YY1 Silencing Induces 5-Fluorouracil-Resistance and BCL2L15 Downregulation in Colorectal Cancer Cells: Diagnostic and Prognostic Relevance
Abstract
1. Introduction
2. Results
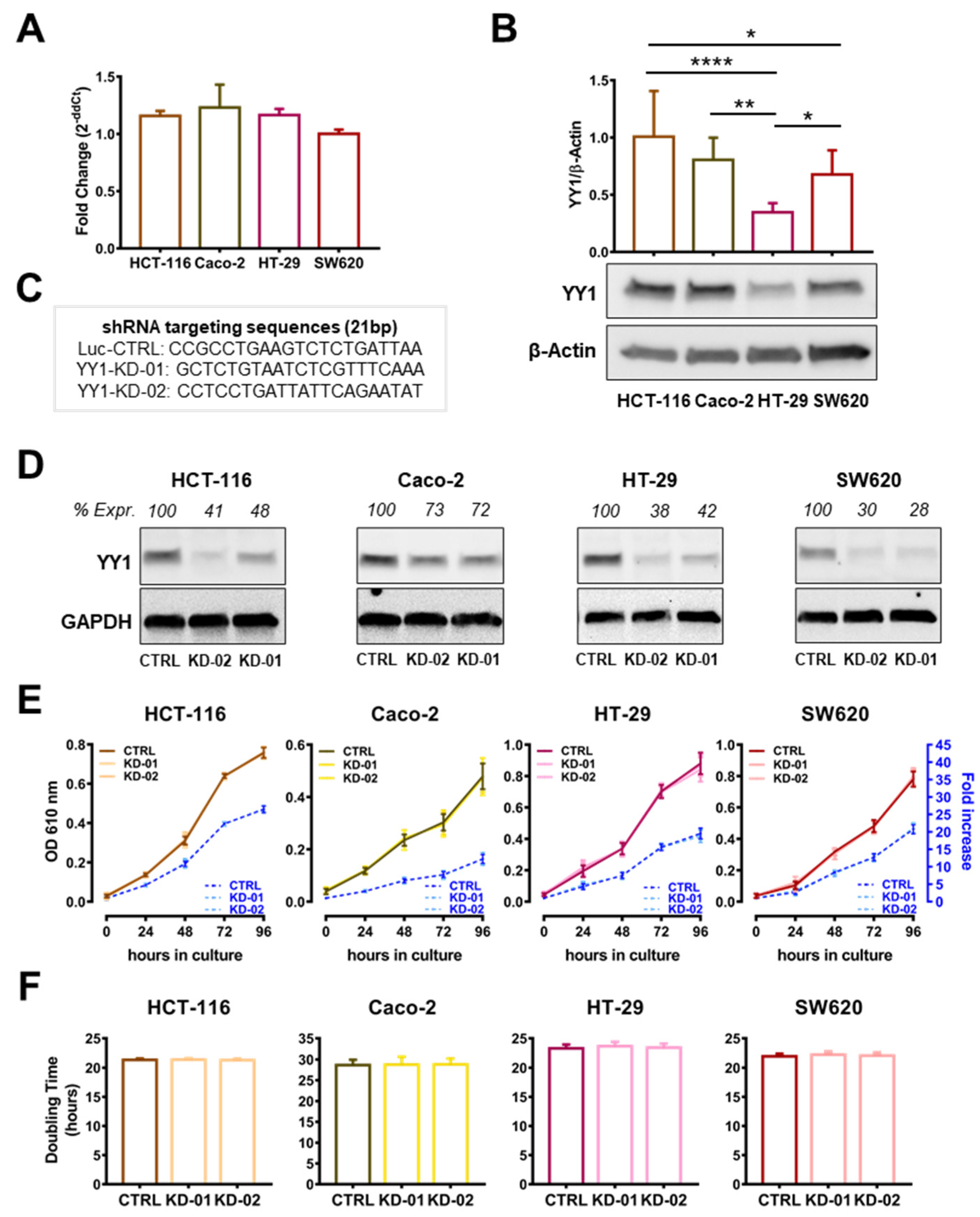
2.1. YY1 Is Heterogeneously Expressed in CRC Cancer Cells and Its Silencing Does Not Affect Cellular Growth and Viability
2.2. YY1 Silencing Protects HT-29 and SW620 CRC Cells against 5-FU-Induced Cytotoxicity
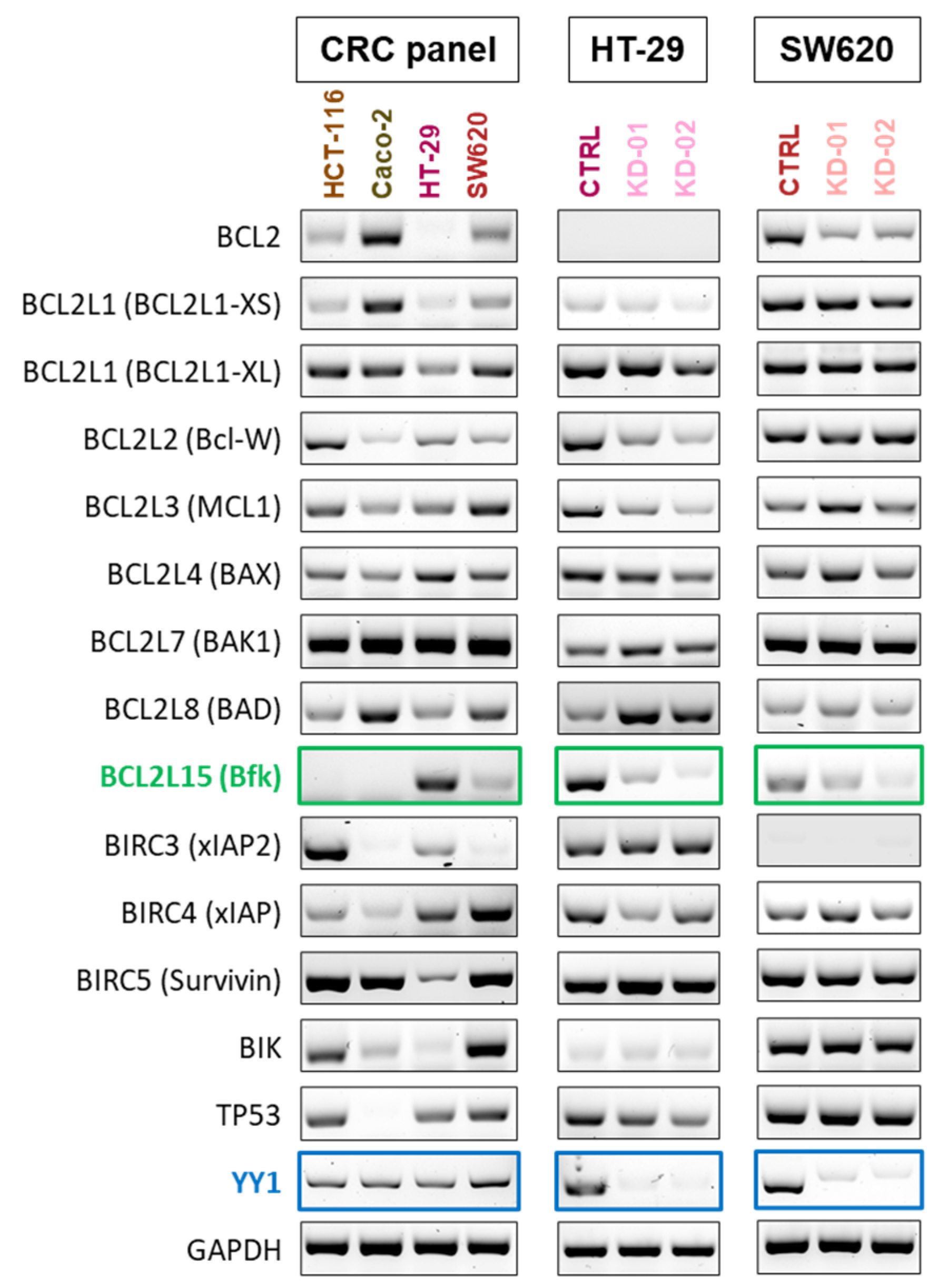
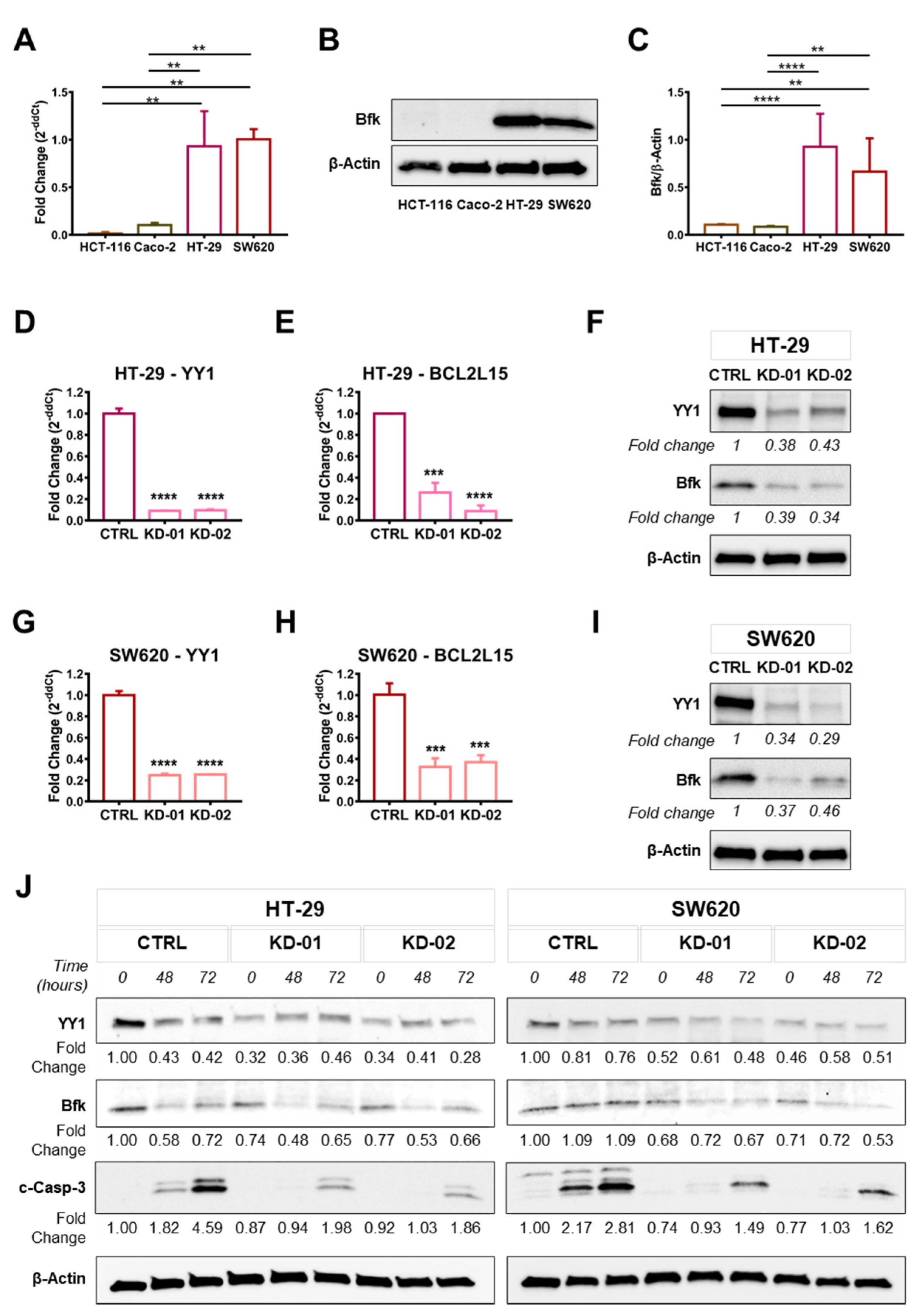
2.3. YY1 Silencing Is Associated with the Downregulation of the Tumor-Suppressor BCL2L15 (Bfk) in HT-29 and SW620 CRC Cells
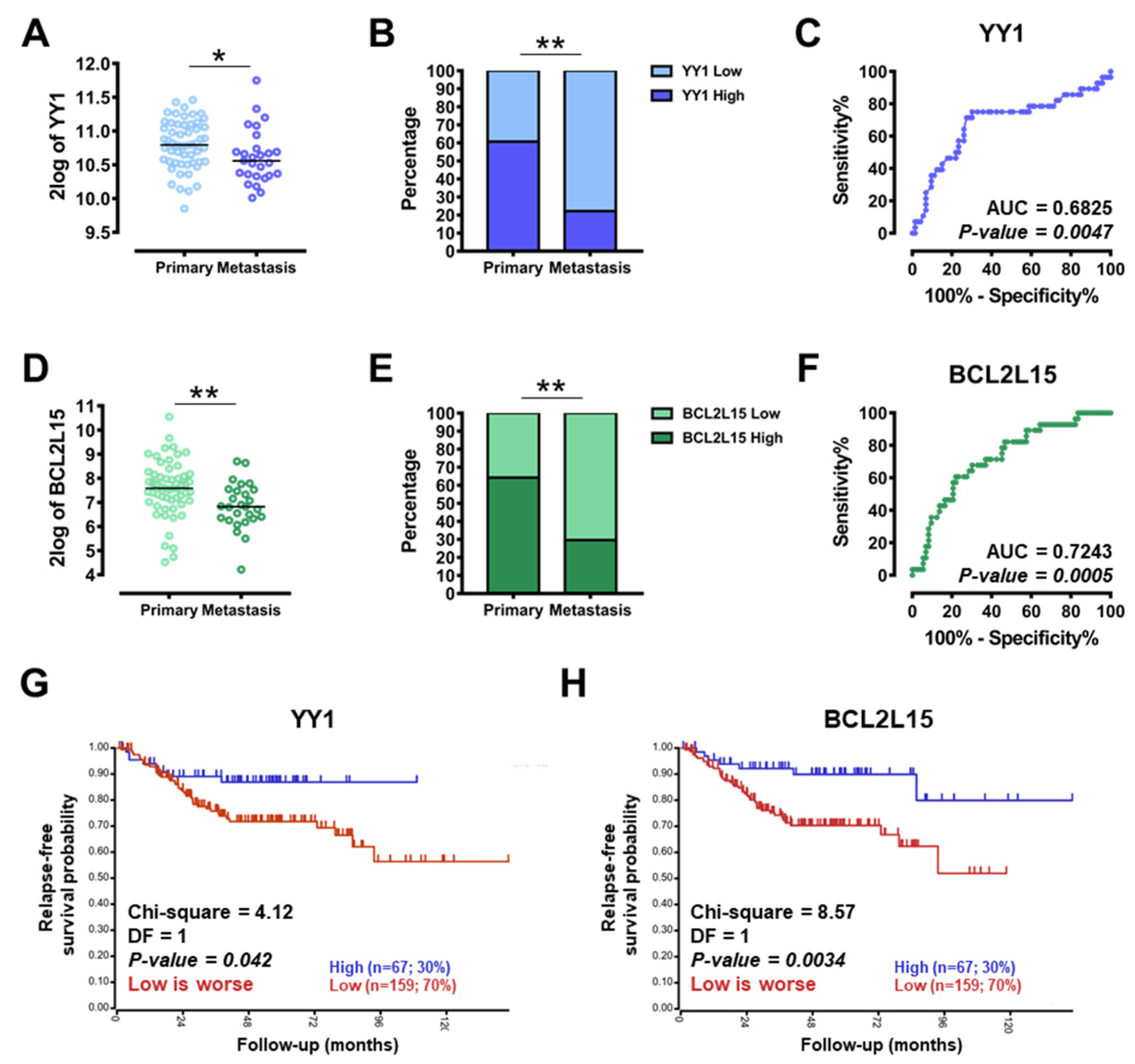
2.4. YY1 and BCL2L15 Are Positive Diagnostic and Prognostic Markers in CRC Patients
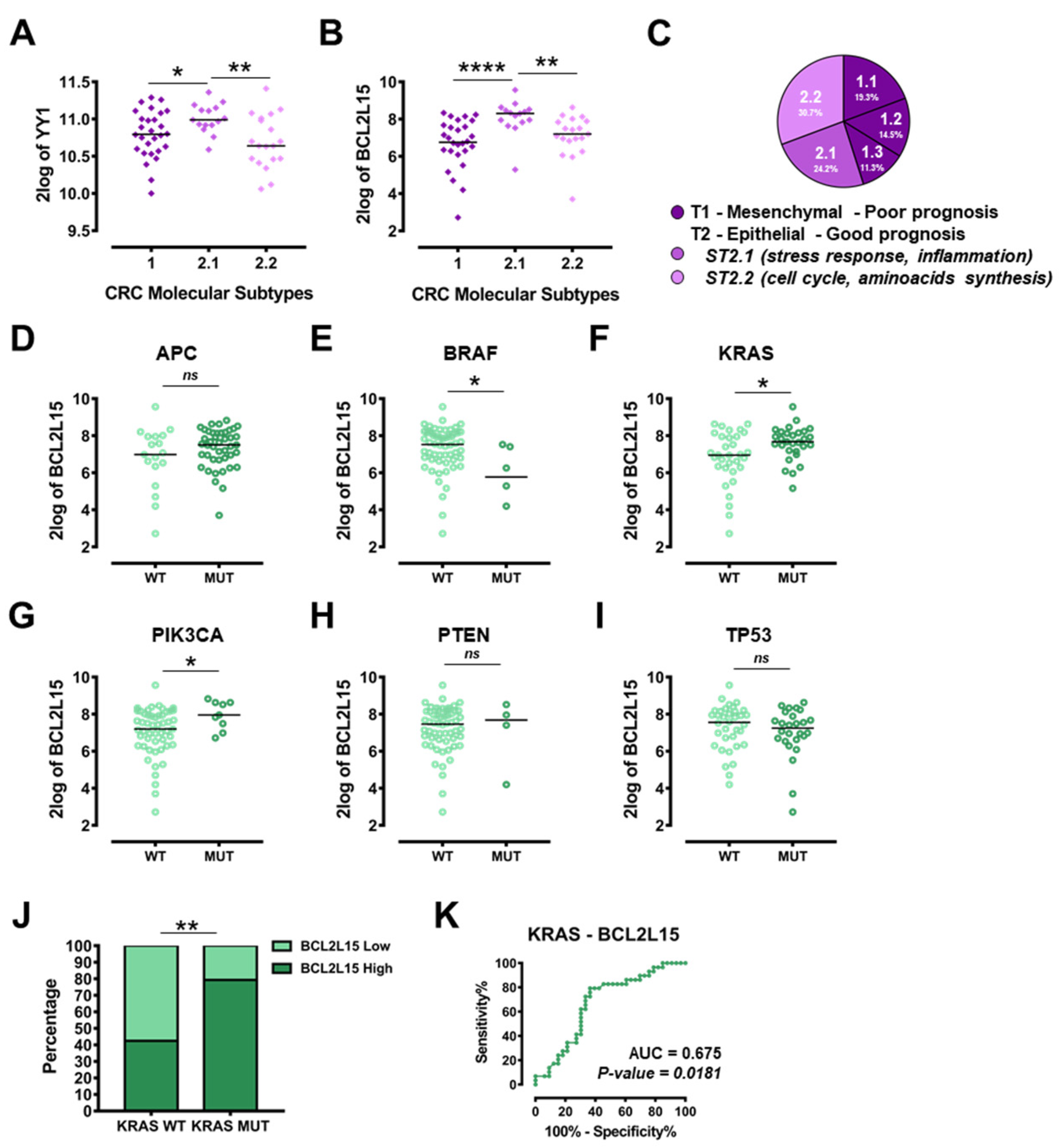
2.5. YY1 and BCL2L15 Expression Is Correlated with Selected CRC Molecular Subtypes and Specific Single-Cell Hierarchical Clustering
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture
4.2. Generation of CRC Cells Constitutively Silenced for YY1
4.3. Cell Viability Assays
4.4. Total RNA Extraction, cDNA Synthesis and Semiquantitative and Quantitative RT-PCR Analyses
4.5. Protein Lysates Preparation, Quantification and Immunoblot Analyses
4.6. Bioinformatic Analyses
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Molinari, C.; Marisi, G.; Passardi, A.; Matteucci, L.; De Maio, G.; Ulivi, P. Heterogeneity in Colorectal Cancer: A Challenge for Personalized Medicine? Int. J. Mol. Sci. 2018, 19, 3733. [Google Scholar] [CrossRef] [PubMed]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Menter, D.G.; Davis, J.S.; Broom, B.M.; Overman, M.J.; Morris, J.; Kopetz, S. Back to the Colorectal Cancer Consensus Molecular Subtype Future. Curr. Gastroenterol. Rep. 2019, 21, 5. [Google Scholar] [CrossRef]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.N.; Fong, M.K.; Jagosky, M. Colorectal Cancer Biomarkers in the Era of Personalized Medicine. J. Pers. Med. 2019, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Akopyan, G.; Garban, H.; Bonavida, B. Transcription factor YY1: Structure, function, and therapeutic implications in cancer biology. Oncogene 2006, 25, 1125–1142. [Google Scholar] [CrossRef]
- Meliala, I.T.S.; Hosea, R.; Kasim, V.; Wu, S. The biological implications of Yin Yang 1 in the hallmarks of cancer. Theranostics 2020, 4, 4183–4200. [Google Scholar] [CrossRef]
- Sarvagalla, S.; Kolapalli, S.P.; Vallabhapurapu, S. The Two Sides of YY1 in Cancer: A Friend and a Foe. Front. Oncol. 2019, 9, 1230. [Google Scholar] [CrossRef]
- Tang, W.; Zhou, W.; Xiang, L.; Wu, X.; Zhang, P.; Wang, J.J.J.J.; Liu, G.; Zhang, W.; Peng, Y.; Huang, X.; et al. The p300/YY1/miR-500a-5p/HDAC2 signalling axis regulates cell proliferation in human colorectal cancer. Nat. Commun. 2019, 10, 663. [Google Scholar] [CrossRef]
- Chinnappan, D.; Xiao, D.; Ratnasari, A.; Andry, C.; King, T.C.; Weber, H.C. Transcription factor YY1 expression in human gastrointestinal cancer cells. Int. J. Oncol. 2009, 34, 1417–1423. [Google Scholar] [PubMed]
- Sui, Y.; Wu, T.; Li, F.; Wang, F.; Cai, Y.; Jin, J. YY1/BCCIP Coordinately Regulates P53-Responsive Element (p53RE)-Mediated Transactivation of p21Waf1/Cip1. Int. J. Mol. Sci. 2019, 20, 2095. [Google Scholar] [CrossRef]
- Fang, Z.; Yang, H.; Chen, D.; Shi, X.; Wang, Q.; Gong, C.; Xu, X.; Liu, H.; Lin, M.; Lin, J.; et al. YY1 promotes colorectal cancer proliferation through the miR-526b-3p/E2F1 axis. Am. J. Cancer Res. 2019, 9, 2679–2692. [Google Scholar] [PubMed]
- Zhang, L.; Dong, X.; Yan, B.; Yu, W.; Shan, L. CircAGFG1 drives metastasis and stemness in colorectal cancer by modulating YY1/CTNNB1. Cell Death Dis. 2020, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Gu, B.; Wang, Y.; Shen, S.; Huang, W. YY1-Induced Upregulation of Long Noncoding RNA ARAP1-AS1 Promotes Cell Migration and Invasion in Colorectal Cancer Through the Wnt/β-Catenin Signaling Pathway. Cancer Biother. Radiopharm. 2019, 34, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Qian, M.; Lu, L.; Chen, Y.; Zhang, X.; Wu, Q.; Liu, Y.; Bian, Z.; Yang, Y.; Guo, S.; et al. O-GlcNAcylation of YY1 stimulates tumorigenesis in colorectal cancer cells by targeting SLC22A15 and AANAT. Carcinogenesis 2019, 40, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, F.; Zhang, J.; Li, J.; Chen, X.; Han, G. LINC00667/miR-449b-5p/YY1 axis promotes cell proliferation and migration in colorectal cancer. Cancer Cell Int. 2020, 20, 322. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, N.N.; Pate, K.T.; Sprowl, S.; Waterman, M.L. A role for YY1 in repression of dominant negative LEF-1 expression in colon cancer. Nucleic Acids Res. 2010, 38, 6375–6388. [Google Scholar] [CrossRef]
- Sui, Y.; Li, F.; Wu, T.; Ding, J.; Lu, Z.; Wang, L.; Yang, Y.; Wang, F.; Zhao, L.; Zhu, H.; et al. BCCIP binds to and activates its promoter in a YY1-dependent fashion in HCT116 cells. FEBS J. 2018, 285, 3026–3040. [Google Scholar] [CrossRef]
- Su, J.; Sui, Y.; Ding, J.; Li, F.; Shen, S.; Yang, Y.; Lu, Z.; Wang, F.; Cao, L.; Liu, X.; et al. Human INO80/YY1 chromatin remodeling complex transcriptionally regulates the BRCA2- and CDKN1A-interacting protein (BCCIP) in cells. Protein Cell 2016, 7, 749–760. [Google Scholar] [CrossRef] [PubMed]
- LIU, X.; CAO, L.; NI, J.; LIU, N.; ZHAO, X.; WANG, Y.; ZHU, L.; WANG, L.; WANG, J.; YUE, Y.; et al. Differential BCCIP gene expression in primary human ovarian cancer, renal cell carcinoma and colorectal cancer tissues. Int. J. Oncol. 2013, 43, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, S.; Falzone, L.; Ligresti, G.; Candido, S.; Garozzo, A.; Magro, G.G.; Bonavida, B.; Libra, M. Role of the Transcription Factor Yin Yang 1 and Its Selectively Identified Target Survivin in High-Grade B-Cells non-Hodgkin Lymphomas: Potential Diagnostic and Therapeutic Targets. Int. J. Mol. Sci. 2020, 21, 6446. [Google Scholar] [CrossRef] [PubMed]
- Schlicker, A.; Beran, G.; Chresta, C.M.; McWalter, G.; Pritchard, A.; Weston, S.; Runswick, S.; Davenport, S.; Heathcote, K.; Castro, D.A.; et al. Subtypes of primary colorectal tumors correlate with response to targeted treatment in colorectal cell lines. BMC Med. Genom. 2012, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Eide, P.W.; Eilertsen, I.A.; Danielsen, S.A.; Eknæs, M.; Hektoen, M.; Lind, G.E.; Lothe, R.A. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis 2013, 2, e71. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.C.G.; Eide, P.W.; Eilertsen, I.A.; Johannessen, B.; Bruun, J.; Danielsen, S.A.; Bjørnslett, M.; Meza-Zepeda, L.A.; Eknæs, M.; Lind, G.E.; et al. Multi-omics of 34 colorectal cancer cell lines—A resource for biomedical studies. Mol. Cancer 2017, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Mouradov, D.; Sloggett, C.; Jorissen, R.N.; Love, C.G.; Li, S.; Burgess, A.W.; Arango, D.; Strausberg, R.L.; Buchanan, D.; Wormald, S.; et al. Colorectal cancer cell lines are representative models of the main molecular subtypes of primary cancer. Cancer Res. 2014, 74, 3238–3247. [Google Scholar] [CrossRef]
- Ronen, J.; Hayat, S.; Akalin, A. Evaluation of colorectal cancer subtypes and cell lines using deep learning. Life Sci. Alliance 2019, 2. [Google Scholar] [CrossRef] [PubMed]
- De Nigris, F.; Zanella, L.; Cacciatore, F.; De Chiara, A.; Fazioli, F.; Chiappetta, G.; Apice, G.; Infante, T.; Monaco, M.; Rossiello, R.; et al. YY1 overexpression is associated with poor prognosis and metastasis-free survival in patients suffering osteosarcoma. BMC Cancer 2011, 11, 472. [Google Scholar] [CrossRef]
- Terreri, S.; Durso, M.; Colonna, V.; Romanelli, A.; Terracciano, D.; Ferro, M.; Perdonà, S.; Castaldo, L.; Febbraio, F.; de Nigris, F.; et al. New Cross-Talk Layer between Ultraconserved Non-Coding RNAs, MicroRNAs and Polycomb Protein YY1 in Bladder Cancer. Genes 2016, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Infante, T.; Mancini, F.P.; Lanza, A.; Soricelli, A.; de Nigris, F.; Napoli, C. Polycomb YY1 is a critical interface between epigenetic code and miRNA machinery after exposure to hypoxia in malignancy. Biochim. Biophys. Acta-Mol. Cell Res. 2015, 1853, 975–986. [Google Scholar] [CrossRef]
- Hafsi, S.; Candido, S.; Maestro, R.; Falzone, L.; Soua, Z.; Bonavida, B.; Spandidos, D.A.; Libra, M. Correlation between the overexpression of Yin Yang 1 and the expression levels of miRNAs in Burkitt’s lymphoma: A computational study. Oncol. Lett. 2016, 11, 1021–1025. [Google Scholar] [CrossRef]
- De Nigris, F.; Crudele, V.; Giovane, A.; Casamassimi, A.; Giordano, A.; Garban, H.J.; Cacciatore, F.; Pentimalli, F.; Marquez-Garban, D.C.; Petrillo, A.; et al. CXCR4/YY1 inhibition impairs VEGF network and angiogenesis during malignancy. Proc. Natl. Acad. Sci. USA 2010, 107, 14484–14489. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, C.E.; Dive, C.; Fletcher, D.J.; Barnes, F.A.; Lobo, A.; Bingle, C.D.; Whyte, M.K.B.; Renshaw, S.A. Expression of pro-apoptotic Bfk isoforms reduces during malignant transformation in the human gastrointestinal tract. FEBS Lett. 2005, 579, 3646–3650. [Google Scholar] [CrossRef] [PubMed]
- Coultas, L.; Pellegrini, M.; Visvader, J.E.; Lindeman, G.J.; Chen, L.; Adams, J.M.; Huang, D.C.S.; Strasser, A. Bfk: A novel weakly proapoptotic member of the Bcl-2 protein family with a BH3 and a BH2 region. Cell Death Differ. 2003, 10, 185–192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ozören, N.; Inohara, N.; Núñez, G. A putative role for human BFK in DNA damage-induced apoptosis. Biotechnol. J. 2009, 4, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, S.; Cheng, J.; Ivanov, K.I.; Zangger, N.; Ceteci, F.; Bernier-Latmani, J.; Milatos, S.; Joseph, J.-M.; Tercier, S.; Bouzourene, H.; et al. PROX1 promotes metabolic adaptation and fuels outgrowth of Wnt(high) metastatic colon cancer cells. Cell Rep. 2014, 8, 1957–1973. [Google Scholar] [CrossRef]
- Affar, E.B.; Gay, F.; Shi, Y.; Liu, H.; Huarte, M.; Wu, S.; Collins, T.; Li, E. Essential dosage-dependent functions of the transcription factor yin yang 1 in late embryonic development and cell cycle progression. Mol. Cell Biol. 2006, 26, 3565–3581. [Google Scholar] [CrossRef]
- Wilkinson, F.H.; Park, K.; Atchison, M.L. Polycomb recruitment to DNA in vivo by the YY1 REPO domain. Proc. Natl. Acad. Sci. USA 2006, 103, 19296–19301. [Google Scholar] [CrossRef]
- Satijn, D.P.E.; Hamer, K.M.; den Blaauwen, J.; Otte, A.P. The Polycomb Group Protein EED Interacts with YY1, and Both Proteins Induce Neural Tissue in XenopusEmbryos. Mol. Cell. Biol. 2001, 21, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Atchison, M.L. Function of YY1 in Long-Distance DNA Interactions. Front. Immunol 2014, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, A.S.; Li, C.H.; Zamudio, A.V.; Sigova, A.A.; Hannett, N.M.; Day, D.S.; Abraham, B.J.; Cohen, M.A.; Nabet, B.; Buckley, D.L.; et al. YY1 Is a Structural Regulator of Enhancer-Promoter Loops. Cell 2017, 171, 1573–1588.e28. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Srivillibhuthur, M.; Joshi, S.; Walton, K.D.; Zhou, A.; Faller, W.J.; Perekatt, A.O.; Sansom, O.J.; Gumucio, D.L.; Xing, J.; et al. A YY1-dependent increase in aerobic metabolism is indispensable for intestinal organogenesis. Development 2016, 143, 3711–3722. [Google Scholar] [CrossRef] [PubMed]
- Perekatt, A.O.; Valdez, M.J.; Davila, M.; Hoffman, A.; Bonder, E.M.; Gao, N.; Verzi, M.P. YY1 is indispensable for Lgr5+ intestinal stem cell renewal. Proc. Natl. Acad. Sci. USA 2014, 111, 7695–7700. [Google Scholar] [CrossRef]
- Kleiman, E.; Jia, H.; Loguercio, S.; Su, A.I.; Feeney, A.J. YY1 plays an essential role at all stages of B-cell differentiation. Proc. Natl. Acad. Sci. USA 2016, 113, E3911–E3920. [Google Scholar] [CrossRef]
- Trabucco, S.E.; Gerstein, R.M.; Zhang, H. YY1 Regulates the Germinal Center Reaction by Inhibiting Apoptosis. J. Immunol. 2016, 197, 1699–1707. [Google Scholar] [CrossRef]
- Onder, T.T.; Kara, N.; Cherry, A.; Sinha, A.U.; Zhu, N.; Bernt, K.M.; Cahan, P.; Marcarci, B.O.; Unternaehrer, J.; Gupta, P.B.; et al. Chromatin-modifying enzymes as modulators of reprogramming. Nature 2012, 483, 598–602. [Google Scholar] [CrossRef]
- Maestro, R.; Dei Tos, A.P.; Hamamori, Y.; Krasnokutsky, S.; Sartorelli, V.; Kedes, L.; Doglioni, C.; Beach, D.H.; Hannon, G.J. Twist is a potential oncogene that inhibits apoptosis. Genes Dev. 1999, 13, 2207–2217. [Google Scholar] [CrossRef]
- Seger, Y.R.; García-Cao, M.; Piccinin, S.; Cunsolo, C.L.; Doglioni, C.; Blasco, M.A.; Hannon, G.J.; Maestro, R. Transformation of normal human cells in the absence of telomerase activation. Cancer Cell 2002, 2, 401–413. [Google Scholar] [CrossRef]
- Hannon, G.J.; Sun, P.; Carnero, A.; Xie, L.Y.; Maestro, R.; Conklin, D.S.; Beach, D. MaRX: An approach to genetics in mammalian cells. Science 1999, 283, 1129–1130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Davis, C.A.; Hitz, B.C.; Sloan, C.A.; Chan, E.T.; Davidson, J.M.; Gabdank, I.; Hilton, J.A.; Jain, K.; Baymuradov, U.K.; Narayanan, A.K.; et al. The Encyclopedia of DNA elements (ENCODE): Data portal update. Nucleic Acids Res. 2018, 46, D794–D801. [Google Scholar] [CrossRef] [PubMed]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M.; Pringle, T.H.; Zahler, A.M.; Haussler, A.D. The Human Genome Browser at UCSC. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Clough, E.; Barrett, T. The Gene Expression Omnibus Database. In Statistical Genomics; Humana Press: New York, NY, USA, 2016; pp. 93–110. [Google Scholar]
- Ochsner, S.A.; Abraham, D.; Martin, K.; Ding, W.; McOwiti, A.; Kankanamge, W.; Wang, Z.; Andreano, K.; Hamilton, R.A.; Chen, Y.; et al. The Signaling Pathways Project, an integrated ‘omics knowledgebase for mammalian cellular signaling pathways. Sci. Data 2019, 6, 252. [Google Scholar] [CrossRef]
- Koster, J. R2: Genomics Analysis and Visualization Platform. Available online: http://r2.amc.nl (accessed on 24 September 2020).
- Lee, H.-O.; Hong, Y.; Etlioglu, H.E.; Cho, Y.B.; Pomella, V.; Van den Bosch, B.; Vanhecke, J.; Verbandt, S.; Hong, H.; Min, J.-W.; et al. Lineage-dependent gene expression programs influence the immune landscape of colorectal cancer. Nat. Genet. 2020, 52, 594–603. [Google Scholar] [CrossRef] [PubMed]
- EMBL-EBI Single Cell Expression Atlas. Available online: https://www.ebi.ac.uk/gxa/sc/home (accessed on 24 September 2020).
- KOBIC User-Friendly InteRface Tool to Explore Cell Atlas (URECA). Available online: http://ureca-singlecell.kr/ (accessed on 24 September 2020).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivarelli, S.; Falzone, L.; Candido, S.; Bonavida, B.; Libra, M. YY1 Silencing Induces 5-Fluorouracil-Resistance and BCL2L15 Downregulation in Colorectal Cancer Cells: Diagnostic and Prognostic Relevance. Int. J. Mol. Sci. 2021, 22, 8481. https://doi.org/10.3390/ijms22168481
Vivarelli S, Falzone L, Candido S, Bonavida B, Libra M. YY1 Silencing Induces 5-Fluorouracil-Resistance and BCL2L15 Downregulation in Colorectal Cancer Cells: Diagnostic and Prognostic Relevance. International Journal of Molecular Sciences. 2021; 22(16):8481. https://doi.org/10.3390/ijms22168481
Chicago/Turabian StyleVivarelli, Silvia, Luca Falzone, Saverio Candido, Benjamin Bonavida, and Massimo Libra. 2021. "YY1 Silencing Induces 5-Fluorouracil-Resistance and BCL2L15 Downregulation in Colorectal Cancer Cells: Diagnostic and Prognostic Relevance" International Journal of Molecular Sciences 22, no. 16: 8481. https://doi.org/10.3390/ijms22168481
APA StyleVivarelli, S., Falzone, L., Candido, S., Bonavida, B., & Libra, M. (2021). YY1 Silencing Induces 5-Fluorouracil-Resistance and BCL2L15 Downregulation in Colorectal Cancer Cells: Diagnostic and Prognostic Relevance. International Journal of Molecular Sciences, 22(16), 8481. https://doi.org/10.3390/ijms22168481









