Dendritic Cells and CCR7 Expression: An Important Factor for Autoimmune Diseases, Chronic Inflammation, and Cancer
Abstract
:1. Introduction
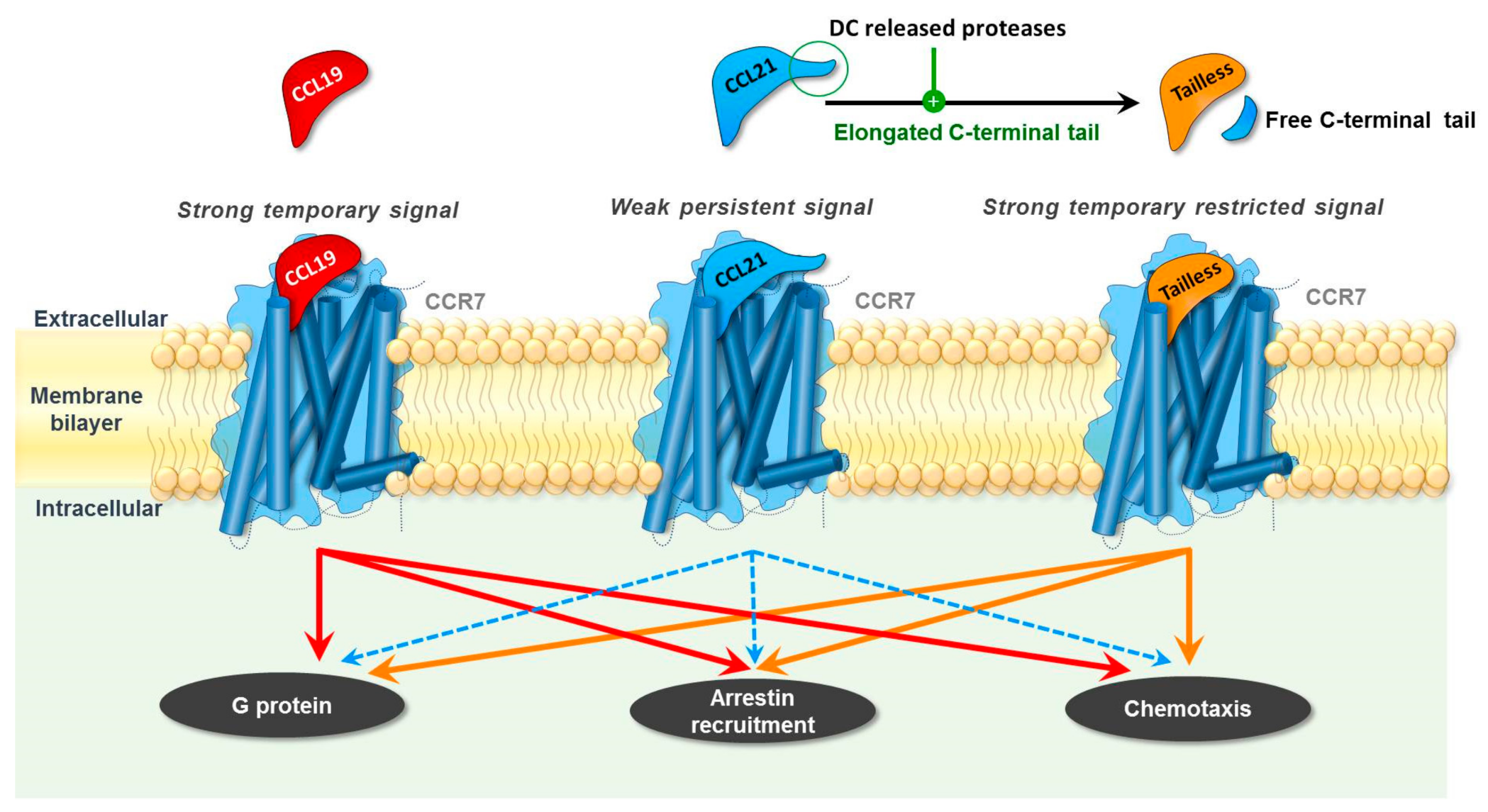
2. Differential Roles of CCR7 Ligands in DC Mobilization
3. DC Maturation and Tissue Egress
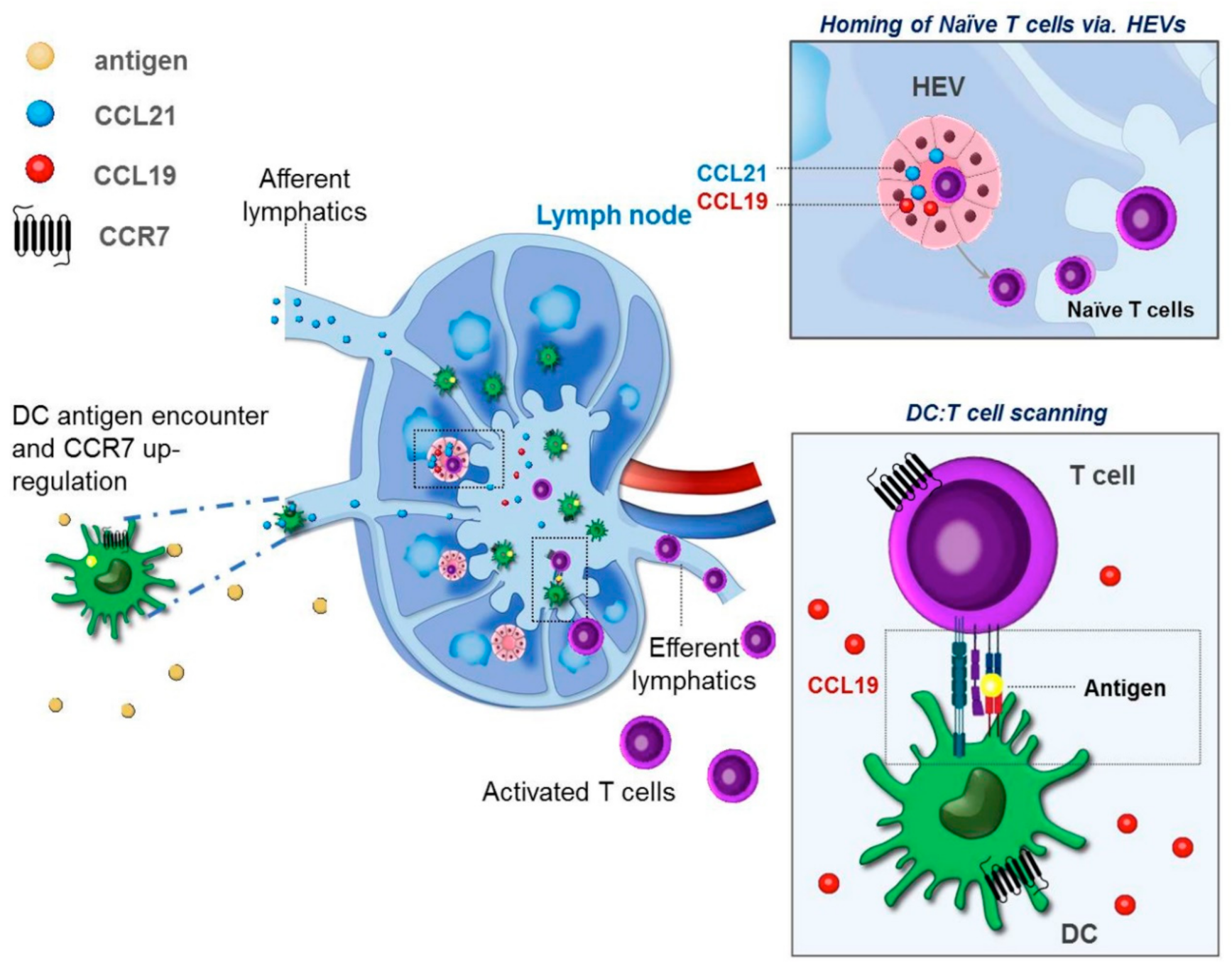
4. CCR7 and Induction of Organ Associated Lymphoid Tissues (ALT)
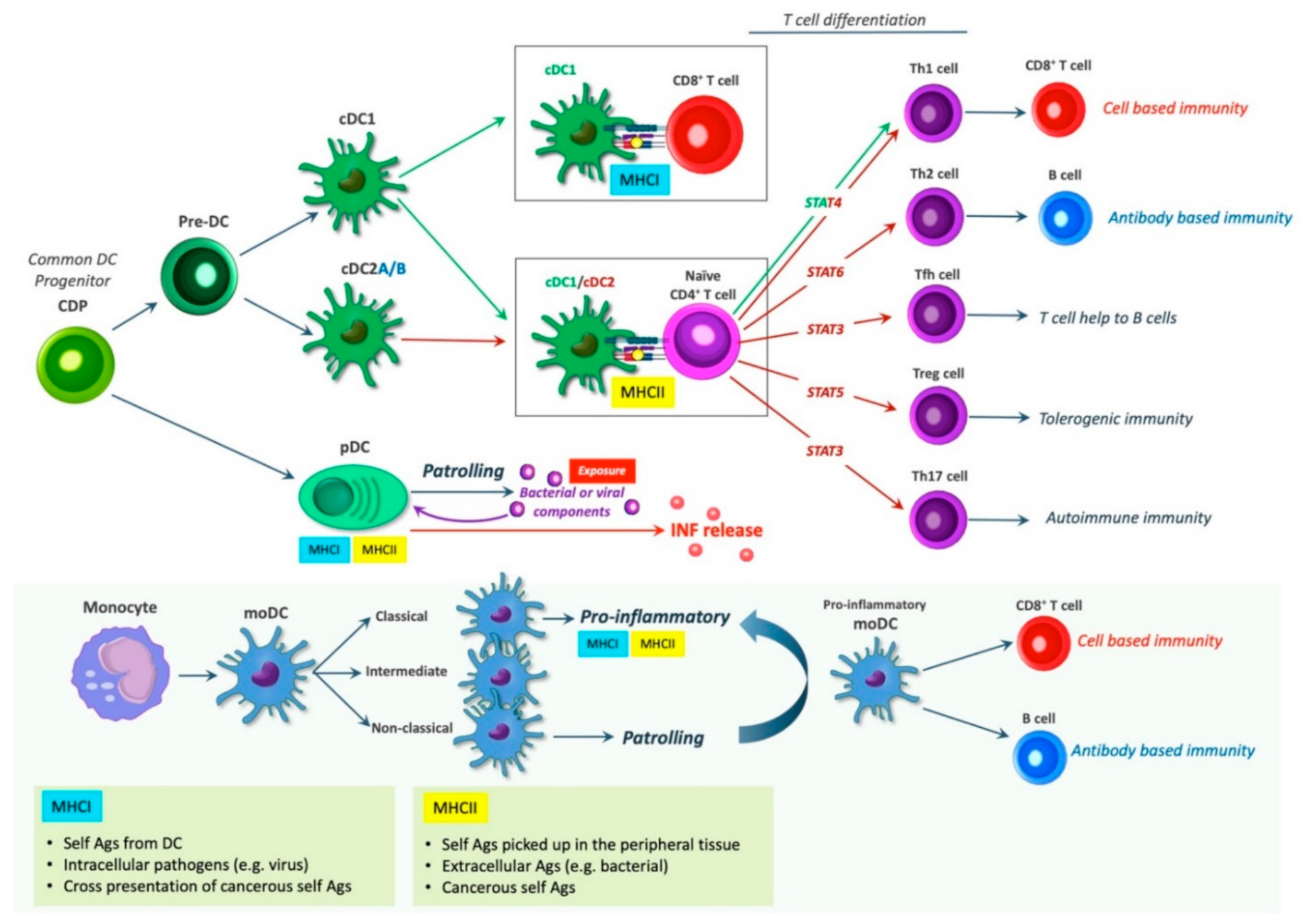
5. DC Subsets and Their Immunological Roles
6. Dendritic Cell Induced T Cell Differentiation
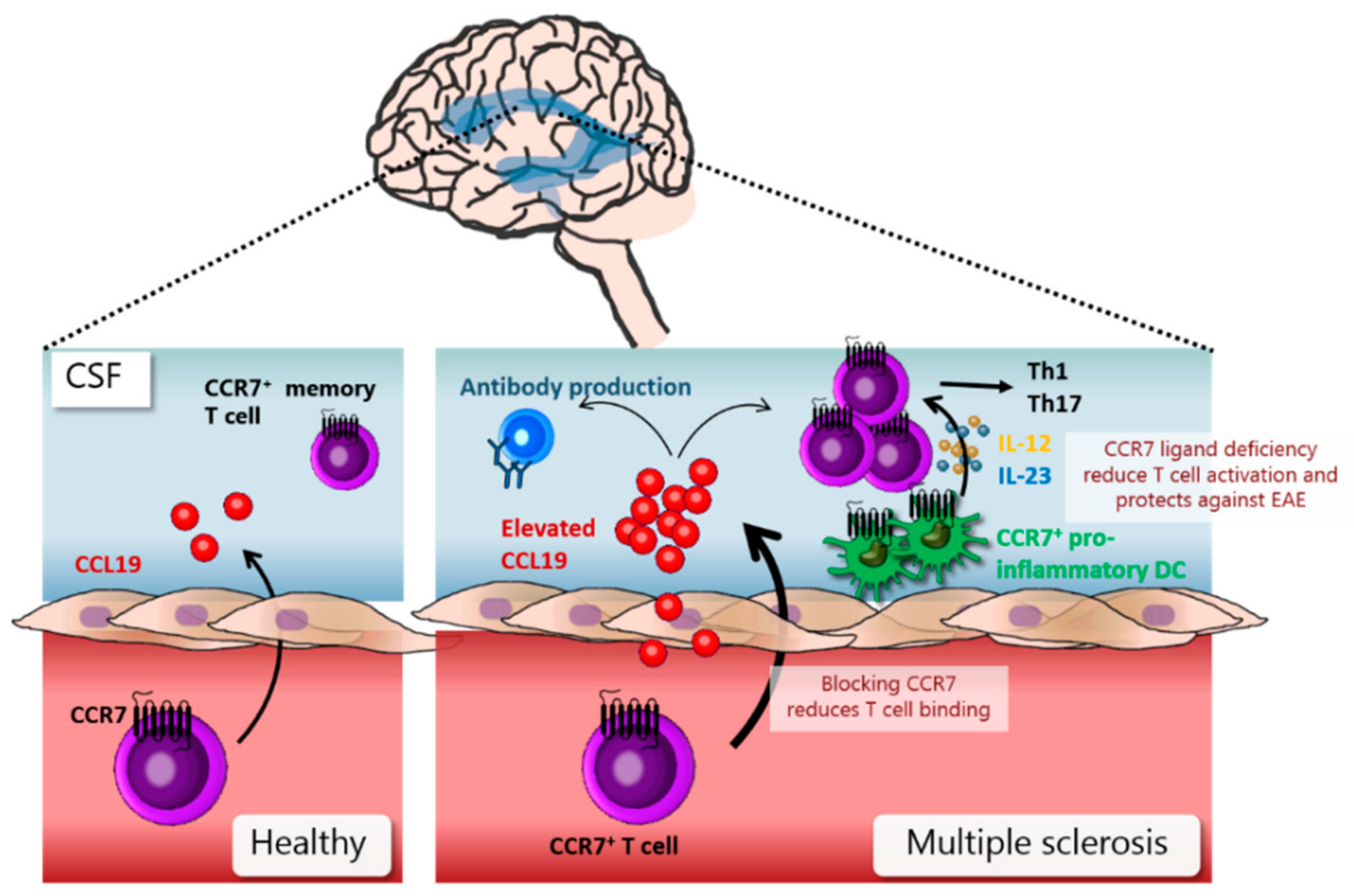
7. DCs in Pathology of Multiple Sclerosis
8. DCs in Initiation and Sustainment of Rheumatoid Arthritis
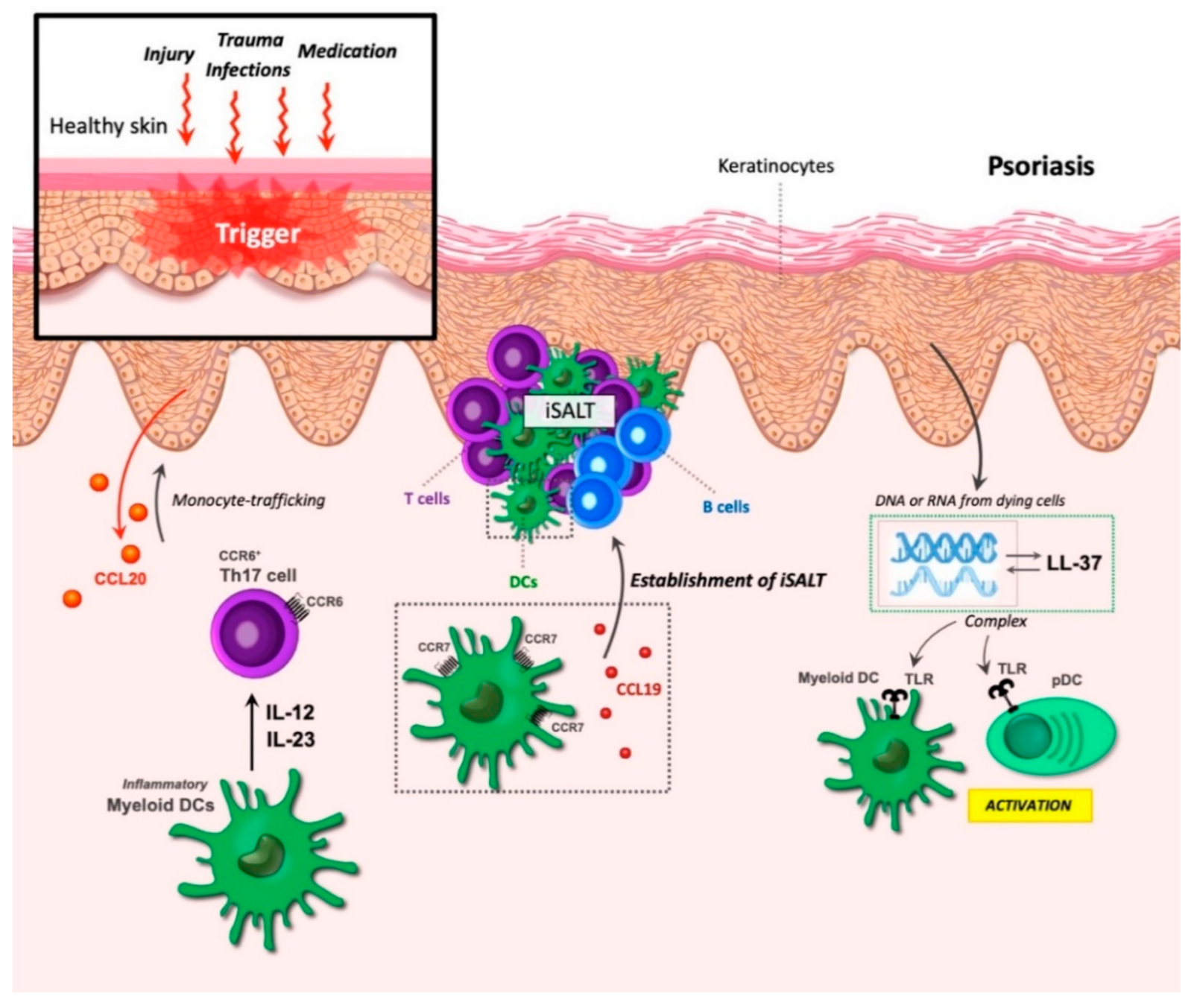
9. DCs in Chronic Inflammation of Psoriasis
10. The Binary Role of CCR7 in Combatting and Progressing Cancer
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Steen, A.; Larsen, O.; Thiele, S.; Rosenkilde, M.M. Biased and G Protein-Independent Signaling of Chemokine Receptors. Front. Immunol. 2014, 5, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and Chemokine Receptors: Positioning Cells for Host Defense and Immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef] [Green Version]
- Förster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef]
- Moschovakis, G.L.; Bubke, A.; Friedrichsen, M.; Ristenpart, J.; Back, J.W.; Falk, C.S.; Kremmer, E.; Förster, R. The chemokine receptor CCR7 is a promising target for rheumatoid arthritis therapy. Cell. Mol. Immunol. 2019, 16, 791–799. [Google Scholar] [CrossRef]
- Belikan, P.; Bühler, U.; Wolf, C.; Pramanik, G.K.; Gollan, R.; Zipp, F.; Siffrin, V. CCR7 on CD4+ T Cells Plays a Crucial Role in the Induction of Experimental Autoimmune Encephalomyelitis. J. Immunol. 2018, 200, 2554–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sozzani, S.; Del Prete, A.; Bosisio, D. Dendritic cell recruitment and activation in autoimmunity. J. Autoimmun. 2017, 85, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Förster, R.; Schubel, A.; Breitfeld, D.; Kremmer, E.; Renner-Müller, I.; Wolf, E.; Lipp, M. CCR7 Coordinates the Primary Immune Response by Establishing Functional Microenvironments in Secondary Lymphoid Organs. Cell 1999, 99, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Ohl, L.; Mohaupt, M.; Czeloth, N.; Hintzen, G.; Kiafard, Z.; Zwirner, J.; Blankenstein, T.; Henning, G.; Förster, R. CCR7 Governs Skin Dendritic Cell Migration under Inflammatory and Steady-State Conditions. Immunity 2004, 21, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Schumann, K.; Lämmermann, T.; Bruckner, M.; Legler, D.F.; Polleux, J.; Spatz, J.P.; Schuler, G.; Forster, R.; Lutz, M.B.; Sorokin, L.; et al. Immobilized Chemokine Fields and Soluble Chemokine Gradients Cooperatively Shape Migration Patterns of Dendritic Cells. Immunity 2010, 32, 703–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luther, S.; Tang, H.L.; Hyman, P.L.; Farr, A.G.; Cyster, J.G. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl. Acad. Sci. USA 2000, 97, 12694–12699. [Google Scholar] [CrossRef] [Green Version]
- Martín-Fontecha, A.; Sebastiani, S.; Höpken, U.E.; Uguccioni, M.; Lipp, M.; Lanzavecchia, A.; Sallusto, F. Regulation of Dendritic Cell Migration to the Draining Lymph Node. J. Exp. Med. 2003, 198, 615–621. [Google Scholar] [CrossRef]
- Sallusto, F.; Palermo, B.; Lenig, D.; Miettinen, M.; Matikainen, S.; Julkunen, I.; Forster, R.; Burgstahler, R.; Lipp, M.; Lanzavecchia, A. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur. J. Immunol. 1999, 29, 1617–1625. [Google Scholar] [CrossRef]
- Jørgensen, A.S.; Adogamhe, P.E.; Laufer, J.M.; Legler, D.F.; Veldkamp, C.T.; Rosenkilde, M.M.; Hjortø, G.M. CCL19 with CCL21-tail displays enhanced glycosaminoglycan binding with retained chemotactic potency in dendritic cells. J. Leukoc. Biol. 2018, 104, 401–411. [Google Scholar] [CrossRef] [Green Version]
- Moussouras, N.A.; Hjortø, G.M.; Peterson, F.C.; Szpakowska, M.; Chevigné, A.; Rosenkilde, M.M.; Volkman, B.F.; Dwinell, M.B. Structural Features of an Extended C-Terminal Tail Modulate the Function of the Chemokine CCL21. Biochemistry 2020, 59, 1338–1350. [Google Scholar] [CrossRef]
- Kiermaier, E.; Moussion, C.; Veldkamp, C.T.; Gerardy-Schahn, R.; De Vries, I.; Williams, L.G.; Chaffee, G.R.; Phillips, A.J.; Freiberger, F.; Imre, R.; et al. Polysialylation controls dendritic cell trafficking by regulating chemokine recognition. Science 2016, 351, 186–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohout, T.A.; Nicholas, S.L.; Perry, S.J.; Reinhart, G.; Junger, S.; Struthers, R.S. Differential Desensitization, Receptor Phosphorylation, β-Arrestin Recruitment, and ERK1/2 Activation by the Two Endogenous Ligands for the CC Chemokine Receptor 7. J. Biol. Chem. 2004, 279, 23214–23222. [Google Scholar] [CrossRef] [Green Version]
- Zidar, D.A.; Violin, J.D.; Whalen, E.J.; Lefkowitz, R.J. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc. Natl. Acad. Sci. USA 2009, 106, 9649–9654. [Google Scholar] [CrossRef] [Green Version]
- Bardi, G.; Lipp, M.; Baggiolini, M.; Loetscher, P. The T cell chemokine receptor CCR7 is internalized on stimulation with ELC, but not with SLC. Eur. J. Immunol. 2001, 31, 3291–3297. [Google Scholar] [CrossRef]
- Hjortø, G.M.; Larsen, O.; Steen, A.; Daugvilaite, V.; Berg, C.; Fares, S.; Hansen, M.; Ali, S.; Rosenkilde, M.M. Differential CCR7 Targeting in Dendritic Cells by Three Naturally Occurring CC-Chemokines. Front. Immunol. 2016, 7, 568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricart, B.G.; John, B.; Lee, O.; Hunter, C.A.; Hammer, D.A. Dendritic Cells Distinguish Individual Chemokine Signals through CCR7 and CXCR4. J. Immunol. 2011, 186, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulvmar, M.H.; Werth, K.; Braun, A.; Kelay, P.; Hub, E.; Eller, K.; Chan, L.; Lucas, B.; Novitzky-Basso, I.; Nakamura, K.; et al. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat. Immunol. 2014, 15, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Bastow, C.R.; Bunting, M.D.; Kara, E.E.; McKenzie, D.R.; Caon, A.; Devi, S.; Tolley, L.; Mueller, S.N.; Frazer, I.H.; Harvey, N.; et al. Scavenging of soluble and immobilized CCL21 by ACKR4 regulates peripheral dendritic cell emigration. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Britschgi, M.R.; Favre, S.; Luther, S.A. CCL21 is sufficient to mediate DC migration, maturation and function in the absence of CCL19. Eur. J. Immunol. 2010, 40, 1266–1271. [Google Scholar] [CrossRef]
- Kaiser, A.; Donnadieu, E.; Abastado, J.-P.; Trautmann, A.; Nardin, A. CC Chemokine Ligand 19 Secreted by Mature Dendritic Cells Increases Naive T Cell Scanning Behavior and Their Response to Rare Cognate Antigen. J. Immunol. 2005, 175, 2349–2356. [Google Scholar] [CrossRef] [Green Version]
- Goulopoulou, S.; McCarthy, C.G.; Webb, R.C. Toll-like Receptors in the Vascular System: Sensing the Dangers Within. Pharmacol. Rev. 2016, 68, 142–167. [Google Scholar] [CrossRef]
- Figdor, C.G.; Van Kooyk, Y.; Adema, G.J. C-type lectin receptors on dendritic cells and langerhans cells. Nat. Rev. Immunol. 2002, 2, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, H.; Akira, S. TLR Signalling and the Function of Dendritic Cells. Chem. Immunol. Allergy 2005, 86, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Tschopp, J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005, 26, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Cella, M.; Danieli, C.; Lanzavecchia, A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: Downregulation by cytokines and bacterial products. J. Exp. Med. 1995, 182, 389–400. [Google Scholar] [CrossRef] [Green Version]
- Zanoni, I.; Granucci, F. Regulation of antigen uptake, migration, and lifespan of dendritic cell by Toll-like receptors. J. Mol. Med. 2010, 88, 873–880. [Google Scholar] [CrossRef]
- Drutman, S.B.; Trombetta, E.S. Dendritic Cells Continue To Capture and Present Antigens after Maturation In Vivo. J. Immunol. 2010, 185, 2140–2146. [Google Scholar] [CrossRef] [Green Version]
- Wilson, N.S.; El-Sukkari, D.; Villadangos, J.A. Dendritic cells constitutively present self antigens in their immature state in vivo and regulate antigen presentation by controlling the rates of MHC class II synthesis and endocytosis. Blood 2004, 103, 2187–2195. [Google Scholar] [CrossRef]
- Wehr, P.; Purvis, H.; Law, S.-C.; Thomas, R. Dendritic cells, T cells and their interaction in rheumatoid arthritis. Clin. Exp. Immunol. 2019, 196, 12–27. [Google Scholar] [CrossRef] [Green Version]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Everts, B.; Amiel, E.; Huang, S.C.-C.; Smith, A.M.; Chang, C.-H.; Lam, W.Y.; Redmann, V.; Freitas, T.C.; Blagih, J.; Van Der Windt, G.J.W.; et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation. Nat. Immunol. 2014, 15, 323–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krawczyk, C.M.; Holowka, T.; Sun, J.; Blagih, J.; Amiel, E.; DeBerardinis, R.J.; Cross, J.; Jung, E.; Thompson, C.B.; Jones, R.G.; et al. Toll-like receptor–induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 2010, 115, 4742–4749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guak, H.; Al Habyan, S.; Ma, E.H.; Aldossary, H.; Al-Masri, M.; Won, S.Y.; Ying, T.; Fixman, E.D.; Jones, R.G.; McCaffrey, L.M.; et al. Glycolytic metabolism is essential for CCR7 oligomerization and dendritic cell migration. Nat. Commun. 2018, 9, 2463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canavan, M.; Marzaioli, V.; McGarry, T.; Bhargava, V.; Nagpal, S.; Veale, D.J.; Fearon, U. Rheumatoid arthritis synovial microenvironment induces metabolic and functional adaptations in dendritic cells. Clin. Exp. Immunol. 2020, 202, 226–238. [Google Scholar] [CrossRef]
- Hauser, M.A.; Schaeuble, K.; Kindinger, I.; Impellizzieri, D.; Krueger, W.A.; Hauck, C.R.; Boyman, O.; Legler, D.F. Inflammation-Induced CCR7 Oligomers Form Scaffolds to Integrate Distinct Signaling Pathways for Efficient Cell Migration. Immunity 2016, 44, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Dieu, M.-C.; Vanbervliet, B.; Vicari, A.; Bridon, J.-M.; Oldham, E.; Aït-Yahia, S.; Brière, F.; Zlotnik, A.; Lebecque, S.; Caux, C. Selective Recruitment of Immature and Mature Dendritic Cells by Distinct Chemokines Expressed in Different Anatomic Sites. J. Exp. Med. 1998, 188, 373–386. [Google Scholar] [CrossRef] [Green Version]
- De Vries, I.J.M.; Krooshoop, D.J.E.B.; Scharenborg, N.M.; Lesterhuis, W.J.; Diepstra, J.H.S.; Van Muijen, G.N.P.; Strijk, S.P.; Ruers, T.J.; Boerman, O.C.; Oyen, W.J.G.; et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003, 63, 12–17. [Google Scholar]
- Lämmermann, T.; Bader, B.L.; Monkley, S.J.; Worbs, T.; Wedlich-Söldner, R.; Hirsch, K.; Keller, M.; Forster, R.; Critchley, D.R.; Fässler, R.; et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 2008, 453, 51–55. [Google Scholar] [CrossRef]
- Weber, M.; Hauschild, R.; Schwarz, J.; Moussion, C.; de Vries, I.; Legler, D.F.; Luther, S.A.; Bollenbach, T.; Sixt, M. Interstitial Dendritic Cell Guidance by Haptotactic Chemokine Gradients. Science 2013, 339, 328–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Li, X.; Zhuang, J.; Han, J.; Luo, G.; Yang, F.; Sun, Y.; Liao, P.; Han, Y.; He, Y.; et al. Blocking Matrix Metalloproteinase-9 Abrogates Collagen-Induced Arthritis via Inhibiting Dendritic Cell Migration. J. Immunol. 2018, 201, 3514–3523. [Google Scholar] [CrossRef] [Green Version]
- Sennikov, S.V.; Falaleeva, S.A.; Shkaruba, N.S.; Chumasova, O.A.; Obleukhova, I.A.; Sizikov, A.E.; Kurilin, V.V. Maturation and cytokine production potential of dendritic cells isolated from rheumatoid arthritis patients peripheral blood and induced in vitro. Hum. Immunol. 2016, 77, 930–936. [Google Scholar] [CrossRef]
- Lorenz, N.; Loef, E.J.; Kelch, I.D.; Verdon, D.J.; Black, M.M.; Middleditch, M.J.; Greenwood, D.; Graham, S.; Brooks, A.E.; Dunbar, P.R.; et al. Plasmin and regulators of plasmin activity control the migratory capacity and adhesion of human T cells and dendritic cells by regulating cleavage of the chemokine CCL21. Immunol. Cell Biol. 2016, 94, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Ansel, K.M.; Ngo, V.; Hyman, P.L.; Luther, S.A.; Forster, R.; Sedgwick, J.D.; Browning, J.; Lipp, M.; Cyster, J.G. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 2000, 406, 309–314. [Google Scholar] [CrossRef]
- Luther, S.; Ansel, K.M.; Cyster, J.G. Overlapping Roles of CXCL13, Interleukin 7 Receptor α, and CCR7 Ligands in Lymph Node Development. J. Exp. Med. 2003, 197, 1191–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohl, L.; Henning, G.; Krautwald, S.; Lipp, M.; Hardtke, S.; Bernhardt, G.; Pabst, O.; Förster, R. Cooperating Mechanisms of CXCR5 and CCR7 in Development and Organization of Secondary Lymphoid Organs. J. Exp. Med. 2003, 197, 1199–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, J.V.; Nombela-Arrieta, C. Chemokine control of lymphocyte trafficking: A general overview. Immunology 2005, 116, 1–12. [Google Scholar] [CrossRef]
- Ruddle, N.H. Basics of Inducible Lymphoid Organs. Curr. Top. Microbiol. Immunol. 2020, 426, 1–19. [Google Scholar]
- Dejardin, E.; Droin, N.M.; Delhase, M.; Haas, E.; Cao, Y.; Makris, C.; Li, Z.-W.; Karin, M.; Ware, C.F.; Green, D.R. The Lymphotoxin-β Receptor Induces Different Patterns of Gene Expression via Two NF-κB Pathways. Immunity 2002, 17, 525–535. [Google Scholar] [CrossRef] [Green Version]
- Hjelmstrom, P.; Fjell, J.; Nakagawa, T.; Sacca, R.; Cuff, C.A.; Ruddle, N.H. Lymphoid Tissue Homing Chemokines Are Expressed in Chronic Inflammation. Am. J. Pathol. 2000, 156, 1133–1138. [Google Scholar] [CrossRef] [Green Version]
- Luther, S.A.; Bidgol, A.; Hargreaves, D.C.; Schmidt, A.; Xu, Y.; Paniyadi, J.; Matloubian, M.; Cyster, J.G. Differing Activities of Homeostatic Chemokines CCL19, CCL21, and CXCL12 in Lymphocyte and Dendritic Cell Recruitment and Lymphoid Neogenesis. J. Immunol. 2002, 169, 424–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonel, G.; Conrad, C.; Laggner, U.; Di Meglio, P.; Grys, K.; McClanahan, T.K.; Blumenschein, W.M.; Qin, J.-Z.; Xin, H.; Oldham, E.; et al. Cutting Edge: A Critical Functional Role for IL-23 in Psoriasis. J. Immunol. 2010, 185, 5688–5691. [Google Scholar] [CrossRef] [Green Version]
- Kerjaschki, D.; Regele, H.M.; Moosberger, I.; Nagy-Bojarski, K.; Watschinger, B.; Soleiman, A.; Birner, P.; Krieger, S.; Hovorka, A.; Silberhumer, G.; et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J. Am. Soc. Nephrol. 2004, 15, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Stranford, S.P.; Ruddle, N.H.P. Follicular dendritic cells, conduits, lymphatic vessels, and high endothelial venules in tertiary lymphoid organs: Parallels with lymph node stroma. Front. Immunol. 2012, 3, 350. [Google Scholar] [CrossRef] [Green Version]
- Amft, N.; Curnow, S.J.; Scheel-Toellner, D.; Devadas, A.; Oates, J.; Crocker, J.; Hamburger, J.; Ainsworth, J.; Mathews, J.; Salmon, M.; et al. Ectopic expression of the B cell–attracting chemokine BCA-1 (CXCL13) on endothelial cells and within lymphoid follicles contributes to the establishment of germinal center–like structures in Sjögren’s syndrome. Arthritis Rheum. 2001, 44, 2633–2641. [Google Scholar] [CrossRef]
- Furtado, G.C.; Marinkovic, T.; Martin, A.P.; Garin, A.; Hoch, B.; Hübner, W.; Chen, B.K.; Genden, E.; Skobe, M.; Lira, S.A. Lymphotoxin beta receptor signaling is required for inflammatory lymphangiogenesis in the thyroid. Proc. Natl. Acad. Sci. USA 2007, 104, 5026–5031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivellese, F.; Pontarini, E.; Pitzalis, C. Tertiary Lymphoid Organs in Rheumatoid Arthritis. Curr. Top. Microbiol. Immunol. 2020, 426, 119–141. [Google Scholar] [CrossRef]
- Takemura, S.; Braun, A.; Crowson, C.S.; Kurtin, P.J.; Cofield, R.H.; O’Fallon, W.M.; Goronzy, J.J.; Weyand, C.M. Lymphoid Neogenesis in Rheumatoid Synovitis. J. Immunol. 2001, 167, 1072–1080. [Google Scholar] [CrossRef] [Green Version]
- Barone, F.; Gardner, D.H.; Nayar, S.; Steinthal, N.; Buckley, C.D.; Luther, S.A. Stromal Fibroblasts in Tertiary Lymphoid Structures: A Novel Target in Chronic Inflammation. Front. Immunol. 2016, 7, 477. [Google Scholar] [CrossRef] [PubMed]
- Halle, S.; Dujardin, H.C.; Bakocevic, N.; Fleige, H.; Danzer, H.; Willenzon, S.; Suezer, Y.; Hämmerling, G.; Garbi, N.; Sutter, G.; et al. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J. Exp. Med. 2009, 206, 2593–2601. [Google Scholar] [CrossRef] [PubMed]
- GeurtsvanKessel, C.H.; Willart, M.A.M.; Bergen, I.M.; Van Rijt, L.S.; Muskens, F.; Elewaut, D.; Osterhaus, A.; Hendriks, R.; Rimmelzwaan, G.F.; Lambrecht, B.N. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus–infected mice. J. Exp. Med. 2009, 206, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef]
- Villani, A.C.; Satija, R.; Reynolds, G.; Sarkizova, S.; Shekhar, K.; Fletcher, J.; Griesbeck, M.; Butler, A.; Zheng, S.; Lazo, S.; et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017, 356, eaah4573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- See, P.; Dutertre, C.-A.; Chen, J.; Günther, P.; McGovern, N.; Irac, S.E.; Gunawan, M.; Beyer, M.; Händler, K.; Duan, K.; et al. Mapping the human DC lineage through the integration of high-dimensional techniques. Science 2017, 356, eaag3009. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.C.; Gudjonson, H.; Pritykin, Y.; Deep, D.; Lavallée, V.-P.; Mendoza, A.; Fromme, R.; Mazutis, L.; Ariyan, C.; Leslie, C.; et al. Transcriptional Basis of Mouse and Human Dendritic Cell Heterogeneity. Cell 2019, 179, 846–863.e24. [Google Scholar] [CrossRef] [Green Version]
- Rojas, I.M.L.; Mok, W.-H.; Pearson, F.E.; Minoda, Y.; Kenna, T.J.; Barnard, R.T.; Radford, K.J. Human Blood CD1c+ Dendritic Cells Promote Th1 and Th17 Effector Function in Memory CD4+ T Cells. Front. Immunol. 2017, 8, 971. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Zhou, Y.J.; Ma, W.; Zhang, W.; Aljoufi, A.; Luh, T.; Lucero, K.; Liang, D.; Thomsen, M.; Bhagat, G.; et al. Lineage specification of human dendritic cells is marked by IRF8 expression in hematopoietic stem cells and multipotent progenitors. Nat. Immunol. 2017, 18, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Scalavino, V.; Liso, M.; Serino, G. Role of microRNAs in the Regulation of Dendritic Cell Generation and Function. Int. J. Mol. Sci. 2020, 21, 1319. [Google Scholar] [CrossRef] [Green Version]
- Naik, S.H.; Sathe, P.; Park, H.-Y.; Metcalf, D.; Proietto, A.I.; Dakic, A.; Carotta, S.; O’Keeffe, M.; Bahlo, M.; Papenfuss, A.; et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat. Immunol. 2007, 8, 1217–1226. [Google Scholar] [CrossRef]
- Onai, N.; Obata-Onai, A.; Schmid, M.A.; Ohteki, T.; Jarrossay, D.; Manz, M.G. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat. Immunol. 2007, 8, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Onai, N.; Kurabayashi, K.; Hosoi-Amaike, M.; Toyama-Sorimachi, N.; Matsushima, K.; Inaba, K.; Ohteki, T. A Clonogenic Progenitor with Prominent Plasmacytoid Dendritic Cell Developmental Potential. Immunity 2013, 38, 943–957. [Google Scholar] [CrossRef] [Green Version]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Dalod, M.; Chelbi, R.; Malissen, B.; Lawrence, T. Dendritic cell maturation: Functional specialization through signaling specificity and transcriptional programming. EMBO J. 2014, 33, 1104–1116. [Google Scholar] [CrossRef]
- Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of Monocytes, Macrophages, and Dendritic Cells. Science 2010, 327, 656–661. [Google Scholar] [CrossRef] [Green Version]
- Weissman, I.L.; Shizuru, J.A. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood 2008, 112, 3543–3553. [Google Scholar] [CrossRef] [Green Version]
- Roberts, E.W.; Broz, M.L.; Binnewies, M.; Headley, M.; Nelson, A.E.; Wolf, D.M.; Kaisho, T.; Bogunovic, D.; Bhardwaj, N.; Krummel, M.F. Critical Role for CD103+/CD141+ Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell 2016, 30, 324–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukowski, S.W.; Rødahl, I.; Kelly, S.; Yu, M.; Gotley, J.; Zhou, C.; Millard, S.; Andersen, S.B.; Christ, A.N.; Belz, G.; et al. Absence of Batf3 reveals a new dimension of cell state heterogeneity within conventional dendritic cells. iScience 2021, 24, 102402. [Google Scholar] [CrossRef]
- Kumar, S.; Jeong, Y.; Ashraf, M.U.; Bae, Y.-S. Dendritic Cell-Mediated Th2 Immunity and Immune Disorders. Int. J. Mol. Sci. 2019, 20, 2159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenbarth, S.C. Dendritic cell subsets in T cell programming: Location dictates function. Nat. Rev. Immunol. 2019, 19, 89–103. [Google Scholar] [CrossRef]
- Min, J.; Yang, D.; Kim, M.; Haam, K.; Yoo, A.; Choi, J.-H.; Schraml, B.; Kim, Y.S.; Kim, D.; Kang, S.-J. Inflammation induces two types of inflammatory dendritic cells in inflamed lymph nodes. Exp. Mol. Med. 2018, 50, e458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.; Liu, Y.-J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Kratofil, R.M.; Kubes, P.; Deniset, J.F. Monocyte Conversion during Inflammation and Injury. Arter. Thromb. Vasc. Biol. 2017, 37, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Solano-Gálvez, S.G.; Tovar-Torres, S.M.; Tron-Gómez, M.S.; Weiser-Smeke, A.E.; Álvarez-Hernández, D.A.; Franyuti-Kelly, G.A.; Tapia-Moreno, M.; Ibarra, A.; Gutiérrez-Kobeh, L.; Vázquez-López, R. Human Dendritic Cells: Ontogeny and Their Subsets in Health and Disease. Med. Sci. 2018, 6, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarif, J.C.; Hernandez, J.R.; Verdone, J.E.; Campbell, S.P.; Drake, C.G.; Pienta, K.J. A phased strategy to differentiate human CD14+ monocytes into classically and alternatively activated macrophages and dendritic cells. Biotechniques 2016, 61, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Holla, S.; Sharma, M.; Vani, J.; Kaveri, S.V.; Balaji, K.N.; Bayry, J. GM-CSF along with IL-4 but not alone is indispensable for the differentiation of human dendritic cells from monocytes. J. Allergy Clin. Immunol. 2014, 133, 1500–1502.e1. [Google Scholar] [CrossRef]
- Jegalian, A.G.; Facchetti, F.; Jaffe, E.S. Plasmacytoid Dendritic Cells. Adv. Anat. Pathol. 2009, 16, 392–404. [Google Scholar] [CrossRef]
- Alcántara-Hernández, M.; Leylek, R.; Wagar, L.E.; Engleman, E.G.; Keler, T.; Marinkovich, M.P.; Davis, M.M.; Nolan, G.P.; Idoyaga, J. High-Dimensional Phenotypic Mapping of Human Dendritic Cells Reveals Interindividual Variation and Tissue Specialization. Immunity 2017, 47, 1037–1050.e6. [Google Scholar] [CrossRef] [Green Version]
- Leylek, R.; Alcántara-Hernández, M.; Lanzar, Z.; Lüdtke, A.; Perez, O.A.; Reizis, B.; Idoyaga, J. Integrated Cross-Species Analysis Identifies a Conserved Transitional Dendritic Cell Population. Cell Rep. 2019, 29, 3736–3750.e8. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Manzotti, C.N.; Liu, M.; Burke, F.; Mead, K.I.; Sansom, D.M. CD86 and CD80 Differentially Modulate the Suppressive Function of Human Regulatory T Cells. J. Immunol. 2004, 172, 2778–2784. [Google Scholar] [CrossRef]
- e Sousa, C.R. Dendritic cells in a mature age. Nat. Rev. Immunol. 2006, 6, 476–483. [Google Scholar] [CrossRef]
- Yamane, H.; Paul, W.E. Early signaling events that underlie fate decisions of naive CD4+ T cells toward distinct T-helper cell subsets. Immunol. Rev. 2013, 252, 12–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of Effector CD4 T Cell Populations. Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyerich, S.; Eyerich, K.; Pennino, D.; Carbone, T.; Nasorri, F.; Pallotta, S.; Cianfarani, F.; Odorisio, T.; Traidl-Hoffmann, C.; Behrendt, H.; et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Investig. 2009, 119, 3573–3585. [Google Scholar] [CrossRef] [Green Version]
- Elyaman, W.; Bradshaw, E.M.; Uyttenhove, C.; Dardalhon, V.; Awasthi, A.; Imitola, J.; Bettelli, E.; Oukka, M.; van Snick, J.; Renauld, J.-C.; et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc. Natl. Acad. Sci. USA 2009, 106, 12885–12890. [Google Scholar] [CrossRef] [Green Version]
- Nowak, E.C.; Weaver, C.T.; Turner, H.; Begum-Haque, S.; Becher, B.; Schreiner, B.; Coyle, A.J.; Kasper, L.H.; Noelle, R.J. IL-9 as a mediator of Th17-driven inflammatory disease. J. Exp. Med. 2009, 206, 1653–1660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, E.H.; Weninger, W.; Hunter, C.A. Trafficking of immune cells in the central nervous system. J. Clin. Investig. 2010, 120, 1368–1379. [Google Scholar] [CrossRef] [Green Version]
- Gold, R.; Jawad, A.; Miller, D.H.; Henderson, D.C.; Fassas, A.; Fierz, W.; Hartung, H.P. Expert opinion: Guidelines for the use of natalizumab in multiple sclerosis patients previously treated with immunomodulating therapies. J. Neuroimmunol. 2007, 187, 156–158. [Google Scholar] [CrossRef]
- De Graaf, M.T.; Smitt, P.A.E.S.; Luitwieler, R.L.; Van Velzen, C.; Broek, P.D.M.V.D.; Kraan, J.; Gratama, J.W. Central memory CD4+ T cells dominate the normal cerebrospinal fluid. Cytom. Part B Clin. Cytom. 2010, 80, 43–50. [Google Scholar] [CrossRef]
- Kivisäkk, P.; Mahad, D.J.; Callahan, M.K.; Sikora, K.; Trebst, C.; Tucky, B.; Wujek, J.; Ravid, R.; Staugaitis, S.M.; Lassmann, H.; et al. Expression of CCR7 in multiple sclerosis: Implications for CNS immunity. Ann. Neurol. 2004, 55, 627–638. [Google Scholar] [CrossRef]
- Pashenkov, M.; Söderström, M.; Link, H. Secondary lymphoid organ chemokines are elevated in the cerebrospinal fluid during central nervous system inflammation. J. Neuroimmunol. 2003, 135, 154–160. [Google Scholar] [CrossRef]
- Alt, C.; Laschinger, M.; Engelhardt, B. Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL 21 (SLC) at the blood-brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyeli. Eur. J. Immunol. 2002, 32, 2133–2144. [Google Scholar] [CrossRef]
- Engelhardt, B. Molecular mechanisms involved in T cell migration across the blood–brain barrier. J. Neural Transm. 2006, 113, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Columba-Cabezas, S.; Elena, B.S.; Aloisi, A.F. Lymphoid Chemokines CCL19 and CCL21 are Expressed in the Central Nervous System During Experimental Autoimmune Encephalomyelitis: Implications for the Maintenance of Chronic Neuroinflammation. Brain Pathol. 2006, 13, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Kivisäkk, P.; Tucky, B.; Wei, T.; Campbell, J.J.; Ransohoff, R.M. Human cerebrospinal fluid contains CD4+ memory T cells expressing gut- or skin-specific trafficking determinants: Relevance for immunotherapy. BMC Immunol. 2006, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Thewissen, K.; Nuyts, A.H.; Deckx, N.; Van Wijmeersch, B.; Nagels, G.; D’Hooghe, M.; Willekens, B.; Cras, P.; Eijnde, B.O.; Goossens, H.; et al. Circulating dendritic cells of multiple sclerosis patients are proinflammatory and their frequency is correlated with MS-associated genetic risk factors. Mult. Scler. J. 2013, 20, 548–557. [Google Scholar] [CrossRef]
- Kuwabara, T.; Ishikawa, F.; Yasuda, T.; Aritomi, K.; Nakano, H.; Tanaka, Y.; Okada, Y.; Lipp, M.; Kakiuchi, T. CCR 7 Ligands Are Required for Development of Experimental Autoimmune Encephalomyelitis through Generating IL-23-Dependent Th17 Cells. J. Immunol. 2009, 183, 2513–2521. [Google Scholar] [CrossRef] [Green Version]
- Koch, A.E. The pathogenesis of rheumatoid arthritis. Am. J. Orthop. 2007, 36 (Suppl. 7), 5–8. [Google Scholar]
- Mellado, M.; Martínez-Muñoz, L.; Cascio, G.; Lucas, P.; Pablos, J.L.; Rodriguez-Frade, J.M. T cell migration in rheumatoid arthritis. Front. Immunol. 2015, 6, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komatsu, N.; Takayanagi, H. Autoimmune Arthritis: The Interface Between the Immune System and Joints. Adv. Immunol. 2012, 115, 45–71. [Google Scholar] [PubMed]
- De Vries, R.R.P.; Huizinga, T.W.J.; Toes, R.E.M. Redefining the HLA and RA association: To be or not to be anti-CCP positive. J. Autoimmun. 2005, 25, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Laganà, B.; Vinciguerra, M.; D’Amelio, R. Modulation of T-Cell Co-Stimulation in Rheumatoid Arthritis. Clin. Drug Investig. 2009, 29, 185–202. [Google Scholar] [CrossRef]
- Page, G.; Lebecque, S.; Miossec, P. Anatomic Localization of Immature and Mature Dendritic Cells in an Ectopic Lymphoid Organ: Correlation with Selective Chemokine Expression in Rheumatoid Synovium. J. Immunol. 2002, 168, 5333–5341. [Google Scholar] [CrossRef] [PubMed]
- Jongbloed, S.L.; Lebre, M.C.; Fraser, A.R.; Gracie, J.A.; Sturrock, R.D.; Tak, P.P.; McInnes, I.B. Enumeration and phenotypical analysis of distinct dendritic cell subsets in psoriatic arthritis and rheumatoid arthritis. Arthritis Res. 2006, 8, R15. [Google Scholar] [CrossRef] [Green Version]
- Canavan, M.; Walsh, A.; Bhargava, V.; Wade, S.; McGarry, T.; Marzaioli, V.; Moran, B.; Biniecka, M.; Convery, H.; Wade, S.; et al. Enriched Cd141+ DCs in the joint are transcriptionally distinct, activated, and contribute to joint pathogenesis. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Itoh, J.; Kinjoh, K.; Ohyama, A.; Nose, M.; Kyogoku, M. Application of two-color immunofluorescence staining to demonstration of T-cells and HLA-DR-bearing cells in rheumatoid synovitis. J. Histochem. Cytochem. 1992, 40, 1675–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dinther-Janssen, A.C.; Pals, S.T.; Scheper, R.; Breedveld, F.; Meijer, C.J. Dendritic cells and high endothelial venules in the rheumatoid synovial membrane. J. Rheumatol. 1990, 17, 11–17. [Google Scholar]
- Radstake, T.R.D.J.; Van Der Voort, R.; ten Brummelhuis, M.; De Waal Malefijt, M.; Looman, M.; Figdor, C.; van den Berg, W.B.; Barrera, P.; Adema, G.J. Increased expression of CCL18, CCL19, and CCL17 by dendritic cells from patients with rheumatoid arthritis, and regulation by Fc gamma receptors. Ann. Rheum. Dis. 2004, 64, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Greenberg, J.D.; Bhardwaj, N. Dendritic cells as targets for therapy in rheumatoid arthritis. Nat. Rev. Rheumatol. 2009, 5, 566–571. [Google Scholar] [CrossRef]
- Wengner, A.M.; Höpken, U.E.; Petrow, P.K.; Hartmann, S.; Schurigt, U.; Bräuer, R.; Lipp, M. CXCR5- and CCR7-dependent lymphoid neogenesis in a murine model of chronic antigen-induced arthritis. Arthritis Rheum. 2007, 56, 3271–3283. [Google Scholar] [CrossRef]
- Schinnerling, K.; Rosas, C.; Soto, L.; Thomas, R.; Aguillón, J.C. Humanized Mouse Models of Rheumatoid Arthritis for Studies on Immunopathogenesis and Preclinical Testing of Cell-Based Therapies. Front. Immunol. 2019, 10, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Santiago-Schwarz, F.; Anand, P.; Liu, S.; Carsons, S.E. Dendritic Cells (DCs) in Rheumatoid Arthritis (RA): Progenitor Cells and Soluble Factors Contained in RA Synovial Fluid Yield a Subset of Myeloid DCs That Preferentially Activate Th1 Inflammatory-Type Responses. J. Immunol. 2001, 167, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Segura, E.; Touzot, M.; Bohineust, A.; Cappuccio, A.; Chiocchia, G.; Hosmalin, A.; Dalod, M.; Soumelis, V.; Amigorena, S. Human Inflammatory Dendritic Cells Induce Th17 Cell Differentiation. Immunity 2013, 38, 336–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada-Capetillo, L.; Hernández-Castro, B.; Monsiváis-Urenda, A.; Alvarez-Quiroga, C.; Layseca-Espinosa, E.; Abud-Mendoza, C.; Baranda, L.; Urzainqui, A.; Sánchez-Madrid, F.; González-Amaro, R. Induction of Th17 Lymphocytes and Treg Cells by Monocyte-Derived Dendritic Cells in Patients with Rheumatoid Arthritis and Systemic Lupus Erythematosus. Clin. Dev. Immunol. 2013, 2013, 584303. [Google Scholar] [CrossRef]
- Van Raemdonck, K.; Umar, S.; Palasiewicz, K.; Volkov, S.; Volin, M.V.; Arami, S.; Chang, H.J.; Zanotti, B.; Sweiss, N.; Shahrara, S. CCL21/CCR7 signaling in macrophages promotes joint inflammation and Th17-mediated osteoclast formation in rheumatoid arthritis. Cell. Mol. Life Sci. 2020, 77, 1387–1399. [Google Scholar] [CrossRef]
- Pickens, S.R.; Chamberlain, N.D.; Volin, M.V.; Pope, R.M.; Ii, A.M.M.; Shahrara, S. Characterization of CCL19 and CCL21 in rheumatoid arthritis. Arthritis Rheum. 2011, 63, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Pickens, S.R.; Chamberlain, N.D.; Volin, M.V.; Pope, R.M.; Talarico, N.E.; Mandelin, A.M.; Shahrara, S. Role of the CCL21 and CCR7 pathways in rheumatoid arthritis angiogenesis. Arthritis Rheum. 2012, 64, 2471–2481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowes, M.A.; Bowcock, A.M.; Krueger, J.G. Pathogenesis and therapy of psoriasis. Nature 2007, 445, 866–873. [Google Scholar] [CrossRef]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of Psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.-M.; Florencia, E.; Prens, E.; et al. Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice Is Mediated via the IL-23/IL-17 Axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Ganguly, D.; Chamilos, G.; Lande, R.; Gregorio, J.; Meller, S.; Facchinetti, V.; Homey, B.; Barrat, F.J.; Zal, T.; Gilliet, M. Self-RNA–antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 2009, 206, 1983–1994. [Google Scholar] [CrossRef]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.-H.; Homey, B.; Cao, W.; Wang, Y.-H.; Su, B.; Nestle, F.O.; et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, H.L.; Kagami, S.; Phillips, K.G.; Kurtz, S.E.; Jacques, S.L.; Blauvelt, A. IL-23–Mediated Psoriasis-Like Epidermal Hyperplasia Is Dependent on IL-17A. J. Immunol. 2011, 186, 1495–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Cesare, A.; Di Meglio, P.; Nestle, F.O. The IL-23/Th17 Axis in the Immunopathogenesis of Psoriasis. J. Investig. Dermatol. 2009, 129, 1339–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papp, K.A.; Leonardi, C.; Menter, A.; Ortonne, J.-P.; Krueger, J.G.; Kricorian, G.; Aras, G.; Li, J.; Russell, C.; Thompson, E.H.; et al. Brodalumab, an Anti–Interleukin-17–Receptor Antibody for Psoriasis. N. Engl. J. Med. 2012, 366, 1181–1189. [Google Scholar] [CrossRef]
- Papp, K.A.; Reid, C.; Foley, P.; Sinclair, R.; Salinger, D.H.; Williams, G.; Dong, H.; Krueger, J.G.; Russell, C.; Martin, D.A. Anti-IL-17 Receptor Antibody AMG 827 Leads to Rapid Clinical Response in Subjects with Moderate to Severe Psoriasis: Results from a Phase I, Randomized, Placebo-Controlled Trial. J. Investig. Dermatol. 2012, 132, 2466–2469. [Google Scholar] [CrossRef] [Green Version]
- Krueger, J.G.; Fretzin, S.; Suárez-Fariñas, M.; Haslett, P.A.; Phipps, K.M.; Cameron, G.S.; McColm, J.; Katcherian, A.; Cueto, I.; White, T.; et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J. Allergy Clin. Immunol. 2012, 130, 145–154.e9. [Google Scholar] [CrossRef] [Green Version]
- Kennedy-Crispin, M.; Billick, E.; Mitsui, H.; Gulati, N.; Fujita, H.; Gilleaudeau, P.; Sullivan-Whalen, M.; Johnson-Huang, L.M.; Suárez-Fariñas, M.; Krueger, J.G. Human Keratinocytes’ Response to Injury Upregulates CCL20 and Other Genes Linking Innate and Adaptive Immunity. J. Investig. Dermatol. 2012, 132, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, T.P.; Zhang, H.H.; Borek, I.; Wolf, P.; Hedrick, M.N.; Singh, S.P.; Kelsall, B.L.; Clausen, B.; Farber, J.M. Monocyte-derived inflammatory Langerhans cells and dermal dendritic cells mediate psoriasis-like inflammation. Nat. Commun. 2016, 7, 13581. [Google Scholar] [CrossRef] [Green Version]
- Suárez-Fariñas, M.; Arbeit, R.; Jiang, W.; Ortenzio, F.S.; Sullivan, T.; Krueger, J.G. Suppression of Molecular Inflammatory Pathways by Toll-Like Receptor 7, 8, and 9 Antagonists in a Model of IL-23-Induced Skin Inflammation. PLoS ONE 2013, 8, e84634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umeyama, L.; Hardianti, B.; Kasahara, S.; Dibwe, D.F.; Awale, S.; Yokoyama, S.; Hayakawa, Y. Anti-inflammatory effects of Morus alba Linne bark on the activation of toll-like receptors and imiquimod-induced ear edema in mice. BMC Complement. Med. Ther. 2021, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.-Y.; Cheng, Y.-G.; Liu, Y.; Tan, J.-Y.; Guan, W.; Guo, S.; Kuang, H.-X.; Liu, Y. Datura Metel L. Ameliorates Imiquimod-Induced Psoriasis-Like Dermatitis and Inhibits Inflammatory Cytokines Production through TLR7/8–MyD88–NF-κB–NLRP3 Inflammasome Pathway. Molecules 2019, 24, 2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.-W.; Chen, Y.-J.; Wang, S.-T.; Ho, L.-W.; Kao, J.-K.; Narita, M.; Takahashi, M.; Wu, C.-Y.; Cheng, H.-Y.; Shieh, J.-J. Azithromycin impairs TLR7 signaling in dendritic cells and improves the severity of imiquimod-induced psoriasis-like skin inflammation in mice. J. Dermatol. Sci. 2016, 84, 59–70. [Google Scholar] [CrossRef]
- Novoszel, P.; Holcmann, M.; Stulnig, G.; Fernandes, C.D.S.; Zyulina, V.; Borek, I.; Linder, M.; Bogusch, A.; Drobits, B.; Bauer, T.; et al. Psoriatic skin inflammation is promoted by c-Jun/AP-1-dependent CCL2 and IL-23 expression in dendritic cells. EMBO Mol. Med. 2021, 13, e12409. [Google Scholar] [CrossRef]
- Mitsui, H.; Suárez-Fariñas, M.; Belkin, D.A.; Levenkova, N.; Fuentes-Duculan, J.; Coats, I.; Fujita, H.; Krueger, J.G. Combined Use of Laser Capture Microdissection and cDNA Microarray Analysis Identifies Locally Expressed Disease-Related Genes in Focal Regions of Psoriasis Vulgaris Skin Lesions. J. Investig. Dermatol. 2012, 132, 1615–1626. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.-G.; Jee, H.; Fuentes-Duculan, J.; Wu, W.H.; Byamba, D.; Kim, D.-S.; Kim, D.Y.; Lew, D.-H.; Yang, W.-I.; Krueger, J.G.; et al. Dermal Clusters of Mature Dendritic Cells and T Cells Are Associated with the CCL20/CCR6 Chemokine System in Chronic Psoriasis. J. Investig. Dermatol. 2014, 134, 1462–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohn, C.; Ober-Blöbaum, J.L.; Haak, S.; Pantelyushin, S.; Cheong, C.; Zahner, S.P.; Onderwater, S.; Kant, M.; Weighardt, H.; Holzmann, B.; et al. Langerinneg conventional dendritic cells produce IL-23 to drive psoriatic plaque formation in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 10723–10728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginhoux, F.; Tacke, F.; Angeli, V.; Bogunovic, M.; Loubeau, M.; Dai, X.-M.; Stanley, E.R.; Randolph, G.J.; Merad, M. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 2006, 7, 265–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seré, K.; Baek, J.-H.; Ober-Blöbaum, J.; Müller-Newen, G.; Tacke, F.; Yokota, Y.; Zenke, M.; Hieronymus, T. Two Distinct Types of Langerhans Cells Populate the Skin during Steady State and Inflammation. Immunity 2012, 37, 905–916. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.A. Skin-Resident T Cells: The Ups and Downs of On Site Immunity. J. Investig. Dermatol. 2010, 130, 362–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comerford, I.; Harata-Lee, Y.; Bunting, M.D.; Gregor, C.; Kara, E.E.; McColl, S.R. A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev. 2013, 24, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Zlotnik, A. Chemokines in neoplastic progression. Semin. Cancer Biol. 2004, 14, 181–185. [Google Scholar] [CrossRef]
- Zlotnik, A. Chemokines and cancer. Int. J. Cancer 2006, 119, 2026–2029. [Google Scholar] [CrossRef] [PubMed]
- Mashino, K.; Sadanaga, N.; Yamaguchi, H.; Tanaka, F.; Ohta, M.; Shibuta, K.; Inoue, H.; Mori, M. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002, 62. [Google Scholar]
- Andre, F.; Cabioglu, N.; Assi, H.; Sabourin, J.C.; Delaloge, S.; Sahin, A.; Broglio, K.; Spano, J.P.; Combadiere, C.; Bucana, C.; et al. Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann. Oncol. 2006, 17, 945–951. [Google Scholar] [CrossRef]
- Nakata, B.; Fukunaga, S.; Noda, E.; Amano, R.; Yamada, N.; Hirakawa, K. Chemokine Receptor CCR7 Expression Correlates with Lymph Node Metastasis in Pancreatic Cancer. Oncology 2008, 74, 69–75. [Google Scholar] [CrossRef]
- Günther, K.; Leier, J.; Henning, G.; Dimmler, A.; Weißbach, R.; Hohenberger, W.; Förster, R. Prediction of lymph node metastasis in colorectal carcinoma by expressionof chemokine receptor CCR7. Int. J. Cancer 2005, 116, 726–733. [Google Scholar] [CrossRef]
- Sperveslage, J.; Frank, S.; Heneweer, C.; Egberts, J.; Schniewind, B.; Buchholz, M.; Bergmann, F.; Giese, N.; Munding, J.; Hahn, S.A.; et al. Lack of CCR7 expression is rate limiting for lymphatic spread of pancreatic ductal adenocarcinoma. Int. J. Cancer 2012, 131, E371–E381. [Google Scholar] [CrossRef]
- Zhao, B.; Cui, K.; Wang, C.-L.; Wang, A.-L.; Zhang, B.; Zhou, W.-Y.; Zhao, W.-H.; Li, S. The chemotactic interaction between CCL21 and its receptor, CCR7, facilitates the progression of pancreatic cancer via induction of angiogenesis and lymphangiogenesis. J. Hepatobiliary Pancreatic Sci. 2011, 18, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Pitkin, L.; Luangdilok, S.; Corbishley, C.; Wilson, P.O.G.; Dalton, P.; Bray, D.; Mady, S.; Williamson, P.; Odutoye, T.; Evans, P.R.; et al. Expression of CC chemokine receptor 7 in tonsillar cancer predicts cervical nodal metastasis, systemic relapse and survival. Br. J. Cancer 2007, 97, 670–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ji, R.; Li, J.; Gu, Q.; Zhao, X.; Sun, T.; Wang, J.; Li, J.; Du, Q.; Sun, B. Correlation effect of EGFR and CXCR4 and CCR7 chemokine receptors in predicting breast cancer metastasis and prognosis. J. Exp. Clin. Cancer Res. 2010, 29, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itakura, M.; Terashima, Y.; Shingyoji, M.; Yokoi, S.; Ohira, M.; Kageyama, H.; Matui, Y.; Yoshida, Y.; Ashinuma, H.; Moriya, Y.; et al. High CC chemokine receptor 7 expression improves postoperative prognosis of lung adenocarcinoma patients. Br. J. Cancer 2013, 109, 1100–1108. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Basheer, H.A.; Ayuso, J.M.; Ahmet, D.; Mazzini, M.; Patel, R.; Shnyder, S.D.; Vinader, V.; Afarinkia, K. Agarose Spot as a Comparative Method for in situ Analysis of Simultaneous Chemotactic Responses to Multiple Chemokines. Sci. Rep. 2017, 7, 1075. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, K.; Brünle, S.; Weinert, T.; Guba, W.; Muehle, J.; Miyazaki, T.; Weber, M.; Furrer, A.; Haenggi, N.; Tetaz, T.; et al. Structural Basis for Allosteric Ligand Recognition in the Human CC Chemokine Receptor 7. Cell 2019, 178, 1222–1230.e10. [Google Scholar] [CrossRef] [Green Version]
- Hillinger, S.; Yang, S.-C.; Zhu, L.; Huang, M.; Duckett, R.; Atianzar, K.; Batra, R.K.; Strieter, R.M.; Dubinett, S.M.; Sharma, S. EBV-Induced Molecule 1 Ligand Chemokine (ELC/CCL19) Promotes IFN-γ-Dependent Antitumor Responses in a Lung Cancer Model. J. Immunol. 2003, 171, 6457–6465. [Google Scholar] [CrossRef] [Green Version]
- Hillinger, S.; Yang, S.-C.; Batra, R.K.; Strieter, R.M.; Weder, W.; Dubinett, S.M.; Sharma, S. CCL19 reduces tumour burden in a model of advanced lung cancer. Br. J. Cancer 2006, 94, 1029–1034. [Google Scholar] [CrossRef] [Green Version]
- Ashour, A.E.; Lin, X.; Wang, X.; Turnquist, H.R.; Burns, N.M.; Tuli, A.; Sadanandam, A.; Suleiman, K.; Singh, R.K.; Talmadge, J.E.; et al. CCL21 is an effective surgical neoadjuvant for treatment of mammary tumors. Cancer Biol. Ther. 2007, 6, 1217–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan-Lai, V.; Kievit, F.M.; Florczyk, S.J.; Wang, K.; Disis, M.L.; Zhang, M. CCL21 and IFNγ Recruit and Activate Tumor Specific T cells in 3D Scaffold Model of Breast Cancer. Anti-Cancer Agents Med. Chem. 2014, 14, 204–210. [Google Scholar] [CrossRef]
- Turnquist, H.R.; Lin, X.; Ashour, A.E.; Hollingsworth, M.A.; Singh, R.K.; Talmadge, J.E.; Solheim, J.C. CCL21 induces extensive intratumoral immune cell infiltration and specific anti-tumor cellular immunity. Int. J. Oncol. 2007, 30, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenton, O.S.; Tibbitt, M.W.; Appel, E.A.; Jhunjhunwala, S.; Webber, M.J.; Langer, R. Injectable Polymer–Nanoparticle Hydrogels for Local Immune Cell Recruitment. Biomacromolecules 2019, 20, 4430–4436. [Google Scholar] [CrossRef]
- Stachowiak, A.N.; Irvine, D.J. Inverse opal hydrogel-collagen composite scaffolds as a supportive microenvironment for immune cell migration. J. Biomed. Mater. Res. Part A 2008, 85, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Kar, U.K.; Srivastava, M.K.; Andersson, Å.; Baratelli, F.; Huang, M.; Kickhoefer, V.; Dubinett, S.M.; Rome, L.H.; Sharma, S. Novel CCL21-Vault Nanocapsule Intratumoral Delivery Inhibits Lung Cancer Growth. PLoS ONE 2011, 6, e18758. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Xing, W.; Peng, J.; Yuan, X.; Zhao, X.; Lei, P.; Li, W.; Wang, M.; Zhu, H.; Huang, B.; et al. Tumor transfected with CCL21 enhanced reactivity and apoptosis resistance of human monocyte-derived dendritic cells. Immunobiology 2008, 213, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-Q.; Sugita, T.; Kanagawa, N.; Iida, K.; Okada, N.; Mizuguchi, H.; Nakayama, T.; Hayakawa, T.; Yoshie, O.; Tsutsumi, Y.; et al. Anti-tumor Responses Induced by Chemokine CCL19 Transfected into an Ovarian Carcinoma Model via Fiber-Mutant Adenovirus Vector. Biol. Pharm. Bull. 2005, 28, 1066–1070. [Google Scholar] [CrossRef] [Green Version]
- Adachi, K.; Kano, Y.; Nagai, T.; Okuyama, N.; Sakoda, Y.; Tamada, K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 2018, 36, 346–351. [Google Scholar] [CrossRef]
- Yang, L.; Huang, C.; Wang, C.; Zhang, S.; Li, Z.; Zhu, Y.; Li, D.; Gao, L.; Ge, Z.; Su, M.; et al. Overexpressed CXCR4 and CCR7 on the surface of NK92 cell have improved migration and anti-tumor activity in human colon tumor model. Anti-Cancer Drugs 2020, 31, 333–344. [Google Scholar] [CrossRef]
- Okada, N.; Mori, N.; Koretomo, R.; Okada, Y.; Nakayama, T.; Yoshie, O.; Mizuguchi, H.; Hayakawa, T.; Nakagawa, S.; Mayumi, T.; et al. Augmentation of the migratory ability of DC-based vaccine into regional lymph nodes by efficient CCR7 gene transduction. Gene Ther. 2004, 12, 129–139. [Google Scholar] [CrossRef]
- Cancel, J.-C.; Crozat, K.; Dalod, M.; Mattiuz, R. Are Conventional Type 1 Dendritic Cells Critical for Protective Antitumor Immunity and How? Front. Immunol. 2019, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen presentation in cancer: Insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 2021, 21, 298–312. [Google Scholar] [CrossRef]
- Lee, J.B.; Ha, S.-J.; Kim, H.R. Clinical Insights into Novel Immune Checkpoint Inhibitors. Front. Pharmacol. 2021, 12, 1074. [Google Scholar] [CrossRef]
- Ma, S.; Li, X.; Wang, X.; Cheng, L.; Li, Z.; Zhang, C.; Ye, Z.; Qian, Q. Current Progress in CAR-T Cell Therapy for Solid Tumors. Int. J. Biol. Sci. 2019, 15, 2548–2560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vonderheide, R.H. The Immune Revolution: A Case for Priming, Not Checkpoint. Cancer Cell 2018, 33, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Teng, M.W.L.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015, 75, 2139–2145. [Google Scholar] [CrossRef] [Green Version]
- Bajor, D.L.; Mick, R.; Riese, M.J.; Huang, A.C.; Sullivan, B.; Richman, L.P.; Torigian, D.A.; George, S.M.; Stelekati, E.; Chen, F.; et al. Long-term outcomes of a phase I study of agonist CD40 antibody and CTLA-4 blockade in patients with metastatic melanoma. OncoImmunology 2018, 7, e1468956. [Google Scholar] [CrossRef] [Green Version]
- Van Willigen, W.W.; Bloemendal, M.; Gerritsen, W.R.; Schreibelt, G.; De Vries, I.J.M.; Bol, K.F. Dendritic Cell Cancer Therapy: Vaccinating the Right Patient at the Right Time. Front. Immunol. 2018, 9, 2265. [Google Scholar] [CrossRef] [PubMed]
- Capelletti, M.; Liegel, J.; Themeli, M.; Mutis, T.; Stroopinsky, D.; Orr, S.; Torres, D.; Morin, A.; Gunset, G.; Ghiasuddin, H.; et al. Potent Synergy between Combination of Chimeric Antigen Receptor (CAR) Therapy Targeting CD19 in Conjunction with Dendritic Cell (DC)/Tumor Fusion Vaccine in Hematological Malignancies. Biol. Blood Marrow Transplant. 2020, 26 (Suppl. 3), S42–S43. [Google Scholar] [CrossRef]
- Anguille, S.; Smits, E.L.; Lion, E.; Van Tendeloo, V.F.; Berneman, Z.N. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014, 15, e257–e267. [Google Scholar] [CrossRef]
- Muthuswamy, R.; Mueller-Berghaus, J.; Haberkorn, U.; Reinhart, T.A.; Schadendorf, D.; Kalinski, P. PGE2 transiently enhances DC expression of CCR7 but inhibits the ability of DCs to produce CCL19 and attract naive T cells. Blood 2010, 116, 1454–1459. [Google Scholar] [CrossRef]
- Hansen, M.; Hjortø, G.M.; Donia, M.; Met, Ö.; Larsen, N.B.; Andersen, M.H.; Straten, P.T.; Svane, I.M. Comparison of clinical grade type 1 polarized and standard matured dendritic cells for cancer immunotherapy. Vaccine 2013, 31, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Bonetto, F.; Srinivas, M.; Weigelin, B.; Cruz, L.J.; Heerschap, A.; Friedl, P.; Figdor, C.; De Vries, I.J.M. A large-scale 19 F MRI-based cell migration assay to optimize cell therapy. NMR Biomed. 2012, 25, 1095–1103. [Google Scholar] [CrossRef]
- Verdijk, P.; Aarntzen, E.H.J.G.; Lesterhuis, W.J.; Boullart, A.C.I.; Kok, E.; Van Rossum, M.M.; Strijk, S.; Eijckeler, F.; Bonenkamp, J.J.; Jacobs, H.; et al. Limited Amounts of Dendritic Cells Migrate into the T-Cell Area of Lymph Nodes but Have High Immune Activating Potential in Melanoma Patients. Clin. Cancer Res. 2009, 15, 2531–2540. [Google Scholar] [CrossRef] [Green Version]
- Verdijk, P.; Aarntzen, E.H.J.G.; Punt, C.J.A.; De Vries, I.J.M.; Figdor, C.G. Maximizing dendritic cell migration in cancer immunotherapy. Expert Opin. Biol. Ther. 2008, 8, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Boudewijns, S.; Koornstra, R.H.T.; Westdorp, H.; Schreibelt, G.; van den Eertwegh, A.J.M.; Foppen, M.H.G.; Haanen, J.B.; de Vries, J.; Figdor, C.G.; Bol, K.F.; et al. Ipilimumab administered to metastatic melanoma patients who progressed after dendritic cell vaccination. OncoImmunology 2016, 5, e1201625. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, C.J.; Das, A.; Liu, G.; Yu, J.S.; Black, K.L. Clinical Responsiveness of Glioblastoma Multiforme to Chemotherapy after Vaccination. Clin. Cancer Res. 2004, 10, 5316–5326. [Google Scholar] [CrossRef] [Green Version]
- Rosengren, S.; Wei, N.; Kalunian, K.C.; Zvaifler, N.J.; Kavanaugh, A.; Boyle, D.L. Elevated autoantibody content in rheumatoid arthritis synovia with lymphoid aggregates and the effect of rituximab. Arthritis Res. Ther. 2008, 10, R105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Nakajima, T.; Goronzy, J.J.; Weyand, C.M. Tissue trafficking patterns of effector memory CD4+ T cells in rheumatoid arthritis. Arthritis Rheum. 2005, 52, 3839–3849. [Google Scholar] [CrossRef]
- Saha, S.; Shukla, A.K. The Inside Story: Crystal Structure of the Chemokine Receptor CCR7 with an Intracellular Allosteric Antagonist. Biochemistry 2019, 59, 12–14. [Google Scholar] [CrossRef] [Green Version]
- Insel, P.A.; Sriram, K.; Gorr, M.W.; Wiley, S.Z.; Michkov, A.; Salmerón, C.; Chinn, A.M. GPCRomics: An Approach to Discover GPCR Drug Targets. Trends Pharmacol. Sci. 2019, 40, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, A.S.; Daugvilaite, V.; De Filippo, K.; Berg, C.; Mavri, M.; Benned-Jensen, T.; Juzenaite, G.; Hjortø, G.; Rankin, S.; Våbenø, J.; et al. Biased action of the CXCR4-targeting drug plerixafor is essential for its superior hematopoietic stem cell mobilization. Commun. Biol. 2021, 4, 569. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandum, E.P.; Jørgensen, A.S.; Rosenkilde, M.M.; Hjortø, G.M. Dendritic Cells and CCR7 Expression: An Important Factor for Autoimmune Diseases, Chronic Inflammation, and Cancer. Int. J. Mol. Sci. 2021, 22, 8340. https://doi.org/10.3390/ijms22158340
Brandum EP, Jørgensen AS, Rosenkilde MM, Hjortø GM. Dendritic Cells and CCR7 Expression: An Important Factor for Autoimmune Diseases, Chronic Inflammation, and Cancer. International Journal of Molecular Sciences. 2021; 22(15):8340. https://doi.org/10.3390/ijms22158340
Chicago/Turabian StyleBrandum, Emma Probst, Astrid Sissel Jørgensen, Mette Marie Rosenkilde, and Gertrud Malene Hjortø. 2021. "Dendritic Cells and CCR7 Expression: An Important Factor for Autoimmune Diseases, Chronic Inflammation, and Cancer" International Journal of Molecular Sciences 22, no. 15: 8340. https://doi.org/10.3390/ijms22158340
APA StyleBrandum, E. P., Jørgensen, A. S., Rosenkilde, M. M., & Hjortø, G. M. (2021). Dendritic Cells and CCR7 Expression: An Important Factor for Autoimmune Diseases, Chronic Inflammation, and Cancer. International Journal of Molecular Sciences, 22(15), 8340. https://doi.org/10.3390/ijms22158340





