Gene Variant of Barrier to Autointegration Factor 2 (Banf2w) Is Concordant with Female Determination in Cichlids
Abstract
1. Introduction
2. Results
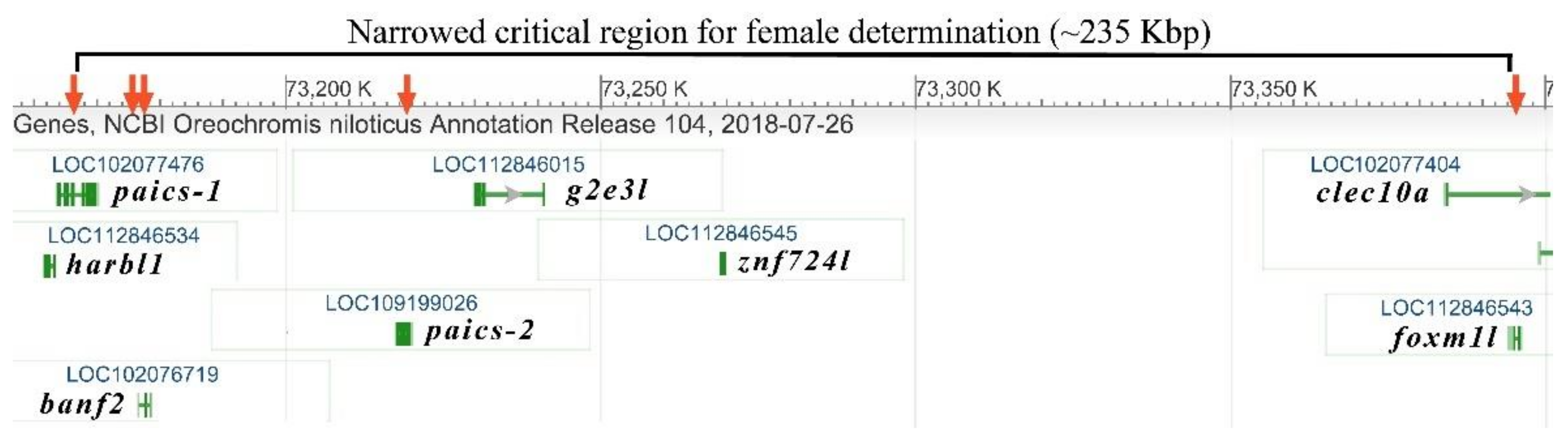
2.1. Assembly of Sequence-Read Contigs for Marker Design and Analysis of Association in the Oa SD Region
2.2. The Gene Models of Banf2 Locus in Oa Hybrid Stock
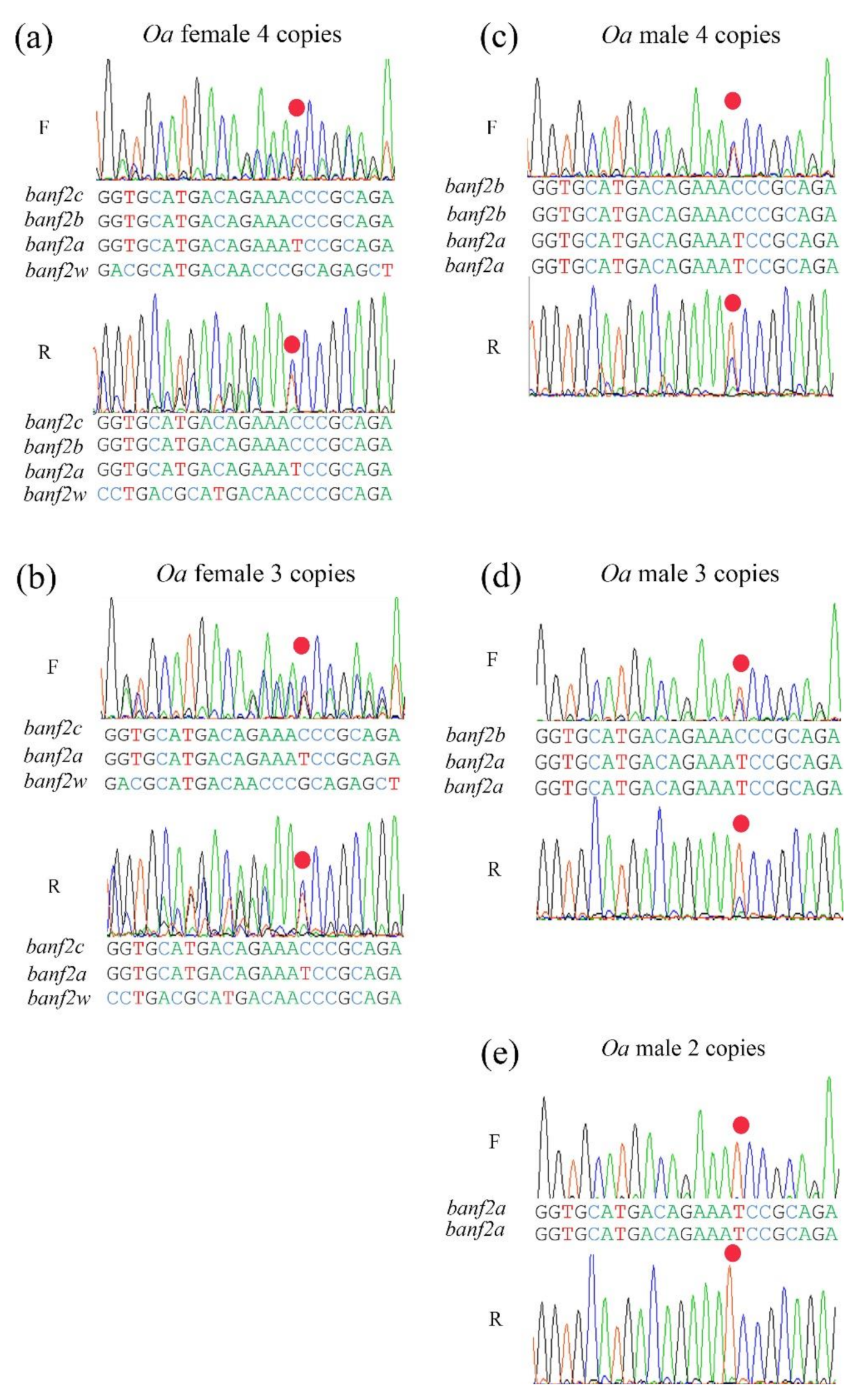
2.3. The Gene Models of Banf2 Locus in Purebred Oa
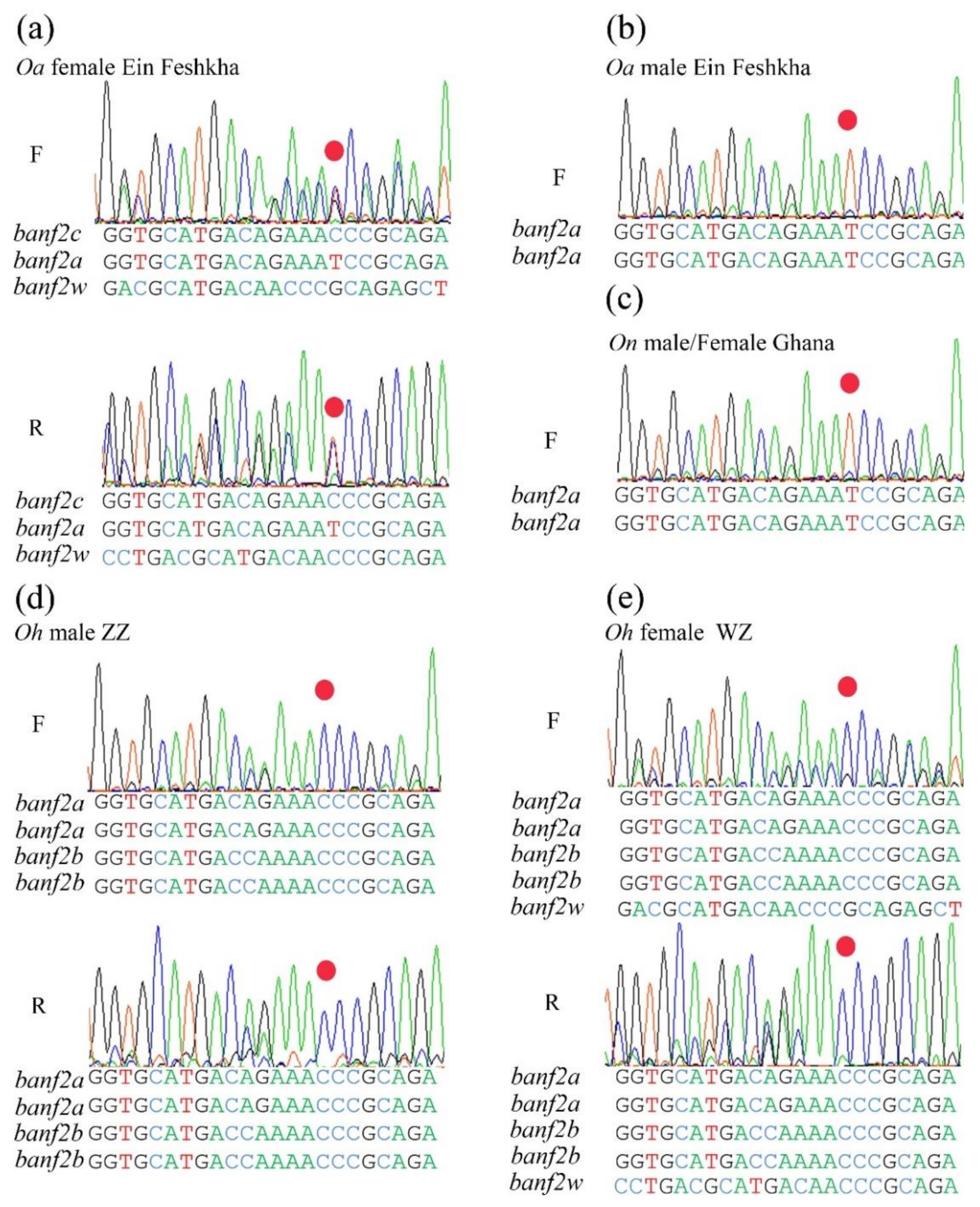
2.4. Banf2w Is Female-Specific in Cichlidae Species with WZ/ZZ SD System on LG3
2.5. Competitive Expression of Banf2w and Other Banf2 Genes in Oa Females
2.6. The Paics Gene Candidates for SD
3. Discussion
4. Materials and Methods
4.1. Fish
4.2. DNA Extraction, PCR Amplification, Agarose Separation and Sanger Sequencing
4.3. Marker Development and Search for Coding Polymorphism in O. aureus
4.4. Copy Number Estimation with Sanger and Construction Genetic Models
4.5. Sequence Alignments
4.6. Expression Analysis
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Herpin, A.; Schartl, M. Vertebrate sex determination: Questioning the hierarchy. FEBS J. 2011, 278, 1001. [Google Scholar] [CrossRef] [PubMed]
- Capel, B. Vertebrate sex determination: Evolutionary plasticity of a fundamental switch. Nat. Rev. Genet. 2017, 18, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.A.M. How to evolve new vertebrate sex determining genes. Dev. Dyn. 2013, 242, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Gubbay, J.; Collignon, J.; Koopman, P.; Capel, B.; Economou, A.; Münsterberg, A.; Vivian, N.; Goodfellow, P.; Lovell-Badge, R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 1990, 346, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.H.; Berta, P.; Palmer, M.S.; Hawkins, J.R.; Griffiths, B.L.; Smith, M.J.; Foster, J.W.; Frischauf, A.-M.; Lovell-Badge, R.; Goodfellow, P.N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 1990, 346, 240–244. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, J.; Bachtrog, D.; An, N.; Huang, Q.; Jarvis, E.D.; Gilbert, M.T.P.; Zhang, G. Complex evolutionary trajectories of sex chromosomes across bird taxa. Science 2014, 346, 1246338. [Google Scholar] [CrossRef]
- Smith, C.A.; Roeszler, K.N.; Ohnesorg, T.; Cummins, D.M.; Farlie, P.G.; Doran, T.J.; Sinclair, A.H. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 2009, 461, 267–271. [Google Scholar] [CrossRef]
- Curzon, A.Y.; Dor, L.; Shirak, A.; Rosenfeld, H.; Ashkenazi, I.M.; Ron, M.; Seroussi, E. A novel c.1759T>G variant in follicle-stimulating hormone-receptor gene is concordant with male determination in the flathead grey mullet (Mugil cephalus). G3 Genes Genomes Genet. 2020, 11, jkaa044. [Google Scholar] [CrossRef]
- Hammerman, I.S.; Avtalion, R.R. Sex determination in Sarotherodon (tilapia). Theor. Appl. Genet. 1979, 55, 177–187. [Google Scholar] [CrossRef]
- Wohlfarth, G.W.; Wedekind, H. The heredity of sex determination in tilapias. Aquaculture 1991, 92, 143–156. [Google Scholar] [CrossRef]
- Koopman, P. The genetics and biology of vertebrate sex determination. Cell 2001, 105, 843–847. [Google Scholar] [CrossRef]
- Graves, J.A.M.; Peichel, C.L. Are homologies in vertebrate sex determination due to shared ancestry or to limited options? Genome Biol. 2010, 11, 205. [Google Scholar] [CrossRef]
- Yano, A.; Guyomard, R.; Nicol, B.; Jouanno, E.; Quillet, E.; Klopp, C.; Cabau, C.; Bouchez, O.; Fostier, A.; Guiguen, Y. An immune-related gene evolved into the master sex-determining gene in rainbow trout, Oncorhynchus mykiss. Curr. Biol. 2012, 22, 1423–1428. [Google Scholar] [CrossRef]
- Yano, A.; Nicol, B.; Jouanno, E.; Quillet, E.; Fostier, A.; Guyomard, R.; Guiguen, Y. The sexually dimorphic on the Y-chromosome gene (sdY) is a conserved male-specific Y-chromosome sequence in many salmonids. Evol. Appl. 2013, 6, 486–496. [Google Scholar] [CrossRef]
- Herpin, A.; Schartl, M. Plasticity of gene-regulatory networks controlling sex determination: Of masters, slaves, usual suspects, newcomers, and usurpators. EMBO Rep. 2015, 16, 1260–1274. [Google Scholar] [CrossRef]
- Bertho, S.; Herpin, A.; Branthonne, A.; Jouanno, E.; Yano, A.; Nicol, B.; Muller, T.; Pannetier, M.; Pailhoux, E.; Miwa, M.; et al. The unusual rainbow trout sex determination gene hijacked the canonical vertebrate gonadal differentiation pathway. Proc. Natl. Acad. Sci. USA 2018, 115, 12781–12786. [Google Scholar] [CrossRef]
- Shirak, A.; Zak, T.; Dor, L.; Benet-Perlberg, A.; Weller, J.I.; Ron, M.; Seroussi, E. Quantitative trait loci on LGs 9 and 14 affect the reproductive interaction between two Oreochromis species, O. niloticus and O. aureus. Heredity 2018, 122, 341–353. [Google Scholar] [CrossRef]
- Megbowon, I.; Mojekwu, T.O. Tilapia sex reversal using methyl testosterone (MT) and its effect on fish, man and environment. Biotechnology 2014, 13, 213–216. [Google Scholar] [CrossRef][Green Version]
- Pruginin, Y.; Rothbard, S.; Wohlfarth, G.; Halevy, A.; Moav, R.; Hulata, G. All-male broods of Tilapia nilotica × T. aurea hybrids. Aquaculture 1975, 6, 11–21. [Google Scholar] [CrossRef]
- Hickling, C.F. The Malacca tilapia hybrids. J. Genet. 1960, 57, 1–10. [Google Scholar] [CrossRef]
- Eknath, A.E.; Bentsen, H.B.; Gjerde, B.; Tayamen, M.M.; Abella, T.A.; Gjedrem, T.; Pullin, R.S.V. Approaches to national fish breeding programs: Pointers from tilapia pilot study. Naga ICLARM Q. 1991, 14, 10–12. Available online: http://aquaticcommons.org/id/eprint/9178 (accessed on 29 June 2021).
- Brawand, D.; Wagner, C.E.; Li, Y.I.; Malinsky, M.; Keller, I.; Fan, S.; Simakov, O.; Ng, A.Y.; Lim, Z.W.; Bezault, E.; et al. The genomic substrate for adaptive radiation in African cichlid fish. Nature 2015, 513, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Ser, J.R.; Roberts, R.B.; Kocher, T.D. Multiple interacting loci control sex determination in lake Malawi Cichlid fishes. Evolution 2010, 64, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Baroiller, J.F.; D’Cotta, H.; Bezault, E.; Wessels, S.; Hoerstgen-Schwark, G. Tilapia sex determination: Where temperature and genetics meet. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 153, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Mair, G.C.; Scott, A.G.; Penman, D.J.; Skibinski, D.O.F.; Beardmore, J.A. Sex determination in the genus Oreochromis—2. Sex reversal, hybridisation, gynogenesis and triploidy in O. aureus Steindachner. Theor. Appl. Genet. 1991, 82, 153–160. [Google Scholar] [CrossRef]
- Curzon, A.Y.; Shirak, A.; Dor, L.; Zak, T.; Perelberg, A.; Seroussi, E.; Ron, M. A duplication of the Anti-Müllerian hormone gene is associated with genetic sex determination of different Oreochromis niloticus strains. Heredity 2020, 125, 317–327. [Google Scholar] [CrossRef]
- Charlesworth, D.; Bergero, R.; Graham, C.; Gardner, J.; Yong, L. Locating the sex determining region of linkage group 12 of guppy (Poecilia reticulata). G3 Genes Genomes Genet. 2020, 10, 3639–3649. [Google Scholar] [CrossRef]
- Dor, L.; Shirak, A.; Kohn, Y.Y.; Gur, T.; Weller, J.I.; Zilberg, D.; Seroussi, E.; Ron, M. Mapping of the sex determining region on linkage group 12 of guppy (Poecilia reticulata). G3 Genes Genomes Genet. 2019, 9, 3867–3875. [Google Scholar] [CrossRef]
- Curzon, A.Y.; Shirak, A.; Zak, T.; Dor, L.; Benet-Perlberg, A.; Naor, A.; Low-Tanne, S.I.; Sharkawi, H.; Ron, M.; Seroussi, E. All-male production by marker-assisted selection for sex determining loci of admixed Oreochromis niloticus and Oreochromis aureus stocks. Anim. Genet. 2021, 52, 361–364. [Google Scholar] [CrossRef]
- Gammerdinger, W.J.; Kocher, T.D. Unusual diversity of sex chromosomes in african cichlid fishes. Genes 2018, 9, 480. [Google Scholar] [CrossRef]
- Gammerdinger, W.J.; Conte, M.A.; Sandkam, B.A.; Penman, D.J.; Kocher, T.D. Characterization of sex chromosomes in three deeply diverged species of Pseudocrenilabrinae (Teleostei: Cichlidae). Hydrobiologia 2019, 832, 397–408. [Google Scholar] [CrossRef]
- Gammerdinger, W.J.; Conte, M.A.; Baroiller, J.-F.; D’Cotta, H.; Kocher, T.D. Comparative analysis of a sex chromosome from the blackchin tilapia, Sarotherodon melanotheron. BMC Genom. 2016, 17, 808. [Google Scholar] [CrossRef]
- Böhne, A.; Weber, A.A.T.; Rajkov, J.; Rechsteiner, M.; Riss, A.; Egger, B.; Salzburger, W. Repeated evolution versus common ancestry: Sex chromosome evolution in the haplochromine cichlid Pseudocrenilabrus philander. Genome Biol. Evol. 2019, 11, 439–458. [Google Scholar] [CrossRef]
- Eshel, O.; Shirak, A.; Dor, L.; Band, M.; Zak, T.; Markovich-Gordon, M.; Chalifa-Caspi, V.; Feldmesser, E.; Weller, J.I.; Seroussi, E.; et al. Identification of male-specific amh duplication, sexually differentially expressed genes and microRNAs at early embryonic development of Nile tilapia (Oreochromis niloticus). BMC Genom. 2014, 15, 774. [Google Scholar] [CrossRef]
- Taslima, K.; Wehner, S.; Taggart, J.B.; de Verdal, H.; Benzie, J.A.H.; Bekaert, M.; McAndrew, B.J.; Penman, D.J. Sex determination in the GIFT strain of tilapia is controlled by a locus in linkage group 23. BMC Genet. 2020, 21, 49. [Google Scholar] [CrossRef]
- Li, M.; Sun, Y.; Zhao, J.; Shi, H.; Zeng, S.; Ye, K.; Jiang, D.; Zhou, L.; Sun, L.; Tao, W.; et al. A tandem duplicate of Anti-Müllerian hormone with a missense SNP on the Y chromosome is esential for male sex determination in Nile tilapia, Oreochromis niloticus. PLoS Genet. 2015, 11, e1005678. [Google Scholar] [CrossRef]
- Cnaani, A.; Kocher, T.D. Sex-linked markers and microsatellite locus duplication in the cichlid species Oreochromis tanganicae. Biol. Lett. 2008, 4, 700–703. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, Z.; Lu, M.; Gaao, F.; Ke, X.; Ma, D.; Huang, Z.; Cao, J.; Wang, M. Screening and identification of a microsatellite marker associated with sex in Wami tilapia, Oreochromis urolepis hornorum. J. Genet. 2016, 95, 283–289. [Google Scholar] [CrossRef]
- Gammerdinger, W.J.; Conte, M.A.; Sandkam, B.A.; Ziegelbecker, A.; Koblmüller, S.; Kocher, T.D. Novel Sex Chromosomes in 3 Cichlid Fishes from Lake Tanganyika. J. Hered. 2018, 109, 489–500. [Google Scholar] [CrossRef]
- Conte, M.A.; Gammerdinger, W.J.; Bartie, K.L.; Penman, D.J.; Kocher, T.D. A high quality assembly of the Nile Tilapia (Oreochromis niloticus) genome reveals the structure of two sex determination regions. BMC Genom. 2017, 18, 341. [Google Scholar] [CrossRef]
- Conte, M.A.; Clark, F.E.; Roberts, R.B.; Xu, L.; Tao, W.; Zhou, Q.; Wang, D.; Kocher, T.D. Origin of a giant sex chromosome. Mol. Biol. Evol. 2020. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, L.; Fan, Z.; Lu, B.; Chen, J.; Tan, D.; Jiang, D.; Tao, W.; Wang, D. Screening and characterization of sex-linked DNA markers and marker-assisted selection in blue tilapia (Oreochromis aureus). Aquaculture 2021, 530, 735934. [Google Scholar] [CrossRef]
- Tao, W.; Xu, L.; Zhao, L.; Zhu, Z.; Wu, X.; Min, Q.; Wang, D.; Zhou, Q. High-quality chromosome-level genomes of two tilapia species reveal their evolution of repeat sequences and sex chromosomes. Mol. Ecol. Resour. 2021, 21, 543–560. [Google Scholar] [CrossRef]
- Lee, B.-Y.; Hulata, G.; Kocher, T.D. Two unlinked loci controlling the sex of blue tilapia (Oreochromis aureus). Heredity 2004, 92, 543–549. [Google Scholar] [CrossRef]
- Jensen, G.L.; Shelton, W.L. Effects of estrogens on Tilapia aurea: Implications for production of monosex genetic male tilapia. Aquaculture 1979, 16, 233–242. [Google Scholar] [CrossRef]
- Avtalion, R.R.; Don, J. Sex-determining genes in tilapia: A model of genetic recombination emerging from sex ratio results of three generations of diploid gynogenetic Oreochromis aureus. J. Fish Biol. 1990, 37, 167–173. [Google Scholar] [CrossRef]
- Graves, J.A.M. Sex chromosome specialization and degeneration in mammals. Cell 2006, 124, 901–914. [Google Scholar] [CrossRef]
- Fraser, J.A.; Heitman, J. Chromosomal sex-determining regions in animals, plants and fungi. Curr. Opin. Genet. Dev. 2005, 15, 645–651. [Google Scholar] [CrossRef]
- Lee, B.Y.; Lee, W.J.; Streelman, J.T.; Carleton, K.L.; Howe, A.E.; Hulata, G.; Slettan, A.; Stern, J.E.; Terai, Y.; Kocher, T.D. A second-generation genetic linkage map of tilapia (Oreochromis spp.). Genetics 2005, 170, 237–244. [Google Scholar] [CrossRef]
- Ocalewicz, K.; Mota-Velasco, J.C.; Campos-Ramos, R.; Penman, D.J. FISH and DAPI staining of the synaptonemal complex of the Nile tilapia (Oreochromis niloticus) allow orientation of the unpaired region of bivalent 1 observed during early pachytene. Chromosom. Res. 2009, 17, 773–782. [Google Scholar] [CrossRef]
- Charlesworth, D.; Charlesworth, B.; Marais, G. Steps in the evolution of heteromorphic sex chromosomes. Heredity 2005, 95, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Seroussi, E. Estimating copy-number proportions: The comeback of Sanger sequencing. Genes 2021, 12, 283. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, E.K.; Chen, T.; Amendola, M.; Van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 2014, 42, e168. [Google Scholar] [CrossRef] [PubMed]
- Shirak, A.; Seroussi, E.; Cnaani, A.; Howe, A.E.; Domokhovsky, R.; Zilberman, N.; Kocher, T.D.; Hulata, G.; Ron, M. Amh and dmrta2 genes map to tilapia (Oreochromis spp.) linkage group 23 within quantitative trait locus regions for sex determination. Genetics 2006, 174, 1573–1581. [Google Scholar] [CrossRef]
- Eshel, O.; Shirak, A.; Weller, J.I.; Hulata, G.; Ron, M. Linkage and physical mapping of sex region on LG23 of Nile tilapia (Oreochromis niloticus). G3 Genes Genomes Genet. 2012, 2, 35–42. [Google Scholar] [CrossRef]
- Eshel, O.; Shirak, A.; Weller, J.I.; Slossman, T.; Hulata, G.; Cnaani, A.; Ron, M. Fine-mapping of a locus on linkage group 23 for sex determination in Nile tilapia (Oreochromis niloticus). Anim. Genet. 2011, 42, 222–224. [Google Scholar] [CrossRef]
- Hattori, R.S.; Murai, Y.; Oura, M.; Masuda, S.; Majhi, S.K.; Sakamoto, T.; Fernandino, J.I.; Somoza, G.M.; Yokota, M.; Strussmann, C.A. A Y-linked anti-Mullerian hormone duplication takes over a critical role in sex determination. Proc. Natl. Acad. Sci. USA 2012, 109, 2955–2959. [Google Scholar] [CrossRef]
- Rondeau, E.B.; Laurie, C.V.; Johnson, S.C.; Koop, B.F. A PCR assay detects a male-specific duplicated copy of Anti-Müllerian hormone (amh) in the lingcod (Ophiodon elongatus). BMC Res. Notes 2016, 9, 230. [Google Scholar] [CrossRef]
- Bej, D.K.; Miyoshi, K.; Hattori, R.S.; Strüssmann, C.A.; Yamamoto, Y. A duplicated, truncated amh gene is involved in male sex determination in an old world Silverside. G3 Genes Genomes Genet. 2017, 7, 2489–2495. [Google Scholar] [CrossRef]
- Pan, Q.; Feron, R.; Yano, A.; Guyomard, R.; Jouanno, E.; Vigouroux, E.; Wen, M.; Busne, J.M.; Bobe, J.; Concordet, J.P.; et al. Identification of the master sex determining gene in Northern pike (Esox lucius) reveals restricted sex chromosome differentiation. PLoS Genet. 2019, 15, e1008013. [Google Scholar] [CrossRef]
- Fraslin, C.; Phocas, F.; Bestin, A.; Charles, M.; Bernard, M.; Krieg, F.; Dechamp, N.; Ciobotaru, C.; Hozé, C.; Petitprez, F.; et al. Genetic determinism of spontaneous masculinisation in XX female rainbow trout: New insights using medium throughput genotyping and whole-genome sequencing. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Ferraresso, S.; Bargelloni, L.; Babbucci, M.; Cannas, R.; Follesa, M.C.; Carugati, L.; Melis, R.; Cau, A.; Koutrakis, M.; Sapounidis, A.; et al. Fshr: A fish sex-determining locus shows variable incomplete penetrance across flathead grey mullet populations. iScience 2021, 24, 101886. [Google Scholar] [CrossRef]
- Turner, M.E.; Ely, D.; Prokop, J.; Milsted, A. Sry, more than testis determination? Am. J. Physiol. Integr. Comp. Physiol. 2011, 301, R561–R571. [Google Scholar] [CrossRef]
- Tifft, K.E.; Segura-Totten, M.; Lee, K.K.; Wilson, K.L. Barrier-to-autointegration factor-like (BAF-L): A proposed regulator of BAF. Exp. Cell Res. 2006, 312, 478–487. [Google Scholar] [CrossRef]
- Maratou, K.; Forster, T.; Costa, Y.; Taggart, M.; Speed, R.M.; Ireland, J.; Teague, P.; Roy, D.; Cooke, H.J. Expression profiling of the developing testis in wild-type and Dazl knockout mice. Mol. Reprod. Dev. 2004, 67, 26–54. [Google Scholar] [CrossRef]
- L’Hôte, D.; Georges, A.; Todeschini, A.L.; Kim, J.H.; Benayoun, B.A.; Bae, J.; Veitia, R.A. Discovery of novel protein partners of the transcription factor FOXL2 provides insights into its physiopathological roles. Hum. Mol. Genet. 2012, 21, 3264–3274. [Google Scholar] [CrossRef][Green Version]
- Penrad-Mobayed, M.; Perrin, C.; Herman, L.; Todeschini, A.; Nigon, F.; Cosson, B.; Caburet, S.; Veitia, R.A. Conventional and unconventional interactions of the transcription factor FOXL2 uncovered by a proteome-wide analysis. FASEB J. 2020, 34, 571–587. [Google Scholar] [CrossRef]
- Margalit, A.; Segura-Totten, M.; Gruenbaum, Y.; Wilson, K.L. Barrier-to-autointegration factor is required to segregate and enclose chromosomes within the nuclear envelope and assemble the nuclear lamina. Proc. Natl. Acad. Sci. USA 2005, 102, 3290–3295. [Google Scholar] [CrossRef]
- Holaska, J.M.; Lee, K.K.; Kowalski, A.K.; Wilson, K.L. Transcriptional repressor germ cell-less (GCL) and barrier to autointegration factor (BAF) compete for binding to emerin in vitro. J. Biol. Chem. 2003, 278, 6969–6975. [Google Scholar] [CrossRef]
- Georges, A.; Auguste, A.; Bessière, L.; Vanet, A.; Todeschini, A.L.; Veitia, R.A. FOXL2: A central transcription factor of the ovary. J. Mol. Endocrinol. 2013, 52, 17–33. [Google Scholar] [CrossRef]
- Benayoun, B.A.; Dipietromaria, A.; Bazin, C.; Veitia, R.A. FOXL2: At the crossroads of female sex determination and ovarian function. Adv. Exp. Med. Biol. 2009, 665, 207–226. [Google Scholar] [CrossRef]
- Windley, S.P.; Wilhelm, D. Signaling pathways involved in mammalian sex determination and gonad development. Sex. Dev. 2016, 9, 297–315. [Google Scholar] [CrossRef]
- McMurray, C.T. Mechanisms of DNA expansion. Chromosoma 1995, 104, 2–13. [Google Scholar] [CrossRef]
- Katju, V.; Bergthorsson, U. Copy-number changes in evolution: Rates, fitness effects and adaptive significance. Front. Genet. 2013, 4, 273. [Google Scholar] [CrossRef]
- Nanda, I.; Kondo, M.; Hornung, U.; Asakawa, S.; Winkler, C.; Shimizu, A.; Shan, Z.; Haaf, T.; Shimizu, N.; Shima, A.; et al. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc. Natl. Acad. Sci. USA 2002, 99, 11778–11783. [Google Scholar] [CrossRef]
- Ventura, T.; Sagi, A. The insulin-like androgenic gland hormone in crustaceans: From a single gene silencing to a wide array of sexual manipulation-based biotechnologies. Biotechnol. Adv. 2012, 30, 1543–1550. [Google Scholar] [CrossRef]
- Bao, L.; Tian, C.; Liu, S.; Zhang, Y.; Elaswad, A.; Yuan, Z.; Khalil, K.; Sun, F.; Yang, Y.; Zhou, T.; et al. The Y chromosome sequence of the channel catfish suggests novel sex determination mechanisms in teleost fish. BMC Biol. 2019, 17, 6. [Google Scholar] [CrossRef]
- Shirak, A.; Dor, L.; Seroussi, E.; Ron, M.; Hulata, G.; Golani, D. DNA barcoding of fish species from the Mediterranean coast of Israel. Mediterr. Mar. Sci. 2016, 17, 459–466. [Google Scholar] [CrossRef][Green Version]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3-new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Dor, L.; Shirak, A.; Gorshkov, S.; Band, M.R.; Korol, A.; Ronin, Y.; Curzon, A.; Hulata, G.; Seroussi, E.; Ron, M. Construction of a microsatellites-based linkage map for the White Grouper (Epinephelus aeneus). G3 Genes Genomes Genet. 2014, 4, 1455–1464. [Google Scholar] [CrossRef]
- Pruitt, K.; Brown, G.; Tatusova, T.; Maglott, D. The Reference Sequence (RefSeq) database. In The NCBI Handbook; National Library of Medicine (US), National Center for Biotechnology Information: Bethesda, MD, USA, 2002; pp. 1–24. Available online: https://www.ncbi.nlm.nih.gov/books/NBK21091/ (accessed on 29 June 2021).



| Marker | Primers | Assay | Accession No. | |
|---|---|---|---|---|
| UNH168 | F | FAM-TAAGAAGGTTAGAAAGAAAGTG | PAGE 1 | G12320 |
| R | TATATAATAATTTCCTAAACGGC | |||
| Paics_long | F | GCGGGAGACTAGCTGCAATA | AGE 2 | NC_031967 |
| R | TTGCAGCACATGGACAGTAG | |||
| Paics_short | F | TCCTCACGTGGAAATCAATG | AGE | OU234059 |
| R | CCACCAGCTGGAAAATCTGT | |||
| Banf2_del | F | CTACTCTGGGGAGGGAGCTG | AGE | OU023198 |
| R | TGACTGTTTGCTCCACTGCT | |||
| Banf2w | F | CTCTGGCCTCCTCGGTCA | HRM 3 | OU019478 |
| R | TGACTGTTTGCTCCACTGCT | |||
| M1_like | F | GGCTAATATTTTGTTGTGTGTAGGG | AGE | NC_031967 XM_025906134 |
| R | AGGAACAACTGCTCTTCAGGA | |||
| Banf2_EX3 | F | CCAATCTTCTTGTTCCTGACC | Sanger sequencing | OU019478 |
| R | GAGGTGCCTCTCAGGTAAAGG | |||
| Intron 1 | Exon | Intron | ||
|---|---|---|---|---|
| No. | Size (bp) | Size (bp) | ||
| GTTAAAGTTCTA | 1 | 119 | AAACAGgttcgtttgc | 1002 |
| ttcttctcagGATGTC | 2 | 127 | AGCAAGgtgcacaaaa | 668 |
| gtgttttcagGCCTCT | 3 | 231 | CTGTGAGGCCCCGCCCCCTTTACCT GAGAGGCACCTCTCAGCTGATTAT GTGCTGAGCATGAATAAA ACATAAATAAAGTTTGTGTGCCGGAG | |
| Marker | Position (Kbp) | Genotypes | Females: Males | p-Value |
|---|---|---|---|---|
| UNH168 | 64,515 | N/N 1 | 5:12 | 6 × 10−12 |
| N/W | 22:2 | |||
| N/Z | 8:29 | |||
| Z/W | 13:0 | |||
| Z/Z | 0:5 | |||
| Paics_long (Paics-1) | 73,159 | F/F 2 | 27:14 | 3.9 × 10−3 |
| F/S | 21:29 | |||
| S/S | 0:5 | |||
| Banf2_del | 73,177 | F/S 2 | 48:1 | 1.5 × 10−26 |
| S/S | 0:47 | |||
| Banf2_w | 73,178 | W/Z | 48:1 | 1.5 × 10−26 |
| Z/Z | 0:47 | |||
| Paics_short (Paics-2) | 73,217 | P 3 | 48:1 | 1.5 × 10−26 |
| A | 0:47 | |||
| M1_like | 73,394 | F/F 2 | 4:5 | 6.8 × 10−3 |
| F/S | 25:10 | |||
| S/S | 19:33 |
| ZZ | WZ | WW | ||
|---|---|---|---|---|
| Observed Distribution | ||||
| 0 | 48 | 0 | ||
| Inheritance Models 1 | Expected Distribution | 2p | ||
| Autosomal locus 3 | 12 | 24 | 12 | 3.8 × 10−11 |
| Lethal WW homozygote 4 | 16 | 32 | 0 | 6.4 × 10−5 |
| Female determining W 5 | 0 | 48 | 0 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curzon, A.Y.; Shirak, A.; Benet-Perlberg, A.; Naor, A.; Low-Tanne, S.I.; Sharkawi, H.; Ron, M.; Seroussi, E. Gene Variant of Barrier to Autointegration Factor 2 (Banf2w) Is Concordant with Female Determination in Cichlids. Int. J. Mol. Sci. 2021, 22, 7073. https://doi.org/10.3390/ijms22137073
Curzon AY, Shirak A, Benet-Perlberg A, Naor A, Low-Tanne SI, Sharkawi H, Ron M, Seroussi E. Gene Variant of Barrier to Autointegration Factor 2 (Banf2w) Is Concordant with Female Determination in Cichlids. International Journal of Molecular Sciences. 2021; 22(13):7073. https://doi.org/10.3390/ijms22137073
Chicago/Turabian StyleCurzon, Arie Yehuda, Andrey Shirak, Ayana Benet-Perlberg, Alon Naor, Shai Israel Low-Tanne, Haled Sharkawi, Micha Ron, and Eyal Seroussi. 2021. "Gene Variant of Barrier to Autointegration Factor 2 (Banf2w) Is Concordant with Female Determination in Cichlids" International Journal of Molecular Sciences 22, no. 13: 7073. https://doi.org/10.3390/ijms22137073
APA StyleCurzon, A. Y., Shirak, A., Benet-Perlberg, A., Naor, A., Low-Tanne, S. I., Sharkawi, H., Ron, M., & Seroussi, E. (2021). Gene Variant of Barrier to Autointegration Factor 2 (Banf2w) Is Concordant with Female Determination in Cichlids. International Journal of Molecular Sciences, 22(13), 7073. https://doi.org/10.3390/ijms22137073






