Review of Dendritic Cells, Their Role in Clinical Immunology, and Distribution in Various Animal Species
Abstract
1. Introduction
1.1. History and Discovery of Dendritic Cells
1.2. The Origin and Anatomical Location of Dendritic Cells
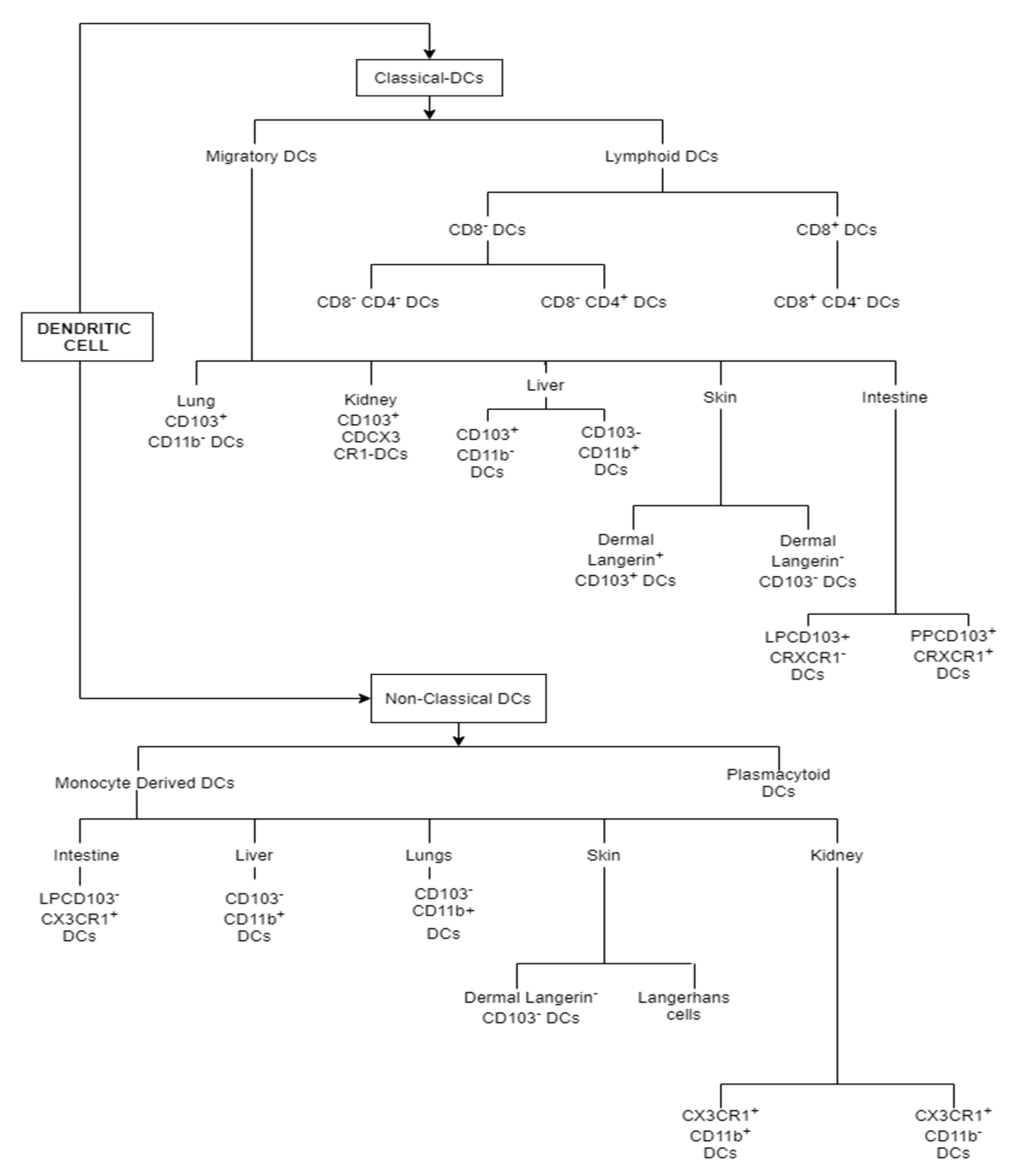
1.3. Types of Dendritic Cell Subsets
1.3.1. Classical DCs (Conventional DCs)
- (1)
- CD8α+ and CD103+ cDCs
- (2)
- CD11b+ cDCs
1.3.2. Non-Classical DCs (Non-Conventional DCs)
- (1)
- Monocyte-Derived DCs
- (2)
- Plasmacytoid Dendritic Cells (pDCs)
- (3)
- Langerhans or Epidermal DCs
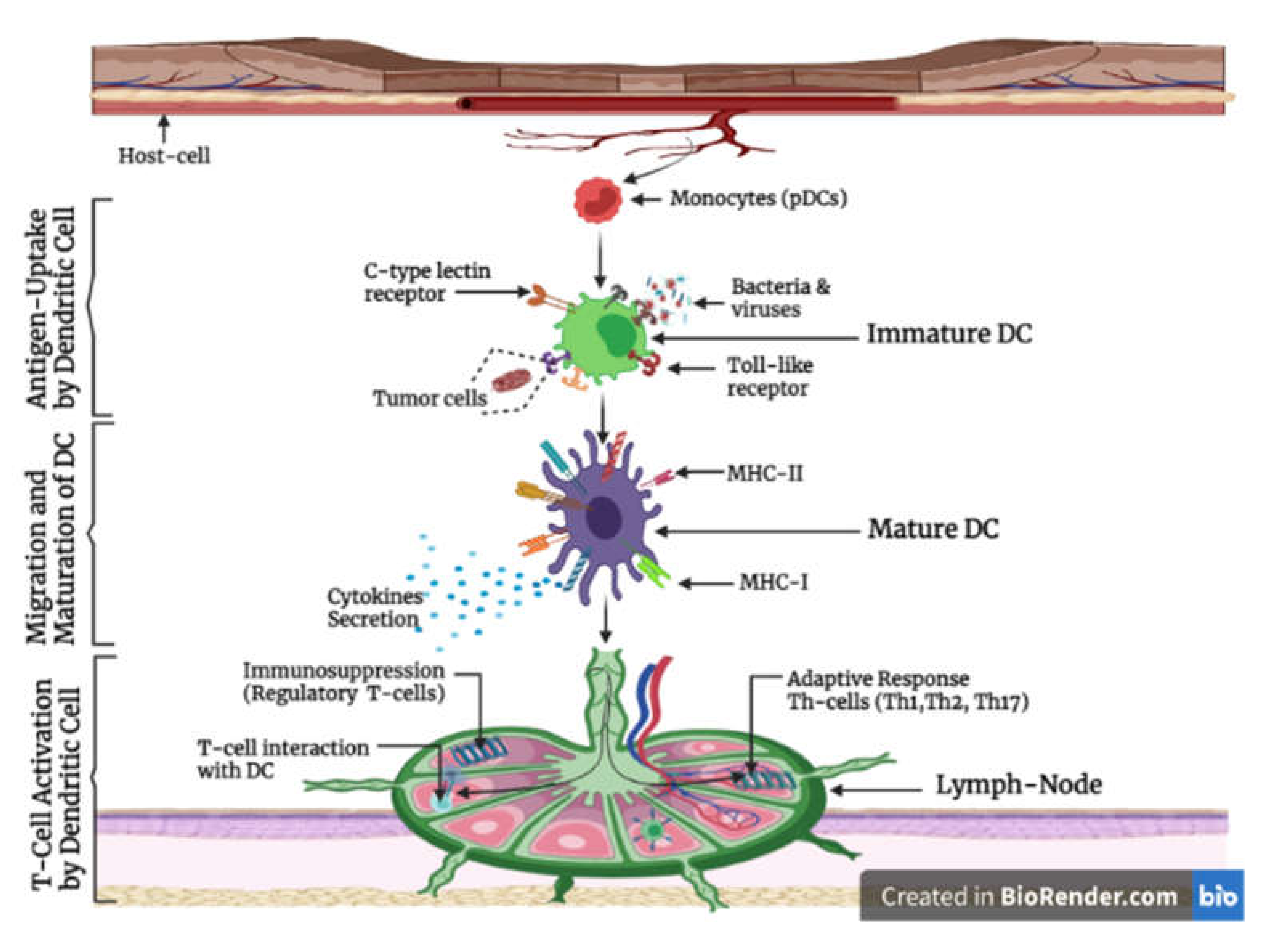
1.4. The Function and Role of Dendritic Cell in Immunity
1.5. Dendritic Cells and Immune Tolerance
1.6. Cytokine Production
2. The Role of Dendritic Cells in Clinical Immunology
2.1. Dendritic Cells in Transplantation
2.2. Dendritic Cells in Autoimmune Disease
2.3. Dendritic Cells in Viral Infection
2.4. Dendritic Cells in Cancer
2.5. Dendritic Cells and Targeted Vaccines
2.5.1. Ex Vivo Antigen-Loaded DC-Based Vaccines
2.5.2. In Vivo DC-Targeted Vaccines
- (1)
- Ligand-Based DC-Targeted Vaccines
- (2)
- Antibody-Based DC-Targeted Vaccines
- (3)
- Delivery System Based DC-Targeted Vaccines
3. Current Dynamic Perspective and Distribution of Dendritic Cells in Human and Various Species of Animal
3.1. Human Dendritic Cells
3.2. Mouse Dendritic Cells
3.2.1. Resident versus Migratory Mouse DCs
3.2.2. Plasmacytoid DCs (pDCs)
3.2.3. Classical DCs
- (1)
- (2)
- IRF4-dependent DCs consist of resident CD8− CD11b+ DCs and migratory CD11b+. They depend on transcriptional factors RelB and IRF4 for their development, and some unique features of IRF4-dependent DCs include specialization in MHC-II-restricted presentation of antigens after pathogen infection or allergen challenges and the induction of Th17 or Th2 in response to the draining of lymph nodes [49].
3.2.4. Langerhans Dendritic Cells (LCs-DCs)
3.3. Rat Dendritic Cells
3.3.1. In Vivo
3.3.2. In Vitro
3.4. Avian Dendritic Cells
3.5. Dog Dendritic Cells
3.6. Cat Dendritic Cells
3.7. Horse Dendritic Cells
3.8. Cattle Dendritic Cells
- (1)
- The cannulation of pseudo-afferent lymphatic ducts after surgical removal of the pre-scapular lymph nodes.
- (2)
- Culture of monocyte-derived DCs in the presence of GM-CSF and IL-4.
3.9. Sheep Dendritic Cells
- (1)
- Lymphadenectomy
- (2)
- In vitro generation of ovine DCs from adherent peripheral blood mononuclear cells (PBMC) using GM-CSF and IL-4
3.10. Pig Dendritic Cells
3.11. Non-Human Primate (NHP) Dendritic Cells
| Spp of Animals: | Human | Mouse | Avian | Dog | Cat | Horse | Cattle | Sheep | Pig | Rat | Monkey | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) DC-Origin | BM-Hp | BM-Hp | BM-Hp | BM-Hp | BM-Hp | BM-Hp | BM-Hp | BM-Hp | BM-Hp | BM-Hp | BM-Hp | [92] |
| CD34+ | CD34+ | Yolk-sac | CD34+ | CD34+ | CD34+ | CD34+ | [93] | |||||
| (2) DC-Location | PBMC | PBMC | B.Fabricius | PBMC | PBMC | PBMC | PBMC | PBMC | PBMC | PBMC | PBMC | [55] |
| Skin | Lymph.N | Peyer’s | Lymph.N | Mucosal | Epidermis | Lymph | Skin | Thymus | Lymph.N | Skin | [94] | |
| Lungs | Skin | Patches | Intestine | tissue | Lungs | Skin | Lymph | Skin | Skin | Lymph | [95] | |
| (3) DC-Functional Characteristics | ||||||||||||
| (a) Antigen intake | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | [96] |
| [97] | ||||||||||||
| (b) Mannose Receptor | High | Low | Low | Low | High | High | High | Low | High | Low | High | [98] |
| [99] | ||||||||||||
| (c) Functional MLR | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | [100] |
| (d) MoDc-Isolation (GM-CSF + IL-4) | Isolated | Isolated | Isolated | Isolated | Isolated | Isolated | Isolated | Isolated | Isolated | Isolated | Isolated | [101] |
| (e) LC Identification. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | [102] |
| [103] | ||||||||||||
| (f) Cytokine Secretion by LPS | IL-6 | IL-1α | IL-1ß, IL-6 | IL-6 | IL-10 | IL-12p35 | IL-1ß | IL-5 | IL-1ß | IL-1b | IL-1ß | [104] |
| IL-12p40 | IL-1ß | IL-8, IL-10 | IL-12 | IL-12 | IL-12p35 | IL-10 | IL-17 | IL-8 | IL-4 | IL-18 | [105] | |
| TNF-α | vIL-12P35 | IL-12ß, IFNγ | IL-17 | B7.1 | IL-12p70 | IL-12p40 | IFNγ | TNFα | TNFα | TNFα | [106] | |
| Spp of Animals: | Human | Mouse | Avian | Dog | Cat | Horse | Refs. |
| DC-Morphology | Irregular surface with numerous Projections cytoplasmic vacuoles | Round cells with irregular dendritic Protrusion | Irregular morphology with Filiform cell process with beaded dendrites | Balloon-like veiled-shape with cytoplasmic Projections | Uniform sized cells with long cytoplasmic Process | Stellate-morphology with long dendrites Birbeck’s granules | [107] |
| [67] | |||||||
| [70] | |||||||
| [65] | |||||||
| Spp of Animals: | Human | Mouse | Avian | Dog | Cat | Horse | Refs. |
| DC-Phenotypes | MHC-II, CD1c | MHC-II | MHC-II | MHC-II, CD86 | MHC-I and II | MHC-I and II | [108] |
| CD11c, CD141, | CD8α, CD11c | CD11c, CD40 | CD1a, CD1c | CD1a, CD1b | CD86, CD11a, | [109] | |
| CD303, CD304 | CD205, CD207 | CD83, CD86 | CD8, CD11a | CD1c, CD11c | CD18, CD4 | [110] | |
| CD370, CD123 | CD40, FLT3L | DEC205 | CD18, CD45 | CD14, CD18 | CD206, CD14 | [75] |
| Spp of Animals: | Cattle | Sheep | Rat | Pig | NHP (Monkey) | Refs. |
| DC-Morphology | Irregular cells with veiled shapes appearance | Cells with veiled-shape Dendritic-like shape appearance | Round cells with irregular outlines and long cell process | Cells with pronounced protrusions with micro-villous projections | Irregular cells with long multiple projections or hairy cytoplasmic projections | [79] |
| [87] | ||||||
| [111] | ||||||
| Spp of Animals: | Cattle | Sheep | Rat | Pig | NHP (Monkey) | Refs. |
| DC-Phenotype | MHC-II, CD11c | MHC-I and II | MHC I and II | SWC3, MHC-I and II | DC-LAMP (CD208) | [74] |
| CD11a, CD11b | CD1b, CD58 | CD54, CD11c | CD1, CD14, CD11a | DEC-205, MHC-I and II | [112] | |
| CD80, CD86, | CD11c, CD205 | CD80, CD86 | CD11b, CD11c, CD18 | vCD11a, CD50,CD54 | [90] | |
| vCD13, CD26, | vCD209 | CD45, α€Intergrin | CD36, CD80, CD86 | CD58, CD83, CD86 | [113] |
4. Conclusions and Future Perspective of Dendritic Cells
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APCs | Antigen-presenting cells |
| BM-Hp | Bone marrow hematopoietic progenitor |
| BF | Bursa of Fabricius |
| CD | Cluster of differentiation |
| cDC | Conventional dendritic cell |
| DCs | Dendritic cell |
| ESAM | Endothelial cell-selective adhesion molecule |
| Flt3L | Fms-like tyrosine kinase-3 Ligand |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| HSC | Hematopoietic stem cells |
| IL | Interleukin |
| IFN | Interferon |
| iNOS | Inducible nitric oxide synthase |
| LCs | Langerhans cells |
| LPS | Lipopolysaccharide |
| LN | Lymph node |
| M-CSF | Monocyte colony-stimulating factor |
| MHC | Major histocompatibility complex |
| MLR | Mixed Lymphocyte Reaction |
| MoDC | Monocyte-derived dendritic cell |
| PAMP | Pathogen-associated molecular pattern |
| PBMC | Peripheral blood mononuclear cells |
| pDC | Plasmacytoid dendritic cell |
| NHP | Non-human primates |
| Spp | Specie |
| SLE | Systemic lupus erythematosus |
References
- Chen, P.; Liu, X.; Sun, Y.; Zhou, P.; Wang, Y.; Zhang, Y. Dendritic Cell Targeted Vaccines: Recent Progresses and Challenges. Hum. Vaccines Immunother. 2016, 12, 612–622. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Umar, S.; Meng, C.; Ullah, Z.; Riaz, F.; Rehman, S.U.; Ding, C. Dendritic Cell Harmonised Immunity to Poultry Pathogens; a Review. Worlds Poult. Sci. J. 2017, 73, 581–590. [Google Scholar] [CrossRef]
- Ricart, B.G. Dendritic Cell Migration and Traction Force Generation in Engineered Microenvironments. Publicly Access. Penn Diss. 2010, 282, 214. [Google Scholar]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The Dendritic Cell Lineage: Ontogeny and Function of Dendritic Cells and Their Subsets in the Steady State and the Inflamed Setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef] [PubMed]
- Shortman, K.; Heath, W.R. The CD8+ Dendritic Cell Subset. Immunol. Rev. 2010, 234, 18–31. [Google Scholar] [CrossRef]
- Edelson, B.T.; Kc, W.; Juang, R.; Kohyama, M.; Benoit, L.A.; Klekotka, P.A.; Moon, C.; Albring, J.C.; Ise, W.; Michael, D.G.; et al. Peripheral CD103+ Dendritic Cells Form a Unified Subset Developmentally Related to CD8alpha+ Conventional Dendritic Cells. J. Exp. Med. 2010, 207, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Crozat, K.; Guiton, R.; Contreras, V.; Feuillet, V.; Dutertre, C.-A.; Ventre, E.; Vu Manh, T.-P.; Baranek, T.; Storset, A.K.; Marvel, J.; et al. The XC Chemokine Receptor 1 Is a Conserved Selective Marker of Mammalian Cells Homologous to Mouse CD8alpha+ Dendritic Cells. J. Exp. Med. 2010, 207, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Kashiwada, M.; Pham, N.-L.L.; Pewe, L.L.; Harty, J.T.; Rothman, P.B. NFIL3/E4BP4 Is a Key Transcription Factor for CD8α+ Dendritic Cell Development. Blood 2011, 117, 6193–6197. [Google Scholar] [CrossRef]
- Arora, P.; Baena, A.; Yu, K.O.A.; Saini, N.K.; Kharkwal, S.S.; Goldberg, M.F.; Kunnath-Velayudhan, S.; Carreño, L.J.; Venkataswamy, M.M.; Kim, J.; et al. A Single Subset of Dendritic Cells Controls the Cytokine Bias of Natural Killer T Cell Responses to Diverse Glycolipid Antigens. Immunity 2014, 40, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Jaitin, D.A.; Kenigsberg, E.; Keren-Shaul, H.; Elefant, N.; Paul, F.; Zaretsky, I.; Mildner, A.; Cohen, N.; Jung, S.; Tanay, A.; et al. Massively Parallel Single-Cell RNA-Seq for Marker-Free Decomposition of Tissues into Cell Types. Science 2014, 343, 776–779. [Google Scholar] [CrossRef]
- Vander Lugt, B.; Khan, A.A.; Hackney, J.A.; Agrawal, S.; Lesch, J.; Zhou, M.; Lee, W.P.; Park, S.; Xu, M.; DeVoss, J.; et al. Transcriptional Programming of Dendritic Cells for Enhanced MHC Class II Antigen Presentation. Nat. Immunol. 2014, 15, 161–167. [Google Scholar] [CrossRef]
- Schlitzer, A.; McGovern, N.; Teo, P.; Zelante, T.; Atarashi, K.; Low, D.; Ho, A.W.S.; See, P.; Shin, A.; Wasan, P.S.; et al. IRF4 Transcription Factor-Dependent CD11b+ Dendritic Cells in Human and Mouse Control Mucosal IL-17 Cytokine Responses. Immunity 2013, 38, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Cheong, C.; Matos, I.; Choi, J.-H.; Dandamudi, D.B.; Shrestha, E.; Longhi, M.P.; Jeffrey, K.L.; Anthony, R.M.; Kluger, C.; Nchinda, G.; et al. Microbial Stimulation Fully Differentiates Monocytes to DC-SIGN/CD209(+) Dendritic Cells for Immune T Cell Areas. Cell 2010, 143, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Plantinga, M.; Guilliams, M.; Vanheerswynghels, M.; Deswarte, K.; Branco-Madeira, F.; Toussaint, W.; Vanhoutte, L.; Neyt, K.; Killeen, N.; Malissen, B.; et al. Conventional and Monocyte-Derived CD11b(+) Dendritic Cells Initiate and Maintain T Helper 2 Cell-Mediated Immunity to House Dust Mite Allergen. Immunity 2013, 38, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Reizis, B.; Bunin, A.; Ghosh, H.S.; Lewis, K.L.; Sisirak, V. Plasmacytoid Dendritic Cells: Recent Progress and Open Questions. Annu. Rev. Immunol. 2011, 29, 163–183. [Google Scholar] [CrossRef]
- Reynolds, G.; Haniffa, M. Human and Mouse Mononuclear Phagocyte Networks: A Tale of Two Species? Front. Immunol. 2015, 6, 330. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Merad, M. Ontogeny and Homeostasis of Langerhans Cells. Immunol. Cell Biol. 2010, 88, 387–392. [Google Scholar] [CrossRef]
- Miller, J.C.; Brown, B.D.; Shay, T.; Gautier, E.L.; Jojic, V.; Cohain, A.; Pandey, G.; Leboeuf, M.; Elpek, K.G.; Helft, J.; et al. Immunological Genome Consortium. Deciphering the Transcriptional Network of the Dendritic Cell Lineage. Nat. Immunol. 2012, 13, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M. Decisions About Dendritic Cells: Past, Present, and Future. Annu. Rev. Immunol. 2012, 30, 1–22. [Google Scholar] [CrossRef]
- Wykes, M.; Macpherson, G. Dendritic Cell–B-Cell Interaction: Dendritic Cells Provide B Cells with CD40-Independent Proliferation Signals and CD40-Dependent Survival Signals. Immunology 2000, 100, 1–3. [Google Scholar] [CrossRef]
- Hilkens, C.M.U.; Isaacs, J.D.; Thomson, A.W. Development of Dendritic Cell-Based Immunotherapy for Autoimmunity. Int. Rev. Immunol. 2010, 29, 156–183. [Google Scholar] [CrossRef] [PubMed]
- Palucka, K.; Banchereau, J. Cancer Immunotherapy via Dendritic Cells. Nat. Rev. Cancer 2012, 12, 265–277. [Google Scholar] [CrossRef]
- Guilliams, M.; Ginhoux, F.; Jakubzick, C.; Naik, S.H.; Onai, N.; Schraml, B.U.; Segura, E.; Tussiwand, R.; Yona, S. Dendritic Cells, Monocytes and Macrophages: A Unified Nomenclature Based on Ontogeny. Nat. Rev. Immunol. 2014, 14, 571–578. [Google Scholar] [CrossRef]
- Celli, S.; Albert, M.L.; Bousso, P. Visualizing the Innate and Adaptive Immune Responses Underlying Allograft Rejection by Two-Photon Microscopy. Nat. Med. 2011, 17, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Vega, A.; Sánchez, E.; Löfgren, S.; Castillejo-López, C.; Alarcón-Riquelme, M.E. Recent Findings on Genetics of Systemic Autoimmune Diseases. Curr. Opin. Immunol. 2010, 22, 698–705. [Google Scholar] [CrossRef]
- Hotta-Iwamura, C.; Tarbell, K.V. Type 1 Diabetes Genetic Susceptibility and Dendritic Cell Function: Potential Targets for Treatment. J. Leukoc. Biol. 2016, 100, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Ferris, S.T.; Carrero, J.A.; Mohan, J.F.; Calderon, B.; Murphy, K.M.; Unanue, E.R. A Minor Subset of Batf3-Dependent Antigen Presenting Cells in Islets of Langerhans Is Essential for the Development of Autoimmune Diabetes. Immunity 2014, 41, 657–669. [Google Scholar] [CrossRef]
- Vervelde, L.; Reemers, S.S.; Van Haarlem, D.A.; Post, J.; Claassen, E.; Rebel, J.M.J.; Jansen, C.A. Chicken Dendritic Cells Are Susceptible to Highly Pathogenic Avian Influenza Viruses Which Induce Strong Cytokine Responses. Dev. Comp. Immunol. 2013, 39, 198–206. [Google Scholar] [CrossRef]
- Waithman, J.; Mintern, J.D. Dendritic Cells and Influenza A Virus Infection. Virulence 2012, 3, 603–608. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mildner, A.; Jung, S. Development and Function of Dendritic Cell Subsets. Immunity 2014, 40, 642–656. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, M.B.; Kacha, A.K.; Kline, J.; Woo, S.-R.; Kranz, D.M.; Murphy, K.M.; Gajewski, T.F. Host Type I IFN Signals Are Required for Antitumor CD8+ T Cell Responses through CD8{alpha}+ Dendritic Cells. J. Exp. Med. 2011, 208, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Paulete, A.R.; Cueto, F.J.; Martínez-López, M.; Labiano, S.; Morales-Kastresana, A.; Rodríguez-Ruiz, M.E.; Jure-Kunkel, M.; Azpilikueta, A.; Aznar, M.A.; Quetglas, J.I.; et al. Cancer Immunotherapy with Immunomodulatory Anti-CD137 and Anti-PD-1 Monoclonal Antibodies Requires BATF3-Dependent Dendritic Cells. Cancer Discov. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Andersen, P.; Woodworth, J.S. Tuberculosis Vaccines--Rethinking the Current Paradigm. Trends Immunol. 2014, 35, 387–395. [Google Scholar] [CrossRef]
- Tacken, P.J.; Torensma, R.; Figdor, C.G. Targeting Antigens to Dendritic Cells in Vivo. Immunobiology 2006, 211, 599–608. [Google Scholar] [CrossRef]
- Bolland, S.; Ravetch, J.V. Inhibitory Pathways Triggered by ITIM-Containing Receptors. Adv. Immunol. 1999, 72, 149–177. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.E.; Titball, R.; Williamson, D. Vaccine Delivery Using Nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef]
- Lee, J.; Breton, G.; Oliveira, T.Y.K.; Zhou, Y.J.; Aljoufi, A.; Puhr, S.; Cameron, M.J.; Sékaly, R.-P.; Nussenzweig, M.C.; Liu, K. Restricted Dendritic Cell and Monocyte Progenitors in Human Cord Blood and Bone Marrow. J. Exp. Med. 2015, 212, 385–399. [Google Scholar] [CrossRef]
- Collin, M.; Bigley, V. Human Dendritic Cell Subsets: An Update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef]
- Breton, G.; Lee, J.; Zhou, Y.J.; Schreiber, J.J.; Keler, T.; Puhr, S.; Anandasabapathy, N.; Schlesinger, S.; Caskey, M.; Liu, K.; et al. Circulating Precursors of Human CD1c+ and CD141+ Dendritic Cells. J. Exp. Med. 2015, 212, 401–413. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, M.; Mok, W.H.; Radford, K.J. Human Dendritic Cell Subsets and Function in Health and Disease. Cell. Mol. Life Sci. CMLS 2015, 72, 4309–4325. [Google Scholar] [CrossRef] [PubMed]
- Halim, T.Y.F.; Hwang, Y.Y.; Scanlon, S.T.; Zaghouani, H.; Garbi, N.; Fallon, P.G.; McKenzie, A.N.J. Group 2 Innate Lymphoid Cells License Dendritic Cells to Potentiate Memory TH2 Cell Responses. Nat. Immunol. 2016, 17, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Haniffa, M.; Collin, M.; Ginhoux, F. Ontogeny and Functional Specialization of Dendritic Cells in Human and Mouse. Adv. Immunol. 2013, 120, 1–49. [Google Scholar] [CrossRef]
- Wilson, N.; El-shukhari, D.; Belz, G.T.; Smith, C.; Steptoe, R.J.; Heath, W.R.; Shortman, K.; Villadangos, J.A. Most Lymphoid Organ Dendritic Cell Types Are Phenotypically and Functionally Immature—ScienceDirect. Blood 2003, 102, 2187–2194. [Google Scholar] [CrossRef]
- Wilson, N.S.; Young, L.J.; Kupresanin, F.; Naik, S.H.; Vremec, D.; Heath, W.R.; Akira, S.; Shortman, K.; Boyle, J.; Maraskovsky, E.; et al. Normal Proportion and Expression of Maturation Markers in Migratory Dendritic Cells in the Absence of Germs or Toll-like Receptor Signaling. Immunol. Cell Biol. 2008, 86, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Gilliet, M.; Cao, W.; Liu, Y.-J. Plasmacytoid Dendritic Cells: Sensing Nucleic Acids in Viral Infection and Autoimmune Diseases. Nat. Rev. Immunol. 2008, 8, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Barragan, L.; Lewis, K.L.; Firner, S.; Thiel, V.; Hugues, S.; Reith, W.; Ludewig, B.; Reizis, B. Plasmacytoid Dendritic Cells Control T-Cell Response to Chronic Viral Infection. Proc. Natl. Acad. Sci. USA 2012, 109, 3012–3017. [Google Scholar] [CrossRef]
- Meredith, M.M.; Liu, K.; Darrasse-Jeze, G.; Kamphorst, A.O.; Schreiber, H.A.; Guermonprez, P.; Idoyaga, J.; Cheong, C.; Yao, K.-H.; Niec, R.E.; et al. Expression of the Zinc Finger Transcription Factor ZDC (Zbtb46, Btbd4) Defines the Classical Dendritic Cell Lineage. J. Exp. Med. 2012, 209, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.W.; Tjota, M.Y.; Clay, B.S.; Vander Lugt, B.; Bandukwala, H.S.; Hrusch, C.L.; Decker, D.C.; Blaine, K.M.; Fixsen, B.R.; Singh, H.; et al. Transcription Factor IRF4 Drives Dendritic Cells to Promote Th2 Differentiation. Nat. Commun. 2013, 4, 2990. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Liu, K.; Helft, J.; Bogunovic, M.; Greter, M.; Hashimoto, D.; Price, J.; Yin, N.; Bromberg, J.; Lira, S.A.; et al. The Origin and Development of Nonlymphoid Tissue CD103+ DCs. J. Exp. Med. 2009, 206, 3115–3130. [Google Scholar] [CrossRef]
- Hubert, F.-X.; Voisine, C.; Louvet, C.; Heslan, M.; Josien, R. Rat Plasmacytoid Dendritic Cells Are an Abundant Subset of MHC Class II+ CD4+CD11b-OX62- and Type I IFN-Producing Cells That Exhibit Selective Expression of Toll-like Receptors 7 and 9 and Strong Responsiveness to CpG. J. Immunol. (Baltim. Md. 1950) 2004, 172, 7485–7494. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.M.; Bonnefont-Rebeix, C.; Rigal, D.; Chabanne, L. Dendritic Cells in Different Animal Species: An Overview. Pathol. Biol. (Paris) 2006. [Google Scholar] [CrossRef] [PubMed]
- Richters, C.D.; Mayen, I.; Havenith, C.E.G.; Beelen, R.H.J.; Kamperdijk, E.W.A. Rat Monocyte-Derived Dendritic Cells Function and Migrate in the Same Way as Isolated Tissue Dendritic Cells. J. Leukoc. Biol. 2002, 71, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Olah, I.; Glick, B. Structure of the Germinal Centers in the Chicken Caecal Tonsil: Light and Electron Microscopic and Autoradiographic Studies. Poult. Sci. 1979, 58, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, A.R.; Yeap, S.K.; Tan, S.W.; Hair-Bejo, M.; Fakurazi, S.; Kaiser, P.; Omar, A.R. In Vitro Characterization of Chicken Bone Marrow-Derived Dendritic Cells Following Infection with Very Virulent Infectious Bursal Disease Virus. Avian Pathol. J. WVPA 2015, 44, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Igyártó, B.-Z.; Magyar, A.; Oláh, I. Origin of Follicular Dendritic Cell in the Chicken Spleen. Cell Tissue Res. 2007, 327, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Gomez Perdiguero, E.; Klapproth, K.; Schulz, C.; Busch, K.; Azzoni, E.; Crozet, L.; Garner, H.; Trouillet, C.; De Bruijn, M.F.; Geissmann, F.; et al. Tissue-Resident Macrophages Originate from Yolk-Sac-Derived Erythro-Myeloid Progenitors. Nature 2015, 518, 547–551. [Google Scholar] [CrossRef]
- Garceau, V.; Smith, J.; Paton, I.R.; Davey, M.; Fares, M.A.; Sester, D.P.; Burt, D.W.; Hume, D.A. Pivotal Advance: Avian Colony-Stimulating Factor 1 (CSF-1), Interleukin-34 (IL-34), and CSF-1 Receptor Genes and Gene Products. J. Leukoc. Biol. 2010, 87, 753–764. [Google Scholar] [CrossRef]
- Garcia-Morales, C.; Rothwell, L.; Moffat, L.; Garceau, V.; Balic, A.; Sang, H.M.; Kaiser, P.; Hume, D.A. Production and Characterisation of a Monoclonal Antibody That Recognises the Chicken CSF1 Receptor and Confirms That Expression Is Restricted to Macrophage-Lineage Cells. Dev. Comp. Immunol. 2014, 42, 278–285. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, T.; Kaiser, P. Chicken CCR6 and CCR7 Are Markers for Immature and Mature Dendritic Cells Respectively. Dev. Comp. Immunol. 2011, 35, 563–567. [Google Scholar] [CrossRef]
- Goodell, E.M.; Blumenstock, D.A.; Bowers, W.E. Canine Dendritic Cells from Peripheral Blood and Lymph Nodes. Vet. Immunol. Immunopathol. 1985, 8, 301–310. [Google Scholar] [CrossRef]
- Fitting, J.; Killian, D.; Junghanss, C.; Willenbrock, S.; Murua Escobar, H.; Lange, S.; Nolte, I.; Barth, S.; Tur, M.K. Generation of Recombinant Antibody Fragments That Target Canine Dendritic Cells by Phage Display Technology. Vet. Comp. Oncol. 2011, 9, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Ricklin Gutzwiller, M.E.; Moulin, H.R.; Zurbriggen, A.; Roosje, P.; Summerfield, A. Comparative Analysis of Canine Monocyte- and Bone-Marrow-Derived Dendritic Cells. Vet. Res. 2010, 41, 40. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Chi, K.-H.; Liao, K.-W.; Liu, C.-C.; Cheng, C.-L.; Lin, Y.-C.; Cheng, C.-H.; Chu, R.-M. Characterization of Canine Monocyte-Derived Dendritic Cells with Phenotypic and Functional Differentiation. Can. J. Vet. Res. 2007, 71, 165–174. [Google Scholar]
- Sugiura, K.; Wijewardana, V.; Fujimoto, M.; Akazawa, T.; Yahata, M.; Mito, K.; Hatoya, S.; Inoue, N.; Inaba, T. Effect of IL-12 on Canine Dendritic Cell Maturation Following Differentiation Induced by Granulocyte-Macrophage CSF and IL-4. Vet. Immunol. Immunopathol. 2010, 137, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rebeix, C.; De Carvalho, C.M.; Bernaud, J.; Chabanne, L.; Marchal, T.; Rigal, D. CD86 Molecule Is a Specific Marker for Canine Monocyte-Derived Dendritic Cells. Vet. Immunol. Immunopathol. 2006, 109, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Bienzle, D.; Reggeti, F.; Clark, M.E.; Chow, C. Immunophenotype and Functional Properties of Feline Dendritic Cells Derived from Blood and Bone Marrow. Vet. Immunol. Immunopathol. 2003, 96, 19–30. [Google Scholar] [CrossRef]
- Sprague, W.S.; Pope, M.; Hoover, E.A. Culture and Comparison of Feline Myeloid Dendritic Cells vs Macrophages. J. Comp. Pathol. 2005, 133, 136–145. [Google Scholar] [CrossRef]
- Nair, S.; McLaughlin, C.; Weizer, A.; Su, Z.; Boczkowski, D.; Dannull, J.; Vieweg, J.; Gilboa, E. Injection of Immature Dendritic Cells into Adjuvant-Treated Skin Obviates the Need for Ex Vivo Maturation. J. Immunol. (Baltim. Md. 1950) 2003, 171, 6275–6282. [Google Scholar] [CrossRef] [PubMed]
- Meyer, W.; Hornickel, I.; Schoennagel, B. A Note on Langerhans Cells in the Oesophagus Epithelium of Domesticated Mammals. Anat. Histol. Embryol. 2010, 39, 160–166. [Google Scholar] [CrossRef]
- Rivera, J.A.; McGuire, T.C. Equine Infectious Anemia Virus-Infected Dendritic Cells Retain Antigen Presentation Capability. Virology 2005, 335, 145–154. [Google Scholar] [CrossRef]
- Kurotaki, T.; Narayama, K.; Oyamada, T.; Yoshikawa, H.; Yoshikawa, T. Ultrastructural Study of Langerhans Cells in Equine Insect Hypersensitivity “Kasen. ” J. Vet. Med. Sci. 2000, 62, 1021–1023. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kratochvílová, L.; Sláma, P. Overview of Bovine Dendritic Cells. Acta Univ. Agric. Silvic. Mendel. Brun. 2018, 66, 815–821. [Google Scholar] [CrossRef]
- Summerfield, A.; Auray, G.; Ricklin, M. Comparative Dendritic Cell Biology of Veterinary Mammals. Annu. Rev. Anim. Biosci. 2015, 3, 533–557. [Google Scholar] [CrossRef]
- Guinan, J.; Lopez, B.S. Generating Bovine Monocyte-Derived Dendritic Cells for Experimental and Clinical Applications Using Commercially Available Serum-Free Medium. Front. Immunol. 2020, 11, 591185. [Google Scholar] [CrossRef]
- Stephens, S.A.; Brownlie, J.; Charleston, B.; Howard, C.J. Differences in Cytokine Synthesis by the Sub-Populations of Dendritic Cells from Afferent Lymph. Immunology 2003, 110, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Schwartz-Cornil, I.; Epardaud, M.; Albert, J.-P.; Bourgeois, C.; Gérard, F.; Raoult, I.; Bonneau, M. Probing Leukocyte Traffic in Lymph from Oro-Nasal Mucosae by Cervical Catheterization in a Sheep Model. J. Immunol. Methods 2005, 305, 152–161. [Google Scholar] [CrossRef]
- Epardaud, M.; Bonneau, M.; Payot, F.; Cordier, C.; Mégret, J.; Howard, C.; Schwartz-Cornil, I. Enrichment for a CD26hi SIRP- Subset in Lymph Dendritic Cells from the Upper Aero-Digestive Tract. J. Leukoc. Biol. 2004, 76, 553–561. [Google Scholar] [CrossRef]
- Chan, S.S.M.; McConnell, I.; Blacklaws, B.A. Generation and Characterization of Ovine Dendritic Cells Derived from Peripheral Blood Monocytes. Immunology 2002, 107, 366–372. [Google Scholar] [CrossRef]
- Bonneau, M.; Epardaud, M.; Payot, F.; Niborski, V.; Thoulouze, M.-I.; Bernex, F.; Charley, B.; Riffault, S.; Guilloteau, L.A.; Schwartz-Cornil, I. Migratory Monocytes and Granulocytes Are Major Lymphatic Carriers of Salmonella from Tissue to Draining Lymph Node. J. Leukoc. Biol. 2006, 79, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Crozat, K.; Tamoutounour, S.; Vu Manh, T.-P.; Fossum, E.; Luche, H.; Ardouin, L.; Guilliams, M.; Azukizawa, H.; Bogen, B.; Malissen, B.; et al. Cutting Edge: Expression of XCR1 Defines Mouse Lymphoid-Tissue Resident and Migratory Dendritic Cells of the CD8α+ Type. J. Immunol. (Baltim. Md. 1950) 2011, 187, 4411–4415. [Google Scholar] [CrossRef]
- Pascale, F.; Pascale, F.; Contreras, V.; Bonneau, M.; Courbet, A.; Chilmonczyk, S.; Bevilacqua, C.; Epardaud, M.; Eparaud, M.; Niborski, V.; et al. Plasmacytoid Dendritic Cells Migrate in Afferent Skin Lymph. J. Immunol. (Baltim. Md. 1950) 2008, 180, 5963–5972. [Google Scholar] [CrossRef]
- Carrasco, C.P.; Rigden, R.C.; Schaffner, R.; Gerber, H.; Neuhaus, V.; Inumaru, S.; Takamatsu, H.; Bertoni, G.; McCullough, K.C.; Summerfield, A. Porcine Dendritic Cells Generated in Vitro: Morphological, Phenotypic and Functional Properties. Immunology 2001, 104, 175–184. [Google Scholar] [CrossRef]
- Summerfield, A.; Guzylack-Piriou, L.; Schaub, A.; Carrasco, C.P.; Tâche, V.; Charley, B.; McCullough, K.C. Porcine Peripheral Blood Dendritic Cells and Natural Interferon-Producing Cells. Immunology 2003, 110, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Marquet, F.; Bonneau, M.; Pascale, F.; Urien, C.; Kang, C.; Schwartz-Cornil, I.; Bertho, N. Characterization of Dendritic Cells Subpopulations in Skin and Afferent Lymph in the Swine Model. PLoS ONE 2011, 6, e16320. [Google Scholar] [CrossRef] [PubMed]
- Maisonnasse, P.; Bouguyon, E.; Piton, G.; Ezquerra, A.; Urien, C.; Deloizy, C.; Bourge, M.; Leplat, J.-J.; Simon, G.; Chevalier, C.; et al. The Respiratory DC/Macrophage Network at Steady-State and upon Influenza Infection in the Swine Biomedical Model. Mucosal Immunol. 2016, 9, 835–849. [Google Scholar] [CrossRef]
- Prasad, S.; Kireta, S.; Leedham, E.; Russ, G.R.; Coates, P.T.H. Propagation and Characterisation of Dendritic Cells from G-CSF Mobilised Peripheral Blood Monocytes and Stem Cells in Common Marmoset Monkeys. J. Immunol. Methods 2010, 352, 59–70. [Google Scholar] [CrossRef]
- Barratt-Boyes, S.M.; Henderson, R.A.; Finn, O.J. Chimpanzee Dendritic Cells with Potent Immunostimulatory Function Can Be Propagated from Peripheral Blood. Immunology 1996, 87, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, L.M.; Grouard-Vogel, G.; Magaletti, D.M.; Doty, R.T.; Andrews, R.G.; Clark, E.A. Isolation and Characterization of Macaque Dendritic Cells from CD34(+) Bone Marrow Progenitors. Cell. Immunol. 1999, 196, 34–40. [Google Scholar] [CrossRef]
- Mehlhop, E.; Villamide, L.A.; Frank, I.; Gettie, A.; Santisteban, C.; Messmer, D.; Ignatius, R.; Lifson, J.D.; Pope, M. Enhanced in Vitro Stimulation of Rhesus Macaque Dendritic Cells for Activation of SIV-Specific T Cell Responses. J. Immunol. Methods 2002, 260, 219–234. [Google Scholar] [CrossRef]
- Coates, P.T.H.; Barratt-Boyes, S.M.; Zhang, L.; Donnenberg, V.S.; O’Connell, P.J.; Logar, A.J.; Duncan, F.J.; Murphey-Corb, M.; Donnenberg, A.D.; Morelli, A.E.; et al. Dendritic Cell Subsets in Blood and Lymphoid Tissue of Rhesus Monkeys and Their Mobilization with Flt3 Ligand. Blood 2003, 102, 2513–2521. [Google Scholar] [CrossRef]
- Puhr, S.; Lee, J.; Zvezdova, E.; Zhou, Y.J.; Liu, K. Dendritic Cell Development-History, Advances, and Open Questions. Semin. Immunol. 2015, 27, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Zmrhal, V.; Slama, P. Immunomodulation of Avian Dendritic Cells under the Induction of Prebiotics. Animals 2020, 10, 698. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Archer, G.E.; Tedder, T.F. Isolation and Generation of Human Dendritic Cells. Curr. Protoc. Immunol. 2012, 99, 7.32.1–7.32.23. [Google Scholar] [CrossRef]
- Junginger, J.; Lemensieck, F.; Moore, P.F.; Schwittlick, U.; Nolte, I.; Hewicker-Trautwein, M. Canine Gut Dendritic Cells in the Steady State and in Inflammatory Bowel Disease. Innate Immun. 2014, 20, 145–160. [Google Scholar] [CrossRef]
- Hutten, T.J.A.; Thordardottir, S.; Fredrix, H.; Janssen, L.; Woestenenk, R.; Tel, J.; Joosten, B.; Cambi, A.; Heemskerk, M.H.M.; Franssen, G.M.; et al. CLEC12A-Mediated Antigen Uptake and Cross-Presentation by Human Dendritic Cell Subsets Efficiently Boost Tumor-Reactive T Cell Responses. J. Immunol. (Baltim. Md. 1950) 2016, 197, 2715–2725. [Google Scholar] [CrossRef] [PubMed]
- Mauel, S.; Steinbach, F.; Ludwig, H. Monocyte-Derived Dendritic Cells from Horses Differ from Dendritic Cells of Humans and Mice. Immunology 2006, 117, 463–473. [Google Scholar] [CrossRef]
- McKenzie, E.J.; Taylor, P.R.; Stillion, R.J.; Lucas, A.D.; Harris, J.; Gordon, S.; Martinez-Pomares, L. Mannose Receptor Expression and Function Define a New Population of Murine Dendritic Cells. J. Immunol. (Baltim. Md. 1950) 2007, 178, 4975–4983. [Google Scholar] [CrossRef]
- Staines, K.; Hunt, L.G.; Young, J.R.; Butter, C. Evolution of an Expanded Mannose Receptor Gene Family. PLoS ONE 2014, 9, e110330. [Google Scholar] [CrossRef]
- Kulakova, N.; Urban, B.; McMichael, A.J.; Ho, L.-P. Functional Analysis of Dendritic Cell–T Cell Interaction in Sarcoidosis. Clin. Exp. Immunol. 2010, 159, 82–86. [Google Scholar] [CrossRef]
- Colić, M.; Jandrić, D.; Stojić-Vukanić, Z.; Antić-Stanković, J.; Popović, P.; Vasilijić, S.; Milosavljević, P.; Balint, B. Differentiation of Human Dendritic Cells from Monocytes in Vitro Using Granulocyte-Macrophage Colony Stimulating Factor and Low Concentration of Interleukin-4. Vojnosanit. Pregl. 2003, 60, 531–538. [Google Scholar] [CrossRef]
- Nagy, N.; Bódi, I.; Oláh, I. Avian Dendritic Cells: Phenotype and Ontogeny in Lymphoid Organs. Dev. Comp. Immunol. 2016, 58, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Clayton, K.; Vallejo, A.F.; Davies, J.; Sirvent, S.; Polak, M.E. Langerhans Cells-Programmed by the Epidermis. Front. Immunol. 2017, 8, 1676. [Google Scholar] [CrossRef]
- Stamatos, N.M.; Carubelli, I.; Van de Vlekkert, D.; Bonten, E.J.; Papini, N.; Feng, C.; Venerando, B.; D’Azzo, A.; Cross, A.S.; Wang, L.-X.; et al. LPS-Induced Cytokine Production in Human Dendritic Cells Is Regulated by Sialidase Activity. J. Leukoc. Biol. 2010, 88, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Van Goor, A.; Slawinska, A.; Schmidt, C.J.; Lamont, S.J. Distinct Functional Responses to Stressors of Bone Marrow Derived Dendritic Cells from Diverse Inbred Chicken Lines. Dev. Comp. Immunol. 2016, 63, 96–110. [Google Scholar] [CrossRef]
- Qu, X.; Cinar, M.U.; Fan, H.; Pröll, M.; Tesfaye, D.; Tholen, E.; Looft, C.; Hölker, M.; Schellander, K.; Uddin, M.J. Comparison of the Innate Immune Responses of Porcine Monocyte-Derived Dendritic Cells and Splenic Dendritic Cells Stimulated with LPS. Innate Immun. 2015, 21, 242–254. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Wu, K.; Azhati, B.; Rexiati, M. Culture and Identification of Mouse Bone Marrow-Derived Dendritic Cells and Their Capability to Induce T Lymphocyte Proliferation. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Fujita, S. Dendritic Cells: Nature and Classification. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2007, 56, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.; Everett, H.; Hamza, E.; Garbani, M.; Gerber, V.; Marti, E.; Steinbach, F. Equine Dendritic Cells Generated with Horse Serum Have Enhanced Functionality in Comparison to Dendritic Cells Generated with Fetal Bovine Serum. BMC Vet. Res. 2016, 12, 254. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.J.; Silveira, P.A.; Hogarth, P.M.; Hart, D.N.J. The Cell Surface Phenotype of Human Dendritic Cells. Semin. Cell Dev. Biol. 2019, 86, 3–14. [Google Scholar] [CrossRef]
- Gnanadevi, R.; Kannan, T.A.; Ushakumary, S.; Ramesh, G. Morphology, Distribution and Functional Significance of Dendritic Cells (DCs) in the Skin of Domestic Animals. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1554–1560. [Google Scholar] [CrossRef][Green Version]
- Akesson, C.P.; McL Press, C.; Espenes, A.; Aleksandersen, M. Phenotypic Characterisation of Intestinal Dendritic Cells in Sheep. Dev. Comp. Immunol. 2008, 32, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Bautista, E.M.; Gregg, D.; Golde, W.T. Characterization and Functional Analysis of Skin-Derived Dendritic Cells from Swine without a Requirement for in Vitro Propagation. Vet. Immunol. Immunopathol. 2002, 88, 131–148. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanna, M.Y.; Yasmin, A.R.; Omar, A.R.; Arshad, S.S.; Mariatulqabtiah, A.R.; Nur-Fazila, S.H.; Mahiza, M.I.N. Review of Dendritic Cells, Their Role in Clinical Immunology, and Distribution in Various Animal Species. Int. J. Mol. Sci. 2021, 22, 8044. https://doi.org/10.3390/ijms22158044
Zanna MY, Yasmin AR, Omar AR, Arshad SS, Mariatulqabtiah AR, Nur-Fazila SH, Mahiza MIN. Review of Dendritic Cells, Their Role in Clinical Immunology, and Distribution in Various Animal Species. International Journal of Molecular Sciences. 2021; 22(15):8044. https://doi.org/10.3390/ijms22158044
Chicago/Turabian StyleZanna, Mohammed Yusuf, Abd Rahaman Yasmin, Abdul Rahman Omar, Siti Suri Arshad, Abdul Razak Mariatulqabtiah, Saulol Hamid Nur-Fazila, and Md Isa Nur Mahiza. 2021. "Review of Dendritic Cells, Their Role in Clinical Immunology, and Distribution in Various Animal Species" International Journal of Molecular Sciences 22, no. 15: 8044. https://doi.org/10.3390/ijms22158044
APA StyleZanna, M. Y., Yasmin, A. R., Omar, A. R., Arshad, S. S., Mariatulqabtiah, A. R., Nur-Fazila, S. H., & Mahiza, M. I. N. (2021). Review of Dendritic Cells, Their Role in Clinical Immunology, and Distribution in Various Animal Species. International Journal of Molecular Sciences, 22(15), 8044. https://doi.org/10.3390/ijms22158044







