Recent Applications of Retro-Inverso Peptides
Abstract
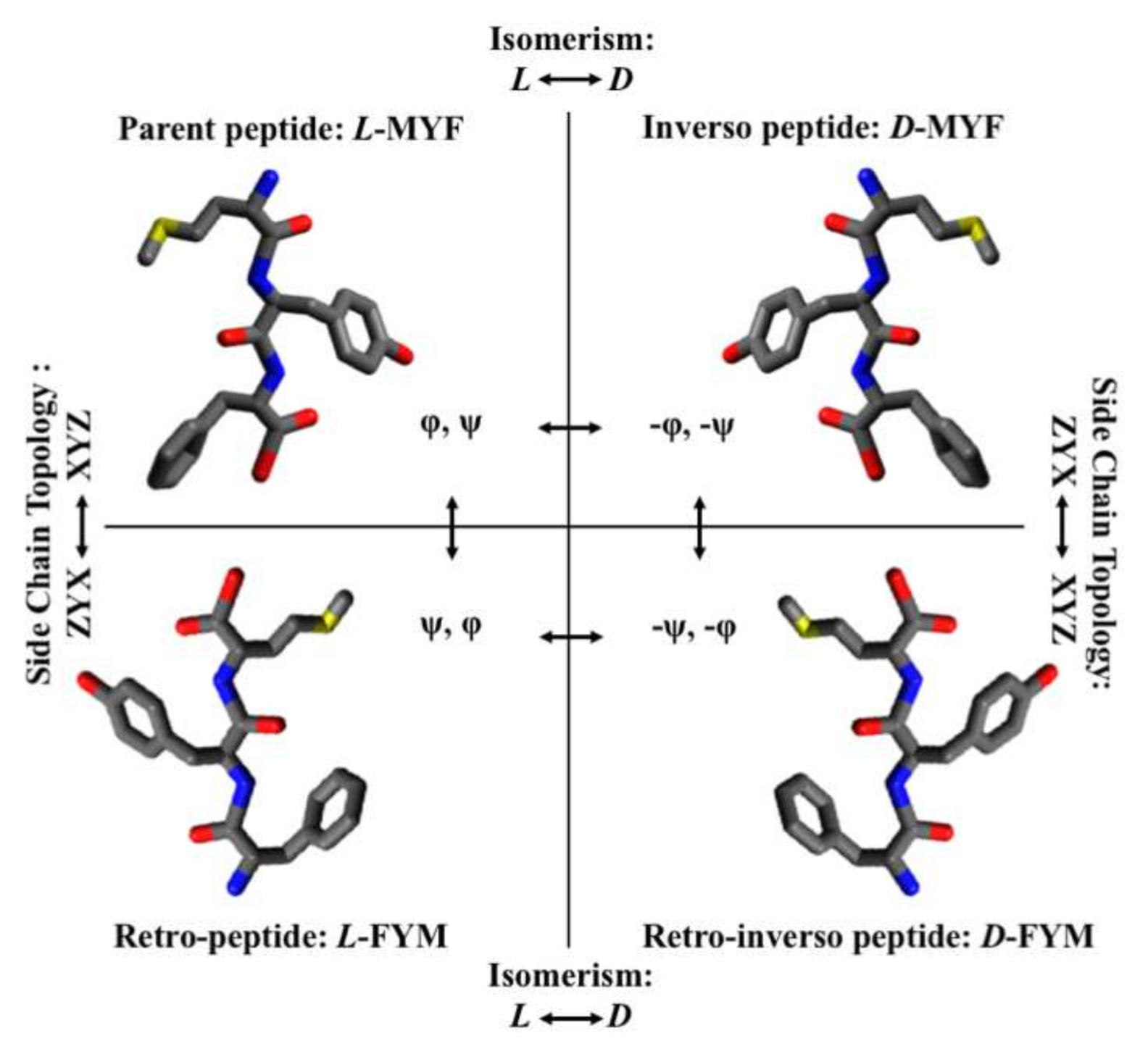
1. Topology, Structural Characteristics
2. Anticancer Applications—Diagnostic
| Name | Sequence 1 | Application | Ref |
|---|---|---|---|
| Anticancer Applications—Diagnostic | |||
| VEGF-P3(CYC) | I76TMQ79CG92IHQGQHPKIRMI80CE93MSF96 * | Inhibition angiogenesis | [38,39] |
| D(LPR) | D(Leu-Pro-Arg) | Inhibition retinal angiogenesis; Diagnostic | [41,42,43] |
| SP5 | PRPSPKMGVSVS * | Drug delivery | [44,45] |
| uPAR88–92 | SRSRY * | Maintaining chemotactic activity and triggers directed cell migration and angiogenesis | [46,47] |
| RI-3 | Ac-D(Tyr- Arg-Aib-Arg)- NH2 | Prevent extracellular invasion by tumor cells | [48] |
| D(RGD) | D(Asp-Gly-Arg) | Diagnostic | [49,50,51] |
| VS | SWFSRHRYSPFAVS * | Glioblastoma multiforme (GBM) | [52,53] |
| VAP | SNTRVAP * | Gliomas, glioma stem cells, vasculogenic mimicry and neovasculature | [54] |
| WSW | SYPGWSW * | Glioma cells and tumor neovasculature | [55] |
| BK | RPPGFSPFR * | Glioma cells | [56] |
| FP21 | YTRDLVYGDPARPGIQGTGTF * | Ovarian cancer | [57,58,59] |
| T7 | HAIYPRH * | Drug delivery | [60] |
| Applications in Immunology | |||
| TG19320 | (rty)4K2KG | IgG binding | [61,62] |
| VSVp | RGYVYQGL * | antigen surface of hepatitis B virus | [63] |
| OVAp | SIINFEKL * | antigen surface of hepatitis B virus | [63] |
| PS1 | HQLDPAFGANSTNPD * | antigen surface of hepatitis B virus | [63] |
| HAI | HAIYPRH * | Crossing BBB | [64] |
| THR | THRPPMWSPVWP * | Crossing BBB | [64] |
| InsB:9–23 | HLVEALYLVCGERGG * | Analogue of diabetogenic islet peptide—prevents T-cell activation in humanized model mice | [65,66] |
| Application in Neurodegenerative Diseases | |||
| Amytrap | WKGEWTGR * | Blocking the oligomerization and aggregation of Aβ1–42 | [67,68,69,70,71] |
| IAPP11–20 | RLANFLVHSS * | Strong inhibitory effects on amylin aggregation in T2DM | [72] |
| β-syn36–45 | GVLYVGSKT * | Reduction of amyloid fibril and oligomer formation | [73] |
| Application in Antimicrobial Antibiotics | |||
| RI1018 | rrwirvavilrv | Preventing formation of Biofilm | [74] |
| RI-JK6 | rivwvrirrwqv | Preventing formation of Biofilm | [74] |
| RI-73 | lwGvwrrvidwlr | Damaging the bacterial membrane | [75] |
| BMAP-28 | GGLRSLGRKILRAWKKYGPIIVPIIRIG * | Broad antimicrobial activities | [76] |
3. Applications in Immunology
4. Applications in Neurodegenerative Diseases
5. Application of RI Peptides as Antimicrobial Antibiotics
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, A.C.; Harris, J.L.; Khanna, K.K.; Hong, J.H. A Comprehensive Review on Current Advances in Peptide Drug Development and Design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar] [CrossRef]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef] [PubMed]
- Corbi-Verge, C.; Garton, M.; Nim, S.; Kim, P.M. Strategies to Develop Inhibitors of Motif-Mediated Protein-Protein Interactions as Drug Leads. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 39–60. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Kreil, G. D-amino acids in animal peptides. Annu. Rev. Biochem. 1997, 66, 337–345. [Google Scholar] [CrossRef]
- Di, L. Strategic Approaches to Optimizing Peptide ADME Properties. AAPS J. 2015, 17, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Fischer, P.M. The design, synthesis and application of stereochemical and directional peptide isomers: A critical review. Curr. Protein Pept. Sci. 2003, 4, 339–356. [Google Scholar] [CrossRef]
- Fletcher, M.D.; Campbell, M.M. Partially Modified Retro-Inverso Peptides: Development, Synthesis, and Conformational Behavior. Chem. Rev. 1998, 98, 763–796. [Google Scholar] [CrossRef] [PubMed]
- Grishin, D.V.; Zhdanov, D.D.; Pokrovskaya, M.V.; Sokolov, N.N. D-amino acids in nature, agriculture and biomedicine. All Life 2020, 13, 11–22. [Google Scholar] [CrossRef]
- Xi, W.; Hansmann, U.H.E. The effect of retro-inverse D-amino acid Abeta-peptides on Abeta-fibril formation. J. Chem. Phys. 2019, 150, 095101. [Google Scholar] [CrossRef]
- Li, C.; Zhan, C.; Zhao, L.; Chen, X.; Lu, W.Y.; Lu, W. Functional consequences of retro-inverso isomerization of a miniature protein inhibitor of the p53-MDM2 interaction. Bioorg. Med. Chem. 2013, 21, 4045–4050. [Google Scholar] [CrossRef]
- Garton, M.; Nim, S.; Stone, T.A.; Wang, K.E.; Deber, C.M.; Kim, P.M. Method to generate highly stable D-amino acid analogs of bioactive helical peptides using a mirror image of the entire PDB. Proc. Natl. Acad Sci. USA 2018, 115, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Rai, J. Peptide and protein mimetics by retro and retroinverso analogs. Chem. Biol. Drug Des. 2019, 93, 724–736. [Google Scholar] [CrossRef]
- Chorev, M. The partial retro-inverso modification: A road traveled together. Biopolymers 2005, 80, 67–84. [Google Scholar] [CrossRef]
- Rai, J. Mini Heme-Proteins: Designability of Structure and Diversity of Functions. Curr. Protein Pept. Sci. 2017, 18, 1132–1140. [Google Scholar] [CrossRef]
- Chorev, M.; Shavitz, R.; Goodman, M.; Minick, S.; Guillemin, R. Partially modified retro-inverso-enkephalinamides: Topochemical long-acting analogs in vitro and in vivo. Science 1979, 204, 1210–1212. [Google Scholar] [CrossRef] [PubMed]
- Ruvo, M.; Fassina, G. End-group modified retro-inverso isomers of tripeptide oxytocin analogues: Binding to neurophysin II and enhancement of its self-association properties. Int. J. Pept. Protein Res. 1995, 45, 356–365. [Google Scholar] [CrossRef]
- Sridhar, S.; Guruprasad, K. Can natural proteins designed with ‘inverted’ peptide sequences adopt native-like protein folds? PLoS ONE 2014, 9, e107647. [Google Scholar] [CrossRef] [PubMed]
- Verdoliva, A.; Ruvo, M.; Cassani, G.; Fassina, G. Topological mimicry of cross-reacting enantiomeric peptide antigens. J. Biol. Chem. 1995, 270, 30422–30427. [Google Scholar] [CrossRef]
- Chorev, M.; Goodman, M. Recent developments in retro peptides and proteins--an ongoing topochemical exploration. Trends Biotechnol. 1995, 13, 438–445. [Google Scholar] [CrossRef]
- Brady, L.; Dodson, G. Drug design. Reflections on a peptide. Nature 1994, 368, 692–693. [Google Scholar] [CrossRef]
- Jameson, B.A.; McDonnell, J.M.; Marini, J.C.; Korngold, R. A rationally designed CD4 analogue inhibits experimental allergic encephalomyelitis. Nature 1994, 368, 744–746. [Google Scholar] [CrossRef]
- Taylor, E.M.; Otero, D.A.; Banks, W.A.; O’Brien, J.S. Retro-inverso prosaptide peptides retain bioactivity, are stable In vivo, and are blood-brain barrier permeable. J. Pharmacol. Exp. Ther. 2000, 295, 190–194. [Google Scholar] [PubMed]
- Banerjee, A.; Raghothama, S.R.; Karle, I.L.; Balaram, P. Ambidextrous molecules: Cylindrical peptide structures formed by fusing left- and right-handed helices. Biopolymers 1996, 39, 279–285. [Google Scholar] [CrossRef]
- Caporale, A.; Biondi, B.; Schievano, E.; Wittelsberger, A.; Mammi, S.; Peggion, E. Structure-function relationship studies of PTH(1-11) analogues containing D-amino acids. Eur. J. Pharmacol. 2009, 611, 1–7. [Google Scholar] [CrossRef]
- Crisma, M.; Bisson, W.; Formaggio, F.; Broxterman, Q.B.; Toniolo, C. Factors governing 3(10)-helix vs alpha-helix formation in peptides: Percentage of C(alpha)-tetrasubstituted alpha-amino acid residues and sequence dependence. Biopolymers 2002, 64, 236–245. [Google Scholar] [CrossRef]
- Wermuth, J.; Goodman, S.L.; Jonczyk, A.; Kessler, H. Stereoisomerism and biological activity of the selective and superactive alpha(v)beta(3) integrin inhibitor cyclo(-RGDfV-) and its retro-inverso peptide. J. Am. Chem. Soc. 1997, 119, 1328–1335. [Google Scholar] [CrossRef]
- Yavari, B.; Mahjub, R.; Saidijam, M.; Raigani, M.; Soleimani, M. The Potential Use of Peptides in Cancer Treatment. Curr. Protein Pept. Sci. 2018, 19, 759–770. [Google Scholar] [CrossRef]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application (Review). Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef]
- Wirtz, D.; Konstantopoulos, K.; Searson, P.C. The physics of cancer: The role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer 2011, 11, 512–522. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Ferrara, J.L.M.; Cooke, K.R.; Teshima, T. The pathophysiology of acute graft-versus-host disease. Int. J. Hematol. 2003, 78, 181–187. [Google Scholar] [CrossRef]
- Kaumaya, P.T.; Foy, K.C. Peptide vaccines and targeting HER and VEGF proteins may offer a potentially new paradigm in cancer immunotherapy. Future Oncol. 2012, 8, 961–987. [Google Scholar] [CrossRef]
- Dass, C.R.; Tran, T.M.; Choong, P.F. Angiogenesis inhibitors and the need for anti-angiogenic therapeutics. J. Dent. Res. 2007, 86, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, J.A.; Nakada, M.T.; Trikha, M.; Lang, Z.; Gordon, M.S.; Jayson, G.C.; Corringham, R.; Prabhakar, U.; Davis, H.M.; Beckman, R.A. Alpha-v integrins as therapeutic targets in oncology. Cancer Investig. 2007, 25, 632–646. [Google Scholar] [CrossRef]
- Ferrara, N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS 2005, 209–231. [Google Scholar] [CrossRef]
- Vicari, D.; Foy, K.C.; Liotta, E.M.; Kaumaya, P.T. Engineered conformation-dependent VEGF peptide mimics are effective in inhibiting VEGF signaling pathways. J. Biol. Chem. 2011, 286, 13612–13625. [Google Scholar] [CrossRef] [PubMed]
- Foy, K.C.; Liu, Z.; Phillips, G.; Miller, M.; Kaumaya, P.T. Combination treatment with HER-2 and VEGF peptide mimics induces potent anti-tumor and anti-angiogenic responses in vitro and in vivo. J. Biol. Chem. 2011, 286, 13626–13637. [Google Scholar] [CrossRef]
- Giordano, R.J.; Cardo-Vila, M.; Lahdenranta, J.; Pasqualini, R.; Arap, W. Biopanning and rapid analysis of selective interactive ligands. Nat. Med. 2001, 7, 1249–1253. [Google Scholar] [CrossRef]
- Giordano, R.J.; Cardo-Vila, M.; Salameh, A.; Anobom, C.D.; Zeitlin, B.D.; Hawked, D.H.; Valente, A.P.; Almeida, F.C.L.; Nor, J.E.; Sidman, R.L.; et al. From combinatorial peptide selection to drug prototype (I): Targeting the vascular endothelial growth factor receptor pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 5112–5117. [Google Scholar] [CrossRef] [PubMed]
- Calvanese, L.; Caporale, A.; Foca, G.; Iaccarino, E.; Sandomenico, A.; Doti, N.; Apicella, I.; Incisivo, G.M.; De Falco, S.; Falcigno, L.; et al. Targeting VEGF receptors with non-neutralizing cyclopeptides for imaging applications. Amino Acids 2018, 50, 321–329. [Google Scholar] [CrossRef]
- Rezazadeh, F.; Sadeghzadeh, N.; Abedi, S.M.; Abediankenari, S. Tc-99m labeled (D)(LPR): A novel retro-inverso peptide for VEGF receptor-1 targeted tumor imaging. Nucl. Med. Biol. 2018, 62–63, 54–62. [Google Scholar] [CrossRef]
- Lee, T.Y.; Lin, C.T.; Kuo, S.Y.; Chang, D.K.; Wu, H.C. Peptide-mediated targeting to tumor blood vessels of lung cancer for drug delivery. Cancer Res. 2007, 67, 10958–10965. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lei, Y.; Wagner, E.; Xie, C.; Lu, W.Y.; Zhu, J.H.; Shen, J.; Wang, J.; Liu, M. Potent Retro-Inverso D-Peptide for Simultaneous Targeting of Angiogenic Blood Vasculature and Tumor Cells. Bioconjugate Chem. 2013, 24, 133–143. [Google Scholar] [CrossRef]
- Bifulco, K.; Longanesi-Cattani, I.; Gala, M.; Di Carluccio, G.; Masucci, M.T.; Pavone, V.; Lista, L.; Arra, C.; Stoppelli, M.P.; Carriero, M.V. The soluble form of urokinase receptor promotes angiogenesis through its Ser(88)-Arg-Ser-Arg-Tyr(92) chemotactic sequence. J. Thromb. Haemost. 2010, 8, 2789–2799. [Google Scholar] [CrossRef]
- Resnati, M.; Pallavicini, I.; Wang, J.M.; Oppenheim, J.; Serhan, C.N.; Romano, M.; Blasi, F. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc. Natl. Acad. Sci. USA 2002, 99, 1359–1364. [Google Scholar] [CrossRef]
- Carriero, M.V.; Bifulco, K.; Ingangi, V.; Costantini, S.; Botti, G.; Ragone, C.; Minopoli, M.; Motti, M.L.; Rea, D.; Scognamiglio, G.; et al. Retro-inverso Urokinase Receptor Antagonists for the Treatment of Metastatic Sarcomas. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef]
- Karimi, H.; Sadeghzadeh, N.; Abediankenari, S.; Rezazadeh, F.; Hallajian, F. Radiochemical Evaluation and In Vitro Assessment of the Targeting Ability of a Novel Tc-99m-HYNIC-RGD for U87MG Human Brain Cancer Cells. Curr. Radiopharm. 2017, 10, 139–144. [Google Scholar] [CrossRef]
- Torabizadeh, S.A.; Abedi, S.M.; Noaparast, Z.; Hosseinimehr, S.J. Comparative assessment of a Tc-99m labeled H1299.2-HYNIC peptide bearing two different co-ligands for tumor-targeted imaging. Bioorgan. Med. Chem. 2017, 25, 2583–2592. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Mei, L.; Yu, Q.W.; Zhang, Q.Y.; Gao, H.L.; Zhang, Z.R.; He, Q. Integrin alpha(v)beta(3) targeting activity study of different retro-inverso sequences of RGD and their potentiality in the designing of tumor targeting peptides. Amino Acids 2015, 47, 2533–2539. [Google Scholar] [CrossRef]
- Ren, Y.C.; Zhan, C.Y.; Gao, J.; Zhang, M.F.; Wei, X.L.; Ying, M.; Liu, Z.N.; Lu, W.Y. A D-Peptide Ligand of Integrins for Simultaneously Targeting Angiogenic Blood Vasculature and Glioma Cells. Mol. Pharmaceut 2018, 15, 592–601. [Google Scholar] [CrossRef]
- van Ommeren, R.; Staudt, M.D.; Xu, H.; Hebb, M.O. Advances in HSP27 and HSP90-targeting strategies for glioblastoma. J. Neuro Oncol. 2016, 127, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Ran, D.N.; Mao, J.N.; Shen, Q.; Xie, C.; Zhan, C.Y.; Wang, R.F.; Lu, W.Y. GRP78 enabled micelle-based glioma targeted drug delivery. J. Control. Release 2017, 255, 120–131. [Google Scholar] [CrossRef]
- Ran, D.N.; Mao, J.N.; Zhan, C.Y.; Xie, C.; Ruan, H.T.; Ying, M.; Zhou, J.F.; Lu, W.L.; Lu, W.Y. D-Retroenantiomer of Quorum-Sensing Peptide-Modified Polymeric Micelles for Brain Tumor-Targeted Drug Delivery. ACS Appl. Mater. Inter. 2017, 9, 25672–25682. [Google Scholar] [CrossRef]
- Xie, Z.X.; Shen, Q.; Xie, C.; Lu, W.Y.; Peng, C.M.; Wei, X.L.; Li, X.; Su, B.X.; Gao, C.L.; Liu, M. Retro-inverso bradykinin opens the door of blood-brain tumor barrier for nanocarriers in glioma treatment. Cancer Lett. 2015, 369, 144–151. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Zhang, M.X.; Wang, J.; Cai, Q.Q.; Zhao, R.; Yu, Y.; Tai, H.Y.; Zhang, X.Y.; Xu, C.J. Retro-inverso follicle-stimulating hormone peptide-mediated polyethylenimine complexes for targeted ovarian cancer gene therapy. Drug Deliv. 2018, 25, 995–1003. [Google Scholar] [CrossRef]
- Zhang, M.X.; Hong, S.S.; Cai, Q.Q.; Zhang, M.; Chen, J.; Zhang, X.Y.; Xu, C.J. Transcriptional control of the MUC16 promoter facilitates follicle-stimulating hormone peptide-conjugated shRNA nanoparticle-mediated inhibition of ovarian carcinoma in vivo. Drug Deliv. 2018, 25, 797–806. [Google Scholar] [CrossRef]
- Hong, S.S.; Zhang, M.X.; Zhang, M.; Yu, Y.; Chen, J.; Zhang, X.Y.; Xu, C.J. Follicle-stimulating hormone peptide-conjugated nanoparticles for targeted shRNA delivery lead to effective gro-alpha silencing and antitumor activity against ovarian cancer. Drug Deliv. 2018, 25, 576–584. [Google Scholar] [CrossRef]
- Tang, J.J.; Wang, Q.T.; Yu, Q.W.; Qiu, Y.; Mei, L.; Wan, D.D.; Wang, X.H.; Li, M.; He, Q. A stabilized retro-inverso peptide ligand of transferrin receptor for enhanced liposome-based hepatocellular carcinoma-targeted drug delivery. Acta Biomater. 2019, 83, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Moiani, D.; Salvalaglio, M.; Cavallotti, C.; Bujacz, A.; Redzynia, I.; Bujacz, G.; Dinon, F.; Pengo, P.; Fassina, G. Structural characterization of a Protein A mimetic peptide dendrimer bound to human IgG. J. Phys. Chem. B 2009, 113, 16268–16275. [Google Scholar] [CrossRef]
- Marino, M.; Ruvo, M.; De Falco, S.; Fassina, G. Prevention of systemic lupus erythematosus in MRL/lpr mice by administration of an immunoglobulin-binding peptide. Nat. Biotechnol. 2000, 18, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.T.; Kaur, K.J.; Singh, K.; Mukherjee, P.; Rajagopal, D.; George, A.; Bal, V.; Rath, S.; Rao, K.V.S.; Salunke, D.M. Mimicry of native peptide antigens by the corresponding retro-inverso analogs is dependent on their intrinsic structure and interaction propensities. J. Immunol. 2003, 170, 1362–1373. [Google Scholar] [CrossRef]
- Lee, J.H.; Engler, J.A.; Collawn, J.F.; Moore, B.A. Receptor mediated uptake of peptides that bind the human transferrin receptor. Eur. J. Biochem. 2001, 268, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Wucherpfennig, K.W.; Wiley, D.C. Structure of a human insulin peptide-HLA-DQ8 complex and susceptibility to type 1 diabetes. Nat. Immunol. 2001, 2, 501–507. [Google Scholar] [CrossRef]
- Nakayama, M.; Abiru, N.; Moriyama, H.; Babaya, N.; Liu, E.; Miao, D.; Yu, L.; Wegmann, D.R.; Hutton, J.C.; Elliott, J.F.; et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 2005, 435, 220–223. [Google Scholar] [CrossRef]
- Gandbhir, O.; Sundaram, P. Pre-Clinical Safety and Efficacy Evaluation of Amytrap, a Novel Therapeutic to Treat Alzheimer’s Disease. J. Alzheimers Dis. Rep. 2019, 3, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Parthsarathy, V.; McClean, P.L.; Holscher, C.; Taylor, M.; Tinker, C.; Jones, G.; Kolosov, O.; Salvati, E.; Gregori, M.; Masserini, M.; et al. A novel retro-inverso peptide inhibitor reduces amyloid deposition, oxidation and inflammation and stimulates neurogenesis in the APPswe/PS1DeltaE9 mouse model of Alzheimer’s disease. PLoS ONE 2013, 8, e54769. [Google Scholar] [CrossRef]
- Gregori, M.; Taylor, M.; Salvati, E.; Re, F.; Mancini, S.; Balducci, C.; Forloni, G.; Zambelli, V.; Sesana, S.; Michael, M.; et al. Retro-inverso peptide inhibitor nanoparticles as potent inhibitors of aggregation of the Alzheimer’s Abeta peptide. Nanomedicine 2017, 13, 723–732. [Google Scholar] [CrossRef]
- Morris, O.; Gregory, J.; Kadirvel, M.; Henderson, F.; Blykers, A.; McMahon, A.; Taylor, M.; Allsop, D.; Allan, S.; Grigg, J.; et al. Development & automation of a novel [(18)F]F prosthetic group, 2-[(18)F]-fluoro-3-pyridinecarboxaldehyde, and its application to an amino(oxy)-functionalised Abeta peptide. Appl. Radiat. Isot. 2016, 116, 120–127. [Google Scholar] [CrossRef]
- Stark, T.; Lieblein, T.; Pohland, M.; Kalden, E.; Freund, P.; Zangl, R.; Grewal, R.; Heilemann, M.; Eckert, G.P.; Morgner, N.; et al. Peptidomimetics That Inhibit and Partially Reverse the Aggregation of Aβ1–42. Biochemistry 2017, 56, 4840–4849. [Google Scholar] [CrossRef]
- Obasse, I.; Taylor, M.; Fullwood, N.J.; Allsop, D. Development of proteolytically stable N-methylated peptide inhibitors of aggregation of the amylin peptide implicated in type 2 diabetes. Interface Focus 2017, 7, 20160127. [Google Scholar] [CrossRef]
- Windisch, M.; Hutter-Paier, B.; Schreiner, E.; Wronski, R. Beta-Synuclein-derived peptides with neuroprotective activity: An alternative treatment of neurodegenerative disorders? J. Mol. NeuroSci 2004, 24, 155–165. [Google Scholar] [CrossRef]
- de la Fuente-Nunez, C.; Reffuveille, F.; Haney, E.F.; Straus, S.K.; Hancock, R.E. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog 2014, 10, e1004152. [Google Scholar] [CrossRef]
- Kumar, P.; Pletzer, D.; Haney, E.F.; Rahanjam, N.; Cheng, J.T.J.; Yue, M.; Aljehani, W.; Hancock, R.E.W.; Kizhakkedathu, J.N.; Straus, S.K. Aurein-Derived Antimicrobial Peptides Formulated with Pegylated Phospholipid Micelles to Target Methicillin-Resistant Staphylococcus aureus Skin Infections. ACS Infect. Dis. 2019, 5, 443–453. [Google Scholar] [CrossRef]
- Lynn, M.A.; Kindrachuk, J.; Marr, A.K.; Jenssen, H.; Pante, N.; Elliott, M.R.; Napper, S.; Hancock, R.E.; McMaster, W.R. Effect of BMAP-28 antimicrobial peptides on Leishmania major promastigote and amastigote growth: Role of leishmanolysin in parasite survival. PLoS Negl. Trop. Dis. 2011, 5, e1141. [Google Scholar] [CrossRef]
- Binetruy-Tournaire, R.; Demangel, C.; Malavaud, B.; Vassy, R.; Rouyre, S.; Kraemer, M.; Plouet, J.; Derbin, C.; Perret, G.; Mazie, J.C. Identification of a peptide blocking vascular endothelial growth factor (VEGF)-mediated angiogenesis. Embo. J. 2000, 19, 1525–1533. [Google Scholar] [CrossRef]
- Blasi, F. uPA, uPAR, PAI-I: Key intersection of proteolytic, adhesive and chemotactic highways? Immunol. Today 1997, 18, 415–417. [Google Scholar] [CrossRef]
- Gargiulo, L.; Longanesi-Cattani, I.; Bifulco, K.; Franco, P.; Raiola, R.; Campiglia, P.; Grieco, P.; Peluso, G.; Stoppelli, M.P.; Carriero, M.V. Cross-talk between fMLP and vitronectin receptors triggered by urokinase receptor-derived SRSRY peptide. J. Biol. Chem. 2005, 280, 25225–25232. [Google Scholar] [CrossRef] [PubMed]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef]
- Trabocchi, A.; Menchi, G.; Danieli, E.; Potenza, D.; Cini, N.; Bottoncetti, A.; Raspanti, S.; Pupi, A.; Guarna, A. Cyclic DGR-peptidomimetic containing a bicyclic reverse turn inducer as a selective alpha(v)beta(5) integrin ligand. Amino Acids 2010, 38, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Winograd-Katz, S.E.; Fassler, R.; Geiger, B.; Legate, K.R. The integrin adhesome: From genes and proteins to human disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Shattil, S.J.; Kim, C.; Ginsberg, M.H. The final steps of integrin activation: The end game. Nat. Rev. Mol. Cell Biol. 2010, 11, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Seguin, L.; Desgrosellier, J.S.; Weis, S.M.; Cheresh, D.A. Integrins and cancer: Regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015, 25, 234–240. [Google Scholar] [CrossRef]
- Leblanc, R.; Lee, S.C.; David, M.; Bordet, J.C.; Norman, D.D.; Patil, R.; Miller, D.; Sahay, D.; Ribeiro, J.; Clezardin, P.; et al. Interaction of platelet-derived autotaxin with tumor integrin alpha(V)beta(3) controls metastasis of breast cancer cells to bone. Blood 2014, 124, 3141–3150. [Google Scholar] [CrossRef]
- Contois, L.W.; Akalu, A.; Caron, J.M.; Tweedie, E.; Cretu, A.; Henderson, T.; Liaw, L.; Friesel, R.; Vary, C.; Brooks, P.C. Inhibition of tumor-associated alpha v beta 3 integrin regulates the angiogenic switch by enhancing expression of IGFBP-4 leading to reduced melanoma growth and angiogenesis in vivo. Angiogenesis 2015, 18, 31–46. [Google Scholar] [CrossRef]
- Kibria, G.; Hatakeyama, H.; Ohga, N.; Hida, K.; Harashima, H. Dual-ligand modification of PEGylated liposomes shows better cell selectivity and efficient gene delivery. J. Control. Release 2011, 153, 141–148. [Google Scholar] [CrossRef]
- Guo, Z.M.; He, B.; Jin, H.W.; Zhang, H.R.; Dai, W.B.; Zhang, L.R.; Zhang, H.; Wang, X.Q.; Wang, J.C.; Zhang, X.; et al. Targeting efficiency of RGD-modified nanocarriers with different ligand intervals in response to integrin alpha v beta 3 clustering. Biomaterials 2014, 35, 6106–6117. [Google Scholar] [CrossRef]
- Caporale, A.; Bolzati, C.; Incisivo, G.M.; Salvarese, N.; Grieco, P.; Ruvo, M. Improved synthesis on solid phase of dithiocarbamic cRGD-derivative and Tc-99m-radiolabelling. J. Pept. Sci. 2019, 25, e3140. [Google Scholar] [CrossRef]
- Flechsig, P.; Lindner, T.; Loktev, A.; Roesch, S.; Mier, W.; Sauter, M.; Meister, M.; Herold-Mende, C.; Haberkorn, U.; Altmann, A. PET/CT Imaging of NSCLC with a alpha(v)beta(6) Integrin-Targeting Peptide. Mol. Imaging Biol. 2019, 21, 973–983. [Google Scholar] [CrossRef]
- Evans, B.J.; King, A.T.; Katsifis, A.; Matesic, L.; Jamie, J.F. Methods to Enhance the Metabolic Stability of Peptide-Based PET Radiopharmaceuticals. Molecules 2020, 25, 2314. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Kountourakis, P.; Kottorou, A.E.; Antonacopoulou, A.G.; Rolfo, C.; Peeters, M.; Kalofonos, H.P. Follicle-Stimulating Hormone Receptor (FSHR): A Promising Tool in Oncology? Mol. Diagn. Ther. 2016, 20, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Perales-Puchalt, A.; Svoronos, N.; Rutkowski, M.R.; Allegrezza, M.J.; Tesone, A.J.; Payne, K.K.; Wickramasinghe, J.; Nguyen, J.M.; O’Brien, S.W.; Gumireddy, K.; et al. Follicle-Stimulating Hormone Receptor Is Expressed by Most Ovarian Cancer Subtypes and Is a Safe and Effective Immunotherapeutic Target. Clin. Cancer Res. 2017, 23, 441–453. [Google Scholar] [CrossRef]
- Huang, R.Q.; Qu, Y.H.; Ke, W.L.; Zhu, J.H.; Pei, Y.Y.; Jiang, C. Efficient gene delivery targeted to the brain using a transferrin-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. FASEB J. 2007, 21, 1117–1125. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.T.; Yan, X.P. Fabrication of Transferrin Functionalized Gold Nanoclusters/Graphene Oxide Nanocomposite for Turn-On Near-Infrared Fluorescent Bioimaging of Cancer Cells and Small Animals. Anal. Chem. 2013, 85, 2529–2535. [Google Scholar] [CrossRef]
- Benkirane, N.; Friede, M.; Guichard, G.; Briand, J.P.; Vanregenmortel, M.H.V.; Muller, S. Antigenicity and Immunogenicity of Modified Synthetic Peptides Containing D-Amino-Acid Residues-Antibodies to a D-Enantiomer Do Recognize the Parent L-Hexapeptide and Reciprocally. J. Biol. Chem. 1993, 268, 26279–26285. [Google Scholar] [CrossRef]
- Guichard, G.; Benkirane, N.; Zederlutz, G.; Vanregenmortel, M.H.V.; Briand, J.P.; Muller, S. Antigenic Mimicry of Natural L-Peptides with Retro-Inverso-Peptidomimetics. Proc. Natl. Acad. Sci. USA 1994, 91, 9765–9769. [Google Scholar] [CrossRef] [PubMed]
- Verdoliva, A.; Ruvo, M.; Villain, M.; Cassani, G.; Fassina, G. Antigenicity of topochemically related peptides. Biochim. Biophys. Acta 1995, 1253, 57–62. [Google Scholar] [CrossRef]
- Weiner, H.L. Oral Tolerance. Proc. Natl. Acad. Sci. USA 1994, 91, 10762–10765. [Google Scholar] [CrossRef]
- Rossi, M.; Manfredi, V.; Ruvo, M.; Fassina, G.; Verdoliva, A. Sequence-simplification and chimeric assembly: New models of peptide antigen modification. Mol. Immunol. 2002, 39, 443–451. [Google Scholar] [CrossRef]
- Fassina, G.; Cassani, G.; Gnocchi, P.; Fornasiero, M.C.; Isetta, A.M. Inhibition of interleukin-2/p55 receptor subunit interaction by complementary peptides. Arch. Biochem. Biophys. 1995, 318, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Arranz-Gibert, P.; Ciudad, S.; Seco, J.; Garcia, J.; Giralt, E.; Teixido, M. Immunosilencing peptides by stereochemical inversion and sequence reversal: Retro-D-peptides. Sci. Rep. 2018, 8, 6446. [Google Scholar] [CrossRef]
- Lombardi, A.; Concepcion, E.; Hou, H.; Arib, H.; Mezei, M.; Osman, R.; Tomer, Y. Retro-inverso D-peptides as a novel targeted immunotherapy for Type 1 diabetes. J. Autoimmun. 2020, 115, 102543. [Google Scholar] [CrossRef] [PubMed]
- Robson, B. Computers and viral diseases. Preliminary bioinformatics studies on the design of a synthetic vaccine and a preventative peptidomimetic antagonist against the SARS-CoV-2 (2019-nCoV, COVID-19) coronavirus. Comput. Biol. Med. 2020, 119, 103670. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Estrada, L.D. Protein misfolding and neurodegeneration. Arch. Neurol. 2008, 65, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-beta and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef]
- Zotova, E.; Bharambe, V.; Cheaveau, M.; Morgan, W.; Holmes, C.; Harris, S.; Neal, J.W.; Love, S.; Nicoll, J.A.; Boche, D. Inflammatory components in human Alzheimer’s disease and after active amyloid-beta42 immunization. Brain 2013, 136, 2677–2696. [Google Scholar] [CrossRef]
- Salloway, S.; Sperling, R.; Fox, N.C.; Blennow, K.; Klunk, W.; Raskind, M.; Sabbagh, M.; Honig, L.S.; Porsteinsson, A.P.; Ferris, S.; et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014, 370, 322–333. [Google Scholar] [CrossRef]
- Resende, R.; Ferreira-Marques, M.; Moreira, P.; Coimbra, J.R.M.; Baptista, S.J.; Isidoro, C.; Salvador, J.A.R.; Dinis, T.C.P.; Pereira, C.F.; Santos, A.E. New BACE1 Chimeric Peptide Inhibitors Selectively Prevent AbetaPP-beta Cleavage Decreasing Amyloid-beta Production and Accumulation in Alzheimer’s Disease Models. J. Alzheimers Dis. 2020, 76, 1317–1337. [Google Scholar] [CrossRef]
- Zheng, X.; Pang, X.; Yang, P.; Wan, X.; Wei, Y.; Guo, Q.; Zhang, Q.; Jiang, X. A hybrid siRNA delivery complex for enhanced brain penetration and precise amyloid plaque targeting in Alzheimer’s disease mice. Acta Biomater. 2017, 49, 388–401. [Google Scholar] [CrossRef]
- Guo, Q.; Xu, S.; Yang, P.; Wang, P.; Lu, S.; Sheng, D.; Qian, K.; Cao, J.; Lu, W.; Zhang, Q. A dual-ligand fusion peptide improves the brain-neuron targeting of nanocarriers in Alzheimer’s disease mice. J. Control. Release 2020, 320, 347–362. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, X.; Guo, Q.; Yang, P.; Pang, X.; Qian, K.; Lu, W.; Zhang, Q.; Jiang, X. Systemic delivery of BACE1 siRNA through neuron-targeted nanocomplexes for treatment of Alzheimer’s disease. J. Control. Release 2018, 279, 220–233. [Google Scholar] [CrossRef]
- Andreetto, E.; Malideli, E.; Yan, L.M.; Kracklauer, M.; Farbiarz, K.; Tatarek-Nossol, M.; Rammes, G.; Prade, E.; Neumuller, T.; Caporale, A.; et al. A Hot-Segment-Based Approach for the Design of Cross-Amyloid Interaction Surface Mimics as Inhibitors of Amyloid Self-Assembly. Angew. Chem. Int. Ed. Engl. 2015, 54, 13095–13100. [Google Scholar] [CrossRef]
- Bakou, M.; Hille, K.; Kracklauer, M.; Spanopoulou, A.; Frost, C.V.; Malideli, E.; Yan, L.M.; Caporale, A.; Zacharias, M.; Kapurniotu, A. Key aromatic/hydrophobic amino acids controlling a cross-amyloid peptide interaction versus amyloid self-assembly. J. Biol. Chem. 2017, 292, 14587–14602. [Google Scholar] [CrossRef]
- Yan, L.M.; Velkova, A.; Tatarek-Nossol, M.; Andreetto, E.; Kapurniotu, A. IAPP mimic blocks Abeta cytotoxic self-assembly: Cross-suppression of amyloid toxicity of Abeta and IAPP suggests a molecular link between Alzheimer’s disease and type II diabetes. Angew. Chem. Int. Ed. Engl. 2007, 46, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Shaltiel-Karyo, R.; Frenkel-Pinter, M.; Egoz-Matia, N.; Frydman-Marom, A.; Shalev, D.E.; Segal, D.; Gazit, E. Inhibiting alpha-synuclein oligomerization by stable cell-penetrating beta-synuclein fragments recovers phenotype of Parkinson’s disease model flies. PLoS ONE 2010, 5, e13863. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides. Int. J. Mol. Sci. 2020, 21, 7047. [Google Scholar] [CrossRef] [PubMed]
- Divyashree, M.; Mani, M.K.; Reddy, D.; Kumavath, R.; Ghosh, P.; Azevedo, V.; Barh, D. Clinical Applications of Antimicrobial Peptides (AMPs): Where do we Stand Now? Protein Pept. Lett. 2020, 27, 120–134. [Google Scholar] [CrossRef]
- Zhang, L.J.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Roncevic, T.; Puizina, J.; Tossi, A. Antimicrobial Peptides as Anti-Infective Agents in Pre-Post-Antibiotic Era? Int. J. Mol. Sci. 2019, 20, 5713. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed Ahmed, M.A.E.-G.; Zhong, L.-L.; Shen, C.; Yang, Y.; Doi, Y.; Tian, G.-B. Colistin and its role in the Era of antibiotic resistance: An extended review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar] [CrossRef]
- de la Fuente-Nunez, C.; Cardoso, M.H.; de Souza Candido, E.; Franco, O.L.; Hancock, R.E. Synthetic antibiofilm peptides. Biochim. Biophys. Acta 2016, 1858, 1061–1069. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Sothiselvam, S.; Lu, T.K.; de la Fuente-Nunez, C. Peptide Design Principles for Antimicrobial Applications. J. Mol. Biol. 2019, 431, 3547–3567. [Google Scholar] [CrossRef]
- Li, F.; Brimble, M. Using chemical synthesis to optimise antimicrobial peptides in the fight against antimicrobial resistance. Pure Appl. Chem. 2019, 91, 181–198. [Google Scholar] [CrossRef]
- Neubauer, D.; Jaśkiewicz, M.; Migoń, D.; Bauer, M.; Sikora, K.; Sikorska, E.; Kamysz, E.; Kamysz, W. Retro analog concept: Comparative study on physico-chemical and biological properties of selected antimicrobial peptides. Amino Acids 2017, 49, 1755–1771. [Google Scholar] [CrossRef]
- Baranska-Rybak, W.; Cirioni, O.; Dawgul, M.; Sokolowska-Wojdylo, M.; Naumiuk, L.; Szczerkowska-Dobosz, A.; Nowicki, R.; Roszkiewicz, J.; Kamysz, W. Activity of Antimicrobial Peptides and Conventional Antibiotics against Superantigen Positive Staphylococcus aureus Isolated from the Patients with Neoplastic and Inflammatory Erythrodermia. Chemother. Res. Pract. 2011, 2011, 270932. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Blecha, F. Antimicrobial peptides and bacteriocins: Alternatives to traditional antibiotics. Anim. Health Res. Rev. 2008, 9, 227–235. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, M.J.; Saugar, J.; Docobo-Perez, F.; de la Torre, B.G.; Pachon-Ibanez, M.E.; Garcia-Curiel, A.; Fernandez-Cuenca, F.; Andreu, D.; Rivas, L.; Pachon, J. Studies on the antimicrobial activity of cecropin A-melittin hybrid peptides in colistin-resistant clinical isolates of Acinetobacter baumannii. J. Antimicrob. Chemother. 2006, 58, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, E.; Greber, K.; Rodziewicz-Motowidlo, S.; Szultka, L.; Lukasiak, J.; Kamysz, W. Synthesis and antimicrobial activity of truncated fragments and analogs of citropin 1.1: The solution structure of the SDS micelle-bound citropin-like peptides. J. Struct. Biol. 2009, 168, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.M.S.; Teo, S.W.; Yong, Y.E.; Ng, F.M.; Lau, Q.Y.; Jureen, R.; Hill, J.; Chia, C.S.B. Preliminary investigations into developing all-D Omiganan for treating Mupirocin-resistant MRSA skin infections. Chem. Biol. Drug Des. 2017, 90, 1155–1160. [Google Scholar] [CrossRef]
- Rubinchik, E.; Dugourd, D.; Algara, T.; Pasetka, C.; Friedland, H.D. Antimicrobial and antifungal activities of a novel cationic antimicrobial peptide, omiganan, in experimental skin colonisation models. Int. J. Antimicrob. Agents 2009, 34, 457–461. [Google Scholar] [CrossRef]
- Melo, M.N.; Dugourd, D.; Castanho, M.A. Omiganan pentahydrochloride in the front line of clinical applications of antimicrobial peptides. Recent Pat. Anti-Infect. Drug Discov. 2006, 1, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Flamm, R.K.; Rhomberg, P.R.; Simpson, K.M.; Farrell, D.J.; Sader, H.S.; Jones, R.N. In vitro spectrum of pexiganan activity when tested against pathogens from diabetic foot infections and with selected resistance mechanisms. Antimicrob. Agents Chemother. 2015, 59, 1751–1754. [Google Scholar] [CrossRef][Green Version]
- Lopez-Medina, E.; Fan, D.; Coughlin, L.A.; Ho, E.X.; Lamont, I.L.; Reimmann, C.; Hooper, L.V.; Koh, A.Y. Candida albicans Inhibits Pseudomonas aeruginosa Virulence through Suppression of Pyochelin and Pyoverdine Biosynthesis. PLoS Pathog. 2015, 11, e1005129. [Google Scholar] [CrossRef]
- Kim, J.B.; Iwamuro, S.; Knoop, F.C.; Conlon, J.M. Antimicrobial peptides from the skin of the Japanese mountain brown frog, Rana ornativentris. J. Pept. Res. 2001, 58, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Kamysz, W.; Mickiewicz, B.; Rodziewicz-Motowidlo, S.; Greber, K.; Okroj, M. Temporin A and its retro-analogues: Synthesis, conformational analysis and antimicrobial activities. J. Pept. Sci. 2006, 12, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.; Ruiz-Gomez, G.; Hill, T.A.; Hoang, H.N.; Fairlie, D.P.; Mason, J.M. Truncated and helix-constrained peptides with high affinity and specificity for the cFos coiled-coil of AP-1. PLoS ONE 2013, 8, e59415. [Google Scholar] [CrossRef] [PubMed]
- Ronga, L.; Langella, E.; Palladino, P.; Marasco, D.; Tizzano, B.; Saviano, M.; Pedone, C.; Improta, R.; Ruvo, M. Does tetracycline bind helix 2 of prion? An integrated spectroscopical and computational study of the interaction between the antibiotic and alpha helix 2 human prion protein fragments. Proteins 2007, 66, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Kaeda, J.; Branford, S.; Rudzki, Z.; Hochhaus, A.; Hensley, M.L.; Gathmann, I.; Bolton, A.E.; van Hoomissen, I.C.; Goldman, J.M.; et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N. Engl. J. Med. 2003, 349, 1423–1432. [Google Scholar] [CrossRef]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Merrifield, R.B.; Juvvadi, P.; Andreu, D.; Ubach, J.; Boman, A.; Boman, H.G. Retro and retroenantio analogs of cecropin-melittin hybrids. Proc. Natl. Acad. Sci. USA 1995, 92, 3449–3453. [Google Scholar] [CrossRef] [PubMed]
- Andreu, D.; Ubach, J.; Boman, A.; Wahlin, B.; Wade, D.; Merrifield, R.B.; Boman, H.G. Shortened cecropin A-melittin hybrids. Significant size reduction retains potent antibiotic activity. FEBS Lett. 1992, 296, 190–194. [Google Scholar] [CrossRef]
- de la Fuente-Nunez, C.; Reffuveille, F.; Fernandez, L.; Hancock, R.E. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar] [CrossRef]
- de la Fuente-Nunez, C.; Reffuveille, F.; Mansour, S.C.; Reckseidler-Zenteno, S.L.; Hernandez, D.; Brackman, G.; Coenye, T.; Hancock, R.E. D-enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem. Biol. 2015, 22, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.M.; McMaster, W.R.; Kamysz, E.; Kamysz, W.; Engman, D.M.; McGwire, B.S. The major surface-metalloprotease of the parasitic protozoan, Leishmania, protects against antimicrobial peptide-induced apoptotic killing. Mol. Microbiol. 2006, 62, 1484–1497. [Google Scholar] [CrossRef]
- Risso, A.; Braidot, E.; Sordano, M.C.; Vianello, A.; Macri, F.; Skerlavaj, B.; Zanetti, M.; Gennaro, R.; Bernardi, P. BMAP-28, an antibiotic peptide of innate immunity, induces cell death through opening of the mitochondrial permeability transition pore. Mol. Cell Biol. 2002, 22, 1926–1935. [Google Scholar] [CrossRef] [PubMed]
- Kindrachuk, J.; Scruten, E.; Attah-Poku, S.; Bell, K.; Potter, A.; Babiuk, L.A.; Griebel, P.J.; Napper, S. Stability, toxicity, and biological activity of host defense peptide BMAP28 and its inversed and retro-inversed isomers. Biopolymers 2011, 96, 14–24. [Google Scholar] [CrossRef]
- Fassina, G.; Verdoliva, A.; Ruvo, M. Antigenic Peptides. U.S. Patent US-5932692-A, 3 August 1999. [Google Scholar]
- Goodman, M.; Chorev, M. On the concept of linear modified retro-peptide structures. Acc. Chem. Res. 1979, 12, 1–7. [Google Scholar] [CrossRef]
- Sakurai, K. A Peptide–Glycolipid Interaction Probed by Retroinverso Peptide Analogues. Chem. Pharm. Bull. 2018, 66, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Nguyen, N.; Hansmann, U.H.E. d-Retro Inverso Amylin and the Stability of Amylin Fibrils. J. Chem. Theory Comput. 2020, 16, 5358–5368. [Google Scholar] [CrossRef] [PubMed]
| Peptide | Sequence | Net Charge | Helicity a | % ACN b | Application | Mechanism of Action | Status | Therapeutic Indication | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| SDS | DPC | |||||||||
| aurein 1.2 | GLFDIIKKIAESF-NH2 * | +1 | < | > | 43.93 | Antimicrobial and anticancer properties | Prerequisite aggregation and carpet-like mechanism | in vitro [129] | [130] | |
| RI-aurein 1.2 | fseaikkiidflg-NH2 ** | > | = | 37.10 | ||||||
| CAMEL | KWKLFKKIGAVLKVL-NH2 * | +6 | = | < | 33.73 | Broad spectrum antibacterial | Bacterial membrane disruption | Preclinical [119] | Bacterial infections [119] | [131,132] |
| RI-CAMEL | lvklvagikkflkwK-NH2 ** | > | = | 30.71 | ||||||
| citropin 1.1 | GLFDVIKKVASVIGGL-NH2 * | +2 | = | = | 42.40 | Broad spectrum antibacterial and anticancer properties | Prerequisite aggregation and carpet-like mechanism | in vitro [129] | [133] | |
| RI-citropin 1.1 | lggivsavkkivdflg-NH2 ** | = | > | 41.28 | ||||||
| Omiganan | ILRWPWWPWRRK-NH2 * | +5 | NO | NO | 32.92 | Broad spectrum antifungal, antibacterial | Bacterial membrane disruption |
|
| [134,135,136] |
| RI-omiganan | krrwpwwpwrli-NH2 ** | NO | NO | 35.48 | ||||||
| Pexiganan | GIGKFLKKAKKFGKAFVKILKK-NH2 * | +10 | = | = | 30.58 | Broad spectrum antibacterial | Bacterial membrane disruption | Phase III complete; rejected, efficacy not superior to current therapies [118] | Infected diabetic foot ulcers [118] | [137,138] |
| RI-pexiganan | kklikvfakgfkkakklfkgig-NH2 ** | < | < | 26.36 | ||||||
| temporin A | FLPLIGRVLSGIL-NH2 * | +2 | < | > | 42.80 | Gram-positive bacteria | Bacterial membrane disruption | Preclinical [119] | Bacterial infections [119] | [139,140] |
| RI-temporin A | ligslvrgilplf-NH2 ** | < | < | 38.91 | ||||||
| Gram-Positive | Gram-Negative | |||||
|---|---|---|---|---|---|---|
| Peptide | E. faecalis 1 PCM 2673 | S. aureus 1 ATCC 25923 | S. pneumoniae ATCC 49619 | E. coli ATCC 25922 | K. pneumoniae 1 ATCC 700603 | P. aeruginosa 1 ATCC 9027 |
| Aurein 1.2 | 64 | 128 | 64 | 128 | 16 | 256 |
| RI-aurein 1.2 | 256 | >256 | 256 | 256 | 128 | >256 |
| CAMEL | 8 | 4 | 0.5 | 2 | 0.125 | 2 |
| RI-CAMEL | 64 | 128 | 128 | 128 | 2 | 8 |
| citropin 1.1 | 32 | 16 | 32 | 32 | 16 | 128 |
| RI-citropin 1.1 | 128 | 64 | 128 | 64 | 32 | >256 |
| Omiganan | 16 | 16 | 8 | 16 | 8 | 16 |
| RI-omiganan | 16 | 8 | 8 | 8 | 4 | 4 |
| Pexiganan | 16 | 8 | 1 | 4 | 1 | 2 |
| RI-pexiganan | 64 | 128 | 4 | 8 | 0.125 | 2 |
| Temporin A | 64 | 4 | >256 | 256 | 128 | >256 |
| RI-temporin A | 256 | 64 | >256 | 256 | 128 | 256 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doti, N.; Mardirossian, M.; Sandomenico, A.; Ruvo, M.; Caporale, A. Recent Applications of Retro-Inverso Peptides. Int. J. Mol. Sci. 2021, 22, 8677. https://doi.org/10.3390/ijms22168677
Doti N, Mardirossian M, Sandomenico A, Ruvo M, Caporale A. Recent Applications of Retro-Inverso Peptides. International Journal of Molecular Sciences. 2021; 22(16):8677. https://doi.org/10.3390/ijms22168677
Chicago/Turabian StyleDoti, Nunzianna, Mario Mardirossian, Annamaria Sandomenico, Menotti Ruvo, and Andrea Caporale. 2021. "Recent Applications of Retro-Inverso Peptides" International Journal of Molecular Sciences 22, no. 16: 8677. https://doi.org/10.3390/ijms22168677
APA StyleDoti, N., Mardirossian, M., Sandomenico, A., Ruvo, M., & Caporale, A. (2021). Recent Applications of Retro-Inverso Peptides. International Journal of Molecular Sciences, 22(16), 8677. https://doi.org/10.3390/ijms22168677









