Identification and Characterization of the Masculinizing Function of the Helicoverpa armigera Masc Gene
Abstract
:1. Introduction
2. Results
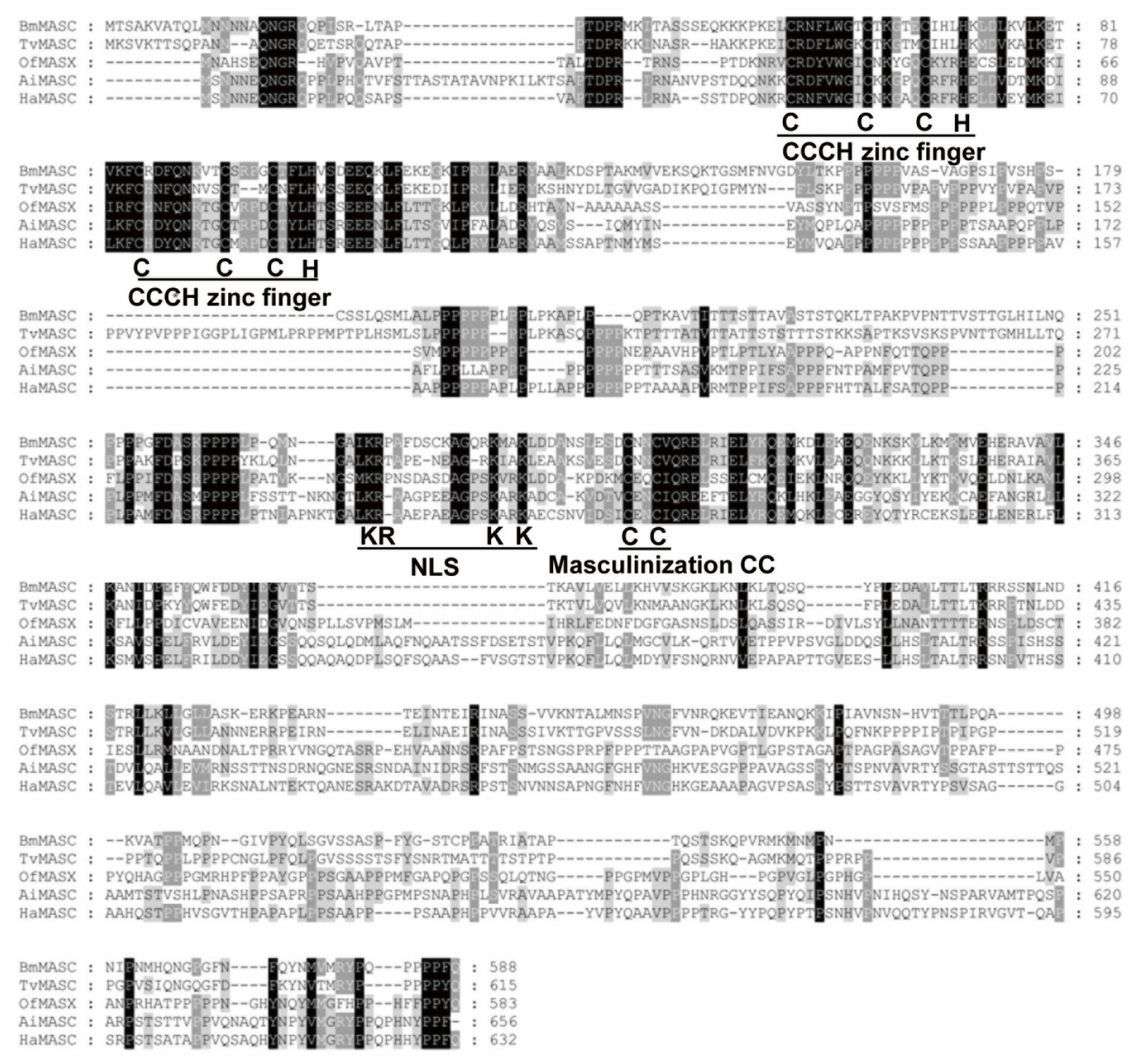
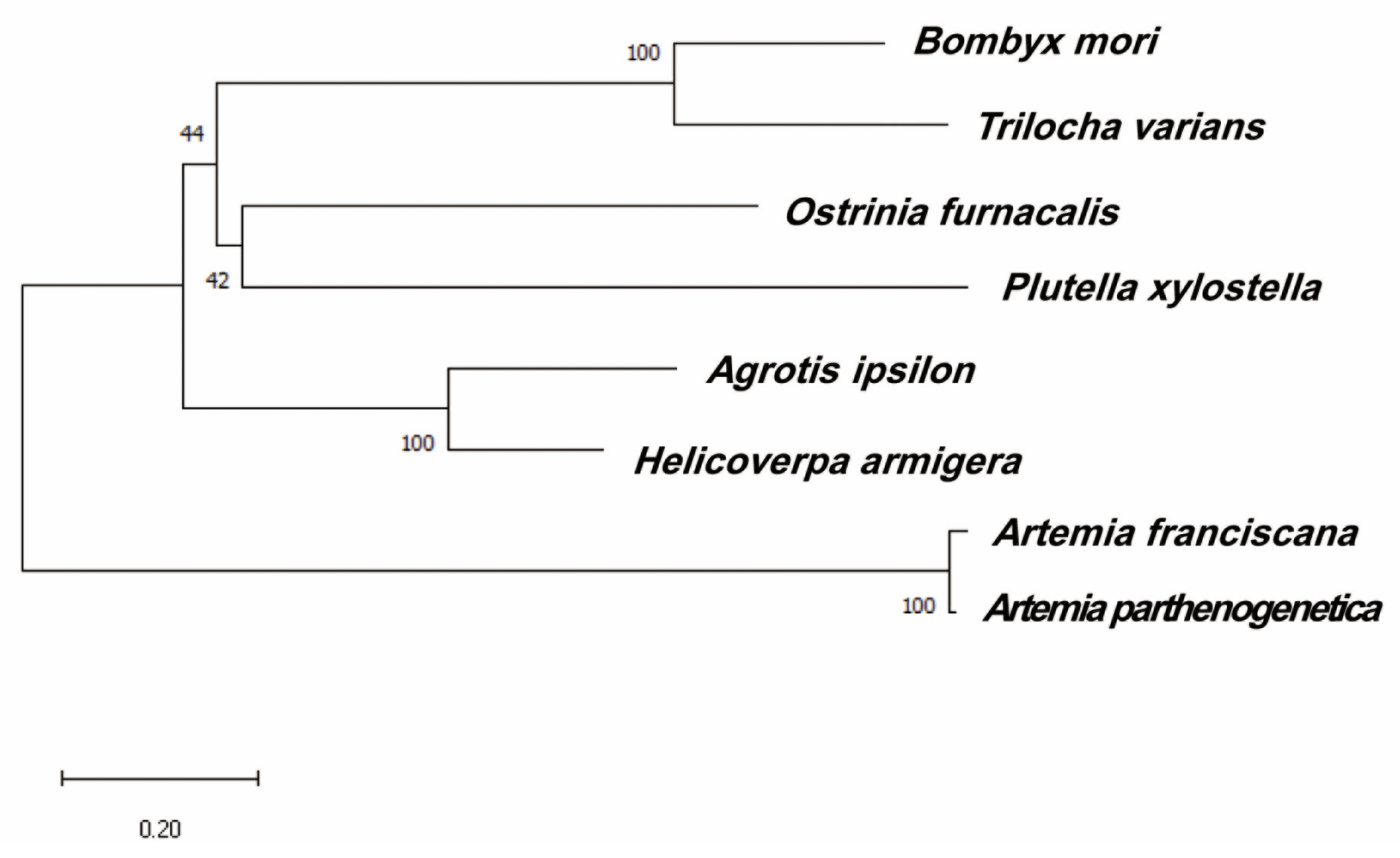
2.1. Cloning and Identification of HaMasc
2.2. Chromosomal Assignment of HaMasc to Z Chromosome
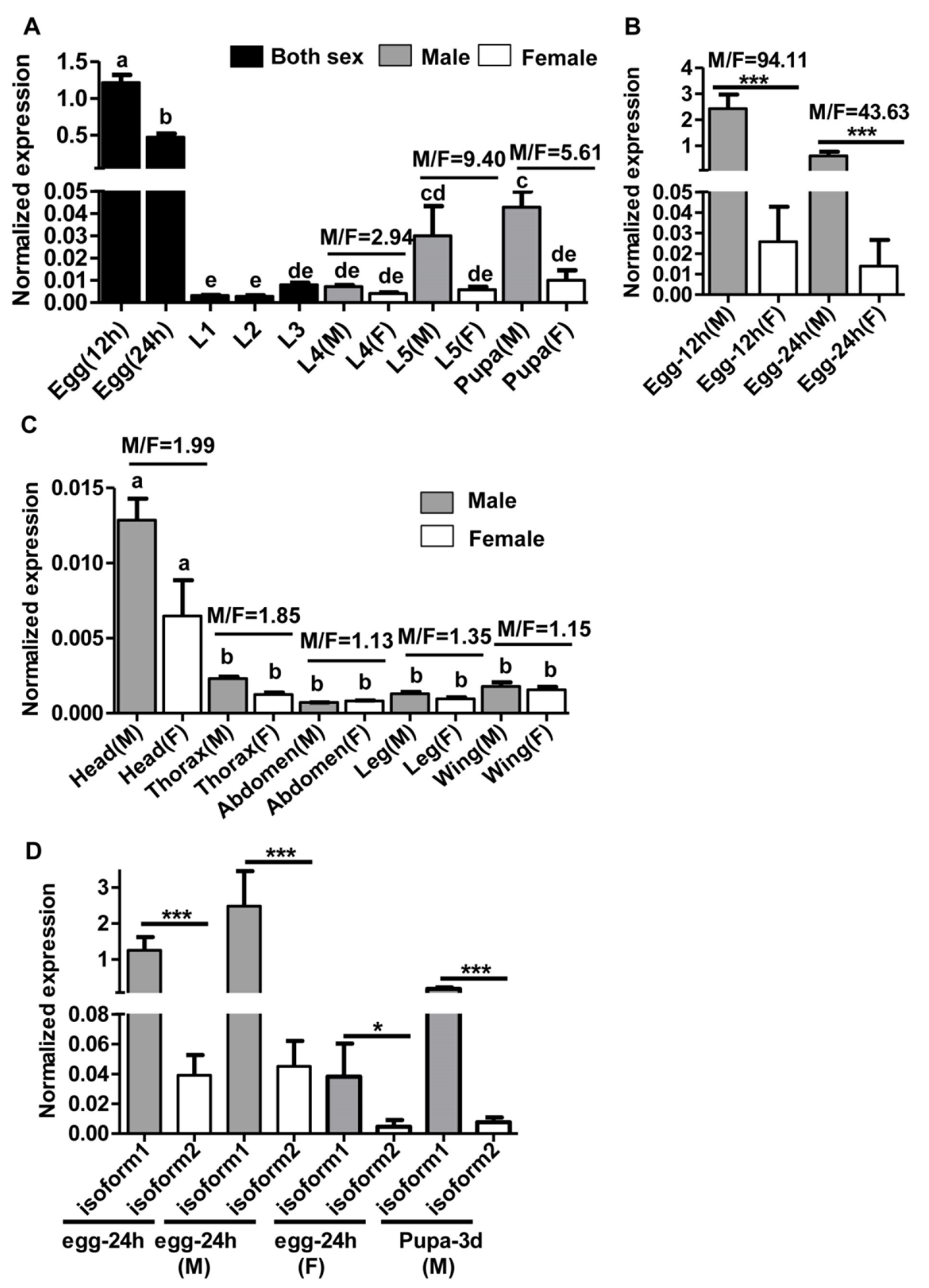
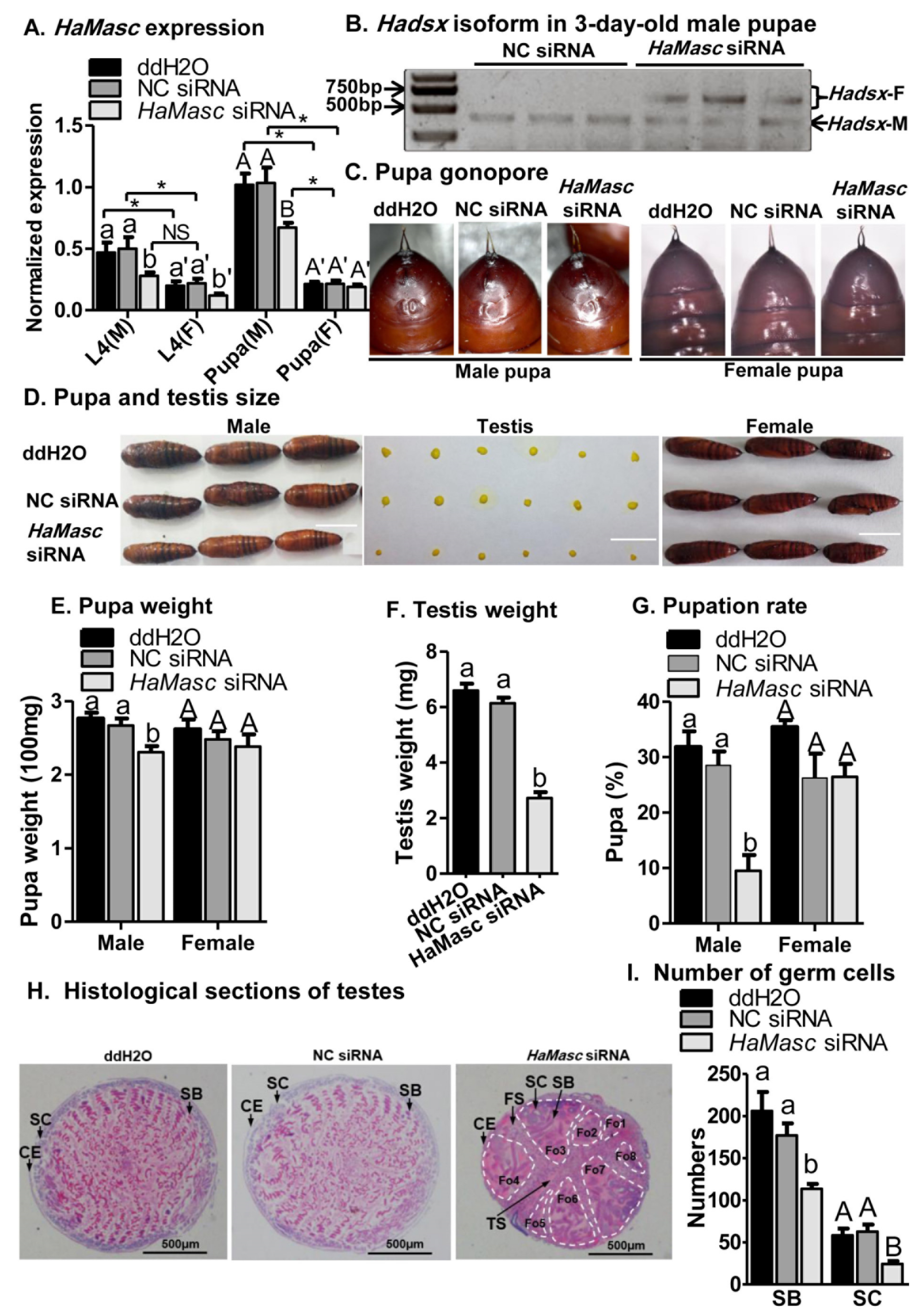
2.3. Sex-, Stage- and Tissue-Specific Expression of HaMasc
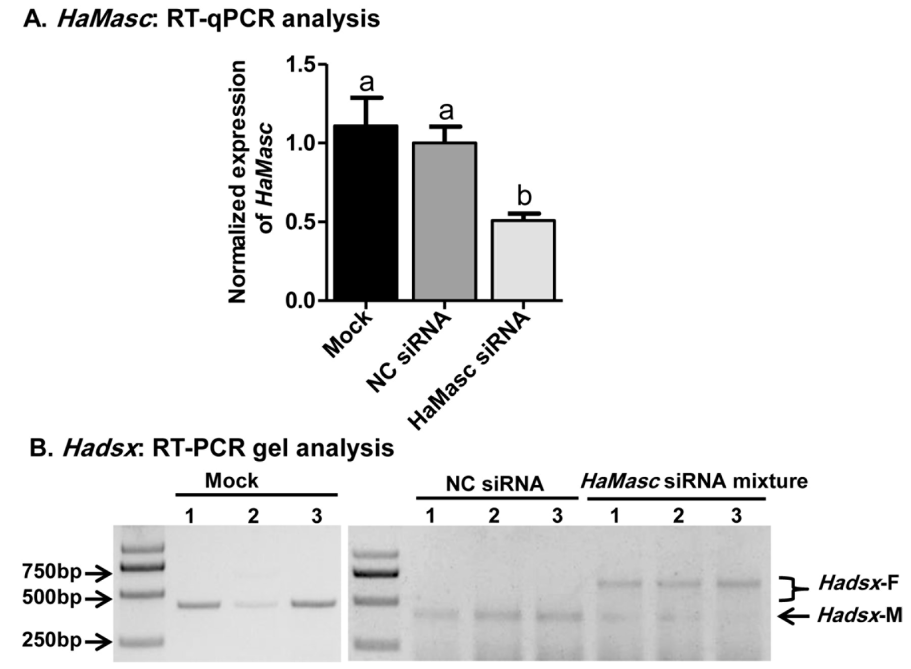
2.4. Suppressing HaMasc Caused Female-Specific Splicing of Hadsx
2.5. Suppressing HaMasc Retarded Male Growth and Testis Development
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. DNA and RNA Isolation and Reverse Transcription
4.3. Cloning of H. armigera Masc cDNA Sequence
4.4. Analysis of HaMasc Sequence
4.5. Genomic PCR and qPCR Analysis of HaMasc
4.6. RT-qPCR Analyses of HaMasc Expression
4.7. RNAi Knockdown of HaMasc in H. armigera Embryo Cell Line and Larvae
4.7.1. RNAi Knockdown of HaMasc in H. armigera Cell Line
4.7.2. RNAi Knockdown of HaMasc in H. armigera Larvae
4.8. Hematoxylin-Eosin (HE) Staining of Pupal Testes
4.9. RT-PCR Gel Analysis of Female and Male-Specific Isoform of Hadsx Transcript
5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salz, H.K. Sex determination in insects: A binary decision based on alternative splicing. Curr. Opin. Genet. Dev. 2011, 21, 395–400. [Google Scholar] [CrossRef] [Green Version]
- Bopp, D.; Saccone, G.; Beye, M. Sex determination in insects: Variations on a common theme. Sex. Dev. 2014, 8, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biedler, J.K.; Tu, Z. Sex Determination in Mosquitoes. Adv. Insect Physiol. 2016, 51, 37–66. [Google Scholar]
- Nagaraju, J.; Gopinath, G.; Sharma, V.; Shukla, J.N. Lepidopteran sex determination: A cascade of surprises. Sex. Dev. 2014, 8, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Salz, H.K.; Erickson, J.W. Sex determination in Drosophila the view from the top. Fly 2010, 4, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Kiuchi, T.; Koga, H.; Kawamoto, M.; Shoji, K.; Sakai, H.; Arai, Y.; Ishihara, G.; Kawaoka, S.; Sugano, S.; Shimada, T.; et al. A single female-specific piRNA is the primary determiner of sex in the silkworm. Nature 2014, 509, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Sawanth, S.K.; Gopinath, G.; Sambrani, N.; Arunkumar, K.P. The autoregulatory loop: A common mechanism of regulation of key sex determining genes in insects. J. Biosci. 2016, 41, 283–294. [Google Scholar] [CrossRef]
- Aryan, A.; Anderson, M.A.E.; Biedler, J.K.; Qi, Y.M.; Overcash, J.M.; Naumenko, A.N.; Sharakhova, M.V.; Mao, C.H.; Adelman, Z.N.; Tu, Z.J. Nix alone is sufficient to convert female Aedes aegypti into fertile males and myo-sex is needed for male flight. Proc. Natl. Acad. Sci. USA 2020, 117, 17702–17709. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, A.S. Moving up the hierarchy: A hypothesis on the evolution of a genetic sex determination pathway. BioEssays News Rev. Mol. Cell. Dev. Biol. 1995, 17, 71–77. [Google Scholar] [CrossRef]
- Hasselmann, M.; Gempe, T.; Schiott, M.; Nunes-Silva, C.G.; Otte, M.; Beye, M. Evidence for the evolutionary nascence of a novel sex determination pathway in honeybees. Nature 2008, 454, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Bachtrog, D.; Mank, J.E.; Peichel, C.L.; Kirkpatrick, M.; Otto, S.P.; Ashman, T.L.; Hahn, M.W.; Kitano, J.; Mayrose, I.; Ming, R.; et al. Sex determination: Why so many ways of doing it? PLoS Biol. 2014, 12, e1001899. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.H.; Sakai, R.K. Triploids and male determination in the mosquito, Anopheles culicifacies. J. Hered 1979, 70, 345–346. [Google Scholar] [CrossRef]
- Willhoeft, U.; Franz, G. Identification of the sex-determining region of the Ceratitis capitata Y chromosome by deletion mapping. Genetics 1996, 144, 737–745. [Google Scholar] [CrossRef]
- Shukla, J.N.; Palli, S.R. Production of all female progeny: Evidence for the presence of the male sex determination factor on the Y chromosome. J. Exp. Biol. 2014, 217, 1653–1655. [Google Scholar] [CrossRef] [Green Version]
- Charlesworth, D.; Mank, J.E. The birds and the bees and the flowers and the trees: Lessons from genetic mapping of sex determination in plants and animals. Genetics 2010, 186, 9–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, A.B.; Basu, S.; Jiang, X.F.; Qi, Y.M.; Timoshevskiy, V.A.; Biedler, J.K.; Sharakhova, M.V.; Elahi, R.; Anderson, M.A.E.; Chen, X.G.; et al. A male-determining factor in the mosquito Anopheles gambiae. Science 2015, 348, 1268–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, A.B.; Papathanos, P.A.; Sharma, A.; Cheng, C.; Akbari, O.S.; Assour, L.; Bergman, N.H.; Cagnetti, A.; Crisanti, A.; Dottorini, T.; et al. Radical remodeling of the Y chromosome in a recent radiation of malaria mosquitoes. Proc. Natl. Acad. Sci. USA 2016, 113, E2114–E2123. [Google Scholar] [CrossRef] [Green Version]
- Krzywinska, E.; Dennison, N.J.; Lycett, G.J.; Krzywinski, J. A maleness gene in the malaria mosquito Anopheles gambiae. Science 2016, 353, 67–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Criscione, F.; Qi, Y.M.; Tu, Z.J. GUY1 confers complete female lethality and is a strong candidate for a male-determining factor in Anopheles stephensi. Elife 2016, 5, e19281. [Google Scholar] [CrossRef]
- Sharma, A.; Heinze, S.D.; Wu, Y.L.; Kohlbrenner, T.; Morilla, I.; Brunner, C.; Wimmer, E.A.; van de Zande, L.; Robinson, M.D.; Beukeboom, L.W.; et al. Male sex in houseflies is determined by Mdmd, a paralog of the generic splice factor gene CWC22. Science 2017, 356, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Meccariello, A.; Salvemini, M.; Primo, P.; Hall, B.; Koskiniot, P.; Dalikova, M.; Gravina, A.; Gucciar, M.A.; Forlenza, F.; Gregoriou, M.E.; et al. Maleness-on-the-Y (MoY) orchestrates male sex determination in major agricultural fruit fly pests. Science 2019, 365, 1457–1460. [Google Scholar] [CrossRef]
- Traut, W.; Sahara, K.; Marec, F. Sex chromosomes and sex determination in Lepidoptera. Sex. Dev. 2007, 1, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Shimada, T. Sex determination in the silkworm, Bombyx mori: A female determinant on the W chromosome and the sex-determining gene cascade. Semin. Cell Dev. Biol. 2007, 18, 379–388. [Google Scholar] [CrossRef]
- Sakai, H.; Sumitani, M.; Chikami, Y.; Yahata, K.; Uchino, K.; Kiuchi, T.; Katsuma, S.; Aoki, F.; Sezutsu, H.; Suzuki, M.G. Transgenic expression of the piRNA-resistant masculinizer gene induces female-specific lethality and partial female-to-male sex reversal in the Silkworm, Bombyx mori. PLoS Genet. 2016, 12, e1006203. [Google Scholar] [CrossRef] [Green Version]
- Sakai, H.; Sakaguchi, H.; Aoki, F.; Suzuki, M.G. Functional analysis of sex-determination genes by gene silencing with LNA-DNA gapmers in the silkworm, Bombyx mori. Mech. Dev. 2015, 137, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.G.; Imanishi, S.; Dohmae, N.; Nishimura, T.; Shimada, T.; Matsumoto, S. Establishment of a novel in vivo sex-specific splicing assay system to identify a trans-acting factor that negatively regulates splicing of Bombyx mori dsx female exons. Mol. Cell Biol. 2008, 28, 333–343. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.G.; Imanishi, S.; Dohmae, N.; Asanuma, M.; Matsumoto, S. Identification of a Male-Specific RNA Binding Protein That regulates sex-specific splicing of Bmdsx by increasing RNA binding activity of BmPSI. Mol. Cell Biol. 2010, 30, 5776–5786. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, J.; Wan, Q.X.; Zhao, Q.; Wang, K.X.; Zha, X.F. Spliceosomal protein gene BmSPX regulates reproductive organ development in Bombyx mori. Int. J. Mol. Sci. 2020, 21, 2579. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.Q.; You, L.; Yan, D.; James, A.A.; Huang, Y.P.; Tan, A.J. Bombyx mori histone methyltransferase BmAsh2 is essential for silkworm piRNA-mediated sex determination. PLoS Genet. 2018, 14, e1007245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, X.C. Preliminary Studies on the Genomic Bases of Polyphagy in Helicoverpa armigera. Ph.D. Dissertation, Chinese Academy of Agriculture Sciences, Beijing, China, 2018. [Google Scholar]
- Lee, J.; Kiuchi, T.; Kawamoto, M.; Shimada, T.; Katsuma, S. Identification and functional analysis of a Masculinizer orthologue in Trilocha varians (Lepidoptera: Bombycidae). Insect Mol. Biol. 2015, 24, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Fukui, T.; Kiuchi, T.; Shoji, K.; Kawamoto, M.; Shimada, T.; Katsuma, S. In Vivo masculinizing function of the Ostrinia furnacalis Masculinizer gene. Biochem. Biophs. Res. Commun. 2018, 503, 1768–1772. [Google Scholar] [CrossRef]
- Wang, Y.H.; Chen, X.E.; Yang, Y.; Xu, J.; Fang, G.Q.; Niu, C.Y.; Huang, Y.P.; Zhan, S. The Masc gene product controls masculinization in the black cutworm, Agrotis ipsilon. Insect Sci. 2019, 26, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Harvey-Samuel, T.; Norman, V.C.; Carter, R.; Lovett, E.; Alphey, L. Identification and characterization of a Masculinizer homologue in the diamondback moth, Plutella xylostella. Insect Mol. Biol. 2020, 29, 231–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Z.Y.; Zhang, Y.K.; Zhang, M.; Huang, J.Y.; Li, C.Y.; Ni, X.Z.; Li, X.C. Characterization of the first W-specific protein-coding gene for sex identification in Helicoverpa armigera. Front. Genet. 2020, 11, 649. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.C.; Dong, J.F.; Tang, Q.B.; Yan, Y.H.; Gelbic, I.; Van Loon, J.J.A.; Wang, C.Z. Hybridization between Helicoverpa armigera and Helicoverpa assulta (Lepidoptera: Noctuidae): Development and morphological characterization of F-1 hybrids. Bull. Entomol. Res. 2005, 95, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Katsuma, S.; Kiuchi, T.; Kawamoto, M.; Fujimoto, T.; Sahara, K. Unique sex determination system in the silkworm, Bombyx mori: Current status and beyond. Proc. Jpn. Acad. B Phys. 2018, 94, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawahara, A.Y.; Breinholt, J.W. Phylogenomics provides strong evidence for relationships of butterflies and moths. Proc. R. Soc. B Biol. Sci. 2014, 281, 1788. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, A.Y.; Plotkin, D.; Espeland, M.; Meusemann, K.; Toussaint, E.F.A.; Donath, A.; Gimnich, F.; Frandsen, P.B.; Zwick, A.; dos Reis, M.; et al. Phylogenomics reveals the evolutionary timing and pattern of butterflies and moths. Proc. Natl. Acad. Sci. USA 2019, 116, 22657–22663. [Google Scholar] [CrossRef] [Green Version]
- Pearson, W.R. An introduction to sequence similarity (“homology”) searching. Curr. Protoc. Bioinform. 2013, 42, 311–318. [Google Scholar] [CrossRef]
- Katsuma, S.; Sugano, Y.; Kiuchi, T.; Shimada, T. Two conserved cysteine residues are required for the masculinizing activity of the Silkworm masc protein. J. Biol. Chem. 2015, 290, 26114–26124. [Google Scholar] [CrossRef] [Green Version]
- Sugano, Y.; Kokusho, R.; Ueda, M.; Fujimoto, M.; Tsutsumi, N.; Shimada, T.; Kiuchi, T.; Katsuma, S. Identification of a bipartite nuclear localization signal in the silkworm Masc protein. FEBS Lett. 2016, 590, 2256–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiuchi, T.; Sugano, Y.; Shimada, T.; Katsuma, S. Two CCCH-type zinc finger domains in the Masc protein are dispensable for masculinization and dosage compensation in Bombyx mori. Insect Biochem. Mol. 2019, 104, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, J.; Wen, M.Y.; Wang, H.; Wang, Y.; Wang, K.X.; Wan, Q.X.; Zha, X.F. A novel splice variant of the masculinizing gene masc with piRNA-cleavage-site defect functions in female external genital development in the Silkworm, Bombyx mori. Biomolecules 2019, 9, 318. [Google Scholar] [CrossRef] [Green Version]
- Rideout, E.J.; Narsaiya, M.S.; Grewal, S.S. The sex determination gene transformer regulates male-female differences in Drosophila body size. PLoS Genet. 2015, 11, e1005683. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Chen, S.; Zeng, B.; James, A.A.; Tan, A.; Huang, Y. Bombyx mori P-element Somatic Inhibitor (BmPSI) Is a key auxiliary factor for Silkworm male sex determination. PLoS Genet. 2017, 13, e1006576. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, Z.; Hu, B.; Yu, Y.; Tan, A. A CCCH zinc finger gene regulates doublesex alternative splicing and male development in Bombyx mori. Insect Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, G.; Arunkumar, K.P.; Mita, K.; Nagaraju, J. Role of Bmznf-2, a Bombyx mori CCCH zinc finger gene, in masculinisation and differential splicing of Bmtra-2. Insect Biochem. Mol. 2016, 75, 32–44. [Google Scholar] [CrossRef]
- Sahara, K.; Yoshido, A.; Kawamura, N.; Ohnuma, A.; Abe, H.; Mita, K.; Oshiki, T.; Shimada, T.; Asano, S.; Bando, H.; et al. W-derived BAC probes as a new tool for identification of the W chromosome and its aberrations in Bombyx mori. Chromosoma 2003, 112, 48–55. [Google Scholar]
- Abe, H.; Mita, K.; Yasukochi, Y.; Oshiki, T.; Shimada, T. Retrotransposable elements on the W chromosome of the silkworm, Bombyx mori. Cytogenet. Genome Res. 2005, 110, 144–151. [Google Scholar] [CrossRef]
- Kawaoka, S.; Kadota, K.; Arai, Y.; Suzuki, Y.; Fujii, T.; Abe, H.; Yasukochi, Y.; Mita, K.; Sugano, S.; Shimizu, K.; et al. The silkworm W chromosome is a source of female-enriched piRNAs. RNA 2011, 17, 2144–2151. [Google Scholar] [CrossRef] [Green Version]
- Waldbauer, G.; Cohen, R.; Friedman, S. An improved procedure for laboratory rearing of the corn earworm, Heliothis zea (Lepidoptera: Noctuidae). Grea Lakes Entomol. 1984, 17, 113–118. [Google Scholar]
- Waldbauer, G.; Friedman, S. Self-Selection of optimal diets by insects. Annu. Rev. Entomol. 1991, 36, 43–63. [Google Scholar] [CrossRef]
- Li, X.C.; Berenbaum, M.R.; Schuler, M.A. Cytochrome P450 and actin genes expressed in Helicoverpa zea and Helicoverpa armigera: Paralogy/orthology identification, gene conversion and evolution. Insect Biochem. Mol. 2002, 32, 311–320. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.P.; Guo, A.Y.; Zhang, H.; Luo, J.C.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.N.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015, 43, 222–226. [Google Scholar] [CrossRef] [Green Version]
- Nicholas, K.; Nicholas, H. Genedoc: A Tool for Editing and Annotating Multiple Sequence Alignments. 1997. Distributed by the authors. Available online: http://www.psc.edu/biomed/genedoc (accessed on 15 October 2006).
- Deng, Z.Y.; Zhang, S.; Gu, S.H.; Ni, X.Z.; Zeng, W.X.; Li, X.C. Useful bicistronic reporter system for studying Poly(A) site-defining cis elements and regulation of alternative polyadenylation. Int. J. Mol. Sci. 2018, 19, 279. [Google Scholar] [CrossRef] [Green Version]
- Zheng, G.; Li, C.; Zhou, H.; Li, S.; Li, G.; Xue, M. Establishment of two new cell lines from the embryonic tissue of Helicoverpa armigera (Lepidoptera: Noctuidae) and their responses to baculovirus infection. Acta Entomol. Sin. 2010, 53, 167–174. [Google Scholar]
- Zhu, B.; Sun, X.; Nie, X.; Liang, P.; Gao, X. MicroRNA-998-3p contributes to Cry1Ac-resistance by targeting ABCC2 in lepidopteran insects. Insect Biochem. Mol. Biol. 2020, 117, 103283. [Google Scholar] [CrossRef]
- Li, S.; Hussain, F.; Unnithan, G.C.; Dong, S.; UlAbdin, Z.; Gu, S.; Mathew, L.G.; Fabrick, J.A.; Ni, X.; Carriere, Y.; et al. A long non-coding RNA regulates cadherin transcription and susceptibility to Bt toxin Cry1Ac in pink bollworm, Pectinophora gossypiella. Pestic. Biochem. Physiol. 2019, 158, 54–60. [Google Scholar] [CrossRef]
- Seroogy, K.; Tsuruo, Y.; Hokfelt, T.; Walsh, J.; Fahrenkrug, J.; Emson, P.C.; Goldstein, M. Further analysis of presence of peptides in dopamine neurons. Cholecystokinin, peptide histidine-isoleucine/vasoactive intestinal polypeptide and substance P in rat supramammillary region and mesencephalon. Exp. Brain Res. 1988, 72, 523–534. [Google Scholar] [PubMed]


| BmMASC | TvMASC | OfMASC | AiMASC | HaMASC | PxMASC | |
|---|---|---|---|---|---|---|
| BmMASC | 100.00 | |||||
| TvMASC | 54.99 | 100.00 | ||||
| OfMASC | 22.48 | 22.29 | 100.00 | |||
| AiMASC | 21.38 | 19.37 | 30.71 | 100.00 | ||
| HaMASC | 25.00 | 22.52 | 34.01 | 62.57 | 100.00 | |
| PxMASC | 19.74 | 17.01 | 22.30 | 19.76 | 21.14 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Z.; Zhang, Y.; Li, Y.; Huang, K.; Chen, X.; Zhang, M.; Huang, J.; Ni, X.; Li, X. Identification and Characterization of the Masculinizing Function of the Helicoverpa armigera Masc Gene. Int. J. Mol. Sci. 2021, 22, 8650. https://doi.org/10.3390/ijms22168650
Deng Z, Zhang Y, Li Y, Huang K, Chen X, Zhang M, Huang J, Ni X, Li X. Identification and Characterization of the Masculinizing Function of the Helicoverpa armigera Masc Gene. International Journal of Molecular Sciences. 2021; 22(16):8650. https://doi.org/10.3390/ijms22168650
Chicago/Turabian StyleDeng, Zhongyuan, Yakun Zhang, Yalu Li, Kaiyuan Huang, Xuewei Chen, Min Zhang, Jinyong Huang, Xinzhi Ni, and Xianchun Li. 2021. "Identification and Characterization of the Masculinizing Function of the Helicoverpa armigera Masc Gene" International Journal of Molecular Sciences 22, no. 16: 8650. https://doi.org/10.3390/ijms22168650
APA StyleDeng, Z., Zhang, Y., Li, Y., Huang, K., Chen, X., Zhang, M., Huang, J., Ni, X., & Li, X. (2021). Identification and Characterization of the Masculinizing Function of the Helicoverpa armigera Masc Gene. International Journal of Molecular Sciences, 22(16), 8650. https://doi.org/10.3390/ijms22168650







