Knockdown of Quinolinate Phosphoribosyltransferase Results in Decreased Salicylic Acid-Mediated Pathogen Resistance in Arabidopsis thaliana
Abstract
:1. Introduction
2. Results
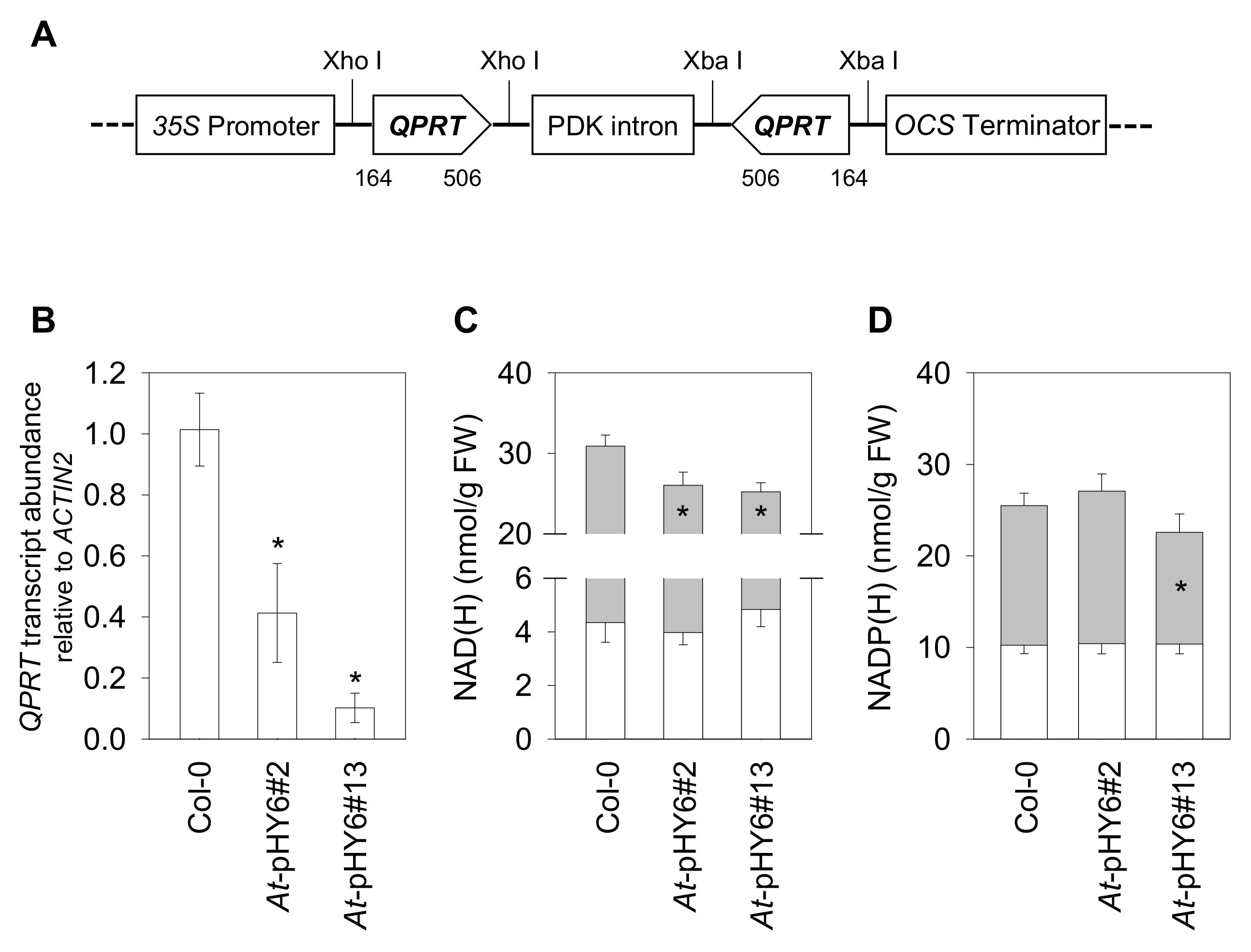
2.1. Generation of Transgenic Plants with Decreased QPRT Expression
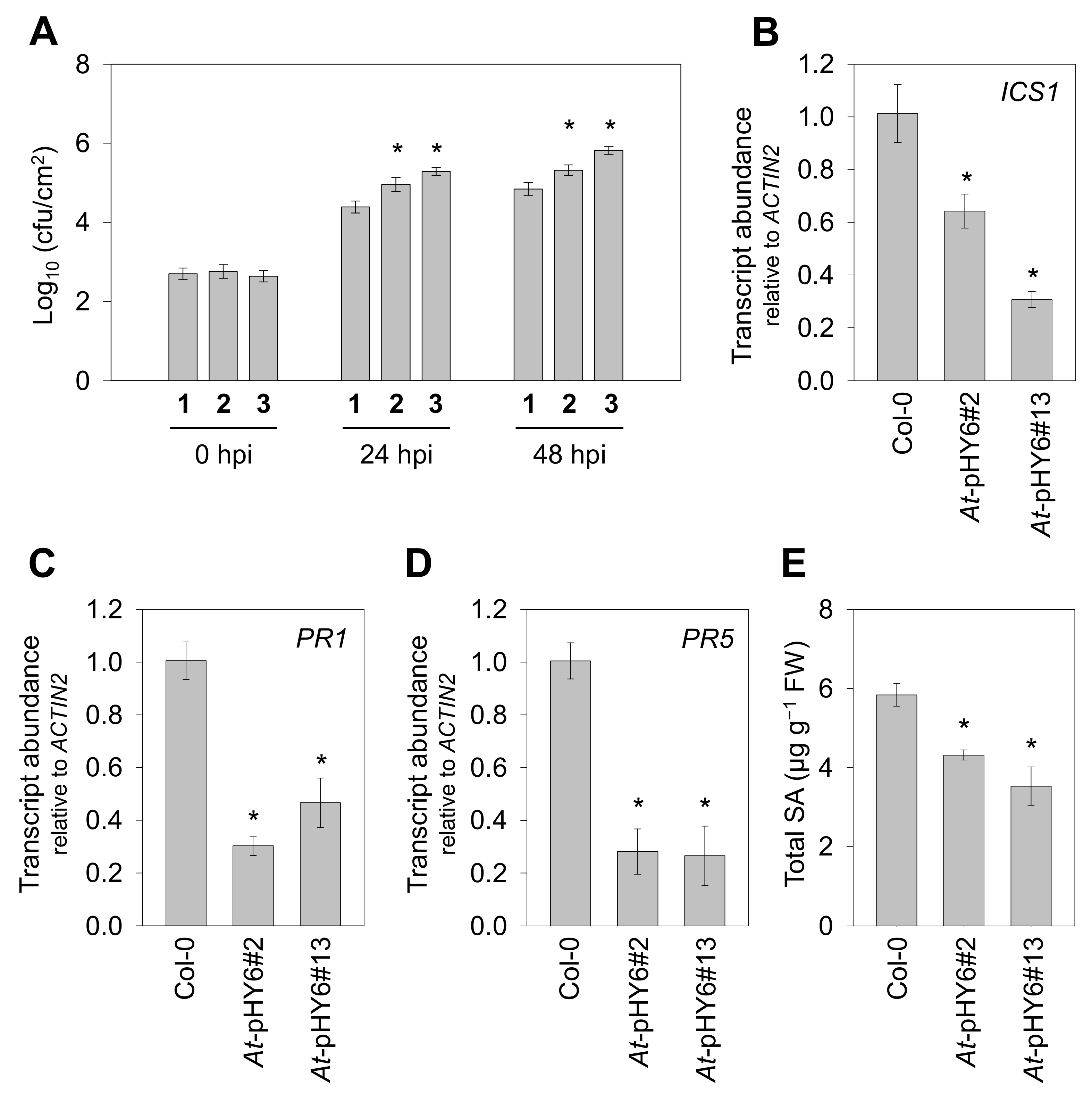
2.2. Decreased QPRT Enhances Sensitivity to Pst-avrRpt2
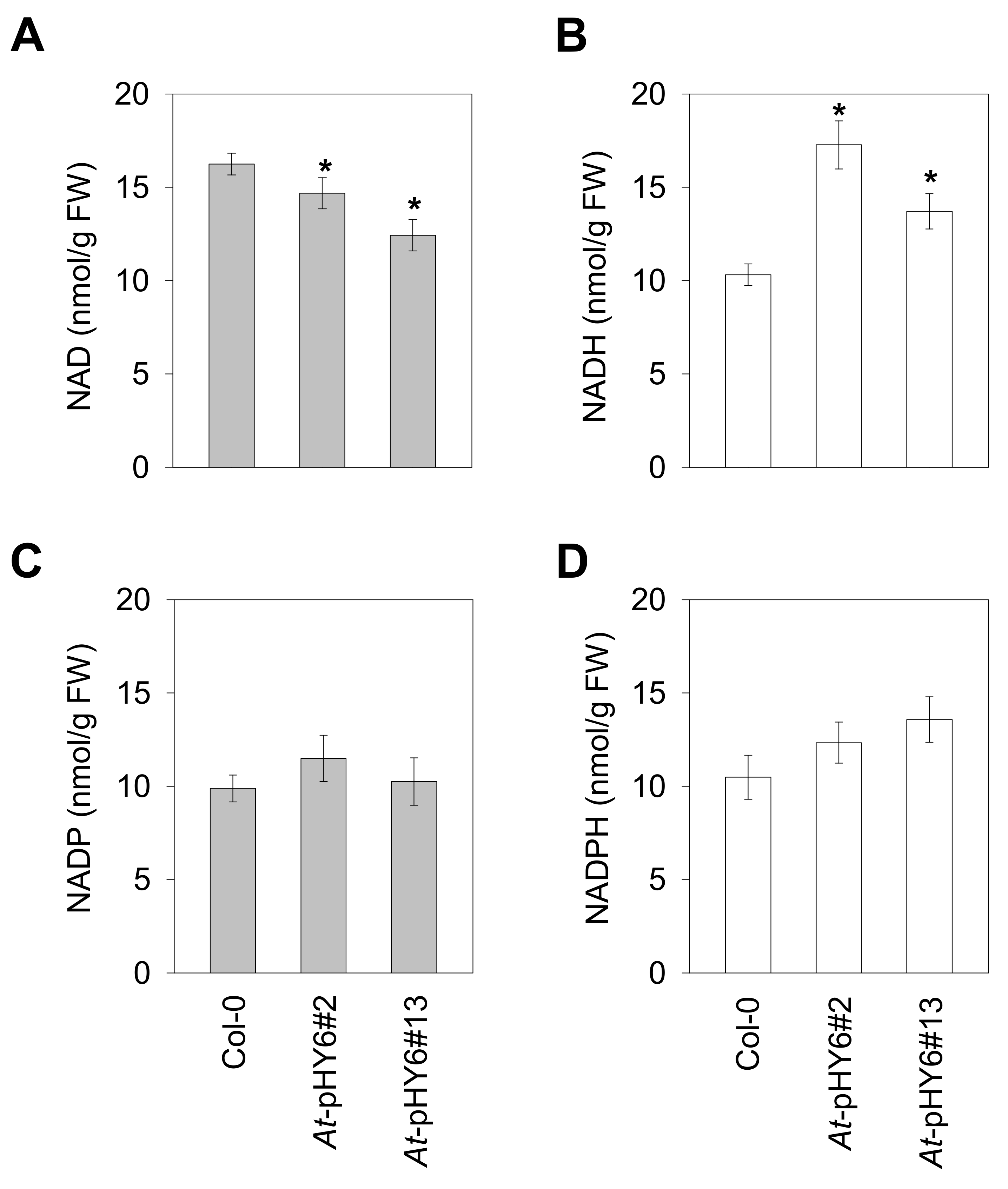
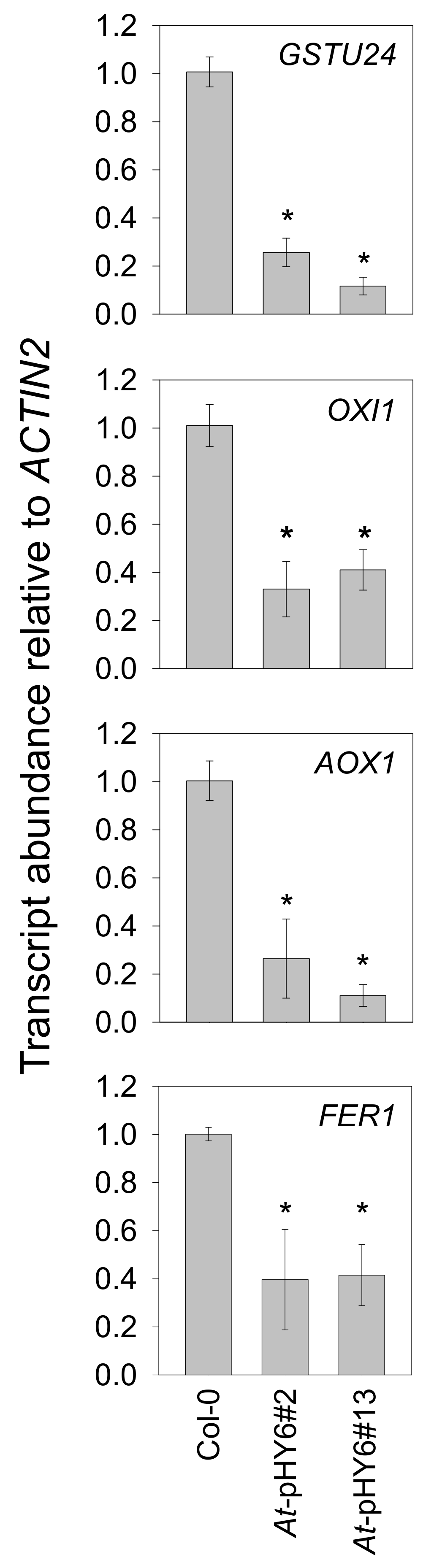
2.3. Decreased QPRT Affects Redox Homeostasis
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Condition
4.2. Generation of RNAi Plants
4.3. Pathogen Test
4.4. RNA Isolation and Quantitative Reverse Transcription-PCR
4.5. Metabolite Measurements
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noctor, G.; Hager, J.; Li, S. Biosynthesis of NAD and its manipulation in plants. Adv. Bot 2011, 58, 153–201. [Google Scholar] [CrossRef]
- Noctor, G.; Queval, G.; Gakière, B. NAD(P) synthesis and pyridine nucleotide cycling in plants and their potential importance in stress conditions. J. Exp. Bot. 2006, 57, 1603–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, J.; Duque, P.; Chua, N. ABA activates ADPR cyclase and cADPR induces a subset of ABA-responsive genes in Arabidopsis. Plant J. 2004, 38, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.G.; Bent, A.F. Poly(ADP-ribosyl)ation in plants. Trends Plant Sci. 2011, 16, 372–380. [Google Scholar] [CrossRef]
- Kupis, W.; Palyga, J.; Tomal, E.; Niewiadomska, E. The role of sirtuins in cellular homeostasis. J. Physiol Biochem 2016, 72, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Imai, S.; Armstrong, C.; Kaeberlein, M.; Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 2000, 403, 795–800. [Google Scholar] [CrossRef]
- Berger, F.; Ramirez-Hernandez, M.H.; Ziegler, M. The new life of a centenarian: Signalling functions of NAD(P). Trends Biochem. Sci. 2004, 29, 111–118. [Google Scholar] [CrossRef]
- Gakière, B.; Fernie, A.R.; Petriacq, P. More to NAD(+) than meets the eye: A regulator of metabolic pools and gene expression in Arabidopsis. Free Radic. Bio. Med. 2018, 122, 86–95. [Google Scholar] [CrossRef]
- Gakière, B.; Hao, J.; de Bont, L.; Pétriacq, P. NAD biosynthesis and signaling in plants. Crit. Rev. Plant Sci. 2018, 37, 1–49. [Google Scholar] [CrossRef]
- Pétriacq, P.; de Bont, L.; Hager, J.; Didierlaurent, L.; Mauve, C.; Guérard, F.; Noctor, G.; Pelletier, S.; Renou, J.; Tcherkez, G.; et al. Inducible NAD overproduction in Arabidopsis alters metabolic pools and gene expression correlated with increased salicylate content and resistance to Pst-AvrRpm1. Plant J. 2012, 70, 650–665. [Google Scholar] [CrossRef] [PubMed]
- Pétriacq, P.; Ton, J.; Patrit, O.; Tcherkez, G.; Gakiere, B. NAD acts as an integral regulator of multiple defense layers. Plant Physiol. 2016, 172, 1465–1479. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, K.; Yoshimura, K.; Harada, K.; Fukusaki, E.; Ogawa, T.; Tamoi, M.; Shigeoka, S. AtNUDX6, an ADP-ribose/NADH pyrophosphohydrolase in Arabidopsis, positively regulates NPR1-dependent salicylic acid signaling. Plant Physiol. 2010, 152, 2000–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, X.; Li, G.J.; Wang, S.B.; Zhu, H.; Zhu, T.; Wang, X.; Xia, Y. AtNUDT7, a negative regulator of basal immunity in Arabidopsis, modulates two distinct defense response pathways and is involved in maintaining redox homeostasis. Plant Physiol. 2007, 145, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Bartsch, M.; Gobbato, E.; Bednarek, P.; Debey, S.; Schultze, J.L.; Bautor, J.; Parker, J.E. Salicylic acid-independent enhanced disease susceptibility1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. Plant Cell 2006, 18, 1038–1051. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, J.P.; Dong, X. Functional characterization of a Nudix hydrolase AtNUDX8 upon pathogen attack indicates a positive role in plant immune responses. PLoS ONE 2014, 9, e114119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Mou, Z. Extracellular pyridine nucleotides induce PR gene expression and disease resistance in Arabidopsis. Plant J. 2009, 57, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, X.; Mou, Z. Comparison of nicotinamide adenine dinucleotide phosphate-induced immune responses against biotrophic and necrotrophic pathogens in Arabidopsis thaliana. Plant Signal. Behav. 2016, 11, e1169358. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, M.; Zhang, X.; Yao, J.; Zhang, Y.; Mou, Z. A lectin receptor kinase as a potential sensor for extracellular nicotinamide adenine dinucleotide in Arabidopsis thaliana. Elife 2017, 6, e25474. [Google Scholar] [CrossRef]
- Wang, C.; Huang, X.; Li, Q.; Zhang, Y.; Li, J.L.; Mou, Z. Extracellular pyridine nucleotides trigger plant systemic immunity through a lectin receptor kinase/BAK1 complex. Nat. Commun. 2019, 10, 4810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, A.; Uenohara, K.; Akita, M.; Hashimoto, T. Early steps in the biosynthesis of NAD in Arabidopsis start with aspartate and occur in the plastid. Plant Physiol. 2006, 141, 851–857. [Google Scholar] [CrossRef] [Green Version]
- Hashida, S.N.; Takahashi, H.; Kawai-Yamada, M.; Uchimiya, H. Arabidopsis thaliana nicotinate/nicotinamide mononucleotide adenyltransferase (AtNMNAT) is required for pollen tube growth. Plant J. 2007, 49, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Macho, A.; Boutrot, F.; Rathjen, J.; Zipfel, C. Aspartate oxidase plays an important role in Arabidopsis stomatal immunity. Plant Physiol. 2012, 159, 1845–1856. [Google Scholar] [CrossRef] [Green Version]
- Murthy, U.M.N.; Ollagnier-de-Choudens, S.; Sanakis, Y.; Abdel-Ghany, S.E.; Rousset, C.; Ye, H.; Fontecave, M.; Pilon-Smits, E.A.H.; Pilon, M. Characterization of Arabidopsis thaliana SufE2 and SufE3: Functions in chloroplast iron-sulfur cluster assembly and Nad synthesis. J. Biol. Chem. 2007, 282, 18254–18264. [Google Scholar] [CrossRef] [Green Version]
- Schippers, J.H.; Nunes-Nesi, A.; Apetrei, R.; Hille, J.; Fernie, A.R.; Dijkwel, P.P. The Arabidopsis onset of leaf death5 mutation of quinolinate synthase affects nicotinamide adenine dinucleotide biosynthesis and causes early ageing. Plant Cell 2008, 20, 2909–2925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, M.; Zhuang, Y.; Li, H.; Li, P.; Huo, H.; Shu, D.; Huang, W.; Wang, S. The cloning and characterization of hypersensitive to salt stress mutant, affected in quinolinate synthase, highlights the involvement of NAD in stress-induced accumulation of ABA and proline. Plant J. 2020, 102, 85–98. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, Z.; Shi, H.; Yao, J.; Liu, X.; Wang, F.; Zeng, L.; Xie, Z.; Zhu, J.K. Reciprocal regulation between nicotinamide adenine dinucleotide metabolism and abscisic acid and stress response pathways in Arabidopsis. PLoS Genet. 2020, 16, e1008892. [Google Scholar] [CrossRef]
- Wang, G.; Pichersky, E. Nicotinamidase participates in the salvage pathway of NAD biosynthesis in Arabidopsis. Plant J. 2007, 49, 1020–1029. [Google Scholar] [CrossRef]
- Hunt, L.; Holdsworth, M.J.; Gray, J.E. Nicotinamidase activity is important for germination. Plant J. 2007, 51, 341–351. [Google Scholar] [CrossRef]
- Sattar, S.; Martinez, M.T.; Ruiz, A.F.; Hanna-Rose, W.; Thompson, G.A. Nicotinamide inhibits aphid fecundity and impacts survival. Sci. Rep. 2019, 9, 19709. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.; Citterico, M.; Kimura, S.; Stevens, D.; Wrzaczek, M.; Gitta Coakerg, G. Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nat. Plants 2021, 7, 403–412. [Google Scholar] [CrossRef]
- Torres, M.A. ROS in biotic interactions. Physiol. Plantarum 2010, 138, 414–429. [Google Scholar] [CrossRef]
- Chaouch, S.; Queval, G.; Noctor, G. AtRbohF is a crucial modulator of defence-associated metabolism and a key actor in the interplay between intracellular oxidative stress and pathogenesis responses in Arabidopsis. Plant J. 2012, 69, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Rentel, M.; Lecourieux, D.; Ouaked, F.; Usher, S.; Petersen, L.; Okamoto, H.; Knight, H.; Peck, S.; Grierson, C.; Hirt, H.; et al. OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 2004, 427, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Vanlerberghe, G.C. Alternative oxidase: A mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int. J. Mol. Sci. 2013, 14, 6805–6847. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.; Briat, J.; Lobréaux, S. Structure and differential expression of the four members of the Arabidopsis thaliana ferritin gene family. Biochem. J. 2001, 359, 575–582. [Google Scholar] [CrossRef]
- Kasimova, M.R.; Grigiene, J.; Krab, K.; Hagedorn, P.H.; Flyvbjerg, H.; Andersen, P.E.; Moller, I.M. The free NADH concentration is kept constant in plant mitochondria under different metabolic conditions. Plant Cell 2006, 18, 688–698. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Mou, Z. Expression of the human NAD(P)-metabolizing ectoenzyme CD38 compromises systemic acquired resistance in Arabidopsis. Mol. Plant Microbe. Interact. 2012, 25, 1209–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.N.; Schwarzländer, M.; Ratcliffe, R.G.; Kruger, N.J. Shining a light on NAD- and NADP-based metabolism in plants. Trends Plant Sci. 2021. [Google Scholar] [CrossRef]
- Mhamdi, A.; Mauve, C.; Gouia, H.; Saindrenan, P.; Hodges, M.; Noctor, G. Cytosolic NADP-dependent isocitrate dehydrogenase contributes to redox homeostasis and the regulation of pathogen responses in Arabidopsis leaves. Plant Cell Environ. 2010, 33, 1112–1123. [Google Scholar] [CrossRef]
- De Vos, M.; Van Oosten, V.; Van Poecke, R.; Van Pelt, J.; Pozo, M.; Mueller, M.; Buchala, A.; Métraux, J.; Van Loon, L.; Dicke, M.; et al. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol. Plant Microbe. Interact. 2005, 18, 923–937. [Google Scholar] [CrossRef] [Green Version]
- Gullner, G.; Komives, T.; Kiraly, L.; Schroder, P. Glutathione S-transferase enzymes in plant-pathogen interactions. Front. Plant Sci. 2018, 9, 1836. [Google Scholar] [CrossRef] [Green Version]
- Petersen, L.N.; Ingle, R.A.; Knight, M.R.; Denby, K.J. OXI1 protein kinase is required for plant immunity against Pseudomonas syringae in Arabidopsis. J. Exp. Bot. 2009, 60, 3727–3735. [Google Scholar] [CrossRef] [Green Version]
- Simons, B.; Millenaar, F.; Mulder, L.; Van Loon, L.; Lambers, H. Enhanced expression and activation of the alternative oxidase during infection of Arabidopsopsis with Pseudomonas syringae pv tomato. Plant Physiol. 1999, 120, 529–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garmier, M.; Priault, P.; Vidal, G.; Driscoll, S.; Djebbar, R.; Boccara, M.; Mathieu, C.; Foyer, C.H.; De Paepe, R. Light and oxygen are not required for harpin-induced cell death. J. Biol. Chem. 2007, 282, 37556–37566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, G.; Ribas-Carbo, M.; Garmier, M.; Dubertret, G.; Rasmusson, A.G.; Mathieu, C.; Foyer, C.H.; De Paepe, R. Lack of respiratory chain complex I impairs alternative oxidase engagement and modulates redox signaling during elicitor-induced cell death in tobacco. Plant Cell 2007, 19, 640–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnaud, N.; Murgia, I.; Boucherez, J.; Briat, J.F.; Cellier, F.; Gaymard, F. An iron-induced nitric oxide burst precedes ubiquitin-dependent protein degradation for Arabidopsis AtFer1 ferritin gene expression. J. Biol. Chem. 2006, 281, 23579–23588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, F.; Rieder, B.; Ventrella, A.; Blanco, E.; Do, P.T.; Nunes-Nesi, A.; Trauth, A.U.; Fiermonte, G.; Tjaden, J.; Agrimi, G.; et al. Molecular identification and functional characterization of Arabidopsis thaliana mitochondrial and chloroplastic NAD+ carrier proteins. J. Biol. Chem. 2009, 284, 31249–31259. [Google Scholar] [CrossRef] [Green Version]
- De Souza Chaves, I.; Feitosa-Araujo, E.; Florian, A.; Medeiros, D.B.; da Fonseca-Pereira, P.; Charton, L.; Heyneke, E.; Apfata, J.A.C.; Pires, M.V.; Mettler-Altmann, T.; et al. The mitochondrial NAD(+) transporter (NDT1) plays important roles in cellular NAD(+) homeostasis in Arabidopsis thaliana. Plant J. 2019, 100, 487–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, L.; He, Y.; Zhao, Y.; Xu, Q.; Wu, J.; Ma, H.; Guo, H.; Bai, L.; Zuo, J.; Zhou, J.M.; et al. Regulation of mitochondrial NAD pool via NAD(+) transporter 2 is essential for matrix NADH homeostasis and ROS production in Arabidopsis. Sci. China Life Sci. 2019, 62, 991–1002. [Google Scholar] [CrossRef]
- Van Roermund, C.W.; Schroers, M.G.; Wiese, J.; Facchinelli, F.; Kurz, S.; Wilkinson, S.; Charton, L.; Wanders, R.J.; Waterham, H.R.; Weber, A.P.; et al. The peroxisomal NAD carrier from Arabidopsis imports NAD in exchange with AMP. Plant Physiol. 2016, 171, 2127–2139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernhardt, K.; Wilkinson, S.; Weber, A.P.; Linka, N. A peroxisomal carrier delivers NAD(+) and contributes to optimal fatty acid degradation during storage oil mobilization. Plant J. 2012, 69, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; You, L.L.; Li, S.C.; Ma, M.Q.; Wu, M.T.; Ma, L.X.; Bock, R.; Chang, L.; Zhang, J. In vivo assembly in Escherichia coli of transformation vectors for plastid genome engineering. Front. Plant Sci. 2017, 8, 1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Henriques, R.; Lin, S.S.; Niu, Q.W.; Chua, N.H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. Oxidative stress and antioxidative systems: Recipes for successful data collection and interpretation. Plant Cell Environ. 2016, 39, 1140–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queval, G.; Noctor, G. A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: Application to redox proWling during Arabidopsis rosette development. Anal. Biochem 2007, 363, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Langlois-Meurinne, M.; Gachon, C.M.; Saindrenan, P. Pathogen-responsive expression of glycosyltransferase genes UGT73B3 and UGT73B5 is necessary for resistance to Pseudomonas syringae pv tomato in Arabidopsis. Plant Physiol. 2005, 139, 1890–1901. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Ding, H.; Deng, Y.; Zhang, J. Knockdown of Quinolinate Phosphoribosyltransferase Results in Decreased Salicylic Acid-Mediated Pathogen Resistance in Arabidopsis thaliana. Int. J. Mol. Sci. 2021, 22, 8484. https://doi.org/10.3390/ijms22168484
Li S, Ding H, Deng Y, Zhang J. Knockdown of Quinolinate Phosphoribosyltransferase Results in Decreased Salicylic Acid-Mediated Pathogen Resistance in Arabidopsis thaliana. International Journal of Molecular Sciences. 2021; 22(16):8484. https://doi.org/10.3390/ijms22168484
Chicago/Turabian StyleLi, Shengchun, Haiyan Ding, Yi Deng, and Jiang Zhang. 2021. "Knockdown of Quinolinate Phosphoribosyltransferase Results in Decreased Salicylic Acid-Mediated Pathogen Resistance in Arabidopsis thaliana" International Journal of Molecular Sciences 22, no. 16: 8484. https://doi.org/10.3390/ijms22168484
APA StyleLi, S., Ding, H., Deng, Y., & Zhang, J. (2021). Knockdown of Quinolinate Phosphoribosyltransferase Results in Decreased Salicylic Acid-Mediated Pathogen Resistance in Arabidopsis thaliana. International Journal of Molecular Sciences, 22(16), 8484. https://doi.org/10.3390/ijms22168484





