Glycerol Improves Skin Lesion Development in the Imiquimod Mouse Model of Psoriasis: Experimental Confirmation of Anecdotal Reports from Patients with Psoriasis
Abstract
1. Introduction
2. Results
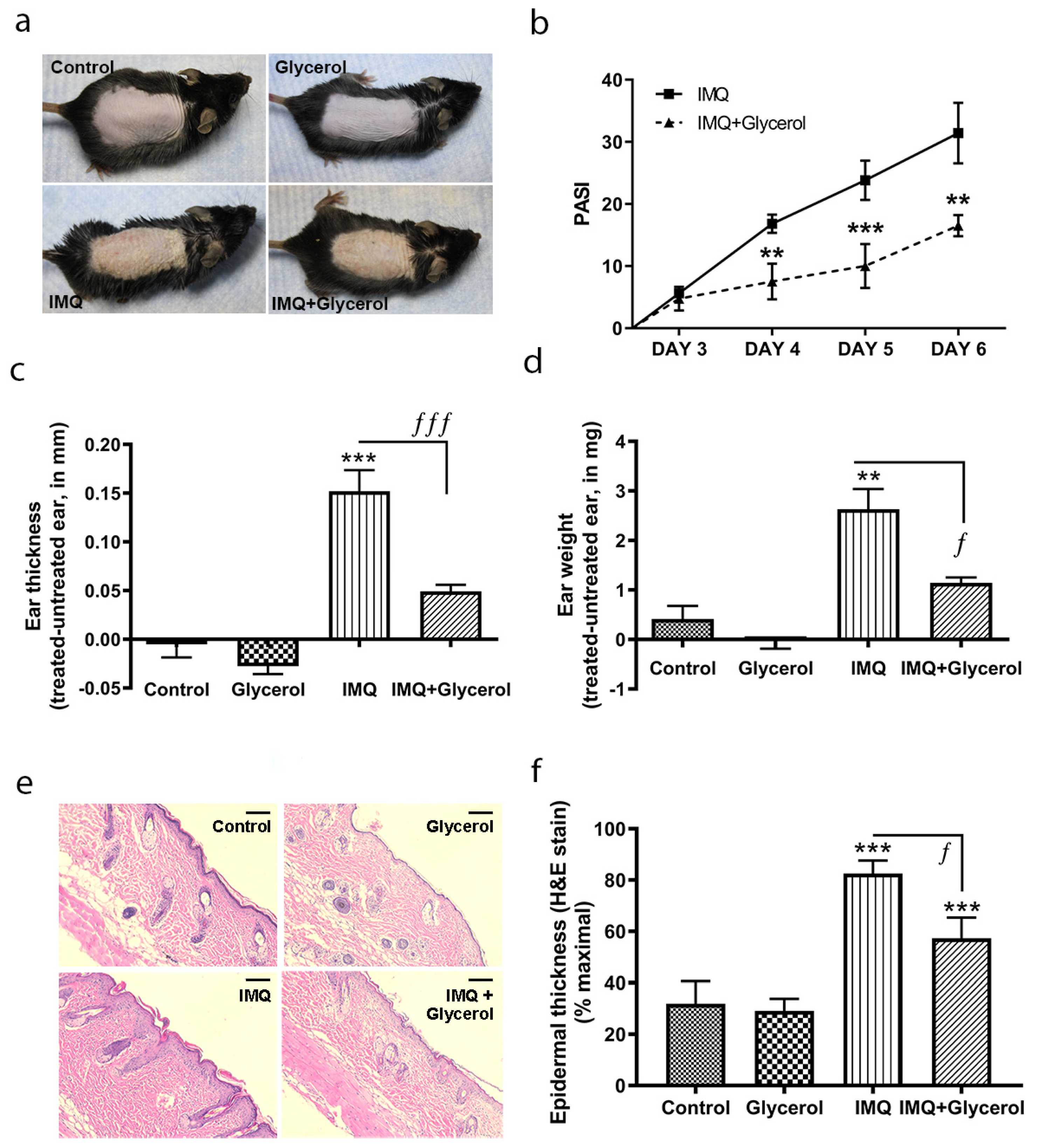
2.1. Topical Application of Glycerol Improved Psoriasiform Lesions, Reducing Inflammation and Epidermal Thickness in the IMQ Mouse Model of Psoriasis in Wild-Type Mice
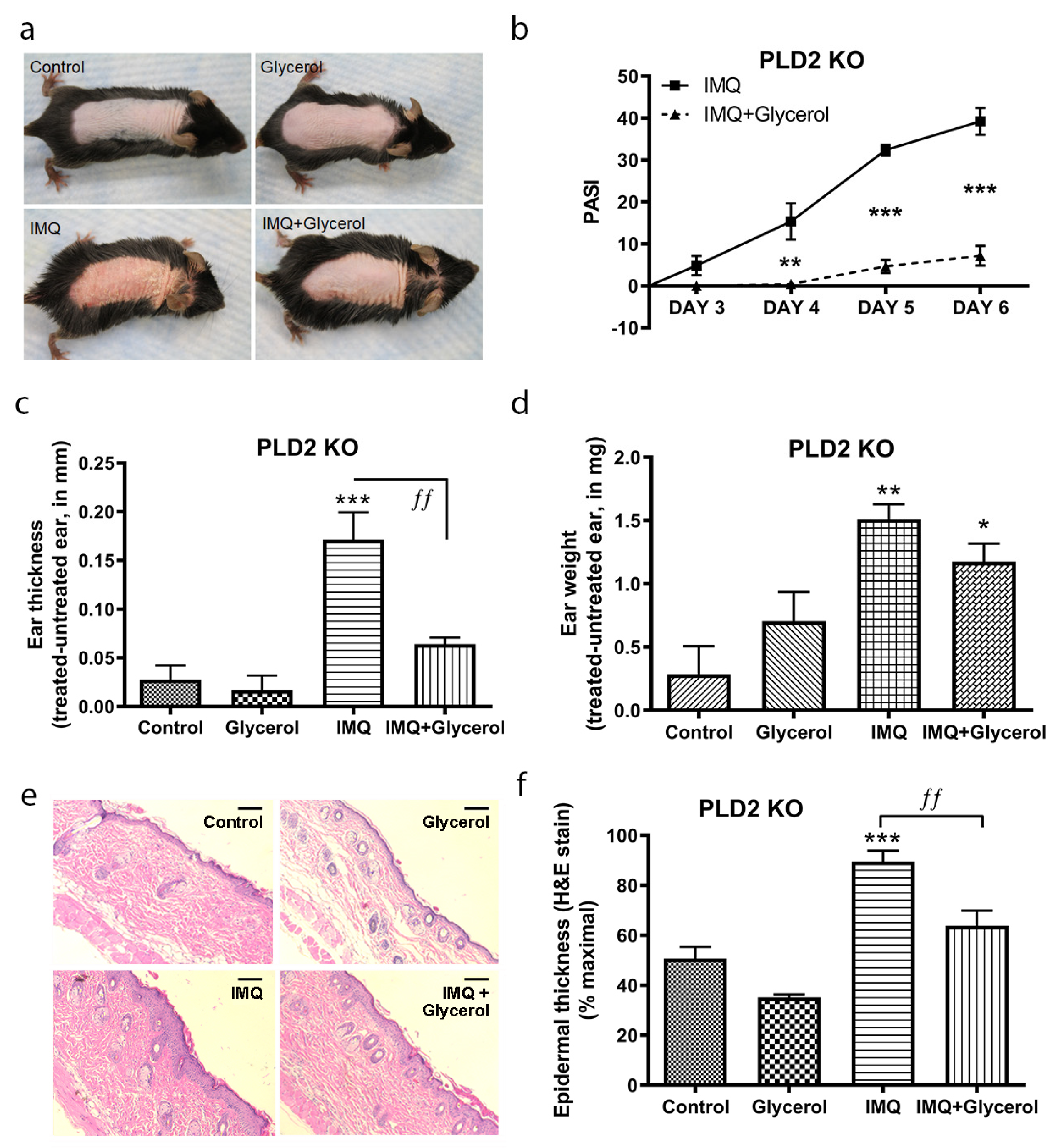
2.2. Topical Glycerol Improved IMQ-Induced Psoriasiform Lesions in PLD2 Knockout Mice
2.3. Oral Glycerol Improved IMQ-Induced Psoriasiform Lesions in Wild-Type C57BL/6 Mice
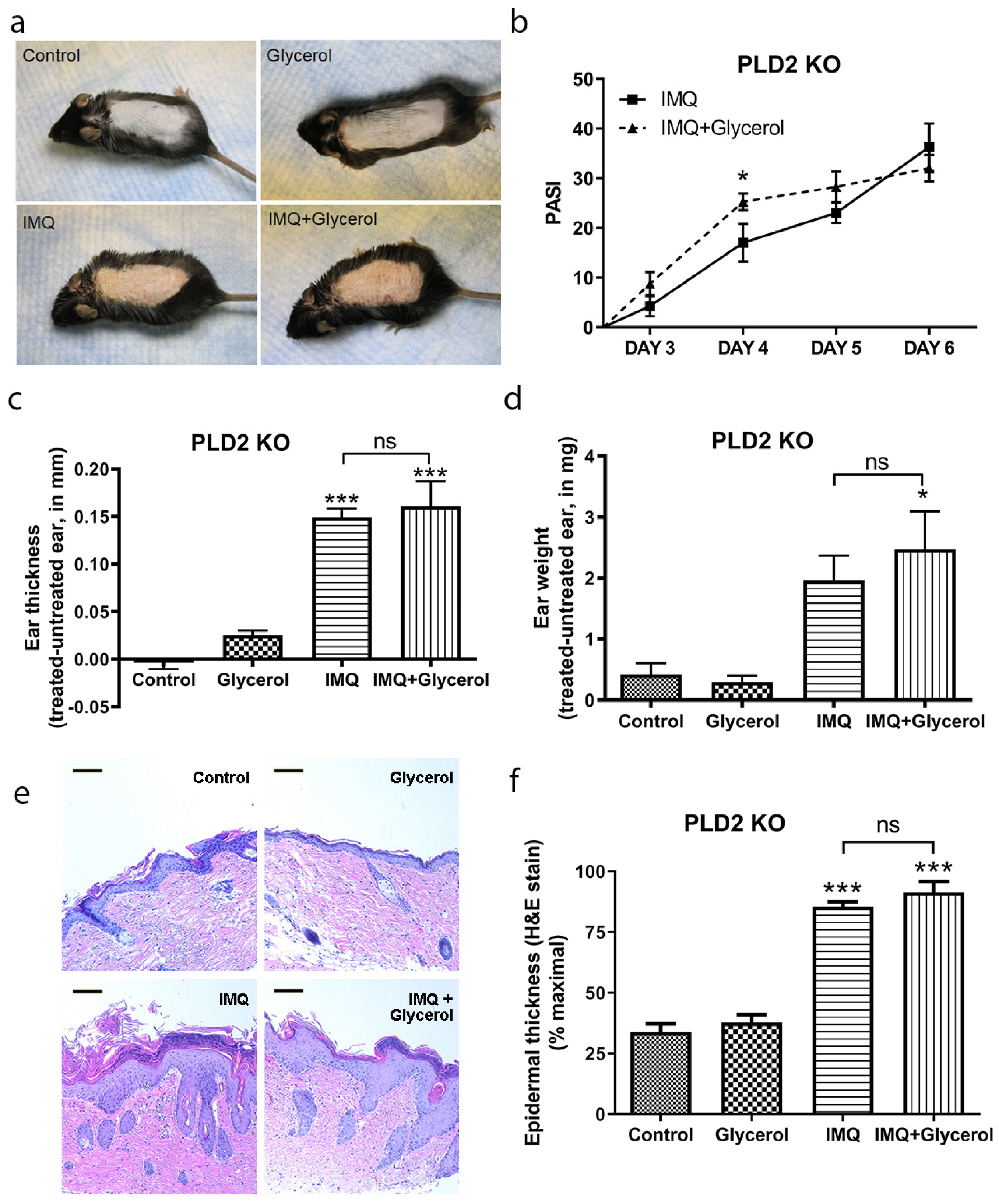
2.4. Oral Glycerol Did Not Improve Psoriasiform Skin Lesions in PLD2 Knockout Mice
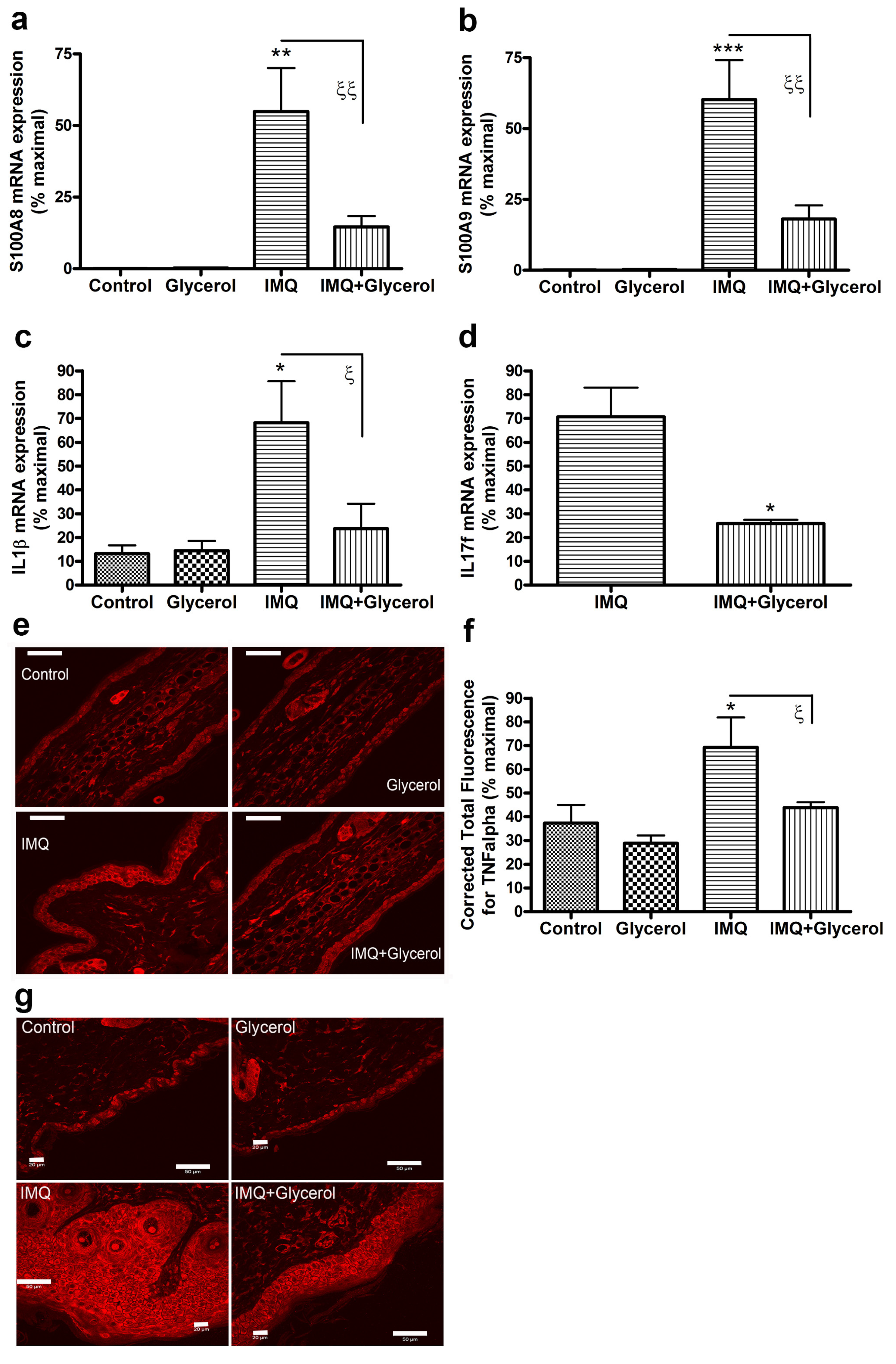
2.5. Oral Glycerol Inhibits the IMQ-Induced Increase in Molecular Markers of Psoriasis in Wild-Type C57BL/6 Mice
3. Discussion
4. Methods
4.1. Animal Experiments
4.2. Histology and Measurement of Epidermal Thickness
4.3. TNFa Immunoreactivity
4.4. RNA Isolation
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naldi, L. Epidemiology of Psoriasis. Curr. Drug Target Inflamm. Allergy 2004, 3, 121–128. [Google Scholar] [CrossRef]
- Stern, R.S.; Nijsten, T.; Feldman, S.; Margolis, D.J.; Rolstad, T. Psoriasis Is Common, Carries a Substantial Burden Even When Not Extensive, and Is Associated with Widespread Treatment Dissatisfaction. J. Investig. Dermatol. Symp. Proc. 2004, 9, 136–139. [Google Scholar] [CrossRef]
- Rapp, S.R.; Feldman, S.R.; Exum, M.; Fleischer, A.B.; Reboussin, D.M. Psoriasis causes as much disability as other major medical diseases. J. Am. Acad. Dermatol. 1999, 41, 401–407. [Google Scholar] [CrossRef]
- Gottlieb, A.B.; Chao, C.; Dann, F. Psoriasis comorbidities. J. Dermatol. Treat. 2008, 19, 5–21. [Google Scholar] [CrossRef]
- Yeung, H.; Takeshita, J.; Mehta, N.N. Psoriasis severity and the prevalence of major medical comorbidity: A population-based study. JAMA Dermatol. 2013, 149, 1173–1179. [Google Scholar] [CrossRef]
- Takeshita, J.; Grewal, S.; Langan, S.M. Psoriasis and comorbid diseases: Epidemiology. J. Am. Acad. Dermatol. 2017, 76, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Mrowietz, U. A molecule solves psoriasis? Systemic therapies for psoriasis inducing interleukin 4 and Th2 responses. J. Mol. Med. 2003, 81, 471–480. [Google Scholar] [CrossRef]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Nograles, K.E.; Davidovici, B.; Krueger, J.G. New insights in the immunologic basis of psoriasis. Semin. Cutan. Med. Surg. 2010, 29, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Russell, C.B.; Martin, D.A.; Towne, J.E.; Krueger, J.G. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013, 34, 174–181. [Google Scholar] [CrossRef]
- Sabat, R.; Wolk, K. Research in practice: IL-22 and IL-20: Significance for epithelial homeostasis and psoriasis pathogenesis. J. Dtsch. Dermatol. Ges. 2011, 9, 518–523. [Google Scholar] [CrossRef]
- Brotas, A.M.; Cunha, J.M.T.; Lago, E.H.J.; Machado, C.C.N.; Carneiro, S.C.D.S. Tumor necrosis factor-alpha and the cytokine network in psoriasis. An. Bras. Dermatol. 2012, 87, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Robertson, A.D.; Wu, J.; Schupp, C.; Lebwohl, M.G. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: Findings from the National Psoriasis Foundation surveys, 2003–2011. JAMA Dermatol. 2013, 149, 1180–1185. [Google Scholar] [CrossRef]
- Maverakis, E.; Bowen, M.P.; Raychaudhuri, S.P.; Sivamani, R.K.; Correa, G.; Ono, Y. Biological therapy of psoriasis. Indian J. Dermatol. 2010, 55, 161–170. [Google Scholar] [CrossRef]
- Fluhr, J.; Darlenski, R.; Surber, C. Glycerol and the skin: Holistic approach to its origin and functions. Br. J. Dermatol. 2008, 159, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Heard, L.K.; Chen, X.; Bollag, W.B. Aquaporins in the Skin. Adv. Exp. Med. Biol. 2017, 969, 173–191. [Google Scholar]
- Hara, M.; Ma, T.; Verkman, A.S. Selectively Reduced Glycerol in Skin of Aquaporin-3-deficient Mice May Account for Impaired Skin Hydration, Elasticity, and Barrier Recovery. J. Biol. Chem. 2002, 277, 46616–46621. [Google Scholar] [CrossRef] [PubMed]
- Fluhr, J.W.; Mao-Qiang, M.; Brown, B.E.; Wertz, P.W.; Crumrine, D.; Sundberg, J.P.; Feingold, K.R.; Elias, P.M. Glycerol Regulates Stratum Corneum Hydration in Sebaceous Gland Deficient (Asebia) Mice. J. Investig. Dermatol. 2003, 120, 728–737. [Google Scholar] [CrossRef]
- Qin, H.; Zheng, X.; Zhong, X.; Shetty, A.K.; Elias, P.M.; Bollag, W.B. Aquaporin-3 in keratinocytes and skin: Its role and interaction with phospholipase D2. Arch. Biochem. Biophys. 2011, 508, 138–143. [Google Scholar] [CrossRef]
- Zheng, X.; Bollag, W.B. Aquaporin 3 colocates with phospholipase D2 in caveolin-rich membrane microdomains and is regulated by keratinocyte differentiation. J. Investig. Dermatol. 2003, 121, 1487–1495. [Google Scholar] [CrossRef]
- Zheng, X.; Ray, S.; Bollag, W.B. Modulation of phospholipase D-mediated phosphatidylglycerol formation by differentiating agents in primary mouse epidermal keratinocytes. Biochim. Biophys. Acta 2003, 1643, 25–36. [Google Scholar] [CrossRef]
- Bollag, W.B.; Xie, D.; Zheng, X.; Zhong, X. A potential role for the phospholipase D2-aquaporin-3 signaling module in early keratinocyte differentiation: Production of a phosphatidylglycerol signaling lipid. J. Investig. Dermatol. 2007, 127, 2823–2831. [Google Scholar] [CrossRef]
- Xie, D.; Seremwe, M.; Edwards, J.G.; Podolsky, R.; Bollag, W.B. Distinct effects of different phosphatidylglycerol species on mouse keratinocyte proliferation. PLoS ONE 2014, 9, e107119. [Google Scholar] [CrossRef]
- Choudhary, V.; Uaratanawong, R.; Patel, R.R.; Patel, H.; Bao, W.; Hartney, B.; Cohen, E.; Chen, X.; Zhong, Q.; Isales, C.M.; et al. Phosphatidylglycerol Inhibits Toll-Like Receptor–Mediated Inflammation by Danger-Associated Molecular Patterns. J. Investig. Dermatol. 2019, 139, 868–877. [Google Scholar] [CrossRef]
- Xie, D.; Choudhary, V.; Seremwe, M.; Edwards, J.G.; Wang, A.; Emmons, A.C.; Bollag, K.A.; Johnson, M.H.; Bollag, W.B. Soy Phosphatidylglycerol Reduces Inflammation in a Contact Irritant Ear Edema Mouse Model In Vivo. J. Pharmacol. Exp. Ther. 2018, 366, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Griffith, S.; Chen, X.; Bollag, W.B. Pathogen-Associated Molecular Pattern-Induced TLR2 and TLR4 Activation Increases Keratinocyte Production of Inflammatory Mediators and is Inhibited by Phosphatidylglycerol. Mol. Pharmacol. 2020, 97, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Van der Fits, L.; Mourits, S.; Voerman, J.S. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Satooka, H.; Watanabe, S. Aquaporin-3-mediated hydrogen peroxide transport is required for NF-kappaB signalling in keratinocytes and development of psoriasis. Nat. Commun. 2015, 6, 7454. [Google Scholar] [CrossRef]
- Hara, M.; Verkman, A.S. Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc. Natl. Acad. Sci. USA 2003, 100, 7360–7365. [Google Scholar] [CrossRef] [PubMed]
- Schonthaler, H.B.; Guinea-Viniegra, J.; Wculek, S.K. S100A8-S100A9 protein complex mediates psoriasis by regulating the expression of complement factor C3. Immunity 2013, 39, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Gudjonsson, J.E.; Elder, J.T. Mouse models: Psoriasis: An epidermal disease after all? Eur. J. Hum. Genet. 2005, 14, 2–4. [Google Scholar] [CrossRef]
- Nakajima, K.; Kanda, T.; Takaishi, M. Distinct roles of IL-23 and IL-17 in the development of psoriasis-like lesions in a mouse model. J. Immunol. 2011, 186, 4481–4489. [Google Scholar] [CrossRef] [PubMed]
- Rácz, E.; Prens, E.; Kurek, D.; Kant, M.; De Ridder, D.; Mourits, S.; Baerveldt, E.M.; Ozgur, Z.; van Ijcken, W.; Laman, J.D.; et al. Effective Treatment of Psoriasis with Narrow-Band UVB Phototherapy Is Linked to Suppression of the IFN and Th17 Pathways. J. Investig. Dermatol. 2011, 131, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.C.; Skokos, D. Th17 response and inflammatory autoimmune diseases. Int. J. Inflam. 2012, 2012, 819467. [Google Scholar] [CrossRef]
- Taniguchi, K.; Arima, K.; Masuoka, M.; Ohta, S.; Shiraishi, H.; Ontsuka, K.; Suzuki, S.; Inamitsu, M.; Yamamoto, K.-I.; Simmons, O.; et al. Periostin controls keratinocyte proliferation and differentiation by interacting with the paracrine IL-1α/IL-6 Loop. J. Investig. Dermatol. 2014, 134, 1295–1304. [Google Scholar] [CrossRef]
- Blauvelt, A.; Chiricozzi, A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.A.; Towne, J.E.; Kricorian, G.; Klekotka, P.; Gudjonsson, J.E.; Krueger, J.G.; Russell, C. The emerging role of IL-17 in the pathogenesis of psoriasis: Preclinical and clinical findings. J. Investig. Dermatol. 2013, 133, 17–26. [Google Scholar] [CrossRef]
- Gordon, K.B.; Blauvelt, A.; Papp, K.A.; Langley, R.G.; Luger, T.; Ohtsuki, M.; Reich, K.; Amato, D.; Ball, S.G.; Braun, D.K.; et al. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 2016, 375, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Swindell, W.R.; Johnston, A.; Carbajal, S.; Han, G.; Wohn, C.; Lu, J.; Xing, X.; Nair, R.P.; Voorhees, J.J.; Elder, J.T.; et al. Genome-Wide expression profiling of five mouse models identifies similarities and differences with human psoriasis. PLoS ONE 2011, 6, e18266. [Google Scholar] [CrossRef] [PubMed]
- Swindell, W.R.; Michaels, K.A.; Sutter, A.J. Imiquimod has strain-dependent effects in mice and does not uniquely model human psoriasis. Genome Med. 2017, 9, 24. [Google Scholar] [CrossRef]
- Korponyai, C.; Szél, E.; Behány, Z.; Varga, E.; Mohos, G.; Dura, Á.; Dikstein, S.; Kemény, L.; Erős, G. Effects of Locally Applied Glycerol and Xylitol on the Hydration, Barrier Function and Morphological Parameters of the Skin. Acta Derm. Venereol. 2017, 97, 182–187. [Google Scholar] [CrossRef]
- Szél, E.; Polyánka, H.; Szabó, K.; Hartmann, P.; Degovics, D.; Balázs, N.I.; Németh, I.; Korponyai, C.; Csányi, E.; Kaszaki, J.; et al. Anti-irritant and anti-inflammatory effects of glycerol and xylitol in sodium lauryl sulphate-induced acute irritation. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2333–2341. [Google Scholar] [CrossRef]
- Breternitz, M.; Kowatzki, D.; Langenauer, M.; Elsner, P.; Fluhr, J. Placebo-Controlled, Double-Blind, Randomized, Prospective Study of a Glycerol-Based Emollient on Eczematous Skin in Atopic Dermatitis: Biophysical and Clinical Evaluation. Ski. Pharmacol. Physiol. 2008, 21, 39–45. [Google Scholar] [CrossRef]
- Asztalos, M.L.; Heller, M.M.; Lee, E.S.; Koo, J. The impact of emollients on phototherapy: A review. J. Am. Acad. Dermatol. 2013, 68, 817–824. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Cavallotti, C.; Berardesca, E. Emollients, moisturizers, and keratolytic agents in psoriasis. Clin. Dermatol. 2008, 26, 380–386. [Google Scholar] [CrossRef]
- Farr, P.M.; Diffey, B.L.; Steele, M.C. A preliminary study on the in vivo transmission of light through psoriatic plaques. Photo-dermatology 1984, 1, 87–90. [Google Scholar] [PubMed]
- Morita, A. Current developments in phototherapy for psoriasis. J. Dermatol. 2018, 45, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Fluhr, J.W.; Gloor, M.; Lehmann, L.; Lazzerinin, S.; Distante, F.; Berardesca, E. Glycerol accelerates recovery of barrier function in vivo. Acta Derm. Venereol. 1999, 79, 418–421. [Google Scholar] [PubMed]
- Segre, J.A. Epidermal barrier formation and recovery in skin disorders. J. Clin. Investig. 2006, 116, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Roberson, E.; Bowcock, A.M. Psoriasis genetics: Breaking the barrier. Trends Genet. 2010, 26, 415–423. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Kiatsurayanon, C.; Chieosilapatham, P.; Ogawa, H. Friends or Foes? Host defense (antimicrobial) peptides and proteins in human skin diseases. Exp. Dermatol. 2017, 26, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Nakahigashi, K.; Kabashima, K.; Ikoma, A.; Verkman, A.S.; Miyachi, Y.; Hara-Chikuma, M. Upregulation of aquaporin-3 is involved in keratinocyte proliferation and epidermal hyperplasia. J. Investig. Dermatol. 2011, 131, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Widyarman, A.S.; Drestia, A.M.; Bachtiar, E.W.; Bachtiar, B.M. The anti-inflammatory effects of glycerol-supplemented probiotic lactobacillus reuteri on infected epithelial cells In vitro. Contemp. Clin. Dent. 2018, 9, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Connor, V. Anti-TNF therapies: A comprehensive analysis of adverse effects associated with immunosuppression. Rheumatol. Int. 2009, 31, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Ghim, J.; Chelakkot, C.; Bae, Y.-S.; Suh, P.-G.; Ryu, S.H. Accumulating insights into the role of phospholipase D2 in human diseases. Adv. Biol. Regul. 2016, 61, 42–46. [Google Scholar] [CrossRef]
- Hong, K.-W.; Jin, H.-S.; Lim, J.-E.; Cho, Y.S.; Go, M.J.; Jung, J.; Lee, J.-E.; Choi, J.; Shin, C.; Hwang, S.-Y.; et al. Non-synonymous single-nucleotide polymorphisms associated with blood pressure and hypertension. J. Hum. Hypertens. 2010, 24, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Voss, K.E.; Bollag, R.J.; Fussell, N.; By, C.; Sheehan, D.J.; Bollag, W.B. Abnormal aquaporin-3 protein expression in hyperproliferative skin disorders. Arch. Dermatol. Res. 2011, 303, 591–600. [Google Scholar] [CrossRef] [PubMed][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhary, V.; Kaddour-Djebbar, I.; Custer, V.E.; Uaratanawong, R.; Chen, X.; Cohen, E.; Yang, R.; Ajebo, E.; Hossack, S.; Bollag, W.B. Glycerol Improves Skin Lesion Development in the Imiquimod Mouse Model of Psoriasis: Experimental Confirmation of Anecdotal Reports from Patients with Psoriasis. Int. J. Mol. Sci. 2021, 22, 8749. https://doi.org/10.3390/ijms22168749
Choudhary V, Kaddour-Djebbar I, Custer VE, Uaratanawong R, Chen X, Cohen E, Yang R, Ajebo E, Hossack S, Bollag WB. Glycerol Improves Skin Lesion Development in the Imiquimod Mouse Model of Psoriasis: Experimental Confirmation of Anecdotal Reports from Patients with Psoriasis. International Journal of Molecular Sciences. 2021; 22(16):8749. https://doi.org/10.3390/ijms22168749
Chicago/Turabian StyleChoudhary, Vivek, Ismail Kaddour-Djebbar, Victoria E. Custer, Rawipan Uaratanawong, Xunsheng Chen, Elyssa Cohen, Rong Yang, Etsubdenk Ajebo, Sarah Hossack, and Wendy B. Bollag. 2021. "Glycerol Improves Skin Lesion Development in the Imiquimod Mouse Model of Psoriasis: Experimental Confirmation of Anecdotal Reports from Patients with Psoriasis" International Journal of Molecular Sciences 22, no. 16: 8749. https://doi.org/10.3390/ijms22168749
APA StyleChoudhary, V., Kaddour-Djebbar, I., Custer, V. E., Uaratanawong, R., Chen, X., Cohen, E., Yang, R., Ajebo, E., Hossack, S., & Bollag, W. B. (2021). Glycerol Improves Skin Lesion Development in the Imiquimod Mouse Model of Psoriasis: Experimental Confirmation of Anecdotal Reports from Patients with Psoriasis. International Journal of Molecular Sciences, 22(16), 8749. https://doi.org/10.3390/ijms22168749





