Stimuli-Responsive Poly(aspartamide) Derivatives and Their Applications as Drug Carriers
Abstract
1. Introduction
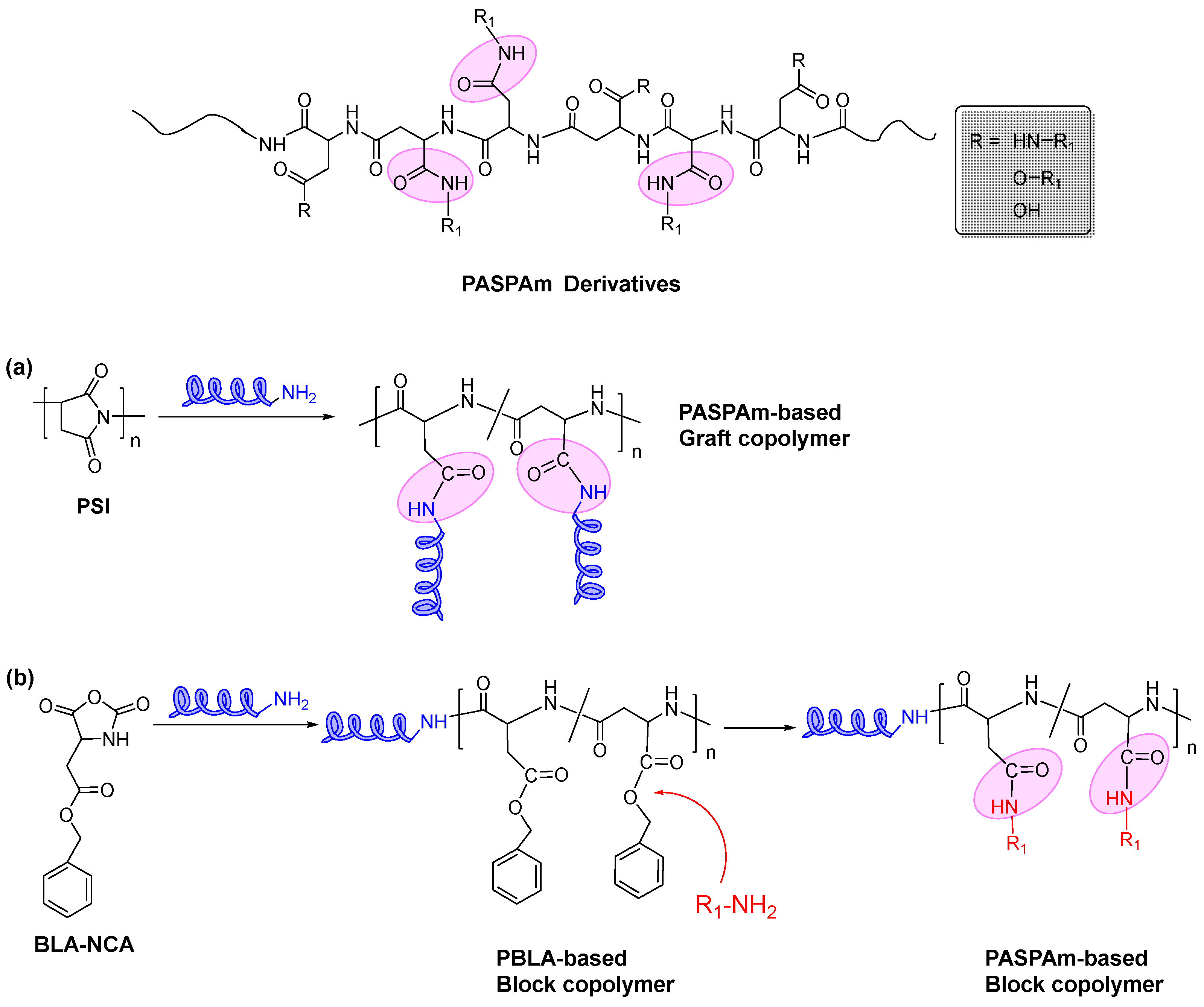
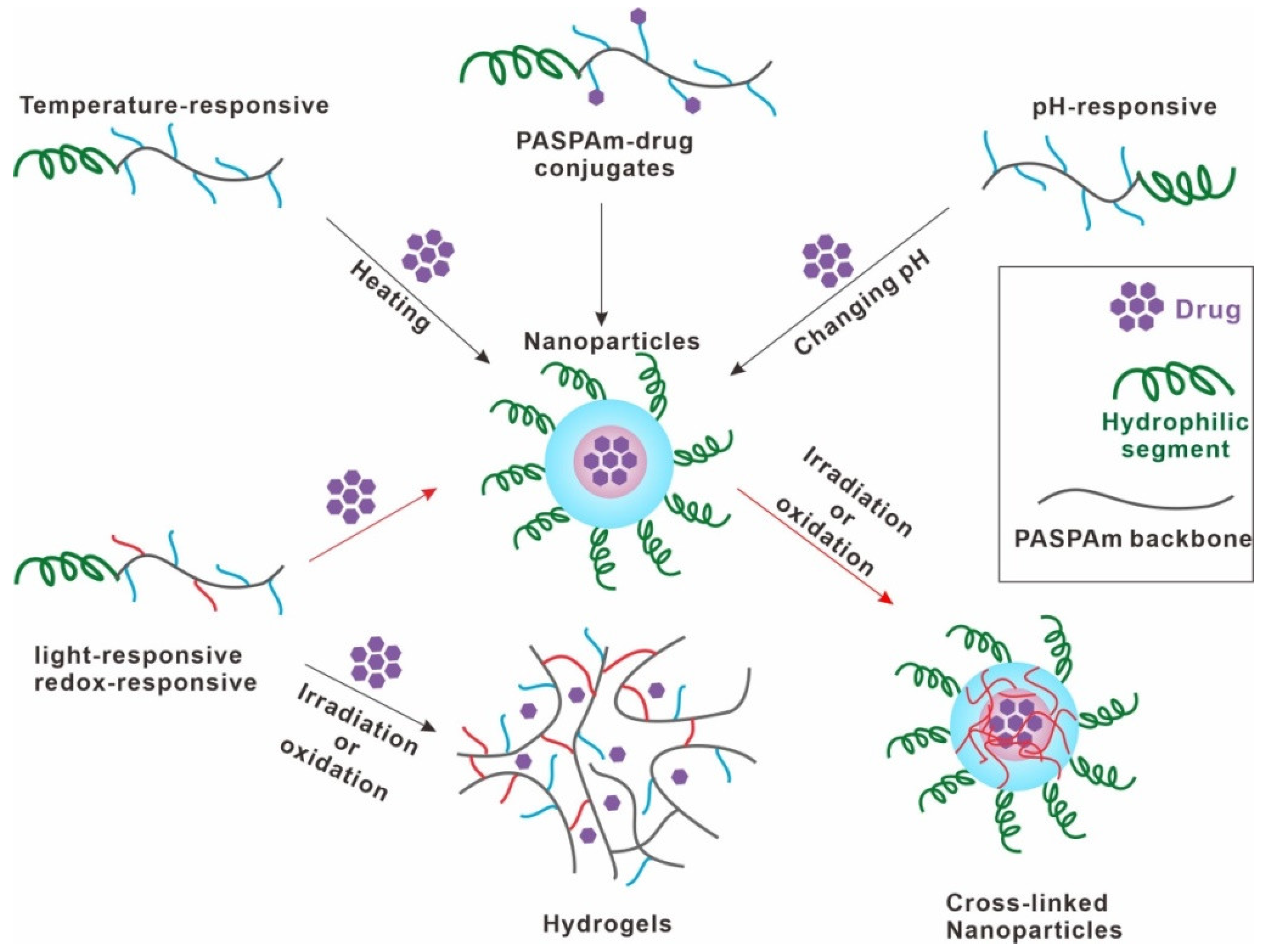
2. Synthesis
2.1. Temperature-Responsive Poly(aspartamide) Derivatives
2.2. pH-Responsive Poly(aspartamide) Derivatives
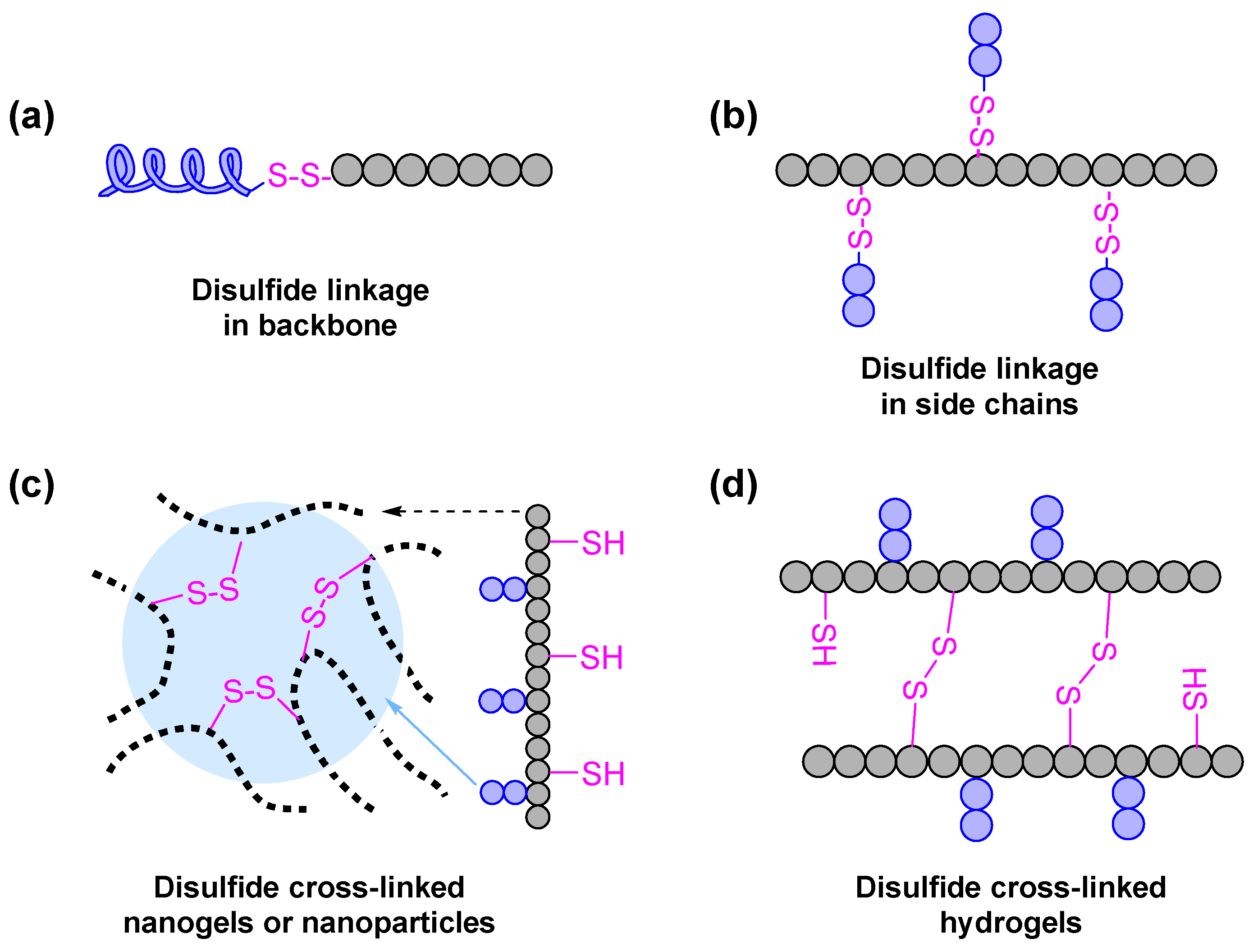
2.3. Redox-Responsive Poly(aspartamide) Derivatives
2.4. Other Stimuli-Responsive Poly(aspartamide) Derivatives
3. Applications
3.1. Drug Loading
3.2. Drug Release
4. Degradation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, S.B.; Sung, M.H.; Uyama, H.; Han, D.K. Poly(glutamic acid): Production, composites, and medical applications of the next-generation biopolymer. Prog. Polym. Sci. 2021, 113, 28. [Google Scholar] [CrossRef]
- Patil, N.A.; Kandasubramanian, B. Functionalized polylysine biomaterials for advanced medical applications: A review. Eur. Polym. J. 2021, 146, 24. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Mhlwatika, Z.; Nwamadi, M.; Balogun, M.O.; Matshe, W.M.R. Synthesis, characterization and in vitro analysis of polymer-based conjugates containing dihydrofolate reductase inhibitors. J. Drug Deliv. Sci. Technol. 2019, 50, 388–401. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A.; Balogun, M.O.; Matshe, W.M.R.; Ray, S.S. Polymer-drug conjugates containing antimalarial drugs and antibiotics. J. Drug Deliv. Sci. Technol. 2019, 53, 12. [Google Scholar] [CrossRef]
- Craparo, E.F.; Drago, S.E.; Mauro, N.; Giammona, G.; Cavallaro, G. Design of New Polyaspartamide Copolymers for siRNA Delivery in Antiasthmatic Therapy. Pharmaceutics 2020, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Kravicz, M.H.; Balogh, D.T.; Kar, M.; Wedepohl, S.; Bentley, M.; Calderon, M. Influence of Alkyl Chains of Modified Polysuccinimide-Based Polycationic Polymers on Polyplex Formation and Transfection. Macromol. Biosci. 2019, 19, 14. [Google Scholar] [CrossRef]
- Naito, M.; Otsu, Y.; Kamegawa, R.; Hayashi, K.; Uchida, S.; Kim, H.J.; Miyata, K. Tunable nonenzymatic degradability of N-substituted polyaspartamide main chain by amine protonation and alkyl spacer length in side chains for enhanced messenger RNA transfection efficiency. Sci. Technol. Adv. Mater. 2019, 20, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chen, S.; Gou, X.; Yang, J.; An, J.; Jin, X.; Yang, Y.-W.; Chen, L.; Gao, H. Biodegradable Supramolecular Materials Based on Cationic Polyaspartamides and Pillar 5 arene for Targeting Gram-Positive Bacteria and Mitigating Antimicrobial Resistance. Adv. Funct. Mater. 2019, 29, 1904683. [Google Scholar] [CrossRef]
- Fiorica, C.; Palumbo, F.S.; Pitarresi, G.; Giammona, G. Photocrosslinkable polyaspartamide/polylactide copolymer and its porous scaffolds for chondrocytes. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Lopukhov, A.V.; Yang, Z.; Haney, M.J.; Bronich, T.K.; Sokolsky-Papkov, M.; Batrakova, E.V.; Klyachko, N.L.; Kabanov, A.V. Mannosylated Cationic Copolymers for Gene Delivery to Macrophages. Macromol. Biosci. 2021, 21, 2000371. [Google Scholar] [CrossRef]
- Perrone, F.; Craparo, E.F.; Cemazar, M.; Kamensek, U.; Drago, S.E.; Dapas, B.; Scaggiante, B.; Zanconati, F.; Bonazza, D.; Grassi, M.; et al. Targeted delivery of siRNAs against hepatocellular carcinoma-related genes by a galactosylated polyaspartamide copolymer. J. Control. Release 2021, 330, 1132–1151. [Google Scholar] [CrossRef]
- Craparo, E.F.; Drago, S.E.; Giammona, G.; Cavallaro, G. Production of polymeric micro- and nanostructures with tunable properties as pharmaceutical delivery systems. Polymer 2020, 200, 9. [Google Scholar] [CrossRef]
- Fiorica, C.; Ventura, C.A.; Pitarresi, G.; Giammona, G. Polyaspartamide based hydrogel with cell recruitment properties for the local administration of hydrophobic anticancer drugs. React. Funct. Polym. 2019, 138, 9–17. [Google Scholar] [CrossRef]
- Fiorica, C.; Palumbo, F.S.; Pitarresi, G.; Giorgi, M.; Calascibetta, F.; Giammona, G. In-situ forming gel-like depot of a polyaspartamide-polylactide copolymer for once a week administration of sulpiride. J. Pharm. Pharmacol. 2015, 67, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, S.; Liu, X.; Xu, Y.; Zhao, J.; Si, X.; Li, H.; Huang, Z.; Wang, Z.; Tang, Z.; et al. Supramolecular Assembled Programmable Nanomedicine As In Situ Cancer Vaccine for Cancer Immunotherapy. Adv. Mater. 2021, 33, 2007293. [Google Scholar] [CrossRef]
- Craparo, E.F.; Cabibbo, M.; Conigliaro, A.; Barreca, M.M.; Musumeci, T.; Giammona, G.; Cavallaro, G. Rapamycin-Loaded Polymeric Nanoparticles as an Advanced Formulation for Macrophage Targeting in Atherosclerosis. Pharmaceutics 2021, 13, 503. [Google Scholar] [CrossRef]
- Negishi, T.; Koizumi, F.; Uchino, H.; Kuroda, J.; Kawaguchi, T.; Naito, S.; Matsumura, Y. NK105, a paclitaxel-incorporating micellar nanoparticle, is a more potent radiosensitising agent compared to free paclitaxel. Br. J. Cancer 2006, 95, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Matsumura, Y.; Suzuki, M.; Shimizu, K.; Goda, R.; Nakamura, I.; Nakatomi, I.; Yokoyama, M.; Kataoka, K.; Kakizoe, T. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br. J. Cancer 2005, 92, 1240–1246. [Google Scholar] [CrossRef]
- Mukai, H.; Kato, K.; Esaki, T.; Ohsumi, S.; Hozomi, Y.; Matsubara, N.; Hamaguchi, T.; Matsumura, Y.; Goda, R.; Hirai, T.; et al. Phase I study of NK105, a nanomicellar paclitaxel formulation, administered on a weekly schedule in patients with solid tumors. Investig. New Drugs 2016, 34, 750–759. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Mukai, H.; Saeki, T.; Ro, J.; Lin, Y.-C.; Nagai, S.E.; Lee, K.S.; Watanabe, J.; Ohtani, S.; Kim, S.B.; et al. A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br. J. Cancer 2019, 120, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Hamaguchi, T.; Ura, T.; Muro, K.; Yamada, Y.; Shimada, Y.; Shirao, K.; Okusaka, T.; Ueno, H.; Ikeda, M.; et al. Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin. Br. J. Cancer 2004, 91, 1775–1781. [Google Scholar] [CrossRef]
- Harada, M.; Bobe, I.; Saito, H.; Shibata, N.; Tanaka, R.; Hayashi, T.; Kato, Y. Improved anti-tumor activity of stabilized anthracycline polymeric micelle formulation, NC-6300. Cancer Sci. 2011, 102, 192–199. [Google Scholar] [CrossRef]
- Takahashi, A.; Yamamoto, Y.; Yasunaga, M.; Koga, Y.; Kuroda, J.-i.; Takigahira, M.; Harada, M.; Saito, H.; Hayashi, T.; Kato, Y.; et al. NC-6300, an epirubicin-incorporating micelle, extends the antitumor effect and reduces the cardiotoxicity of epirubicin. Cancer Sci. 2013, 104, 920–925. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Adelnia, H.; Tran, H.D.N.; Little, P.J.; Blakey, I.; Ta, H.T. Poly(aspartic acid) in Biomedical Applications: From Polymerization, Modification, Properties, Degradation, and Biocompatibility to Applications. ACS Biomater. Sci. Eng. 2021, 7, 2083–2105. [Google Scholar] [CrossRef]
- Jalalvandi, E.; Shavandi, A. Polysuccinimide and its derivatives: Degradable and water soluble polymers (review). Eur. Polym. J. 2018, 109, 43–54. [Google Scholar] [CrossRef]
- Gu, X.; Wang, J.; Liu, X.; Zhao, D.; Wang, Y.; Gao, H.; Wu, G. Temperature-responsive drug delivery systems based on polyaspartamides with isopropylamine pendant groups. Soft Matter 2013, 9, 7267–7273. [Google Scholar] [CrossRef]
- Vega-Chacon, J.; Piazza, R.D.; Costa Marques, R.F.; Elaissari, A.; Jafelicci, M. The influence of pH, hydrolysis and degree of substitution on the temperature-sensitive properties of polyaspartamides. Polym. Int. 2019, 68, 88–93. [Google Scholar] [CrossRef]
- Liu, N.; Li, B.; Gong, C.; Liu, Y.; Wang, Y.; Wu, G. A pH- and thermo-responsive poly(amino acid)-based drug delivery system. Colloids Surf. B Biointerfaces 2015, 136, 562–569. [Google Scholar] [CrossRef]
- Moon, J.R.; Park, Y.H.; Kim, J.-H. Synthesis and characterization of novel thermo- and pH-responsive copolymers based on amphiphilic polyaspartamides. J. Appl. Polym. Sci. 2009, 111, 998–1004. [Google Scholar] [CrossRef]
- Moon, J.; Kim, J.-H. Preparation of Biodegradable Thermo-responsive Polyaspartamides with N-Isopropylamine Pendent Groups (I). Bull. Korean Chem. Soc. 2006, 27, 1981–1984. [Google Scholar] [CrossRef][Green Version]
- Tachibana, Y.; Kurisawa, M.; Uyama, H.; Kakuchi, T.; Kobayashi, S. Biodegradable thermoresponsive poly(amino acid)s. Chem. Commun. 2003, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhou, J.; Wang, P. A novel cross-linkable, polyaspartamide derivative-containing cinnamoyl groups with temperature and pH dual stimuli-responsiveness. Iran. Polym. J. 2019, 28, 157–166. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, G.; Li, L.; Yu, H.; Liu, J.; Wang, C.; Chu, Y.; Zhuo, R.; Jiang, X. Temperature and pH Dual-Sensitive Polyaspartamide Derivatives for Antitumor Drug Delivery. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 879–888. [Google Scholar] [CrossRef]
- Du, X.; Jiang, Y.; Zhuo, R.; Jiang, X. Thermosensitive and Photocleavable Polyaspartamide Derivatives for Drug Delivery. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2855–2863. [Google Scholar] [CrossRef]
- Hsu, S.-P.; Chu, I.-M.; Yang, J.-D. Thermo- and pH-Responsive Polymersomes of Poly(α,β-N-substituted-DL-aspartamide)s. J. Appl. Polym. Sci. 2012, 125, 133–144. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, X.; Zhuo, R. Biodegradable and thermosensitive polyaspartamide derivatives bearing aromatic structures. Mater. Lett. 2014, 121, 78–80. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.; Chu, Y.; Ma, Y.; Zhuo, R.; Jiang, X. Facile synthesis of thermosensitive functional polyaspartamide derivatives by click chemistry. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1296–1303. [Google Scholar] [CrossRef]
- Czaderna-Lekka, A.; Kozanecki, M.; Matusiak, M.; Kadlubowski, S. Phase transitions of poly(oligo(ethylene glycol) methyl ether methacrylate)-water systems. Polymer 2021, 212, 12. [Google Scholar] [CrossRef]
- Zuppardi, F.; Malinconico, M.; D’Agosto, F.; D’Ayala, G.G.; Cerruti, P. Well-Defined Thermo-Responsive Copolymers Based on Oligo(Ethylene Glycol) Methacrylate and Pentafluorostyrene for the Removal of Organic Dyes from Water. Nanomaterials 2020, 10, 1779. [Google Scholar] [CrossRef]
- Zhou, C.P.; Shi, Z.W.; Xu, F.; Ling, Y.; Tang, H.Y. Preparation and properties of thermo- and pH-responsive polypeptide bearing OEG and aldehyde pendants. Colloid Polym. Sci. 2020, 298, 1293–1302. [Google Scholar] [CrossRef]
- Cheng, Y.; He, C.; Xiao, C.; Ding, J.; Zhuang, X.; Chen, X. Versatile synthesis of temperature-sensitive polypeptides by click grafting of oligo(ethylene glycol). Polym. Chem. 2011, 2, 2627–2634. [Google Scholar] [CrossRef]
- Zhang, G.; Bao, C.; Yi, H. Investigation of the Temperature Responsive Behaviors of Novel Polyaspartamide Derivatives Bearing Alkyl Ether-type Pendants. Arab. J. Chem. 2021, 14, 103287. [Google Scholar] [CrossRef]
- Lagant, P.; Vergoten, G.; Loucheux, C.; Fleury, G. Study of Poly(L-aspartic acid). I. Laser Raman Spectrometry. Polym. J. 1979, 11, 345–351. [Google Scholar] [CrossRef][Green Version]
- Németh, C.; Gyarmati, B.; Abdullin, T.; László, K.; Szilágyi, A. Poly(aspartic acid) with adjustable pH-dependent solubility. Acta Biomater. 2017, 49, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Oh, Y.T.; Lee, K.S.; Yun, J.M.; Park, B.T.; Oh, K.T. Development of a pH-sensitive polymer using poly(aspartic acid-graft-imidazole)-block-poly(ethylene glycol) for acidic pH targeting systems. Macromol. Res. 2011, 19, 453–460. [Google Scholar] [CrossRef]
- Chu, Y.; Yu, H.; Ma, Y.; Zhang, Y.; Chen, W.; Zhang, G.; Wei, H.; Zhang, X.; Zhuo, R.; Jiang, X. Synthesis and characterization of biodegradable pH and reduction dual-sensitive polymeric micelles for doxorubicin delivery. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1771–1780. [Google Scholar] [CrossRef]
- Gong, C.; Shan, M.; Li, B.; Wu, G. A pH and redox dual stimuli-responsive poly(amino acid) derivative for controlled drug release. Colloids Surf. B Biointerfaces 2016, 146, 396–405. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.-H.; Kim, D. pH-Sensitive Amphiphilic Biodegradable Graft Co-Polymer Aggregates Based on Polyaspartamide for Intracellular Delivery. J. Biomater. Sci. Polym. Ed. 2012, 23, 1255–1269. [Google Scholar] [CrossRef]
- Wang, L.; Luan, J.; Du, L.; Li, L.; Liu, J.; Liu, Z.; Zhuo, R. Polyaspartamide-based multi-responsive micelle with sheddable shell for antitumor drug delivery. RSC Adv. 2016, 6, 111161–111169. [Google Scholar] [CrossRef]
- Seo, K.; Chung, S.W.; Byun, Y.; Kim, D. Paclitaxel loaded nano-aggregates based on pH sensitive polyaspartamide amphiphilic graft copolymers. Int. J. Pharm. 2012, 424, 26–32. [Google Scholar] [CrossRef]
- Lin, W.; Kim, D. pH-Sensitive Micelles with Cross-Linked Cores Formed from Polyaspartamide Derivatives for Drug Delivery. Langmuir 2011, 27, 12090–12097. [Google Scholar] [CrossRef]
- Seo, K.; Kim, D. pH-dependent hemolysis of biocompatible imidazole-grafted polyaspartamide derivatives. Acta Biomater. 2010, 6, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Ngoc Bich, T.; Kim, J.Y.; Kim, Y.-C.; Kim, Y.J.; Kim, J.-H. CO2-responsive swelling behavior and metal-ion adsorption properties in novel histamine-conjugated polyaspartamide hydrogel. J. Appl. Polym. Sci. 2016, 133, 43305. [Google Scholar] [CrossRef]
- Moon, J.R.; Jeon, Y.S.; Zrinyi, M.; Kim, J.-H. pH-Responsive PEGylated nanoparticles based on amphiphilic polyaspartamide: Preparation, physicochemical characterization and in vitro evaluation. Polym. Int. 2013, 62, 1218–1224. [Google Scholar] [CrossRef]
- Gu, X.; Wang, J.; Wang, Y.; Wang, Y.; Gao, H.; Wu, G. Layer-by-layer assembled polyaspartamide nanocapsules for pH-responsive protein delivery. Colloids Surf. B Biointerfaces 2013, 108, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.R.; Kim, M.W.; Kim, D.; Jeong, J.H.; Kim, J.-H. Synthesis and self-assembly behavior of novel polyaspartamide derivatives for anti-tumor drug delivery. Colloid Polym. Sci. 2011, 289, 63–71. [Google Scholar] [CrossRef]
- Moon, J.R.; Kim, J.-H. Biodegradable stimuli-responsive hydrogels based on amphiphilic polyaspartamides with tertiary amine pendent groups. Polym. Int. 2010, 59, 630–636. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, X.; Zhuo, R. Microwave-assisted solid-phase synthesis of pH-responsive polyaspartamide derivatives. Carbohydr. Polym. 2012, 89, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Kim, D. Folate-PEG/Hyd-curcumin/C18-g-PSI micelles for site specific delivery of curcumin to colon cancer cells via Wnt/beta-catenin signaling pathway. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 464–471. [Google Scholar] [CrossRef]
- Lu, C.; Wang, X.; Wu, G.; Wang, J.; Wang, Y.; Gao, H.; Ma, J. An injectable and biodegradable hydrogel based on poly(alpha,beta-aspartic acid) derivatives for localized drug delivery. J. Biomed. Mater. Res. Part A 2014, 102, 628–638. [Google Scholar] [CrossRef]
- Lim, C.; Cho, E.-B.; Kim, D. pH-triggered intracellular release of doxorubicin from polyaspartamide-encapsulated mesoporous silica nanoparticles. Korean J. Chem. Eng. 2019, 36, 166–172. [Google Scholar] [CrossRef]
- Yu, H.; Sun, J.; Zhang, Y.; Zhang, G.; Chu, Y.; Zhuo, R.; Jiang, X. pH- and beta-Cyclodextrin-Responsive Micelles Based on Polyaspartamide Derivatives as Drug Carrier. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1387–1395. [Google Scholar] [CrossRef]
- Sonawane, S.J.; Kalhapure, R.S.; Govender, T. Hydrazone linkages in pH responsive drug delivery systems. Eur. J. Pharm. Sci. 2017, 99, 45–65. [Google Scholar] [CrossRef]
- Liu, N.; Han, J.M.; Zhang, X.C.; Yang, Y.; Liu, Y.; Wang, Y.M.; Wu, G.L. pH-responsive zwitterionic polypeptide as a platform for anti-tumor drug delivery. Colloids Surf. B Biointerfaces 2016, 145, 401–409. [Google Scholar] [CrossRef]
- Lim, C.W.; Kim, D. Bone targeting nano-aggregates prepared from self-assembled polyaspartamide graft copolymers for pH sensitive DOX delivery. Biomater. Sci. 2021, 9, 1660–1667. [Google Scholar] [CrossRef]
- Van Tran Thi, T.; Lim, C.W.; Park, J.H.; Ahn, C.-H.; Kim, D. Self-assembled nanoaggregates based on polyaspartamide graft copolymers for pH-controlled release of doxorubicin. J. Mater. Chem. B 2015, 3, 2978–2985. [Google Scholar] [CrossRef]
- Nguyen, M.P.; Thuy, V.T.; Kim, D. Integration of iron oxide nanoparticles and polyaspartamide biopolymer for MRI image contrast enhancement and an efficient drug-delivery system in cancer therapy. Nanotechnology 2020, 31, 11. [Google Scholar] [CrossRef]
- Jang, J.; Cha, C. Multivalent Polyaspartamide Cross-Linker for Engineering Cell-Responsive Hydrogels with Degradation Behavior and Tunable Physical Properties. Biomacromolecules 2018, 19, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Sanjoh, M.; Hiki, S.; Lee, Y.; Oba, M.; Miyata, K.; Ishii, T.; Kataoka, K. pDNA/poly(L-lysine) Polyplexes Functionalized with a pH-Sensitive Charge-Conversional Poly(aspartamide) Derivative for Controlled Gene Delivery to Human Umbilical Vein Endothelial Cells. Macromol. Rapid Commun. 2010, 31, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Pittella, F.; Nomoto, T.; Takemoto, H.; Nishiyama, N.; Miyata, K.; Kataoka, K. Fine-Tuning of Charge-Conversion Polymer Structure for Efficient Endosomal Escape of siRNA-Loaded Calcium Phosphate Hybrid Micelles. Macromol. Rapid Commun. 2014, 35, 1211–1215. [Google Scholar] [CrossRef]
- Cao, L.; Xiao, Y.; Lu, W.; Liu, S.; Gan, L.; Yu, J.; Huang, J. Nanomicelle drug with acid-triggered doxorubicin release and enhanced cellular uptake ability based on mPEG-graft-poly(N-(2-aminoethyl)-L-aspartamide)-hexahydrophthalic acid copolymers. J. Biomater. Appl. 2018, 32, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.R.; MacKrell, E.J.; Forsthoefel, C.P.; Jensen, S.P.; Chen, M.; Moore, G.A.; He, Z.L.; Sumerlin, B.S. Biodegradable and pH-Responsive Nanoparticles Designed for Site-Specific Delivery in Agriculture. Biomacromolecules 2015, 16, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, Y.; Huang, Y.; He, Y.; Xu, Y.; Lu, W.; Yu, J. Poly(ethylene glycol) shell-sheddable TAT-modified core cross-linked nano-micelles: TAT-enhanced cellular uptake and lysosomal pH-triggered doxorubicin release. Colloids Surf. B Biointerfaces 2020, 188, 110772. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, Y.; Yang, Q.; Li, C.; Hennink, W.E.; Zhuo, R.; Jiang, X. Reduction biodegradable brushed PDMAEMA derivatives synthesized by atom transfer radical polymerization and click chemistry for gene delivery. Acta Biomater. 2013, 9, 7758–7766. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Yang, Q.; Zhuo, R.; Jiang, X. Disulfide-Containing Brushed Polyethylenimine Derivative Synthesized by Click Chemistry for Nonviral Gene Delivery. Bioconjug. Chem. 2012, 23, 1290–1299. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Q.; Bi, B.; Xu, L.; Liu, J.; Zhuo, R.; Jiang, X. A bioreducible supramolecular nanoparticle gene delivery system based on cyclodextrin-conjugated polyaspartamide and adamantyl-terminated polyethylenimine. J. Mater. Chem. B 2018, 6, 797–808. [Google Scholar] [CrossRef]
- Park, C.W.; Yang, H.-M.; Woo, M.-A.; Lee, K.S.; Kim, J.-D. Completely disintegrable redox-responsive poly(amino acid) nanogels for intracellular drug delivery. J. Ind. Eng. Chem. 2017, 45, 182–188. [Google Scholar] [CrossRef]
- Birhan, Y.S.; Darge, H.F.; Hanurry, E.Y.; Andrgie, A.T.; Mekonnen, T.W.; Chou, H.-Y.; Lai, J.-Y.; Tsai, H.-C. Fabrication of Core Crosslinked Polymeric Micelles as Nanocarriers for Doxorubicin Delivery: Self-Assembly, In Situ Diselenide Metathesis and Redox-Responsive Drug Release. Pharmaceutics 2020, 12, 580. [Google Scholar] [CrossRef]
- Gyarmati, B.; Vajna, B.; Nemethy, A.; Laszlo, K.; Szilagyi, A. Redox- and pH-Responsive Cysteamine-Modified Poly(aspartic acid) Showing a Reversible SolGel Transition. Macromol. Biosci. 2013, 13, 633–640. [Google Scholar] [CrossRef]
- Krisch, E.; Gyarmati, B.; Barczikai, D.; Lapeyre, V.; Szilágyi, B.Á.; Ravaine, V.; Szilágyi, A. Poly(aspartic acid) hydrogels showing reversible volume change upon redox stimulus. Eur. Polym. J. 2018, 105, 459–468. [Google Scholar] [CrossRef]
- Krisch, E.; Messager, L.; Gyarmati, B.; Ravaine, V.; Szilágyi, A. Redox- and pH-Responsive Nanogels Based on Thiolated Poly(aspartic acid). Macromol. Mater. Eng. 2016, 301, 260–266. [Google Scholar] [CrossRef]
- Szilágyi, B.Á.; Gyarmati, B.; Horvát, G.; Laki, Á.; Budai-Szűcs, M.; Csányi, E.; Sandri, G.; Bonferoni, M.C.; Szilágyi, A. The effect of thiol content on the gelation and mucoadhesion of thiolated poly(aspartic acid). Polym. Int. 2017, 66, 1538–1545. [Google Scholar] [CrossRef]
- Park, C.W.; Yang, H.-M.; Lee, K.S.; Kim, J.-D. Disulfide and β-sheet stabilized poly(amino acid) nanovesicles for intracellular drug delivery. J. Ind. Eng. Chem. 2017, 56, 277–284. [Google Scholar] [CrossRef]
- Gyarmati, B.; Mammadova, A.; Barczikai, D.; Stankovits, G.; Misra, A.; Alavijeh, M.S.; Varga, Z.; László, K.; Szilágyi, A. Side group ratio as a novel means to tune the hydrolytic degradation of thiolated and disulfide cross-linked polyaspartamides. Polym. Degrad. Stab. 2021, 188, 109577. [Google Scholar] [CrossRef]
- Budai-Szűcs, M.; Kiss, E.L.; Szilágyi, B.Á.; Szilágyi, A.; Gyarmati, B.; Berkó, S.; Kovács, A.; Horvát, G.; Aigner, Z.; Soós, J.; et al. Mucoadhesive Cyclodextrin-Modified Thiolated Poly(aspartic acid) as a Potential Ophthalmic Drug Delivery System. Polymers 2018, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, S.; Zhang, W. A New Family of Thermo-, pH-, and CO2-Responsive Homopolymers of Poly[Oligo(ethylene glycol) (N-dialkylamino) methacrylate]s. Macromolecules 2017, 50, 4686–4698. [Google Scholar] [CrossRef]
- Eskandari, P.; Roghani-Mamaqani, H.; Salami-Kalajahi, M.; Abousalman-Rezvani, Z. Modification of cellulose nanocrystal with dual temperature- and CO2-responsive block copolymers for ion adsorption applications. J. Mol. Liq. 2020, 310, 113234. [Google Scholar] [CrossRef]
- Furusho, Y.; Endo, T. Reversible capture and release of carbon dioxide by binary system of polyamidine and polyethylene glycol. Polym. Bull. 2017, 74, 1207–1219. [Google Scholar] [CrossRef]
- Schattling, P.; Pollmann, I.; Theato, P. Synthesis of CO2-responsive polymers by post-polymerization modification. React. Funct. Polym. 2014, 75, 16–21. [Google Scholar] [CrossRef]
- Bich Ngoc, T.; Bui, Q.T.; Jeon, Y.S.; Park, H.S.; Kim, J.-H. Preparation and characterization of CO2-responsive poly(amino acid) derivatives with guanidine group. Polym. Bull. 2015, 72, 2605–2620. [Google Scholar] [CrossRef]
- Wei, J.; Sun, J.; Yang, X.; Ji, S.; Wei, Y.; Li, Z. Self-crosslinking assemblies with tunable nanostructures from photoresponsive polypeptoid-based block copolymers. Polym. Chem. 2020, 11, 337–343. [Google Scholar] [CrossRef]
- Jia, F.; Wang, Y.; Wang, H.; Jin, Q.; Cai, T.; Chen, Y.; Ji, J. Light cross-linkable and pH de-cross-linkable drug nanocarriers for intracellular drug delivery. Polym. Chem. 2015, 6, 2069–2075. [Google Scholar] [CrossRef]
- Park, J.H.; Moon, J.R.; Hong, K.H.; Kim, J.-H. Photo-crosslinked polyaspartamide hybrid gel containing thermo-responsive Pluronic triblock copolymer. J. Polym. Res. 2011, 18, 273–278. [Google Scholar] [CrossRef]
- Cao, H.; Ma, X.Y.; Sun, S.H.; Su, H.J.; Tan, T.W. A new photocrosslinkable hydrogel based on a derivative of polyaspartic acid for the controlled release of ketoprofen. Polym. Bull. 2010, 64, 623–632. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, X.; Zhuo, R. Biodegradable and thermosensitive micelles of amphiphilic polyaspartamide derivatives containing aromatic groups for drug delivery. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3917–3924. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, X. Temperature Responsive Nanoparticles Based on PEGylated Polyaspartamide Derivatives for Drug Delivery. Polymers 2019, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.D.; Park, J.H.; Bhang, S.H.; Kim, J.H. Synthesis and characterization of novel multi-hydroxy polyaspartamide derivative and its crosslinked hydrogels. React. Funct. Polym. 2020, 147, 9. [Google Scholar] [CrossRef]
- Gong, C.; Lu, C.C.; Li, B.Q.; Shan, M.; Wu, G.L. Injectable dopamine-modified poly(alpha,beta-aspartic acid) nanocomposite hydrogel as bioadhesive drug delivery system. J. Biomed. Mater. Res. Part A 2017, 105, 1000–1008. [Google Scholar] [CrossRef]
- Wang, B.; Jeon, Y.S.; Park, H.S.; Kim, J.-H. Self-healable mussel-mimetic nanocomposite hydrogel based on catechol-containing polyaspartamide and graphene oxide. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 160–170. [Google Scholar] [CrossRef]
- Nguyen, H.L.N.; Kim, J.H. Stimuli-sensitive complexation and the strongly adhesive antibacterial gel from biocompatible PolyAspAm(EA/EDA) and tannic acid. Int. J. Polym. Mater. Polym. Biomater. 2020, 8. [Google Scholar] [CrossRef]
- Hladysh, S.; Oleshchuk, D.; Dvorakova, J.; Golunova, A.; Salek, P.; Panek, J.; Janouskova, O.; Kankova, D.; Pavlova, E.; Proks, V. Zwitterionic polyaspartamides based on L-lysine side-chain moieties: Synthesis, nonfouling properties and direct/indirect nanogel preparation. Eur. Polym. J. 2021, 148, 110347. [Google Scholar] [CrossRef]
- Han, X.; Meng, X.; Wu, Z.; Wu, Z.; Qi, X. Dynamic imine bond cross-linked self-healing thermosensitive hydrogels for sustained anticancer therapy via intratumoral injection. Mater. Sci. Eng. C 2018, 93, 1064–1072. [Google Scholar] [CrossRef]
- Kim, S.; Nishimoto, S.K.; Bumgardner, J.D.; Haggard, W.O.; Gaber, M.W.; Yang, Y. A chitosan/β-glycerophosphate thermo-sensitive gel for the delivery of ellagic acid for the treatment of brain cancer. Biomaterials 2010, 31, 4157–4166. [Google Scholar] [CrossRef]
- Shriky, B.; Kelly, A.; Isreb, M.; Babenko, M.; Mahmoudi, N.; Rogers, S.; Shebanova, O.; Snow, T.; Gough, T. Pluronic F127 thermosensitive injectable smart hydrogels for controlled drug delivery system development. J. Colloid Interface Sci. 2020, 565, 119–130. [Google Scholar] [CrossRef]
- Justus, C.; Dong, L.; Yang, L. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front. Physiol. 2013, 4, 354. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Wang, S.; Low, P.S. Measurement of endosome pH following folate receptor-mediated endocytosis. Biochim. Biophys. Acta BBA Mol. Cell Res. 1996, 1312, 237–242. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Glutathione and modulation of cell apoptosis. Biochim. Biophys. Acta BBA Mol. Cell Res. 2012, 1823, 1767–1777. [Google Scholar] [CrossRef]
- Mukai, H.; Kogawa, T.; Matsubara, N.; Naito, Y.; Sasaki, M.; Hosono, A. A first-in-human Phase 1 study of epirubicin-conjugated polymer micelles (K-912/NC-6300) in patients with advanced or recurrent solid tumors. Investig. New Drugs 2017, 35, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.P.; Goel, S.; Chow, W.; Braiteh, F.; Singh, A.S.; Olson, J.E.G.; Osada, A.; Bobe, I.; Riedel, R.F. A Phase 1b Dose Escalation Trial of NC-6300 (Nanoparticle Epirubicin) in Patients with Advanced Solid Tumors or Advanced, Metastatic, or Unresectable Soft-tissue Sarcoma. Clin. Cancer Res. 2020, 26, 4225–4232. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Wang, Y.F.; Zhang, T.Y.; Pu, X.H.; Zong, L.L.; Zhu, H.Y.; Zhao, L.L.; Feng, B. Redox-Responsive Disulfide Bond-Bridged mPEG-PBLA Prodrug Micelles for Enhanced Paclitaxel Biosafety and Antitumor Efficacy. Front. Oncol. 2019, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Li, B.; Lin, L.; Huang, J.; An, Y.; Huang, W.; Zhou, Z.; Wang, Y.; Shuai, X.; Zhu, K. A reduction and pH dual-sensitive nanodrug for targeted theranostics in hepatocellular carcinoma. Biomater. Sci. 2020, 8, 3485–3499. [Google Scholar] [CrossRef]
- Tabata, K.; Abe, H.; Doi, Y. Microbial Degradation of Poly(aspartic acid) by Two Isolated Strains of Pedobacter sp. and Sphingomonas sp. Biomacromolecules 2000, 1, 157–161. [Google Scholar] [CrossRef]
- Drobník, J.; Saudek, V.; Vlasák, J.; Kálal, J. Polyaspartamide—A potential drug carrier. J. Polym. Sci. Polym. Symp. 1979, 66, 65–74. [Google Scholar] [CrossRef]
- Tabata, K.; Kajiyama, M.; Hiraishi, T.; Abe, H.; Yamato, I.; Doi, Y. Purification and Characterization of Poly(aspartic acid) Hydrolase from Sphingomonas sp. KT-1. Biomacromolecules 2001, 2, 1155–1160. [Google Scholar] [CrossRef]
- Wei, J.; Xue, M.; Li, C.; Cao, H.; Tan, T. Effect of enzyme and mechanical stirring on the degradation of polyaspartic acid hydro-gel. Prog. Nat. Sci. Mater. Int. 2015, 25, 425–429. [Google Scholar] [CrossRef]
- Nakato, T.; Yoshitake, M.; Matsubara, K.; Tomida, M.; Kakuchi, T. Relationships between Structure and Properties of Poly(aspartic acid)s. Macromolecules 1998, 31, 2107–2113. [Google Scholar] [CrossRef]
- Alford, D.D.; Wheeler, A.P.; Pettigrew, C.A. Biodegradation of thermally synthesized polyaspartate. J. Environ. Polym. Degrad. 1994, 2, 225–236. [Google Scholar] [CrossRef]
- Juriga, D.; Zrínyi, M. Biodegradation of Poly(aspartamide) Based Hydrogels. Macromol. Symp. 2019, 385, 1800194. [Google Scholar] [CrossRef]
- Juriga, D.; Nagy, K.; Jedlovszky-Hajdú, A.; Perczel-Kovách, K.; Chen, Y.M.; Varga, G.; Zrínyi, M. Biodegradation and Osteosarcoma Cell Cultivation on Poly(aspartic acid) Based Hydrogels. ACS Appl. Mater. Interfaces 2016, 8, 23463–23476. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chau, M.; Boyle, A.J.; Liu, P.; Niehoff, A.; Weinrich, D.; Reilly, R.M.; Winnik, M.A. Effect of Pendant Group Structure on the Hydrolytic Stability of Polyaspartamide Polymers under Physiological Conditions. Biomacromolecules 2012, 13, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
| Temperature Responsive PASPAm Derivatives | Composition of Side Chain | R1/R2 | LCST (°C) | Ref | |
|---|---|---|---|---|---|
| R1 | R2 | ||||
| PAIPAHA | 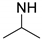 | NH-(CH2)6OH | 55/45 | 30 | [27] |
| PAIPAPA | 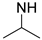 | NH-(CH2)3OH | 55/45 | 40 | [27] |
| PAA-TS | 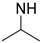 | OH | 69/31 | 38 a | [28] |
| 73/27 | 40 a | ||||
| 74/26 | 45 a | ||||
| 75/25 | 47 a | ||||
| 77/23 | 49 a | ||||
| 78/22 | 56 a | ||||
| 80/20 | 62 a | ||||
| PSI-TS | 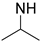 | - | 69/31 | 34 a | [28] |
| mPEG-hyd-PDAHy | 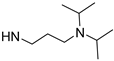 | 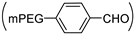 NH-NH2 | 80/(9)20 | 40 | [29] |
| PolyAspAm(HA/NIPEDA) | 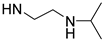 | NH-(CH2)5CH3 | 57/43 | 24 | [30] |
| 58/42 | 30 | ||||
| 59/41 | 39 | ||||
| 62/38 | 52 | ||||
| PolyAspAm(OA/NIPEDA) | 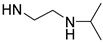 | NH-(CH2)7CH3 | 57/43 | 22 | [30] |
| 59/41 | 36 | ||||
| 60.5/39.5 | 43 | ||||
| 62/38 | 49 | ||||
| PolyAspAm(LA/NIPEDA) | 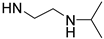 | NH-(CH2)11CH3 | 59/41 | 22 | [30,31] |
| 61/39 | 37 | ||||
| 64/36 | 50 | ||||
| PAspAm(C5OH/C6OH) | NH-(CH2)5OH | NH-(CH2)6OH | 50/50 | 23 | [32] |
| 60/40 | 29 | ||||
| 70/30 | 37 | ||||
| 80/20 | 44 | ||||
| PAspAm(C4OH/C6OH) | NH-(CH2)4OH | NH-(CH2)6OH | 30/70 | 53 | [36] |
| 25/75 | 40 | ||||
| 20/80 | 38 | ||||
| 15/85 | 32 | ||||
| 10/90 | 28 | ||||
| phe-g-PHPA | 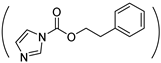 | NH-(CH2)5OH | (2)/100 | 54 | [37] |
| (6)/100 | 32 | ||||
| (11)/100 | 20 | ||||
| (12)/100 | 14 | ||||
| (13)/100 | 9 | ||||
| Phe/DEAE-g-PHPA | 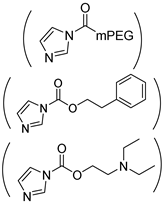 | NH-(CH2)5OH | (0/22.2/30.3)/100 | 35.7 b | [34] |
| (0/26.6/28.3)/100 | 31.5 b | ||||
| (0/37.6/27.3)/100 | 22.5 b | ||||
| (0/39.6/19.0)/100 | 29.5 | ||||
| (0/53.6/12.0)/100 | 45.8 | ||||
| (5/34.6/12.7)/100 | 28 b | ||||
| (5/42.4/10.5)/100 | 15 b | ||||
| NB-g-PHPA | 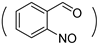 | NH-(CH2)5OH | (4)/100 | 32 | [35] |
| (7.5)/100 | 21 | ||||
| (10)/100 | 11 | ||||
| NB-g-PHPA-g-mPEG | 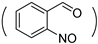 NH-(CH2)5OH | NH-mPEG | (12)98.5/1.5 | 25 | [35] |
| (17)98.5/1.5 | 8 | ||||
| P(Asp-Az)x-HPA | NH-(CH2)2N3 | NH-(CH2)5OH | 40/60 | 63 | [33] |
| 39/61 | 58 | [38] | |||
| 56/44 | 29 | ||||
| P(Asp-Az)x-HPA-PEA | 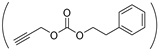 NH-(CH2)2N3 | NH-(CH2)5OH | (5)39/61 | 19 | [38] |
| (9.9)39/61 | 4 | ||||
| P(Asp-Az)x-HPA-IMZ |  NH-(CH2)2N3 | NH-(CH2)5OH | (5)39/61 | 22 | [38] |
| (9.7)39/61 | 10 | ||||
| P(HPA-Az)-CA | 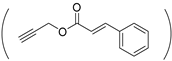 NH-(CH2)2N3 | NH-(CH2)5OH | (2.5)40/60 | 47 | [33] |
| (4.3)40/60 | 40 | ||||
| PASP-OCx | NH-(CH2)3OCH2CH3 | - | 100/- | 54 | [43] |
| NH-(CH2)2OCH2CH3 | - | 100/- | 87 | ||
| pH Responsive PASPAm Derivatives | Linkages | Formation of Linkages between | Ref | |
|---|---|---|---|---|
| A | B | |||
| NC-6300 | Hydrazone | Hydrazide group of PEG polyaspartate block copolymer | Ketone group of EPI | [23] |
| Cross-linked nanocapsules with PADH and PACA | Hydrazone | Hydrazide group of PADH | Aldehyde groups of PACA | [56] |
| PALHy-hyd-DOX | Hydrazone | Hydrazide group of PALHy | Ketone group of DOX | [65] |
| ALN-PEG/C-18/HYD-DOX-g-PASPAM | Hydrazone | Hydrazide group of PASPAM | Ketone group of DOX | [66] |
| MPEG/CA10/DOX-g-PASPAM | Hydrazone | Hydrazide group of MPEG/Hyd/CA10-g-PASPAM | Ketone group of DOX | [67] |
| PA | Hydrazone | Hydrazide group of biotin-PEG/C18-PSI/Hydrazine | Ketone group of DOX | [68] |
| PEG/Hyd-Curcumin/C18-g-PSI (NFA-Cur) | Hydrazone | Hydrazide group of PEG/C18-g-PSI | Ketone group of Curcumin | [60] |
| Injectable PAsp hydrogel | Hydrazone | Hydrazide group of PAHy | Aldehyde groups of PAAld | [61] |
| PHHZA-linked alginate hydrogels | Hydrazone | Hydrazide group of PHHZA | Aldehyde groups of oxidized alginate | [69] |
| PHEDA-linked alginate hydrogels | Imine | Amino group of PHEDA | Aldehyde groups of oxidized alginate | [69] |
| Pasp-EDA-g-Ad/mPEG | Imine | Amino group of Pasp-EDA | Aldehyde group of 4-adamantane carboxylate benzaldehyde | [63] |
| PAsp(DET-Aco) | cis-Aconitic amide | Amino group of PAsp(DET) | Anhydride group of cis-aconitic anhydride | [70] |
| PEG-PAsp(DET-ACO) | cis-Aconitic amide | Amino group of PEG-PAsp(DET) | Anhydride group of cis-aconitic anhydride | [71] |
| PEG-PAsp(DET-PMM) | 2-propionic-3-methyl maleic amide | Amino group of PEG-PAsp(DET) | Anhydride group of 2-propionic-3-methyl maleic anhydride | [71] |
| mPEG-g-P(ae-Asp)-Hap | β-carboxylic amide | Amino group of PEG-g-P(ae-Asp) | Anhydride group of hexahydrophthalic anhydride | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Yi, H.; Bao, C. Stimuli-Responsive Poly(aspartamide) Derivatives and Their Applications as Drug Carriers. Int. J. Mol. Sci. 2021, 22, 8817. https://doi.org/10.3390/ijms22168817
Zhang G, Yi H, Bao C. Stimuli-Responsive Poly(aspartamide) Derivatives and Their Applications as Drug Carriers. International Journal of Molecular Sciences. 2021; 22(16):8817. https://doi.org/10.3390/ijms22168817
Chicago/Turabian StyleZhang, Guangyan, Hui Yi, and Chenhui Bao. 2021. "Stimuli-Responsive Poly(aspartamide) Derivatives and Their Applications as Drug Carriers" International Journal of Molecular Sciences 22, no. 16: 8817. https://doi.org/10.3390/ijms22168817
APA StyleZhang, G., Yi, H., & Bao, C. (2021). Stimuli-Responsive Poly(aspartamide) Derivatives and Their Applications as Drug Carriers. International Journal of Molecular Sciences, 22(16), 8817. https://doi.org/10.3390/ijms22168817






