins; RBOHs, respiratory burst oxidase homologs; ROS, reactive oxygen species; and SnRK2, SNF1-related protein kinase 2.
4.1. Role of ROS during Low Temperatures Stress
Like in heat stress, low or freezing temperatures lead to ROS formation, and an excess accumulation of ROS in cell membranes induces oxidative stress. Low temperatures induce an enhanced rate of oxygenation reactions in the chloroplasts and produce a higher glycolate content. The glycolate is then converted to glyoxylate in the peroxisomes, producing H2O2 as a byproduct [89]. A higher accumulation of H2O2 results in activation of the ROS scavenging system through the conversion of GSH (reduced glutathione) to GSSG (oxidized glutathione) by the enzyme glutathione peroxidase (GPX) and glutathione S-transferase (GST), thus protecting plants from cold-induced oxidative stress [88,89].
In rice, the ROS-mediated gene expression and upregulation of antioxidant-related metabolites were shown to be involved in attaining chilling tolerance [165]. For example, metabolomic studies using rice varieties, such as japonica, indica, or nipponbare, at low temperatures showed a higher accumulation of MDA contents and H2O2 levels compared to their controls, revealing a ROS-dominated rice adaptation mechanism to low temperature environments [165]. Furthermore, the gene expression in response to cold stress may be regulated by redox signaling, as several defense genes containing antioxidant-responsive elements in their promoter regions [88]. More recently, the proteomics analysis in Brassica napus L. showed that the molecular mechanism of enhanced cold tolerance was achieved through ROS scavenging via the metabolic pathways [166].
4.2. Role of Hormones during Low Temperature Stress
4.2.1. Role of ABA during Low Temperatures
As in other abiotic stress responses, cold stress responses are mediated by both the individual and combined actions of plant hormones. ABA is one of the key hormones involved in cold stress acclimation in plants [17]. ABA can induce an increase in the transcript levels of CBF genes by binding to the CRT/DRE element and activating CBF or induce COR gene expression [167]. All signaling components of the ABA signaling pathways were shown to be important players in attaining a cold tolerance. For example, OPEN STOMATA1 (OST1), an SNF1-related protein kinases2 (SnRK2) protein kinase family member, is a central regulator of the cold signaling pathway in Arabidopsis. The cold activated protein kinase SnRK2/OST1 phosphorylates ICE1, which prevents ICE1 degradation mediated by HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENE15 (HOS1) and subsequently promotes the stability and binding of ICE to the CBF promoters, leading to COR gene expression, thereby enhancing the freezing tolerance (Figure 3) [168,169]. A mutant analysis indicated that the transgenic plants overexpressing OST1 exhibit an enhanced freezing tolerance, while the ost1 mutants exhibited a freezing hypersensitivity [168]. In parallel to these observations, the crosstalk of ABA-induced OST1 along with MAPKs was also shown to phosphorylate ICE1 to induce cold stress responses [170]. Likewise, during cold or ABA treatments, a type 2C phosphatase ABA INSENSITIVE1 (ABI1) was shown to partially inhibit OST1, and the loss-of-function mutants of abi1 showed an enhanced tolerance to freezing stress, highlighting the importance of protein phosphatases in inducing cold stress acclimation [168].
4.2.2. Role of JA during Low Temperatures
During low temperatures, the synthesized JA activates the JA receptor COI1 (CORONATINE-INSENSITIVE 1) and promotes the degradation of JAZ (JASMONATE-ZIM-DOMAIN) proteins via the ubiquitin/26S proteasome pathway. The negative regulator of the JA-signaling pathway, JAZ1, physically interacts and represses the transcriptional function of ICE1, activating the ICE-CBF transcriptional regulation cascade, followed by the expressions of cold-regulated genes to improve the plant freezing tolerance [15,171,172]. In addition, the increased expression of JA biosynthesis and signaling genes were observed in rice plants when they were exposed to cold treatment [173]. In contrast, the plants showed a hypersensitive response to freezing stress when the JA signaling pathway was inhibited [174]. Experiments using apple seedlings indicated that MIEL1 (MYB30-Interacting E3 Ligase1) and JAZ proteins coregulated the JA-mediated cold stress tolerance via the B-box protein BBX37-ICE1-CBF module that aided in cold stress tolerance [174]. Furthermore, JA or its precursors such as OPDA or MeJA are well-known to induce ROS production, and plants treated with MeJA showed a higher antioxidant capacity, thus enhancing the cold tolerance. More recently, it was shown that cyclic nucleotide-gated channels and glutamate receptor-like channels (GLRs) play a role in inducing cold stress acclimation by increasing the endogenous jasmonate levels under cold stress [175,176]. The plant hormone GA promotes the accumulation of DELLAs that regulate the induction of CBF3 through ICE1 via JAZs, suggesting a possible crosstalk between JA and GA in attaining an acclimation to cold [177].
4.2.3. Role of ET during Low Temperatures
The plant hormone ET acts as a negative regulator of cold stress responses (Figure 3) [178]. In the ET signaling pathway, one of the primary negative regulators of the CBF expression is the TF ETHYLENE INSENSITIVE3 (EIN3). The EIN3 is degraded by two F-box proteins (EIN3-BINDING F-BOX 1 (EBF1) and EBF2) via the 26S proteasome pathway enhancing the stability of PHYTOCHROME-INTERACTING FACTOR 3 (PIF3), thus repressing the activity of CBF genes during cold stress [179,180]. A transcriptomic study using ERF mutants in Arabidopsis showed that two genes, ERF102 and ERF103, are involved in the cold signaling pathway [181]. In addition, higher ROS accumulation in erf105 plants suggests a role for ERF105 in regulating ROS homeostasis, which is important for the cold stress response [17,182]. As in heat stress, miRNAs are also involved in cold stress tolerance via ET signaling. For example, reports on Poncirus trifoliata showed that ptr-miR396b positively regulates cold tolerance through reducing the 1-AMINOCYCLOPROPANE-1-CARBOXYLIC ACID OXIDASE (ACO) transcript levels and repressing ethylene synthesis and leads to enhanced ROS scavenging, thus inducing cold tolerance [165].
4.2.4. Role of BRs during Low Temperatures
In contrast to ET, the plant hormone BR positively regulates the freezing tolerance. Studies using BR signaling mutants in Arabidopsis showed that TFs BRZ1 and BES1 regulate the CBF expression by binding to the promoters of these genes, thus resulting in a freezing tolerance [108,183]. Since BRs are shown to be specific regulators of the CBF-COR pathway, these authors suggest that CBF genes may constitute a central node of hormone crosstalk during cold stress response [108]. In tomatoes, BRs were shown to positively regulate chilling tolerance via a signaling cascade involving RBOH1, GLUTAREDOXIN (GRX), and 2-Cys Prx. The BR-induced chilling tolerance was associated with an increase in the transcripts of the RBOH1 and GRX genes, increasing the ratio of reduced/oxidized 2-cysteine peroxiredoxin (2-Cys Prx) and activation of the antioxidant enzymes [184]. Furthermore, in response to chilling stress, BRs were shown to act as positive regulators of photoprotection by inducing ROS production. The leucine-rich repeat receptor-like kinase, BZR1, induces the transcription of an NADPH oxidase-encoding gene, RBOH1, leading to a higher accumulation of apoplastic H2O2. The presence of ROS induces a cyclic electron flow, non-photochemical quenching (NPQ), accumulation of photoprotective proteins, and increased activity of several antioxidant enzymes, thus inducing photoprotection under low temperatures [30]. Furthermore, the BR-induced production of antioxidant enzymes aid in maintaining the ROS homeostasis and induces stress tolerance following cold stress [23,185]. The exogenous application of BRs leads to enhanced antioxidant capacity, orchestrating the alleviation of ROS burst-induced oxidative damage [186]. The exogenous application of BRs also increases the expression of CBF genes that control a significant portion of COR genes and are required for the adequate development of cold acclimation response [183,187].
4.3. Hormone and ROS Crosstalk during Low Temperatures Stress
Some of the primary hormones inducing stress responses to low temperatures include ABA and BRs (Figure 3). The signaling components of these hormones, OST1 or BZR1/BES1, regulate the TF activity of ICE1 and induce the expression of CBF genes. ABA and BRs are known to trigger H2O2 production via the RBOHs, resulting in enhanced cold tolerance and acclimation response [17,27,29,108]. A recent study demonstrated that the exogenous application of ABA enhanced cold tolerance by increasing the antioxidant enzymes like CAT, SOD, and APX [188]. Likewise, in a BR signaling pathway, BZR1 was shown to activate RBOH1 resulting in the production of ROS [30,189]. In line with these observations, many studies indicated that priming plants with H2O2 improves plant’s antioxidant capacity and induces cold tolerance [27,190].
Cold stress increases the endogenous levels of JA in plants. During this process, the inducer of the ICE1-CBF regulatory cascade plays a key role in the regulation of cold stress tolerance [174]. JA has also been shown to counteract chilling stress by inducing ROS scavenging enzymes. Although the precise mechanism and pathways of the interaction between JA and ROS during low temperatures remains to be clarified, a few studies suggest that treatments of cold-stored lemon with MeJA together with SA increased its chilling tolerance by inhibiting the activity of polyphenol oxidase (PPO) and peroxidase (POD) [191,192,193].
Many ET-response TFs regulate ROS homeostasis and function in cold tolerance [194]. For instance, a study on ethylene-responsive factor in tobacco reported that overexpressing MfERF1 showed higher activities of antioxidants such as Zn-SOD, CAT1, CAT2, and resulted in increased tolerance to freezing and chilling in transgenic tobacco, whereas KO mutants showed reduced freezing tolerance. ET also induces or transcriptionally inhibits the expression of CBF/DREB1 via EIN3, a negative regulator of ethylene signaling (Figure 3) [178]. Parallel studies using ERF109 from Poncirus trifoliata (L.) showed that ERF109 binds to the promoter and regulates the expression of the POD-encoding gene (PtrPrx1), a class III peroxidase, to maintain a robust antioxidant capacity for effectively scavenging ROS, thus conferring cold tolerance [195]. Taken together, these studies highlight the role of hormones, ROS, and the intrinsic coordination between them, to induce tolerance to temperature stress. Plant hormones and ROS not only interact with each other, but also with other key signaling components, including NO, Ca2+, kinases, phosphatases, TFs, and various other metabolites, to orchestrate appropriate molecular, metabolic, and physiological acclimation responses that regulate acclimation responses to high or low-temperature stress.
5. Conclusions and Perspectives
To overcome the effects of temperature fluctuations, plants have evolved sophisticated acclimation mechanisms, including signal transduction pathways that activate stress-specific TFs, genes, signaling molecules, and enhance antioxidant activity. Plant hormones are developmental regulators that control many cellular, biochemical, and physiological responses throughout the plant life cycle. Stress perception by receptors triggers changes in the hormone levels, which activate stress-specific signal transduction cascades to regulate the expression of stress-specific TFs and their related genes. Part of their response also includes interaction with other hormones and many signaling molecules, including ROS. Hormones trigger ROS production (via RBOHs), and ROS, in turn, can impact hormone biosynthesis and allocation. The variations in the pattern of ROS/hormone signatures after early stages of stress are known to be one of the key processes influencing different stress response networks, thus attaining stress acclimation. Hence, identifying these hormone-ROS-regulated signaling pathways will aid in a better understanding of the molecular mechanism and help in developing improved stress-tolerant plants.
During temperature fluctuations, one of the most toxic byproducts of the plant’s metabolism is the generation of ROS. Paradoxically, ROS also serve as signaling molecules involved in the long-distance signaling and stress acclimation process. Due to its metabolic and toxic nature, many studies suggest that genetic manipulation of genes involved in ROS metabolism or antioxidants and scavenging enzymes could increase tolerance to temperatures stress. Alternatively, insights into the mechanism of thermal tolerance through high-throughput techniques point towards the importance of exogenous application of lower concentrations of ROS or stress-specific hormones to induce higher sustainability during extreme temperatures. Engineering plants to synthesize these compounds may offer an alternate way of increasing thermotolerance among important crop species. Thus, identifying the role of ROS and hormone integration in the avenue of stress combination is an area of potential future research. Increasing evidence shows that priming plants with heat can improve their photosynthetic capacity, stomatal conductance, and develop a higher antioxidant capacity to avoid ROS-induced damages. Lastly, biotechnology approaches including genome-wide association studies, quantitative trait loci mapping, transcriptomics, metabolomics, and proteomics, followed by selective breeding or genetic engineering, can greatly aid in identifying the nature of the signaling cascades and the utilization of specific genes in coping with high or low temperatures. Once these molecular principles have been established, conventional plant breeding approaches including backcrossing and pedigree, or population breeding can be employed to develop high-yielding and thermotolerant plants that can adapt to climate change.
With the rapid changes in global temperatures, temperature stress is expected to be one of the primary abiotic stresses determining plant health. Temperatures, either high or low, and in their extremes, adversely affect all developmental processes in plants, from seed germination to cell death. They also negatively influence the plant’s physiological processes, including transpiration, photosynthesis, respiration, and cellular structures. At the cellular level, they disrupt various signal transduction pathways and generate and alter the levels of toxic metabolites, resulting in lower crop yield or death. Plant responses to these temperature fluctuations include stress perception via thermal sensors, alterations in the signaling pathways affecting plant redox and hormone levels, and changes in the activity of stress-specific TFs and genes. Many studies have independently identified various mechanisms that govern a plant’s response to temperature changes. However, a complete understanding of temperature stress tolerance mechanisms involving plant hormones and secondary metabolites such as ROS had remained elusive. Here we have provided a comprehensive view of recent work on the roles of plant hormones, ROS, and their integration in achieving plant tolerance to high and low temperatures.
Author Contributions
A.R.D. drafted the manuscript. A.R.D., T.J.T., G.A.T., W.M., and J.-G.C. revised the manuscript. All authors contributed to the article and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Center for Bioenergy Innovation, the Plant-Microbe Interfaces Scientific Focus Area, and the Genomics-Enabled Plant Biology for Determination of Gene Function program by the Office of Biological and Environmental Research in the U.S. Department of Energy Office of Science. The Oak Ridge National Laboratory is managed by UT-Battelle, LLC for the U.S. Department of Energy under contract DE-AC05-00OR22725.
Notice
This manuscript has been authored by UT-Battelle, LLC under Contract No. DE-AC05-00OR22725 with the U.S. Department of Energy. The United States Government retains, and the publisher, by accepting the article for publication, acknowledges, that the United States Government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. The Department of Energy will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan accessed on 18 July 2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ma, X.; Su, Z.; Ma, H. Molecular genetic analyses of abiotic stress responses during plant reproductive development. J. Exp. Bot. 2020, 71, 2870–2885. [Google Scholar] [CrossRef] [PubMed]
- Horton, D.; Johnson, N.C.; Singh, D.; Swain, D.; Rajaratnam, B.; Diffenbaugh, N. Contribution of changes in atmospheric circulation patterns to extreme temperature trends. Nat. Cell Biol. 2015, 522, 465–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [Green Version]
- Delmotte, M.V.; Zhai, P.; Pörtner, H.O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Oki, W.; Péan, C.; Pidcock, R.; et al. (Eds.) IPCC Global Warming of 1.5 °C—An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways; IPPC: Geneva, Switzerland, 2018; Volume 2. [Google Scholar]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop. J. 2016, 4, 162–176. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Janni, M.; Gullì, M.; Maestri, E.; Marmiroli, M.; Valliyodan, B.; Nguyen, H.T.; Marmiroli, N. Molecular and genetic bases of heat stress responses in crop plants and breeding for increased resilience and productivity. J. Exp. Bot. 2020, 71, 3780–3802. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Moriondo, M.; Giannakopoulos, C.; Bindi, M. Climate change impact assessment: The role of climate extremes in crop yield simulation. Clim. Chang. 2011, 104, 679–701. [Google Scholar] [CrossRef]
- Quint, M.; Delker, C.; Franklin, K.A.; Wigge, P.; Halliday, K.; Van Zanten, M. Molecular and genetic control of plant thermomorphogenesis. Nat. Plants 2016, 2, 15190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Vu, L.D.; Gevaert, K.; De Smet, I. Feeling the Heat: Searching for Plant Thermosensors. Trends Plant Sci. 2019, 24, 210–219. [Google Scholar] [CrossRef]
- Liu, H.-C.; Charng, Y.-Y. Acquired thermotolerance independent of heat shock factor A1 (HsfA1), the master regulator of the heat stress response. Plant Signal. Behav. 2012, 7, 547–550. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Bautista, N.; Fernández-Calvino, L.; Muñoz, A.; Toribio, R.; Mock, H.P.; Castellano, M.M. HOP family plays a major role in long-term acquired thermotolerance in Arabidopsis. Plant Cell Environ. 2018, 41, 1852–1869. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Liu, D.; Chong, K. Cold signaling in plants: Insights into mechanisms and regulation. J. Integr. Plant Biol. 2018, 60, 745–756. [Google Scholar] [CrossRef] [Green Version]
- Chinnusamy, V.; Zhu, J.K.; Zhu, J.K. Cold Stress Regulation of Gene Expression in Plants. Trends Plant. Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Espinoza, V.A.; López-Climent, M.F.; Casaretto, J.A.; Egomez-Cadenas, A. Water Stress Responses of Tomato Mutants Impaired in Hormone Biosynthesis Reveal Abscisic Acid, Jasmonic Acid and Salicylic Acid Interactions. Front. Plant Sci. 2015, 6, 997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozga, J.A.; Kaur, H.; Savada, R.P.; Reinecke, D.M. Hormonal regulation of reproductive growth under normal and heat-stress conditions in legume and other model crop species. J. Exp. Bot. 2016, 68, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Blumwald, E. The Roles of ROS and ABA in Systemic Acquired Acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Mhamdi, A.; Van Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.-J.; Zhou, Y.-H.; Shi, K.; Zhou, J.; Foyer, C.H.; Yu, J.-Q. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 2015, 66, 2839–2856. [Google Scholar] [CrossRef] [Green Version]
- Mignolet-Spruyt, L.; Xu, E.; Idänheimo, N.; Hoeberichts, F.A.; Mühlenbock, P.; Brosche, M.; Van Breusegem, F.; Kangasjärvi, J. Spreading the News: Subcellular and Organellar Reactive Oxygen Species Production and Signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanojia, A.; Dijkwel, P.P. Abiotic Stress Responses are Governed by Reactive Oxygen Species and Age. Annu. Plant Rev. Online 2018, 1, 295–326. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Hormonal impact on photosynthesis and photoprotection in plants. Plant Physiol. 2021, 185, 1500–1522. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Shi, K.; Xia, X.J.; Zhou, Y.H.; Yu, J.Q. Hydrogen peroxide is involved in the cold acclimation-induced chilling tolerance of tomato plants. Plant Physiol. Biochem. 2012, 60, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, J.; Li, X.; Xia, X.-J.; Zhou, Y.-H.; Shi, K.; Chen, Z.; Yu, J.-Q. H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J. Exp. Bot. 2014, 65, 4371–4383. [Google Scholar] [CrossRef]
- Lv, B.; Tian, H.; Zhang, F.; Liu, J.; Lu, S.; Bai, M.; Li, C.; Ding, Z. Brassinosteroids regulate root growth by controlling reactive oxygen species homeostasis and dual effect on ethylene synthesis in Arabidopsis. PLoS Genet. 2018, 14, e1007144. [Google Scholar] [CrossRef]
- Fang, P.; Yan, M.; Chi, C.; Wang, M.; Zhou, Y.; Zhou, J.; Shi, K.; Xia, X.; Foyer, C.H.; Yu, J. Brassinosteroids Act as a Positive Regulator of Photoprotection in Response to Chilling Stress. Plant Physiol. 2019, 180, 2061–2076. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant. Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [Green Version]
- Kollist, H.; Zandalinas, S.I.; Sengupta, S.; Nuhkat, M.; Kangasjärvi, J.; Mittler, R. Rapid Responses to Abiotic Stress: Priming the Landscape for the Signal Transduction Network. Trends Plant. Sci. 2019, 24, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- I Zandalinas, S.; Sengupta, S.; Burks, D.; Azad, R.K.; Mittler, R. Identification and characterization of a core set of ROS wave-associated transcripts involved in the systemic acquired acclimation response of Arabidopsis to excess light. Plant J. 2019, 98, 126–141. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Fichman, Y.; Mittler, R. Rapid systemic signaling during abiotic and biotic stresses: Is the ROS wave master of all trades? Plant J. 2020, 102, 887–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The Plant NADPH Oxidase RBOHD Mediates Rapid Systemic Signaling in Response to Diverse Stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilroy, S.; Suzuki, N.; Miller, G.; Choi, W.-G.; Toyota, M.; Devireddy, A.R.; Mittler, R. A tidal wave of signals: Calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 2014, 19, 623–630. [Google Scholar] [CrossRef]
- Gilroy, S.; Białasek, M.; Suzuki, N.; Górecka, M.; Devireddy, A.R.; Karpiński, S.; Mittler, R. ROS, Calcium, and Electric Signals: Key Mediators of Rapid Systemic Signaling in Plants. Plant Physiol. 2016, 171, 1606–1615. [Google Scholar] [CrossRef]
- Karpinski, S.; Reynolds, H.; Karpinska, B.; Wingsle, G.; Creissen, G.; Mullineaux, P. Systemic Signaling and Acclimation in Response to Excess Excitation Energy in Arabidopsis. Science 1999, 284, 654–657. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Salazar, C.; Mondal, H.A.; Shulaev, E.; Cortes, D.F.; Shuman, J.L.; Luo, X.; Shah, J.; Schlauch, K.; et al. Temporal-Spatial Interaction between Reactive Oxygen Species and Abscisic Acid Regulates Rapid Systemic Acclimation in Plants. Plant Cell 2013, 25, 3553–3569. [Google Scholar] [CrossRef] [Green Version]
- Devireddy, A.R.; Arbogast, J.; Mittler, R. Coordinated and rapid whole-plant systemic stomatal responses. New Phytol. 2020, 225, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fichman, Y.; Devireddy, A.R.; Sengupta, S.; Azad, R.K.; Mittler, R. Systemic signaling during abiotic stress combination in plants. Proc. Natl. Acad. Sci. USA 2020, 117, 13810–13820. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Gorsuch, P.A.; Sargeant, A.W.; Penfield, S.D.; Quick, W.P.; Atkin, O.K. Systemic low temperature signaling in Arabidopsis. Plant Cell Physiol. 2010, 51, 1488–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, M.J.; Choi, W.-G.; Gilroy, S.; Morris, R.J. A ROS-Assisted Calcium Wave Dependent on the AtRBOHD NADPH Oxidase and TPC1 Cation Channel Propagates the Systemic Response to Salt Stress. Plant Physiol. 2016, 171, 1771–1784. [Google Scholar] [CrossRef] [Green Version]
- Finka, A.; Cuendet, A.F.H.; Maathuis, F.J.; Saidi, Y.; Goloubinoff, P. Plasma Membrane Cyclic Nucleotide Gated Calcium Channels Control Land Plant Thermal Sensing and Acquired Thermotolerance. Plant Cell 2012, 24, 3333–3348. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-T.; Gao, F.; Li, G.-L.; Han, J.-L.; Liu, D.-L.; Sun, D.-Y.; Zhou, R.-G. The calmodulin-binding protein kinase 3 is part of heat-shock signal transduction inArabidopsis thaliana. Plant J. 2008, 55, 760–773. [Google Scholar] [CrossRef]
- Tian, S.; Wang, X.; Li, P.; Wang, H.; Ji, H.; Xie, J.; Qiu, Q.; Shen, D.; Dong, H. Plant Aquaporin AtPIP1;4 Links Apoplastic H2O2 Induction to Disease Immunity Pathways. Plant Physiol. 2016, 171, 1635–1650. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.-H.; Domijan, M.; Klose, C.; Biswas, S.; Ezer, D.; Gao, M.; Khattak, A.K.; Box, M.S.; Charoensawan, V.; Cortijo, S.; et al. Phytochromes function as thermosensors inArabidopsis. Science 2016, 354, 886–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free. Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chaouch, S.; Mhamdi, A.; Queval, G.; Zechmann, B.; Noctor, G. Functional Analysis of Arabidopsis Mutants Points to Novel Roles for Glutathione in Coupling H2O2 to Activation of Salicylic Acid Accumulation and Signaling. Antioxidants Redox Signal. 2013, 18, 2106–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passaia, G.; Margis-Pinheiro, M. Glutathione peroxidases as redox sensor proteins in plant cells. Plant Sci. 2015, 234, 22–26. [Google Scholar] [CrossRef]
- König, J.; Muthuramalingam, M.; Dietz, K.-J. Mechanisms and dynamics in the thiol/disulfide redox regulatory network: Transmitters, sensors and targets. Curr. Opin. Plant Biol. 2012, 15, 261–268. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Narayanan, S.; Tamura, P.J.; Roth, M.R.; Prasad, P.V.; Welti, R. Wheat leaf lipids during heat stress: I. High day and night temperatures result in major lipid alterations. Plant Cell Environ. 2016, 39, 787–803. [Google Scholar] [CrossRef] [Green Version]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Awasthi, R.; Gaur, P.; Turner, N.; Vadez, V.; Siddique, K.; Nayyar, H. Effects of individual and combined heat and drought stress during seed filling on the oxidative metabolism and yield of chickpea (Cicer arietinum) genotypes differing in heat and drought tolerance. Crop. Pasture Sci. 2017, 68, 823–841. [Google Scholar] [CrossRef]
- Liu, J.; Hasanuzzaman, M.; Wen, H.; Zhang, J.; Peng, T.; Sun, H.; Zhao, Q. High temperature and drought stress cause abscisic acid and reactive oxygen species accumulation and suppress seed germination growth in rice. Protoplasma 2019, 256, 1217–1227. [Google Scholar] [CrossRef]
- Larkindale, J.; Hall, J.D.; Knight, M.; Vierling, E. Heat Stress Phenotypes of Arabidopsis Mutants Implicate Multiple Signaling Pathways in the Acquisition of Thermotolerance. Plant Physiol. 2005, 138, 882–897. [Google Scholar] [CrossRef] [Green Version]
- de Pinto, M.C.; Locato, V.; Paradiso, A.; De Gara, L. Role of redox homeostasis in thermo-tolerance under a climate change scenario: Fig. 1. Ann. Bot. 2015, 116, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Davletova, S.; Rizhsky, L.; Liang, H.; Shengqiang, Z.; Oliver, D.J.; Coutu, J.; Shulaev, V.; Schlauch, K.; Mittler, R. Cytosolic Ascorbate Peroxidase 1 Is a Central Component of the Reactive Oxygen Gene Network of Arabidopsis. Plant Cell 2005, 17, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, G.; Suzuki, N.; Rizhsky, L.; Hegie, A.; Koussevitzky, S.; Mittler, R. Double Mutants Deficient in Cytosolic and Thylakoid Ascorbate Peroxidase Reveal a Complex Mode of Interaction between Reactive Oxygen Species, Plant Development, and Response to Abiotic Stresses. Plant Physiol. 2007, 144, 1777–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakuma, Y.; Maruyama, K.; Qin, F.; Osakabe, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc. Natl. Acad. Sci. USA 2006, 103, 18822–18827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persak, H.; Pitzschke, A. Dominant Repression by Arabidopsis Transcription Factor MYB44 Causes Oxidative Damage and Hypersensitivity to Abiotic Stress. Int. J. Mol. Sci. 2014, 15, 2517–2537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohama, N.; Kusakabe, K.; Mizoi, J.; Zhao, H.; Kidokoro, S.; Koizumi, S.; Takahashi, F.; Ishida, T.; Yanagisawa, S.; Shinozaki, K.; et al. The Transcriptional Cascade in the Heat Stress Response of Arabidopsis Is Strictly Regulated at the Level of Transcription Factor Expression. Plant Cell 2016, 28, 181–201. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Howell, S. Heat Stress Responses and Thermotolerance in Maize. Int. J. Mol. Sci. 2021, 22, 948. [Google Scholar] [CrossRef]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional Regulatory Network of Plant Heat Stress Response. Trends Plant Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef]
- Volkov, R.; Panchuk, I.; Mullineaux, P.M.; Schöffl, F. Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol. Biol. 2006, 61, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-H.; Park, Y.-J.; Park, C.-M. Light Primes the Thermally Induced Detoxification of Reactive Oxygen Species During Development of Thermotolerance in Arabidopsis. Plant Cell Physiol. 2018, 60, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Legris, M.; Klose, C.; Burgie, E.S.; Rojas, C.C.R.; Neme, M.; Hiltbrunner, A.; Wigge, P.A.; Schäfer, E.; Vierstra, R.D.; Casal, J.J. Phytochrome B integrates light and temperature signals in Arabidopsis. Science 2016, 354, 897–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legris, M.; Nieto, C.; Sellaro, R.; Prat, S.; Casal, J.J. Perception and signalling of light and temperature cues in plants. Plant J. 2017, 90, 683–697. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.J.; Jung, J.H.; Park, C.M. The Arabidopsis Thaliana RNA-Binding Protein FCA Regulates Thermotolerance by Modulating the Detoxification of Reactive Oxygen Species. New Phytol. 2015, 205, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Lu, X.; Zeng, H.; Zhang, Y.; Zhu, J. Heat Stress Induction of MiR398 Triggers a Regulatory Loop That Is Critical for Thermotolerance in Arabidopsis. Plant. J. 2013, 74, 840–851. [Google Scholar] [CrossRef]
- Zhao, J.; He, Q.; Chen, G.; Wang, L.; Jin, B. Regulation of Non-coding RNAs in Heat Stress Responses of Plants. Front. Plant Sci. 2016, 7, 1213. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, X.; Yang, J.; He, Y. Natural antisense transcripts of MIR398 genes suppress microR398 processing and attenuate plant thermotolerance. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Fang, Y.; Liao, K.; Du, H.; Xu, Y.; Song, H.; Li, X.; Xiong, L. A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. J. Exp. Bot. 2015, 66, 6803–6817. [Google Scholar] [CrossRef] [Green Version]
- Kaya, H.; Nakajima, R.; Iwano, M.; Kanaoka, M.; Kimura, S.; Takeda, S.; Kawarazaki, T.; Senzaki, E.; Hamamura, Y.; Higashiyama, T.; et al. Ca2+-Activated Reactive Oxygen Species Production by Arabidopsis RbohH and RbohJ Is Essential for Proper Pollen Tube Tip Growth. Plant Cell 2014, 26, 1069–1080. [Google Scholar] [CrossRef] [Green Version]
- Muhlemann, J.K.; Younts, T.L.B.; Muday, G.K. Flavonols control pollen tube growth and integrity by regulating ROS homeostasis during high-temperature stress. Proc. Natl. Acad. Sci. USA 2018, 115, E11188–E11197. [Google Scholar] [CrossRef] [Green Version]
- Qi, C.; Lin, X.; Li, S.; Liu, L.; Wang, Z.; Li, Y.; Bai, R.; Xie, Q.; Zhang, N.; Ren, S.; et al. SoHSC70 positively regulates thermotolerance by alleviating cell membrane damage, reducing ROS accumulation, and improving activities of antioxidant enzymes. Plant Sci. 2019, 283, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Wollenweber, B.; Jiang, D. Multiple Heat Priming Enhances Thermo-Tolerance to a Later High Temperature Stress via Improving Subcellular Antioxidant Activities Inwheat Seedlings. Plant. Physiol. Biochem. 2014, 74, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ma, C.; Huang, Z.; Abid, M.; Jiang, S.; Dai, T.; Zhang, W.; Ma, S.; Jiang, D.; Han, X. Heat Priming During Early Reproductive Stages Enhances Thermo-Tolerance to Post-anthesis Heat Stress via Improving Photosynthesis and Plant Productivity in Winter Wheat (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 805. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, L.; Rusalepp, L.; Kaurilind, E.; Sulaiman, H.Y.; Püssa, T.; Niinemets, Ü. Heat priming improved heat tolerance of photosynthesis, enhanced terpenoid and benzenoid emission and phenolics accumulation in Achillea millefolium. Plant Cell Environ. 2021, 44, 2365–2385. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Li, X.; dos Santos, T.M.; Rosenqvist, E.; Ottosen, C.-O. Combined high light and heat stress induced complex response in tomato with better leaf cooling after heat priming. Plant Physiol. Biochem. 2020, 151, 1–9. [Google Scholar] [CrossRef]
- Kocsy, G.; Tóth, B.; Berzy, T.; Szalai, G.; Jednákovits, A.; Galiba, G. Glutathione reductase activity and chilling tolerance are induced by a hydroxylamine derivative BRX-156 in maize and soybean. Plant Sci. 2001, 160, 943–950. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Stockinger, E.J.; Gilmour, S.J.; Thomashow, M.F. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. USA 1997, 94, 1035–1040. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two Transcription Factors, DREB1 and DREB2, with an EREBP/AP2 DNA Binding Domain Separate Two Cellular Signal Transduction Pathways in Drought- and Low-Temperature-Responsive Gene Expression, Respectively, in Arabidopsis. Plant. Cell. 1998, 10, 1391–1406. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 2014, 21, 133–139. [Google Scholar] [CrossRef]
- Yoshida, T.; Obata, T.; Feil, R.; Lunn, J.E.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Fernie, A.R. The Role of Abscisic Acid Signaling in Maintaining the Metabolic Balance Required for Arabidopsis Growth under Nonstress Conditions. Plant Cell 2019, 31, 84–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, L.; Liu, Y.-Q.; Chen, D.-Y.; Xue, X.-Y.; Mao, Y.-B.; Chen, X.-Y. Arabidopsis Transcription Factors SPL1 and SPL12 Confer Plant Thermotolerance at Reproductive Stage. Mol. Plant 2017, 10, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, R.; Li, Y.; Wang, W.; Tai, F.; Xue, R.; Li, C. Heat shock protein 70 regulates the abscisic acid-induced antioxidant response of maize to combined drought and heat stress. Plant Growth Regul. 2009, 60, 225–235. [Google Scholar] [CrossRef]
- Hsieh, E.-J.; Cheng, M.-C.; Lin, T.-P. Functional characterization of an abiotic stress-inducible transcription factor AtERF53 in Arabidopsis thaliana. Plant Mol. Biol. 2013, 82, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-J.; Chen, D.; Mclntyre, C.L.; Dreccer, M.F.; Zhang, Z.-B.; Drenth, J.; Kalaipandian, S.; Chang, H.; Xue, G.-P. Heat shock factor C2a serves as a proactive mechanism for heat protection in developing grains in wheat via an ABA-mediated regulatory pathway. Plant Cell Environ. 2018, 41, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ahammed, G.J.; Zhang, Y.Q.; Zhang, G.Q.; Sun, Z.H.; Zhou, J.; Zhou, Y.H.; Xia, X.J.; Yu, J.Q.; Shi, K. Carbon dioxide enrichment alleviates heat stress by improving cellular redox homeostasis through an ABA-independent process in tomato plants. Plant Biol. 2015, 17, 81–89. [Google Scholar] [CrossRef]
- Driedonks, N.; Xu, J.; Peters, J.; Park, S.; Rieu, I. Multi-Level Interactions Between Heat Shock Factors, Heat Shock Proteins, and the Redox System Regulate Acclimation to Heat. Front. Plant Sci. 2015, 6, 999. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-C.; Niu, C.-Y.; Yang, C.-R.; Jinn, T.-L. The heat-stress factor HSFA6b connects ABA signaling and ABA-mediated heat responses. Plant Physiol. 2016, 172, 1182–1199. [Google Scholar] [CrossRef] [PubMed]
- Balfagón, D.; Zandalinas, S.I.; Gómez-Cadenas, A. High temperatures change the perspective: Integrating hormonal responses in citrus plants under co-occurring abiotic stress conditions. Physiol. Plant. 2019, 165, 183–197. [Google Scholar] [CrossRef]
- Monte, I.; Kneeshaw, S.; Franco-Zorrilla, J.M.; Chini, A.; Zamarreño, A.M.; García-Mina, J.M.; Solano, R. An Ancient COI1-Independent Function for Reactive Electrophilic Oxylipins in Thermotolerance. Curr. Biol. 2020, 30, 962–971.e3. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.M.; Cristescu, S.M.; Miersch, O.; Harren, F.; Wasternack, C.; Mur, L. Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytol. 2009, 182, 175–187. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Zahid, Z.; Mubarik, M.S.; Javed, R.; Siddiqui, M.H.; Hasanuzzaman, M. Jasmonic Acid: A Key Frontier in Conferring Abiotic Stress Tolerance in Plants. Plant. Cell Rep. 2020, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shiroto, Y.; Kishitani, S.; Ito, Y.; Toriyama, K. Enhanced heat and drought tolerance in transgenic rice seedlings overexpressing OsWRKY11 under the control of HSP101 promoter. Plant Cell Rep. 2009, 28, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, X.; Chen, L.; Huang, W.; Yu, D. Functional characterization of Arabidopsis thaliana WRKY39 in heat stress. Mol. Cells 2010, 29, 475–483. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Li, C.; Wang, H.; Guo, Z. WRKY transcription factors: Evolution, binding, and action. Phytopathol. Res. 2019, 1, 13. [Google Scholar] [CrossRef]
- Li, H.; Ye, K.; Shi, Y.; Cheng, J.; Zhang, X.; Yang, S. BZR1 Positively Regulates Freezing Tolerance via CBF-Dependent and CBF-Independent Pathways in Arabidopsis. Mol. Plant 2017, 10, 545–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibañez, C.; Delker, C.; Martinez, C.; Bürstenbinder, K.; Janitza, P.; Lippmann, R.; Ludwig, W.; Sun, H.; James, G.V.; Klecker, M.; et al. Brassinosteroids Dominate Hormonal Regulation of Plant Thermomorphogenesis via BZR1. Curr. Biol. 2018, 28, 303–310.e3. [Google Scholar] [CrossRef]
- Martínez, C.; Espinosa-Ruíz, A.; Lucas, M.; Bernardo-García, S.; Franco-Zorrilla, J.M.; Prat, S. PIF 4-induced BR Synthesis Is Critical to Diurnal and Thermomorphogenic Growth. EMBO J. 2018, 37, e99552. [Google Scholar] [CrossRef]
- Lv, M.; Li, J. Molecular Mechanisms of Brassinosteroid-Mediated Responses to Changing Environments in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 2737. [Google Scholar] [CrossRef] [Green Version]
- Setsungnern, A.; Muñoz, P.; Pérez-Llorca, M.; Müller, M.; Thiravetyan, P.; Munné-Bosch, S. A defect in BRI1-EMS-SUPPRESSOR 1 (bes1)-mediated brassinosteroid signaling increases photoinhibition and photo-oxidative stress during heat stress in Arabidopsis. Plant Sci. 2020, 296, 110470. [Google Scholar] [CrossRef]
- Sadura, I.; Janeczko, A. Physiological and molecular mechanisms of brassinosteroid-induced tolerance to high and low temperature in plants. Biol. Plant. 2018, 62, 601–616. [Google Scholar] [CrossRef] [Green Version]
- Sadura, I.; Libik-Konieczny, M.; Jurczyk, B.; Gruszka, D.; Janeczko, A. HSP Transcript and Protein Accumulation in Brassinosteroid Barley Mutants Acclimated to Low and High Temperatures. Int. J. Mol. Sci. 2020, 21, 1889. [Google Scholar] [CrossRef] [Green Version]
- Bajguz, A. Isolation and characterization of brassinosteroids from algal cultures of Chlorella vulgaris Beijerinck (Trebouxiophyceae). J. Plant Physiol. 2009, 166, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, J.; Ye, N.; Zhang, H.; Tan, M.; Jiang, M. Nitric Oxide Mediates Brassinosteroid-Induced ABA Biosynthesis Involved in Oxidative Stress Tolerance in Maize Leaves. Plant Cell Physiol. 2010, 52, 181–192. [Google Scholar] [CrossRef]
- Yao, Y.; He, R.J.; Xie, Q.L.; Zhao, X.H.; Deng, X.M.; He, J.B.; Song, L.; He, J.; Marchant, A.; Chen, X.Y.; et al. ETHYLENE RESPONSE FACTOR 74 (ERF74) Plays an Essential Role in Controlling a Respiratory Burst Oxidase Homolog D (RbohD)-Dependent Mechanism in Response to Different Stresses in Arabidopsis. New Phytol. 2017, 213, 1667–1681. [Google Scholar] [CrossRef]
- Xie, Z.; Nolan, T.; Jiang, H.; Yin, Y. AP2/ERF Transcription Factor Regulatory Networks in Hormone and Abiotic Stress Responses in Arabidopsis. Front. Plant Sci. 2019, 10, 228. [Google Scholar] [CrossRef] [Green Version]
- Sewelam, N.; Kazan, K.; Thomas-Hall, S.R.; Kidd, B.N.; Manners, J.M.; Schenk, P.M. Ethylene Response Factor 6 Is a Regulator of Reactive Oxygen Species Signaling in Arabidopsis. PLoS ONE 2013, 8, e70289. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.; Munné-Bosch, S. Ethylene Response Factors: A Key Regulatory Hub in Hormone and Stress Signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Zhao, X.; Bürger, M.; Wang, Y.; Chory, J. Two interacting ethylene response factors regulate heat stress response. Plant Cell 2021, 33, 338–357. [Google Scholar] [CrossRef]
- Clarke, S.; Mur, L.; Wood, J.E.; Scott, I.M. Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance inArabidopsis thaliana. Plant J. 2004, 38, 432–447. [Google Scholar] [CrossRef]
- Wang, L.-J.; Fan, L.; Loescher, W.; Duan, W.; Liu, G.-J.; Cheng, J.-S.; Luo, H.-B.; Li, S.-H. Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol. 2010, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, H.; Hou, P.; Su, X.; Zhao, P.; Zhao, H.; Liu, S. Foliar-applied salicylic acid alleviates heat and high light stress induced photoinhibition in wheat (Triticum aestivum) during the grain filling stage by modulating the psbA gene transcription and antioxidant defense. Plant Growth Regul. 2014, 73, 289–297. [Google Scholar] [CrossRef]
- Duan, J.; Lee, K.P.; Dogra, V.; Zhang, S.; Liu, K.; Caceres-Moreno, C.; Lv, S.; Xing, W.; Kato, Y.; Sakamoto, W.; et al. Impaired PSII Proteostasis Promotes Retrograde Signaling via Salicylic Acid. Plant Physiol. 2019, 180, 2182–2197. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhou, L.; Liu, J.; Cao, Z.; Du, X.; Huang, F.; Pan, G.; Cheng, F. Involvement of CAT in the detoxification of HT-induced ROS burst in rice anther and its relation to pollen fertility. Plant Cell Rep. 2018, 37, 741–757. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, C.; Chen, T.; Zhang, X.; Tao, L.; Fu, G. Salicylic acid reverses pollen abortion of rice caused by heat stress. BMC Plant Biol. 2018, 18, 245. [Google Scholar] [CrossRef]
- Wassie, M.; Zhang, W.; Zhang, Q.; Ji, K.; Cao, L.; Chen, L. Exogenous salicylic acid ameliorates heat stress-induced damages and improves growth and photosynthetic efficiency in alfalfa (Medicago sativa L.). Ecotoxicol. Environ. Saf. 2020, 191, 110206. [Google Scholar] [CrossRef]
- Rai, K.; Pandey, N.; Rai, S.P. Salicylic acid and nitric oxide signaling in plant heat stress. Physiol. Plant. 2019, 168, 241–255. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef] [Green Version]
- Hare, P.D.; Cress, W.A.; Van Staden, J. The involvement of cytokinins in plant responses to environmental stress. Plant Growth Regul. 1997, 23, 79–103. [Google Scholar] [CrossRef]
- Černý, M.; Jedelský, P.L.; Novák, J.; Schlosser, A.; Brzobohatý, B. Cytokinin Modulates Proteomic, Transcriptomic and Growth Responses to Temperature Shocks in Arabidopsis. Plant. Cell Environ. 2014, 37, 1641–1655. [Google Scholar] [CrossRef] [PubMed]
- Skalák, J.; Cerný, M.; Jedelský, P.; Dobrá, J.; Ge, E.; Novák, J.; Hronková, M.; Dobrev, P.; Vanková, R.; Brzobohatý, B. Stimulation of Ipt Overexpression as a Tool to Elucidate the Role of Cytokinins in High Temperature Responses of Arabidopsis Thaliana. J. Exp. Bot. 2016, 67, 2861–2873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmülling, T. Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environ. 2019, 42, 998–1018. [Google Scholar] [CrossRef]
- Prerostova, S.; Dobrev, P.I.; Kramna, B.Z.; Gaudinova, A.; Knirsch, V.; Spichal, L.; Zatloukal, M.; Vankova, R. Heat Acclimation and Inhibition of Cytokinin Degradation Positively Affect Heat Stress Tolerance of Arabidopsis. Front. Plant Sci. 2020, 11, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talaat, N.B. Role of reactive oxygen species signaling in plant growth and development. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; Wiley: New York, NY, USA, 2019; pp. 225–266. [Google Scholar] [CrossRef]
- Tognetti, V.B.; Mühlenbock, P.; Van Breusegem, F. Stress homeostasis—The redox and auxin perspective. Plant Cell Environ. 2012, 35, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Prodhan, M.Y.; Munemasa, S.; Nahar, M.N.-E.-N.; Nakamura, Y.; Murata, Y. Guard cell salicylic acid signaling is integrated into abscisic acid signaling via the Ca2+/CPK-dependent pathway. Plant Physiol. 2018, 178, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA Transport and Plant Water Stress Responses. Trends Plant Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, P.; Singh, S.; Mishra, R.K.; Singh, V.P.; Prasad, S.M. Reactive oxygen species signaling and stomatal movement: Current updates and future perspectives. Redox Biol. 2017, 11, 213–218. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Tian, Y.; Fan, M.; Qin, Z.; Lv, H.; Wang, M.; Zhang, Z.; Zhou, W.; Zhao, N.; Li, X.; Han, C.; et al. Hydrogen peroxide positively regulates brassinosteroid signaling through oxidation of the BRASSINAZOLE-RESISTANT1 transcription factor. Nat. Commun. 2018, 9, 1063. [Google Scholar] [CrossRef]
- Yan, M.; Xie, D.; Cao, J.; Xia, X.; Shi, K.; Zhou, Y.; Zhou, J.; Foyer, C.H.; Yu, J. Brassinosteroid-mediated reactive oxygen species are essential for tapetum degradation and pollen fertility in tomato. Plant J. 2020, 102, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Qin, K.; Song, X.; Zhang, Q.; Zhou, Y.; Xia, X.; Yu, J. BZR1 Transcription Factor Regulates Heat Stress Tolerance Through FERONIA Receptor-Like Kinase-Mediated Reactive Oxygen Species Signaling in Tomato. Plant Cell Physiol. 2018, 59, 2239–2254. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Silva, H.; Klessig, D. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 1993, 262, 1883–1886. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Vã¡squez, A.; Salinas, P.; Holuigue, L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front. Plant Sci. 2015, 6, 171. [Google Scholar] [CrossRef] [Green Version]
- Kumazaki, A.; Suzuki, N. Enhanced tolerance to a combination of heat stress and drought in Arabidopsis plants deficient in ICS1 is associated with modulation of photosynthetic reaction center proteins. Physiol. Plant. 2018, 165, 232–246. [Google Scholar] [CrossRef]
- Jahan, M.S.; Wang, Y.; Shu, S.; Zhong, M.; Chen, Z.; Wu, J.; Sun, J.; Guo, S. Exogenous salicylic acid increases the heat tolerance in Tomato (Solanum lycopersicum L.) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci. Hortic. 2019, 247, 421–429. [Google Scholar] [CrossRef]
- Torun, H.; Novák, O.; Mikulík, J.; Pěnčík, A.; Strnad, M.; Ayaz, F.A. Timing-dependent effects of salicylic acid treatment on phytohormonal changes, ROS regulation, and antioxidant defense in salinized barley (Hordeum vulgare L.). Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Liu, J.; Shi, Y.; Yang, S. Insights into the regulation of C-repeat binding factors in plant cold signaling. J. Integr. Plant Biol. 2018, 60, 780–795. [Google Scholar] [CrossRef]
- Rihan, H.Z.; Al-Issawi, M.; Fuller, M. Advances in physiological and molecular aspects of plant cold tolerance. J. Plant Interactions 2017, 12, 143–157. [Google Scholar] [CrossRef]
- Liu, H.; Yu, C.; Li, H.; Ouyang, B.; Wang, T.; Zhang, J.; Wang, X.; Ye, Z. Overexpression of ShDHN, a dehydrin gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses in tomato. Plant Sci. 2015, 231, 198–211. [Google Scholar] [CrossRef]
- Shi, Y.; Ding, Y.; Yang, S. Molecular Regulation of CBF Signaling in Cold Acclimation. Trends Plant Sci. 2018, 23, 623–637. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Xiao, J.; Bao, F. Effects of Chilling on the Structure, Function and Development of Chloroplasts. Front. Plant Sci. 2018, 9, 1715. [Google Scholar] [CrossRef] [Green Version]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Schulz, E.; Tohge, T.; Zuther, E.; Fernie, A.R.; Hincha, D.K. Natural variation in flavonol and anthocyanin metabolism during cold acclimation in Arabidopsis thaliana accessions. Plant Cell Environ. 2015, 38, 1658–1672. [Google Scholar] [CrossRef]
- Hoermiller, I.I.; Nägele, T.; Augustin, H.; Stutz, S.; Weckwerth, W.; Heyer, A.G. Subcellular reprogramming of metabolism during cold acclimation in Arabidopsis thaliana. Plant Cell Environ. 2017, 40, 602–610. [Google Scholar] [CrossRef]
- Leuendorf, J.E.; Frank, M.; Schmülling, T. Acclimation, priming and memory in the response of Arabidopsis thaliana seedlings to cold stress. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, K.; Renhu, N.; Naito, M.; Nakamura, A.; Shiba, H.; Yamamoto, T.; Suzaki, T.; Iida, H.; Miura, K. Ca2+-permeable mechanosensitive channels MCA1 and MCA2 mediate cold-induced cytosolic Ca2+ increase and cold tolerance in Arabidopsis. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Lv, X.; Li, H.; Chen, X.; Xiang, X.; Guo, Z.; Yu, J.; Zhou, Y. The role of calcium-dependent protein kinase in hydrogen peroxide, nitric oxide and ABA-dependent cold acclimation. J. Exp. Bot. 2018, 69, 4127–4139. [Google Scholar] [CrossRef]
- Shi, Y.; Ding, Y.; Yang, S. Cold Signal Transduction and its Interplay with Phytohormones During Cold Acclimation. Plant Cell Physiol. 2015, 56, 7–15. [Google Scholar] [CrossRef]
- Knight, M.; Knight, H. Low-temperature perception leading to gene expression and cold tolerance in higher plants. New Phytol. 2012, 195, 737–751. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. COLD1 Confers Chilling Tolerance in Rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef] [Green Version]
- Zarka, D.G.; Vogel, J.; Cook, D.; Thomashow, M.F. Cold Induction of Arabidopsis CBF Genes Involves Multiple ICE (Inducer of CBF Expression) Promoter Elements and a Cold-Regulatory Circuit That Is Desensitized by Low Temperature. Plant Physiol. 2003, 133, 910–918. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Luo, W.; Zhao, Y.; Xu, Y.; Song, S.; Chong, K. Comparative metabolomic analysis reveals a reactive oxygen species-dominated dynamic model underlying chilling environment adaptation and tolerance in rice. New Phytol. 2016, 211, 1295–1310. [Google Scholar] [CrossRef]
- Mi, W.; Liu, Z.; Jin, J.; Dong, X.; Xu, C.; Zou, Y.; Xu, M.; Zheng, G.; Cao, X.; Fang, X.; et al. Comparative proteomics analysis reveals the molecular mechanism of enhanced cold tolerance through ROS scavenging in winter rapeseed (Brassica napus L.). PLoS ONE 2021, 16, e0243292. [Google Scholar] [CrossRef] [PubMed]
- Knight, H.; Zarka, D.G.; Okamoto, H.; Thomashow, M.F.; Knight, M.R. Abscisic Acid Induces CBF Gene Transcription and Subsequent Induction of Cold-Regulated Genes via the CRT Promoter Element. Plant Physiol. 2004, 135, 1710–1717. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Li, H.; Zhang, X.; Xie, Q.; Gong, Z.; Yang, S. OST1 Kinase Modulates Freezing Tolerance by Enhancing ICE1 Stability in Arabidopsis. Dev. Cell 2015, 32, 278–289. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Jia, Y.; Shi, Y.; Zhang, X.; Song, C.; Gong, Z.; Yang, S. OST 1-mediated BTF 3L phosphorylation positively regulates CBF s during plant cold responses. EMBO J. 2018, 37, e98228. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ding, Y.; Shi, Y.; Zhang, X.; Zhang, S.; Gong, Z.; Yang, S. MPK3- and MPK6-Mediated ICE1 Phosphorylation Negatively Regulates ICE1 Stability and Freezing Tolerance in Arabidopsis. Dev. Cell 2017, 43, 630–642.e4. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, L.; Wang, F.; Yu, D. Jasmonate Regulates the INDUCER of CBF expression–C-repeat binding factor/dre binding Factor1 Cascade and Freezing Tolerance in Arabidopsis. Plant Cell 2013, 25, 2907–2924. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Jiang, Y.; Han, X.; Wang, H.; Pan, J.; Yu, D. Jasmonate regulates leaf senescence and tolerance to cold stress: Crosstalk with other phytohormones. J. Exp. Bot. 2017, 68, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, H.; Xiong, L. Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front. Plant Sci. 2013, 4, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, J.; Wang, X.; Zhang, X.; You, C.; Hao, Y. Apple B-box Protein BBX37 Regulates Jasmonic Acid Mediated Cold Tolerance through the JAZ-BBX37-ICE1-CBF Pathway and Undergoes MIEL1-mediated Ubiquitination and Degradation. New Phytol. 2021, 229, 2707–2729. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Luo, L.; Wei, J.; Chen, Q.; Yang, Y.; Hu, X.; Kong, X. The Glutamate Receptors AtGLR1.2 and AtGLR1.3 Increase Cold Tolerance by Regulating Jasmonate Signaling in Arabidopsis Thaliana. Biochem. Biophys. Res. Commun. 2018, 506, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, X.; Lv, X.; Ahammed, G.J.; Guo, Z.; Qi, Z.; Yu, J.; Zhou, Y. Tomato GLR3.3 and GLR3.5 mediate cold acclimation-induced chilling tolerance by regulating apoplastic H2O2 production and redox homeostasis. Plant Cell Environ. 2019, 42, 3326–3339. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, H.; Wei, D.; Ma, H.; Lin, J. Arabidopsis CBF3 and DELLAs positively regulate each other in response to low temperature. Sci. Rep. 2017, 7, 39819. [Google Scholar] [CrossRef] [Green Version]
- Robison, J.D.; Yamasaki, Y.; Randall, S.K. The Ethylene Signaling Pathway Negatively Impacts CBF/DREB-Regulated Cold Response in Soybean (Glycine max). Front. Plant Sci. 2019, 10, 121. [Google Scholar] [CrossRef] [Green Version]
- An, F.; Zhao, Q.; Ji, Y.; Li, W.; Jiang, Z.; Yu, X.; Zhang, C.; Han, Y.; He, W.; Liu, Y.; et al. Ethylene-Induced Stabilization of ETHYLENE INSENSITIVE3 and EIN3-LIKE1 Is Mediated by Proteasomal Degradation of EIN3 Binding F-Box 1 and 2 That Requires EIN2 in Arabidopsis. Plant Cell 2010, 22, 2384–2401. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Shi, Y.; Zhang, X.; Xin, X.; Qi, L.; Guo, H.; Li, J.; Yang, S. PIF3 is a negative regulator of the CBF pathway and freezing tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, E6695–E6702. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Lee, C.; Doherty, C.J.; Gilmour, S.J.; Kim, Y.; Thomashow, M.F. Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J. 2015, 82, 193–207. [Google Scholar] [CrossRef] [Green Version]
- Bolt, S.; Zuther, E.; Zintl, S.; Hincha, D.K.; Schmülling, T. ERF105 Is a Transcription Factor Gene of Arabidopsis Thaliana Required for Freezing Tolerance and Cold Acclimation. Plant. Cell Environ. 2017, 40, 108–112. [Google Scholar] [CrossRef]
- Barrero-Gil, J.; Salinas, J. CBFs at the Crossroads of Plant Hormone Signaling in Cold Stress Response. Mol. Plant 2017, 10, 542–544. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.J.; Fang, P.P.; Guo, X.; Qian, X.J.; Zhou, J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Brassinosteroid-Mediated Apoplastic H2O2-Glutaredoxin 12/14 Cascade Regulates Antioxidant Capacity in Response to Chilling in Tomato. Plant. Cell Environ. 2018, 41, 1052–1064. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, V.E.; Poppenberger, B. Modes of Brassinosteroid Activity in Cold Stress Tolerance. Front. Plant Sci. 2020, 11, 5982. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Campbell, L.; Turner, S. Secondary cell walls: Biosynthesis and manipulation. J. Exp. Bot. 2016, 67, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Eremina, M.; Unterholzner, S.J.; Rathnayake, A.I.; Castellanos, M.; Khan, M.; Kugler, K.G.; May, S.T.; Mayer, K.F.X.; Rozhon, W.; Poppenberger, B. Brassinosteroids participate in the control of basal and acquired freezing tolerance of plants. Proc. Natl. Acad. Sci. USA 2016, 113, E5982–E5991. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Cang, J.; Lu, Q.; Fan, B.; Xu, Q.; Li, W.; Wang, X. ABA enhanced cold tolerance of wheat ‘dn1’ via increasing ROS scavenging system. Plant Signal. Behav. 2020, 15, 1780403. [Google Scholar] [CrossRef]
- Xia, X.J.; Wang, Y.J.; Zhou, Y.H.; Tao, Y.; Mao, W.H.; Shi, K.; Asami, T.; Chen, Z.; Yu, J.Q. Reactive Oxygen Species Are Involved in Brassinosteroid-Induced Stress Tolerance in Cucumber. Plant Physiol. 2009, 150, 801–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baier, M.; Bittner, A.; Prescher, A.; Van Buer, J. Preparing plants for improved cold tolerance by priming. Plant Cell Environ. 2019, 42, 782–800. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Wang, K.; Jin, P.; Rui, H. Methyl jasmonate reduces chilling injury and enhances antioxidant enzyme activity in postharvest loquat fruit. Food Chem. 2009, 115, 1458–1463. [Google Scholar] [CrossRef]
- Siboza, X.I.; Bertling, I.; Odindo, A.O. Salicylic acid and methyl jasmonate improve chilling tolerance in cold-stored lemon fruit (Citrus limon). J. Plant Physiol. 2014, 171, 1722–1731. [Google Scholar] [CrossRef]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klay, I.; Gouia, S.; Liu, M.; Mila, I.; Khoudi, H.; Bernadac, A.; Bouzayen, M.; Pirrello, J. Ethylene Response Factors (ERF) are differentially regulated by different abiotic stress types in tomato plants. Plant Sci. 2018, 274, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Dai, W.; Du, J.; Ming, R.; Dahro, B.; Liu, J. ERF109 of trifoliate orange (Poncirus trifoliata (L.) Raf.) contributes to cold tolerance by directly regulating expression ofPrx1involved in antioxidative process. Plant Biotechnol. J. 2019, 17, 1316–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).