Abstract
Uterine leiomyomas represent the most common benign gynecologic tumor. These hormone-dependent smooth-muscle formations occur with an estimated prevalence of ~70% among women of reproductive age and cause symptoms including pain, abnormal uterine bleeding, infertility, and recurrent abortion. Despite the prevalence and public health impact of uterine leiomyomas, available treatments remain limited. Among the potential causes of leiomyomas, early hormonal exposure during periods of development may result in developmental reprogramming via epigenetic changes that persist in adulthood, leading to disease onset or progression. Recent developments in unbiased high-throughput sequencing technology enable powerful approaches to detect driver mutations, yielding new insights into the genomic instability of leiomyomas. Current data also suggest that each leiomyoma originates from the clonal expansion of a single transformed somatic stem cell of the myometrium. In this review, we propose an integrated cellular and molecular view of the origins of leiomyomas, as well as paradigm-shifting studies that will lead to better understanding and the future development of non-surgical treatments for these highly frequent tumors.
1. Introduction
Uterine leiomyomas (uLM), also known as fibroids or uterine myomas, are the most important benign neoplastic threat to women’s health, with an estimated lifetime incidence of up to 70% [1,2,3]. Clinically, they are the most common cause of hysterectomy and a major source of infertility and abnormal uterine bleeding; thus, this condition significantly affects patients’ quality of life, as well as exerting significant economic impacts on healthcare systems worldwide [4,5,6].
Although current medical approaches to the diagnosis of uLM are based mainly on imaging and histological assumptions, molecular tools are gaining relevance as an alternative to conventional strategies in all clinical fields [7,8,9]. Likewise, considerable progress in understanding the origin and development of this highly prevalent condition has been made in the last decade. In this regard, hormonal processes, genetic predisposition, somatic alterations (point mutations and chromosomal abnormalities), epigenetic disruptions and the cellular origin of uLM will be discussed in this comprehensive review to share up-to-date knowledge and new concepts regarding the basic molecular biology and pathophysiology of this condition.
Recent advances related to the identification of biomarkers for early and differential diagnosis of uLM that support current histology-based classification will be discussed, as well as the detection of targetable pathways to develop novel and improved options for clinical management of these benign tumors.
2. Uterine Leiomyoma Etiopathology
2.1. Hormone Features
Despite the extensive clinical evidence regarding the hormonal influences on uLM development, the functional role of estrogens and progesterone remains unclear [10,11,12,13,14]. Early menarche age is associated with a higher risk of developing uLM due to the longer duration of exposure to estradiol and progesterone, which are reduced during menopause [15,16,17,18]. Conversely, increased parity seems to reduce the risk of fibroid development, while nulliparity is related to higher risk of uLM. This situation may seem ambiguous since there are high levels of circulating estrogens and progesterone of placental origin; however, this paradox may be due to differentiation of the myometrium during pregnancy [19]. This process makes the tissue less susceptible to the action of growth factors [20] and genetic mutations that trigger uLM tumors. Furthermore, uLM present before a pregnancy are reduced in volume after the gestation, suggesting apoptosis induced by postpartum remodeling and ischemia during delivery [21].
Estrogen, together with estrogen receptor-a (ERa), renders leiomyomas uLM responsive to progesterone by inducing progesterone receptor (PR) expression (Figure 1). PR binds to tens of thousands of DNA sites in leiomyoma smooth muscle cells to regulate multiple genes and promote proliferation, survival, and abnormal production of extracellular matrix. This occurs mainly during the reproductive years, while low hormone levels due to menopause or GnRH analogue-therapies are responsible for tumor degeneration [22,23].
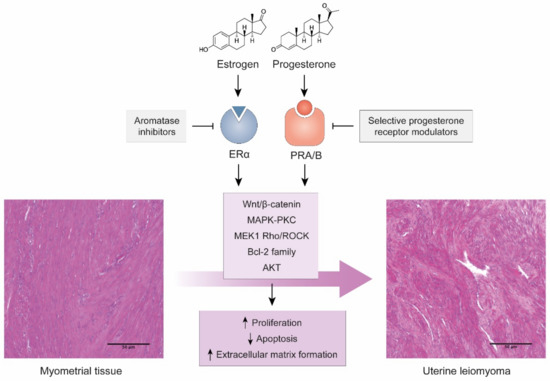
Figure 1.
Hormonal action on myometrial tissue as a cause of uLM. Images of H&E-stained tissues from our group’s archives. Scale bars represent 100 µm.
At the tissue level, high expression levels of progesterone receptor in the myometrium are also associated with increased risk of developing uLM [24], while progesterone is essential for the growth and maintenance of uLM [25]. Several mechanisms have been proposed for the relevance of progesterone in uLM, including that it induces the expression of proliferation genes such as the BCL-2 family [26,27] and induces specific pathways such as the AKT pathway [28] or the MEK1/2 Rho/Rock pathways in response to mechanical signaling [29]. In fact, progesterone receptors are one of the most common therapeutic targets in uLM management due to the use of selective progesterone receptor modulators, including ulipristal acetate (UPA) or mifepristone, that can inhibit proliferation, increase apoptosis, and reduce tumor growth and symptoms [30,31].
The interaction between progesterone and estrogens, their respective receptors, and other paracrine signals is also key in the etiopathogenesis of uLM. Progesterone may suppress estrogen receptors [32], but estrogen disruptions can alter the function of both estrogen and progesterone-associated genes and pathways [33]. Estradiol, the main form of estrogen, can trigger production of specific growth factors, mainly PDGF, through the MAPK-PKC pathway, leading to increased proliferation or immortalization [34,35,36]. Estradiol can also activate the Wnt/β-Catenin pathway through ERα to promote proliferation [37]. Similar to progesterone, inhibition of estrogen activity has been proposed, mainly by using aromatase inhibitors [38].
These findings show that progesterone and estrogen play a key role in uLM pathogenesis; however, further research on their involvement is needed to explore new therapeutic approaches that exploit the specific mechanisms of action of these ovarian steroid hormones. These novel fibroid therapies hold immense promise for shifting mainstream treatment of uLM from the surgical domain to the realm of orally administered medicines.
2.2. Genetics
Like most tumors, uLM have complex genetic backgrounds, including germline alterations and somatic mutations (point mutations and chromosomal abnormalities). These changes may be both the causes and consequences of the mechanisms behind the development of these uterine tumors (Figure 2).
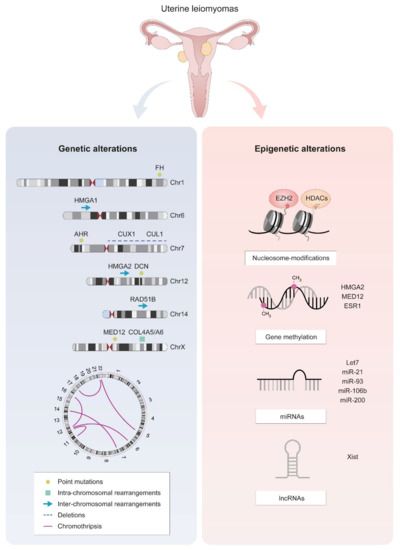
Figure 2.
The most frequent molecular alterations in uLM cells. Genetic alterations are represented on ideograms of the most frequently affected chromosomes, along with a scheme of HMGA1/2-RAD51B and a circos plot representing chromothripsis. Epigenetic alterations include disrupted nucleosome modification, gene methylation, and altered expression of non-coding RNAs.
2.2.1. Inherited Susceptibility
Although leiomyomas frequently appear spontaneously, there is a heritable component in their development with heritability values ranging from 8% to 70% [39,40,41]. Some of the first works that attributed some degree of heritability to leiomyoma development were twin studies that observed higher concordance levels between monozygotic twins than between dizygotic twins [39,42].
Family-based association studies have also found that the incidence of leiomyomas was higher among first-degree related women than among unrelated individuals [43,44,45]. Further, familial prevalence of fibroids was significantly associated with higher frequency of specific symptomatology [46]. One of the most relevant examples of familial association in uLM is hereditary leiomyomatosis and renal cell carcinoma (HLRCC), an autosomal dominant cancer syndrome characterized by skin leiomyomas, uterine fibroids, and kidney tumors [47]. HLRCC is caused by germline mutations in the fumarate hydratase (FH) gene, which encodes an enzyme involved in the tricarboxylic acid cycle with a tumor suppressor role [48,49]. In HLRCC, biallelic inactivation of FH causes deficient oxidative phosphorylation, which can produce the high levels of ATP required for rapid cell proliferation in tumoral cells through the Warburg effect [50].
Lastly, genome-wide association studies (GWAS) have sought genetic variants that may predispose women to uLM. Some studies found distinct susceptibility loci depending on the race/ethnicity of the patients, which explains the differences in incidence rates between populations such as the increased risk of uLM in women of African ancestry [51,52,53,54].
Although it is challenging to find specific genes involved in uLM development due to these differences in susceptibility loci between races/ethnicities, specific associations have been found between genes, and not only related to uLM risk but also uLM size [55,56]. So far, reported genes associated with uLM include ODFC3, BET1L, RIC8A, SIRT3, SLK, OBFC1, TNRC6B, FASN and HMGA2; however, the specific mechanisms underlying the associations remain unclear [57]. One GWAS also associated candidate genes for age of menarche to uLM, suggesting a possible explanation for increased uLM risk in women with early menarche age [58].
2.2.2. Point Mutations
Besides germline mutations that may predispose individuals to uLM, these tumors also accumulate several somatic mutations that usually affect a widely researched subset of genes and genomic regions. One of the most relevant genes affected by somatic mutations is MED12, which is mutated in up to 70% of uLM [59,60,61,62].
In normal physiological conditions, MED12 belongs to the mediator kinase module, which is a regulator of RNA polymerase II-mediated transcription. Binding of MED12 activates the catalytic core of this module, CKD8, by placing an “activation helix” and preventing the binding of kinase inhibitors, which allows precise transcription regulation [63]. However, MED12 mutations or altered expression are widely reported in many human diseases, including behavioral disorders and several cancer types such as breast, prostate, colon, ovarian and lung cancer [64].
In uLM specifically, MED12 mutations frequently occur in codon 44 of exon 2 [65] and less frequently in exon 1 [66], usually in the absence of any other recurrent mutations (Figure 2). This finding indicates that MED12 alterations alone may be sufficient to cause tumor development [65]. Although less frequent, a hotspot for small deletions in MED12 has also been detected in uLM, which may be caused by non-canonical DNA structures located in this hotspot [67].
One of the driver mechanisms behind MED12 mutations is disruption of the MED12–CDK8 interaction, which can lead to altered expression profiles of many genes due to altered transcription regulation [61]. Further, gain-of-function mutations in MED12 may cause genomic instability [68], possibly by inducing the Wnt4/β-Catenin pathway, which may induce cell proliferation, tumorigenicity and impaired autophagy through mTOR signaling [69,70].
In addition to its role as a driver gene, MED12 alterations are associated with clinical features of uLM including tumor size, conventional histology, and subserosal location as well as diagnosis of multiple vs. single uLM [71,72]. Moreover, the number of MED12-positive fibroids is inversely related to parity, whereas the number of mutation-negative tumors is positively associated with a history of pelvic inflammatory disease [73].
Other genes are reported to accumulate specific point mutations in uLM, including genes involved in the cell cycle and tumor suppression such as CAPRIN1, DCN, and AHR [74] as well as specific mitochondrial genes [75].
2.2.3. Chromosomal Abnormalities
uLM also display alterations that affect whole genes or larger chromosomal regions (Figure 2). Aberrations are found in several different chromosomes, primarily chromosomes 7, 12, 14, and 15 [67,76,77,78]. Such cytogenetic alterations are proposed as criteria to divide uLM into subgroups with different molecular and clinical features [79]. In general, tumor size is increased in cytogenetically abnormal fibroids [80,81], while deletions in specific regions such as chromosome 1p may be associated with histopathological variants of uLM and with prognosis [82].
- Chromothripsis
Chromosomal rearrangements in uLM sometimes present as numerous deletions with many breakpoints affecting limited genomic regions in only one of the homologous chromosomes, a process called chromothripsis [83]. The causes behind this type of complex chromosomal rearrangement remain unclear, although different mechanisms, such as chromosome pulverization, abortion of apoptosis, and telomere shortening, are proposed as drivers in different cancer types [84].
In uLM, chromothripsis appears to be more moderate than in other cancer types (Figure 2), which is why it is also referred to as a chromothripsis-like phenomenon [85]. This type of event is not associated with specific MED12 mutations or other alterations and is limited to up to 4 chromosomes, although the proportion of uLM presenting chromothripsis-like profiles varies from 20% to 42% [86,87,88]. As expected, the specific chromosomes affected by this phenomenon also vary between studies since chromothripsis occurs due to apparently random processes that cause breakages in different chromosomes. Although chromothripsis was initially associated with malignant processes, the detection of rearrangements in numerous genomic regions in uLM indicate that it may be one of the mechanisms in the etiopathology of this tumor [89].
- Target genes of chromosomal alterations
While chromosomal aberrations can happen anywhere in the genome in uLM, specific genes are frequently affected by these types of events, leading to disrupted biological processes that may drive tumorigenesis (Figure 2).
Rearrangements in the 12q14–15 region in fibroids are reported to increase expression of the HMGA2 gene [90,91]. Altered expression of this protein, which is involved in transcription regulation, has been associated with leiomyoma development through different mechanisms including angiogenesis [92], ERα-mediated cell proliferation [93], and homologous recombination DNA repair, since one of the preferential translocation partners of HMGA2 is the DNA repair gene RAD51B [94,95]. Another member of the family, HMGA1, is also affected in uLM by rearrangements targeting region 6p21. HMGA1 and HMGA2 may have a similar physiological role, which explains why disruptions of both genes result in similar consequences [96,97].
Conversely, while deletions in the 7q22 region have been associated with uLM [98,99], the affected target gene has not been conclusively identified. While these deletions alter expression of several genes such as LHFPL3, LAMB1, or HPB1 [100,101,102], one of the most accepted target genes is CUX1. 7q deletions can result in both increased and reduced expression of CUX1, suggesting a tumor suppressor or oncogenic role [103,104] that may cause uLM development in a process similar to RAD51B [105].
Lastly, deletions in the X chromosome affecting the collagen IV genes COL4A5 and COL4A6 associated with Alport syndrome have also been associated with development of smooth muscle tumors (diffuse leiomyomatosis), including uterine leiomyomas [106,107,108].
2.3. Epigenetics
In humans, epigenetic mechanisms, which affect gene expression, play a crucial role in leiomyoma formation [109,110]. The main epigenetic mechanisms include regulation by DNA methylation, histone modifications, microRNAs (miRNAs), and long-noncoding RNAs (lncRNAs) (Figure 2).
DNA methylation is essential for normal development and aberrations in this process affecting key embryonic development genes such as FOXO1, TERT, and WNT4 [111], which are associated with hypermethylation of tumor suppressor genes and/or hypomethylation of oncogenes, could contribute to tumorigenesis [112,113]. Specifically, uLM are associated with alterations of DNA methylation, increased mRNA expression of estrogen receptor 1 (ESR1; [114]), and DNA methyltransferases in tumor samples compared to the normal myometrium [115,116,117,118]. Recently, it has also been demonstrated that upregulation of HMGA2 expression does not always appear to be dependent on translocation but is associated with hypomethylation in the HMGA2 gene [119]. Additionally, DNA methylation and MED12 mutation together constitute a complex regulatory network that affects progesterone/PR-mediated RANKL gene expression [120,121], with an important role in activating stem cell proliferation and leiomyoma development [121]. Together, these findings suggest that DNA methylation might play a key role in the pathogenesis of uterine leiomyoma by altering the normal myometrial mRNA expression profile. Further studies involving epigenetic modulators such as 5′-Aza-Cytidine could lead to novel treatments that slow tumor growth by depleting the stem cells through specific demethylation mechanisms [93,122].
Other epigenetic alterations include methylation and acetylation of the histone tails, with enhancer of zeste homolog 2 (EZH2) and histone deacetylases (HDACs) being the main enzymes involved in these processes related to uterine leiomyomas [109]. While histone methylation can determine either activation or repression of gene transcription, histone acetylation only regulates gene activation [123,124]. Particularly, in uLM, EZH2 methylation silences gene function [125,126], though HDACs are involved in the regulation of tumor suppressor gene KLF11, which is diminished in these benign tumors [127].
Lastly, it is important to note the significant role of microRNAs from the let7, miR-21, miR-93, miR-106b, and miR-200 families in the epigenetic mechanisms contributing to the proliferation, inflammation, angiogenesis, and synthesis of extracellular matrix components. Thus, they contribute to uLM development through relevant signaling pathways such as Wnt/β-catenin and Wnt/MAPK [128,129,130,131,132]. Recently, expression of lncRNAs (RNA transcripts of more than 200 nucleotides) such as XIST was reported to be altered in uLM leiomyomas compared to myometrium [133].
Advancing our understanding of the role of epigenetic regulations in the risk of uLM and their contribution to tumorigenesis (probably associated with a hyperestrogenized phenotype during myometrial development) will help to identify novel therapeutic options for uterine leiomyoma [134].
2.4. Cell Origin
Considering that uLM are monoclonal tumors [135], it is possible that dysregulation of committed cells that acquire stem-like features could be responsible for this benign condition (Figure 3).
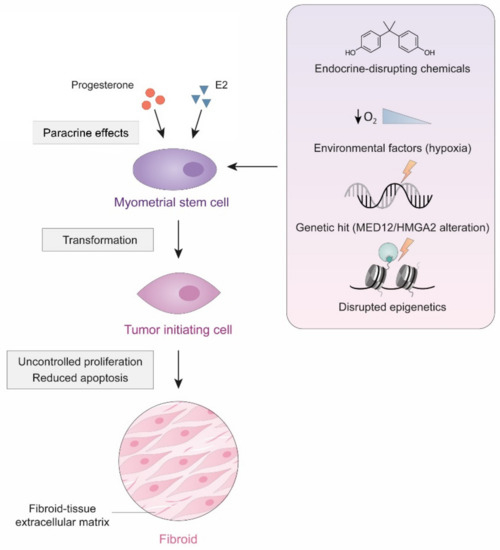
Figure 3.
Cellular origin of uLM. Specific environmental and genetic changes may cause the transformation of myometrial stem cells into tumor initiating cells, that, through uncontrolled proliferation and reduced apoptosis, may eventually give rise to a uLM tumor.
Indeed, cells with stem or progenitor characteristics can be isolated from myometrial and leiomyoma tissues [136,137]. Interestingly, these cells seem to have low levels of sex steroid hormone receptors but require these steroids for tumor growth, suggesting that an additional paracrine mechanism, exerted by mature surrounding cells that express these receptors, is necessary for the transformation of these cells [138]. In fact, early-life exposure to endocrine-disrupting chemicals such as xenoestrogens can alter the characteristics of progenitor cells, causing them to become tumor cells [139].
Moreover, progenitor cells from uLM, but not from the myometrium, carry MED12 mutations, indicating that at least one genetic hit may transform a myometrial stem cell into a tumor-initiating cell and give rise to these benign tumors [140,141]. Mutations in myometrial cells that affect the expression of the HMGA2 gene are also reported, leading to the formation of leiomyoma-like tissue in xenograft models [142]. Although alterations in MED12 and HMGA2 seem to be mutually exclusive [143], whether these genetic alterations induce the transformation of myometrial stem cells or maintain already existing leiomyoma stem-progenitor cells remains unclear.
Ultimately, other environmental and epigenetic conditions such as uterine hypoxia or aberrant methylation could play a critical role in leiomyoma development and growth from the cellular point of view [93,144,145].
In summary, while further characterization of the cellular origin of uLM is needed, these myometrial stem/progenitor cells offer novel and powerful potential targets for therapeutic or preventative strategies. Because transformation of myometrial stem cells into pre-fibroids seems to be a widespread if not ubiquitous process, future interventions will probably target the growth acceleration phase of fibroid development.
3. Molecular Tools in Clinical Management of Uterine Leiomyomas
Given the relevance of molecular processes in the development of uterine leiomyomas, there is a broad spectrum of molecular tools and resources that could be employed to develop better detection, classification, and treatment methods (Table 1). These promising approaches may help to improve the outcome and quality of life of symptomatologic patients and reduce costs and possible complications.

Table 1.
Molecular tools in clinical management of uterine leiomyomas. Possible applications include discovery of biomarkers for detection, classification based on gene expression profiling or pathway-targeting for treatment.
3.1. Detection–Putative Biomarkers
Clinically, tumor biomarkers could be useful for the identification of women at higher risk of developing fast growing leiomyomas as well as to distinguish this benign condition from other pathological conditions. Several biomarkers show increased serum levels in women with uLM compared to women without uLM, including leptin, VEGF, ghrelin, and CA125 [146,147,148]. However, only some of these show significant differences in healthy and fibroid patients and could be used in clinical practice. Potential serum biomarkers for uLM include proteins involved in oxidative stress, such as vitamin D3 or AOPP [149,150,151,152], inflammatory processes, such as YKL-40 [153] and TRADD [154], and angiogenesis, such as TGF-β and LRG1 [155].
Further, biomarkers could be used to distinguish uLM from other uterine malignancies to improve prognosis and treatment, for instance, in deciding whether the uterus needs to be completely resected. Differential diagnosis of uLM and uterine sarcomas could be based on GDF-15 [156], and CA125 [157] serum levels, although their applicability is limited since increased expression of these biomarkers is more typical of high-grade tumors. Similarly, HLA-G levels differ between patients with uLM and patients with ovarian endometriosis, although these levels are greatly dependent on the phase of the menstrual cycle, which limits their clinical potential [158]. Although many different biomarkers of uLM have been reported, still none of them are currently used in clinical practice. This highlights the need for further research and validation of these tools and their clinical applicability.
3.2. Classification–Gene Expression Profiling
Although classification of uLM is based mainly on their localization and histopathological features, efforts are focused on developing a more precise new classification system that may aid diagnostic and therapeutic decision making.
Molecular classification of uLM has, so far, relied on dividing them into different categories based on their mutational status for the main “driver genes” (MED12, FH, HMGA2), which are usually mutually exclusive [159,160]. However, some uLM do not fall within any of these categories, or could belong to more than one category, for example, by presenting both MED12 and HMGA2 alterations [161,162,163]. This demonstrates that further delineation of the features of these subtypes and an improved classification system are necessary.
Gene expression profiling could be used not only to unravel the specific mechanisms behind the pathogenesis of the main molecular subtypes of uLM, but also to define new subgroups based on their transcriptomic signatures. Transcriptomic analysis clustered uLM samples based on their driver mutations [95], although functional analysis of dysregulated genes showed that the involved cellular processes were in fact very similar in molecular subtypes, e.g., mitosis, cell cycle, and angiogenesis [119].
Lastly, transcriptomic analysis of tissue biopsies could also be applied to the differential diagnosis of myometrial tumors to accurately distinguish leiomyomas from leiomyosarcomas [164,165].
3.3. Treatment–Targetable Dysregulated Pathways
Recent omic approaches have generated large amounts of information about the ethiopathology of uLM. This opens a new field that may allow clinicians to choose better treatments based on the specific molecular pathways that are altered in each uLM. Although many different pathways are altered in uLM, in this review we will focus on three we have found to be most relevant from a therapeutic point of view.
3.3.1. Wnt/β-Catenin Pathway
Disruptions in Wnt/β-catenin signaling, a conserved pathway that regulates proliferation, migration, and tissue homeostasis among other processes, have been reported in different cancer types, including breast and gynecological cancers such as ovarian cancer [166]. In uLM, activation of this pathway stimulates proliferation and stem cell function [167], while inhibiting it can lead to reduced cell growth [132]. Besides, environmental conditions such as hypoxia or serum starvation may inhibit this pathway in cultured human leiomyoma cells, and induce their transdifferentiation, leading to the development of lipoleiomyomas, a less prevalent variant of uLM with a high content in adipocytes [168].
The importance of this pathway in the development and progression of uLM makes it of special therapeutic interest. In fact, vitamin D3 has already been proposed as a potential Wnt4/AKT/β-catenin inhibitor that could be used to reduce leiomyoma growth and proliferation [169,170].
3.3.2. PI3K/AKT Pathway
The PI3K/AKT pathway is also frequently altered in different cancer types, where disruptions in the function of phosphatidylinositol-3 kinases, AKT, or mTOR can result in increased or uncontrolled cell survival and growth [171].
In uLM, over-activation of this pathway or its components are associated with growth and development through different genes such as GSK3, PTEN, AKT, CCND2, and P27 [172,173,174,175]. This pathway is inhibited by widely used therapeutic options for uLM such as GnRH analogues [176] and ulipristal acetate [169].
3.3.3. TGF-β Pathway
Transforming growth factor β is one of the most relevant cytokines involved in the proliferation and differentiation of myometrial tissue [177]. Overexpression of this cytokine and alteration of the TGF-β pathway were reported in different studies [178,179,180,181]. Accordingly, therapies that decrease TGF-β signaling, including GnRH analogues [182] and ulipristal acetate [183,184], succeed in reducing uLM growth and symptomatology.
4. Clinical Translation and Future Research
Recent molecular studies based on genomic and transcriptomic approaches have revealed driver mutations, aberrant regulatory programs, and disease subtypes for major human myometrial tumors [119,165,185]. However, these studies were focused on the whole tumor mass, which implies certain limitations due to tumor heterogeneity, which can hide critical differences between cells within populations [186,187]. In this sense, intratumoral heterogeneity represents one of the greatest challenges in tumor biology and oncology. In fact, interactions between cells, as well as local and autonomous mechanisms within the tumor microenvironment could be essential for better understanding the origin and evolution of uterine fibroids. In addition, the local and autonomous mechanisms of cells could play a key role in tumor development and growth.
To further examine the function and regulation of these cell populations, unbiased genome-wide studies of their gene expression are needed. In the last decade, the exponential growth of single-cell transcriptomic technologies enables processing hundreds of thousands of cells at an affordable cost and time [188,189,190,191]. One of these technologies, “Smart-seq2”, can generate quantitative and reproducible data since it has improved reverse transcription and pre-amplification to increase the throughput and the length of cDNA libraries generated from single cells [188]. Recently, standardization of droplet genomic methods democratized this technology, making it possible to reduce the batch effects when integrating data obtained from different geographic locations. These developments led to the first atlases of human tissues, which have provided significant information about multiple biological systems [192,193,194]. Full characterization of the individual cell types present in human myometrium and leiomyoma would help to clarify the fundamental physiological and pathological processes involved in uLM and further improve disease-related diagnosis and treatment.
Complementary approaches based on targeted nanoparticles could also have promising applications for the detection and therapy of this benign condition [128,195]. Modern studies have assessed nanomaterial-mediated delivery of anti-tumor cytokines [196] or adenovirus [197]. This delivery method results in increased apoptosis and suppressed tumor proliferation compared with other medical treatments. Thus, it could be interesting to consider the use of dedicated systems to deliver specific drugs for targeted non-invasive treatment of this pathology instead of surgery.
Finally, CRISPR/Cas9-mediated silencing of the AP-1 subunits in human uterine smooth muscle cells revealed that the loss of AP-1 can trigger large-scale changes in gene expression and acetylation, which could explain the wide transcriptional changes seen in leiomyoma pathogenesis [198,199]. Consequently, the CRISPR/Cas9 system could be used to correct specific mutations. In fact, in the upcoming decade, genome editing tools could help to accelerate therapeutic directions that specifically target the molecular mechanisms of uLM.
In summary, these and other novel techniques will be the next steps toward a better understanding of uLM from a research and clinical perspective (Figure 4).
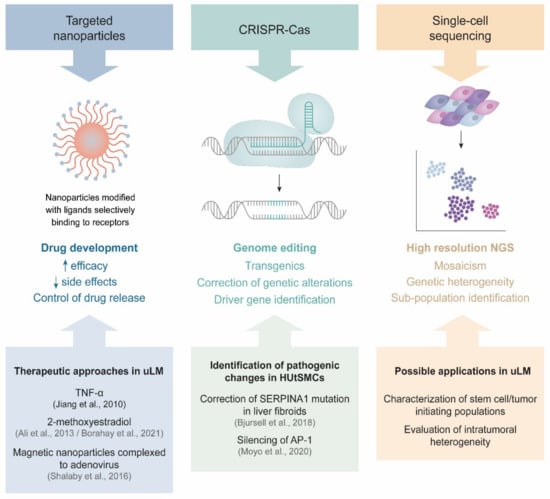
Figure 4.
New techniques in uLM research. Each technique and its features are displayed, with relevant publications or future directions in uLM research.
5. Conclusions
Despite being benign tumors of myometrial origin, uterine leiomyomas have a considerable impact on women’s quality of life and on healthcare systems. Research in this field is growing rapidly, and the increasing novel molecular and cellular insights regarding this condition will allow scientists and clinicians to better understand the etiopathology of uLM.
These tumors are greatly influenced by levels of sex hormones that may trigger specific changes in an already altered genetic, transcriptomic, and proteomic environment. Uterine leiomyomas display great genetic complexity, ranging from germinal mutations that predispose women to developing uLM, to somatic alterations such as point mutations in the MED12 gene or copy number variants and chromosomal aberrations that affect different regions, to epigenetic alterations, which complicates the utilization and interpretation of multi-omic information related to these tumors. Additionally, although it is widely accepted that uLM have monoclonal origins, the processes behind the transformation of myometrial stem cells into tumor-initiating cells remain elusive, further complicating our comprehension of uLM etiopathology.
In brief, understanding all the mechanisms behind uLM through the integration of differential cellular/molecular characteristics could facilitate the discovery of tumor-specific biomarkers, as well as the development of a more suitable uLM subclassification system, and therapeutic approaches specifically targeting the affected pathways. Application of current novel techniques and procedures such as single-cell sequencing or CRISPR-Cas genome editing to uLM research will further our understanding of the tumorigenic processes and improve the clinical management of this benign condition.
Author Contributions
Conceptualization, A.M.; writing—original draft preparation, A.M. and A.M.-L.; writing—review and editing, A.M. and C.S.; supervision, A.M. and C.S.; project administration, A.M. and C.S.; funding acquisition, A.M. and A.M.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Miguel Servet Spanish Program Grant CP19/00162 and Health Research Funds PI20/00942 from the Carlos III Institute, Spain (A.M.), as well as a FDEGENT/2019/010 PhD Training Grant for Valencian Entities (A.M.-L.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank Marta Gálvez Viedma for their support.
Conflicts of Interest
C.S. is the Founder and Head of the Scientific Advisory Board of Igenomix. The remaining authors declare no conflict of interest.
References
- Baird, D.; Dunson, D.; Hill, M.; Cousins, D.; Schectman, J. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am. J. Obstet. Gynecol. 2003, 188, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A.; Cookson, C.L.; Gandolfo, R.A.; Schulze-Rath, R. Epidemiology of uterine fibroids: A systematic review. BJOG 2017, 124, 1501–1512. [Google Scholar] [CrossRef]
- Marsh, E.E.; Ekpo, G.E.; Cardozo, E.R.; Brocks, M.; Dune, T.; Cohen, L.S. Racial differences in fibroid prevalence and ultrasound findings in asymptomatic young women (18–30 years old): A pilot study. Fertil. Steril. 2013, 99, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Marsh, E.E.; Al-Hendy, A.; Kappus, D.; Galitsky, A.; Stewart, E.A.; Kerolous, M. Burden, Prevalence, and Treatment of Uterine Fibroids: A Survey of U.S. Women. J. Women’s Health 2018, 27, 1359–1367. [Google Scholar] [CrossRef]
- Spies, J.B.; Myers, E.R.; Worthington-Kirsch, R.; Mulgund, J.; Goodwin, S.; Mauro, M. The FIBROID Registry: Symptom and quality-of-life status 1 year after therapy. Obstet. Gynecol. 2005, 106, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Downes, E.; Sikirica, V.; Gilabert-Estelles, J.; Bolge, S.C.; Dodd, S.L.; Maroulis, C.; Subramanian, D. The burden of uterine fibroids in five European countries. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 152, 96–102. [Google Scholar] [CrossRef]
- Friedman, A.A.; Letai, A.; Fisher, D.E.; Flaherty, K.T. Precision medicine for cancer with next-generation functional diagnostics. Nat. Rev. Cancer 2015, 15, 747–756. [Google Scholar] [CrossRef]
- Prasad, V.; Fojo, T.; Brada, M. Precision oncology: Origins, optimism, and potential. Lancet Oncol. 2016, 17, e81–e86. [Google Scholar] [CrossRef]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Lethaby, A.; Vollenhoven, B. Fibroids (uterine myomatosis, leiomyomas). BMJ Clin. Evid. 2015, 2015, 814. [Google Scholar]
- Marsh, E.E.; Bulun, S.E. Steroid hormones and leiomyomas. Obstet. Gynecol. Clin. N. Am. 2006, 33, 59–67. [Google Scholar] [CrossRef]
- Deng, L.; Wu, T.; Chen, X.Y.; Xie, L.; Yang, J. Selective estrogen receptor modulators (SERMs) for uterine leiomyomas. Cochrane Database Syst. Rev. 2012, 10, Cd005287. [Google Scholar] [CrossRef] [PubMed]
- Tristan, M.; Orozco, L.J.; Steed, A.; Ramírez-Morera, A.; Stone, P. Mifepristone for uterine fibroids. Cochrane Database Syst. Rev. 2012, 8, Cd007687. [Google Scholar] [CrossRef] [PubMed]
- Sangkomkamhang, U.S.; Lumbiganon, P.; Pattanittum, P. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy). Cochrane Database Syst. Rev. 2020, 11, Cd008994. [Google Scholar] [CrossRef]
- D’Aloisio, A.A.; Baird, D.D.; DeRoo, L.A.; Sandler, D.P. Association of intrauterine and early-life exposures with diagnosis of uterine leiomyomata by 35 years of age in the Sister Study. Environ. Health Perspect. 2010, 118, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Palmer, J.R.; Harlow, B.L.; Spiegelman, D.; Stewart, E.A.; Adams-Campbell, L.L.; Rosenberg, L. Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African-American women: A prospective study. Am. J. Epidemiol. 2004, 159, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Velez Edwards, D.R.; Baird, D.D.; Hartmann, K.E. Association of age at menarche with increasing number of fibroids in a cohort of women who underwent standardized ultrasound assessment. Am. J. Epidemiol. 2013, 178, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Laughlin-Tommaso, S.K. Epidemiology of Uterine Fibroids: From Menarche to Menopause. Clin. Obstet. Gynecol. 2016, 59, 2–24. [Google Scholar] [CrossRef]
- Ciavattini, A.; Di Giuseppe, J.; Stortoni, P.; Montik, N.; Giannubilo, S.R.; Litta, P.; Islam, M.S.; Tranquilli, A.L.; Reis, F.M.; Ciarmela, P. Uterine fibroids: Pathogenesis and interactions with endometrium and endomyometrial junction. Obstet. Gynecol. Int. 2013, 2013, 173184. [Google Scholar] [CrossRef]
- Reis, F.M.; Bloise, E.; Ortiga-Carvalho, T.M. Hormones and pathogenesis of uterine fibroids. Best Pract. Res. Clin. Obstet. Gynecol. 2016, 34, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, S.K.; Herring, A.H.; Savitz, D.A.; Olshan, A.F.; Fielding, J.R.; Hartmann, K.E.; Baird, D.D. Pregnancy-related fibroid reduction. Fertil. Steril. 2010, 94, 2421–2423. [Google Scholar] [CrossRef] [PubMed]
- Maruo, T.; Ohara, N.; Wang, J.; Matsuo, H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum. Reprod. Update 2004, 10, 207–220. [Google Scholar] [CrossRef]
- Sabry, M.; Al-Hendy, A. Innovative oral treatments of uterine leiomyoma. Obstet. Gynecol. Int. 2012, 2012, 943635. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.; Laknaur, A.; Al-Hendy, A.; Yang, Q. Myometrial progesterone hyper-responsiveness associated with increased risk of human uterine fibroids. BMC Womens Health 2019, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Ishi, K.; Serna, V.A.; Kakazu, R.; Bulun, S.E.; Kurita, T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 2010, 151, 2433–2442. [Google Scholar] [CrossRef]
- Yin, P.; Lin, Z.; Cheng, Y.H.; Marsh, E.E.; Utsunomiya, H.; Ishikawa, H.; Xue, Q.; Reierstad, S.; Innes, J.; Thung, S.; et al. Progesterone receptor regulates Bcl-2 gene expression through direct binding to its promoter region in uterine leiomyoma cells. J. Clin. Endocrinol. Metab. 2007, 92, 4459–4466. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, Q.; Wang, Z.; Liu, P.; Cui, S. Progesterone receptor antagonist provides palliative effects for uterine leiomyoma through a Bcl-2/Beclin1-dependent mechanism. Biosci. Rep. 2019, 39, BSR20190094. [Google Scholar] [CrossRef]
- Hoekstra, A.V.; Sefton, E.C.; Berry, E.; Lu, Z.; Hardt, J.; Marsh, E.; Yin, P.; Clardy, J.; Chakravarti, D.; Bulun, S.; et al. Progestins activate the AKT pathway in leiomyoma cells and promote survival. J. Clin. Endocrinol. Metab. 2009, 94, 1768–1774. [Google Scholar] [CrossRef]
- Cordeiro Mitchell, C.N.; Islam, M.S.; Afrin, S.; Brennan, J.; Psoter, K.J.; Segars, J.H. Mechanical stiffness augments ligand-dependent progesterone receptor B activation via MEK 1/2 and Rho/ROCK-dependent signaling pathways in uterine fibroid cells. Fertil. Steril. 2021, 116, 255–265. [Google Scholar] [CrossRef]
- Engman, M.; Granberg, S.; Williams, A.R.; Meng, C.X.; Lalitkumar, P.G.; Gemzell-Danielsson, K. Mifepristone for treatment of uterine leiomyoma. A prospective randomized placebo controlled trial. Hum. Reprod. 2009, 24, 1870–1879. [Google Scholar] [CrossRef]
- Murji, A.; Whitaker, L.; Chow, T.L.; Sobel, M.L. Selective progesterone receptor modulators (SPRMs) for uterine fibroids. Cochrane Database Syst. Rev. 2017, 4, Cd010770. [Google Scholar] [CrossRef] [PubMed]
- Hodges, L.C.; Houston, K.D.; Hunter, D.S.; Fuchs-Young, R.; Zhang, Z.; Wineker, R.C.; Walker, C.L. Transdominant suppression of estrogen receptor signaling by progesterone receptor ligands in uterine leiomyoma cells. Mol. Cell. Endocrinol. 2002, 196, 11–20. [Google Scholar] [CrossRef]
- Hassan, M.H.; Salama, S.A.; Arafa, H.M.; Hamada, F.M.; Al-Hendy, A. Adenovirus-mediated delivery of a dominant-negative estrogen receptor gene in uterine leiomyoma cells abrogates estrogen- and progesterone-regulated gene expression. J. Clin. Endocrinol. Metab. 2007, 92, 3949–3957. [Google Scholar] [CrossRef]
- Barbarisi, A.; Petillo, O.; Di Lieto, A.; Melone, M.A.; Margarucci, S.; Cannas, M.; Peluso, G. 17-beta estradiol elicits an autocrine leiomyoma cell proliferation: Evidence for a stimulation of protein kinase-dependent pathway. J. Cell. Physiol. 2001, 186, 414–424. [Google Scholar] [CrossRef]
- Hermon, T.L.; Moore, A.B.; Yu, L.; Kissling, G.E.; Castora, F.J.; Dixon, D. Estrogen receptor alpha (ERalpha) phospho-serine-118 is highly expressed in human uterine leiomyomas compared to matched myometrium. Virchows Arch. 2008, 453, 557–569. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, N.; Guan, Q.; Zheng, L.; Qu, X.; Dai, H.; Cheng, Z. Estrogen-mediated activation of fibroblasts and its effects on the fibroid cell proliferation. Transl. Res. 2014, 163, 232–241. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, G.; Li, B.; Qu, J.; Zhang, Y. LncRNA APTR Promotes Uterine Leiomyoma Cell Proliferation by Targeting ERα to Activate the Wnt/β-Catenin Pathway. Front. Oncol. 2021, 11, 536346. [Google Scholar] [CrossRef]
- Song, H.; Lu, D.; Navaratnam, K.; Shi, G. Aromatase inhibitors for uterine fibroids. Cochrane Database Syst. Rev. 2013, 10, Cd009505. [Google Scholar] [CrossRef]
- Luoto, R.; Kaprio, J.; Rutanen, E.M.; Taipale, P.; Perola, M.; Koskenvuo, M. Heritability and risk factors of uterine fibroids--the Finnish Twin Cohort study. Maturitas 2000, 37, 15–26. [Google Scholar] [CrossRef]
- Ge, T.; Chen, C.Y.; Neale, B.M.; Sabuncu, M.R.; Smoller, J.W. Phenome-wide heritability analysis of the UK Biobank. PLoS Genet. 2017, 13, e1006711. [Google Scholar] [CrossRef]
- Bray, M.J.; Davis, L.K.; Torstenson, E.S.; Jones, S.H.; Edwards, T.L.; Edwards, D.R.V. Estimating Uterine Fibroid SNP-Based Heritability in European American Women with Imaging-Confirmed Fibroids. Hum. Hered. 2019, 84, 73–81. [Google Scholar] [CrossRef]
- Snieder, H.; MacGregor, A.J.; Spector, T.D. Genes control the cessation of a woman’s reproductive life: A twin study of hysterectomy and age at menopause. J. Clin. Endocrinol. Metab. 1998, 83, 1875–1880. [Google Scholar] [CrossRef]
- Vikhlyaeva, E.M.; Khodzhaeva, Z.S.; Fantschenko, N.D. Familial predisposition to uterine leiomyomas. Int. J. Gynecol. Obstet. 1995, 51, 127–131. [Google Scholar] [CrossRef]
- Sato, F.; Mori, M.; Nishi, M.; Kudo, R.; Miyake, H. Familial aggregation of uterine myomas in Japanese women. J. Epidemiol. 2002, 12, 249–253. [Google Scholar] [CrossRef]
- Van Voorhis, B.J.; Romitti, P.A.; Jones, M.P. Family history as a risk factor for development of uterine leiomyomas. Results of a pilot study. J. Reprod. Med. 2002, 47, 663–669. [Google Scholar] [PubMed]
- Okolo, S.O.; Gentry, C.C.; Perrett, C.W.; Maclean, A.B. Familial prevalence of uterine fibroids is associated with distinct clinical and molecular features. Hum. Reprod. 2005, 20, 2321–2324. [Google Scholar] [CrossRef] [PubMed]
- Ooi, A. Advances in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) research. Semin. Cancer Biol. 2020, 61, 158–166. [Google Scholar] [CrossRef]
- Launonen, V.; Vierimaa, O.; Kiuru, M.; Isola, J.; Roth, S.; Pukkala, E.; Sistonen, P.; Herva, R.; Aaltonen, L.A. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 3387–3392. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, I.P.; Alam, N.A.; Rowan, A.J.; Barclay, E.; Jaeger, E.E.; Kelsell, D.; Leigh, I.; Gorman, P.; Lamlum, H.; Rahman, S.; et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet. 2002, 30, 406–410. [Google Scholar] [CrossRef]
- Linehan, W.M.; Rouault, T.A. Molecular pathways: Fumarate hydratase-deficient kidney cancer--targeting the Warburg effect in cancer. Clin. Cancer Res. 2013, 19, 3345–3352. [Google Scholar] [CrossRef]
- Cha, P.C.; Takahashi, A.; Hosono, N.; Low, S.K.; Kamatani, N.; Kubo, M.; Nakamura, Y. A genome-wide association study identifies three loci associated with susceptibility to uterine fibroids. Nat. Genet. 2011, 43, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Ruiz-Narvaez, E.A.; Palmer, J.R.; Cozier, Y.C.; Tandon, A.; Patterson, N.; Radin, R.G.; Rosenberg, L.; Reich, D. African ancestry and genetic risk for uterine leiomyomata. Am. J. Epidemiol. 2012, 176, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Bondagji, N.S.; Morad, F.A.; Al-Nefaei, A.A.; Khan, I.A.; Elango, R.; Abdullah, L.S.; Al-Mansouri, N.M.; Sabir, J.; Banaganapalli, B.; Edris, S.; et al. Replication of GWAS loci revealed the moderate effect of TNRC6B locus on susceptibility of Saudi women to develop uterine leiomyomas. J. Obstet. Gynecol. Res. 2017, 43, 330–338. [Google Scholar] [CrossRef]
- Eggert, S.L.; Huyck, K.L.; Somasundaram, P.; Kavalla, R.; Stewart, E.A.; Lu, A.T.; Painter, J.N.; Montgomery, G.W.; Medland, S.E.; Nyholt, D.R.; et al. Genome-wide linkage and association analyses implicate FASN in predisposition to Uterine Leiomyomata. Am. J. Hum. Genet. 2012, 91, 621–628. [Google Scholar] [CrossRef]
- Aissani, B.; Zhang, K.; Wiener, H. Evaluation of GWAS candidate susceptibility loci for uterine leiomyoma in the multi-ethnic NIEHS uterine fibroid study. Front. Genet. 2015, 6, 241. [Google Scholar] [CrossRef] [PubMed]
- Aissani, B.; Zhang, K.; Wiener, H. Genetic determinants of uterine fibroid size in the multiethnic NIEHS uterine fibroid study. Int. J. Mol. Epidemiol. Genet. 2015, 6, 9–19. [Google Scholar]
- Gallagher, C.S.; Morton, C.C. Genetic Association Studies in Uterine Fibroids: Risk Alleles Presage the Path to Personalized Therapies. Semin. Reprod. Med. 2016, 34, 235–241. [Google Scholar] [CrossRef]
- Ponomarenko, I.; Reshetnikov, E.; Polonikov, A.; Verzilina, I.; Sorokina, I.; Yermachenko, A.; Dvornyk, V.; Churnosov, M. Candidate Genes for Age at Menarche Are Associated With Uterine Leiomyoma. Front. Genet. 2020, 11, 512940. [Google Scholar] [CrossRef]
- Makinen, N.; Mehine, M.; Tolvanen, J.; Kaasinen, E.; Li, Y.; Lehtonen, H.J.; Gentile, M.; Yan, J.; Enge, M.; Taipale, M.; et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 2011, 334, 252–255. [Google Scholar] [CrossRef]
- Lee, M.; Cheon, K.; Chae, B.; Hwang, H.; Kim, H.K.; Chung, Y.J.; Song, J.Y.; Cho, H.H.; Kim, J.H.; Kim, M.R. Analysis of MED12 Mutation in Multiple Uterine Leiomyomas in South Korean patients. Int. J. Med. Sci. 2018, 15, 124–128. [Google Scholar] [CrossRef]
- Park, M.J.; Shen, H.; Spaeth, J.M.; Tolvanen, J.H.; Failor, C.; Knudtson, J.F.; McLaughlin, J.; Halder, S.K.; Yang, Q.; Bulun, S.E.; et al. Oncogenic exon 2 mutations in Mediator subunit MED12 disrupt allosteric activation of cyclin C-CDK8/19. J. Biol. Chem. 2018, 293, 4870–4882. [Google Scholar] [CrossRef] [PubMed]
- Ajabnoor, G.M.A.; Mohammed, N.A.; Banaganapalli, B.; Abdullah, L.S.; Bondagji, O.N.; Mansouri, N.; Sahly, N.N.; Vaidyanathan, V.; Bondagji, N.; Elango, R.; et al. Expanded Somatic Mutation Spectrum of MED12 Gene in Uterine Leiomyomas of Saudi Arabian Women. Front. Genet. 2018, 9, 552. [Google Scholar] [CrossRef]
- Klatt, F.; Leitner, A.; Kim, I.V.; Ho-Xuan, H.; Schneider, E.V.; Langhammer, F.; Weinmann, R.; Müller, M.R.; Huber, R.; Meister, G.; et al. A precisely positioned MED12 activation helix stimulates CDK8 kinase activity. Proc. Natl. Acad. Sci. USA 2020, 117, 2894–2905. [Google Scholar] [CrossRef]
- Srivastava, S.; Kulshreshtha, R. Insights into the regulatory role and clinical relevance of mediator subunit, MED12, in human diseases. J. Cell. Physiol. 2021, 236, 3163–3177. [Google Scholar] [CrossRef]
- Mäkinen, N.; Vahteristo, P.; Bützow, R.; Sjöberg, J.; Aaltonen, L.A. Exomic landscape of MED12 mutation-negative and -positive uterine leiomyomas. Int. J. Cancer. 2014, 134, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Kampjarvi, K.; Park, M.J.; Mehine, M.; Kim, N.H.; Clark, A.D.; Butzow, R.; Bohling, T.; Bohm, J.; Mecklin, J.P.; Jarvinen, H.; et al. Mutations in Exon 1 highlight the role of MED12 in uterine leiomyomas. Hum. Mutat. 2014, 35, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.C.; Kim, T.M.; Dreyfuss, J.M.; Somasundaram, P.; Christacos, N.C.; Rousselle, M.; Quade, B.J.; Park, P.J.; Stewart, E.A.; Morton, C.C. Expression profiling of uterine leiomyomata cytogenetic subgroups reveals distinct signatures in matched myometrium: Transcriptional profilingof the t(12;14) and evidence in support of predisposing genetic heterogeneity. Hum. Mol. Genet. 2012, 21, 2312–2329. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Shin, Y.H.; Yatsenko, S.A.; Castro, C.A.; Surti, U.; Rajkovic, A. Med12 gain-of-function mutation causes leiomyomas and genomic instability. J. Clin. Investig. 2015, 125, 3280–3284. [Google Scholar] [CrossRef]
- Al-Hendy, A.; Laknaur, A.; Diamond, M.P.; Ismail, N.; Boyer, T.G.; Halder, S.K. Silencing Med12 Gene Reduces Proliferation of Human Leiomyoma Cells Mediated via Wnt/β-Catenin Signaling Pathway. Endocrinology 2017, 158, 592–603. [Google Scholar] [CrossRef]
- El Andaloussi, A.; Al-Hendy, A.; Ismail, N.; Boyer, T.G.; Halder, S.K. Introduction of Somatic Mutation in MED12 Induces Wnt4/β-Catenin and Disrupts Autophagy in Human Uterine Myometrial Cell. Reprod. Sci. 2020, 27, 823–832. [Google Scholar] [CrossRef]
- Asano, R.; Asai-Sato, M.; Matsukuma, S.; Mizushima, T.; Taguri, M.; Yoshihara, M.; Inada, M.; Fukui, A.; Suzuki, Y.; Miyagi, Y.; et al. Expression of erythropoietin messenger ribonucleic acid in wild-type MED12 uterine leiomyomas under estrogenic influence: New insights into related growth disparities. Fertil. Steril. 2019, 111, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, H.R.; Sarvilinna, N.S.; Sjöberg, J.; Kämpjärvi, K.; Pitkänen, E.; Vahteristo, P.; Mäkinen, N.; Aaltonen, L.A. MED12 mutation frequency in unselected sporadic uterine leiomyomas. Fertil. Steril. 2014, 102, 1137–1142. [Google Scholar] [CrossRef]
- Heinonen, H.R.; Pasanen, A.; Heikinheimo, O.; Tanskanen, T.; Palin, K.; Tolvanen, J.; Vahteristo, P.; Sjöberg, J.; Pitkänen, E.; Bützow, R.; et al. Multiple clinical characteristics separate MED12-mutation-positive and -negative uterine leiomyomas. Sci. Rep. 2017, 7, 1015. [Google Scholar] [CrossRef]
- Yatsenko, S.A.; Mittal, P.; Wood-Trageser, M.A.; Jones, M.W.; Surti, U.; Edwards, R.P.; Sood, A.K.; Rajkovic, A. Highly heterogeneous genomic landscape of uterine leiomyomas by whole exome sequencing and genome-wide arrays. Fertil. Steril. 2017, 107, 457–466. [Google Scholar] [CrossRef]
- Shaik, N.A.; Lone, W.G.; Khan, I.A.; Vaidya, S.; Rao, K.P.; Kodati, V.L.; Hasan, Q. Detection of somatic mutations and germline polymorphisms in mitochondrial DNA of uterine fibroids patients. Genet. Test. Mol. Biomark. 2011, 15, 537–541. [Google Scholar] [CrossRef]
- Dal Cin, P.; Moerman, P.; Deprest, J.; Brosens, I.; Van den Berghe, H. A new cytogenetic subgroup in uterine leiomyoma is characterized by a deletion of the long arm of chromosome 3. Genes Chromosomes Cancer 1995, 13, 219–220. [Google Scholar] [CrossRef]
- Vanharanta, S.; Wortham, N.C.; Laiho, P.; Sjoberg, J.; Aittomaki, K.; Arola, J.; Tomlinson, I.P.; Karhu, A.; Arango, D.; Aaltonen, L.A. 7q deletion mapping and expression profiling in uterine fibroids. Oncogene 2005, 24, 6545–6554. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, A.A. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: Leiomyoma. Cancer Genet. Cytogenet. 2005, 158, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Surti, U. Subgroups of uterine leiomyomas based on cytogenetic analysis. Hum. Pathol. 1991, 22, 1009–1016. [Google Scholar] [CrossRef]
- Rein, M.S.; Friedman, A.J.; Barbieri, R.L.; Pavelka, K.; Fletcher, J.A.; Morton, C.C. Cytogenetic abnormalities in uterine leiomyomata. Obstet. Gynecol. 1991, 77, 923–926. [Google Scholar]
- El-Gharib, M.N.; Elsobky, E.S. Cytogenetic aberrations and the development of uterine leiomyomata. J. Obstet. Gynecol. Res. 2010, 36, 101–107. [Google Scholar] [CrossRef]
- Hodge, J.C.; Pearce, K.E.; Clayton, A.C.; Taran, F.A.; Stewart, E.A. Uterine cellular leiomyomata with chromosome 1p deletions represent a distinct entity. Am. J. Obstet. Gynecol. 2014, 210, 572.e1–572.e7. [Google Scholar] [CrossRef]
- Stephens, P.J.; Greenman, C.D.; Fu, B.; Yang, F.; Bignell, G.R.; Mudie, L.J.; Pleasance, E.D.; Lau, K.W.; Beare, D.; Stebbings, L.A.; et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 2011, 144, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Koltsova, A.S.; Pendina, A.A.; Efimova, O.A.; Chiryaeva, O.G.; Kuznetzova, T.V.; Baranov, V.S. On the Complexity of Mechanisms and Consequences of Chromothripsis: An Update. Front. Genet. 2019, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Mehine, M.; Mäkinen, N.; Heinonen, H.R.; Aaltonen, L.A.; Vahteristo, P. Genomics of uterine leiomyomas: Insights from high-throughput sequencing. Fertil. Steril. 2014, 102, 621–629. [Google Scholar] [CrossRef]
- Mehine, M.; Kaasinen, E.; Makinen, N.; Katainen, R.; Kampjarvi, K.; Pitkanen, E.; Heinonen, H.R.; Butzow, R.; Kilpivaara, O.; Kuosmanen, A.; et al. Characterization of uterine leiomyomas by whole-genome sequencing. N. Engl. J. Med. 2013, 369, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, C.; Saager, C.; Mechtersheimer, G.; Koczan, D.; Helmke, B.; Bullerdiek, J. Malignant transformation of uterine leiomyoma to myxoid leiomyosarcoma after morcellation associated with ALK rearrangement and loss of 14q. Oncotarget 2018, 9, 27595–27604. [Google Scholar] [CrossRef][Green Version]
- Pendina, A.A.; Koltsova, A.S.; Efimova, O.A.; Malysheva, O.V.; Osinovskaya, N.S.; Sultanov, I.Y.; Tikhonov, A.V.; Shved, N.Y.; Chiryaeva, O.G.; Simareva, A.D.; et al. Case of chromothripsis in a large solitary non-recurrent uterine leiomyoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 219, 134–136. [Google Scholar] [CrossRef]
- Markowski, D.N.; Bullerdiek, J. Chromothripsis in uterine leiomyomas. N. Engl. J. Med. 2013, 369, 2160. [Google Scholar] [CrossRef]
- Gross, K.L.; Neskey, D.M.; Manchanda, N.; Weremowicz, S.; Kleinman, M.S.; Nowak, R.A.; Ligon, A.H.; Rogalla, P.; Drechsler, K.; Bullerdiek, J.; et al. HMGA2 expression in uterine leiomyomata and myometrium: Quantitative analysis and tissue culture studies. Genes Chromosomes Cancer 2003, 38, 68–79. [Google Scholar] [CrossRef]
- Klemke, M.; Meyer, A.; Nezhad, M.H.; Bartnitzke, S.; Drieschner, N.; Frantzen, C.; Schmidt, E.H.; Belge, G.; Bullerdiek, J. Overexpression of HMGA2 in uterine leiomyomas points to its general role for the pathogenesis of the disease. Genes Chromosomes Cancer 2009, 48, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiang, W.; Griffin, B.B.; Gao, T.; Chakravarti, D.; Bulun, S.; Kim, J.J.; Wei, J.J. HMGA2-mediated tumorigenesis through angiogenesis in leiomyoma. Fertil. Steril. 2020, 114, 1085–1096. [Google Scholar] [CrossRef]
- Liu, B.; Chen, G.; He, Q.; Liu, M.; Gao, K.; Cai, B.; Qu, J.; Lin, S.; Geng, A.; Li, S.; et al. An HMGA2-p62-ERα axis regulates uterine leiomyomas proliferation. FASEB J. 2020, 34, 10966–10983. [Google Scholar] [CrossRef] [PubMed]
- Schoenmakers, E.F.; Huysmans, C.; Van de Ven, W.J. Allelic knockout of novel splice variants of human recombination repair gene RAD51B in t(12;14) uterine leiomyomas. Cancer Res. 1999, 59, 19–23. [Google Scholar]
- Mehine, M.; Kaasinen, E.; Heinonen, H.R.; Makinen, N.; Kampjarvi, K.; Sarvilinna, N.; Aavikko, M.; Vaharautio, A.; Pasanen, A.; Butzow, R.; et al. Integrated data analysis reveals uterine leiomyoma subtypes with distinct driver pathways and biomarkers. Proc. Natl. Acad. Sci. USA 2016, 113, 1315–1320. [Google Scholar] [CrossRef]
- Sornberger, K.S.; Weremowicz, S.; Williams, A.J.; Quade, B.J.; Ligon, A.H.; Pedeutour, F.; Vanni, R.; Morton, C.C. Expression of HMGIY in three uterine leiomyomata with complex rearrangements of chromosome 6. Cancer Genet. Cytogenet. 1999, 114, 9–16. [Google Scholar] [CrossRef]
- Nezhad, M.H.; Drieschner, N.; Helms, S.; Meyer, A.; Tadayyon, M.; Klemke, M.; Belge, G.; Bartnitzke, S.; Burchardt, K.; Frantzen, C.; et al. 6p21 rearrangements in uterine leiomyomas targeting HMGA1. Cancer Genet. Cytogenet. 2010, 203, 247–252. [Google Scholar] [CrossRef]
- Vanni, R.; Schoenmakers, E.F.; Andria, M. Deletion 7q in uterine leiomyoma: Fluorescence in situ hybridization characterization on primary cytogenetic preparations. Cancer Genet. Cytogenet. 1999, 113, 183–187. [Google Scholar] [CrossRef]
- Vanharanta, S.; Wortham, N.C.; Langford, C.; El-Bahrawy, M.; van der Spuy, Z.; Sjöberg, J.; Lehtonen, R.; Karhu, A.; Tomlinson, I.P.; Aaltonen, L.A. Definition of a minimal region of deletion of chromosome 7 in uterine leiomyomas by tiling-path microarray CGH and mutation analysis of known genes in this region. Genes Chromosomes Cancer 2007, 5, 451–458. [Google Scholar] [CrossRef]
- Saito, E.; Okamoto, A.; Saito, M.; Shinozaki, H.; Takakura, S.; Yanaihara, N.; Ochiai, K.; Tanaka, T. Genes associated with the genesis of leiomyoma of the uterus in a commonly deleted chromosomal region at 7q22. Oncol. Rep. 2005, 13, 469–472. [Google Scholar]
- Ptacek, T.; Song, C.; Walker, C.L.; Sell, S.M. Physical mapping of distinct 7q22 deletions in uterine leiomyoma and analysis of a recently annotated 7q22 candidate gene. Cancer Genet. Cytogenet. 2007, 174, 116–120. [Google Scholar] [CrossRef]
- Hodge, J.C.; Park, P.J.; Dreyfuss, J.M.; Assil-Kishawi, I.; Somasundaram, P.; Semere, L.G.; Quade, B.J.; Lynch, A.M.; Stewart, E.A.; Morton, C.C. Identifying the molecular signature of the interstitial deletion 7q subgroup of uterine leiomyomata using a paired analysis. Genes Chromosomes Cancer 2009, 48, 865–885. [Google Scholar] [CrossRef]
- Zeng, W.R.; Scherer, S.W.; Koutsilieris, M.; Huizenga, J.J.; Filteau, F.; Tsui, L.C.; Nepveu, A. Loss of heterozygosity and reduced expression of the CUTL1 gene in uterine leiomyomas. Oncogene 1997, 14, 2355–2365. [Google Scholar] [CrossRef][Green Version]
- Moon, N.S.; Rong Zeng, W.; Premdas, P.; Santaguida, M.; Bérubé, G.; Nepveu, A. Expression of N-terminally truncated isoforms of CDP/CUX is increased in human uterine leiomyomas. Int. J. Cancer 2002, 100, 429–432. [Google Scholar] [CrossRef]
- Schoenmakers, E.F.; Bunt, J.; Hermers, L.; Schepens, M.; Merkx, G.; Janssen, B.; Kersten, M.; Huys, E.; Pauwels, P.; Debiec-Rychter, M.; et al. Identification of CUX1 as the recurrent chromosomal band 7q22 target gene in human uterine leiomyoma. Genes Chromosomes Cancer 2013, 52, 11–23. [Google Scholar] [CrossRef]
- Zhou, J.; Mochizuki, T.; Smeets, H.; Antignac, C.; Laurila, P.; de Paepe, A.; Tryggvason, K.; Reeders, S.T. Deletion of the paired alpha 5(IV) and alpha 6(IV) collagen genes in inherited smooth muscle tumors. Science 1993, 261, 1167–1169. [Google Scholar] [CrossRef]
- Heidet, L.; Dahan, K.; Zhou, J.; Xu, Z.; Cochat, P.; Gould, J.D.; Leppig, K.A.; Proesmans, W.; Guyot, C.; Guillot, M.; et al. Deletions of both alpha 5(IV) and alpha 6(IV) collagen genes in Alport syndrome and in Alport syndrome associated with smooth muscle tumours. Hum. Mol. Genet. 1995, 4, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Nozu, K.; Minamikawa, S.; Yamada, S.; Oka, M.; Yanagita, M.; Morisada, N.; Fujinaga, S.; Nagano, C.; Gotoh, Y.; Takahashi, E.; et al. Characterization of contiguous gene deletions in COL4A6 and COL4A5 in Alport syndrome-diffuse leiomyomatosis. J. Hum. Genet. 2017, 62, 733–735. [Google Scholar] [CrossRef]
- Yang, Q.; Mas, A.; Diamond, M.P.; Al-Hendy, A. The Mechanism and Function of Epigenetics in Uterine Leiomyoma Development. Reprod. Sci. 2016, 23, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Vergara, D.; Favilli, A.; La Rosa, V.L.; Tinelli, A.; Gerli, S.; Noventa, M.; Vitagliano, A.; Triolo, O.; Rapisarda, A.M.C.; et al. Epigenetic and genetic landscape of uterine leiomyomas: A current view over a common gynecological disease. Arch. Gynecol. Obstet. 2017, 296, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Välimäki, N.; Kuisma, H.; Pasanen, A.; Heikinheimo, O.; Sjöberg, J.; Bützow, R.; Sarvilinna, N.; Heinonen, H.R.; Tolvanen, J.; Bramante, S.; et al. Genetic predisposition to uterine leiomyoma is determined by loci for genitourinary development and genome stability. Elife 2018, 7, e37110. [Google Scholar] [CrossRef]
- Martinez-Jimenez, C.P.; Sandoval, J. Epigenetic crosstalk: A molecular language in human metabolic disorders. Front. Biosci. Sch. 2015, 7, 46–57. [Google Scholar]
- Miozzo, M.; Vaira, V.; Sirchia, S.M. Epigenetic alterations in cancer and personalized cancer treatment. Future Oncol. 2015, 11, 333–348. [Google Scholar] [CrossRef]
- Asada, H.; Yamagata, Y.; Taketani, T.; Matsuoka, A.; Tamura, H.; Hattori, N.; Ohgane, J.; Shiota, K.; Sugino, N. Potential link between estrogen receptor-alpha gene hypomethylation and uterine fibroid formation. Mol. Hum. Reprod. 2008, 14, 539–545. [Google Scholar] [CrossRef]
- Navarro, A.; Yin, P.; Monsivais, D.; Lin, S.M.; Du, P.; Wei, J.J.; Bulun, S.E. Genome-wide DNA methylation indicates silencing of tumor suppressor genes in uterine leiomyoma. PLoS ONE 2012, 7, e33284. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, R.; Sato, S.; Yamagata, Y.; Asada, H.; Tamura, I.; Lee, L.; Okada, M.; Tamura, H.; Takaki, E.; Nakai, A.; et al. Genome-wide DNA methylation analysis reveals a potential mechanism for the pathogenesis and development of uterine leiomyomas. PLoS ONE 2013, 8, e66632. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, Y.; Maekawa, R.; Asada, H.; Taketani, T.; Tamura, I.; Tamura, H.; Ogane, J.; Hattori, N.; Shiota, K.; Sugino, N. Aberrant DNA methylation status in human uterine leiomyoma. Mol. Hum. Reprod. 2009, 15, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Styer, A.K.; Rueda, B.R. The Epidemiology and Genetics of Uterine Leiomyoma. Best Pract. Res. Clin. Obstet. Gynecol. 2016, 34, 3–12. [Google Scholar] [CrossRef]
- George, J.W.; Fan, H.; Johnson, B.; Carpenter, T.J.; Foy, K.K.; Chatterjee, A.; Patterson, A.L.; Koeman, J.; Adams, M.; Madaj, Z.B.; et al. Integrated Epigenome, Exome, and Transcriptome Analyses Reveal Molecular Subtypes and Homeotic Transformation in Uterine Fibroids. Cell Rep. 2019, 29, 4069–4085.e4066. [Google Scholar] [CrossRef]
- Kol’tsova, A.S.; Pendina, A.A.; Efimova, O.A.; Kaminskaya, A.N.; Tikhonov, A.V.; Osinovskaya, N.S.; Sultanov, I.Y.; Shved, N.Y.; Kakhiani, M.I.; Baranov, V.S. Differential DNA Hydroxymethylation in Human Uterine Leiomyoma Cells Depending on the Phase of Menstrual Cycle and Presence of MED12 Gene Mutations. Bull. Exp. Biol. Med. 2017, 163, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yin, P.; Kujawa, S.A.; Coon, J.S.t.; Okeigwe, I.; Bulun, S.E. Progesterone receptor integrates the effects of mutated MED12 and altered DNA methylation to stimulate RANKL expression and stem cell proliferation in uterine leiomyoma. Oncogene 2019, 38, 2722–2735. [Google Scholar] [CrossRef]
- Vaiman, D. Towards an Epigenetic Treatment of Leiomyomas? Endocrinology 2020, 161, bqaa172. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.; Helin, K. Histone methyltransferases in cancer. Semin. Cell Dev. Biol. 2010, 21, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Crabtree, G.R. Chromatin remodelling during development. Nature 2010, 463, 474–484. [Google Scholar] [CrossRef]
- Cao, R.; Wang, L.; Wang, H.; Xia, L.; Erdjument-Bromage, H.; Tempst, P.; Jones, R.S.; Zhang, Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 2002, 298, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Viré, E.; Brenner, C.; Deplus, R.; Blanchon, L.; Fraga, M.; Didelot, C.; Morey, L.; Van Eynde, A.; Bernard, D.; Vanderwinden, J.M.; et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature 2006, 439, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.H.; Torng, P.L.; Hsiao, S.M.; Jeng, Y.M.; Chen, M.W.; Chen, C.A. Histone deacetylase 6 regulates estrogen receptor alpha in uterine leiomyoma. Reprod. Sci. 2011, 18, 755–762. [Google Scholar] [CrossRef]
- Borahay, M.A.; Al-Hendy, A.; Kilic, G.S.; Boehning, D. Signaling Pathways in Leiomyoma: Understanding Pathobiology and Implications for Therapy. Mol. Med. 2015, 21, 242–256. [Google Scholar] [CrossRef]
- Wang, J.; Bhutani, M.; Pathak, A.K.; Lang, W.; Ren, H.; Jelinek, J.; He, R.; Shen, L.; Issa, J.P.; Mao, L. Delta DNMT3B variants regulate DNA methylation in a promoter-specific manner. Cancer Res. 2007, 67, 10647–10652. [Google Scholar] [CrossRef]
- Karmon, A.E.; Cardozo, E.R.; Rueda, B.R.; Styer, A.K. MicroRNAs in the development and pathobiology of uterine leiomyomata: Does evidence support future strategies for clinical intervention? Hum. Reprod. Update 2014, 20, 670–687. [Google Scholar] [CrossRef]
- Segars, J.; Al-Hendy, A. Uterine Leiomyoma: New Perspectives on an Old Disease. Semin. Reprod. Med. 2017, 35, 471–472. [Google Scholar] [CrossRef]
- Ono, M.; Yin, P.; Navarro, A.; Moravek, M.B.; Coon, V.J.; Druschitz, S.A.; Gottardi, C.J.; Bulun, S.E. Inhibition of canonical WNT signaling attenuates human leiomyoma cell growth. Fertil. Steril. 2014, 101, 1441–1449. [Google Scholar] [CrossRef]
- Chuang, T.D.; Rehan, A.; Khorram, O. Functional role of the long noncoding RNA X-inactive specific transcript in leiomyoma pathogenesis. Fertil. Steril. 2021, 115, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Ciarmela, P.; Petraglia, F. New epigenetic mechanism involved in leiomyoma formation. Fertil. Steril. 2021, 115, 94–95. [Google Scholar] [CrossRef]
- Linder, D.; Gartler, S.M. Glucose-6-phosphate dehydrogenase mosaicism: Utilization as a cell marker in the study of leiomyomas. Science 1965, 150, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Maruyama, T.; Masuda, H.; Kajitani, T.; Nagashima, T.; Arase, T.; Ito, M.; Ohta, K.; Uchida, H.; Asada, H.; et al. Side population in human uterine myometrium displays phenotypic and functional characteristics of myometrial stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 18700–18705. [Google Scholar] [CrossRef] [PubMed]
- Mas, A.; Cervello, I.; Gil-Sanchis, C.; Faus, A.; Ferro, J.; Pellicer, A.; Simon, C. Identification and characterization of the human leiomyoma side population as putative tumor-initiating cells. Fertil. Steril. 2012, 98, 741–751.e746. [Google Scholar] [CrossRef] [PubMed]
- Moravek, M.B.; Yin, P.; Ono, M.; Coon, V.J.S.; Dyson, M.T.; Navarro, A.; Marsh, E.E.; Chakravarti, D.; Kim, J.J.; Wei, J.-J.; et al. Ovarian steroids, stem cells and uterine leiomyoma: Therapeutic implications. Hum. Reprod. Update 2015, 21, 1–12. [Google Scholar] [CrossRef]
- Mas, A.; Stone, L.; O’Connor, P.M.; Yang, Q.; Kleven, D.; Simon, C.; Walker, C.L.; Al-Hendy, A. Developmental Exposure to Endocrine Disruptors Expands Murine Myometrial Stem Cell Compartment as a Prerequisite to Leiomyoma Tumorigenesis. Stem. Cells 2017, 35, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Qiang, W.; Serna, V.A.; Yin, P.; Coon, J.S.t.; Navarro, A.; Monsivais, D.; Kakinuma, T.; Dyson, M.; Druschitz, S.; et al. Role of stem cells in human uterine leiomyoma growth. PLoS ONE 2012, 7, e36935. [Google Scholar] [CrossRef] [PubMed]
- Mas, A.; Cervello, I.; Gil-Sanchis, C.; Simon, C. Current understanding of somatic stem cells in leiomyoma formation. Fertil. Steril. 2014, 102, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Mas, A.; Cervelló, I.; Fernández-Álvarez, A.; Faus, A.; Díaz, A.; Burgués, O.; Casado, M.; Simón, C. Overexpression of the truncated form of High Mobility Group A proteins (HMGA2) in human myometrial cells induces leiomyoma-like tissue formation. Mol. Hum. Reprod. 2015, 21, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, E.; Qiang, W.; Zhang, Q.; Espona-Fiedler, M.; Druschitz, S.; Liu, Y.; Mittal, K.; Kong, B.; Kurita, T.; Wei, J.J. MED12 and HMGA2 mutations: Two independent genetic events in uterine leiomyoma and leiomyosarcoma. Mod. Pathol. 2014, 27, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yi, T.; Shen, K.; Zhang, B.; Huang, F.; Zhao, X. Hypoxia: The driving force of uterine myometrial stem cells differentiation into leiomyoma cells. Med. Hypotheses 2011, 77, 985–986. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Ono, M.; Yoshimura, Y. Somatic stem cells in the myometrium and in myomas. Semin. Reprod. Med. 2013, 31, 77–81. [Google Scholar] [CrossRef]
- Chen, D.C.; Liu, J.Y.; Wu, G.J.; Ku, C.H.; Su, H.Y.; Chen, C.H. Serum vascular endothelial growth factor165 levels and uterine fibroid volume. Acta Obstet. Gynecol. Scand. 2005, 84, 317–321. [Google Scholar] [CrossRef]
- Dingiloglu, B.S.; Gungor, T.; Ozdal, B.; Cavkaytar, S.; Bilge, U.; Mollamahmutoglu, L. Serum leptin levels in women with uterine leiomyomas. Taiwan, J. Obstet. Gynecol. 2007, 46, 33–37. [Google Scholar] [CrossRef][Green Version]
- Levy, G.; Hill, M.J.; Plowden, T.C.; Catherino, W.H.; Armstrong, A.Y. Biomarkers in uterine leiomyoma. Fertil. Steril. 2013, 99, 1146–1152. [Google Scholar] [CrossRef]
- Lin, C.P.; Chen, Y.W.; Liu, W.H.; Chou, H.C.; Chang, Y.P.; Lin, S.T.; Li, J.M.; Jian, S.F.; Lee, Y.R.; Chan, H.L. Proteomic identification of plasma biomarkers in uterine leiomyoma. Mol. Biosyst. 2012, 8, 1136–1145. [Google Scholar] [CrossRef]
- Santulli, P.; Borghese, B.; Lemaréchal, H.; Leconte, M.; Millischer, A.E.; Batteux, F.; Chapron, C.; Borderie, D. Increased serum oxidative stress markers in women with uterine leiomyoma. PLoS ONE 2013, 8, e72069. [Google Scholar] [CrossRef]
- Caglayan, A.; Katlan, D.C.; Tuncer, Z.S.; Yuce, K.; Sayal, H.B.; Kocer-Gumusel, B. Assessment of oxidant-antioxidant status alterations with tumor biomarkers and reproductive system hormones in uterine MYOMAS. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 229, 1–7. [Google Scholar] [CrossRef]
- Ciebiera, M.; Włodarczyk, M.; Słabuszewska-Jóźwiak, A.; Nowicka, G.; Jakiel, G. Influence of vitamin D and transforming growth factor β3 serum concentrations, obesity, and family history on the risk for uterine fibroids. Fertil. Steril. 2016, 106, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, J.; Wei, H. Serum YKL-40 Level Positively Correlates With Uterine Leiomyomas. Reprod. Sci. 2016, 23, 1559–1564. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, D.; Sheng, J.; Luo, L.; Zhang, W. Identification of TRADD as a potential biomarker in human uterine leiomyoma through iTRAQ based proteomic profiling. Mol. Cell. Probes. 2017, 36, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Kamalipooya, S.; Zarezadeh, R.; Latifi, Z.; Nouri, M.; Fattahi, A.; Salemi, Z. Serum transforming growth factor β and leucine-rich α-2-glycoprotein 1 as potential biomarkers for diagnosis of uterine leiomyomas. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102037. [Google Scholar] [CrossRef]
- Trovik, J.; Salvesen, H.B.; Cuppens, T.; Amant, F.; Staff, A.C. Growth differentiation factor-15 as biomarker in uterine sarcomas. Int. J. Gynecol. Cancer 2014, 24, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Juang, C.M.; Yen, M.S.; Horng, H.C.; Twu, N.F.; Yu, H.C.; Hsu, W.L. Potential role of preoperative serum CA125 for the differential diagnosis between uterine leiomyoma and uterine leiomyosarcoma. Eur. J. Gynaecol. Oncol. 2006, 27, 370–374. [Google Scholar]
- Basta, P.; Mach, P.; Pitynski, K.; Bednarek, W.; Klimek, M.; Zietek, J.; Zajac, K.; Wicherek, L. Differences in the blood serum levels of soluble HLA-G concentrations between the menstrual cycle phases and menopause in patients with ovarian endometriosis and uterine leiomyoma. Neuro-Endocrinol. Lett. 2009, 30, 91–98. [Google Scholar]
- Makinen, N.; Kampjarvi, K.; Frizzell, N.; Butzow, R.; Vahteristo, P. Characterization of MED12, HMGA2, and FH alterations reveals molecular variability in uterine smooth muscle tumors. Mol. Cancer 2017, 16, 101. [Google Scholar] [CrossRef]
- Kämpjärvi, K.; Mäkinen, N.; Mehine, M.; Välipakka, S.; Uimari, O.; Pitkänen, E.; Heinonen, H.R.; Heikkinen, T.; Tolvanen, J.; Ahtikoski, A.; et al. MED12 mutations and FH inactivation are mutually exclusive in uterine leiomyomas. Br. J. Cancer 2016, 114, 1405–1411. [Google Scholar] [CrossRef]
- Markowski, D.N.; Bartnitzke, S.; Löning, T.; Drieschner, N.; Helmke, B.M.; Bullerdiek, J. MED12 mutations in uterine fibroids--their relationship to cytogenetic subgroups. Int. J. Cancer 2012, 131, 1528–1536. [Google Scholar] [CrossRef]
- Holzmann, C.; Markowski, D.N.; Bartnitzke, S.; Koczan, D.; Helmke, B.M.; Bullerdiek, J. A rare coincidence of different types of driver mutations among uterine leiomyomas (UL). Mol. Cytogenet. 2015, 8, 76. [Google Scholar] [CrossRef]
- Galindo, L.J.; Hernández-Beeftink, T.; Salas, A.; Jung, Y.; Reyes, R.; de Oca, F.M.; Hernández, M.; Almeida, T.A. HMGA2 and MED12 alterations frequently co-occur in uterine leiomyomas. Gynecol. Oncol. 2018, 150, 562–568. [Google Scholar] [CrossRef]
- Adams, C.L.; Dimitrova, I.; Post, M.D.; Gibson, L.; Spillman, M.A.; Behbakht, K.; Bradford, A.P. Identification of a novel diagnostic gene expression signature to discriminate uterine leiomyoma from leiomyosarcoma. Exp. Mol. Pathol. 2019, 110, 104284. [Google Scholar] [CrossRef]
- Mas, A.; Simon, C. Molecular differential diagnosis of uterine leiomyomas and leiomyosarcomas. Biol. Reprod. 2019, 101, 1115–1123. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yin, P.; Dotts, A.J.; Kujawa, S.A.; Coon, V.J.; Wei, J.J.; Chakravarti, D.; Bulun, S.E. Activation of protein kinase B by WNT4 as a regulator of uterine leiomyoma stem cell function. Fertil. Steril. 2020, 114, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Tsuda, Y.; Yabuki, K.; Shiba, E.; Uchihashi, K.; Matsuyama, A.; Fujino, Y.; Hachisuga, T.; Hisaoka, M. Inhibition of WNT/β-catenin signaling under serum starvation and hypoxia induces adipocytic transdifferentiation in human leiomyoma cells. Lab. Investig. 2018, 98, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Al-Hendy, A.; Diamond, M.P.; Boyer, T.G.; Halder, S.K. Vitamin D3 Inhibits Wnt/β-Catenin and mTOR Signaling Pathways in Human Uterine Fibroid Cells. J. Clin. Endocrinol. Metab. 2016, 101, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Corachán, A.; Ferrero, H.; Aguilar, A.; Garcia, N.; Monleon, J.; Faus, A.; Cervelló, I.; Pellicer, A. Inhibition of tumor cell proliferation in human uterine leiomyomas by vitamin D via Wnt/β-catenin pathway. Fertil. Steril. 2019, 111, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; De Santis, M.C.; Braccini, L.; Gulluni, F.; Hirsch, E. PI3K/AKT signaling pathway and cancer: An updated review. Ann. Med. 2014, 46, 372–383. [Google Scholar] [CrossRef]
- Kovács, K.A.; Lengyel, F.; Vértes, Z.; Környei, J.L.; Gocze, P.M.; Sumegi, B.; Szabó, I.; Vértes, M. Phosphorylation of PTEN (phosphatase and tensin homologue deleted on chromosome ten) protein is enhanced in human fibromyomatous uteri. J. Steroid. Biochem. Mol. Biol. 2007, 103, 196–199. [Google Scholar] [CrossRef]
- Karra, L.; Shushan, A.; Ben-Meir, A.; Rojansky, N.; Klein, B.Y.; Shveiky, D.; Levitzki, R.; Ben-Bassat, H. Changes related to phosphatidylinositol 3-kinase/Akt signaling in leiomyomas: Possible involvement of glycogen synthase kinase 3alpha and cyclin D2 in the pathophysiology. Fertil. Steril. 2010, 93, 2646–2651. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sasson, H.; Ben-Meir, A.; Shushan, A.; Karra, L.; Rojansky, N.; Klein, B.Y.; Levitzki, R.; Ben-Bassat, H. All-trans-retinoic acid mediates changes in PI3K and retinoic acid signaling proteins of leiomyomas. Fertil. Steril. 2011, 95, 2080–2086. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, Y.S.; Park, J.H.; Kim, H.; Lee, I.; Won, Y.B.; Yun, B.H.; Seo, S.K.; Lee, B.S.; Cho, S. MiR-150-5p May Contribute to Pathogenesis of Human Leiomyoma via Regulation of the Akt/p27(Kip1) Pathway In Vitro. Int. J. Mol. Sci. 2019, 20, 2684. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, G.; Miele, C.; Pellicano, M.; Trencia, A.; Ferraioli, M.; Paturzo, F.; Tommaselli, G.A.; Beguinot, F.; Nappi, C. Molecular mechanisms involved in GnRH analogue-related apoptosis for uterine leiomyomas. Mol. Hum. Reprod. 2004, 10, 43–48. [Google Scholar] [CrossRef][Green Version]
- Ciebiera, M.; Włodarczyk, M.; Wrzosek, M.; Męczekalski, B.; Nowicka, G.; Łukaszuk, K.; Słabuszewska-Jóźwiak, A.; Jakiel, G. Role of Transforming Growth Factor β in Uterine Fibroid Biology. Int. J. Mol. Sci. 2017, 18, 2435. [Google Scholar] [CrossRef]
- Lee, B.S.; Nowak, R.A. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-beta 3 (TGF beta 3) and altered responses to the antiproliferative effects of TGF beta. J. Clin. Endocrinol. Metab. 2001, 86, 913–920. [Google Scholar] [CrossRef]
- Luo, X.; Ding, L.; Xu, J.; Chegini, N. Gene expression profiling of leiomyoma and myometrial smooth muscle cells in response to transforming growth factor-beta. Endocrinology 2005, 146, 1097–1118. [Google Scholar] [CrossRef] [PubMed]
- Wolańska, M.; Bańkowski, E. Transforming growth factor beta and platelet-derived growth factor in human myometrium and in uterine leiomyomas at various stages of tumour growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 130, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wu, Y.; Lu, Q.; Zhang, P.; Ren, M. Transforming growth factor-β signaling pathway cross-talking with ERα signaling pathway on regulating the growth of uterine leiomyoma activated by phenolic environmental estrogens in vitro. Tumour. Biol. 2016, 37, 455–462. [Google Scholar] [CrossRef]
- Chegini, N.; Luo, X.; Ding, L.; Ripley, D. The expression of Smads and transforming growth factor beta receptors in leiomyoma and myometrium and the effect of gonadotropin releasing hormone analogue therapy. Mol. Cell. Endocrinol. 2003, 209, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Włodarczyk, M.; Wrzosek, M.; Słabuszewska-Jóźwiak, A.; Nowicka, G.; Jakiel, G. Ulipristal acetate decreases transforming growth factor β3 serum and tumor tissue concentrations in patients with uterine fibroids. Fertil. Steril. 2018, 109, 501–507.e502. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.D.; Malik, M.; Britten, J.; Parikh, T.; Cox, J.; Catherino, W.H. Ulipristal acetate decreases active TGF-β3 and its canonical signaling in uterine leiomyoma via two novel mechanisms. Fertil. Steril. 2019, 111, 806–815.e801. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, P.; Mughal, S.S.; Sanders, M.A.; Hubschmann, D.; Chung, I.; Deeg, K.I.; Wong, S.H.; Rabe, S.; Hlevnjak, M.; Zapatka, M.; et al. Integrative genomic and transcriptomic analysis of leiomyosarcoma. Nat. Commun. 2018, 9, 144. [Google Scholar] [CrossRef]
- Stratton, K.L.; Chang, S.S. Locally advanced prostate cancer: The role of surgical management. BJU Int. 2009, 104, 449–454. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef]
- Picelli, S.; Björklund, Å.K.; Faridani, O.R.; Sagasser, S.; Winberg, G.; Sandberg, R. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat. Methods 2013, 10, 1096–1098. [Google Scholar] [CrossRef]
- Navin, N.E. The first five years of single-cell cancer genomics and beyond. Genome Res. 2015, 25, 1499–1507. [Google Scholar] [CrossRef]
- Tanay, A.; Regev, A. Scaling single-cell genomics from phenomenology to mechanism. Nature 2017, 541, 331–338. [Google Scholar] [CrossRef]
- Svensson, V.; Vento-Tormo, R.; Teichmann, S.A. Exponential scaling of single-cell RNA-seq in the past decade. Nat. Protoc. 2018, 13, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, J.R.; Bambah-Mukku, D.; Eichhorn, S.W.; Vaughn, E.; Shekhar, K.; Perez, J.D.; Rubinstein, N.D.; Hao, J.; Regev, A.; Dulac, C.; et al. Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 2018, 362, eaau5324. [Google Scholar] [CrossRef]
- Angelidis, I.; Simon, L.M.; Fernandez, I.E.; Strunz, M.; Mayr, C.H.; Greiffo, F.R.; Tsitsiridis, G.; Ansari, M.; Graf, E.; Strom, T.M.; et al. An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat. Commun. 2019, 10, 963. [Google Scholar] [CrossRef]
- Plasschaert, L.W.; Žilionis, R.; Choo-Wing, R.; Savova, V.; Knehr, J.; Roma, G.; Klein, A.M.; Jaffe, A.B. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 2018, 560, 377–381. [Google Scholar] [CrossRef]
- Ali, H.; Kilic, G.; Vincent, K.; Motamedi, M.; Rytting, E. Nanomedicine for uterine leiomyoma therapy. Ther. Deliv. 2013, 4, 161–175. [Google Scholar] [CrossRef]
- Jiang, J.; Bischof, J. Effect of timing, dose and interstitial versus nanoparticle delivery of tumor necrosis factor alpha in combinatorial adjuvant cryosurgery treatment of ELT-3 uterine fibroid tumor. Cryo. Lett. 2010, 31, 50–62. [Google Scholar]
- Shalaby, S.M.; Khater, M.K.; Perucho, A.M.; Mohamed, S.A.; Helwa, I.; Laknaur, A.; Lebedyeva, I.; Liu, Y.; Diamond, M.P.; Al-Hendy, A.A. Magnetic nanoparticles as a new approach to improve the efficacy of gene therapy against differentiated human uterine fibroid cells and tumor-initiating stem cells. Fertil. Steril. 2016, 105, 1638–1648.e1638. [Google Scholar] [CrossRef]
- Moyo, M.B.; Parker, J.B.; Chakravarti, D. Altered chromatin landscape and enhancer engagement underlie transcriptional dysregulation in MED12 mutant uterine leiomyomas. Nat. Commun. 2020, 11, 1019. [Google Scholar] [CrossRef] [PubMed]
- Grossman, S.R.; Zhang, X.; Wang, L.; Engreitz, J.; Melnikov, A.; Rogov, P.; Tewhey, R.; Isakova, A.; Deplancke, B.; Bernstein, B.E.; et al. Systematic dissection of genomic features determining transcription factor binding and enhancer function. Proc. Natl. Acad. Sci. USA 2017, 114, e1291–e1300. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).