Apheresis Platelet Rich-Plasma for Regenerative Medicine: An In Vitro Study on Osteogenic Potential
Abstract
:1. Introduction
2. Results
2.1. Study Population
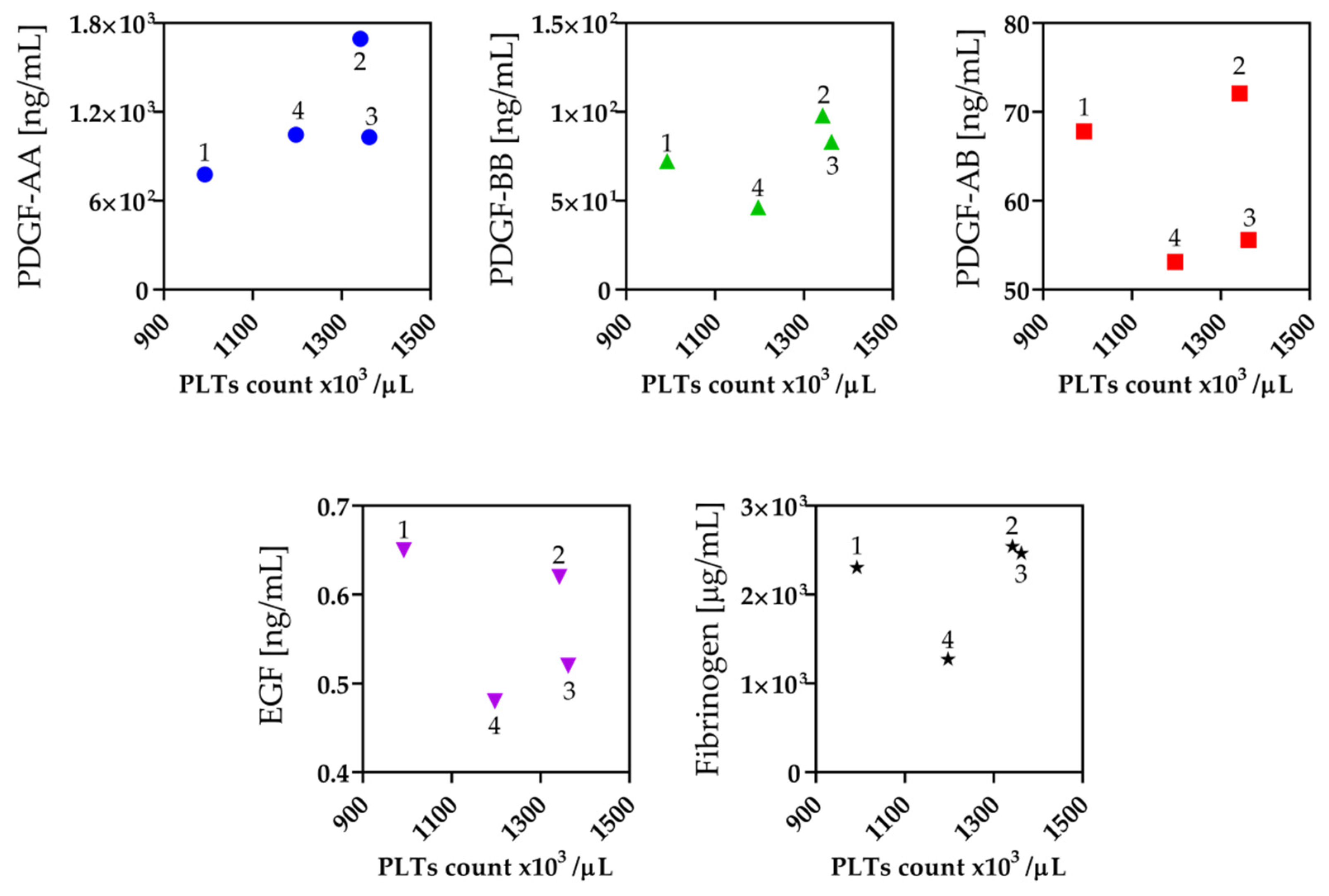
2.2. Platelet Count
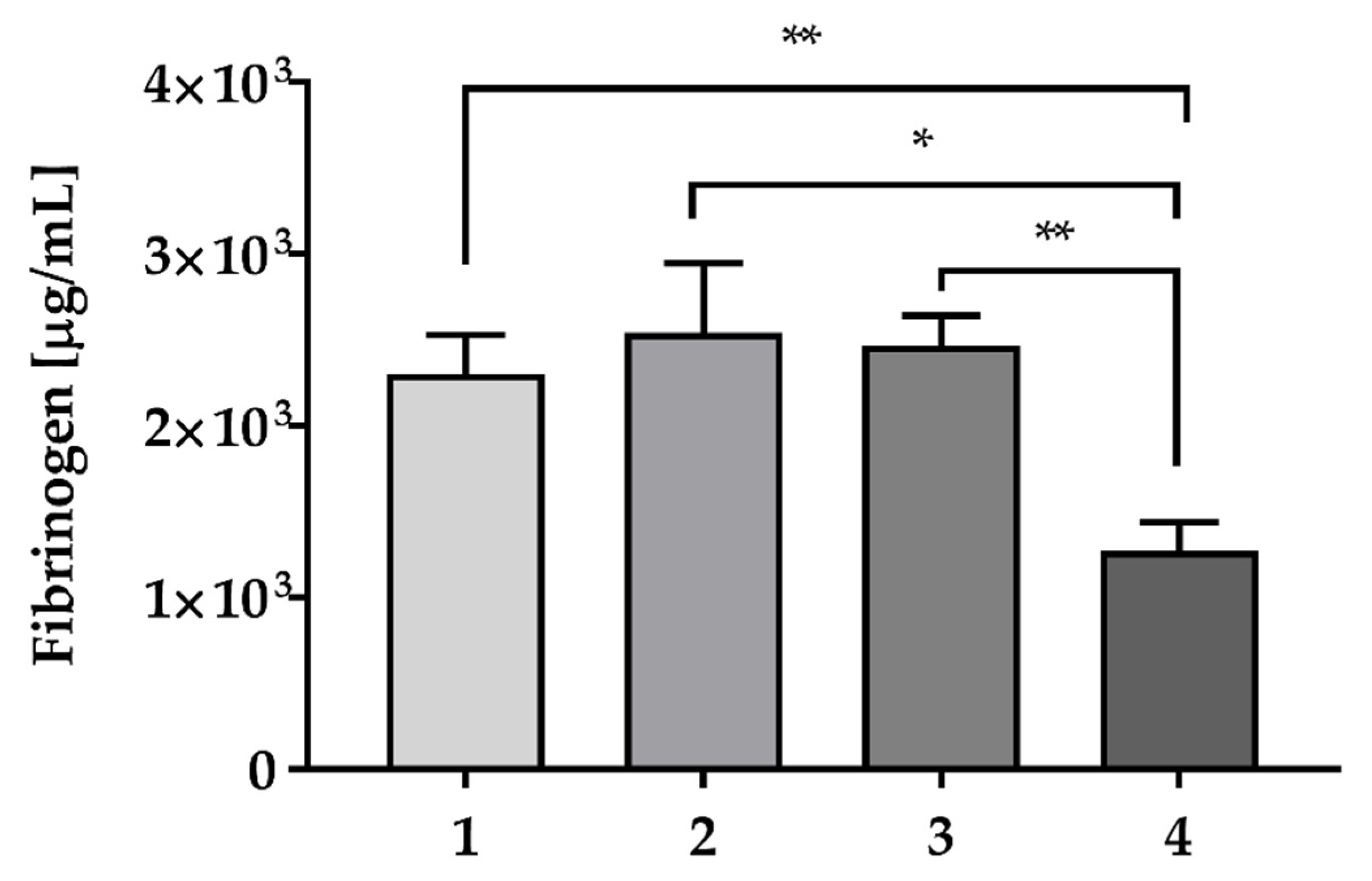
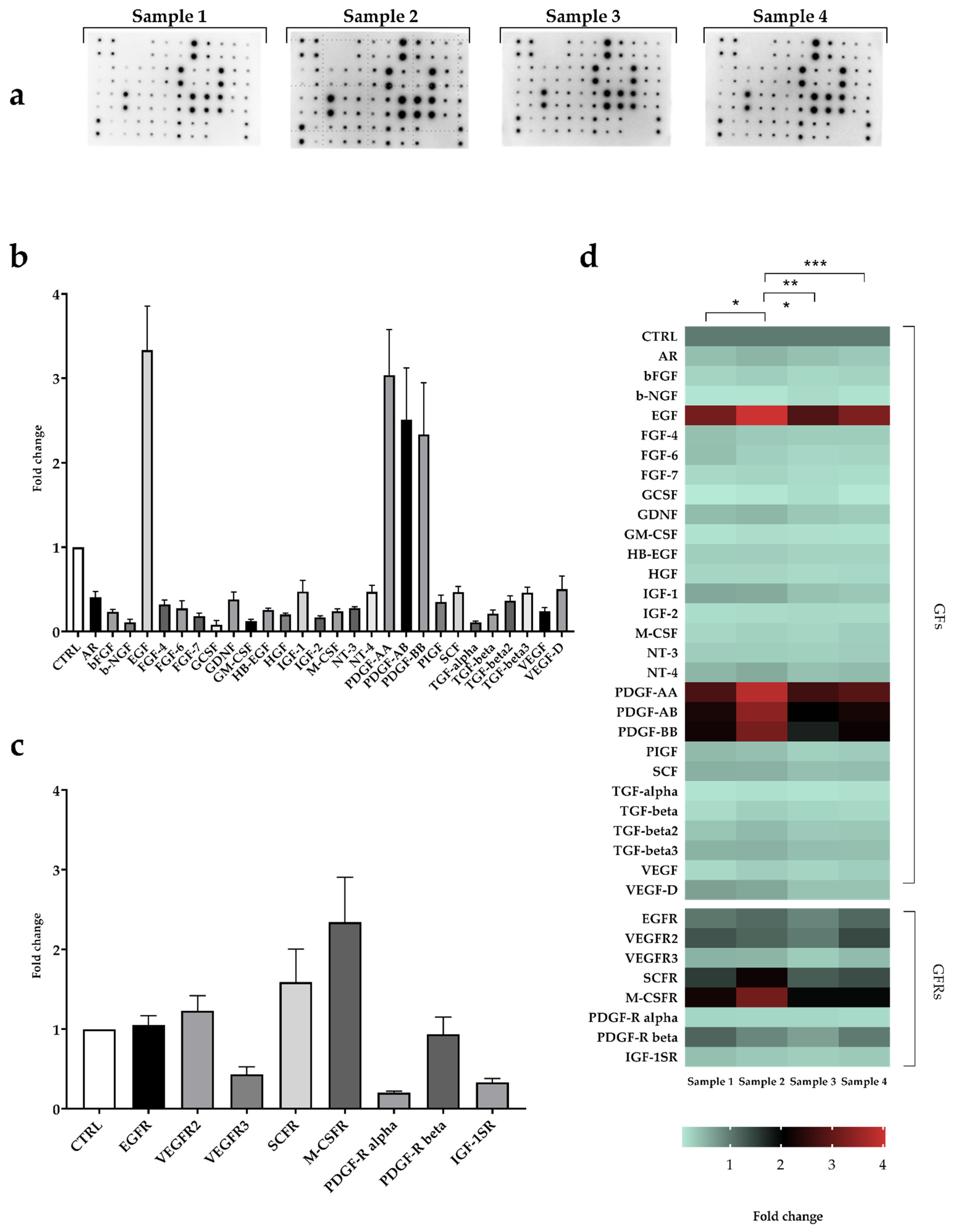
2.3. Fibrinogen and Growth Factors Quantification
2.4. In Vitro Evaluation of Osteogenic Potential
2.4.1. Cell Proliferation
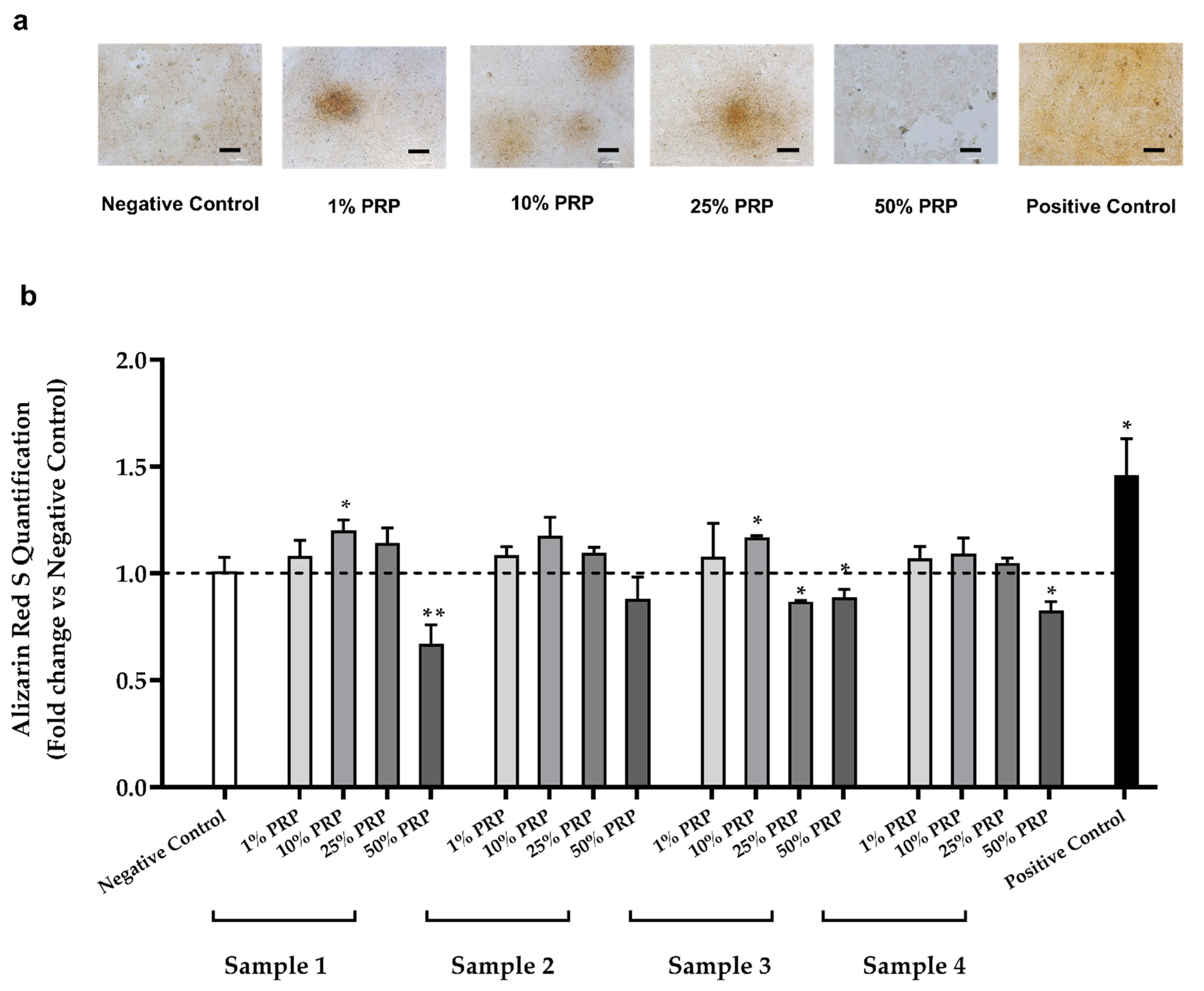
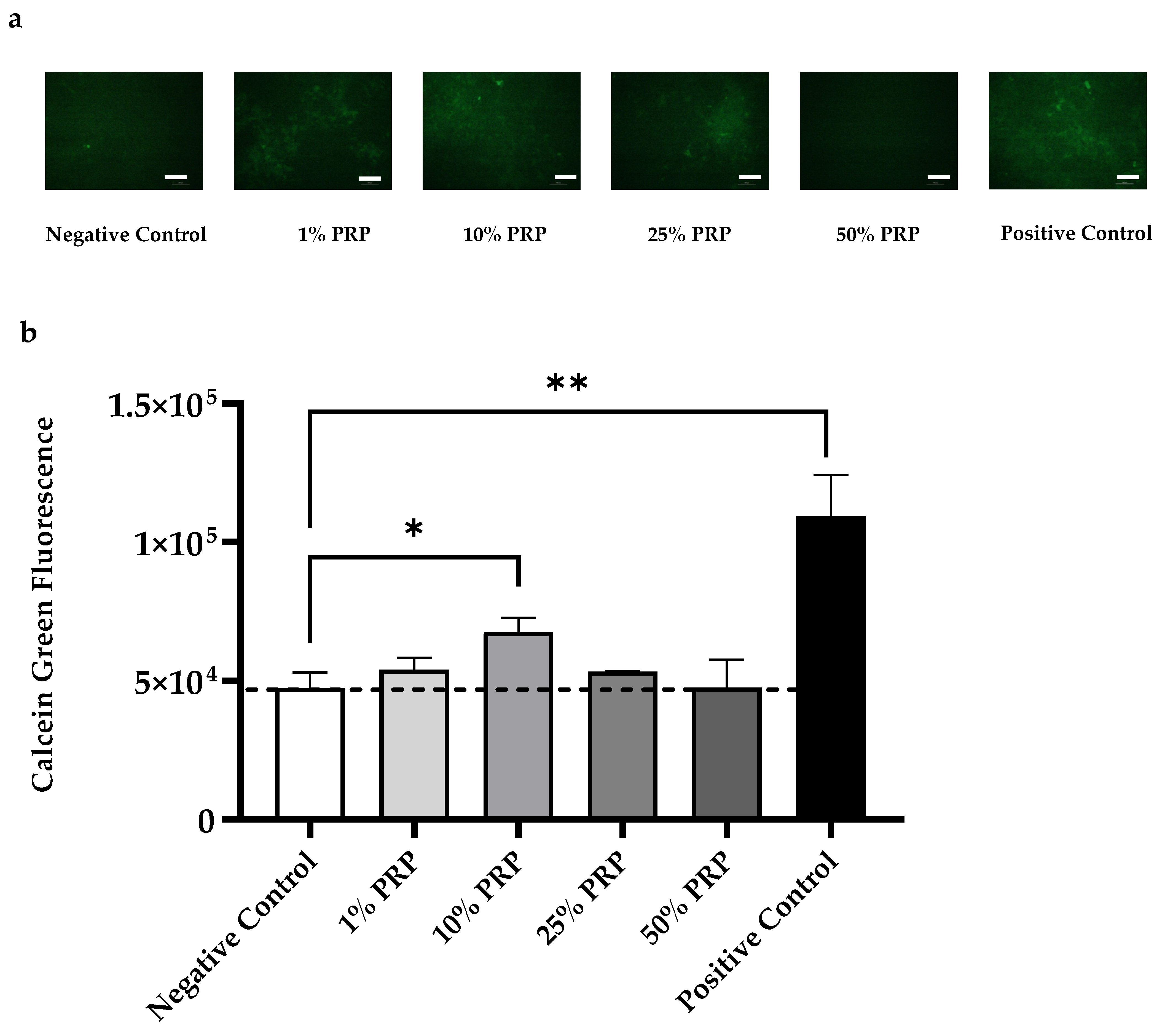
2.4.2. Mineralized Bone Matrix Deposition
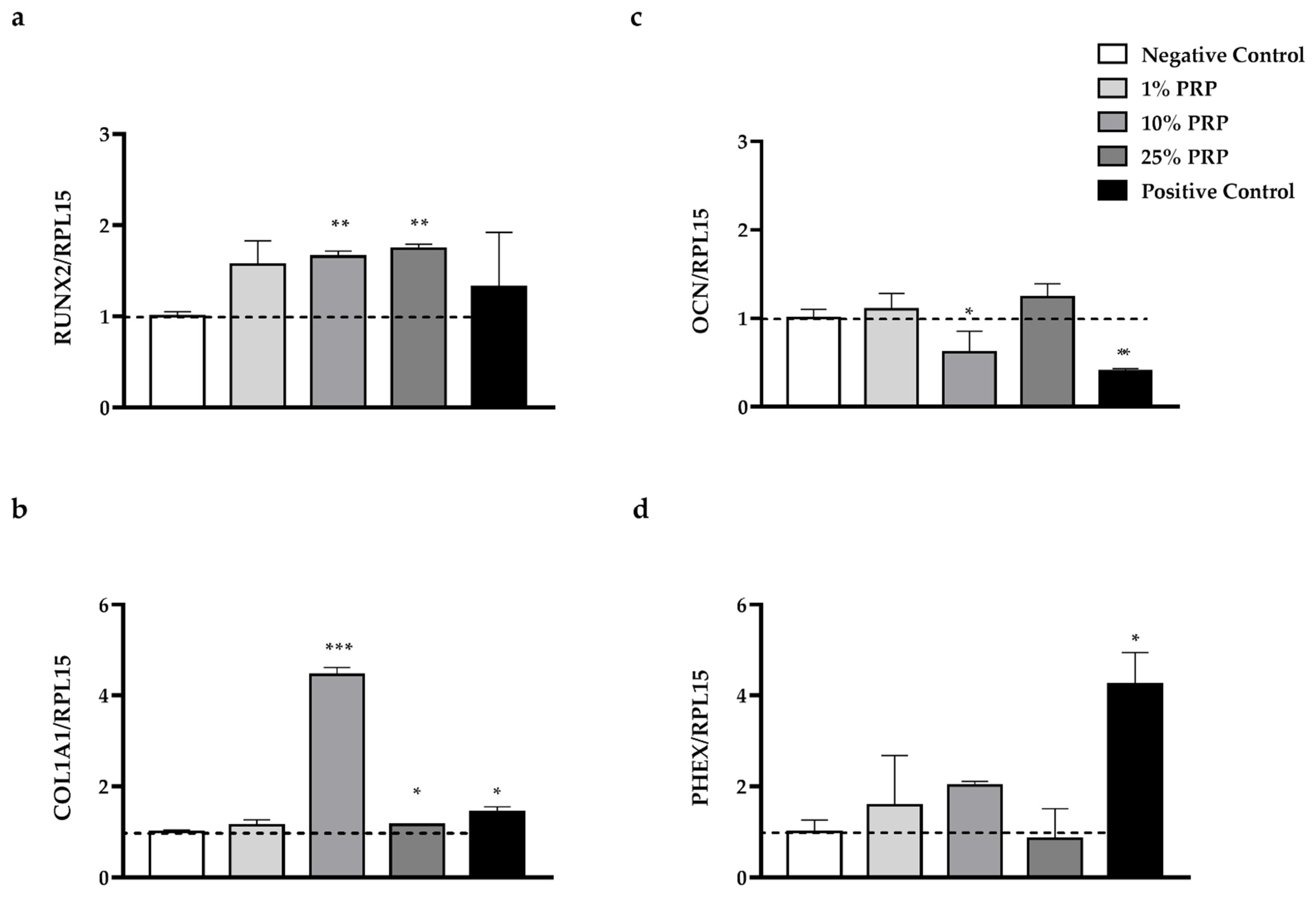
2.4.3. Gene Expression
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. PRP Samples Collection
4.3. Human Growth Factor Antibody Array
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Cell Culture
4.6. Resazurin Assay
4.7. Alizarin Red Assay
4.8. Calcein Green Staining
4.9. Gene Expression Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VEGF | Vascular endothelial growth factor |
| PDGF | Platelet-derived growth factor |
| TGF-β | Transforming growth factor β |
| EGF | Epidermal growth factor |
| IGF | Insulinlike growth factor |
| PRP | Platelet-Rich Plasma |
| GFs | Growth Factors |
| WB-PRP | Whole Blood PRP |
| RUNX2 | Runt-related transcription factor 2 |
| COL1A1 | alpha-1 type I collagen |
| OCN | Osteocalcin |
| PHEX | Phosphate-regulating neutral endopeptidase, X-linked |
| RBCs | Red blood cells |
| PLTs | Platelets |
| GFRs | Growth Factors Receptors |
| MCSFR | Macrophage colony-stimulating factor receptor |
| SCSFR | Stem Cell Factor Receptor |
| HSAB | Human Serum AB |
| HGF | Hepatocyte growth factor |
| ACD-A | Acid Citrate Dextrose Solution A |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal Bovine Serum |
| PBS | Phosphate-buffered saline |
Appendix A
| Study Population | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Sex | F | F | M | M |
| Characteristics | ||||
| Age (years) | 21 | 38 | 59 | 56 |
| Systolic blood pressure | 115 | 110 | 120 | 140 |
| Diastolic blood pressure | 75 | 70 | 80 | 90 |
| Weight (Kg) | 78 | 65 | 75 | 87 |
| WBC (103/µL) | 5.7 | 4.3 | 5.8 | 4.8 |
| RBC (106/µL) | 4.8 | 4.8 | 5.1 | 5.6 |
| MCV (fL) | 84.1 | 83.5 | 85.2 | 87.2 |
| MCH (pg) | 26.9 | 26.3 | 30.1 | 29.2 |
| MCHC (g/dL) | 32 | 31.4 | 35.3 | 33.5 |
| Haematocrit (HCT, %) | 40.1 | 40.0 | 43.4 | 48.6 |
| Haemoglobin (g/dL) | 12.8 | 12.6 | 15.3 | 16.3 |
| Platelets (103 µL) | 300 | 256 | 252 | 249 |
| MPV (fL) | 7.9 | 7.8 | 6.4 | 7.9 |
| Apheresis procedure | ||||
| Total volume of processed blood (mL) | 1933 | 1879 | 2260 | 2068 |
| Number of cycles | 4 | 4 | 5 | 5 |
| ACD-A volume (mL) | 258 | 252 | 297 | 273 |
References
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Bigham-Sadegh, A.; Oryan, A. Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures. Int. Wound J. 2015, 12, 238–247. [Google Scholar] [CrossRef]
- Bodine, P.V.; Komm, B.S. Tissue culture models for studies of hormone and vitamin action in bone cells. Vitam. Horm. 2002, 64, 101–151. [Google Scholar] [CrossRef] [PubMed]
- Schaffler, M.B.; Cheung, W.Y.; Majeska, R.; Kennedy, O. Osteocytes: Master orchestrators of bone. Calcif. Tissue Int. 2014, 94, 5–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 2, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Bigham-Sadegh, A. Bone morphogenetic proteins: A powerful osteoinductive compound with non-negligible side effects and limitations. Biofactors 2014, 40, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.R.; Daluiski, A.; Einhorn, T.A. The role of growth factors in the repair of bone. Biology and clinical applications. J. Bone Jt. Surg. Am. 2002, 84, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Tejero, R.; Zalduendo, M.M.; Orive, G. Plasma rich in growth factors promotes bone tissue regeneration by stimulating proliferation, migration, and autocrine secretion in primary human osteoblasts. J. Periodontol. 2013, 84, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Shin, Y.W.; Yang, K.H.; Kim, S.B.; Yoo, M.J.; Han, S.K.; Im, S.A.; Won, Y.D.; Sung, Y.B.; Jeon, T.S.; et al. A multi-center, randomized, clinical study to compare the effect and safety of autologous cultured osteoblast (Ossron) injection to treat fractures. BMC Musculoskelet. Disord. 2009, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, N.S.; Mohamed, M.R.; Ali, M.A.M.; Aglan, H.A.; Amr, K.S.; Ahmed, H.H. Osteoblast-based therapy-A new approach for bone repair in osteoporosis: Pre-clinicals. Tissue Eng. Regen. Med. 2020, 17, 363–373. [Google Scholar] [CrossRef]
- Noh, J.Y.; Yang, Y.; Jung, H. Molecular mechanisms and emerging therapeutics for osteoporosis. Int. J. Mol. Sci. 2020, 21, 7623. [Google Scholar] [CrossRef]
- Mokhtari, H.; Montaseri, A.; Mojaddadi, A.; Zonouzi, H.R.; Karimiyan, N.; Arami, S. Effect of Platelet-Rich Plasma on differentiation of osteoblasts and osteoclasts in the presence of three-dimensional scaffold. Pharm. Sci. 2018, 24, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Li, F.X.; Li, Y.; Qiao, C.W.; Zhu, J.; Chen, J.; Zhang, P.Y. Topical use of platelet-rich plasma can improve the clinical outcomes after total knee arthroplasty: A systematic review and meta-analysis of 1316 patients. Int. J. Surg. 2017, 38, 109–116. [Google Scholar] [CrossRef]
- Steller, D.; Herbst, N.; Pries, R.; Juhl, D.; Hakim, S.G. Positive impact of Platelet-rich plasma and Platelet-rich fibrin on viability, migration and proliferation of osteoblasts and fibroblasts treated with zoledronic acid. Sci. Rep. 2019, 9, 8310. [Google Scholar] [CrossRef] [Green Version]
- Colciago, A.; Celotti, F.; Casati, L.; Giancola, R.; Castano, S.M.; Antonini, G.; Sacchi, M.C.; Negri-Cesi, P. In Vitro Effects of PDGF Isoforms (AA, BB, AB and CC) on migration and proliferation of SaOS-2 osteoblasts and on migration of human osteoblasts. Int. J. Biomed. Sci. 2009, 5, 380–389. [Google Scholar] [PubMed]
- Del Angel-Mosqueda, C.; Gutiérrez-Puente, Y.; López-Lozano, A.P.; Romero-Zavaleta, R.E.; Mendiola-Jiménez, A.; Medina-De la Garza, C.E.; Márquez-M, M.; de la Garza-Ramos, M.A. Epidermal growth factor enhances osteogenic differentiation of dental pulp stem cells in vitro. Head Face Med. 2015, 11, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Lin, Y.; Hu, X.; Zhang, Y.; Wu, H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 707–713. [Google Scholar] [CrossRef]
- Zumstein, M.A.; Berger, S.; Schober, M.; Boileau, P.; Nyffeler, R.W.; Horn, M.; Dahinden, C.A. Leukocyte- and platelet-rich fibrin (L-PRF) for long-term delivery of growth factor in rotator cuff repair: Review, preliminary results and future directions. Curr. Pharm. Biotechnol. 2012, 13, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Sitek, P.; Wysocka-Wycisk, A.; Kępski, F.; Król, D.; Bursig, H.; Dyląg, S. PRP-fibrinogen gel-like chondrocyte carrier stabilized by TXA-preliminary study. Cell Tissue Bank. 2013, 14, 133–140. [Google Scholar] [CrossRef]
- Martino, M.M.; Briquez, P.S.; Ranga, A.; Lutolf, M.P.; Hubbell, J.A. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc. Natl. Acad. Sci. USA 2013, 110, 4563–4568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graziani, F.; Ivanovski, S.; Cei, S.; Ducci, F.; Tonetti, M.; Gabriele, M. The in vitro effect of different PRPconcentrations on osteoblasts and fibroblasts. Clin. Oral Implant. Res. 2006, 17, 212–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oryan, A.; Alidadi, S.; Moshiri, A. Platelet-rich plasma for bone healing and regeneration. Expert Opin. Biol. Ther. 2016, 16, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Agostini, F.; Abbruzzese, L.; Toffola, R.T.; Zanolin, S.; Suine, C.; Mazzucato, M. Growth factor release from platelet concentrates: Analytic quantification and characterization for clinical applications. Vox Sang. 2013, 105, 129–136. [Google Scholar] [CrossRef]
- Agostini, F.; Polesel, J.; Battiston, M.; Lombardi, E.; Zanolin, S.; da Ponte, A.; Astori, G.; Durante, C.; Mazzucato, M. Standardization of platelet release products for clinical applications in cell therapy: A mathematical approach. J. Transl. Med. 2017, 15, 107. [Google Scholar] [CrossRef]
- Camargo, P.M.; Lekovic, V.; Weinlaender, M.; Vasilic, N.; Madzarevic, M.; Kenney, E.B. Platelet-rich plasma and bovine porous bone mineral combined with guided tissue regeneration in the treatment of intrabony defects in humans. J. Periodontal. Res. 2002, 37, 300–306. [Google Scholar] [CrossRef]
- Dallari, D.; Savarino, L.; Stagni, C.; Cenni, E.; Cenacchi, A.; Fornasari, P.M.; Albisinni, U.; Rimondi, E.; Baldini, N.; Giunti, A. Enhanced tibial osteotomy healing with use of bone grafts supplemented with platelet gel or platelet gel and bone marrow stromal cells. J. Bone Jt. Surg. Am. 2007, 89, 2413–2420. [Google Scholar] [CrossRef]
- Divieti Pajevic, P. New and Old Osteocytic Cell Lines and 3D Models. Curr. Osteoporos. Rep. 2020, 18, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Billiard, J.; Moran, R.A.; Whitley, M.Z.; Chatterjee-Kishore, M.; Gillis, K.; Brown, E.L.; Komm, B.S.; Bodine, P.V.N. Transcriptional profiling of human osteoblast differentiation. J. Cell. Biochem. 2003, 89, 389–400. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Systematic Review—The potential implications of different Platelet-Rich-Plasma (PRP) concentrations in regenerative medicine for tissue repair. Int. J. Mol. Sci. 2020, 21, 5702. [Google Scholar] [CrossRef]
- Aprili, G.; Gandini, G.; Guaschino, R.; Mazzucco, L.; Salvaneschi, L.; Vaglio, S. SIMTI Working Group. SIMTI recommendations on blood components for non-transfusional use. Blood Transfus. 2013, 11, 611–622. [Google Scholar] [CrossRef]
- Singh, R.P.; Marwaha, N.; Malhotra, P.; Dash, S. Quality assessment of platelet concentrates prepared by platelet rich plasma-platelet concentrate, buffy coat poor-platelet concentrate (BC-PC) and apheresis-PC methods. Asian J. Transfus. Sci. 2009, 3, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Marck, R.E.; Gardien, K.L.M.; Vlig, M.; Breederveld, R.S.; Middelkoop, E. Growth factor quantification of Platelet-Rich Plasma in burn patients compared to matched healthy volunteers. Int. J. Mol. Sci. 2019, 20, 288. [Google Scholar] [CrossRef] [Green Version]
- Castillo, T.N.; Pouliot, M.; Kim, H.J.; Dragoo, J.L. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am. J. Sports Med. 2011, 39, 266–271. [Google Scholar] [CrossRef]
- Serafini, G.; Lopreiato, M.; Lollobrigida, M.; Lamazza, L.; Mazzucchi, G.; Fortunato, L.; Mariano, A.; Scotto d’Abusco, A.; Fontana, M.; De Biase, A. Platelet Rich Fibrin (PRF) and Its Related Products: Biomolecular Characterization of the Liquid Fibrinogen. J. Clin. Med. 2020, 9, 1099. [Google Scholar] [CrossRef]
- Kidwai, F.; Edwards, J.; Zou, L.; Kaufman, D.S. Fibrinogen Induces RUNX2 Activity and Osteogenic Development from Human Pluripotent Stem Cells. Stem Cells 2016, 34, 2079–2089. [Google Scholar] [CrossRef] [Green Version]
- Krüger, J.P.; Freymannx, U.; Vetterlein, S.; Neumann, K.; Endres, M.; Kaps, C. Bioactive factors in platelet-rich plasma obtained by apheresis. Transfus Med. Hemother. 2013, 40, 432–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ten Dijke, P.; Fu, J.; Schaap, P.; Roelen, B.A. Signal transduction of bone morphogenetic proteins in osteoblast differentiation. J. Bone Jt. Surg. Am. 2003, 85 (Suppl. 3), 34–38. [Google Scholar] [CrossRef]
- Strandberg, G.; Sellberg, F.; Sommar, P.; Ronaghi, M.; Lubenow, N.; Knutson, F.; Berglund, D. Standardizing the freeze-thaw preparation of growth factors from platelet lysate. Transfusion 2017, 57, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.E.; Gisbert, S.; Palazón, A.; Alio, J.L. Quantification of growth factors and fibronectin in diverse preparations of Platelet-Rich Plasma for the treatment of ocular surface disorders (E-PRP). Transl. Vis. Sci. Technol. 2020, 9, 22. [Google Scholar] [CrossRef]
- Alliston, T.; Choy, L.; Ducy, P.; Karsenty, G.; Derynck, R. TGF-β-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J. 2001, 20, 2254–2272. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-S.; Kim, H.-J.; Li, Q.-L.; Chi, X.-Z.; Ueta, C.; Komori, T.; Wozney, J.M.; Kim, E.-G.; Choi, J.-Y.; Ryoo, H.-M. Runx2 is a common target of transforming growth factor β1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol. Cell. Biol. 2000, 20, 8783–8792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Medina, T.; Vaquette, C.; Ivanovski, S. Systematic comparison of the effect of four clinical-grade platelet rich hemoderivatives on osteoblast behaviour. Int. J. Mol. Sci. 2019, 20, 6243. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.B.; Chen, E.H.; Lynch, S.E. A review of the e_ects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthr. Cartil. 2006, 14, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Oneto, P.; Zubiry, P.R.; Schattner, M.; Etulain, J. Anticoagulants interfere with the angiogenic and regenerative responses mediated by platelets. Front. Bioeng. Biotechnol. 2020, 8, 223. [Google Scholar] [CrossRef]
- Ma, C.; Tian, X.; Kim, J.P.; Xie, D.; Ao, X.; Shan, D.; Lin, Q.; Hudock, M.R.; Bai, X.; Yang, J. Citrate-based materials fuel human stem cells by metabonegenic regulation. PNAS 2018, 115, E11741–E11750. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, T.M.; Jensen, E.D.; Westendorf, J.J. Runx2: A master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res. C Embryo Today 2005, 75, 213–225. [Google Scholar] [CrossRef]
- Cazzaniga, A.; Maier, J.A.M.; Castiglioni, S. Impact of simulated microgravity on human bone stem 502 cells: New hints for space medicine. Biochem. Biophys. Res. Commun. 2016, 473, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; de Crombrugghe, B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003, 19, 458–466. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kronenberg, H. Minireview: Transcriptional regulation in development of bone. Endocrinology 2005, 146, 1012–1017. [Google Scholar] [CrossRef]
- Dallas, S.L.; Prideaux, M.; Bonewald, L.F. The osteocyte: An endocrine cell ... and more. Endocr. Rev. 2013, 34, 658–690. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, Z.Y.; Wang, Y.P.; Wu, Z.H.; Yu, B. Dynamic expression profiles of marker genes in osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Chin. Med. Sci. J. 2015, 30, 108–113. [Google Scholar] [CrossRef]
- Westbroek, I.; De Rooij, K.E.; Nijweide, P.J. Osteocyte-specific monoclonal antibody MAb OB7.3 is directed against Phex protein. J. Bone Miner. Res. 2002, 17, 845–853. [Google Scholar] [CrossRef]
- Aubin, J.E. Regulation of osteoblast formation and function. Rev. Endocr. Metab. Disord. 2001, 2, 81–94. [Google Scholar] [CrossRef]
- Franz-Odendaal, T.A.; Hall, B.K.; Witten, P.E. Buried alive: How osteoblasts become osteocytes. Dev. Dyn. 2006, 235, 176–190. [Google Scholar] [CrossRef]
- Tsao, Y.T.; Huang, Y.J.; Wu, H.H.; Liu, Y.A.; Liu, Y.S.; Lee, O.K. Osteocalcin Mediates Biomineralization during Osteogenic Maturation in Human Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2017, 18, 159. [Google Scholar] [CrossRef]
- Fujisawa, R.; Tamura, M. Acidic bone matrix proteins and their roles in calcification. Front. Biosci. (Landmark Ed.) 2012, 17, 1891–1903. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, Y.; Shi, B.; Cheng, X. A platelet-derived growth factor releasing chitosan/coral composite scaffold for periodontal tissue engineering. Biomaterials 2007, 28, 1515–1522. [Google Scholar] [CrossRef]
- Glueck, M.; Gardner, O.; Czekanska, E.; Alini, M.; Stoddart, M.J.; Salzmann, G.M.; Schmal, H. Induction of osteogenic differentiation in human mesenchymal stem cells by crosstalk with osteoblasts. BioRes. Open Access 2015, 4, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.S.; Song, I.H.; Park, S.Y.; Sung, M.C.; Ahn, M.W.; Song, K.E. Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J. Lab. Med. 2011, 31, 212–218. [Google Scholar] [CrossRef]
- Larsson, A.; Carlsson, L.; Gordh, T.; Lind, A.-L.; Thulin, M.; Kamali-Moghaddam, M. The effects of age and gender on plasma levels of 63 cytokines. J. Immunol. Methods 2015, 425, 58–61. [Google Scholar] [CrossRef]
- O’Donnell, C.; Migliore, E.; Grandi, F.C.; Koltsov, J.; Lingampalli, N.; Cisar, C.; Indelli, P.F.; Sebastiano, V.; Robinson, W.H.; Bhutani, N.; et al. Platelet-rich plasma (PRP) from older males with knee osteoarthritis depresses chondrocyte metabolism and upregulates inflammation. J. Orthop. Res. 2019, 37, 1760–1770. [Google Scholar] [CrossRef]
- Evanson, J.R.; Guyton, M.K.; Oliver, D.L.; Hire, J.M.; Topolski, R.L.; Zumbrun, S.D.; McPherson, J.C.; Bojescul, J.A. Gender and age differences in growth factor concentrations from platelet-rich plasma in adults. Mil. Med. 2014, 179, 799–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, D.; Bilbao, A.M.; Beitia, M.; Garate, A.; Sánchez, P.; González-Burguera, I.; Isasti, A.; López De Jesús, M.; Zuazo-Ibarra, J.; Montilla, A.; et al. Effects of Platelet-Rich Plasma on Cellular Populations of the Central Nervous System: The Influence of Donor Age. Int. J. Mol. Sci. 2021, 22, 1725. [Google Scholar] [CrossRef]
- Bodine, P.V.; Vernon, S.K.; Komm, B.S. Establishment and hormonal regulation of a conditionally transformed preosteocytic cell line from adult human bone. Endocrinology 1996, 137, 4592–4604. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Toscani, D.; Palumbo, C.; Dalla Palma, B.; Ferretti, M.; Bolzoni, M.; Marchica, V.; Sena, P.; Martella, E.; Mancini, C.; Ferri, V. The proteasome inhibitor bortezomib maintains osteocyte viability in multiple myeloma patients by reducing both apoptosis and autophagy: A new function for proteasome inhibitors. J. Bone Miner. Res. 2016, 31, 815–827. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, N.; Colla, S.; Morandi, F.; Lazzaretti, M.; Sala, R.; Bonomini, S.; Grano, M.; Colucci, S.; Svaldi, M.; Rizzoli, V. Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood 2005, 106, 2472–2483. [Google Scholar] [CrossRef]
- Negri, F.; Bozzetti, C.; Pedrazzi, G.; Azzoni, C.; Bottarelli, L.; Squadrilli, A.; Lagrasta, C.; Tamagnini, I.; Bisagni, A.; Ragazzi, M. High levels of Notch intracellular cleaved domain are associated with stemness and reduced bevacizumab efficacy in patients with advanced colon cancer. Oncol. Rep. 2019, 42, 2750–2758. [Google Scholar] [CrossRef] [PubMed]

| Sample | PLTs Recovery (103/µL) | Enrichment | |
|---|---|---|---|
| Whole Blood | PRP | ||
| 1 | 300 | 992 | 3.3 |
| 2 | 256 | 1342 | 5.4 |
| 3 | 252 | 1362 | 5.4 |
| 4 | 249 | 1197 | 4.8 |
| Growth Factors (ng/mL) | ||||
|---|---|---|---|---|
| PRP | PDGF-AA | PDGF-AB | PDGF-BB | EGF |
| 1 | 777.25 ± 147.07 | 67.82 ± 2.16 | 72.29 ± 4.98 | 0.65 ± 0.05 * |
| 2 | 1694.25 ± 311.23 ** | 72.10 ± 2.53 *** | 98.10 ± 12.13 | 0.62 ± 0.13 |
| 3 | 1030.07± 62.74 | 55.58 ± 5.26 | 83.18 ± 12.26 | 0.52 ± 0.03 |
| 4 | 1046.06 ± 176.25 | 53.10 ± 3.42 | 46.26 ± 8.18 *** | 0.48 ± 0.37 |
| GFs (ng/mL) | ||||
|---|---|---|---|---|
| Dilution | PDGF-AA | PDGF-AB | PDGF-BB | EGF |
| 1% | 11.36 ± 3.9 | 0.61 ± 0.1 | 0.74 ± 0.2 | 5.65 ± 0.82 (pg/mL) |
| 10% | 113.67 ± 39 | 6.17 ± 0.9 | 7.45 ± 2.2 | 0.056 ± 0.0 |
| 25% | 284.18 ± 97 | 15.43 ± 2.3 | 18.62 ± 5.4 | 0.14 ± 0.02 |
| 50% | 568.37 ± 195.0 | 30.87 ± 4.6 | 37.25 ± 10.9 | 0.28 ± 0.04 |
| Gene Name | Gene Bank Number | Forward Primer | Reverse Primer | Efficiency | Product Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|---|---|
| COL1A1 | NM_000088 | GTCGAGGGCCAAGACGAAG | CAGATCACGTCATCGCACAAC | 1.07 | 143 | 59 |
| OCN | NM_199173 | TCACACTCCTCGCCCTAT | GTCAGCCAACTCGTCACA | 1.12 | 245 | 60 |
| PHEX | NM_000444 | AGGCATCACATTCACCAACAAC | GCACCATTGACCCTAAACTGAG | 0.82 | 138 | 59 |
| RPL15 | NM_002948 | GCAGCCATCAGGTAAGCCAAG | AGCGGACCCTCAGAAGAAAGC | 0.94 | 100 | 59 |
| RUNX2 | NM_004348 | CCAGGCAGGCACAGTCTTC | GTCAGAGGTGGCAGTGTCATC | 1.13 | 182 | 59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pulcini, S.; Merolle, L.; Marraccini, C.; Quartieri, E.; Mori, D.; Schiroli, D.; Berni, P.; Iotti, B.; Di Bartolomeo, E.; Baricchi, R.; et al. Apheresis Platelet Rich-Plasma for Regenerative Medicine: An In Vitro Study on Osteogenic Potential. Int. J. Mol. Sci. 2021, 22, 8764. https://doi.org/10.3390/ijms22168764
Pulcini S, Merolle L, Marraccini C, Quartieri E, Mori D, Schiroli D, Berni P, Iotti B, Di Bartolomeo E, Baricchi R, et al. Apheresis Platelet Rich-Plasma for Regenerative Medicine: An In Vitro Study on Osteogenic Potential. International Journal of Molecular Sciences. 2021; 22(16):8764. https://doi.org/10.3390/ijms22168764
Chicago/Turabian StylePulcini, Stefano, Lucia Merolle, Chiara Marraccini, Eleonora Quartieri, Daniele Mori, Davide Schiroli, Pamela Berni, Barbara Iotti, Erminia Di Bartolomeo, Roberto Baricchi, and et al. 2021. "Apheresis Platelet Rich-Plasma for Regenerative Medicine: An In Vitro Study on Osteogenic Potential" International Journal of Molecular Sciences 22, no. 16: 8764. https://doi.org/10.3390/ijms22168764
APA StylePulcini, S., Merolle, L., Marraccini, C., Quartieri, E., Mori, D., Schiroli, D., Berni, P., Iotti, B., Di Bartolomeo, E., Baricchi, R., Sala, R., & Pertinhez, T. A. (2021). Apheresis Platelet Rich-Plasma for Regenerative Medicine: An In Vitro Study on Osteogenic Potential. International Journal of Molecular Sciences, 22(16), 8764. https://doi.org/10.3390/ijms22168764






