Dual Nature of Relationship between Mycobacteria and Cancer
Abstract
1. Introduction
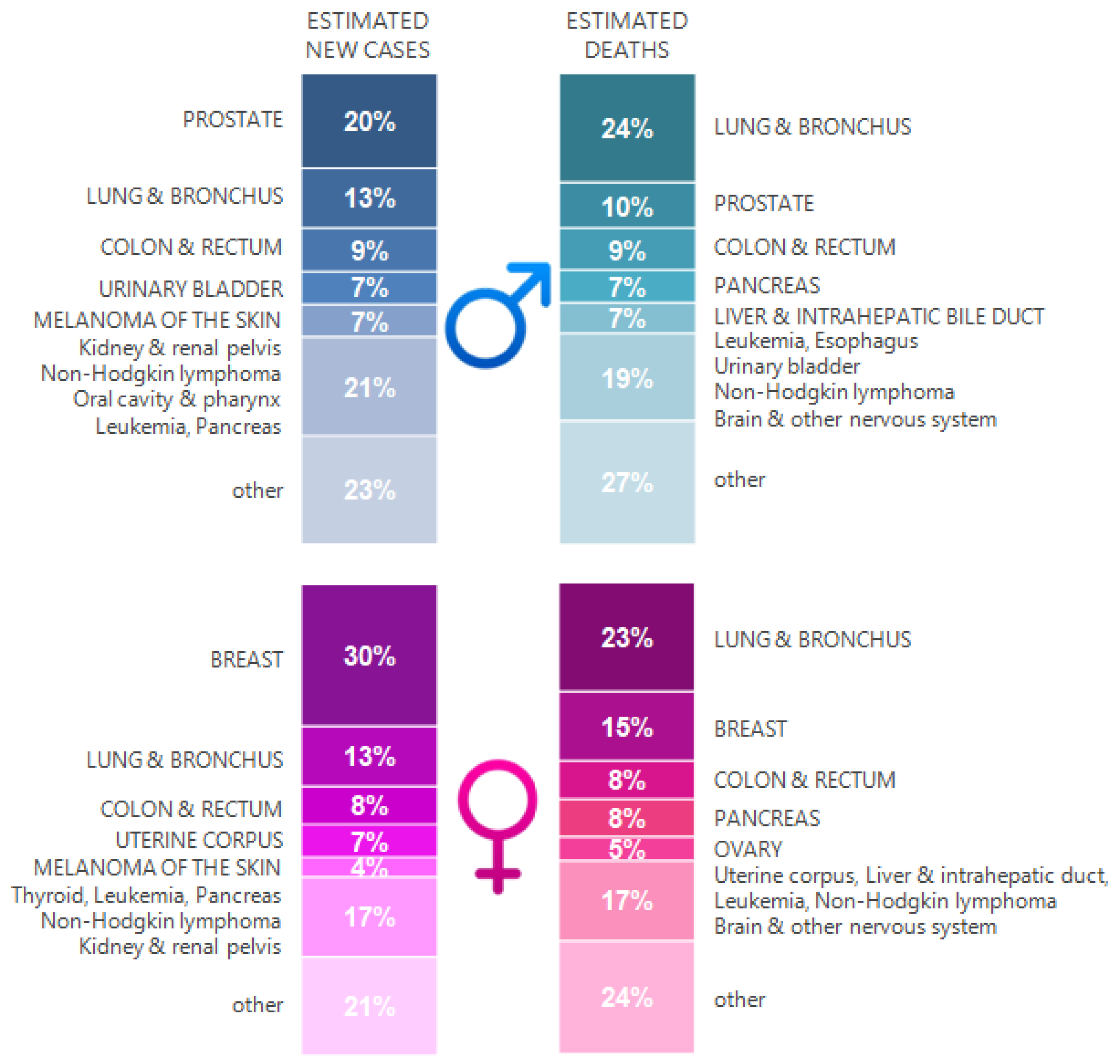
2. TB and Lung Cancer Epidemiology
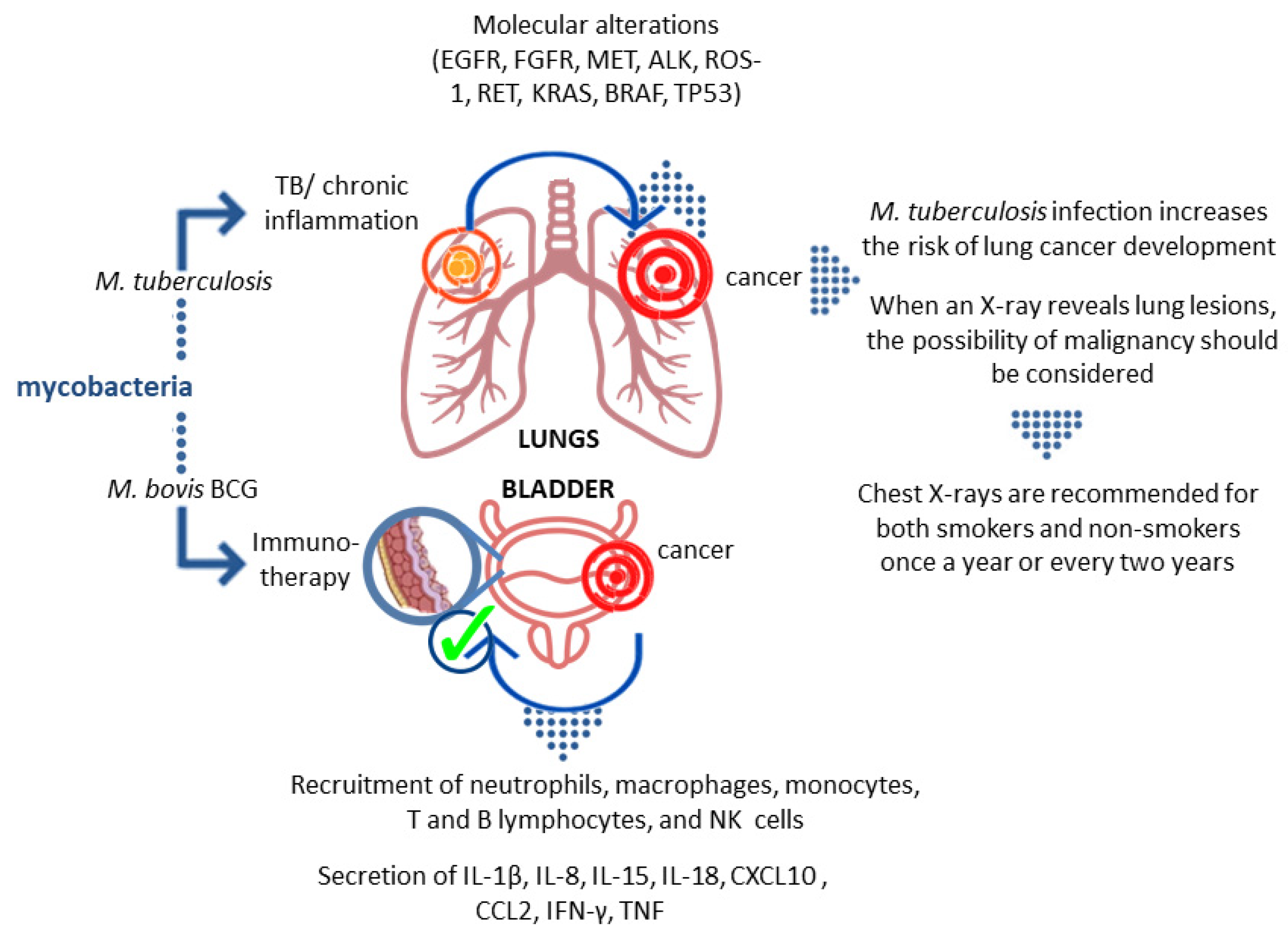
3. Association of M.tb Infection with Malignancy Development
4. Mycobacteria as Causative Agents of Cancer
5. Mycobacteria as Therapeutic Agents
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Houben, R.M.; Dodd, P.J. The global burden of latent tuberculosis infection: A re-estimation using mathematical modelling. PLoS Med. 2016, 13, e1002152. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Tuberculosis Report. 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf (accessed on 30 June 2021).
- Di Gennaro, F.; Gualano, G.; Timelli, L.; Vittozzi, P.; Di Bari, V.; Libertone, R.; Cerva, C.; Pinnarelli, L.; Nisii, C.; Ianniello, S.; et al. Increase in Tuberculosis Diagnostic Delay during First Wave of the COVID-19 Pandemic: Data from an Italian Infectious Disease Referral Hospital. Antibiotics 2021, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Religioni, U. Cancer incidence and mortality in Poland. Clin. Epidemiol Glob. Health 2020, 8, 329–334. [Google Scholar] [CrossRef]
- Obecny Stan Zwalczania Nowotworów w Polsce. 2014. Available online: http://www.walkazrakiem.pl/sites/default/files/library/files/obecny_stan_zwalczania_nowotworow_w_polsce_10-07-2014_2.pdf (accessed on 30 June 2021).
- Ren, H.; Xu, D.; Shi, X.; Xu, J.; Zhuang, D.; Yang, G. Characterization of gastric cancer and its relation to environmental factors: A case study in Shenqiu County, China. Int. J. Env. Health Res. 2016, 26, 1369–1619. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Wojciechowska, U.; Didkowska, J.; Michałek, I.; Olasek, P.; Ciuba, A. Cancer in Poland in 2018. In Raport Narodowego Instytutu Onkologii; Narodowego Instytutu Onkologii: Warsaw, Poland, 2020. [Google Scholar]
- Modlińska, A.; Kowalczyk, A. Rak płuca–epidemiologia, obraz kliniczny oraz społeczne następstwa choroby. Psychoonkologia 2016, 20, 57–65. [Google Scholar] [CrossRef]
- Giovino, G.A.; Mirza, S.A.; Samet, J.M.; Gupta, P.C.; Jarvis, M.; Bhala, N.; Peto, R.; Zatonski, W.; Hsia, J.; Morton, J.; et al. Tobacco use in 3 billion individuals from 16 countries: An analysis of nationally representative cross-sectional household surveys. Lancet 2012, 380, 668–679. [Google Scholar] [CrossRef]
- Stiles, B.M.; Rahouma, M.; Hussein, M.K.; Nasar, A.; Nguyen, A.B.; Harrison, S.; Lee, B.; Port, J.L.; Altorki, N.K. Never smokers with resected lung cancer: Different demographics, similar survival. Eur. J. Cardio-Thorac Surg. 2018, 53, 842–848. [Google Scholar] [CrossRef]
- Saito, S.; Espinoza-Mercado, F.; Liu, H.; Sata, N.; Cui, X.; Soukiasian, H.J. Current status of research and treatment for non-small cell lung cancer in never-smoking females. Cancer Biol. Ther. 2017, 18, 359–368. [Google Scholar] [CrossRef]
- Barrera-Rodriguez, R.; Morales-Fuentes, J. Lung cancer in women. Lung Cancer 2012, 3, 79–89. [Google Scholar]
- Samet, J.M.; Wiggins, C.L.; Humble, C.G.; Pathak, D.R. Cigarette smoking and lung cancer in New Mexico. Am. Rev. Respir Dis. 1988, 137, 1110–1113. [Google Scholar] [CrossRef] [PubMed]
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef]
- Seo, S.; Ha, W.H.; Kang, J.K.; Lee, D.; Park, S.; Kwon, T.E.; Jin, Y.W. Health effects of exposure to radon: Implications of the radon bed mattress incident in Korea. Epidemiol. Health 2019, 41, e2019004. [Google Scholar] [CrossRef]
- Hammond, E.C.; Selikoff, I.J.; Seidman, H. Asbestos exposure, cigarette smoking, and death rates. Ann. N. Y. Acad. Sci. 1979, 330, 473–490. [Google Scholar] [CrossRef]
- Alberg, A.J.; Brock, M.V.; Ford, J.G.; Samet, J.M.; Spivack, S.D. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed.: American college of chest physicians evidence-based clinical practice guidelines. Chest 2013, 143, e1S–e29S. [Google Scholar] [CrossRef]
- Corrales, L.; Rosell, R.; Cardona, A.F.; Martín, C.; Zatarain-Barrón, Z.L.; Arrieta, O. Lung cancer in never smokers: The role of different risk factors other than tobacco smoking. Crit. Rev. Oncol. Hematol. 2020, 148, 102895. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.M.; Kim, E.H. Human papillomavirus infection and risk of lung cancer in never-smokers and women: An ‘adaptive’ meta-analysis. Epidemiol. Health 2015, 37, e2015052. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, L.; Jaxmar, T.; Casadio, C.; Gariglio, M.; Manna, A.; D’Antonio, D.; Syrjanen, K.; Favalli, C.; Ciotti, M. Detection of oncogenic viruses (SV40, BKV, JCV, HCMV, HPV) and p53 codon 72 polymorphism in lung carcinoma. Lung Cancer 2007, 57, 273–281. [Google Scholar] [CrossRef]
- de Groot, P.M.; Wu, C.C.; Carter, B.W.; Munden, R.F. The epidemiology of lung cancer. Transl. Lung Cancer Res. 2018, 7, 220–233. [Google Scholar] [CrossRef]
- Zheng, H.; Aziz, H.A.; Nakanishi, Y.; Masuda, S.; Saito, H.; Tsuneyama, K.; Takano, Y. Oncogenic role of JC virus in lung cancer. J. Pathol. 2007, 212, 306–315. [Google Scholar] [CrossRef]
- Qu, Y.-L.; Liu, J.; Zhang, L.-X.; Wu, C.-M.; Chu, A.-J.; Wen, B.-L.; Ma, C.; Yan, X.-Y.; Zhang, X.; Wang, D.-M.; et al. Asthma and the risk of lung cancer: A meta-analysis. Oncotarget 2017, 8, 11614–11620. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.R.; McLaughlin, J.R.; Hung, R.J. Previous lung diseases and lung cancer risk: A systematic review and meta-analysis. PLoS ONE 2011, 6, e17479. [Google Scholar] [CrossRef]
- Yu, Y.H.; Liao, C.C.; Hsu, W.H.; Chen, H.J.; Liao, W.C.; Muo, C.H.; Sung, F.C.; Chen, C.Y. Increased lung cancer risk among patients with pulmonary tuberculosis: A population cohort study. J. Thorac. Oncol. 2011, 6, 32–37. [Google Scholar] [CrossRef]
- Seo, G.H.; Kim, M.J.; Seo, S.; Hwang, B.; Lee, E.; Yun, Y.; Choi, M.; Kim, M.; Kim, J.W.; Kim, E.S.; et al. Can-cer-specific incidence rates of tuberculosis: A 5-year nationwide population-based study in a country with an intermediate tu-berculosis burden. Medicine 2016, 95, e4919. [Google Scholar] [CrossRef] [PubMed]
- Vento, S.; Lanzafame, M. Tuberculosis and cancer: A complex and dangerous liaison. Lancet Oncol. 2011, 12, 520–522. [Google Scholar] [CrossRef]
- Simonsen, D.F.; Farkas, D.K.; Horsburgh, C.R.; Thomsen, R.W.; Sørensen, H.T. Increased risk of active tuberculosis after cancer diagnosis. J. Infect. 2017, 74, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-Y.; Li, X.-L.; Yu, X.-S.; Guan, P.; Yin, Z.-H.; He, Q.-C.; Zhou, B.-S. Facts and fiction of the relationship between preexisting tuberculosis and lung cancer risk: A systematic review. Int. J. Cancer 2009, 125, 2936–2944. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.M.; Roh, Y.H.; Lim, D.; Kong, H.J.; Cho, H.; Hwangbo, B.; Won, Y.J.; Jung, K.W.; Oh, K. Pulmonary tuberculosis is associated with elevated risk of lung cancer in Korea: The nationwide cohort study. J. Cancer 2020, 11, 1899–1906. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Hu, H.-Y.; Pu, C.-Y.; Huang, N.; Shen, H.-C.; Li, C.-P.; Chou, Y.-J. Aerodigestive tract, lung and haematological cancers are risk factors for tuberculosis: An 8-year population-based study. Int. J. Tuberc. Lung Dis. 2011, 15, 125–130. [Google Scholar] [PubMed]
- Kim, H.-R.; Hwang, S.S.; Ro, Y.K.; Jeon, C.H.; Ha, D.Y.; Park, S.J.; Lee, C.-H.; Lee, S.-M.; Yoo, C.-G.; Kim, Y.W.; et al. Solid-organ malignancy as a risk factor for tuberculosis. Respirology 2008, 13, 413–419. [Google Scholar] [CrossRef]
- Skowroński, M.; Iwanik, K.; Halicka, A.; Barinow-Wojewódzki, A. Squamous cell lung cancer in a male with pulmonary tuberculosis. Pneumonol. Alergol. Pol. 2015, 83, 298–302. [Google Scholar] [CrossRef]
- Cukic, V. The association between lung carcinoma and tuberculosis. Med. Arch. 2017, 71, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Engels, E.A.; Shen, M.; Chapman, R.S.; Pfeiffer, R.M.; Yu, Y.Y.; He, X.; Lan, Q. Tuberculosis and subsequent risk of lung cancer in Xuanwei, China. Int. J. Cancer 2009, 124, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Cicènas, S.; Vencevičius, V. Lung cancer in patients with tuberculosis. World J. Surg. Oncol. 2007, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, M.; Kant, S.; Bhaskar, R. Pulmonary tuberculosis as differential diagnosis of lung cancer. South Asian J. Cancer 2012, 1, 36–42. [Google Scholar] [CrossRef]
- Fried, L.E.; Arbiser, J.L. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid Redox Signal. 2009, 11, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Liuzzo, G.; Trotta, F.; Pedicino, D. Interleukin-17 in atherosclerosis and cardiovascular disease: The good, the bad, and the unknown. Eur. Heart J. 2013, 34, 556–559. [Google Scholar] [CrossRef]
- Mariani, F.; Bocchino, M.; Cappelli, G.; Persechini, T.; Colizzi, V.; Bonanno, E.; Ponticiello, A.; Sanduzzi, A. Tuberculosis and lung cancer. An interesting case study. Monaldi Arch. Chest Dis 2001, 56, 30–32. [Google Scholar] [PubMed]
- Çiçek, Y.; Kosar, P.A.; Öztürk, Ö. Molecular genetics of lung cancer. Eurasian J. Pulmonol. 2018, 20, 111–117. [Google Scholar] [CrossRef]
- McCusker, C.; Warrington, R. Primary immunodeficiency. Allergy Asthma Clin. Immunol. 2011, 7 Suppl 1, S11. [Google Scholar] [CrossRef]
- Hauck, F.; Gennery, A.R.; Seidel, M.G. Editorial: The relationship between cancer predisposition and primary immunodeficiency. Front. Immunol. 2019, 30, 1781. [Google Scholar] [CrossRef] [PubMed]
- Boisson-Dupuis, S.; Bustamante, J.; El-Baghdadi, J.; Camcioglu, Y.; Parvaneh, N.; El Azbaoui, S.; Agader, A.; Hassani, A.; El Hafidi, N.; Mrani, N.A.; et al. Inherited and acquired immunodeficiencies underlying tuberculosis in childhood. Immunol. Rev. 2015, 264, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, J.; Boisson-Dupuis, S.; Abel, L.; Casanova, J.L. Mendelian susceptibility to mycobacterial disease: Genetic, immunological, and clinical features of inborn errors of IFN-γ immunity. Semin. Immunol. 2014, 26, 454–470. [Google Scholar] [CrossRef]
- Swinson, S.; Hall, G.; Pollard, A.J. Reactivation of the bacille Calmette-Guerin scar following immune reconstitution during treatment of infant acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 2004, 26, 112–115. [Google Scholar] [CrossRef]
- Arlotta, A.; Cefalo, M.G.; Maurizi, P.; Ruggiero, A.; Dodi, I.; Riccardi, R. Critical pulmonary infection due to nontuberculous mycobacterium in pediatric leukemia: Report of a difficult diagnosis and review of pediatric series. J. Pediatr. Hematol. Oncol. 2014, 36, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Claass, A.; Claviez, A.; Westphal, E.; Rusch-Gerdes, S.; Schneppenheim, R. First case of disseminated Mycobacterium avium infection following chemotherapy for childhood acute myeloid leukemia. Infection 1995, 23, 301–302. [Google Scholar] [CrossRef]
- Gupta, S.; Basu, A.K.; Guliani, A.; Dhamija, A. Hemophagocytic lymphohistiocytosis complicating nontuberculous mycobacterial infection. Lung India 2019, 36, 266–267. [Google Scholar] [PubMed]
- Valdez, J.M.; Scheinberg, P.; Young, N.S.; Walsh, T.J. Infections in patients with aplastic anemia. Semin. Hematol. 2009, 46, 269–276. [Google Scholar] [CrossRef]
- Patel, R.; Roberts, G.D.; Keating, M.R.; Paya, C.V. Infections due to nontuberculous mycobacteria in kidney, heart, and liver transplant recipients. Clin. Infect. Dis. 1994, 19, 263–273. [Google Scholar] [CrossRef]
- Searle, E.; Patel, H.; Vilar, F.J.; Gharib, M.; Turner, A.J.; Batra, G.; Wynn, R.F. Inflammatory BCG adenitis associated with immune reconstitution following allogeneic haematopoietic stem cell transplant in infancy. Pediatr. Blood Cancer 2010, 54, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Unal, E.; Yen, C.; Saiman, L.; George, D.; Della-Latta, P.; van de Ven, C.; Morris, E.; Bradley, M.B.; Del Toro, G.; Garvin, J.; et al. A low incidence of nontuberculous mycobacterial infections in pediatric hematopoietic stem cell transplantation recipients. Biol. Blood Marrow Transplant. 2006, 12, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Tshitenge, S.; Ogunbanjo, G.A.; Citeya, A. A mortality review of tuberculosis and HIV co-infected patients in Mahalapye, Botswana: Does cotrimoxazole preventive therapy and/or antiretroviral therapy protect against death? Afr. J. Prim. Health Care Fam. Med. 2018, 10, e1–e5. [Google Scholar] [CrossRef]
- Walker, N.F.; Meintjes, G.; Wilkinson, R.J. HIV-1 and the immune response to TB. Future Virol. 2013, 8, 57–80. [Google Scholar] [CrossRef]
- Lapinel, N.C.; Jolley, S.E.; Ali, J.; Welsh, D.A. Prevalence of non-tuberculous mycobacteria in HIV-infected patients admitted to hospital with pneumonia. Int. J. Tuberc Lung Dis. 2019, 23, 491–497. [Google Scholar] [CrossRef]
- Ames, B.N.; Gold, L.S.; Willett, W.C. The causes and prevention of cancer. Proc. Natl. Acad. Sci. USA 1995, 92, 5258–5265. [Google Scholar] [CrossRef]
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a key event in cancer development. Mol. Cancer Res. 2006, 4, 221–233. [Google Scholar] [CrossRef]
- Wu, C.Y.; Hu, H.Y.; Pu, C.Y.; Huang, N. Pulmonary tuberculosis increases the risk of lung cancer: A Population-based cohort study. Cancer 2011, 117, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.T.; Almenoff, P.L. Pulmonary mycobacterial infections associated with neoplasia. Semin. Respir. Infect. 1992, 7, 104–113. [Google Scholar]
- Alavanja, M.C.; Brownson, R.C.; Boice, J.D., Jr.; Hock, E. Preexisting lung disease and lung cancer among nonsmoking women. Am. J. Epidemiol. 1992, 136, 623–632. [Google Scholar] [CrossRef]
- Lande, L.; Gogoi, R.; Yankowski, C.; Stampler, K.; Sawicki, K.; Daum, G.; Peterson, D.D.; Sawicki, J. Association between pulmonary Mycobacterium avium complex infection and lung cancer. J. Thorac. Oncol. 2012, 7, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.R.; Etuaful, S.N.; Amofah, G.; Adjei, O.; Lucas, S.; Wansbrough-Jones, M.H. Squamous cell carcinoma secondary to Buruli ulcer. Trans. R Soc. Trop. Med. Hyg. 1999, 93, 63–64. [Google Scholar] [CrossRef]
- Stanley, S.A.; Cox, J.S. Host-pathogen interactions during Mycobacterium tuberculosis infections. Curr. Top. Microbiol. Immunol. 2013, 374, 211–241. [Google Scholar] [PubMed]
- Ehrt, S.; Schnappinger, D. Mycobacterial survival strategies in the phagosome: Defence against host stresses. Cell Microbiol. 2009, 11, 1170–1178. [Google Scholar] [CrossRef]
- Guirado, E.; Schlesinger, L.S. Modeling the Mycobacterium tuberculosis granuloma—the critical battlefield in host immunity and disease. Front. Immunol. 2013, 4, 98. [Google Scholar] [CrossRef]
- Dalgleish, A.G.; O’Byrne, K.J. Chronic immune activation and inflammation in the pathogenesis of AIDS and cancer. Adv. Cancer Res. 2002, 84, 231–276. [Google Scholar]
- Melnikova, V.O.; Ananthaswamy, H.N. Cellular and molecular events leading to the development of skin cancer. Mutat. Res. 2005, 571, 91–106. [Google Scholar] [CrossRef]
- Azad, N.; Rojanasakul, Y.; Vallyathan, V. Inflammation and lung cancer: Roles of reactive oxygen/nitrogen species. J. Toxicol. Environ. Health B Crit. Rev. 2008, 11, 1–15. [Google Scholar] [CrossRef]
- Bowden, G.T. Prevention of non-melanoma skin cancer by targeting ultraviolet-b-light signalling. Nat. Rev. Cancer 2004, 4, 23–35. [Google Scholar] [CrossRef]
- Broussard, G.W.; Norris, M.B.; Schwindt, A.R.; Fournie, J.W.; Winn, R.N.; Kent, M.L.; Ennis, D.G. Chronic Mycobacterium marinum infection acts as a tumor promoter in Japanese Medaka (Oryziaslatipes). Comp. Biochem. Physiol. C Toxicol. Pharm. 2009, 149, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Teventiyanon, T.; Ratanaharathorn, V.; Leoparait, J. Mucoepidermoid carcinoma of the lung presenting as cavitary lesion. J. Med. Assoc. Thail. 2004, 87, 988–991. [Google Scholar]
- Yilmaz, A.; Gungor, S.; Damadoglu, E.; Axoy, F.; Aibatly, A. Coexisting bronchial carcinoid tumor and pulmonary tuberculosis in the same lobe: A case report. Tuberk Toraks 2004, 52, 369–372. [Google Scholar]
- Weitzman, S.A.; Weitberg, A.B.; Clark, E.P.; Stossel, T.P. Phagocytes as carcinogens: Malignant transformation produced by human neutrophils. Science 1985, 227, 1231–1233. [Google Scholar] [CrossRef]
- Shacter, E.; Beecham, E.J.; Covey, J.M.; Kohn, K.W.; Potter, M. Activated neutrophils induce prolonged DNA damage in neighboring cells. Carcinogenesis 1988, 9, 2297–2304. [Google Scholar] [CrossRef]
- Wakabayashi, N.; Itoh, K.; Wakabayashi, J.; Motohashi, H.; Noda, S.; Takahashi, S.; Imakado, S.; Kotsuji, T.; Otsuka, F.; Roop, D.R.; et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 2003, 35, 238–245. [Google Scholar] [CrossRef]
- Zhuang, J.C.; Lin, D.; Lin, C.; Jethwaney, D.; Wogan, G.N. Genotoxicity associated with NO production in macrophages and co-cultured target cells. Free Radic. Biol. Med. 2002, 33, 94–102. [Google Scholar] [CrossRef]
- Kim, M.Y.; Wogan, G.N. Mutagenesis of thesupFGene of pSP189 Replicating in AD293 Cells Cocultivated with Activated Macrophages: Roles of Nitric Oxide and Reactive Oxygen Species. Chem. Res. Toxicol. 2006, 19, 1483–1491. [Google Scholar] [CrossRef]
- Molina-Romero, C.; Arrieta, O.; Hernaández-Pando, R. Tuberculosis and lung cancer. Salud Publica Mex. 2019, 61, 286–291. [Google Scholar] [CrossRef]
- Ardies, C.M. Inflammation as cause for scar cancers of the lung. Integr. Cancer Ther. 2003, 2, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Brenner, A.V.; Zuoyuan, W.; Kleinerman, R.A.; Wang, L.; Zhang, S.; Metayer, C.; Chen, K.; Lei, S.; Cui, H.; Lubin, J.H. Previous pulmonary diseases and risk of lung cancer in Gansu Province, China. Int. J. Epidemiol. 2001, 30, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Shiels, M.S.; Albanes, D.; Virtamo, J.; Engels, E.A. Increased risk of lung cancer in men with tuberculosis in the alpha-tocopherol, beta-carotene cancer prevention study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 672–678. [Google Scholar] [CrossRef]
- Brenner, D.R.; Boffetta, P.; Duell, E.J.; Bickeböller, H.; Rosenberger, A.; McCormack, V.; Muscat, J.E.; Yang, P.; Wichmann, H.-E.; Brueske-Hohlfeld, I.; et al. Previous Lung Diseases and Lung Cancer Risk: A Pooled Analysis From the International Lung Cancer Consortium. Am. J. Epidemiol. 2012, 176, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Everatt, R.; Kuzmickiene, I.; Davidaviciene, E.; Cicenas, S. Incidence of lung cancer among patients with tuberculosis: A nationwide cohort study in Lithuania. Int. J. Tuberc Lung Dis. 2016, 20, 757–763. [Google Scholar] [CrossRef]
- Denholm, R.; Schüz, J.; Straif, K.; Stücker, I.; Jockel, K.-H.; Brenner, D.R.; De Matteis, S.; Boffetta, P.; Guida, F.; Brüske, I.; et al. Is Previous Respiratory Disease a Risk Factor for Lung Cancer? Am. J. Respir. Crit. Care Med. 2014, 190, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.C. Does tuberculosis increase the risk of lung cancer? Int. J. Tuberc Lung Dis. 2016, 20, 712. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Pinsky, P.F.; Caporaso, N.E.; Chatterjee, N.; Baumgarten, M.; Langenberg, P.; Furuno, J.P.; Lan, Q.; Engels, E.A. Lung, cancer risk following detection of pulmonary scarring by chest radiography in the prostate, lung, colorectal, and ovarian cancer screening trial. Arch. Intern. Med. 2008, 168, 2326–2332. [Google Scholar] [CrossRef]
- Dacosta, N.A.; Kinare, S.G. Association of lung carcinoma and tuberculosis. J. Postgrad. Med. 1991, 37, 185–189. [Google Scholar] [PubMed]
- Nalbandian, A.; Yan, B.S.; Pichugin, A.; Bronson, R.T.; Kramnik, I. Lung carcinogenesis induced by chronic tuberculosis infection: The experimental model and genetic control. Oncogene 2009, 28, 1928–1938. [Google Scholar] [CrossRef]
- Cao, S.; Li, J.; Lu, J.; Zhong, R.; Zhong, H. Mycobacterium tuberculosis antigens repress Th1 immune response suppression and promote lung cancer metastasis through PD-1/PDl-1 signaling pathway. Cell Death Dis. 2019, 10, 44. [Google Scholar] [CrossRef]
- Holla, S.; Ghorpade, D.S.; Singh, V.; Bansal, K.; Balaji, K.N. Mycobacterium bovis BCG promotes tumor cell survival from tumor necrosis factor-α-induced apoptosis. Mol. Cancer 2014, 13, 210. [Google Scholar] [CrossRef]
- Ratoosh, S.L.; Cohen, P.R.; Troncoso, P. Cutaneous-malignancy and leprosy. Report of a patient with Mycobacterium leprae and basal cell carcinoma concurrently present in the same lesion. J. Derm. Surg. Oncol. 1994, 20, 613–618. [Google Scholar] [CrossRef]
- Cope, R.B.; Stang, B.; Valentine, B.A.; Bermudez, L.E. Topical exposure to exogenous ultraviolet-irradiated urocanic acid enhances Mycobacterium ulcerans infection in a Crl:IAF(HA)-hrBR hairless guinea-pig model of Buruli ulcer disease. Photodermatol. Photoimmunol. Photomed. 2004, 20, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Richardus, J.H.; Smith, T.C. Squamous cell carcinoma in chronic ulcers in leprosy: A review of 38 consecutive cases. Lepr. Rev. 1991, 62, 381–388. [Google Scholar] [CrossRef]
- Asenjo, M.M.; Martín Guerra, J.M.; López Pedreira, M.R.; Prieto de Paula, J.M. Mycobacterium xenopi and squamous cell carcinoma of the lung. Arch. Bronconeumol. 2017, 53, 698–700. [Google Scholar] [CrossRef]
- Pierce, E.S. Could Mycobacterium avium subspecies paratuberculosis cause Crohn’s disease, ulcerative colitis…and colorectal cancer? Infect. Agents Cancer 2018, 13, 1. [Google Scholar] [CrossRef]
- Alsaif, H.S.; Hassan, A.; Refai, O.; Awary, K.; Kussaibi, H.; Ismail, M.H.; Alghnimi, I. Concomitant hepatic tuberculosis and hepatocellular carcinoma: A case report and review of the literature. BMC Surg. 2021, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- El-Mahallawy, H.A.; Eissa, S.A.; Refeh, N.G.; Salem, A.E.S.; Eissa, S.A.; Allian, S.A. Tuberculosis in cancer patients: Role of newer techniques in relation to conventional diagnostic methods. J. Adv. Res. 2010, 1, 157–162. [Google Scholar] [CrossRef]
- Laake, A.M.; Liappis, A.P.; Guy, E.; Kerr, G.; Benator, D.A. Tuberculosis reactivation in hepatocellular carcinoma: Association with transarterial chemoembolization. Infect. Dis. 2015, 47, 267–270. [Google Scholar] [CrossRef]
- He, W.; Zhang, X.; Li, W.; Kong, C.; Wang, Y.; Zhu, L.; Xu, R.; Deng, G.; Zhang, R. Activated pulmonary tuberculosis in a patient with melanoma during PD-1 inhibition: A case report. Onco. Targets Ther. 2018, 11, 7423–7427. [Google Scholar] [CrossRef] [PubMed]
- Suliman, A.M.; Bek, S.A.; Elkhatim, M.S.; Husain, A.A.; Mismar, A.Y.; Sharaf, E.M.Z.; Lengyel, Z.; Elazzazy, S.; Rasul, K.I.; Omar, N.E. Tuberculosis following programmed cell death receptor-1 (PD-1) inhibitor in a patient with non-small cell lung cancer. Case report and literature review. Cancer Immunol. Immunother. 2020, 70, 935–944. [Google Scholar] [CrossRef]
- Lim, Y.P.; Lin, C.L.; Hung, D.Z.; Lin, Y.N.; Kao, C.H. Anti-tuberculosis treatments and risk of hepatocellular carcinoma in tuberculosis patients with liver cirrhosis: A population-based case–control study. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Anguille, S.; Smits, E.L.; Lion, E.; van Tendeloo, V.F.; Berneman, Z.N. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014, 15, 257–267. [Google Scholar] [CrossRef]
- Bickels, J.; Kollender, Y.; Merinsky, O.; Meller, I. Coley’s toxin: Historical perspective. Isr. Med. Assoc. J. 2002, 4, 471–472. [Google Scholar]
- McCarthy, E.F. The toxin of William, B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar]
- Kawai, K.; Miyazaki, J.; Joraku, A.; Nishiyama, H.; Akaza, H. Bacillus Calmette–Guerin (BCG) immunotherapy for bladder cancer: Current understanding and perspectives on engineered BCG vaccine. Cancer Sci. 2013, 104, 22–27. [Google Scholar] [CrossRef]
- Delogu, G.; Fadda, G. The quest for a new vaccine against tuberculosis. J. Infect. Dev. Ctries. 2009, 3, 5–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McShane, H. Vaccine strategies against tuberculosis. Swiss Med. Wkly. 2009, 139, 156–160. [Google Scholar]
- Salyers, A.A.; Whitt, D.D. Gruźlica: Powrót danego wroga. In Mikrobiologia. Różnorodność, Chorobotwórczość i Środowisko; Markiewicz, Z., Ed.; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2003; pp. 336–340. [Google Scholar]
- Blok, B.A.; Arts, R.J.; van Crevel, R.; Benn, C.S.; Netea, M.G. Trained innate immunity as underlying mechanism for the long-term, nonspecific effects of vaccines. J. Leukocyt. Biol. 2015, 98, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Garly, M.L.; Martins, C.L.; Balé, C.; Baldé, M.A.; Hedegaard, K.L.; Gustafson, P.; Lisse, I.M.; Whittle, H.C.; Aaby, P. BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non-specific beneficial effect of BCG? Vaccine 2003, 21, 2782–2790. [Google Scholar] [CrossRef]
- Roth, A.; Gustafson, P.; Nhaga, A.; Djana, Q.; Poulsen, A.; Garly, M.L.; Jensen, H.; Sodemann, M.; Rodriques, A.; Aaby, P. BCG vaccination scar associated with better childhood survival in Guinea-Bissau. Int. J. Epidemiol. 2005, 34, 540–547. [Google Scholar] [CrossRef]
- Aaby, P.; Roth, A.; Ravn, H.; Napirna, B.M.; Rodrigues, A.; Lisse, I.M.; Stensballe, L.; Diness, B.R.; Lausch, K.R.; Lund, N. Randomized trial of BCG vaccination at birth to low-birth-weight children: Beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 2011, 204, 245–252. [Google Scholar] [CrossRef]
- Biering-Sørensen, S.; Aaby, P.; Napirna, B.M.; Roth, A.; Ravn, H.; Rodrigues, A.; Whittle, H.; Benn, C.S. Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guérin vaccination at first health center contact. Pediatr. Infect. Dis. J. 2012, 31, 306–308. [Google Scholar] [CrossRef]
- van ’t Wout, J.W.; Poell, R.; van Furth, R. The role of BCG/PPD-activated macrophages in resistance against systemic candidiasis in mice. Scand. J. Immunol. 1992, 36, 713–719. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: Program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.; Jacobs, C.; Van Loenhout, J.; Xavier, R.J.; Aaby, P.; Van Der Meer, J.W.; et al. Long-Lasting Effects of BCG Vaccination on Both Heterologous Th1/Th17 Responses and Innate Trained Immunity. J. Innate Immun. 2014, 6, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Kiselyov, A.; Bunimovich-Mendrazitsky, S.; Startsev, V. Treatment of non-muscle invasive bladder cancer with Bacillus Calmette-Guerin (BCG): Biological markers and simulation studies. BBA Clin. 2015, 4, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Pearl, R. Cancer and tuberculosis. Am. J. Epidemiol. 1929, 9, 97–159. [Google Scholar] [CrossRef]
- Kamat, A.M.; Lamm, D.L. Immunotherapy for bladder cancer. Curr. Urol. Rep. 2001, 2, 62–69. [Google Scholar] [CrossRef]
- Lamm, D.L. Bacillus Calmette-Guerin immunotherapy for bladder cancer. J. Urol. 1985, 134, 40–46. [Google Scholar] [CrossRef]
- Ayati, M.; Nowroozi, M.R.; Mortazavi, A.; Ohadian Moghadam, S.; Ghorani, H. Management of hepatic granulomatous tuberculosis after BCG therapy for bladder cancer. Urol. Case Rep. 2017, 13, 158–159. [Google Scholar] [CrossRef]
- Fujita, K.; Yamamoto, Y.; Kanai, O.; Okamura, M.; Nakatani, K.; Mio, T. Development of Mycobacterium avium complex lung disease in patients with lung cancer on immune checkpoint inhibitors. Open Forum Infect. Dis. 2020, 7, ofaa067. [Google Scholar] [CrossRef]
- Bereta, M.; Hayhurst, A.; Gajda, M.; Chorobik, P.; Targosz, M.; Marcinkiewicz, J.; Kaufman, H.L. Improving tumor targeting and therapeutic potential of Salmonella VNP20009 by displaying cell surface CEA-specific antibodies. Vaccine 2007, 22, 4183. [Google Scholar] [CrossRef] [PubMed]
- Pettenati, C.; Ingresoll, M.A. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat. Rev. Urol. 2018, 15, 615–625. [Google Scholar] [CrossRef]
- Guallar-Garrido, S.; Julián, E. Bacillus Calmette-Guérin (BCG) therapy for bladder cancer: An update. ImmunoTargets Ther. 2020, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Unda-Urzaiz, M.; Cozar-Olmos, J.M.; Miñana-Lopez, B.; Camarero-Jimenez, J.; Brugarolas-Rossello, X.; Zubiaur-Libano, C.; Ribal-Caparros, M.J.; Suarez-Charneco, A.J.; Rodriguez-Tesedo, V.; Chantada-Abal, V.; et al. Safety and efficacy of various strains of bacille Calmette-Guérin in the treatment of bladder tumours in standard clinical practice. Actas Urol. Esp. 2018, 42, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, W.; Matuszewski, M.; Poletajew, S.; Grzegrzółka, J.; Zdrojowy, R.; Kołodziej, A. Are there differences in toxicity and efficacy between various bacillus Calmette-Guerin strains in bladder cancer patients? Analysis of 844 patients. Urol. Int. 2018, 101, 277–284. [Google Scholar] [CrossRef]
- D’Andrea, D.; Soria, F.; Abufaraj, M.; Pones, M.; Gontero, P.; Machado, A.T.; Waksman, R.; Enikeev, D.; Glybochko, P.V.; Adonias, S.P.; et al. Comparative Effectiveness of Intravesical BCG-Tice and BCG-Moreau in Patients With Non–muscle-invasive Bladder Cancer. Clin. Genitourin. Cancer 2020, 18, 20–25.e2. [Google Scholar] [CrossRef]
- Rentsch, C.A.; Birkhäuser, F.D.; Biot, C.; Gsponer, J.R.; Bisiaux, A.; Wetterauer, C.; Lagranderie, M.; Marchal, G.; Orgeur, M.; Bouchier, C.; et al. Bacillus Calmette-Guérin Strain Differences Have an Impact on Clinical Outcome in Bladder Cancer Immunotherapy. Eur. Urol. 2014, 66, 677–688. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fol, M.; Koziński, P.; Kulesza, J.; Białecki, P.; Druszczyńska, M. Dual Nature of Relationship between Mycobacteria and Cancer. Int. J. Mol. Sci. 2021, 22, 8332. https://doi.org/10.3390/ijms22158332
Fol M, Koziński P, Kulesza J, Białecki P, Druszczyńska M. Dual Nature of Relationship between Mycobacteria and Cancer. International Journal of Molecular Sciences. 2021; 22(15):8332. https://doi.org/10.3390/ijms22158332
Chicago/Turabian StyleFol, Marek, Piotr Koziński, Jakub Kulesza, Piotr Białecki, and Magdalena Druszczyńska. 2021. "Dual Nature of Relationship between Mycobacteria and Cancer" International Journal of Molecular Sciences 22, no. 15: 8332. https://doi.org/10.3390/ijms22158332
APA StyleFol, M., Koziński, P., Kulesza, J., Białecki, P., & Druszczyńska, M. (2021). Dual Nature of Relationship between Mycobacteria and Cancer. International Journal of Molecular Sciences, 22(15), 8332. https://doi.org/10.3390/ijms22158332





