Protective Effect of Ciclopirox against Ovariectomy-Induced Bone Loss in Mice by Suppressing Osteoclast Formation and Function
Abstract
:1. Introduction
2. Results
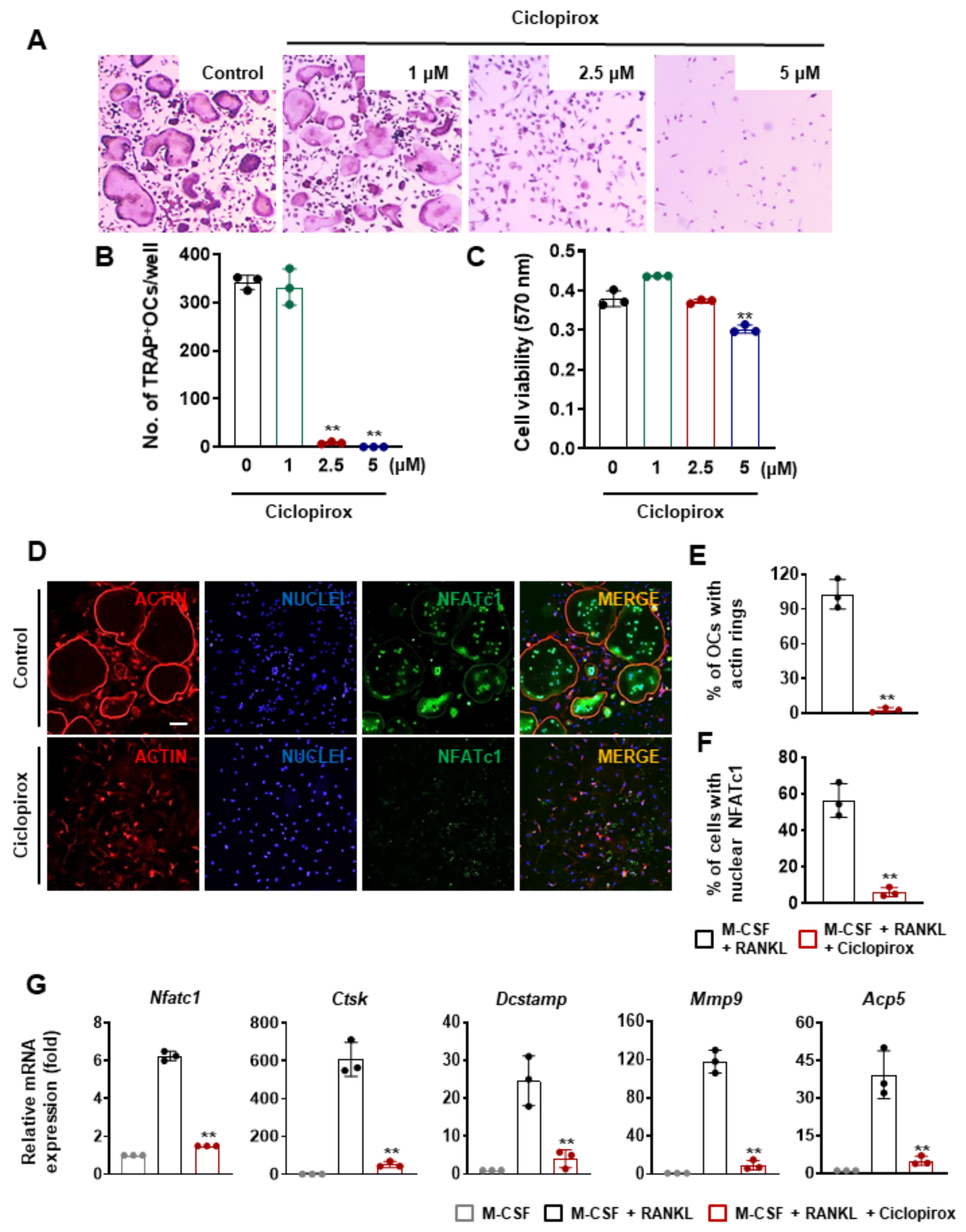
2.1. Effect of Ciclopirox on RANKL-Induced Osteoclast Differentiation
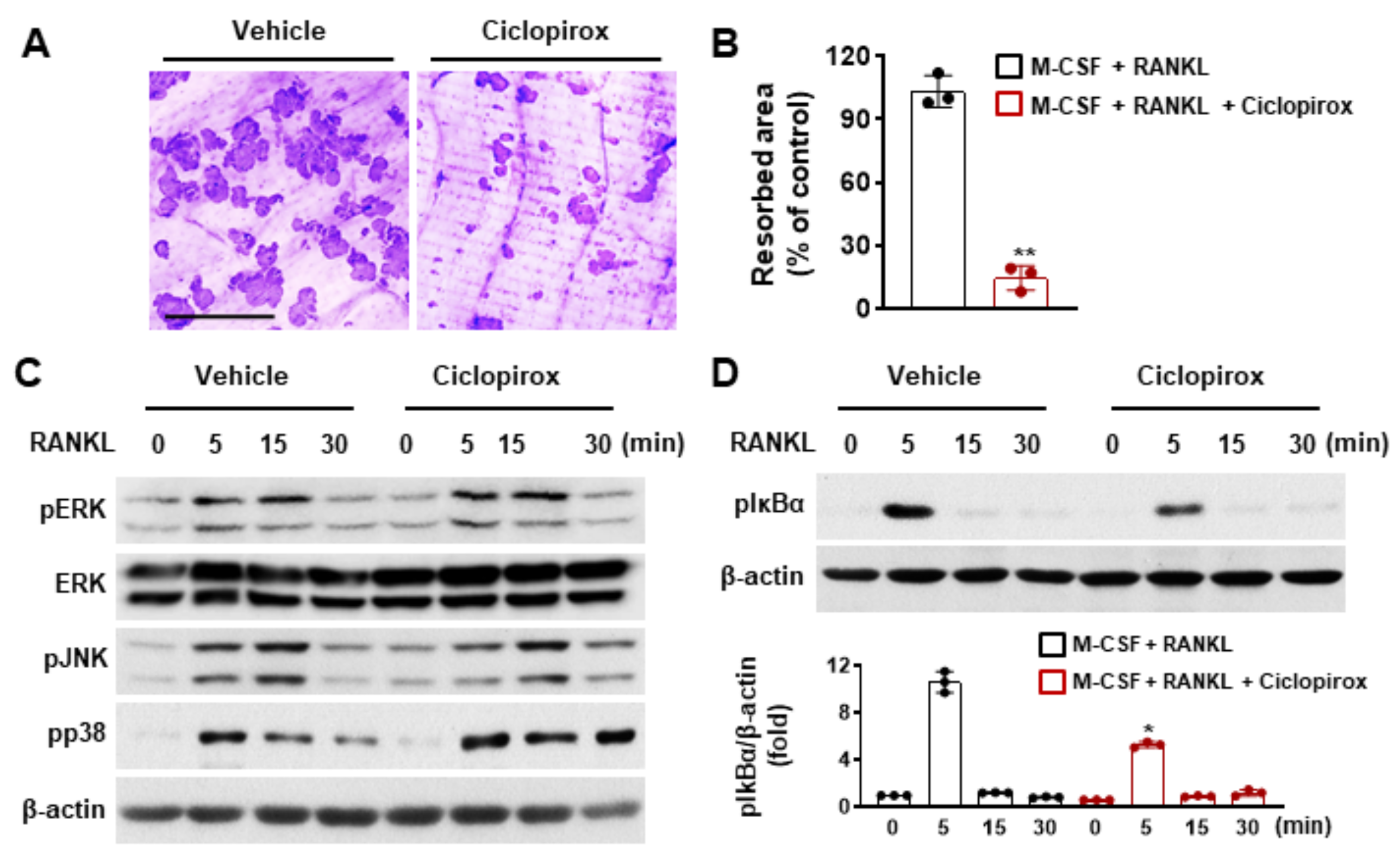
2.2. Effect of Ciclopirox on Osteoclast Function
2.3. Effect of Ciclopirox on RANKL-Mediated Signaling Pathways
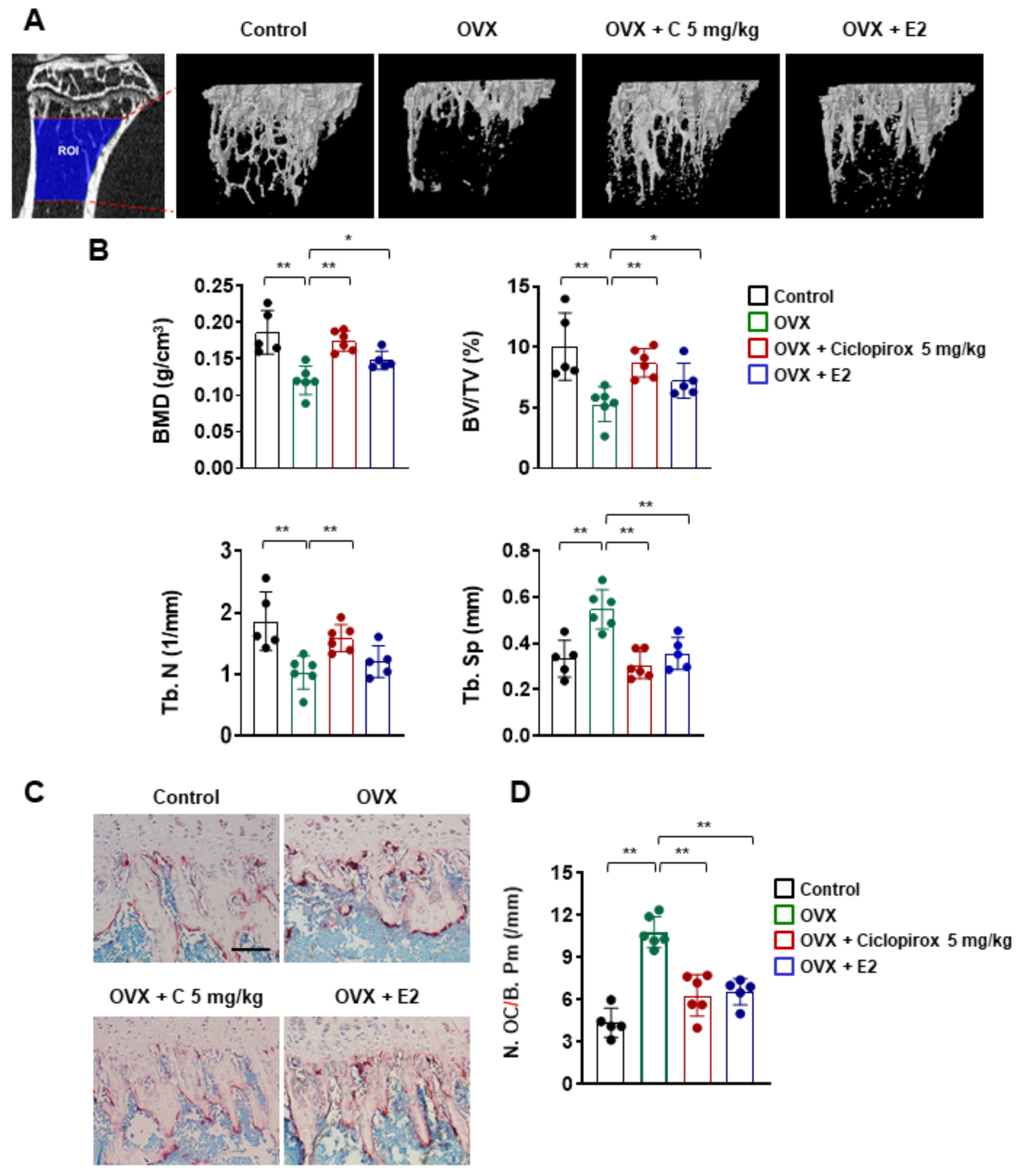
2.4. Effect of Ciclopirox on OVX-Induced Bone Loss
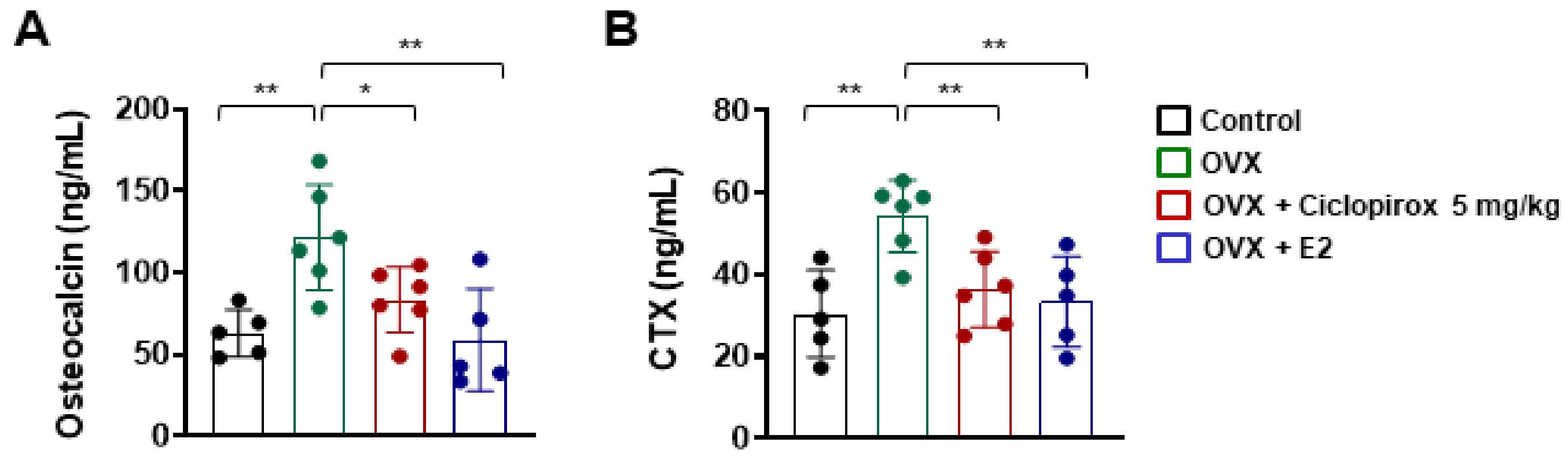
2.5. Effect of Ciclopirox on Serum Levels of Bone Turnover Markers
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Animals
4.3. Osteoclast Differentiation
4.4. Cell Viability Assay
4.5. Quantitative Real-Time PCR
4.6. Immunoblotting
4.7. Bone Resorption Assay
4.8. Immunofluorescence Staining
4.9. Ovariectomy Animal Model
4.10. Micro-CT and Histomorphometric Analysis
4.11. Measurements of Bone Turnover Markers
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, X.; Teitelbaum, S.L. Osteoclasts: New Insights. Bone Res. 2013, 1, 11–26. [Google Scholar]
- Purdue, P.E.; Koulouvaris, P.; Potter, H.G.; Nestor, B.J.; Sculco, T.P. The Cellular and Molecular Biology of Periprosthetic Osteolysis. Clin. Orthop. Relat. Res. 2007, 454, 251–261. [Google Scholar] [CrossRef]
- Autio, K.A.; Farooki, A.; Glezerman, I.; Chan, A.; Schneider, C.W.; Barr, H.C.; Seyboth, B.M.; Kampel, L.J.; Danila, D.C.; Rathkopf, D.; et al. Severe Hypocalcemia Associated With Denosumab in Metastatic Castration-Resistant Prostate Cancer: Risk Factors and Precautions for Treating Physicians. Clin. Genitourin. Cancer 2015, 13, e305–e309. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.P.; Yeo, I.; So, S.-Y.; Kim, K.; Moon, Y.-W.; Park, Y.-S.; Lim, S.-J. Atypical Femoral Shaft Fractures in Female Bisphosphonate Users Were Associated with an Increased Anterolateral Femoral Bow and a Thicker Lateral Cortex: A Case-Control Study. BioMed Res. Int. 2017, 2017, 5932496. [Google Scholar] [CrossRef]
- Darnay, B.G.; Haridas, V.; Ni, J.; Moore, P.A.; Aggarwal, B.B. Characterization of the intracellular domain of receptor activator of NF-kappaB (RANK). Interaction with tumor necrosis factor receptor-associated factors and activation of NF-kappab and c-Jun N-terminal kinase. J. Biol. Chem. 1998, 273, 20551–20555. [Google Scholar] [CrossRef] [Green Version]
- Wong, B.R.; Josien, R.; Lee, S.Y.; Vologodskaia, M.; Steinman, R.M.; Choi, Y. The TRAF family of signal transducers mediates NF-kappaB activation by the TRANCE receptor. J. Biol. Chem. 1998, 273, 28355–28359. [Google Scholar] [CrossRef] [Green Version]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.-I.; et al. Induction and Activation of the Transcription Factor NFATc1 (NFAT2) Integrate RANKL Signaling in Terminal Differentiation of Osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef]
- Shen, T.; Huang, S. Repositioning the Old Fungicide Ciclopirox for New Medical Uses. Curr. Pharm. Des. 2016, 22, 4443–4450. [Google Scholar] [CrossRef]
- Shen, T.; Shang, C.; Zhou, H.; Luo, Y.; Barzegar, M.; Odaka, Y.; Wu, Y.; Huang, S. Ciclopirox inhibits cancer cell proliferation by suppression of Cdc25A. Genes Cancer 2017, 8, 505–516. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.K.; Plott, T. Ciclopirox: A broad-spectrum antifungal with antibacterial and anti-inflammatory properties. Int. J. Dermatol. 2004, 43, 3–8. [Google Scholar] [CrossRef]
- Kokjohn, K.; Bradley, M.; Griffiths, B.; Ghannoum, M. Evaluation ofin vitroactivity of ciclopirox olamine, butenafine HCl and econazole nitrate against dermatophytes, yeasts and bacteria. Int. J. Dermatol. 2003, 42, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, Y.; McDermott, S.P.; Wang, X.; Gronda, M.; Venugopal, A.; Wood, T.E.; Hurren, R.; Datti, A.; Batey, R.; Wrana, J.; et al. Chelation of intracellular iron with the antifungal agent ciclopirox olamine induces cell death in leukemia and myeloma cells. Blood 2009, 114, 3064–3073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Shen, T.; Luo, Y.; Liu, L.; Chen, W.; Xu, B.; Han, X.; Pang, J.; Rivera, C.A.; Huang, S. The antitumor activity of the fungicide ciclopirox. Int. J. Cancer 2010, 127, 2467–2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, S.H.; Nauta, A.; Morrison, S.D.; Zhou, H.; Zimmermann, A.; Gurtner, G.C.; Ding, S.; Longaker, M.T. Antimycotic Ciclopirox Olamine in the Diabetic Environment Promotes Angiogenesis and Enhances Wound Healing. PLoS ONE 2011, 6, e27844. [Google Scholar] [CrossRef] [Green Version]
- Lau, W.Y.; Li, A.K.C.; Chu, K.W.; Yuen, W.K.; Poon, G.P.; Hwang, J.S.T. Operative choledochoscopy in patients with acute cholangitis: A prospective, randomized study. J. Br. Surg. 1991, 78, 1226–1229. [Google Scholar] [CrossRef]
- Tan, T.; Marín-García, J.; Damle, S.; Weiss, H.R. Hypoxia-inducible factor-1 improves inotropic responses of cardiac myocytes in ageing heart without affecting mitochondrial activity. Exp. Physiol. 2010, 95, 712–722. [Google Scholar] [CrossRef]
- Weir, S.J.; Patton, L.; Castle, K.; Rajewski, L.; Kasper, J.; Schimmer, A.D. The repositioning of the anti-fungal agent ciclopirox olamine as a novel therapeutic agent for the treatment of haematologic malignancy. J. Clin. Pharm. Ther. 2010, 36, 128–134. [Google Scholar] [CrossRef]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Cooper, C.; Rizzoli, R.; Reginster, J.Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2013, 24, 23–57. [Google Scholar] [CrossRef] [Green Version]
- Voss, P.J.; Steybe, D.; Poxleitner, P.; Schmelzeisen, R.; Münzenmayer, C.; Fuellgraf, H.; Stricker, A.; Semper-Hogg, W. Osteonecrosis of the jaw in patients transitioning from bisphosphonates to denosumab treatment for osteoporosis. Odontology 2018, 106, 469–480. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, H.S.; Yeon, J.-T.; Choi, S.-W.; Chun, C.H.; Kwak, H.B.; Oh, J. GM-CSF Regulates Fusion of Mononuclear Osteoclasts into Bone-Resorbing Osteoclasts by Activating the Ras/ERK Pathway. J. Immunol. 2009, 183, 3390–3399. [Google Scholar] [CrossRef] [Green Version]
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K.; et al. DC-STAMP is essential for cell–cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005, 202, 345–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Brooks, P.J.; Jang, J.J.; Silver, A.S.; Arora, P.D.; McCulloch, C.A.; Glogauer, M. Role of actin filaments in fusopod formation and osteoclastogenesis. Biochim. Biophys. Acta (BBA) Bioenerg. 2015, 1853, 1715–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltel, F.; Destaing, O.; Bard, F.; Eichert, D.; Jurdic, P. Apatite-mediated Actin Dynamics in Resorbing Osteoclasts. Mol. Biol. Cell 2004, 15, 5231–5241. [Google Scholar] [CrossRef] [PubMed]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; Wagner, E.F.; Mak, T.W.; Serfling, E.; et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 2005, 202, 1261–1269. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, Y.; Matsuo, K. Molecular mechanisms of triggering, amplifying and targeting RANK signaling in osteoclasts. World J. Orthop. 2012, 3, 167–174. [Google Scholar] [CrossRef]
- Takatsuna, H.; Asagiri, M.; Kubota, T.; Oka, K.; Osada, T.; Sugiyama, C.; Saito, H.; Aoki, K.; Ohya, K.; Takayanagi, H.; et al. Inhibition of RANKL-induced osteoclastogenesis by (-)-DHMEQ, a novel NF-kappaB inhibitor, through downregulation of NFATc1. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2005, 20, 653–662. [Google Scholar] [CrossRef]
- Iotsova, V.; Caamano, J.; Loy, J.; Yang, Y.; Lewin, A.; Bravo, R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat. Med. 1997, 3, 1285–1289. [Google Scholar] [CrossRef]
- Komori, T. Animal models for osteoporosis. Eur. J. Pharmacol. 2015, 759, 287–294. [Google Scholar] [CrossRef]
- Riggs, B.L. The mechanisms of estrogen regulation of bone resorption. J. Clin. Investig. 2000, 106, 1203–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihn, H.J.; Kim, J.A.; Lim, S.; Nam, S.-H.; Hwang, S.H.; Lim, J.; Kim, G.-Y.; Choi, Y.H.; Jeon, Y.-J.; Lee, B.-J.; et al. Fermented Oyster Extract Prevents Ovariectomy-Induced Bone Loss and Suppresses Osteoclastogenesis. Nutrients 2019, 11, 1392. [Google Scholar] [CrossRef] [Green Version]
- Jilka, R.L.; Hangoc, G.; Girasole, G.; Passeri, G.; Williams, D.C.; Abrams, J.S.; Boyce, B.; Broxmeyer, H.; Manolagas, S.C. Increased osteoclast development after estrogen loss: Mediation by interleukin-6. Science 1992, 257, 88–91. [Google Scholar] [CrossRef]
- Kim, T.-H.; Jung, J.W.; Ha, B.G.; Hong, J.M.; Park, E.K.; Kim, H.-J.; Kim, S.-Y. The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J. Nutr. Biochem. 2011, 22, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.; Yuen, T.; Sun, L.; Rosen, C.J. Regulation of Skeletal Homeostasis. Endocr. Rev. 2018, 39, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Ihn, H.J.; Kim, K.; Cho, H.S.; Park, E.K. Pentamidine Inhibits Titanium Particle-Induced Osteolysis In Vivo and Receptor Activator of Nuclear Factor-kappaB Ligand-Mediated Osteoclast Differentiation In Vitro. Tissue Eng. Regen. Med. 2019, 16, 265–273. [Google Scholar] [CrossRef]
- Yoon, J.-Y.; Baek, C.-W.; Kim, H.-J.; Kim, E.-J.; Byeon, G.-J. Remifentanil Negatively Regulates RANKL-Induced Osteoclast Differentiation and Bone Resorption by Inhibiting c-Fos/NFATc1 Expression. Tissue Eng. Regen. Med. 2018, 15, 333–340. [Google Scholar] [CrossRef]
- Lim, S.; Kim, J.A.; Lee, T.; Lee, D.; Nam, S.-H.; Lim, J.; Park, E.K. Stimulatory Effects of KPR-A148 on Osteoblast Differentiation and Bone Regeneration. Tissue Eng. Regen. Med. 2019, 16, 405–413. [Google Scholar] [CrossRef]
- Ihn, H.J.; Lee, T.; Lee, D.; Bae, J.-S.; Kim, S.-H.; Jang, I.H.; Bae, Y.C.; Shin, H.-I.; Park, E.K. Inhibitory Effect of KP-A038 on Osteoclastogenesis and Inflammatory Bone Loss Is Associated With Downregulation of Blimp1. Front. Pharmacol. 2019, 10, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihn, H.J.; Kim, T.H.; Kim, K.; Kim, G.Y.; Jeon, Y.J.; Choi, Y.H.; Bae, J.S.; Kim, J.E.; Park, E.K. 2-O-digalloyl-1,3,4,6-tetra-O-galloyl-beta-D-glucose isolated from Galla Rhois suppresses osteoclast differentiation and function by inhibiting NF-kappaB signaling. BMB Rep. 2019, 52, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Alpermann, H.G.; Schutz, E. Studies on the pharmacology and toxicology of ciclopiroxolamine (author’s transl). Arzneimittel-Forschung 1981, 31, 1328–1332. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ihn, H.J.; Lim, J.; Kim, K.; Nam, S.-H.; Lim, S.; Lee, S.J.; Bae, J.-S.; Kim, T.H.; Kim, J.-E.; Baek, M.-C.; et al. Protective Effect of Ciclopirox against Ovariectomy-Induced Bone Loss in Mice by Suppressing Osteoclast Formation and Function. Int. J. Mol. Sci. 2021, 22, 8299. https://doi.org/10.3390/ijms22158299
Ihn HJ, Lim J, Kim K, Nam S-H, Lim S, Lee SJ, Bae J-S, Kim TH, Kim J-E, Baek M-C, et al. Protective Effect of Ciclopirox against Ovariectomy-Induced Bone Loss in Mice by Suppressing Osteoclast Formation and Function. International Journal of Molecular Sciences. 2021; 22(15):8299. https://doi.org/10.3390/ijms22158299
Chicago/Turabian StyleIhn, Hye Jung, Jiwon Lim, Kiryeong Kim, Sang-Hyeon Nam, Soomin Lim, Su Jeong Lee, Jong-Sup Bae, Tae Hoon Kim, Jung-Eun Kim, Moon-Chang Baek, and et al. 2021. "Protective Effect of Ciclopirox against Ovariectomy-Induced Bone Loss in Mice by Suppressing Osteoclast Formation and Function" International Journal of Molecular Sciences 22, no. 15: 8299. https://doi.org/10.3390/ijms22158299
APA StyleIhn, H. J., Lim, J., Kim, K., Nam, S.-H., Lim, S., Lee, S. J., Bae, J.-S., Kim, T. H., Kim, J.-E., Baek, M.-C., Bae, Y. C., & Park, E. K. (2021). Protective Effect of Ciclopirox against Ovariectomy-Induced Bone Loss in Mice by Suppressing Osteoclast Formation and Function. International Journal of Molecular Sciences, 22(15), 8299. https://doi.org/10.3390/ijms22158299







